Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
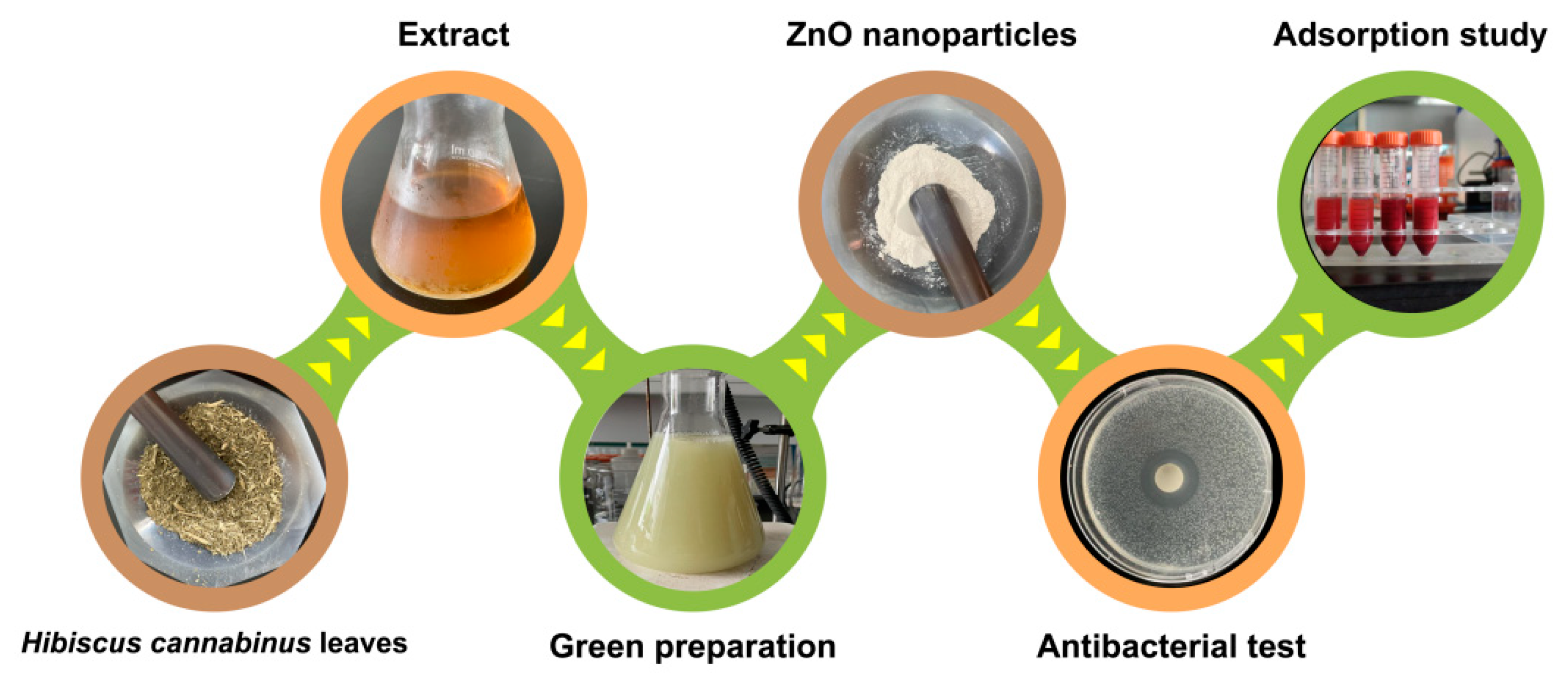
2.2. Green Preparation of ZnO Nanoparticles
2.3. Characterizations
2.4. Adsorption of Dyes
2.5. Antioxidant Activity
2.6. Antibacterial Activity
3. Results and Discussion
3.1. Characterizations of ZnO Nanoparticles
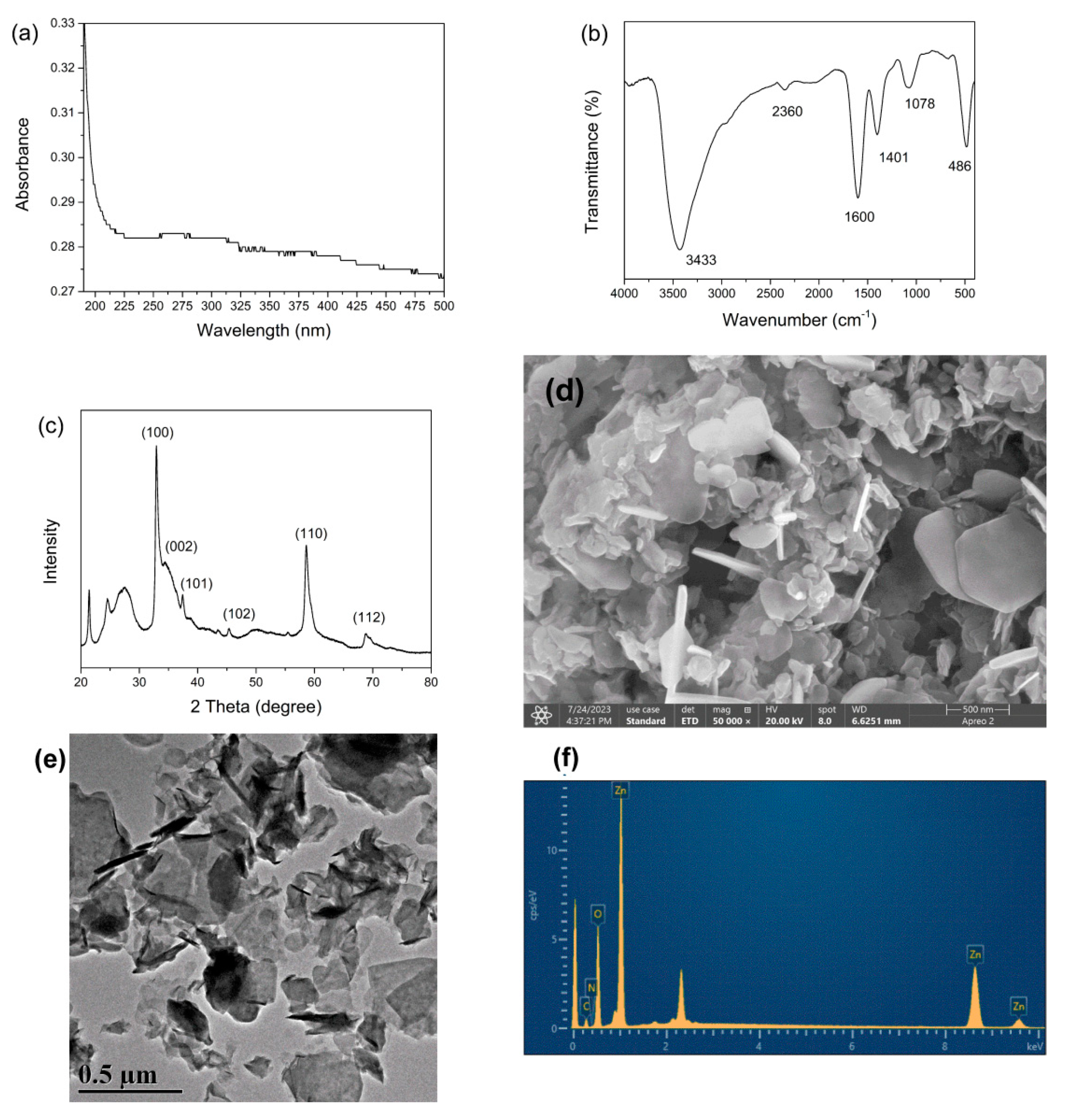
3.1.1. UV–Vis Spectroscopic Analysis
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
3.1.4. Morphology Analysis
3.1.5. Elemental Composition Analysis
3.2. GC–MS Analysis of Hibiscus cannabinus Leaf Extracts
3.3. Impact of Different Parameters on the Adsorption of the CR Dye
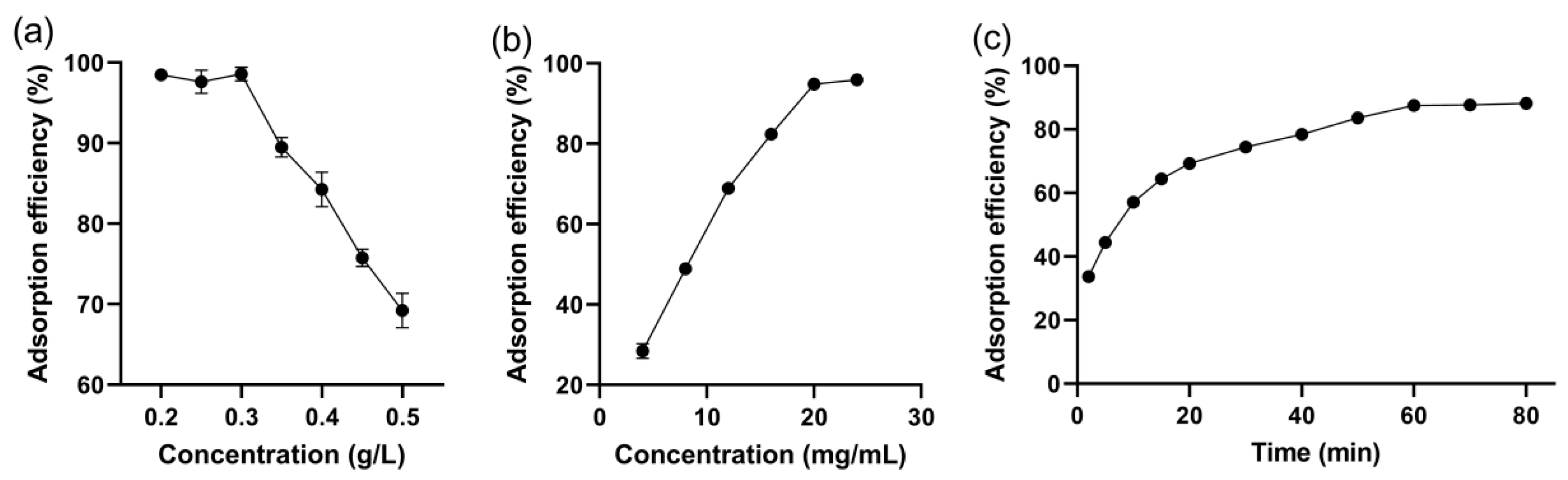
3.3.1. Effect of the Initial Dye Concentration
3.3.2. Effect of Adsorbent Dosage
3.3.3. Effect of Contact Time
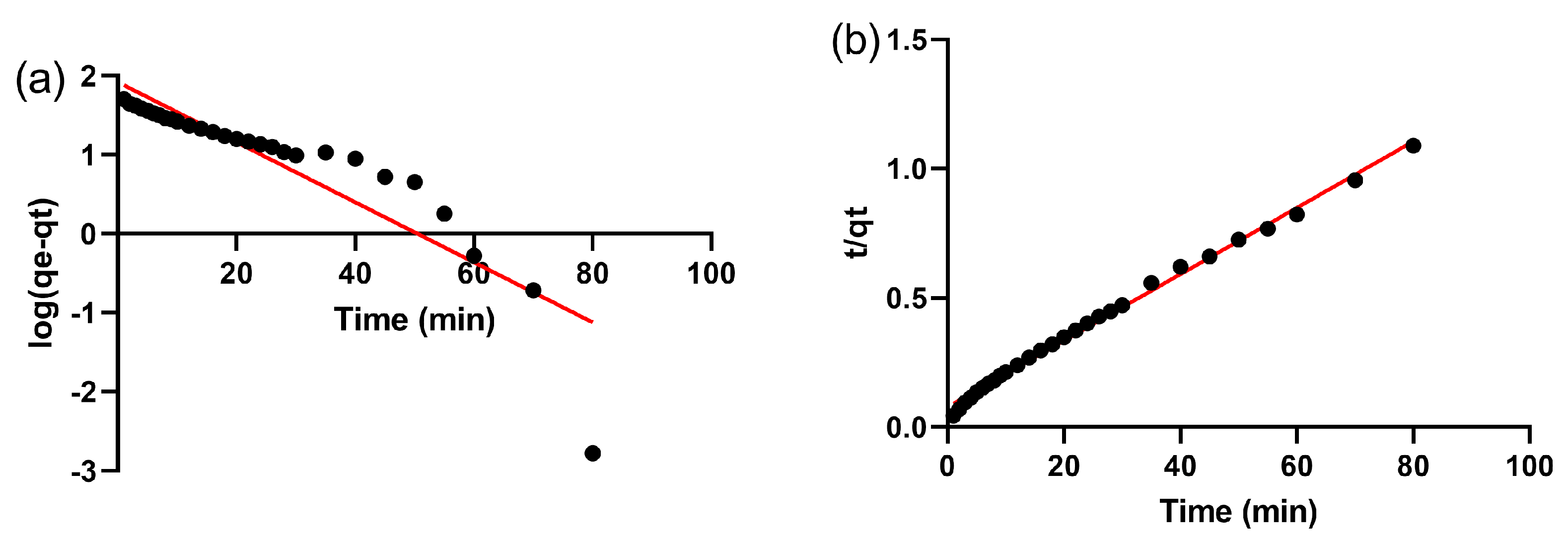
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
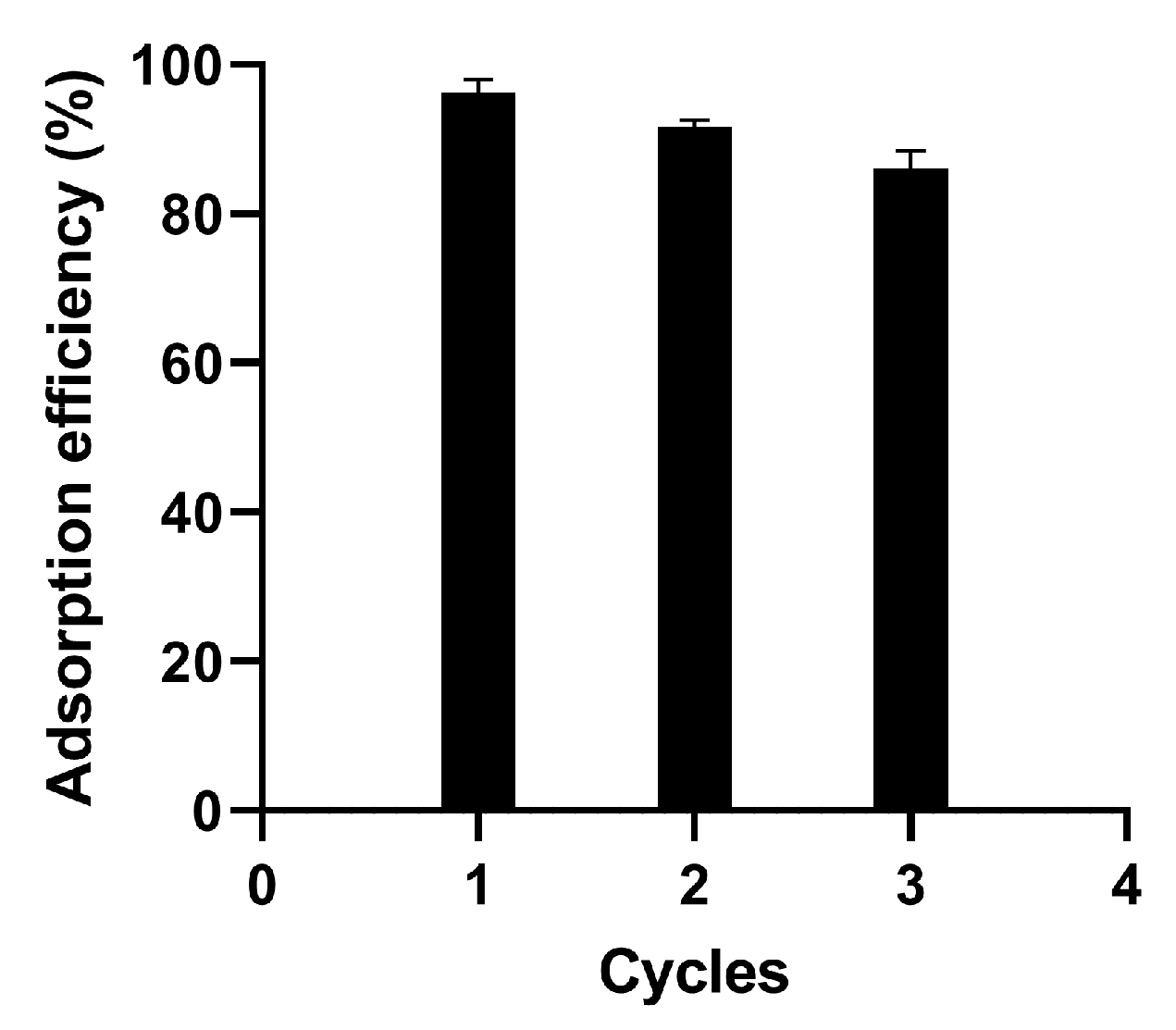
3.6. Reusability Study
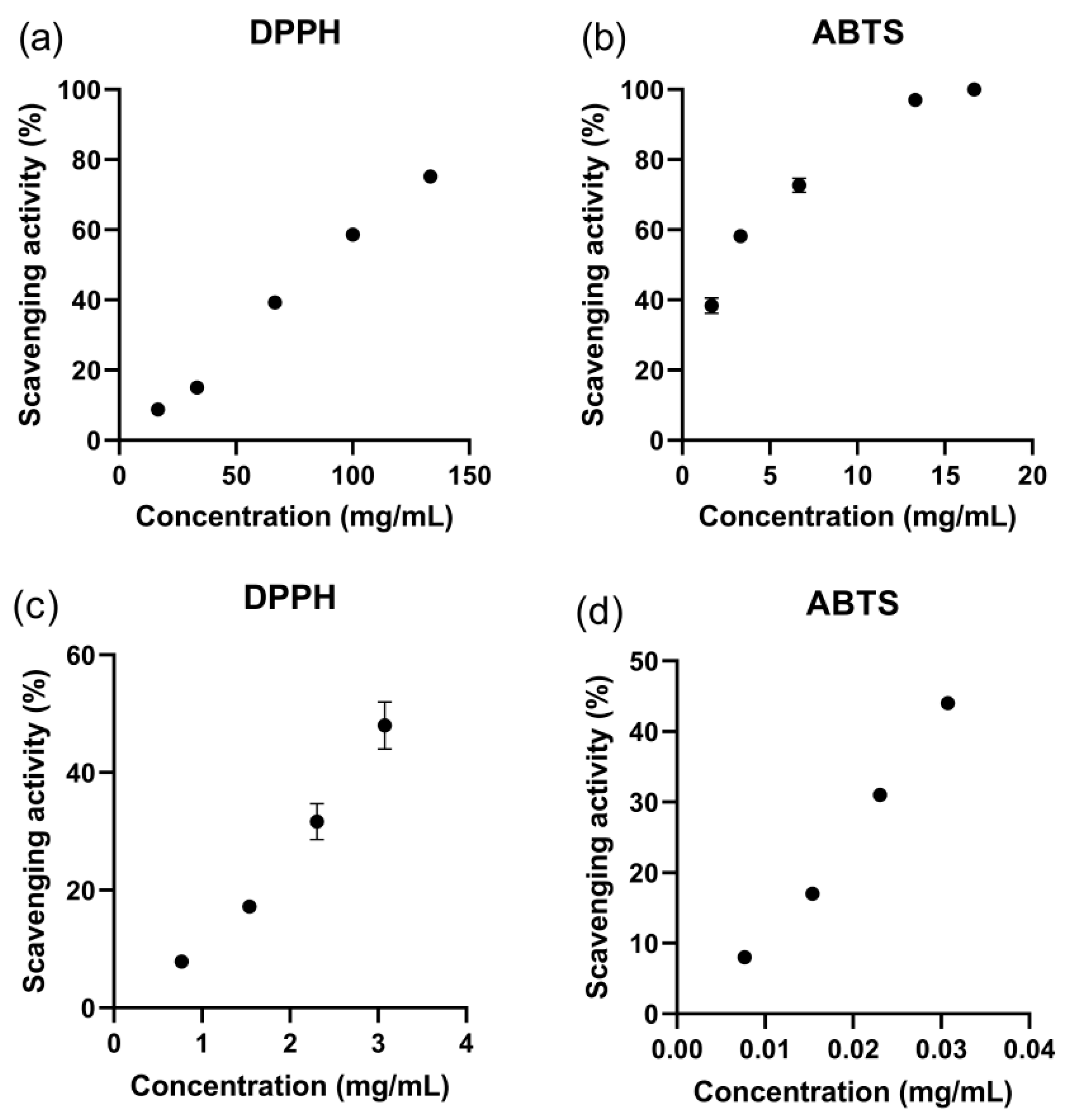
3.7. Antioxidant Activity of ZnO Nanoparticles
3.8. Antibacterial Activity of ZnO Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majeed, K.; Ambreen, J.; Khan, S.A.; Muhammad, S.; Shah, A.A.; Bhatti, M.A.; Batool, S.S.; Farooq, M.; Shah Bukhari, S.N.; Chandio, A.D.; et al. Effective Removal of Methylene Blue by Mn3O4/NiO Nanocomposite under Visible Light. Separations 2023, 10, 200. [Google Scholar] [CrossRef]
- Iqbal, M.; Hanif, M.A.; Rashid, U.; Jilani, M.I.; Alharthi, F.A.; Kazerooni, E.A. Optimization and Kinetic Study of Treating Dye-Contaminated Wastewater Using Bio-Composite Synthesized from Natural Waste. Separations 2023, 10, 386. [Google Scholar] [CrossRef]
- Ahmad, A.; Kamaruddin, M.A.; Hps, A.K.; Yahya, E.B.; Muhammad, S.; Rizal, S.; Ahmad, M.I.; Surya, I.; Abdullah, C.K. Recent Advances in Nanocellulose Aerogels for Efficient Heavy Metal and Dye Removal. Gels 2023, 9, 416. [Google Scholar] [CrossRef]
- Xue, X.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for Water Pollution Monitoring and Remediation. Environ. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Haitosa, H.H.; Chen, X.; Wu, Y.-N. Recent Progress on Plant Extract-Mediated Biosynthesis of ZnO-Based Nanocatalysts for Environmental Remediation: Challenges and Future Outlooks. Adv. Colloid Interface Sci. 2023, 317, 102931. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kataria, N.; Garg, V.K. Green Fabrication of ZnO Nanoparticles Using Eucalyptus spp. Leaves Extract and Their Application in Wastewater Remediation. Chemosphere 2020, 247, 125803. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Salam, M.A. Green Synthesis of Zinc Oxide Nanoparticles by Ziziphus Jujuba Leaves Extract: Environmental Application, Kinetic and Thermodynamic Studies. J. Phys. Chem. Solids 2021, 158, 110237. [Google Scholar] [CrossRef]
- Batra, V.; Kaur, I.; Pathania, D.; Sonu; Chaudhary, V. Efficient Dye Degradation Strategies Using Green Synthesized ZnO-Based Nanoplatforms: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100314. [Google Scholar] [CrossRef]
- Dangana, R.S.; George, R.C.; Shittu, U.O.; Agboola, F.K. Facile Biosynthesis, Characterisation and Biotechnological Application of ZnO Nanoparticles Mediated by Leaves of Cnidoscolus Aconitifolius. Artif. Cells Nanomed. Biotechnol. 2023, 51, 309–317. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Osman, A.; El-Saber, M.M.; Camele, I.; Abbas, E. Antifungal Activity of Green and Chemically Synthesized ZnO Nanoparticles against Alternaria Citri, the Causal Agent Citrus Black Rot. Plant Pathol. J. 2023, 39, 265–274. [Google Scholar] [CrossRef]
- Rini, A.; Linda, T.; Hamzah, Y.; Umar, L.; Sari, M.; Rati, Y. Antibacterial Activity of Green Synthesized ZnO Nano-Flower Using Pineapple Peel Extract. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2023, 14, 025008. [Google Scholar] [CrossRef]
- Abid, S.; Munir, R.; Sayed, M.; Nadeem, R.; Muneer, A.; Zahid, M.; Yaseen, M.; Siddiqua, U.; Noreen, S. Synthesis of Zinc Oxide, Ferric, Cu Nano Particles by Almond Shells, Sugar Cane Bagasse, Eggshells, and Their Application as Catalyst for Dye Reactive Red 195 (RR 195) Removal. Catal. Surv. Asia 2023, 1–17. [Google Scholar] [CrossRef]
- Loh, X.; Ooi, Z.; Teoh, Y.; Shuit, S. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Peppermint Tea (Mentha Piperita) Dregs Extract and Their Photocatalytic Performance. Environ. Prog. Sustain. Energy 2023, e14202. [Google Scholar] [CrossRef]
- Jalali, T.; Ghanavati, F.; Osfouri, S. Green Synthesis of ZnO Nanoparticles Using Marine Brown Algae (Cystoseira) Extract Comprising Sol-Gel, and Combustion Techniques Based on Dye-Sensitized Solar Cells Application. Int. J. Mod. Phys. B 2023, 2450178. [Google Scholar] [CrossRef]
- Mushtaq, W.; Ishtiaq, M.; Maqbool, M.; Mazhar, M.W.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Green Synthesis of Zinc Oxide Nanoparticles Using Viscum Album Extracts: Unveiling Bioactive Compounds, Antibacterial Potential, and Antioxidant Activities. Plants 2023, 12, 2130. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Rafii, M.Y.; Misran, A.B.; Berahim, Z.; Ahmad, Z.; Khan, M.M.H.; Oladosu, Y.; Arolu, F. Kenaf (Hibiscus cannabinus L.): A Promising Fiber Crop with Potential for Genetic Improvement Utilizing Both Conventional and Molecular Approaches. J. Nat. Fibers 2023, 20, 2145410. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Rafii, M.Y.; Misran, A.B.; Berahim, Z.; Ahmad, Z.; Khan, M.M.H.; Oladosu, Y. Heterosis and Combining Ability Estimate on Yield and Yield-Related Traits in a Half Diallel Crosses of Kenaf (Hibiscus cannabinus L.) in Malaysia. J. Nat. Fibers 2023, 20, 2192541. [Google Scholar] [CrossRef]
- Ayadi, R.; Hanana, M.; Mzid, R.; Hamrouni, L.; Khouja, M.L.; Salhi Hanachi, A. Hibiscus cannabinus L.—Kenaf: A Review Paper. J. Nat. Fibers 2017, 14, 466–484. [Google Scholar] [CrossRef]
- Liu, H.; Ying, Q.; Li, C.; Norra, S.; Lichtfouse, E. Enhanced Removal of Antimony in Dyeing Wastewater by Mixing Fe3O4 with Manganese Sand Filter Material. Water Environ. Res. 2020, 92, 1208–1213. [Google Scholar] [CrossRef]
- Nho, S.W.; Cui, X.; Kweon, O.; Jin, J.; Chen, H.; Moon, M.S.; Kim, S.-J.; Cerniglia, C.E. Phylogenetically Diverse Bacteria Isolated from Tattoo Inks, an Azo Dye-Rich Environment, Decolorize a Wide Range of Azo Dyes. Ann. Microbiol. 2021, 71, 35. [Google Scholar] [CrossRef]
- Alshehrei, F. Role of Microorganisms in Biodegradation of Food Additive Azo Dyes: A Review. Afr. J. Biotechnol. 2020, 19, 799–805. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green Synthesis of ZnO Nanoparticles Using Tecoma Castanifolia Leaf Extract: Characterization and Evaluation of Its Antioxidant, Bactericidal and Anticancer Activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, L.; Zhao, Y.; Yu, Z. Fabrication of Zinc Oxide/Polypyrrole Nanocomposites for Brilliant Green Removal from Aqueous Phase. Arab. J. Sci. Eng. 2019, 44, 111–121. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, P.; Wang, Z.; Jiang, C.; Zeng, Q.; Shen, C.; Wu, Y.; Liu, L.; Yi, Y.; Zhu, H.; et al. Explore the Effect of the Structure-Activity Relationship and Dose-Effect Relationship on the Antioxidant Activity of Licorice Flavonoids. J. Mol. Struct. 2023, 1292, 136101. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Xiao, A.; Huang, S.; Li, D. Screening and Analysis of Xanthine Oxidase Inhibitors in Jute Leaves and Their Protective Effects against Hydrogen Peroxide-Induced Oxidative Stress in Cells. Open Chem. 2020, 18, 1481–1494. [Google Scholar] [CrossRef]
- Bourhia, M.; Bouothmany, K.; Bakrim, H.; Hadrach, S.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Albadr, N.A.; Gmouh, S.; Laglaoui, A.; et al. Chemical Profiling, Antioxidant, Antiproliferative, and Antibacterial Potentials of Chemically Characterized Extract of Citrullus colocynthis L. Seeds. Separations 2021, 8, 114. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum Tamala Leaf Extract and Its Promising Effect towards the Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Lakshmi Ranganatha, V.; Ramu, R.; Udayabhanu; Nagaraju, G. Facile Microwave-Assisted Green Synthesis of ZnO Nanoparticles: Application to Photodegradation, Antibacterial and Antioxidant. J. Mater. Sci. Mater. Electron. 2020, 31, 1004–1021. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-Inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Venkidasamy, B.; Khan, A.; Dulta, K.; Kumar Chauhan, P.; Kumar, D.; Shin, D.-S. In Vitro Antidiabetic, Antioxidant, Antimicrobial, and Cytotoxic Activity of Murraya Koenigii Leaf Extract Intercedes ZnO Nanoparticles. Luminescence 2023, 38, 1139–1148. [Google Scholar] [CrossRef]
- Saad Algarni, T.; Abduh, N.A.Y.; Al Kahtani, A.; Aouissi, A. Photocatalytic Degradation of Some Dyes under Solar Light Irradiation Using ZnO Nanoparticles Synthesized from Rosmarinus Officinalis Extract. Green Chem. Lett. Rev. 2022, 15, 460–473. [Google Scholar] [CrossRef]
- Li, W.; You, Q.; Zhang, J.; Li, W.; Xu, H. Green Synthesis of Antibacterial LFL-ZnO Using L. Plantarum Fermentation Liquid Assisted by Ultrasound-Microwave. J. Alloys Compd. 2023, 947, 169697. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Shanmugam, V.K. Functionalization of Zinc Oxide Nanoparticles Using Mucuna Pruriens and Its Antibacterial Activity. Surf. Interfaces 2020, 19, 100521. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green Synthesis of Zinc Oxide Nanoparticles: A Comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Hasan, I.M.A.; Tawfik, A.R.; Assaf, F.H. GC/MS Screening of Buckthorn Phytochemicals and Their Use to Synthesize ZnO Nanoparticles for Photocatalytic Degradation of Malachite Green Dye in Water. Water Sci. Technol. 2021, 85, 664–684. [Google Scholar] [CrossRef]
- Ko, G.-A.; Shrestha, S.; Kim Cho, S. Sageretia Thea Fruit Extracts Rich in Methyl Linoleate and Methyl Linolenate Downregulate Melanogenesis via the Akt/GSK3β Signaling Pathway. Nutr. Res. Pract. 2018, 12, 3–12. [Google Scholar] [CrossRef]
- Pejin, B.; Savic, A.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Radotic, K.; Mojovic, M. Further In Vitro Evaluation of Antiradical and Antimicrobial Activities of Phytol. Nat. Prod. Res. 2014, 28, 372–376. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The Story of Beta-Sitosterol- A Review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Ranjan, S.; Kalaiselvi, V.; Dasgupta, N.; Chen, J.; Durán-Lara, E.F. Biogenic Preparation and Characterization of ZnO Nanoparticles from Natural Polysaccharide Azadirachta indica L. (Neem Gum) and Its Clinical Implications. J. Clust. Sci. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Nayak, A.; Sahoo, J.K.; Sahoo, S.K.; Sahu, D. Removal of Congo Red Dye from Aqueous Solution Using Zinc Oxide Nanoparticles Synthesised from Ocimum sanctum (Tulsi Leaf): A Green Approach. Int. J. Environ. Anal. Chem. 2022, 102, 7889–7910. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Yusof, N.; Mohd Yusop, M.Z.; Hamdan, R.; Awang, N.A.; Ismail, N.H.; Rosman, N.; Sazali, N.; Ismail, A.F. Pb(II) Removal and Its Adsorption from Aqueous Solution Using Zinc Oxide/Graphene Oxide Composite. Chem. Eng. Commun. 2021, 208, 646–660. [Google Scholar] [CrossRef]
- Arab, C.; El Kurdi, R.; Patra, D. Effect of PH on the Removal of Anionic and Cationic Dyes Using Zinc Curcumin Oxide Nanoparticles as Adsorbent. Mater. Chem. Phys. 2022, 277, 125504. [Google Scholar] [CrossRef]
- Marahel, F.; Ghaedi, M.; Ansari, A. Zinc Oxide Nanoparticles Loaded on Activated Carbon and Its Application for Adsorption Removal of Uric Acid. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 1387–1395. [Google Scholar] [CrossRef]
- Eleryan, A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Hassaan, M.A.; Elkatory, M.R.; Ragab, S.; Osibote, O.A.; Kusuma, H.S.; El Nemr, A. Adsorption of Direct Blue 106 Dye Using Zinc Oxide Nanoparticles Prepared via Green Synthesis Technique. Environ. Sci. Pollut. Res. 2023, 30, 69666–69682. [Google Scholar] [CrossRef]
- Phan, D.-N.; Rebia, R.A.; Saito, Y.; Kharaghani, D.; Khatri, M.; Tanaka, T.; Lee, H.; Kim, I.-S. Zinc Oxide Nanoparticles Attached to Polyacrylonitrile Nanofibers with Hinokitiol as Gluing Agent for Synergistic Antibacterial Activities and Effective Dye Removal. J. Ind. Eng. Chem. 2020, 85, 258–268. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Byrappa, K.; Surendra, D.M.; Yallappa, S.; Hungund, B. Surfactant Assisted Solvothermal Synthesis of ZnO Nanoparticles and Study of Their Antimicrobial and Antioxidant Properties. J. Mater. Sci. Technol. 2018, 34, 1035–1043. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Khan, N.; Saad Umar, M.; Owais, M.; Shamsuzzaman. Synthesis of Steroidal Dihydropyrazole Derivatives Using Green ZnO NPs and Evaluation of Their Anticancer and Antioxidant Activity. Steroids 2022, 188, 109113. [Google Scholar] [CrossRef]
- Krishnan, B.R.; Ramesh, M.; Selvakumar, M.; Karthick, S.; Sasikumar, A.; Geerthi, D.V.; Senthilkumar, N. A Facile Green Approach of Cone-like ZnO NSs Synthesized Via Jatropha Gossypifolia Leaves Extract for Photocatalytic and Biological Activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4441–4451. [Google Scholar] [CrossRef]
- Velsankar, K.; Venkatesan, A.; Muthumari, P.; Suganya, S.; Mohandoss, S.; Sudhahar, S. Green Inspired Synthesis of ZnO Nanoparticles and Its Characterizations with Biofilm, Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activities. J. Mol. Struct. 2022, 1255, 132420. [Google Scholar] [CrossRef]
- Ganesan, V.; Hariram, M.; Vivekanandhan, S.; Muthuramkumar, S. Periconium sp. (Endophytic Fungi) Extract Mediated Sol-Gel Synthesis of ZnO Nanoparticles for Antimicrobial and Antioxidant Applications. Mater. Sci. Semicond. Process. 2020, 105, 104739. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Ahmad, N.; Khan, M.M. Antioxidant and Antibacterial Studies of Phytogenic Fabricated ZnO Using Aqueous Leaf Extract of Ziziphus mauritiana Lam. Chem. Pap. 2021, 75, 3295–3308. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; da Silva, A.A.; da Silva, B.D.; Neto, L.T.; Tessaro, L.; Furtado, C.R.G.; de Sousa, A.M.F.; Carvalho, N.M.F.; Conte-Junior, C.A. Eco-Friendly Synthesis of ZnO Nanomaterial from Green Tea Extract: Photocatalytic, Antibacterial and Antioxidant Potential. Biomass Convers. Biorefin. 2023, 1–15. [Google Scholar] [CrossRef]
- Ali, A.H. Experimental Investigations on Effects of ZnO NPS and Annona Muricata Extract for in Vitro and in Vivo Antibacterial Activity. Mater. Today Proc. 2022, 57, 527–530. [Google Scholar] [CrossRef]
- Hameed, H.; Waheed, A.; Sharif, M.S.; Saleem, M.; Afreen, A.; Tariq, M.; Kamal, A.; Al-onazi, W.A.; Al Farraj, D.A.; Ahmad, S.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles from Green Algae and Their Assessment in Various Biological Applications. Micromachines 2023, 14, 928. [Google Scholar] [CrossRef]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green Route for the Synthesis of Zinc Oxide Nanoparticles from Melia azedarach Leaf Extract and Evaluation of Their Antioxidant and Antibacterial Activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
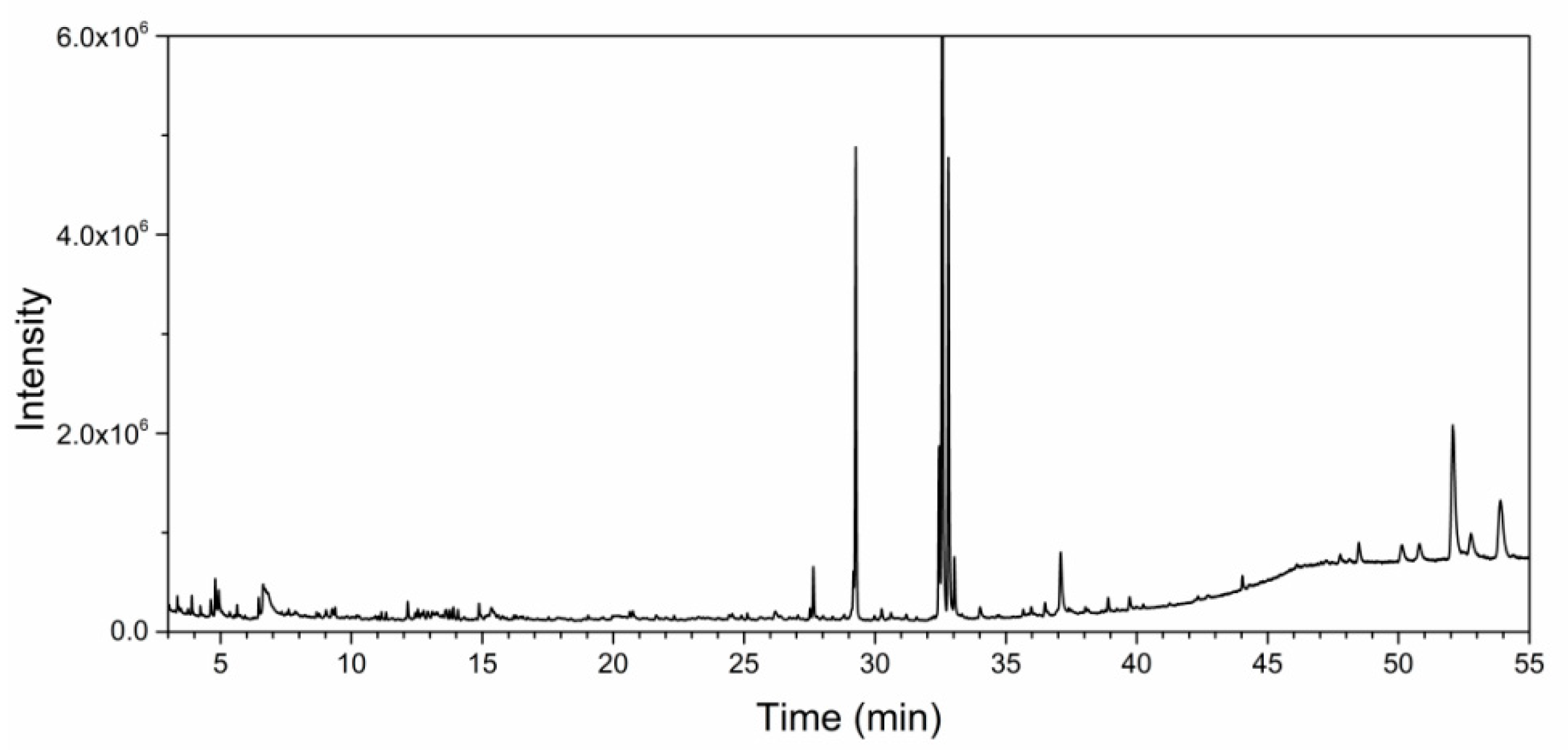



| Rt (min) | Mw | Molecular Formula | Molecular Name |
|---|---|---|---|
| 4.795 | 84.0 | C4H4O2 | Butenolide |
| 4.954 | 88.1 | C4H8O2 | Acetaldol |
| 6.799 | 92.0 | C3H8O3 | Glycerin |
| 12.156 | 198.2 | C14H30 | Tetradecane |
| 27.660 | 268.3 | C18H36O | Hexahydrofarnesyl acetone |
| 29.289 | 270.3 | C17H34O2 | Methyl hexadecanoate |
| 32.438 | 294.3 | C19H34O2 | Methyl linoleate |
| 32.597 | 292.2 | C19H32O2 | Methyl linolenate |
| 32.820 | 296.3 | C20H40O | Phytol |
| 33.068 | 438.8 | C29H58O2 | Methyl montanate |
| 37.121 | 281.3 | C18H35NO | Oleamide |
| 52.122 | 414.4 | C29H50O | β-Sitosterol |
| 53.961 | 426.4 | C30H50O | Amyrin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Cao, X.; Chen, C.; Liao, L.; Yuan, S.; Huang, S. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity. Separations 2023, 10, 466. https://doi.org/10.3390/separations10090466
Yang X, Cao X, Chen C, Liao L, Yuan S, Huang S. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity. Separations. 2023; 10(9):466. https://doi.org/10.3390/separations10090466
Chicago/Turabian StyleYang, Xitao, Xuan Cao, Chenxiao Chen, Liping Liao, Sitian Yuan, and Siqi Huang. 2023. "Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity" Separations 10, no. 9: 466. https://doi.org/10.3390/separations10090466
APA StyleYang, X., Cao, X., Chen, C., Liao, L., Yuan, S., & Huang, S. (2023). Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity. Separations, 10(9), 466. https://doi.org/10.3390/separations10090466





