Isolation and Chemotaxonomic Implications of Tenelloside, a Novel Unusual C-Glycosyl Flavanone from Phyllanthus tenellus Roxb. in Tenerife Island
Abstract
:1. Introduction
2. Results and Discussion
3. Methods and Materials
3.1. Plant Material
3.2. Compound Isolation
3.3. NMR and Mass Spectrometry (MS/MS and High-Resolution MS)
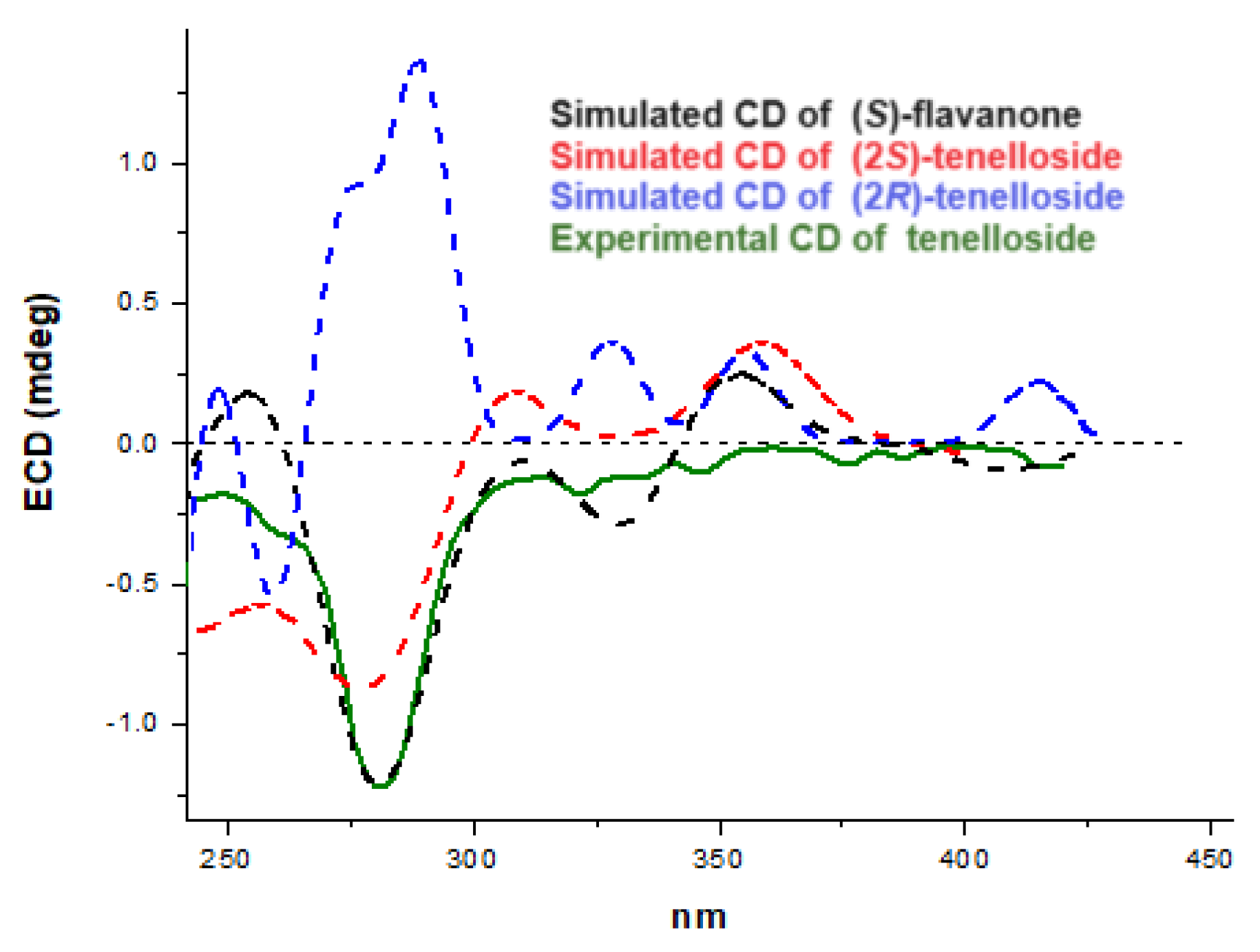
3.4. Calculated and Experimental Electronic Circular Dichroism (ECD)
- Conformational search: Molecular mechanics (MM) calculations were performed on flavanone in the (S)-configuration, as well as on the structures of 1 with both the (2S)- and (2R)-configurations. The search identified the most representative conformations within an energetic window of 3 kcal/mol. For flavanone, 8 conformations were selected; for (2S)-tellenoside, 24 conformations were selected; and for (2R)-tellenoside, 29 conformations were selected.
- Energetic stability assessment: The energetic stability of all the conformations selected in step 1 was assessed using the Hartree–Fock 3-21G level of theory as a single-point energy evaluation.
- ECD simulation: Quantum-mechanical simulations of electronic circular dichroism (ECD) were performed for the most stable conformations of flavanone and the ones of one in both the (2S)- and (2R)-configurations.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B. Ethnopharmacology and Drug Discovery. J. Ethnopharmacol. 2005, 100, 50–52. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, J.; Qin, J.; Yang, M. Screening Techniques for the Identification of Bioactive Compounds in Natural Products. J. Pharm. Biomed. Anal. 2019, 168, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Conrado, A.B.; Mosca, L.; Fontana, M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxidative Med. Cell. Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Swamy, M.K.; Sinniah, U.R. Natural Bio-Active Compounds. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Springer: Singapore, 2019; pp. 1–608. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid. Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef] [PubMed]
- Gowrishanker, B.; Vivekanandan, O.S. In Vivo Studies of a Crude Extract of Phyllanthus amarus L. in Modifying the Genotoxicity Induced in Vicia faba L. by Tannery Effluents. Mutat. Res. Toxicol. 1994, 322, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Yong, C.Y.; Faruq, U.; Ong, H.K.; Amin, Z.B.M.; Ho, W.Y.; Sharifudin, S.; Jaganath, I.B. In Vivo Toxicity and Antioxidant of Pressurize Hot Water Phyllanthus tenellus Roxb. Extracts. BMC Complement. Med. Ther. 2021, 21, 86. [Google Scholar] [CrossRef]
- Ignácio, S.R.N.; Ferreira, J.L.P.; Almeida, M.B.; Kubelka, C.F. Nitric Oxide Production by Murine Peritoneal Macrophages In Vitro and In Vivo Treated with Phyllanthus tenellus Extracts. J. Ethnopharmacol. 2001, 74, 181–187. [Google Scholar] [CrossRef]
- Hidayah, N.; Jusoh, M.; Subki, A.; Keong Yeap, S.; Yap, K.C.; Jaganath, I.B. Pressurized Hot Water Extraction of Hydrosable Tannins from Phyllanthus tenellus Roxb. BMC Chem. 2019, 13, 134. [Google Scholar] [CrossRef]
- Nikule, H.A.; Nitnaware, K.M.; Chambhare, M.R.; Kadam, N.S.; Borde, M.Y.; Nikam, T.D. In-Vitro Propagation, Callus Culture and Bioactive Lignan Production in Phyllanthus tenellus Roxb: A New Source of Phyllanthin, Hypophyllanthin and Phyltetralin. Sci. Rep. 2020, 10, 10668. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Peng, W.-H.; Cheng, H.-Y.; Chen, F.-N.; Lai, M.-T.; Chiu, T.-H. Hepatoprotective Effect of Phyllanthus in Taiwan on Acute Liver Damage Induced by Carbon Tetrachloride. Am. J. Chin. Med. 2006, 34, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ihantola-Vormisto, A.; Summanen, J.; Kankaanranta, H.; Vuorela, H.; Asmawi, Z.M.; Moilanen, E. Anti-Inflammatory Activity of Extracts from Leaves of Phyllanthus emblica. Planta Medica 1997, 63, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Franke, K.; Villani, C.; Mosca, L.; D’Erme, M.; Frischbutter, S.; Brandt, W.; Sanchez-Lamar, A.; Wessjohann, L. Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol. Molecules 2019, 24, 2855. [Google Scholar] [CrossRef] [PubMed]
- Bililign, T.; Griffith, B.R.; Thorson, J.S. Structure, Activity, Synthesis and Biosynthesis of Aryl-C-Glycosides. Nat. Prod. Rep. 2005, 22, 742–760. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Nidetzky, B. C-Ribosylating Enzymes in the (Bio)Synthesis of C-Nucleosides and C-Glycosylated Natural Products. ACS Catal. 2023, 13, 15910–15938. [Google Scholar] [CrossRef]
- Chua, L.S.; Abdullah, F.I.; Awang, M.A. Potential of Natural Bioactive C-Glycosyl Flavones for Antidiabetic Properties. Stud. Nat. Prod. Chem. 2020, 64, 241–261. [Google Scholar] [CrossRef]
- Tegl, G.; Nidetzky, B. Leloir Glycosyltransferases of Natural Product C-Glycosylation: Structure, Mechanism and Specificity. Biochem. Soc. Trans. 2020, 48, 1583–1598. [Google Scholar] [CrossRef]
- Franz, G.; Grun, M. Chemistry, Occurrence and Biosynthesis of C-Glycosyl Compounds in Plants. Planta Medica 1983, 47, 131–140. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C-Glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef]
- Khamar, H.; Benkhnigue, O.; Douira, A.; Zidane, L.; Touhami, A.O. Phyllanthus tenellus Roxb. (Phyllanthaceae), a Newly Naturalising Species in Morocco. Check List 2022, 18, 411–417. [Google Scholar] [CrossRef]
- Tureček, F.; Hanus, V. Retro-Diels-Alder Reaction in Mass Spectrometry. Mass Spectrom. Rev. 1984, 3, 85–152. [Google Scholar] [CrossRef]
- Forkmann, G.; Heller, W. Biosynthesis of Flavonoids. Compr. Nat. Prod. Chem. 1999, 1, 713–748. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Avula, B.; Lee, J.; Upton, R.; Khan, I.A. Chemical Characterization and Quantitative Determination of Flavonoids and Phenolic Acids in Yerba Santa (Eriodictyon Spp.) Using UHPLC/DAD/Q-ToF. J. Pharm. Biomed. Anal. 2023, 234, 115570. [Google Scholar] [CrossRef] [PubMed]
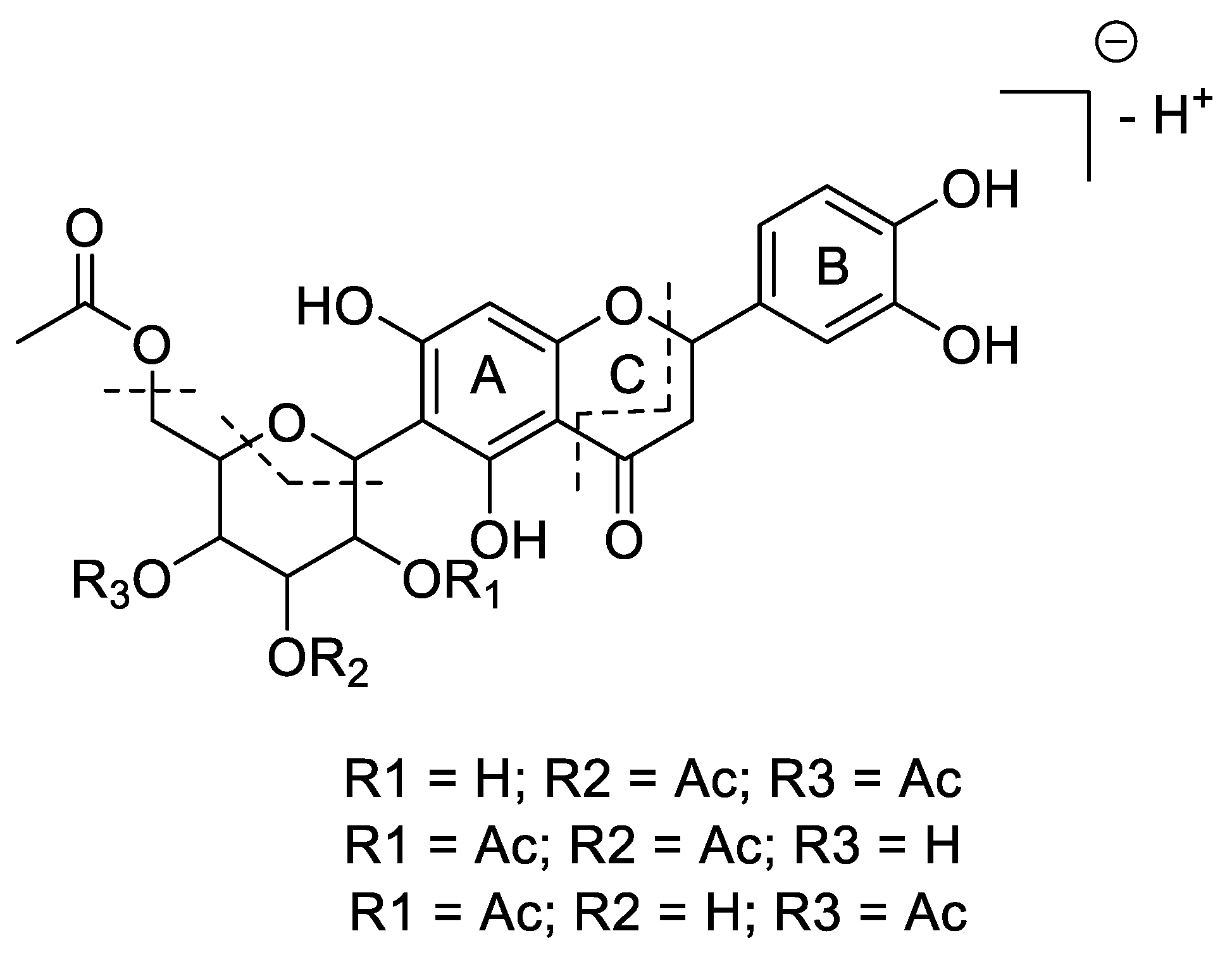
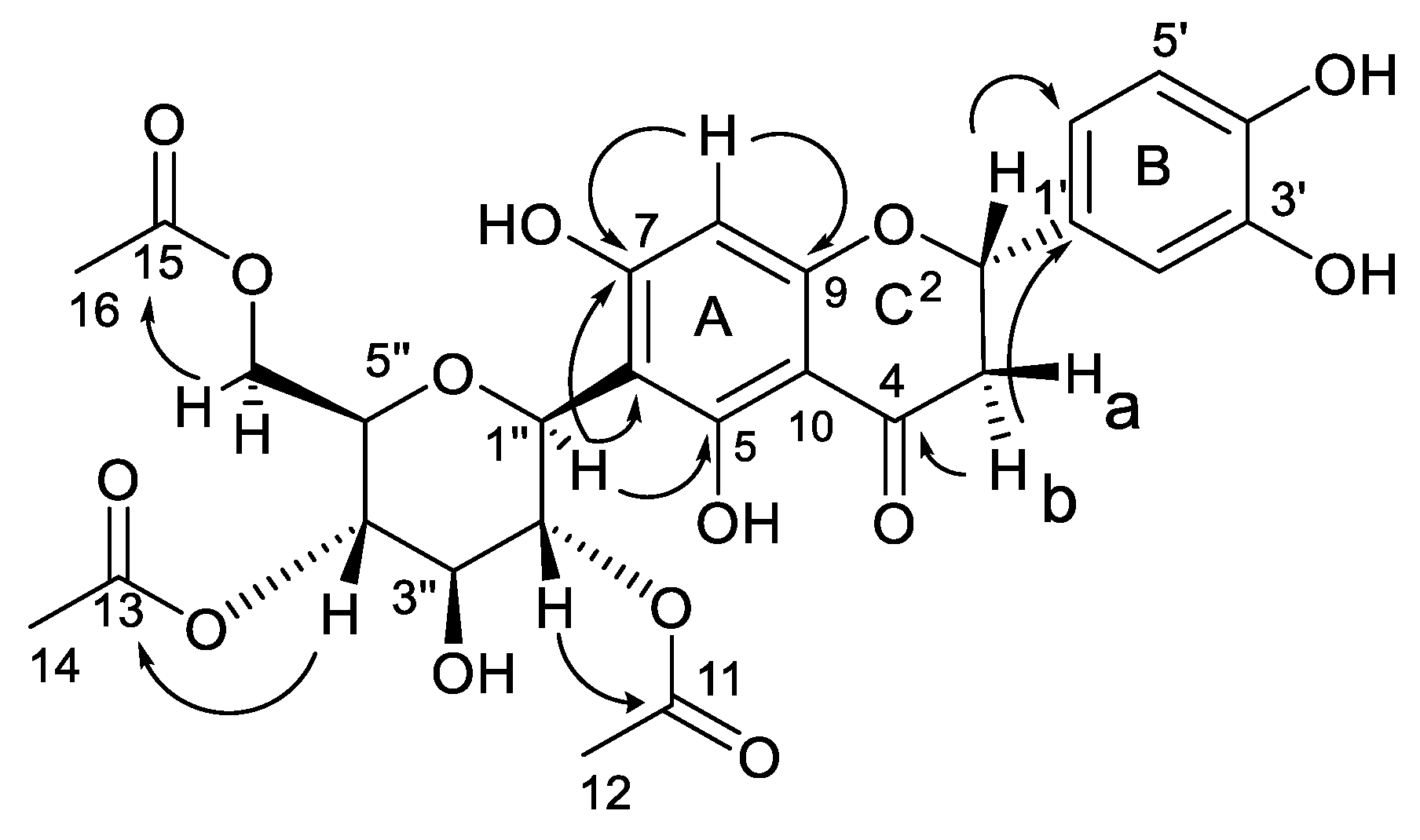
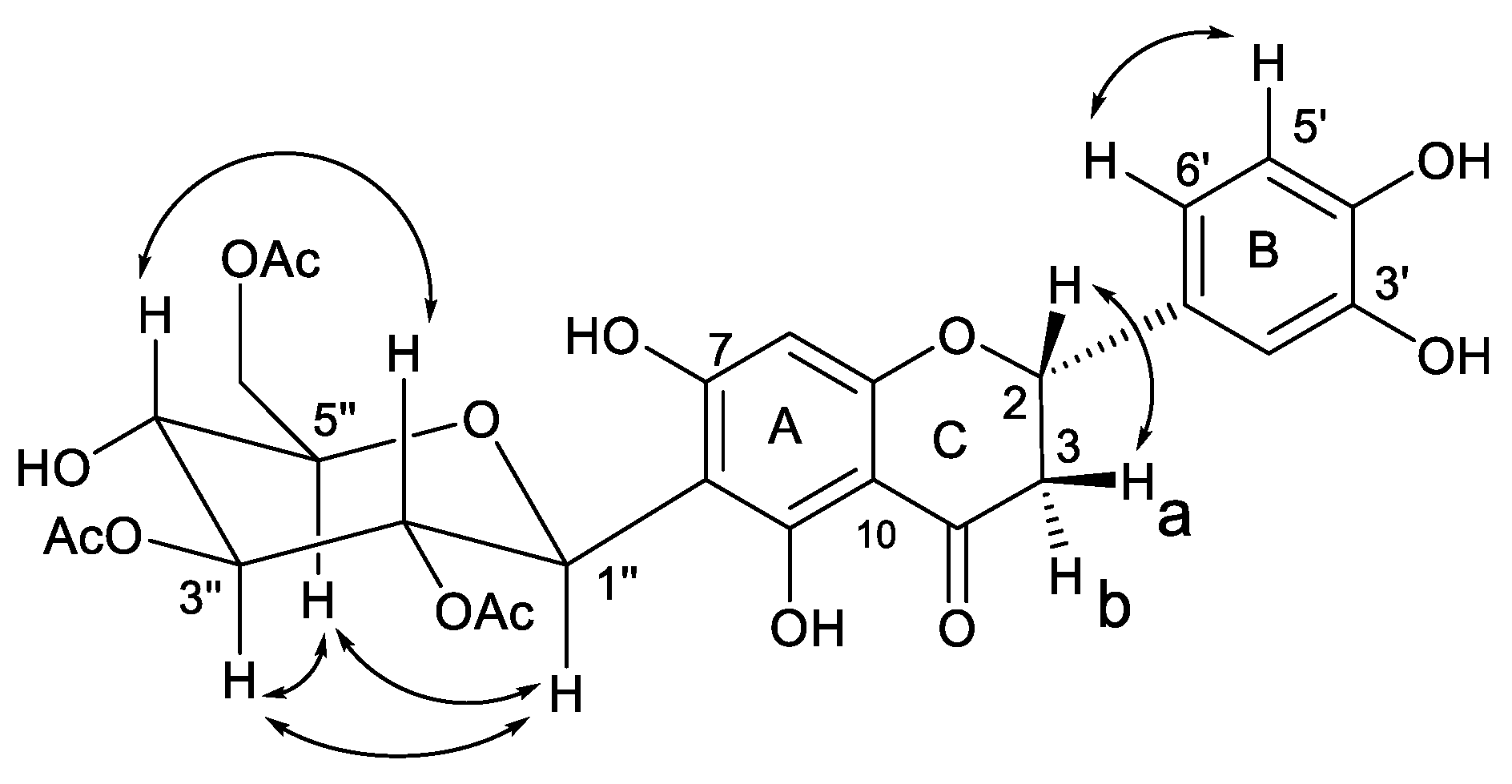
| Nr. | δC | Multiplicity | δH (J in Hz) | HMBC |
|---|---|---|---|---|
| 2 | 76.9 | CH | 5.72 (dd; 2.7, 13.7) | C4; C1′; C5′ |
| 3 | 43.3 | CH2 | Ha 3.00 (dd; 2.7, 17.2) Hb 2.78 (dd; 13.7, 17.2) | C2 Hb; C4 Hb Ha; C1′ Hb; C2′Ha |
| 4 | 199.0 | C | ||
| 5 | 165.0 | C | ||
| 6 | 103.7 | C | ||
| 7 | 166.6 | C | ||
| 8 | 96.0 | CH | 5.91 (s) | C4; C7; C9; C1″ |
| 9 | 165.2 | C | ||
| 10 | 103.6 | C | ||
| 1′ | 119.0 | C | ||
| 2′ | 103.4 | CH | 6.33 (d; 2.04) | C1′; C3′; C4′; C6′ |
| 3′ | 155.9 | C | ||
| 4′ | 159.2 | C | ||
| 5′ | 127.7 | CH | 7.57 (d; 8.44) | C2; C3′; C4′ |
| 6′ | 107.9 | CH | 6.48 (dd; 2.04, 8.4) | C1′; C2′; C4′ |
| 1″ | 73.1 | CH | 4.91 c | C5; C6; C7; C2″; C3″; C5″ |
| 2″ | 72.9 | CH | 5.69 (t; 9.6) | C6; C11; C1″; C3″ |
| 3″ | 75.2 | CH | 3.78 (t; 9.4) | C4″; C5″ |
| 4″ | 72.2 | CH | 4.98 (t; 9.6) | C13 |
| 5″ | 77.5 | CH | 3.75 (m) | |
| 6″ | 64.3 | CH2 | Ha 4.28 (dd; 5.0, 12.6) Hb 4.12 (d; 12.6) | C4″; C5″; C15 |
| 11 | 171.7 | C | ||
| 12 | 20.6 | CH3 | 1.82 (s) | |
| 13 | 171.9 | C | ||
| 14 | 20.9 | CH3 | 2.09 (s) c | |
| 15 | 172.8 | C | ||
| 16 | 21 | CH3 | 2.09 (s) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francioso, A.; Jiménez Díaz, I.A.; Reyes, C.P.; González Montelongo, C.; Pierini, M.; Villani, C.; Bazzocchi, I.L. Isolation and Chemotaxonomic Implications of Tenelloside, a Novel Unusual C-Glycosyl Flavanone from Phyllanthus tenellus Roxb. in Tenerife Island. Separations 2024, 11, 15. https://doi.org/10.3390/separations11010015
Francioso A, Jiménez Díaz IA, Reyes CP, González Montelongo C, Pierini M, Villani C, Bazzocchi IL. Isolation and Chemotaxonomic Implications of Tenelloside, a Novel Unusual C-Glycosyl Flavanone from Phyllanthus tenellus Roxb. in Tenerife Island. Separations. 2024; 11(1):15. https://doi.org/10.3390/separations11010015
Chicago/Turabian StyleFrancioso, Antonio, Ignacio Antonio Jiménez Díaz, Carolina Pérez Reyes, Cristina González Montelongo, Marco Pierini, Claudio Villani, and Isabel López Bazzocchi. 2024. "Isolation and Chemotaxonomic Implications of Tenelloside, a Novel Unusual C-Glycosyl Flavanone from Phyllanthus tenellus Roxb. in Tenerife Island" Separations 11, no. 1: 15. https://doi.org/10.3390/separations11010015
APA StyleFrancioso, A., Jiménez Díaz, I. A., Reyes, C. P., González Montelongo, C., Pierini, M., Villani, C., & Bazzocchi, I. L. (2024). Isolation and Chemotaxonomic Implications of Tenelloside, a Novel Unusual C-Glycosyl Flavanone from Phyllanthus tenellus Roxb. in Tenerife Island. Separations, 11(1), 15. https://doi.org/10.3390/separations11010015










