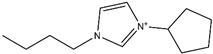
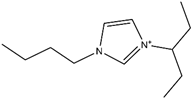
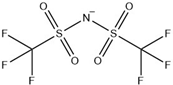
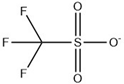
Abstract
Ionic liquids (ILs) are the safest solvent in various high-temperature applications due to their non-flammable properties. In order to obtain their thermal stability properties, thermogravimetric analysis (TGA) is extensively used to analyze the kinetics of the thermal decomposition process. This review summarizes the different kinetics analysis methods and finds the isoconversional methods are superior to the Arrhenius methods in calculating the activation energy, and two tools—the compensation effect and master plots—are suggested for the calculation of the pre-exponential factor. With both parameters, the maximum operating temperature (MOT) can be calculated to predict the thermal stability in long-term runnings. The collection of thermal stability data of ILs with divergent cations and anions shows the structure of cations such as alkyl side chains, functional groups, and alkyl substituents will affect the thermal stability, but their influence is less than that of anions. To develop ILs with superior thermal stability, dicationic ILs (DILs) are recommended, and typically, [C4(MIM)2][NTf2]2 has a decomposition temperature as high as 468.1 °C. For the convenience of application, thermal stability on the decomposition temperature and thermal decomposition activation energy of 130 ILs are summarized at the end of this manuscript.
1. Introduction
Ionic liquids (ILs) are molten salts at room temperature composed of organic cations and organic/inorganic anions [1,2]. ILs have been described as “designer solvents” because their physicochemical property can be regulated or tailor-made by changing their constituents or the structures of the pairs of the ions [3,4,5]. Previous research has proved that ILs have many advantages, including non-volatility, non-flammability, high thermal and chemical stability, wide electrochemical window, tunable miscibility, and good extraction capability, which are not attained for the volatile organic solvents [6,7,8]. As a result of these features and advantages, ILs are used in a wide range of applications such as catalyst [9,10,11,12,13,14], pre-treatment of biomass [15,16,17], absorbent [18,19,20], gas sensors [21], electrolyte [22,23,24], and membrane separation [25,26,27]. Among all of the applications, high-temperature utilization accounts for the vast majority, including high-temperature lubricants [28,29,30], solvents for high-temperature organic reactions [31], heat-transfer fluids [32,33], and thermal energy storage [34,35]. Although ILs are generally considered to be thermally stable, yet their stability is influenced seriously by a lot of factors.
So far, the thermal stability of ILs has been studied by many techniques, such as UV (ultraviolet)–vis spectroscopy [36], flame ionization detection (FID) [37,38], and mass spectrometry (MS) [39,40,41]. Moreover, in some studies, ILs are heated isothermally in a furnace and open to air to simulate the real environment [42,43]. In order to quantitatively evaluate the thermal stability of ILs, parameters accounting for short-term and long-term thermal stability are obtained by thermogravimetric analysis (TGA), and the most representative ones are the onset decomposition temperature Tonset and Tz/y (decomposition degree z in a selected time y), respectively. Although many studies have indicated Tonset obtained by dynamic TGA overestimates the thermal stability [36,39,44], it is still widely used to express the stability of ILs from divergent papers [45,46]. Despite the long-term isothermal experiments can more accurately reflect the real stability of ILs [44,47,48,49], but it should be noted that the time in these experiments is still far less than the heat exposure time in many real applications. To provide the method in predicting long-term thermal stability, different models have been developed [50,51,52].
To solve the problem when the entire sample cannot be decomposed [47,48], non-isothermal TGA is developed as the most popular method to determine the kinetic parameters in the thermal decomposition process, and the combination of TGA data allows researchers to calculate the activation energy and pre-exponential factor in this process. Arrhenius methods are widely used in kinetics analysis due to their simple calculation process. However, some recent studies have used the isoconversional methods to calculate the activation energy [44,53,54], while the methods of compensation effect and master plots provide the pre-exponential factor calculation [55,56,57].
The thermal stability of ILs is mainly determined by the structure of anions and cations, and anions usually play a major role [46,58,59]. However, more studies are focused on the modification of cations, including alkyl chain length, functional groups, and alkyl substituents to improve the thermal stability of ILs [60,61]. Other conditions, such as gas atmosphere, heating rates, and impurities, also influence the thermal stability measurements [45,62]. Among which, the heating rate has the most significant impact on the TGA results, and the difference in Tonset obtained at 1 °C/min and 20 °C/min is even up to 100 °C [44]. Finally, ILs mixtures and dicationic ILs (DILs) are introduced as potential applications for the further development of high-temperature ILs.
2. Measurement of Thermal Stability
2.1. Short-Term Thermal Stability
Dynamic TGA is applied in the study for short-term thermal stability measurement, and the most common heating rate is 10 °C/min [36,39,42,63,64,65,66]. Effects of different heating rates, at 5, 10, 15, 20 °C/min are available in previous studies, and it should be noted that the faster the heating rate is, the more overestimated the thermal stability of ILs is, and the corresponding dynamic TGA curve will move to the right accordingly. [18,39,49,53,57].
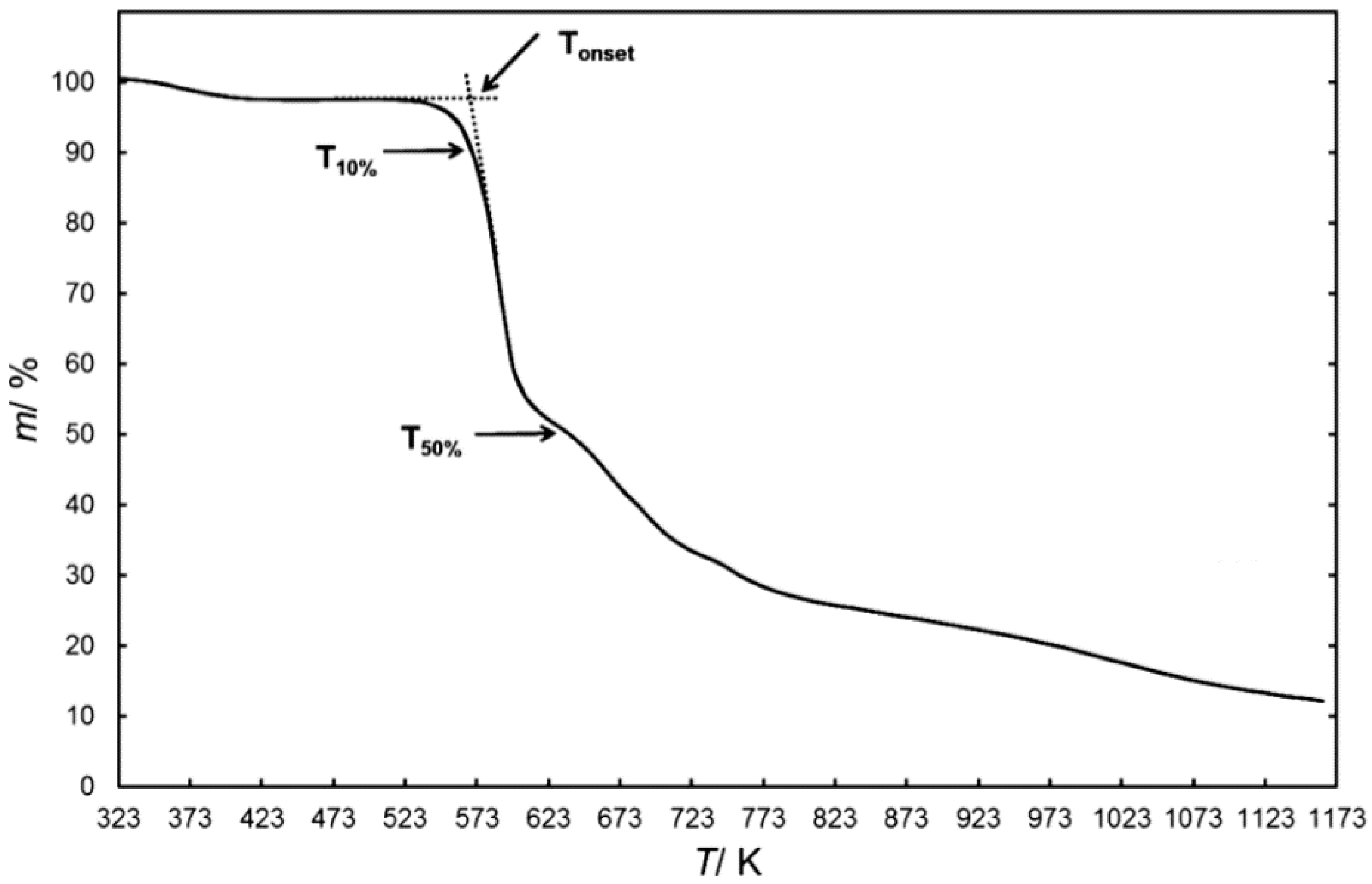
Tonset, which is basically known as the short-term thermal stability, is determined by dynamic TGA. Moreover, it is a value calculated by the thermal analysis software, which is defined as the intersection of the baseline of zero weight loss and the tangent of the weight versus temperature curve as decomposition occurs [45]. Therefore, the temperature at which the sample begins to decompose is lower than Tonset. Tz (decomposition degree z) is also a parameter to characterize the short-term thermal stability, which directly shows the temperatures at different decomposition degrees [55,63,67,68,69]. Both parameters are illustrated clearly in Figure 1. In fact, the difference between Tonset and T50% or T10% is a measure of the decomposition rate, and the lower the temperature difference between Tonset and T50% or T10%, the lower the stability of the ionic liquid [70].
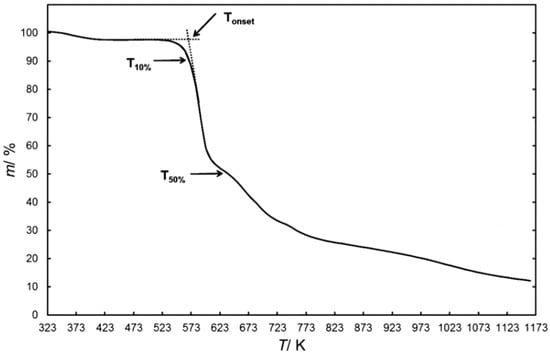
Figure 1.
Thermal parameters of ionic liquids (ILs) obtained from dynamic thermogravimetric analysis (TGA) curves [70].
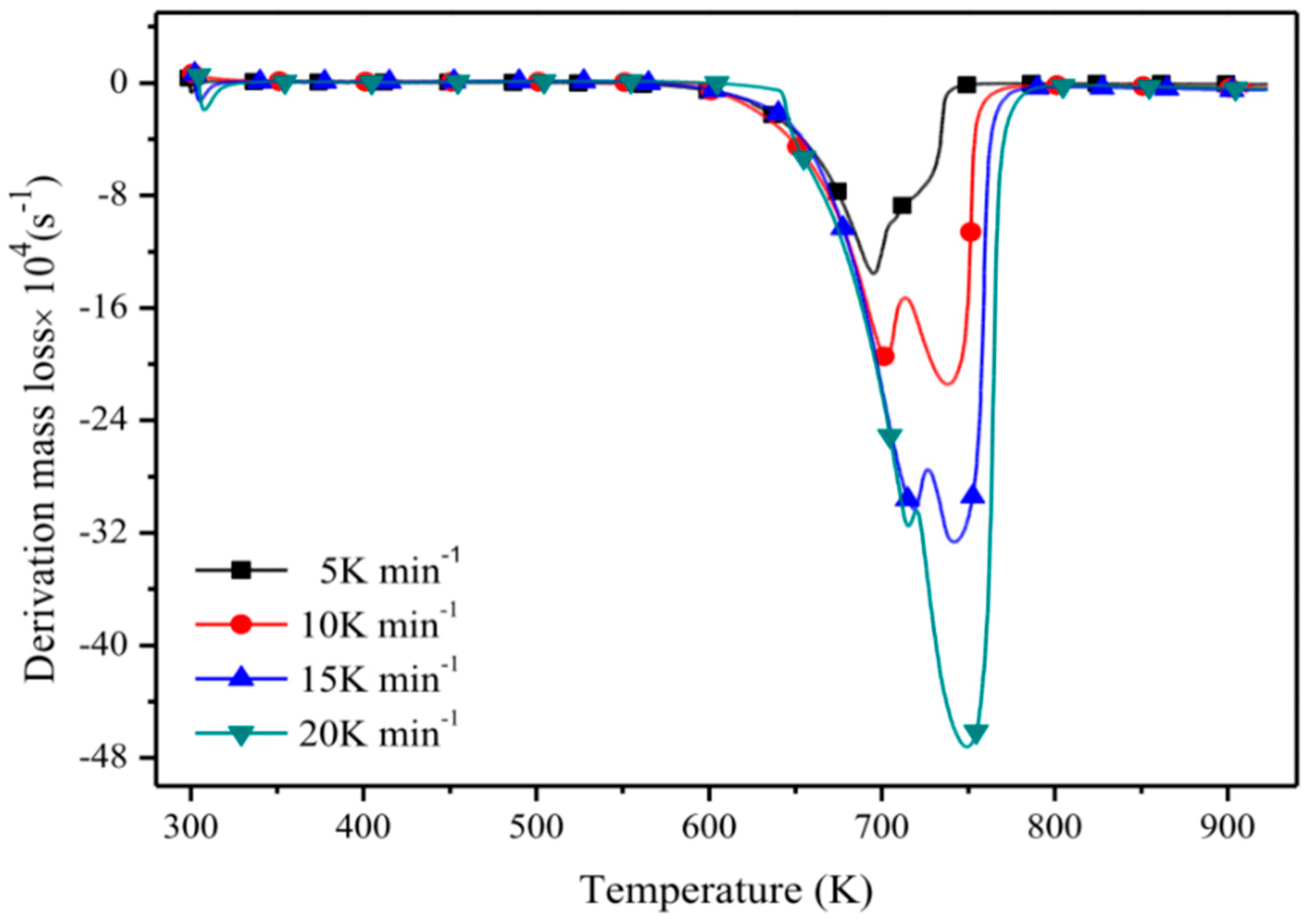
Another method to investigate the short-term thermal stability called derivative thermogravimetry (DTG) is shown in Figure 2. It determines the temperature of maximum degradation Tpeak [42,64,71,72,73], i.e., if there is one more peak in the DTG curve, the temperature corresponding to the highest peak will be selected [74]. The number of peaks in a DTG curve is also an important parameter. Most ILs have only one peak, indicating the decomposition process is a simple one-step process [44], and two peaks in some DTG curves correspond to two different degradation processes in the sample [63].
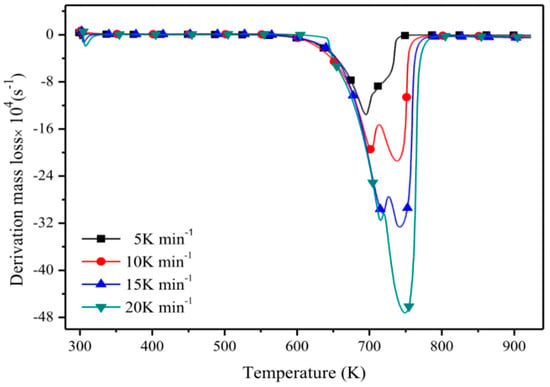
Figure 2.
Derivative thermogravimetry (DTG) curves of [C4MIM][NTf2] [18].
2.2. Long-Term Thermal Stability
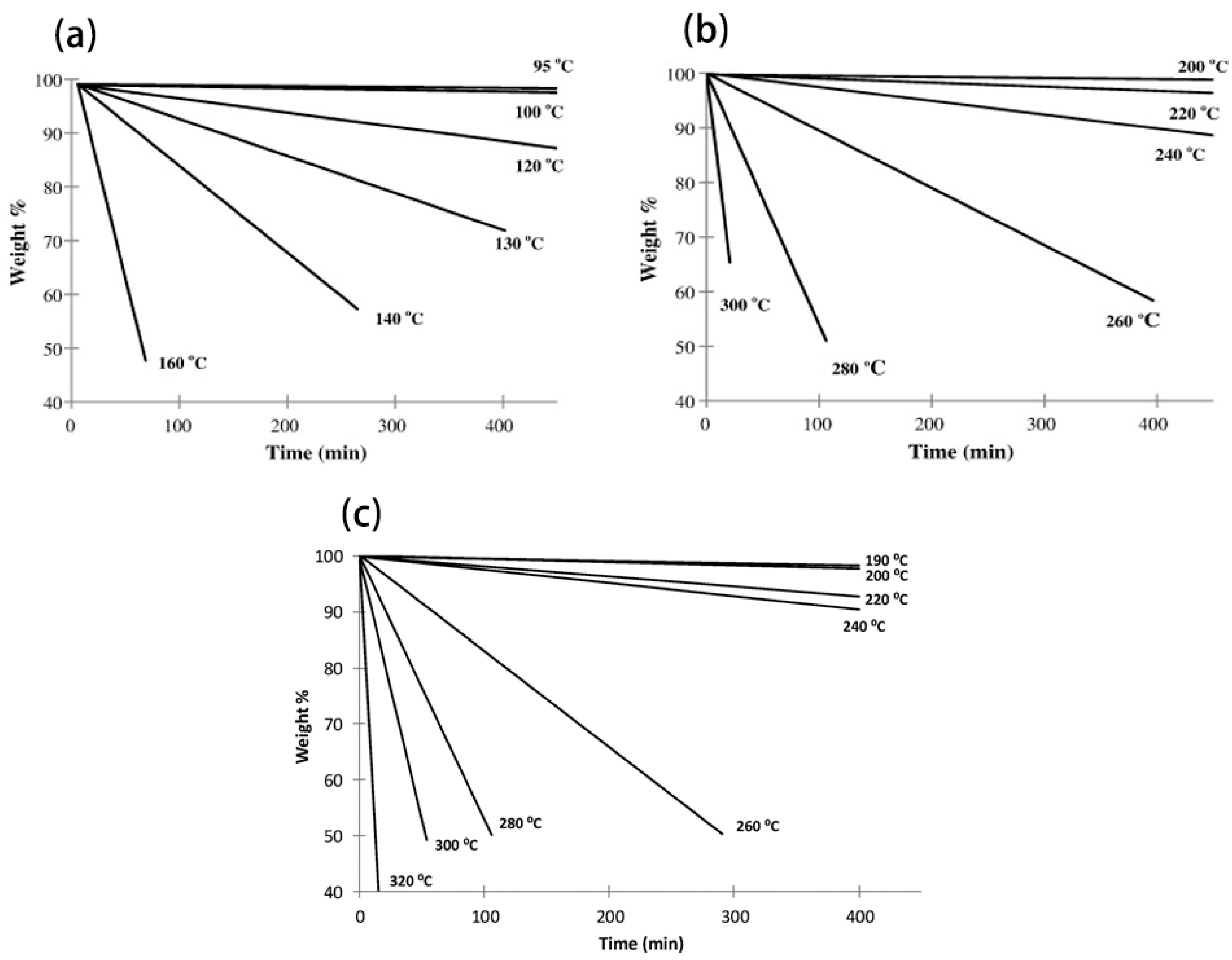
Although Tonset is widely used to describe the thermal stability of ILs, it is not a suitable parameter for long-term industrial applications. To establish the correlation between the operating temperature and time, it is necessary to investigate the long-term thermal stability of ILs through isothermal TGA [36,75,76]. In this method, samples are heated at a fixed temperature varying from an hour to tens of hours, then Tz/y is determined [49,57,77,78,79]. As shown in Figure 3, more than three temperatures are usually used in the analysis [75,78], and the interval between different temperatures is determined by Tonset [74]. However, it is much shorter than the cycle of industrial runnings. Neither extrapolating experimental data nor extending the heating time of isothermal TGA seems to be the best solution. Therefore, some means predicting the long-term thermal stability of ILs have been proposed.
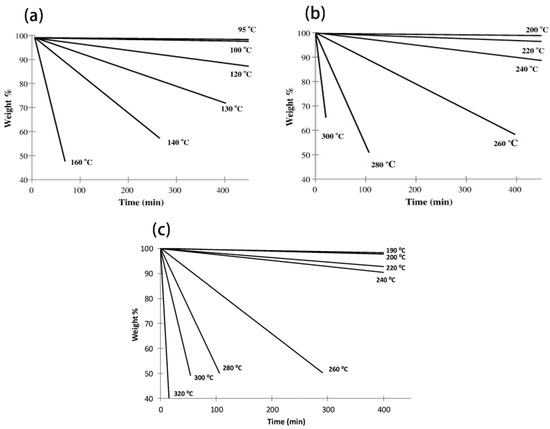
Figure 3.
Isothermal TGA for (a) 4-methyl-1-propyl-1,2,4-triazolium iodide, (b) 4-methyl-1-propyl-1,2,4-triazolium triflate, and (c) 1-butyl-4-methyl-1,2,4-triazolium [NTf2] in nitrogen atmosphere [75,78].
Seeberger et al. [50] proposed using the maximum operating temperature (MOT) to measure the long-term thermal stability at 1% decomposition degree, which is
where denotes the pre-exponential factor, is the activation energy, is the universal gas constant, and is the maximum operation time. MOT has been used to predict the long-term thermal stability successfully in several recent studies [56,71,73]. Moreover, MOT is also applied in the prediction of the thermal stability of ILs mixtures [80].
To estimate the maximum time for ILs used under a specific temperature, Salgado et al. [51] proposed an exponential function of the temperature, similar to Cao and Mu [74], which is
where is the time in minutes, and are the fitting parameters, and is the scanning temperature in K. From isothermal TGA, that each IL takes to decompose to a certain percentage of mass, is determined at different . This equation can quantitatively describe the relationship between the decomposition temperature and the decomposition time of ILs under a certain degree of decomposition. With this method, T0.01/10h, T0.05/10h, and T0.1/10h have been correlated, and T0.01/10h was given by Wooster et al. [52] according to
where is the temperature at which the first appreciable weight loss occurs. The results of calculated by Equation (2) is higher than that by Equation (3), which attributes to the different experimental conditions [51]. In another research, the above three methods are used to calculate of some aprotic ILs [63]. The experiments prove that the results obtained by Wooster’s method (Equation (3)) are the highest, followed by Salgado’s method (Equation (2)), and those calculated by Seeberger’s method (Equation (1)) are the lowest.
2.3. Factors Affecting TGA Results
2.3.1. Heating Rates
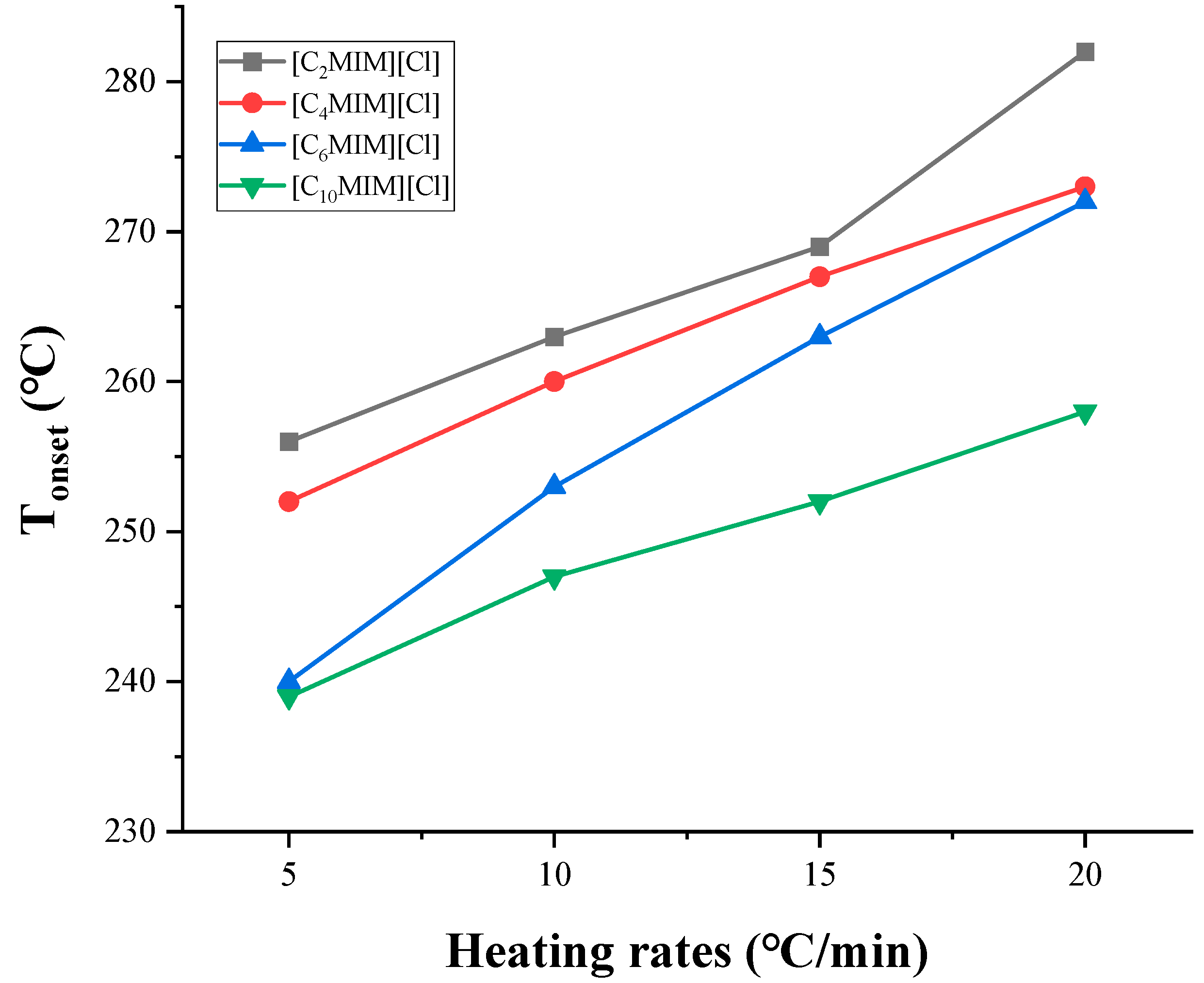
In dynamic TGA, Tonset varies significantly with different heating rates, since when the temperature rises rapidly, it will easily exceed the onset decomposition temperature, resulting in a mass loss that cannot be measured correctly [50]. As shown in Figure 4, compared with a slower heating rate, the higher Tonset is obtained at a faster heating rate [57]. For [C2MIM][NTf2] and [C3MIM][NTf2], it is found that the difference of Tonset obtained at 1 °C/min and 20 °C/min is 100 °C [44]. This trend has also been found in more investigations [39,54]. Therefore, it is necessary to pay attention to the heating rate when comparing Tonset by different authors. As a criterion, most of the literature known to date gives the Tonset value at 10 °C/min.
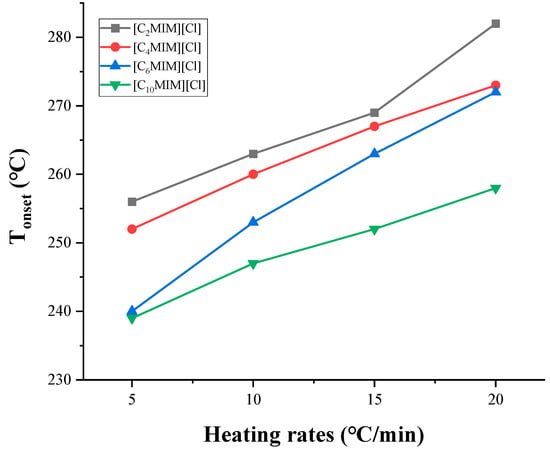
Figure 4.
Tonset measured at different heating rates [57].
2.3.2. Gas Atmosphere
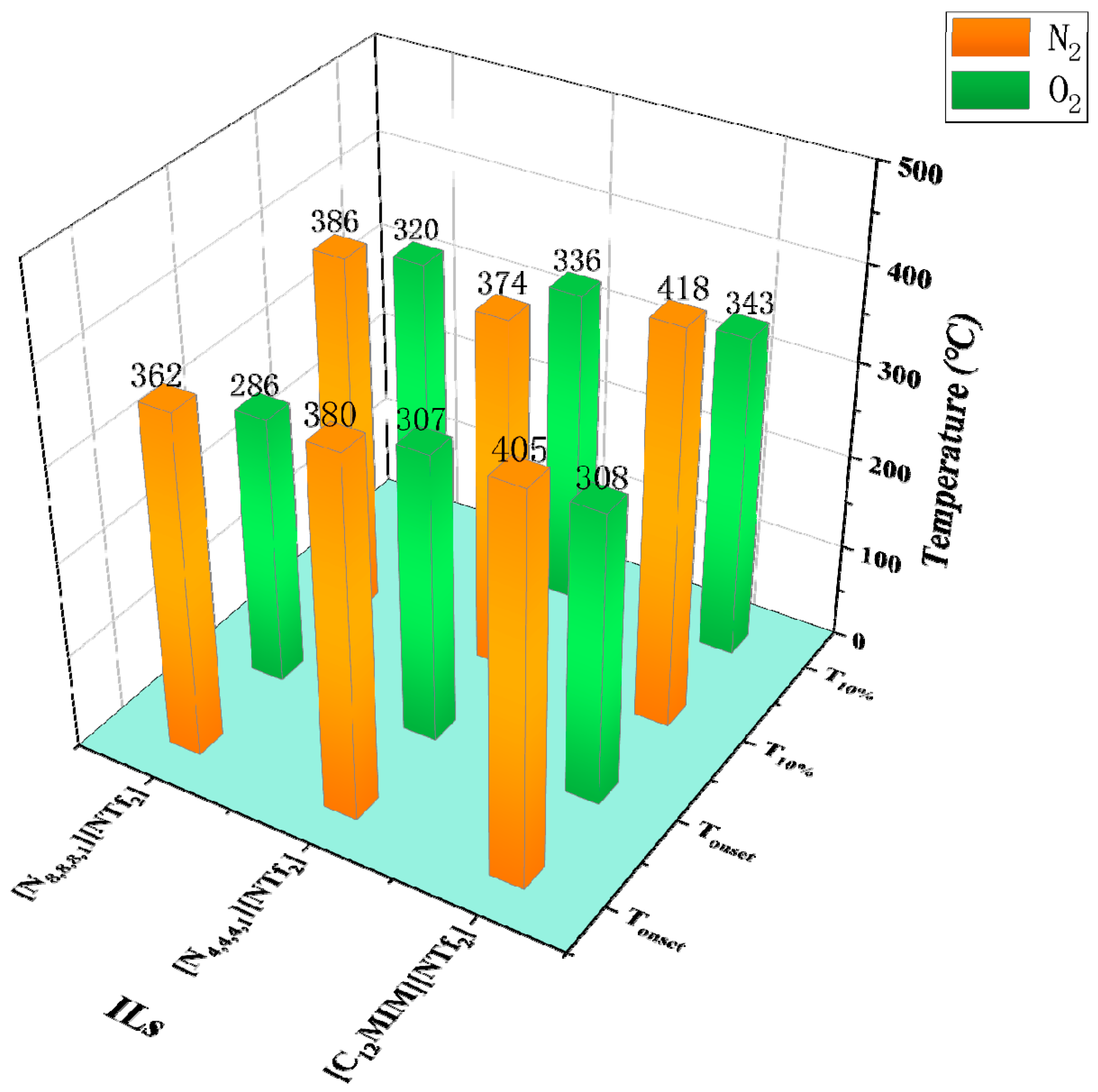
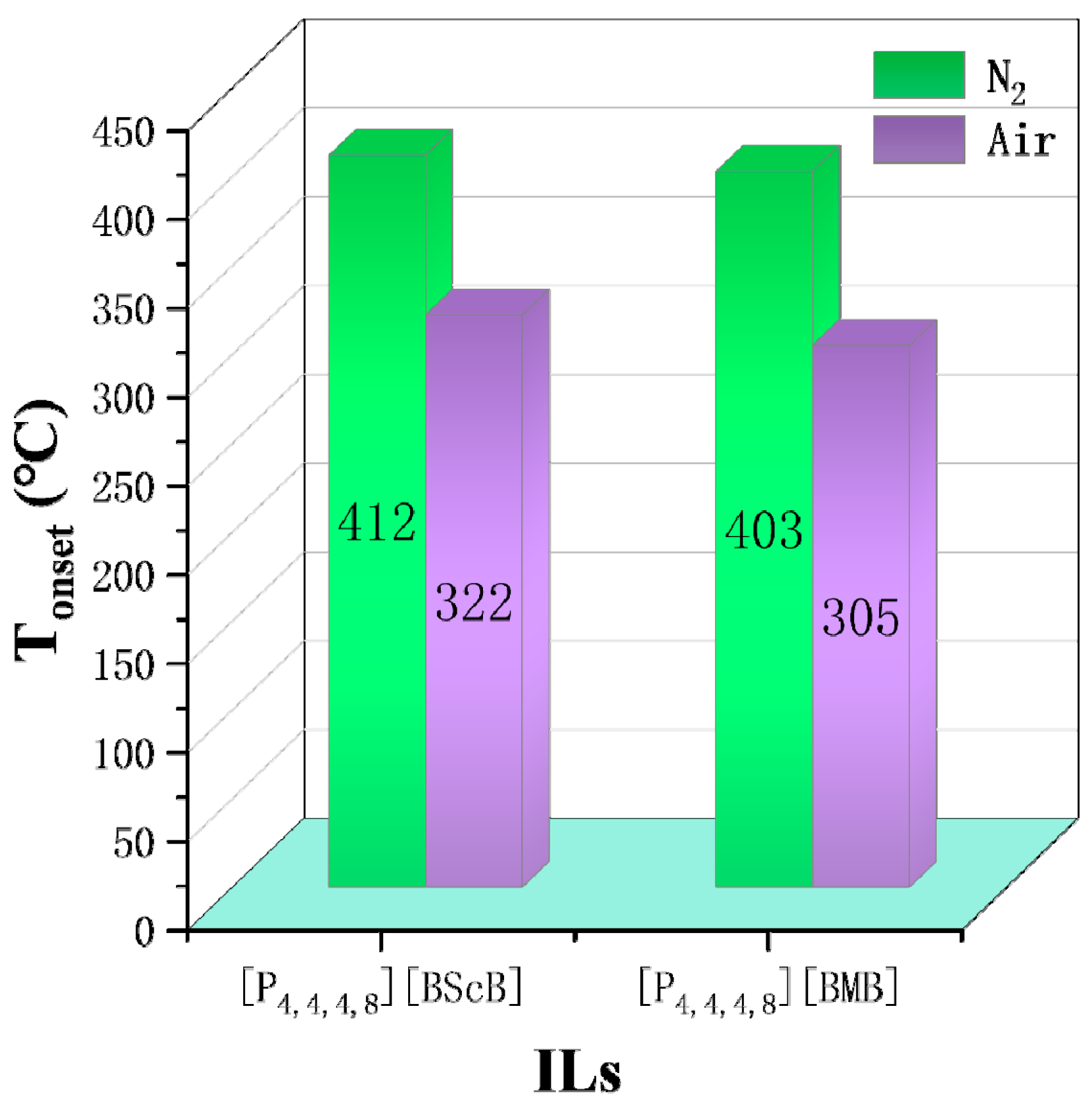
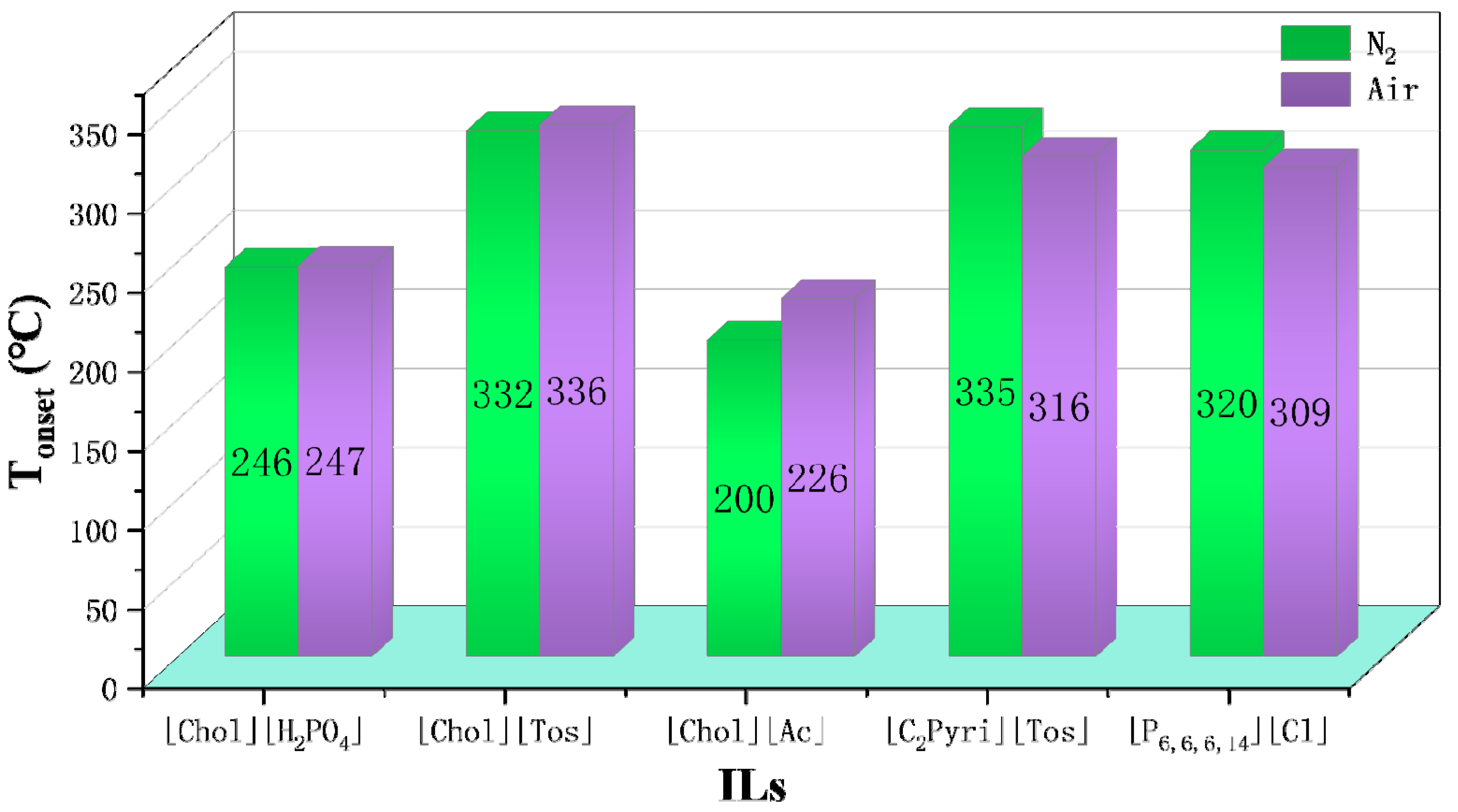
N2 is often chosen as the gas atmosphere in TGA, while it is just a special case in industrial applications. Therefore, it is necessary to figure out the influence of different gas atmospheres on the thermal stability of ILs. As shown by Götz et al., the TGA results of [P14,6,6,6][NTf2] indicate the mass loss in H2 is much higher than that in N2 at the same temperature, i.e., H2 accelerates the decomposition of [P14,6,6,6][NTf2] [6]. Figure 5 shows some Tonset and T10% obtained in both N2 and O2 atmospheres. The results reveal that the Tonset and T10% of [NTf2]− ILs obtained in O2 are lower by 38 °C to 97 °C than those obtained in N2, indicating the reactive atmosphere reduces their thermal stability [49]. The effect of air on thermal stability is similar to that of O2. As shown in Figure 6, [P4,4,4,8][BScB] and [P4,4,4,8][BMB] are more thermally stable in N2 than in air [81], and the same results are observed in another study [55]. However, according to Figure 7, the Tonset of some choline, pyridinium, and phosphonium ILs are not significantly affected by gas atmospheres, and the maximum temperature difference is only 26 °C [40,73].
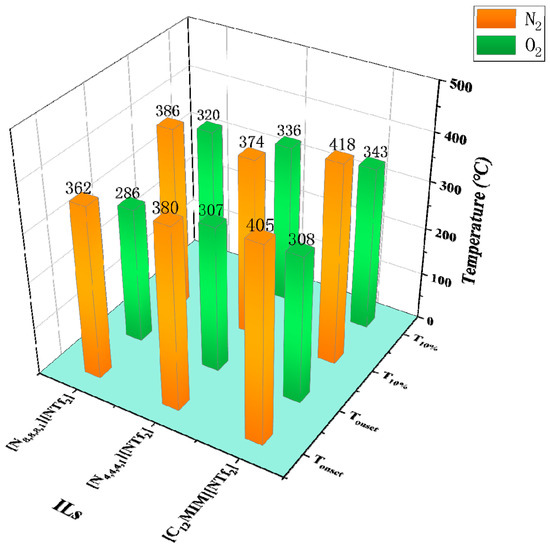
Figure 5.
Tonset and T10% of [NTf2]− ILs in N2 and O2 at a heating rate of 10 °C/min [49].
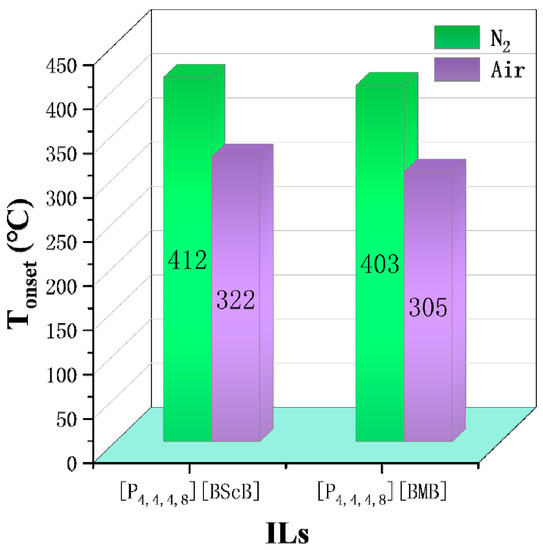
Figure 6.
Tonset of [P4,4,4,8][BScB] and [P4,4,4,8][BMB] in N2 and air at a heating rate of 10 °C/min [81].
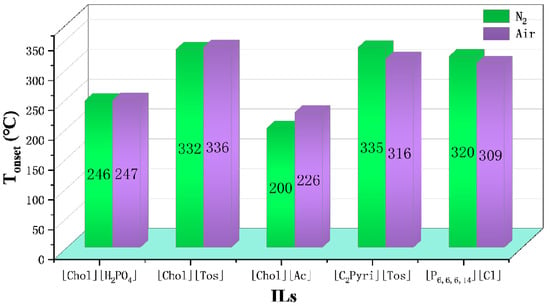
Figure 7.
Tonset of choline, pyridinium, and phosphonium ILs in N2 and air at a heating rate of 10 °C/min [40,73]. Reproduced with permission from Clio Deferm, Physical Chemistry Chemical Physics; published by Royal Society of Chemistry, 2018; reproduced with permission from J.J.Parajó, The Journal of Chemical Thermodynamics; published by Elsevier, 2020.
2.3.3. Impurities
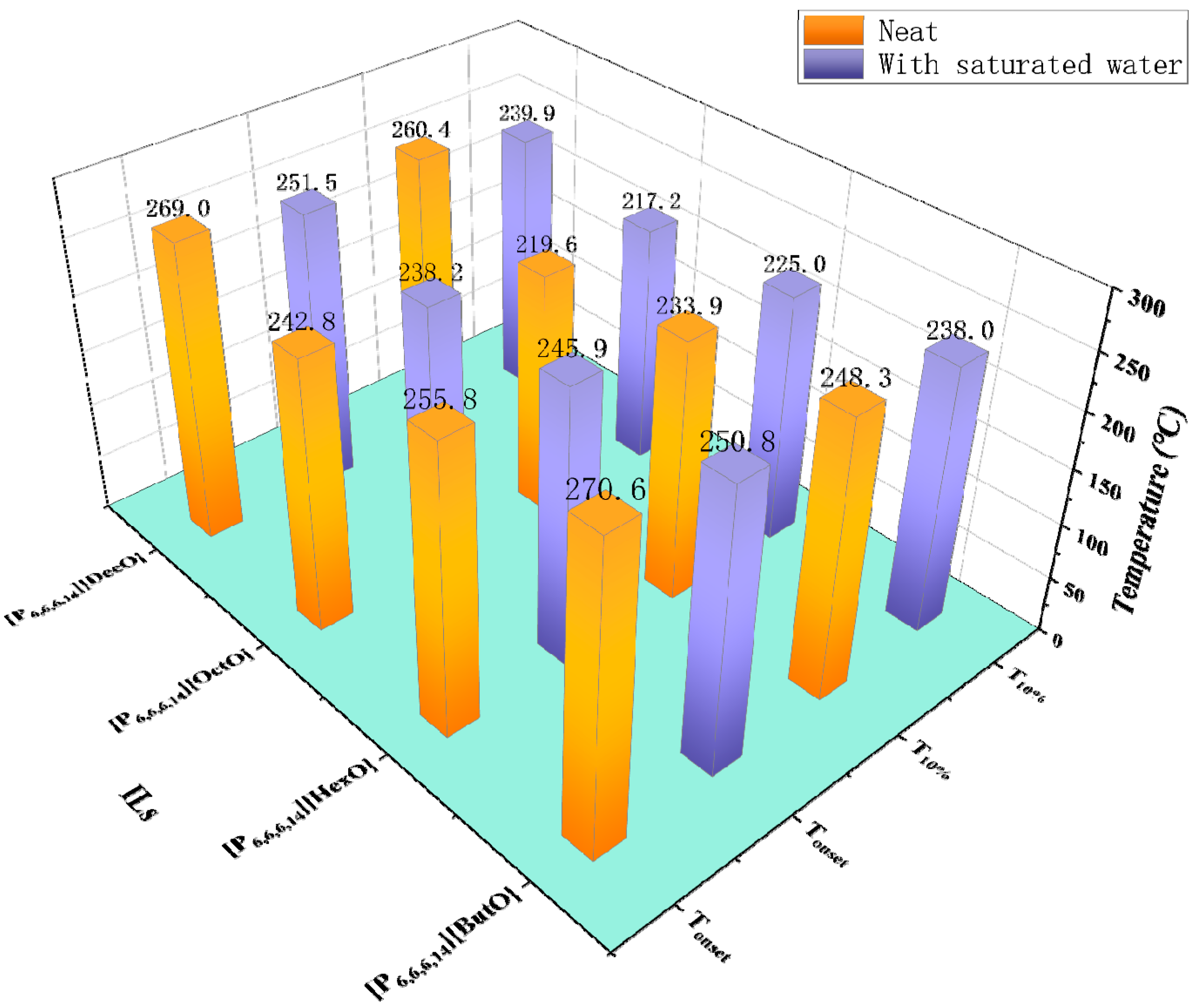
In most cases, water has a wide range of values from 30 ppm to 21,000 ppm [51,66,73]. For different kinds of ILs, water has different effects on their thermal stability. On one hand, water hinders the degradation reaction of [C2MIM][Ac] and [C4MIM][Ac], as shown by Williams et al. that the mass of [C2MIM][Ac] and [C4MIM][Ac] decreased significantly in an isothermal TGA at 150 °C, but the time-lapsed changed in the UV–vis spectra monitored over 24 h showed that mixtures of [C2MIM][Ac] and [C4MIM][Ac] with water did not display any significant decomposition at 150 °C [36]. The observed results are in line with the research of [C2MIM][AcO] and [C4MIM][AcO] [82]. On the other hand, the Tonset of the quaternary phosphonium carboxylate ILs decreases by 19.8 °C at most when the water content is saturated, as shown in Figure 8 [83]. Moreover, the TGA curves of two protic ILs, [DEMA][HSO4] and [DEMA][CF3COO], decrease markedly with the increase of water content [84]. However, for [C1OC2C1Py][NTf2], [C1OC2C1Py][(C2F5)3PF3], and [P6,6,6,14][(C2F5)3PF3], differences of Tonset between water saturation and supply conditions are lower than the expanded uncertainties of the apparatus [63].
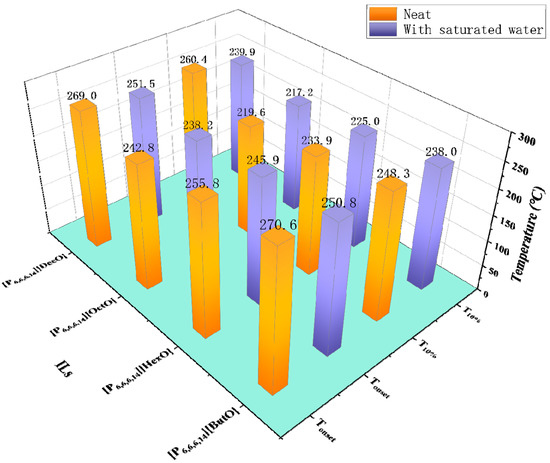
Figure 8.
Tonset and T10% of neat ILs and mixtures with water at a heating rate of 5 °C/min in nitrogen atmosphere [83].
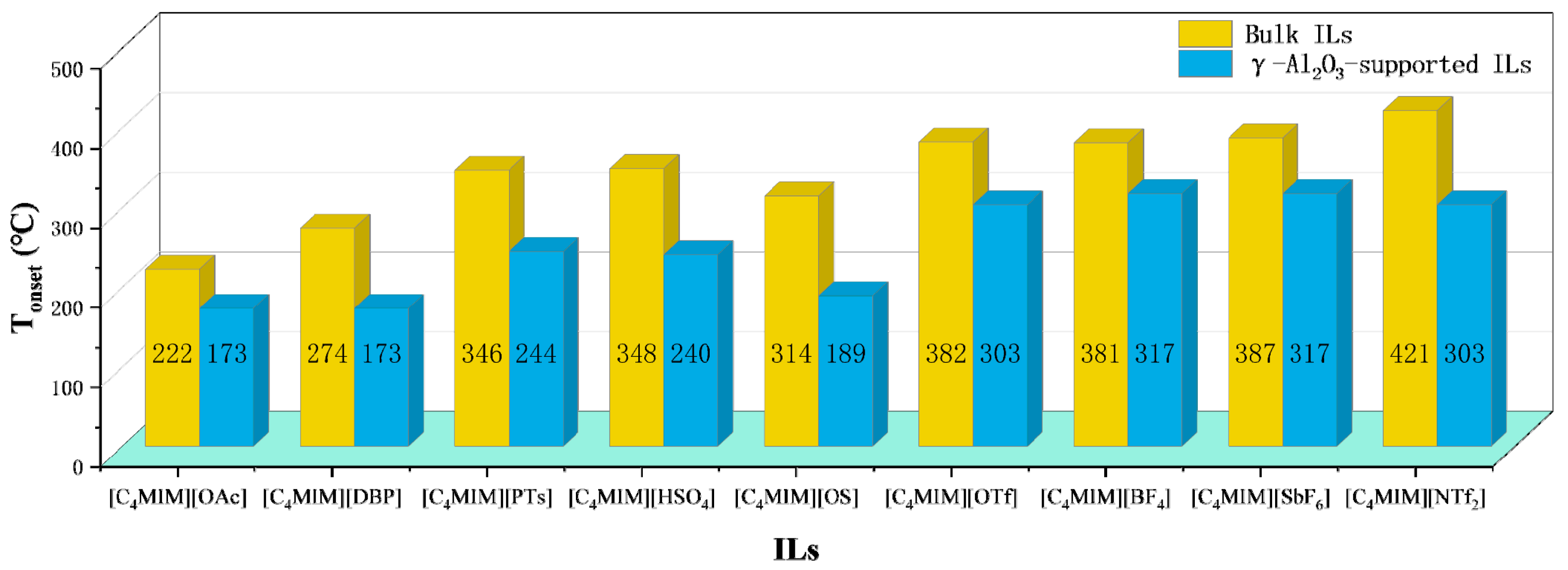
In addition to water, the influence of inorganic salts and metal oxides on thermal stability has also been studied. Adding CuO to [C2MIM][Ac] results in an exothermic reaction and lowers the decomposition temperature. It is assumed that CuO decomposes ester compounds produced from [C2MIM][Ac] as it might accelerate the decomposition of organic compounds [85]. In addition, results summarized in Figure 9 show Tonset of bulk ILs are significantly higher than the Tonset of γ-Al2O3-supported ILs, indicating the interactions of ILs with γ-Al2O3 control their thermal stability limits [58]. Putting 20% NH4Cl into [BMMIM][Cl] reduces the thermal stability [86], and Tonset of commercial [P6,6,6,14][Cl] is 8 °C lower than its pure counterpart [40]. Both studies mean the existence of impurities alters the thermal stability of ILs.
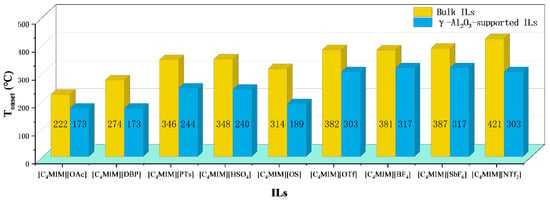
Figure 9.
Tonset of Bulk and γ-Al2O3-supported ILs at a heating rate of 10 °C/min in nitrogen atmosphere [58].
3. Kinetics of Thermal Decomposition
The kinetic data in the thermal decomposition process can be obtained based on isothermal and non-isothermal TGAs. Generally, the rate of thermal decomposition can be expressed by the following formula:
where is the rate constant, is determined by the kinetic models as shown in Table 1, and , , and are the initial mass, mass at certain time, and terminal mass of the sample, respectively. is defined as the fraction of the total mass loss in the process, ranging from 0 (no mass loss) to 1 (complete mass loss).

Table 1.
Some of the models used in thermal decomposition kinetics of ILs. ().
The correlation between the rate constant and the temperature is defined by the Arrhenius equation as
where denotes the pre-exponential factor, is the activation energy, is the universal gas constant, and refers to the temperature.
Combining Equation (4) and Equation (6) yields
If the heating rate is constant, then Equation (7) can be transformed into Equation (8) as follows:
The calculation of Arrhenius parameters, E and A, will be introduced in the following sections.
3.1. Isoconversional Methods
The isoconversional methods are based on the isoconversional principle, meaning that the reaction rate is only a function of temperature at a constant extent of conversion [41] and the reaction model is independent of temperature [87]. In addition, these methods require that the decomposition process can be approximated as a single-step kinetic process, that is to say, activation energy calculated by isoconversional methods does not vary significantly with [88]. According to this principle, the isoconversional values of activation energy can be evaluated without assuming or determining any particular form of the reaction model, so the isoconversional methods are frequently called “model-free” methods. Currently, these methods have been classified into differential isoconversional methods and integral isoconversional methods.
3.1.1. Differential Isoconversional Methods
Taking the logarithm on both sides of Equation (8), the most representative differential isoconversional method is obtained according to Friedman [89]
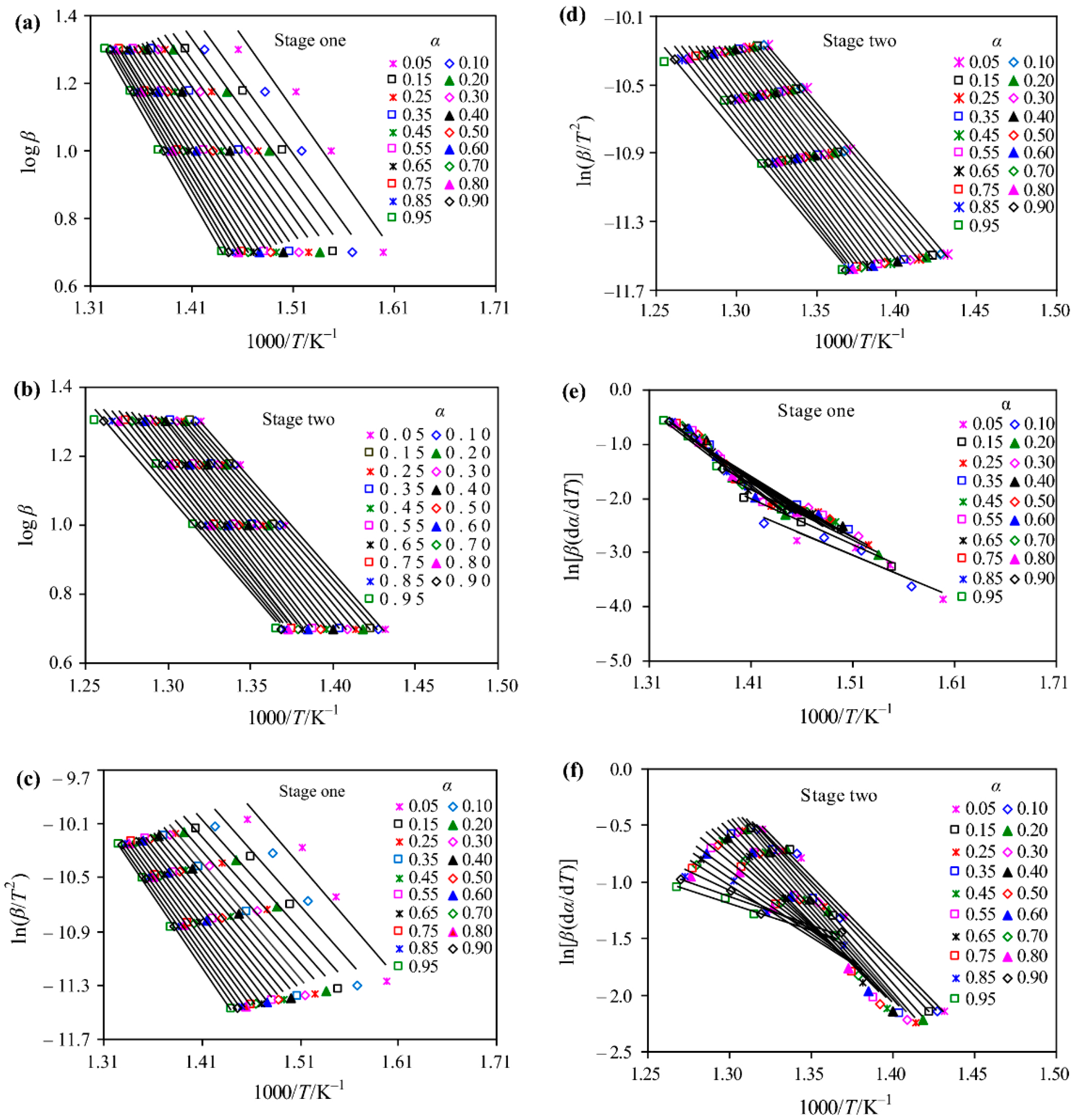
where the subscript denotes individual heating rate, and is the temperature at which the extent of conversion is reached under th heating rate. The activation energy corresponding to each conversion can be obtained easily by using DTG and TGA data. As shown in Figure 10, in order to better characterize the thermal decomposition process of [C4MIM][PF6] in the nitrogen atmosphere, the thermal decomposition is divided into two steps. The activation energy value calculated by the Friedman method suggests that the linearity of the result is relatively poor in the fitting process and the activation energy varies significantly with [55]. In addition, a similar conclusion has also been drawn in other investigations [90]. In the non-isothermal kinetic analysis of imidazolium [NTf2] ILs, it is found that the activation energy calculated by the Friedman method has the largest variation range [44].
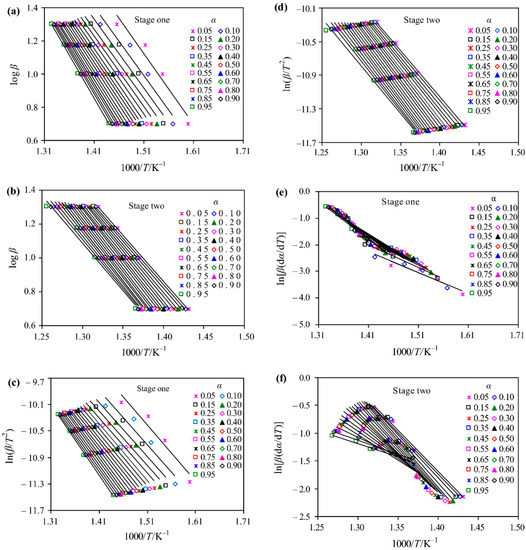
Figure 10.
Linear Arrhenius plots for [C4MIM][PF6] in a nitrogen atmosphere.(a), (b) Flynn–Wall–Ozawa (FWO) method log β versus 1000/T; (c), (d) Coats–Redfern (CR) method ln(β/T2) versus 1000/T and; (e), (f) Friedman method ln[β(dα/dt)] versus 1000/T [55]. Reproduced with permission from Zhen Huang, Journal of Thermal Analysis and Calorimetry; published by Springer Nature, 2019.
3.1.2. Integral Isoconversional Methods
By integrating Equation (7), the following equation can be obtained as
In the case of constant heating rate, Equation (10) can be converted into
According to the isoconversional principle, Equation (10) can be converted into Equation (12) under the isothermal condition as follows:
where indicates isothermal temperature, and is the time to reach a given extent of conversion in the th isothermal experiment. Apart from the study by Williams et al. that used this method to calculate the activation energy and pre-exponential factor values of 1-alkyl-3-methylimidazolium chloride ILs [57], no other literature is known using this method to analyze the thermal degradation kinetics of ILs.
Compared with isothermal experiments, the constant heating rate (=constant) is more popular in isoconversional kinetic analysis [18,39,49,53,56,57]. There is no analytical solution to the integral of Equation (11), so a series of integral isoconversional methods using different approximations have appeared. Many integral isoconversional methods can be expressed in the following form [91]:
Flynn–Wall–Ozawa (FWO) method [92,93], Kissinger–Akahira–Sunose (KAS) [94] method, which is also known as Coats–Redfern (CR) method [95], and Starink method [96] are all derived based on Equations (14)–(16), respectively. Then the activation energy values can be calculated from the slope of plots.
Table 2 indicates that the activation energy difference of [C4MIM][NTf2] calculated by the KAS method, FWO method, and Starink method is less than 5.4 kJ/mol, and the correlation coefficients (R²) is all greater than 0.97 [18]. The calculated results are in line with many other studies [44,53,54]. Therefore, as recommended by ICTAC (the International Confederation for Thermal Analysis and Calorimetry), there is no need to perform kinetic analysis in multiple forms of integral isoconversional methods [88].

Table 2.
Correlation coefficient and activation energy for the [C4MIM][NTf2] calculated using different integral isoconversional methods [18].
The numerical integration developed by Vyazovkin et al. improves the calculation accuracy [97,98,99]. For a series of different heating rates, the activation energy values can be obtained by minimizing the following function:
where the subscript denotes different heating programs, and the subscript is used to denote all heating rates other than . In order to reduce error as much as possible, the temperature integral is calculated over a small segment [87] as
Table 3 shows that the activation energy values calculated by the Vyazovkin method are similar to the results of the KAS and Starink methods, indicating that the latter two simple integration methods are precise enough in most cases [55,57], and the Vyazovkin method is a better choice when the activation energy values vary with obviously [88].

Table 3.
The activation energy () values determined from the Kissinger–Akahira–Sunose (KAS) method, Vyazovkin method, Starink method, and max-rate Starink method. Reproduced with permission from Zhen Huang, Journal of Thermal Analysis and Calorimetry; published by Springer Nature, 2019; reproduced with permission from Michael L.Williams, Thermochimica Acta; published by Elsevier, 2020.
3.1.3. Maximum-Rate Methods
When the maximum rate of decomposition of ILs is achieved, in Equation (13) can be replaced by [91]. This class of methods will be indicated by adding “max rate” before the name. In the max-rate Starink method, the activation energy of 1-alkyl-3-methylimidazolium chloride can be calculated, which is similar to that calculated by the Vyazovkin method and Starink method, as shown in Table 4 [57]. However, the activation energy of [C4MPy][NTf2] calculated by is about 20 kJ/mol higher than that calculated by [53].

Table 4.
The activation energy values determined from the integral isoconversional methods and maximum-rate methods. Reproduced with permission from Khurrum Shehzad Quraishi, Journal of Thermal Analysis and Calorimetry; published by Springer Nature, 2017; reproduced with permission from Michael L.Williams, Thermochimica Acta; published by Elsevier, 2020.
3.2. Arrhenius Methods
According to the data of isothermal TGA, Arrhenius methods directly use Equation (6) to calculate the values of activation energy and pre-exponential factor. Due to the simplicity of the calculation process, Arrhenius methods have been used extensively [39,44,71,100,101]. By assuming the decomposition is of zero-order or first-order, the rate constant can be calculated.
For zero-order reactions
Equation (19) can be transformed into
where is sample mass, and is obtained as the slope of a linear fitting of mass loss versus time for every isothermal TGA. The activation energy and pre-exponential factor can be derived from Equation (20). Several studies have applied this method to calculate the activation energy and pre-exponential factor [47,74,75,78,101]. As shown in Table 5, Parajó et al. used both the isoconversional methods and the zero-order Arrhenius method to calculate the activation energies of several imidazolium [NTf2] ILs, and they found some activation energies calculated by the non-isothermal methods are about 20 kJ/mol lower than those calculated by the isothermal method [44].

Table 5.
Activation energies for the selected ILs by the different methods in the air atmosphere [44].
For the first-order reaction
Equation (21) can be transformed into
Table 6 shows the values of activation energy and pre-exponential factor calculated by the first-order Arrhenius method, and these values are quite different from other calculation results. In the studies of Efimova et al., the activation energy values are calculated using the isoconversional methods, and then Equation (22) is used to calculate the pre-exponential factor values [39,71].

Table 6.
Activation energy and pre-exponential factor calculated by the first-order Arrhenius method, zero-order Arrhenius method, and KAS method. Reproduced with permission from Wenlong Wang, Chemical Engineering Journal; published by Elsevier, 2017; reproduced with permission from Juan J. Parajó, The Journal of Chemical Thermodynamics; published by Elsevier, 2017; reproduced with permission from Yuanyuan Cao, Industrial & Engineering Chemistry Research; published by American Chemical Society, 2014; reproduced with permission from Florian Heym, Chemie Ingenieur Technik; published by Wiley, 2015.
3.3. Determining the Pre-Exponential Factor
Despite the isoconversional methods are convenient to calculate the values of activation energy without the reaction model, it is difficult to obtain the values of the pre-exponential factor. For single-step reactions, the pre-exponential factors can be determined by the following means when using model-free methods [88].
3.3.1. Using Compensation Effect
Different pairs of the Arrhenius parameters and can be obtained by substituting different and experimental data into Equation (8). Although the Arrhenius parameters vary widely with , according to the compensation effect, they conform to the following correlation [88]:
where and are the parameters of the compensation effect and are determined by fitting the pairs of and at different into Equation (23). Then for the single-step reaction, the average pre-exponential factor can be obtained by substituting the average activation energy determined in non-isothermal experiments into Equation (23). Using the compensation effect, Haung et al. and Jiang et al. calculated the pre-exponential factors of [C4MIM][PF6] and [C4MIM][DBP], respectively [55,56]. The pre-exponential factors of [C4MIM][PF6] ranged from to min−1, while those calculated by the Arrhenius method are min−1 [74].
3.3.2. Using Master Plots
Using a non-isothermal TGA, after determining the activation energy values of the reaction, the reaction mechanism can be simply and accurately determined by plotting master plots or [102,103]. The function has the following form:
After determining and at different , it is possible to plot the experimental values of against . Because is an unknown constant, the shape of the theoretical master plots is the same as . The experimental master plots are normalized to vary from 0 to 1, then compared with the theoretical shape of in different kinetic models in Table 1, and the reaction model of thermal decomposition can be determined. Finally, can be easily obtained from Equation (24).
Another function has the following form:
The temperature integral in can be approximately expressed by the following formula [97]:
where
Combining Equations (7) and (26) followed by some rearrangement, the following equation is obtained:
where is an experimental value. It has been proved has a negligible influence on the shape of experimental master plots [102,103]. So the correlation between experimental and can be determined from the data of non-isothermal TGA and DTG. Theoretical master plots can be drawn according to different kinetic models in Table 1, and the suitable reaction models can be determined by normalizing experimental and the theoretical ones in different kinetic models from 0 to 1 and comparing the normalized results.
After the reaction model has been established, Williams et al. calculated the pre-exponential factor values from Equation (24). By comparing the pre-exponential factor values of [C4MIM][Cl] calculated by several different methods in Table 7, it can be found that results calculated by master plots [57] are close to those calculated by the zero-order Arrhenius method [74], and those calculated by the first-order Arrhenius method [39] are much lower.

Table 7.
Pre-exponential factor values of [BMIM][Cl] in different investigations. Reproduced with permission from Anastasia Efimova, The Journal of Physical Chemistry B; published by American Chemical Society, 2018; reproduced with permission from Michael L.Williams, Thermochimica Acta; published by Elsevier, 2020; reproduced with permission from Yuanyuan Cao, Industrial & Engineering Chemistry Research; published by American Chemical Society, 2014.
4. Influence of Chemical Structure on Thermal Stability
4.1. Influence of Alkyl Chain Length
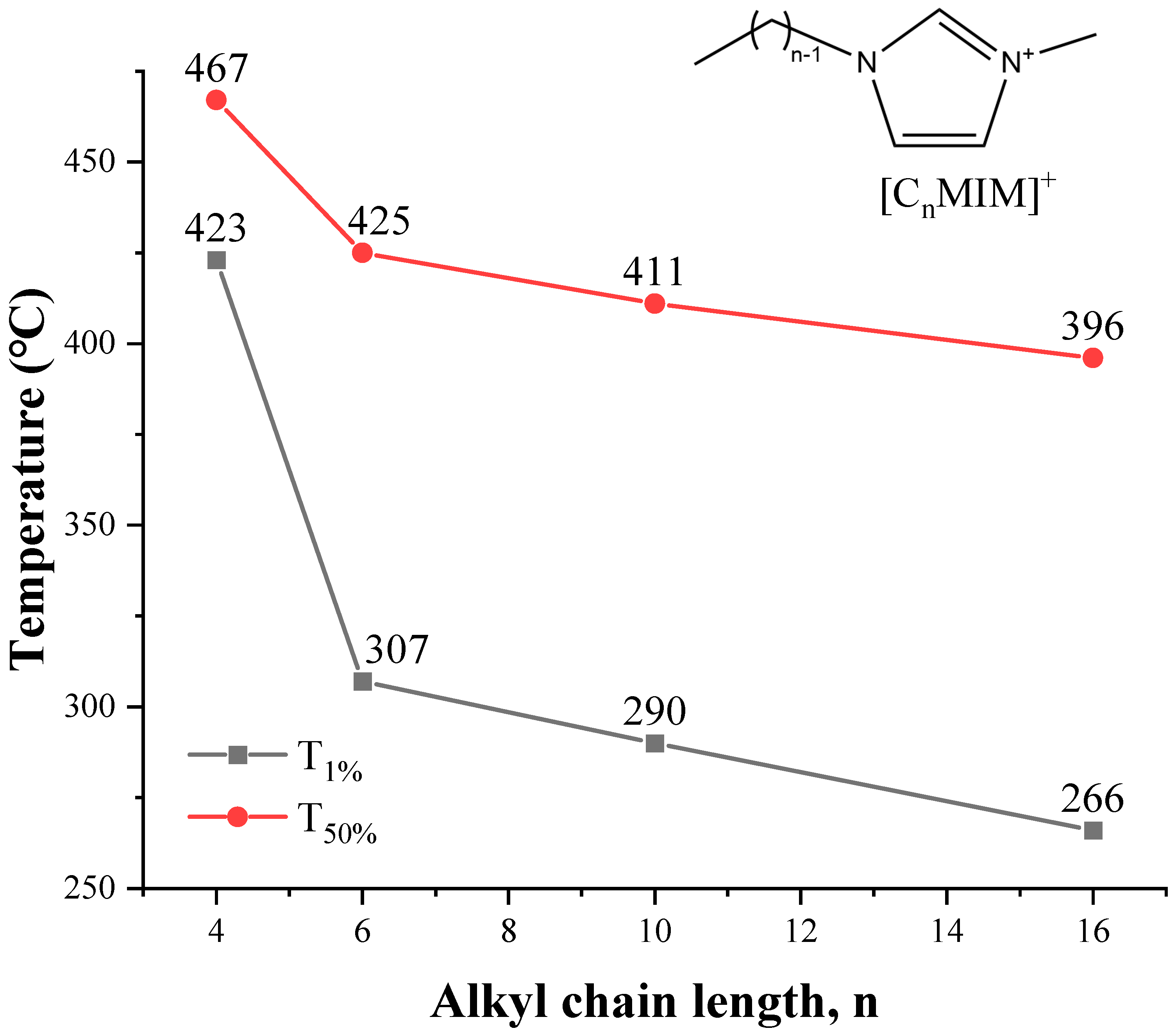
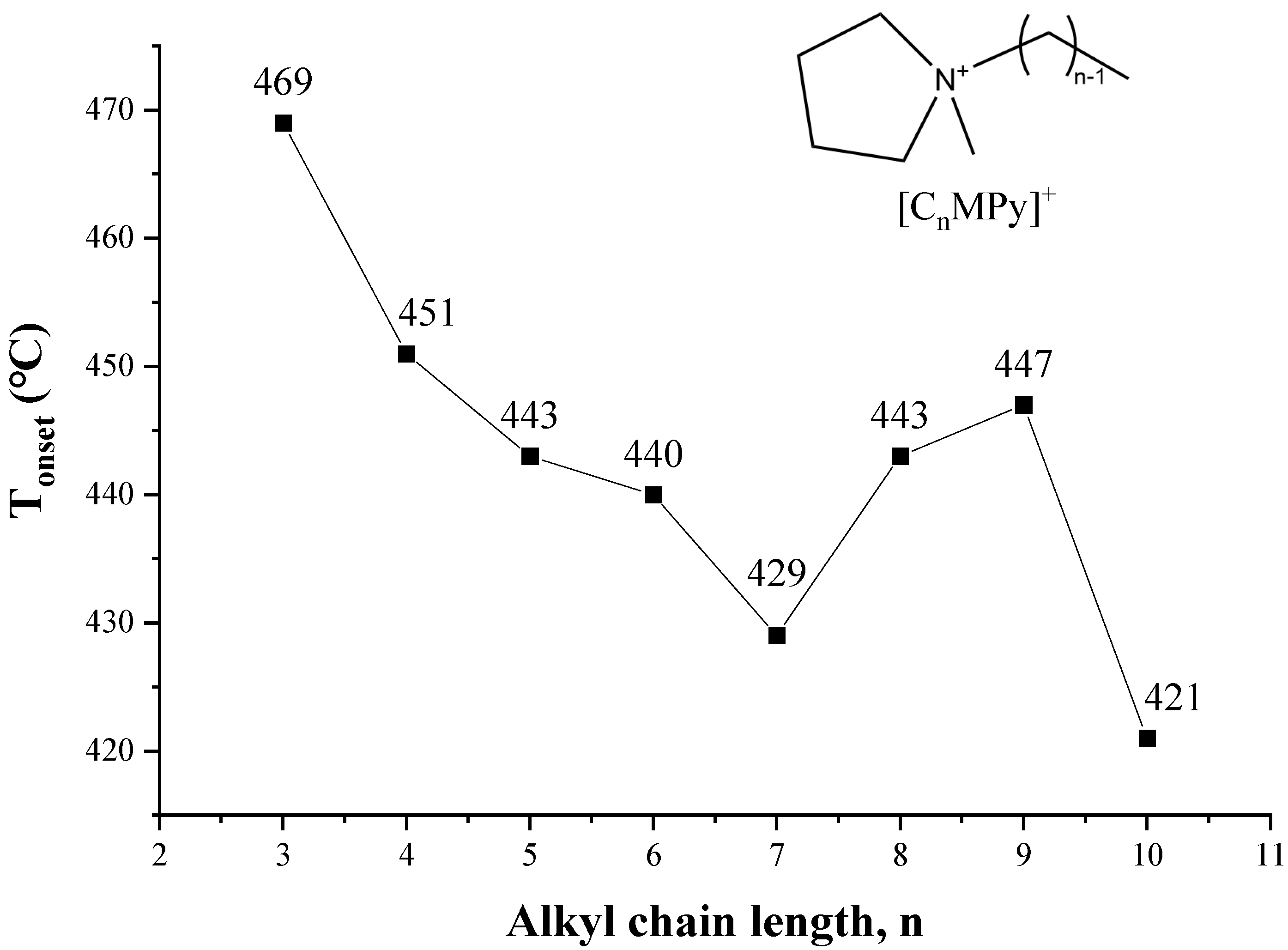
As shown in Figure 11, a method based on the cations exchange of ILs with sodium montmorillonite clay (MMT−Na+) is adopted to estimate thermal stability from [C2MIM]+ to [C16MIM]+ [104], and the T1% and T50% decrease with the increase of alkyl chain length. The same conclusion is obtained from quantum chemistry calculation [104]. The thermal stability of 1-alkyl-4-methyl-1,2,4-triazolium iodides shows a small but consistent decrease as the chain length is increased from butyl to dodecyl [105]. For the pyrrolidinium [NTf2] ILs in Figure 12, although Tonset of [C8MPy][NTf2] and [C9MPy][NTf2] are higher than [C7MPy][NTf2], it should be stressed that the longer chain length has resulted in lower thermal stability on the whole, and the difference of Tonset between [C3MPy]+ and [C10MPy]+ is up to 48 °C [72]. Moreover, the thermal decomposition temperatures of [CnIMBS][HSO4] generally decrease with the increase of chain length and the decomposition temperature decreases from 311 to 253 °C when n increases from 1 to 16. This trend has also been observed in some studies of [CnMIM][Cl] [57], [N8,8,8,n][BScB] [106], and [PP1n][NTf2] [107]. Generally, longer the chain length usually results in lower thermal stability, which is proved by more and more investigations [46,53].
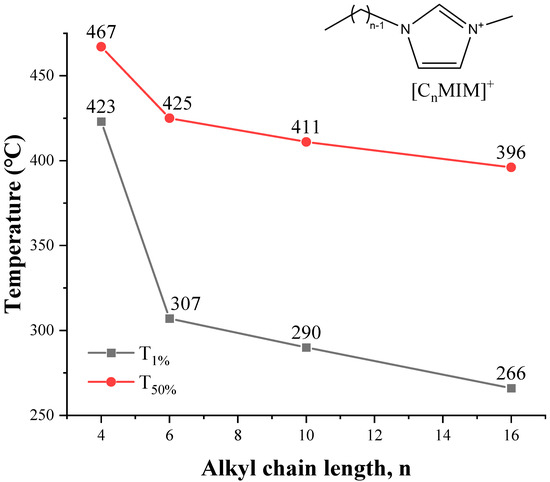
Figure 11.
Thermal stability of MMT–[CnMIM]+ clays at a heating rate of 10 °C/min in nitrogen atmosphere [104]. (n = 4, 6, 10, 16)
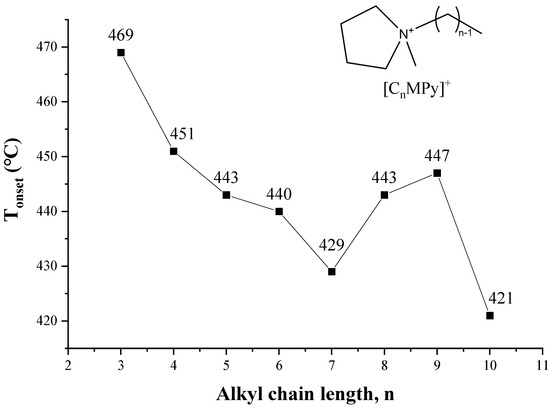
Figure 12.
Tonset of [CnMPy][NTf2] at a heating rate of 10 °C/min in nitrogen atmosphere [72]. (n = 3–10) Reproduced with permission from Gebrekidan Gebresilassie Eshetu, ChemSusChem; published by Wiley, 2017.
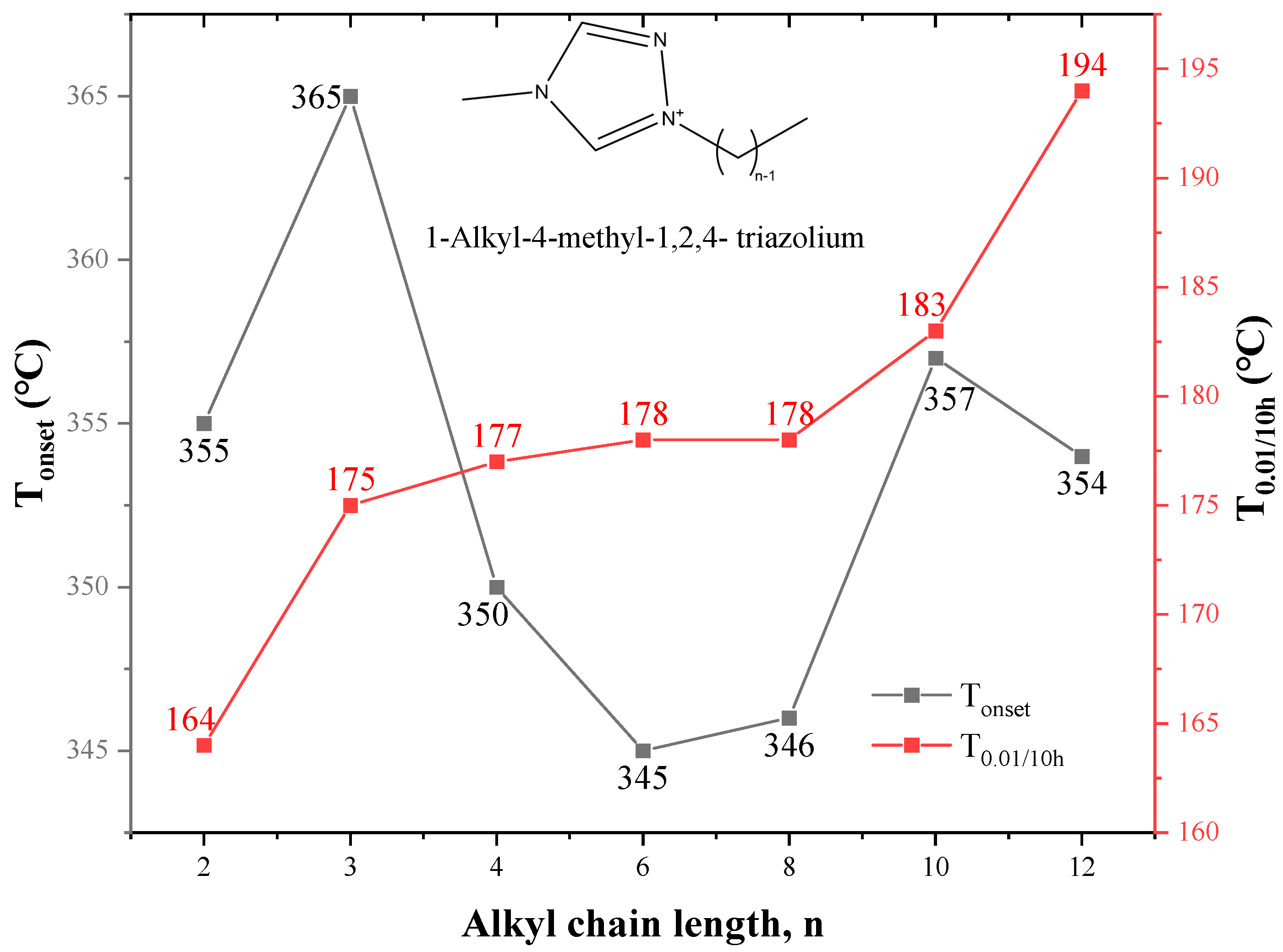
However, there are some exceptions. Thermal stability of 1-alkyl-4-methyl-1,2,4- triazolium [NTf2] ILs in Figure 13 reveals that there is no correlation between Tonset and alkyl chain length, and T0.01/10h instead increases with the increase of alkyl chain length [75]. The same results are obtained for some 3,5-dimethylpyrazolum ILs and some paramagnetic ILs [108,109]. In the study of amino acid ILs, [N1,1,14,2O12][Lys] with the long-chain alkyl group on the side of nitrogen has higher thermal stability than [N1,1,6,2O12][Lys] under the isothermal condition, while both longer and shorter alkyl side chains harm the thermal stability under the non-isothermal condition [77].
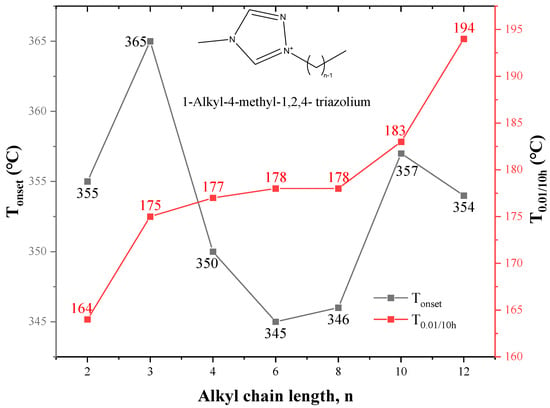
Figure 13.
Thermal Properties of 1-alkyl-4-methyl-1,2,4-triazolium [NTf2] ILs at a heating rate of 10 °C/min in nitrogen atmosphere [75] (n = 1, 2, 3, 5, 7, 9, 11).
The influence of alkyl chain length on thermal stability can be explained as follows:
- (1)
- Increasing the alkyl chain length weakens the bond between the alkyl chain and the cation such as imidazolium and ammonium, making it more vulnerable to attack and therefore more readily thermally decomposed [53,106];
- (2)
- Carbocations and carbon radicals with longer alkyl chains are more stable and easier to leave on heating, and thus ILs with longer alkyl chains favor the decomposition phenomenon [72];
- (3)
- For choline based amino acid ILs, longer alkyl chains on cation create strong hydrophobic interaction with gradually decreased hydrogen bond interaction due to bulkier cationic size. Upon decreasing alkyl chain length, the reverse effect renders lowering the overall thermal stability of the ILs [77].
4.2. Influence of Functionalization and Alkyl Substituents
Comparing the decomposition temperature of some imidazolium ILs and amino acid ILs with different functional groups in Table 8, the functionalization usually decreases the thermal stability of ILs. [C2MMIM][BF4] and [C2MIM][Br] are more stable than [C2NH2MMIM][BF4] and [C2NH2MIM][Br] respectively. The reduced thermal stability is explained by the introduction of amine to the imidazolium cation, which makes the nearby carbon atom more positively charged and easier to be attacked by the anion [101]. Aromatic functionality also decreases the thermal stability of imidazolium [NTf2] ILs, and the Tonset of IL with two benzyls is higher than that with naphthylmethyl [110].
Hydroxyl functionalization has different effects on the thermal stability of ILs. The decomposition temperatures of [CnOHMMIM][NTf2] are lower than [CnMMIM][NTf2] (n = 2, 3, 4, 6, 8) and the maximum difference can reach 48 °C [111]. Similarly, the substitution of the alkyl side chain to the hydroxyl group in [EMIM][BF4] and [C2MIM][C4F9SO3] reduces their thermal stability [74,112], which can be attributed to the higher chemical activity and easier decomposition of the hydroxyethyl group [61]. However, Tonset of [C3OHMIM][Cl] (295 °C) [113] are much higher than [C3MIM][Cl] (246 °C) [57], and hydroxyl functionalization also improves the thermal stability of [EMIM][Ac] and [C2MIM][C4F9CO2] [74,112]. It could be speculated that the hydrogen bonding interaction between the hydroxyethyl group and anion results in this trend, and the higher intramolecular hydrogen bond interaction stabilizes ILs and block thermal decomposition reaction to some extent [74].

Table 8.
Thermal stability of ILs with different functional groups at a heating rate of 10 °C/min in an inert atmosphere.
Table 8.
Thermal stability of ILs with different functional groups at a heating rate of 10 °C/min in an inert atmosphere.
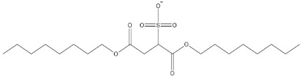
| ILs | Structure of Cations | Tonset/°C | Reference |
|---|---|---|---|
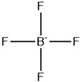
| [C2NH2MMIM][BF4] | 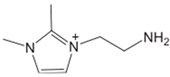 | 284 | [101] |
| [C2MMIM][BF4] | 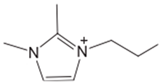 | 391 | [101] |
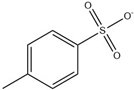
| [C2NH2MIM][Br] | 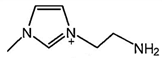 | 222 | [113] |
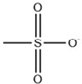
| [C2MIM][Br] | 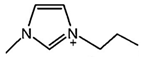 | 300 | [71] |
| [C7MIM][NTf2] | 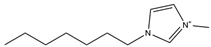 | 437 | [110] |
| [BnzMIM][NTf2] | 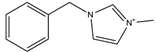 | 421 | [110] |
| [(Bnz)2IM][NTf2] | 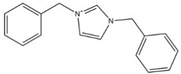 | 410 | [110] |
| [NapmMIM][NTf2] | 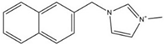 | 406 | [110] |
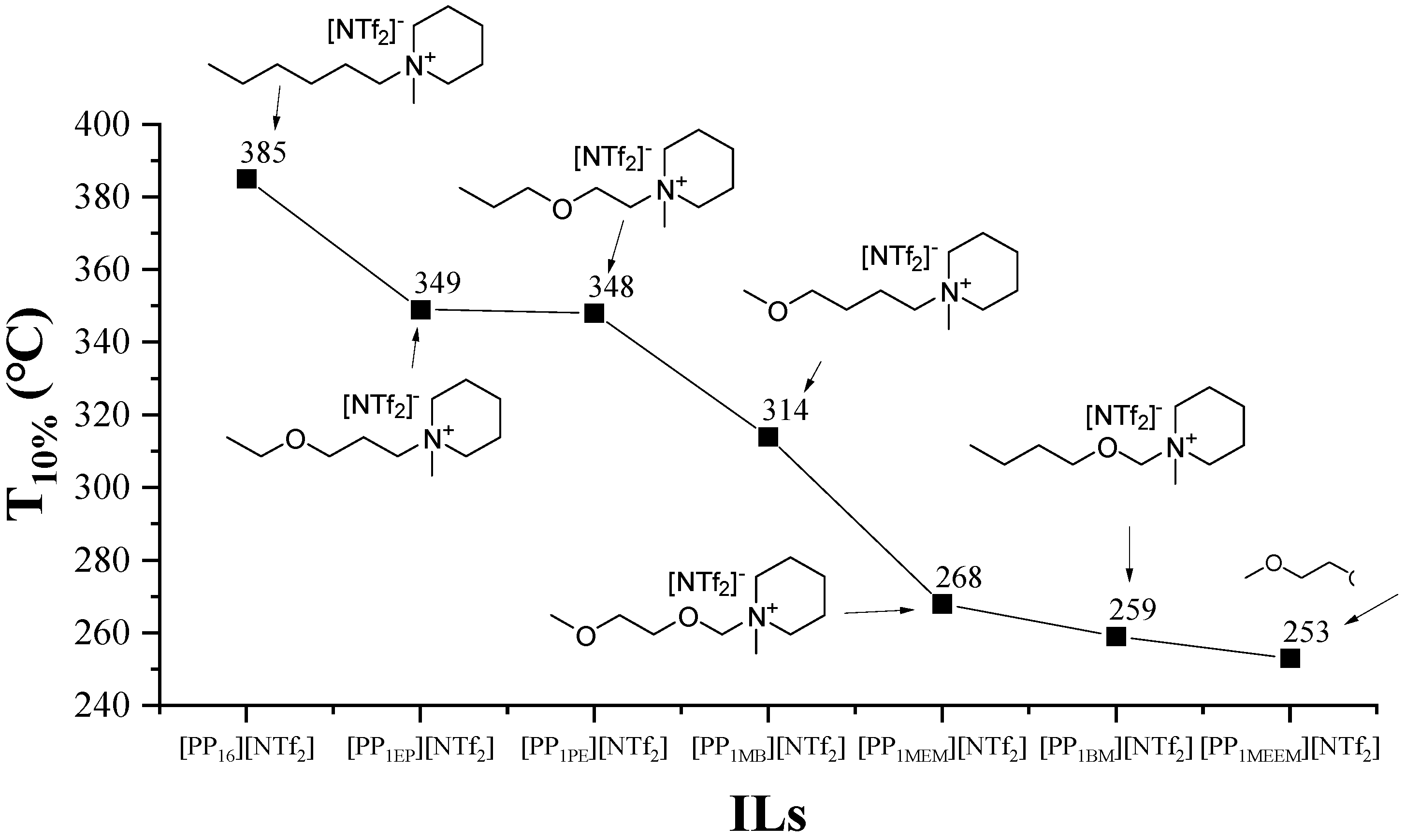
Moreover, ether in alkyl side chains is favorable for thermal decomposition. As the number of introduced ether increases, the thermal stability tends to decrease [66,114,115,116] because the introduction of oxygen atoms weakens the interaction between the cation and anion [77,115]. Specifically, Figure 14 demonstrates that the oxygen atom at the β-position significantly decreases the thermal stability of ILs, which can be explained by retro-alkylation of piperidinium ILs into 1-methyl piperidine and the corresponding oxocarbenium ion intermediates at elevated temperature [117]. In addition, the length of the O-alkyl chain also influences thermal stability. However, it is interesting that the increase of the length of the oxygen alkyl chain improves the thermal stability in isothermal TGA, while the conclusion obtained by non-isothermal TGA is opposite [77]. This contradiction between the results of non-isothermal TGA and isothermal TGA has also been found in other studies [44,57,75,78,110].
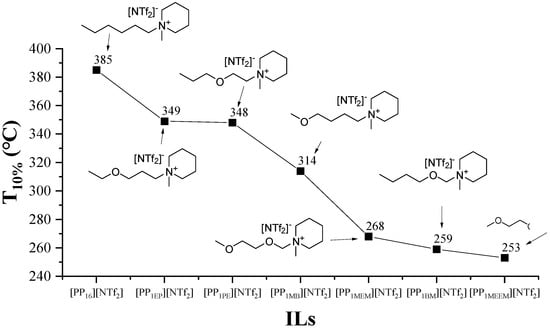
Figure 14.
Thermal stability of piperidinium ILs with an oxygen atom-containing alkyl side chain at a heating rate of 10 °C/min in argon atmosphere [117]. Reproduced with permission from T. Nokami, Faraday Discussions; published by Royal Society of Chemistry, 2018.
In addition to functional groups, the alkyl substituents also affect thermal stability. Ngo et al. [118] found that the thermal stability of the imidazolium ILs is improved by increasing the degree of substitution of hydrogen by alkyl groups on the imidazolium ring, the potential energy barrier for an attack is increased. Methyl substitution in C2 (the carbon atom between two nitrogen atoms in the imidazolium ring) enhances the thermal stability [45,74,119]. C-2H acidity of the imidazolium ring is one of the structural factors determining short-term thermal stability because the most acidic proton on the imidazolium cation locates at C2 [58].
Moreover, Table 9 shows the bonding of the alkyl chain via tertiary carbon atom decreases the thermal stability of the IL, compared to those isomers, in which the alkyl is connected with the secondary carbon atom to the imidazolium ring [66,120]. The potential energy barrier of the decomposition reaction decreases because the decomposition products originating from tertiary carbon can be easily stabilized [66,72].

Table 9.
T5% of several isomeric and quasi-isomeric ILs at a heating rate of 10 °C/min in argon atmosphere [66].
4.3. Influence of Anions and Cations
Anions play a major role in determining thermal stability, which has been confirmed in many studies [46,58,59]. For 1,2,4-triazolium ILs, the stability increases in the order [NTf2]− > [OTf]− > [OTs]− > [BF4]− > [OMs]− > [I]− > [NO3]− (Table 10). For tributyltetradecyl phosphonium ILs, the stability increases in the order [PFOS]− > [NPf2]− > [NTf2]− > [FeCl4]− > [AOT]− > [OS]− (Table 11). For nitrile-functionalized azepanium ILs, the stability increases in the order [OTf]− > [PTS]− > [CH3SO4]− > [OMs]− > [Br]− > [TFA]− (Table 12) For choline based amino acid ILs, the stability increases in the order [Tau]− > [Threo]− > [β-ala]− [77]. For fatty acid ILs, the stability increases in the order oleate > linoleate > caprylate > caproate [121]. The influence of anions on the thermal stability for other cations has been compared in detail by Xue et al. [122]. In general, ILs with [NTf2]−, [BF4]− or [PF6]− perform better in heat resistance, and imidazolium, pyrrolidinium, and pyridinium are considered as cations with excellent thermal stability [34,63,122].

Table 10.
Tonset of 1,2,4-triazolium ILs at a heating rate of 10 °C/min in nitrogen atmosphere [78].

Table 11.
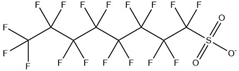
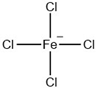
T5% of tributyltetradecyl phosphonium ILs at a heating rate of 10 °C/min in nitrogen atmosphere [37].

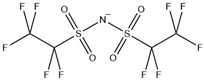
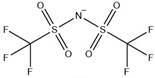
Table 12.
The thermal decomposition temperature of nitrile-functionalized azepanium ILs at a heating rate of 10 °C/min in an inert atmosphere [123].
5. Thermal Stability of Some Novel ILs
5.1. ILs Mixtures
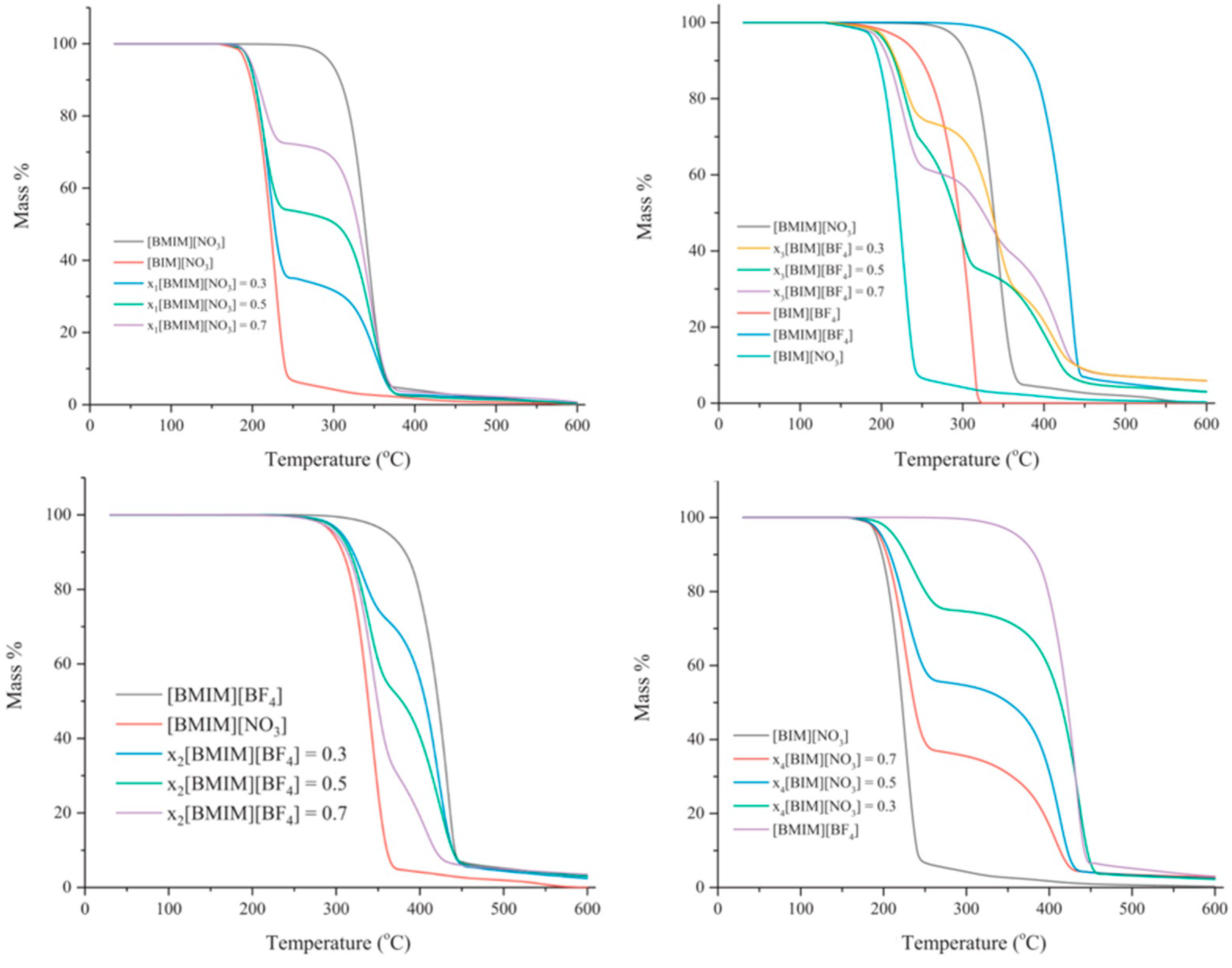
Preparing IL mixtures is a simpler and more promising approach compared with developing new ILs. Figure 15 shows TGA curves of binary mixtures with different proportions of [C4IM][BF4], [C4IM][NO3], [C4MIM][BF4] and [C4MIM][NO3] [124]. It can be concluded that the thermal stability of binary mixtures is determined by the ILs which could be reconstituted by all ions in the mixture [125]. In other words, the anions and cations can combine freely in the mixture [111,112].
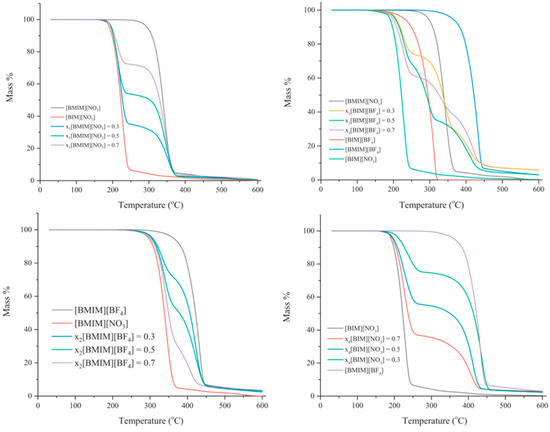
Figure 15.
TGA curves of binary mixtures with different ILs and proportions at a heating rate of 10 °C/min in nitrogen atmosphere [124].
In order to predict the thermal stability of mixtures, Navarro et al. [126] proposed a method to obtain the mass loss of IL mixtures at different temperatures by using pure ILs TGA data as
where is the mass that the mixture would lose in an ideal case at each temperature, denotes the experimental mass loss of species in forming the mixture at a certain temperature, refers to the mass fraction of the lth component in the mixture, and is the number of compounds involved in the mixture. This method has successfully predicted the thermal stability of several IL binary mixtures [80,126,127]. However, for the mixture of [4C4MPyr][NTf2] and [C2MIM][EtSO4], the model cannot predict its thermal stability well, and this may be related to the strong interaction between [EtSO4]− anion and the other ions in the mixture [128].
In addition, the study has revealed that mixing certain ILs in an appropriate ratio can improve thermal stability. TGA data of mixtures of [C4MIM][NTf2] and [C8MIM][AcO] in different proportions proves that when the mole fraction of [C8MIM][AcO] is between 0.1 and 0.3, T5% and T10% are significantly higher than the values predicted by Equation (30)[64]. The results of nuclear magnetic resonance (NMR) indicate that the synergistic role of hydrogen bond and electrostatic interactions are proposed as the main reason for the improvement of the thermal stability of the IL mixtures.
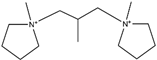
5.2. Dicationic ILs
Due to their unique properties, dicationic ILs (DILs) have been widely used in thermal storage [67,129], organic synthesis [130], anti-corrosion [131], or as surface-active agents [132], and they are less toxic than their monocationic counterparts [133].
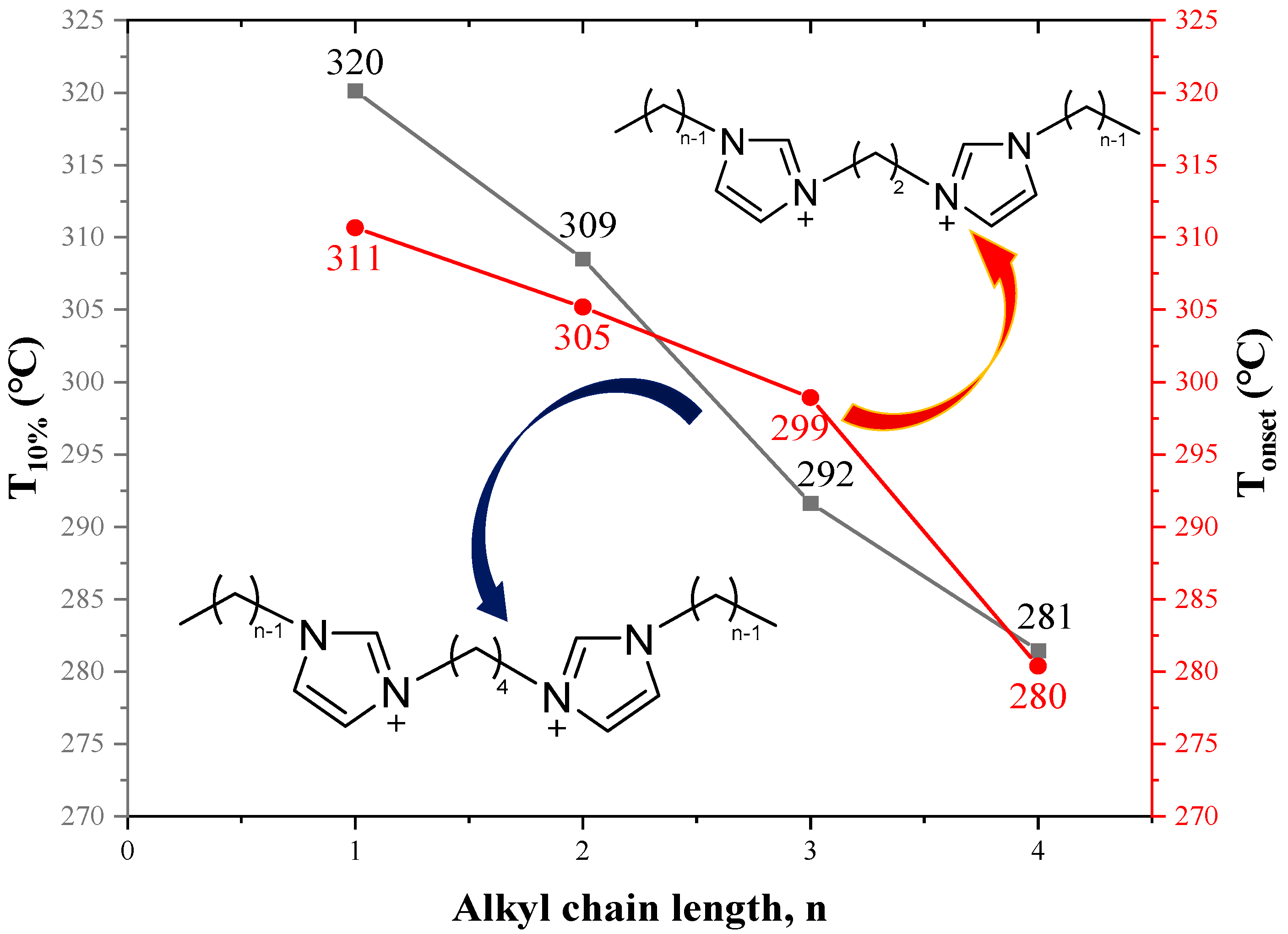
The Tonset of monocationic ILs [C10MIM][Br], [C12MIM][Br] and [C14MIM][Br], and dicationic ILs [C10(MIM)2][Br]2, [C12(MIM)2][Br]2 and [C14(MIM)2][Br]2, are shown in Figure 16. The values of DILs are 30-40 °C higher than their monocationic counterparts [134,135,136]. Other studies have obtained similar conclusions, which are ascribed to higher charges, higher molecular weight, and greater intermolecular interactions of DILs [6,129,137]. Due to their stronger electrostatic interactions, DILs often demonstrate higher melting points than monocationic ILs, which limits their applications [69]. Therefore, numerous methods of lowering melting points have been proposed, such as adjusting alkane linkage chain length, changing cationic head groups and anions, mixing DILs with the same cationic head groups and anion but different alkane linkage chains [37], utilizing branched alkane linkage chains [69] and designing unsymmetrical DILs [138].
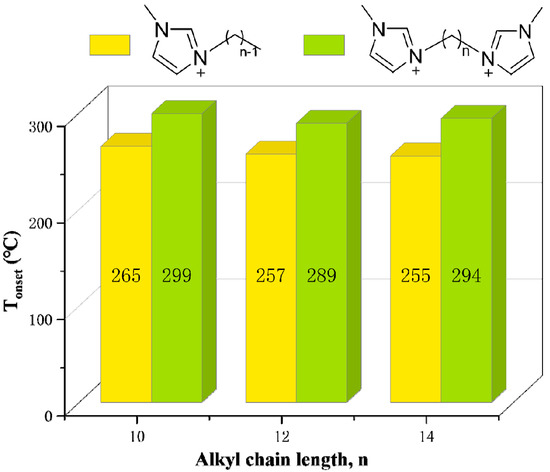
Figure 16.
Tonset of [CnMIM][Br] and [Cn(MIM)2][Br]2 obtained by TGA at a heating rate of 10 °C/min in nitrogen atmosphere [134] (n = 10, 12, 14).
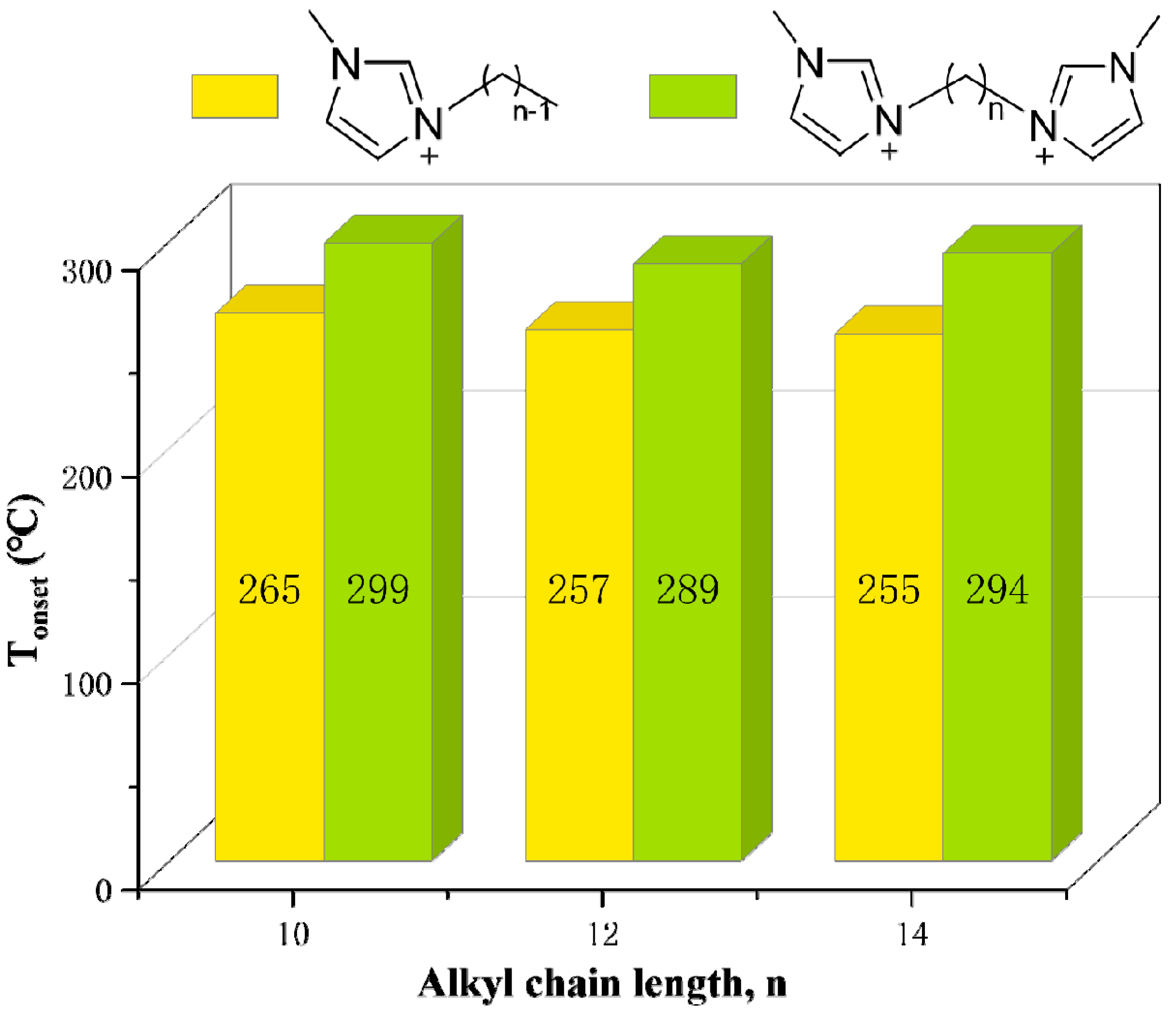
As shown in Table 13, when the linkage chain is the same, the thermal stability of DILs with cationic head groups of (MMIM), (C1Py), and (PC3C3C3) is significantly different. Moreover, Figure 17 suggests that the longer the alkyl side chains are, the lower the thermal stability of DILs are. The reduced thermal stability is attributed to the decreasing symmetry of cations with the increase of the number of carbon atoms in the alkyl side chains, which hinders the crystal effective accumulation [129,139]. Table 14 demonstrates that the decomposition temperatures of DILs with some functional groups are higher than those of DILs without corresponding functional groups. This might be explained by the appearance of complex functional groups increasing the strength of intermolecular interaction and the energy of chemical bonds [139].

Table 13.
Thermal stability of dicationic ILs (DILs) containing different cationic head groups at a heating rate of 10 °C/min in nitrogen atmosphere [69].
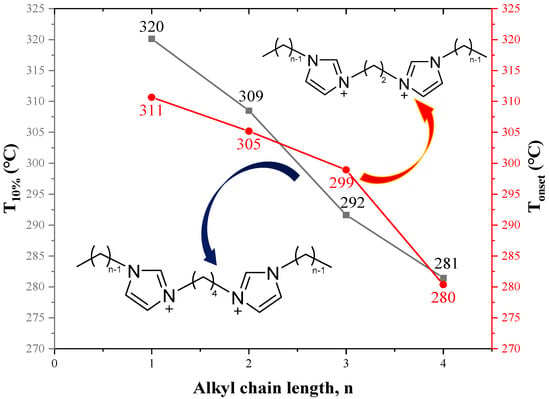
Figure 17.
Thermal decomposition temperature of [C2(CnIM)2][Br]2 and [C4(CnIM)2][Br]2 at a heating rate of 10 °C/min in nitrogen atmosphere [129,139] (n = 1–4).

Table 14.
Tonset of DILs with different functional groups at a heating rate of 10 °C/min in nitrogen atmosphere [139].
The change of the linkage chain between the two cationic head groups also has an important influence on thermal stability. The linkage chain is usually a straight-chain alkyl, and a longer linkage chain leads to worse thermal stability within a certain range, while a further increase in chain length leads to the decrease of thermal stability [37]. DILs with the branched linkage chain are developed due to reducing the high melting point of DILs with a straight linkage chain. Although the thermal stability of DILs containing the branched alkyl linkage chain is lower than their linear counterparts, it should be noted that this discrepancy is acceptable when the length of the linkage chain reaches C5. Therefore, the thermal stability of DILs can be tuned by branching linkage chains [69]. Recently, DILs with m-xylyl, pyridine functional groups, and dioxane linkage chain have been synthesized, and all of them have a low melting point and high thermal stability [140,141,142]. Similar to monocationic ILs, anions have a major influence in the thermal stability of DILs, and Table 15 reveals that the thermal stability order is as follows: [NTf2]− > [PF6]− >[BF4]− > [OTf]− > [Cl]− [129,141]. The [NTf2]− DILs also have high thermal stability and low melting point [37,138,140]. Generally, the influence factors of DILs structure on thermal stability are similar to monocationic ILs. Therefore, the properties of DILs that have not been determined by experiments can be inferred from the known monocationic ILs.

Table 15.
The decomposition temperature of DILs with different anions at a heating rate of 10 °C/min in nitrogen atmosphere [129,141]. Reproduced with permission from Jianlian Liu, Journal of Molecular Liquids; published by Elsevier, 2020; reproduced with permission from Coby J. Clarke, ACS Sustainable Chemistry & Engineering; published by American Chemical Society, 2020.
6. Summary
The methods measuring the thermal stability of ILs are discussed. Although Tonset overestimates the thermal stability, it is still used as a universal parameter in different investigations. It should be noted that when using Tonset to compare the thermal stability it is necessary to determine whether the experimental conditions (atmosphere type, heating rate, etc.) are the same. Although Tz/y is defined as a parameter to measure the long-term thermal stability of ILs, it is clear that this parameter cannot meet the required time of industrial running. Thus, a series of methods have been proposed to predict the thermal stability of ILs over a longer period. Among them, MOT is considered a highly accurate method, and the activation energy and pre-exponential factor values in the thermal decomposition process are required for calculating MOT.
Different means to study the thermal degradation kinetics of ILs are elaborated. After extensively analyzing the kinetic data from several investigations, it can be concluded that the isoconversional methods have been widely used to calculate the activation energy values, and the results of integral isoconversional methods are more accurate than those of differential methods. In addition, maximum-rate methods and Arrhenius methods are used in many research studies due to their simple calculation process, although both methods are not as precise as isoconversional methods. The Arrhenius method is also used to calculate the pre-exponential factor. However, compensation effect or master plots are seen lately as reliable tools for calculating pre-exponential factor values.
Thermal stability data of divergent ILs are summarized in Table 16 according to the types of anions, and this table reveals many structural factors affecting thermal stability, in which the modification of cations is the prime research object. For cations, a longer alkyl side chain, functionalization, and the bonding of alkyl chain via tertiary carbon atom all reduce the thermal stability of ILs. In the process of etherification, the number and position of oxygen atoms and the length of the O–alkyl chain have evident effects on thermal stability. Moreover, due to the high acidity of the C2 proton, methyl substitution in the C2 position on the imidazolium ring can improve the thermal stability. Although there are many methods to change the thermal stability by tuning cations, it should be stressed that the type of anions plays a major role in determining thermal stability. Generally, some specific anions such as [NTf2]−, [BF4]−, and [PF6]− and cations such as imidazolium, pyrrolidinium, and pyridinium make the ILs more stable at high temperatures.

Table 16.
Decomposition temperature and activation energy of some ILs and DILs.
Finally, the thermal stability of some novel ILs is introduced to provide more choices in high-temperature applications. In ILs mixtures, the anions and cations can combine freely. It is also feasible to predict the thermal stability of ILs mixtures by using TGA data of pure ILs. DILs have better thermal stability than their monocationic counterparts, and the influence factors of DILs structure on thermal stability are similar to monocationic ILs. Moreover, due to the stronger electrostatic interactions, DILs have a higher melting point, while adjusting the length of the linkage chain and the type of anions can obtain DILs with low melting point and high thermal stability.
Author Contributions
Conceptualization, C.X. and Z.C.; writing—review and editing, C.X.; review and editing, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21676085) and the National Key R&D Program of China (No. 2019YFC1906705).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| A | Pre-exponential factor, min−1 |
| Isoconversional values of pre-exponential factor, min−1 | |
| E | Activation energy, kJ mol−1 |
| Isoconversional values of the activation energy, kJ mol−1 | |
| Kinetic model function | |
| Integral kinetic model function | |
| Rate constant | |
| Number of compounds presented in the mixture | |
| Mass at a certain time | |
| End mass of the sample | |
| Initial mass | |
| Experimental mass loss of each IL forming the mixture at each temperature | |
| Mixture mass lose in an ideal case at each temperature | |
| MOT | Maximum operating temperature, °C |
| R | Universal gas constant, 8.314 J mol−1 K−1 |
| tmax | Maximum operation time |
| Tonset | Onset decomposition temperature, °C |
| Tpeak | Temperature of maximum degradation, °C |
| T50% | Temperature at the decomposition degree of 50%, °C |
| Tz | Temperature at the decomposition degree z, °C |
| T0.01/10h | Temperature at decomposition degree of 0.01 within 10 h, °C |
| Tz/y | Temperature at decomposition degree z within the selected time y, °C |
| Temperature at which the first appreciable weight loss occurs, K | |
| Each IL mass fraction in the mixture | |
| Greek Symbols | |
| α | Extent of conversion |
| β | Heating rate, °C min−1 |
| Subscripts | |
| α | Different extent of conversion |
| i | Different temperature programs |
| All heating programs other than | |
References
- Egorova, K.S.; Ananikov, V.P. Fundamental Importance of Ionic Interactions in the Liquid Phase: A Review of Recent Studies of Ionic Liquids in Biomedical and Pharmaceutical Applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, W.; Theyssen, N.; Chen, C.; Hou, Z. Temperature-Responsive Ionic Liquids: Fundamental Behaviors and Catalytic Applications. Chem. Rev. 2017, 117, 6881–6928. [Google Scholar] [CrossRef]
- Boruń, A. Conductance and Ionic Association of Selected Imidazolium Ionic Liquids in Various Solvents: A Review. J. Mol. Liq. 2019, 276, 214–224. [Google Scholar] [CrossRef]
- Ichikawa, T.; Kato, T.; Ohno, H. Dimension Control of Ionic Liquids. Chem. Commun. 2019, 55, 8205–8214. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, Y.; Wang, Y.; Wang, X. Viscosity of Typical Room-Temperature Ionic Liquids: A Critical Review. J. Phys. Chem. Ref. Data 2019, 48, 033101. [Google Scholar] [CrossRef]
- Götz, M.; Reimert, R.; Bajohr, S.; Schnetzer, H.; Wimberg, J.; Schubert, T.J.S. Long-Term Thermal Stability of Selected Ionic Liquids in Nitrogen and Hydrogen Atmosphere. Thermochim. Acta 2015, 600, 82–88. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Rybińska-Fryca, A.; Mikolajczyk, A.; Łuczak, J.; Paszkiewicz-Gawron, M.; Paszkiewicz, M.; Zaleska-Medynska, A.; Puzyn, T. How Thermal Stability of Ionic Liquids Leads to More Efficient TiO2-Based Nanophotocatalysts: Theoretical and Experimental Studies. J. Colloid Interface Sci. 2020, 572, 396–407. [Google Scholar] [CrossRef]
- Elhamifar, D.; Kazempoor, S.; Karimi, B. Amine-Functionalized Ionic Liquid-Based Mesoporous Organosilica as a Highly Efficient Nanocatalyst for the Knoevenagel Condensation. Catal. Sci. Technol. 2016, 6, 4318–4326. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M. Covalently Supported Ionic Liquid Phases: An Advanced Class of Recyclable Catalytic Systems. ChemCatChem 2016, 8, 664–684. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Jang, H.B.; Im, S.; Song, M.J.; Kim, S.-Y.; Park, S.-W.; Chi, D.Y.; Song, C.E.; Lee, S. SN2 Fluorination Reactions in Ionic Liquids: A Mechanistic Study towards Solvent Engineering. Org. Biomol. Chem. 2010, 9, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, S.; Pégot, B.; Marrot, J.; Magnier, E. Solvent Free Nucleophilic Introduction of Fluorine with [Bmim][F]. Tetrahedron Lett. 2014, 55, 826–829. [Google Scholar] [CrossRef]
- Newington, I.; Perez-Arlandis, J.M.; Welton, T. Ionic Liquids as Designer Solvents for Nucleophilic Aromatic Substitutions. Org. Lett. 2007, 9, 5247–5250. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Ullah, Z.; Nasrullah, A.; Khan, M.I.; Gonfa, G.; Ahmad, P.; Muhammad, N. Kinetics and Thermodynamic Parameters of Ionic Liquid Pretreated Rubber Wood Biomass. J. Mol. Liq. 2016, 223, 754–762. [Google Scholar] [CrossRef]
- Reina, L.; Botto, E.; Mantero, C.; Moyna, P.; Menéndez, P. Production of Second Generation Ethanol Using Eucalyptus Dunnii Bark Residues and Ionic Liquid Pretreatment. Biomass Bioenergy 2016, 93, 116–121. [Google Scholar] [CrossRef]
- Gregorio, G.F.D.; Weber, C.C.; Gräsvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic Insights into Lignin Depolymerisation in Acidic Ionic Liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Grimes, S.; Cai, H.; Zhang, M. Study on the Absorbability, Regeneration Characteristics and Thermal Stability of Ionic Liquids for VOCs Removal. Chem. Eng. J. 2017, 328, 353–359. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, L.; Jiang, J. CO2 Capture with Complex Absorbent of Ionic Liquid, Surfactant and Water. J. Environ. Chem. Eng. 2015, 3, 227–232. [Google Scholar] [CrossRef]
- Faghihi-Zarandi, A.; Shirkhanloo, H.; Jamshidzadeh, C. A New Method for Removal of Hazardous Toluene Vapor from Air Based on Ionic Liquid-Phase Adsorbent. Int. J. Environ. Sci. Technol. 2019, 16, 2797–2808. [Google Scholar] [CrossRef]
- Gębicki, J.; Kloskowski, A.; Chrzanowski, W.; Stepnowski, P.; Namiesnik, J. Application of Ionic Liquids in Amperometric Gas Sensors. Crit. Rev. Anal. Chem. 2016, 46, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zhang, Z.; Li, X.; Yang, L.; Tachibana, K.; Hirano, S. Polymer Electrolytes Based on Dicationic Polymeric Ionic Liquids: Application in Lithium Metal Batteries. J. Mater. Chem. A 2014, 3, 170–178. [Google Scholar] [CrossRef]
- Varela, J.C.; Sankar, K.; Hino, A.; Lin, X.; Chang, W.; Coker, D.; Grinstaff, M. Piperidinium Ionic Liquids as Electrolyte Solvents for Sustained High Temperature Supercapacitor Operation. Chem. Commun. 2018, 54, 5590–5593. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, D.; Stępniak, I.; Galiński, M. Acetate- and Lactate-Based Ionic Liquids: Synthesis, Characterisation and Electrochemical Properties. J. Mol. Liq. 2018, 264, 233–241. [Google Scholar] [CrossRef]
- Wang, X.; Jin, M.; Li, Y.; Zhao, L. The Influence of Various Ionic Liquids on the Properties of SPEEK Membrane Doped with Mesoporous Silica. Electrochim. Acta 2017, 257, 290–300. [Google Scholar] [CrossRef]
- Wu, H.; Shen, F.; Wang, J.; Wan, Y. Membrane Fouling in Vacuum Membrane Distillation for Ionic Liquid Recycling: Interaction Energy Analysis with the XDLVO Approach. J. Membr. Sci. 2018, 550, 436–447. [Google Scholar] [CrossRef]
- Pizzoccaro-Zilamy, M.-A.; Drobek, M.; Petit, E.; Totée, C.; Silly, G.; Guerrero, G.; Cowan, M.G.; Ayral, A.; Julbe, A. Initial Steps toward the Development of Grafted Ionic Liquid Membranes for the Selective Transport of CO2. Ind. Eng. Chem. Res. 2018, 57, 16027–16040. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Cai, M.; Zhou, F.; Liu, W. Investigation of the Lubricity and Antiwear Behavior of Guanidinium Ionic Liquids at High Temperature. Tribol. Int. 2017, 114, 65–76. [Google Scholar] [CrossRef]
- Lee, J.; Yeo, C.-D.; Hu, Z.; Thalangama-Arachchige, V.D.; Kaur, J.; Quitevis, E.L.; Kumar, G.; Koh, Y.P.; Simon, S. Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures. Metals 2019, 9, 1180. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ding, Q.; Feng, D.; Qin, B.; Hu, L. The Corrosion and Lubrication Properties of 2-Mercaptobenzothiazole Functionalized Ionic Liquids for Bronze. Tribol. Int. 2017, 114, 121–131. [Google Scholar] [CrossRef]
- Kaur, N. Green Synthesis of Three- to Five-Membered O-Heterocycles Using Ionic Liquids. Synth. Commun. 2018, 48, 1588–1613. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic Properties and Thermal Stability of Ionic Liquid-Based Nanofluids Containing Graphene as Advanced Heat Transfer Fluids for Medium-to-High-Temperature Applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Li, B.; Sundén, B. A Review on Molten-Salt-Based and Ionic-Liquid-Based Nanofluids for Medium-to-High Temperature Heat Transfer. J. Therm. Anal. Calorim. 2019, 136, 1037–1051. [Google Scholar] [CrossRef]
- Lamas, A.; Brito, I.; Salazar, F.; Graber, T.A. Synthesis and Characterization of Physical, Thermal and Thermodynamic Properties of Ionic Liquids Based on [C12mim] and [N444H] Cations for Thermal Energy Storage. J. Mol. Liq. 2016, 224, 999–1007. [Google Scholar] [CrossRef]
- Mehrkesh, A.; Karunanithi, A.T. Optimal Design of Ionic Liquids for Thermal Energy Storage. Comput. Chem. Eng. 2016, 93, 402–412. [Google Scholar] [CrossRef]
- Williams, M.L.; Holahan, S.P.; McCorkill, M.E.; Dickmann, J.S.; Kiran, E. Thermal and Spectral Characterization and Stability of Mixtures of Ionic Liquids [EMIM]Ac and [BMIM]Ac with Ethanol, Methanol, and Water at Ambient Conditions and at Elevated Temperatures and Pressures. Thermochim. Acta 2018, 669, 126–139. [Google Scholar] [CrossRef]
- Patil, R.A.; Talebi, M.; Xu, C.; Bhawal, S.S.; Armstrong, D.W. Synthesis of Thermally Stable Geminal Dicationic Ionic Liquids and Related Ionic Compounds: An Examination of Physicochemical Properties by Structural Modification. Chem. Mater. 2016, 28, 4315–4323. [Google Scholar] [CrossRef]
- Odugbesi, G.A.; Nan, H.; Soltani, M.; Davis, J.H.; Anderson, J.L. Ultra-High Thermal Stability Perarylated Ionic Liquids as Gas Chromatographic Stationary Phases for the Selective Separation of Polyaromatic Hydrocarbons and Polychlorinated Biphenyls. J. Chromatogr. A 2019, 1604, 460466. [Google Scholar] [CrossRef] [PubMed]
- Efimova, A.; Varga, J.; Matuschek, G.; Saraji-Bozorgzad, M.R.; Denner, T.; Zimmermann, R.; Schmidt, P. Thermal Resilience of Imidazolium-Based Ionic Liquids—Studies on Short- and Long-Term Thermal Stability and Decomposition Mechanism of 1-Alkyl-3-Methylimidazolium Halides by Thermal Analysis and Single-Photon Ionization Time-of-Flight Mass Spectrometry. J. Phys. Chem. B 2018, 122, 8738–8749. [Google Scholar] [CrossRef]
- Deferm, C.; van den Bossche, A.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Thermal Stability of Trihexyl(Tetradecyl)Phosphonium Chloride. Phys. Chem. Chem. Phys. 2018, 20, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Talebi, M.; Berthod, A.; Armstrong, D.W. Dicationic Ionic Liquid Thermal Decomposition Pathways. Anal. Bioanal. Chem. 2018, 410, 4645–4655. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Siu, B.; Salter, E.A.; Wierzbicki, A.; West, K.N.; Davis, J.H. Synthesis, Thermal Stability, and Computed Bond Dissociation Energies of Tetraarylphosphonium-Based Mesothermal Ionic Liquids Bearing a Quinoline Ring System. Tetrahedron Lett. 2017, 58, 4628–4631. [Google Scholar] [CrossRef]
- Siu, B.; Cassity, C.G.; Benchea, A.; Hamby, T.; Hendrich, J.; Strickland, K.J.; Wierzbicki, A.; Sykora, R.E.; Salter, E.A.; O’Brien, R.A.; et al. Thermally Robust: Triarylsulfonium Ionic Liquids Stable in Air for 90 Days at 300 °C. RSC Adv. 2017, 7, 7623–7630. [Google Scholar] [CrossRef]
- Parajó, J.J.; Teijeira, T.; Fernández, J.; Salgado, J.; Villanueva, M. Thermal Stability of Some Imidazolium [NTf 2 ] Ionic Liquids: Isothermal and Dynamic Kinetic Study through Thermogravimetric Procedures. J. Chem. Thermodyn. 2017, 112, 105–113. [Google Scholar] [CrossRef]
- Maton, C.; Vos, N.D.; Stevens, C.V. Ionic Liquid Thermal Stabilities: Decomposition Mechanisms and Analysis Tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Al-Sallami, W.; Parsaeian, P.; Neville, A. An Appraisal of the Thermal Decomposition Mechanisms of ILs as Potential Lubricants. Lubr. Sci. 2019, 31, 229–238. [Google Scholar] [CrossRef]
- Salgado, J.; Villanueva, M.; Parajó, J.J.; Fernández, J. Long-Term Thermal Stability of Five Imidazolium Ionic Liquids. J. Chem. Thermodyn. 2013, 65, 184–190. [Google Scholar] [CrossRef]
- Blanco, D.; Bartolomé, M.; Ramajo, B.; Viesca, J.L.; González, R.; Hernández Battez, A. Isoconversional Kinetic Analysis Applied to Five Phosphonium Cation-Based Ionic Liquids. Thermochim. Acta 2017, 648, 62–74. [Google Scholar] [CrossRef]
- Blanco, D.; Oulego, P.; Ramos, D.; Fernández, B.; Cuetos, J.M. Model-Free Kinetics Applied to Evaluate the Long-Term Thermal Stability of Three [NTf2] Anion-Based Ionic Liquids. Thermochim. Acta 2017, 656, 70–84. [Google Scholar] [CrossRef]
- Seeberger, A.; Andresen, A.-K.; Jess, A. Prediction of Long-Term Stability of Ionic Liquids at Elevated Temperatures by Means of Non-Isothermal Thermogravimetrical Analysis. Phys. Chem. Chem. Phys. 2009, 11, 9375. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.; Parajó, J.J.; Fernández, J.; Villanueva, M. Long-Term Thermal Stability of Some 1-Butyl-1-Methylpyrrolidinium Ionic Liquids. J. Chem. Thermodyn. 2014, 74, 51–57. [Google Scholar] [CrossRef]
- Wooster, T.J.; Johanson, K.M.; Fraser, K.J.; MacFarlane, D.R.; Scott, J.L. Thermal Degradation of Cyano Containing Ionic Liquids. Green Chem. 2006, 8, 691. [Google Scholar] [CrossRef]
- Quraishi, K.S.; Bustam, M.A.; Krishnan, S.; Khan, M.I.; Wilfred, C.D.; Lévêque, J.-M. Thermokinetics of Alkyl Methylpyrrolidinium [NTf2] Ionic Liquids: Effect of Alkyl Chain on Thermal Stability. J. Therm. Anal. Calorim. 2017, 129, 261–270. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z.; Khan, A.S. Thermal Stability and Kinetic Study of Benzimidazolium Based Ionic Liquid. Procedia Eng. 2016, 148, 215–222. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Lu, T.; Nong, D.; Zhao, J.; Wei, M.; Teng, L.; Teng, L. Isoconversional Kinetic Analysis of Thermal Decomposition of 1-Butyl-3-Methylimidazolium Hexafluorophosphate under Inert Nitrogen and Oxidative Air Atmospheres. J. Therm. Anal. Calorim. 2019, 140, 695–712. [Google Scholar] [CrossRef]
- Jiang, H.-C.; Lin, W.-C.; Hua, M.; Pan, X.-H.; Shu, C.-M.; Jiang, J.-C. Analysis of Thermal Stability and Pyrolysis Kinetic of Dibutyl Phosphate-Based Ionic Liquid through Thermogravimetry, Gas Chromatography/Mass Spectrometry, and Fourier Transform Infrared Spectrometry. J. Therm. Anal. Calorim. 2019, 138, 489–499. [Google Scholar] [CrossRef]
- Williams, M.L.; Dickmann, J.S.; McCorkill, M.E.; Hassler, J.C.; Kiran, E. The Kinetics of Thermal Decomposition of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquids under Isothermal and Non-Isothermal Conditions. Thermochim. Acta 2020, 685, 178509. [Google Scholar] [CrossRef]
- Akçay, A.; Balci, V.; Uzun, A. Structural Factors Controlling Thermal Stability of Imidazolium Ionic Liquids with 1- n -Butyl-3-Methylimidazolium Cation on γ-Al 2 O 3. Thermochim. Acta 2014, 589, 131–136. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Villemin, D.; Didi, M.A. Synthesis of Bisimidazolium–Ionic Liquids: Characterization, Thermal Stability and Application to Bentonite Intercalation. J. Taibah Univ. Sci. 2016, 10, 168–180. [Google Scholar] [CrossRef]
- Mezzetta, A.; Łuczak, J.; Woch, J.; Chiappe, C.; Nowicki, J.; Guazzelli, L. Surface Active Fatty Acid ILs: Influence of the Hydrophobic Tail and/or the Imidazolium Hydroxyl Functionalization on Aggregates Formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Song, Y.; Xia, Y.; Liu, Z. Influence of Cation Structure on Physicochemical and Antiwear Properties of Hydroxyl-Functionalized Imidazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids. Tribol. Trans. 2012, 55, 738–746. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Thermal Stability of Ionic Liquids. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2020; pp. 1–13. ISBN 978-981-10-6739-6. [Google Scholar]
- Parajó, J.J.; Villanueva, M.; Otero, I.; Fernández, J.; Salgado, J. Thermal Stability of Aprotic Ionic Liquids as Potential Lubricants. Comparison with Synthetic Oil Bases. J. Chem. Thermodyn. 2018, 116, 185–196. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, L.; Zhai, S.; Zhao, D.; Sun, J.; An, Q. Hydrogen Bond Promoted Thermal Stability Enhancement of Acetate Based Ionic Liquid. Chin. J. Chem. Eng. 2020, S1004954120300847. [Google Scholar] [CrossRef]
- Monteiro, B.; Maria, L.; Cruz, A.; Carretas, J.M.; Marçalo, J.; Leal, J.P. Thermal Stability and Specific Heats of Coordinating Ionic Liquids. Thermochim. Acta 2020, 684, 178482. [Google Scholar] [CrossRef]
- Rotrekl, J.; Jandová, V.; Storch, J.; Velíšek, P.; Cuřínová, P.; Schwarz, J.; Wagner, Z.; Bendová, M. Thermal Properties of Novel Oligoether-Substituted Ionic Liquids and the Influence of Alkyl-Substituent Isomery. Fluid Phase Equilibria 2020, 514, 112561. [Google Scholar] [CrossRef]
- Kuhn, B.L.; Osmari, B.F.; Heinen, T.M.; Bonacorso, H.G.; Zanatta, N.; Nielsen, S.O.; Ranathunga, D.T.S.; Villetti, M.A.; Frizzo, C.P. Dicationic Imidazolium-Based Dicarboxylate Ionic Liquids: Thermophysical Properties and Solubility. J. Mol. Liq. 2020, 308, 112983. [Google Scholar] [CrossRef]
- Thasneema, K.K.; Thayyil, M.S.; Rosalin, T.; Elyas, K.K.; Dipin, T.; Sahu, P.K.; Krishna Kumar, N.S.; Saheer, V.C.; Messali, M.; Hadda, T.B. Thermal and Spectroscopic Investigations on Three Phosphonium Based Ionic Liquids for Industrial and Biological Applications. J. Mol. Liq. 2020, 307, 112960. [Google Scholar] [CrossRef]
- Talebi, M.; Patil, R.A.; Armstrong, D.W. Physicochemical Properties of Branched-Chain Dicationic Ionic Liquids. J. Mol. Liq. 2018, 256, 247–255. [Google Scholar] [CrossRef]
- Navarro, P.; Larriba, M.; Rojo, E.; García, J.; Rodríguez, F. Thermal Properties of Cyano-Based Ionic Liquids. J. Chem. Eng. Data 2013, 58, 2187–2193. [Google Scholar] [CrossRef]
- Efimova, A.; Pfützner, L.; Schmidt, P. Thermal Stability and Decomposition Mechanism of 1-Ethyl-3-Methylimidazolium Halides. Thermochim. Acta 2015, 604, 129–136. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Jeong, S.; Pandard, P.; Lecocq, A.; Marlair, G.; Passerini, S. Comprehensive Insights into the Thermal Stability, Biodegradability, and Combustion Chemistry of Pyrrolidinium-Based Ionic Liquids. ChemSusChem 2017, 10, 3146–3159. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Troncoso, J.; Salgado, J. Thermophysical Properties of Choline and Pyridinium Based Ionic Liquids as Advanced Materials for Energy Applications. J. Chem. Thermodyn. 2020, 141, 105947. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- De la Hoz, A.T.; Brauer, U.G.; Miller, K.M. Physicochemical and Thermal Properties for a Series of 1-Alkyl-4-Methyl-1,2,4-Triazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids. J. Phys. Chem. B 2014, 118, 9944–9951. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Vilas, M.; Verdía, P.; Villanueva, M.; Salgado, J.; Tojo, E. Long-Term Thermal Stabilities of Ammonium Ionic Liquids Designed as Potential Absorbents of Ammonia. RSC Adv. 2015, 5, 41278–41284. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shah, F.U. Thermal Stability of Choline Based Amino Acid Ionic Liquids. J. Mol. Liq. 2018, 266, 597–602. [Google Scholar] [CrossRef]
- Brauer, U.G.; De la Hoz, A.T.; Miller, K.M. The Effect of Counteranion on the Physicochemical and Thermal Properties of 4-Methyl-1-Propyl-1,2,4-Triazolium Ionic Liquids. J. Mol. Liq. 2015, 210, 286–292. [Google Scholar] [CrossRef]
- Feng, W.; Lu, Y.; Chen, Y.; Lu, Y.; Yang, T. Thermal Stability of Imidazolium-Based Ionic Liquids Investigated by TG and FTIR Techniques. J. Therm. Anal. Calorim. 2016, 125, 143–154. [Google Scholar] [CrossRef]
- Navarro, P.; Larriba, M.; García, J.; Rodríguez, F. Thermal Stability and Specific Heats of {[Emim][DCA]+[Emim][TCM]} Mixed Ionic Liquids. Thermochim. Acta 2014, 588, 22–27. [Google Scholar] [CrossRef]
- Shah, F.U.; Khan, I.A.; Johansson, P. Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids. Molecules 2020, 25, 2388. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Li, K.; An, Q.; Ma, H.; Yang, L.; Wei, L. Water Addition Enhanced Thermal Stability of Alkylimidazolium Acetate in Ionosolv Treatment of Lignin. Int. J. Biol. Macromol. 2019, 141, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Oster, K.; Goodrich, P.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. A New Insight into Pure and Water-Saturated Quaternary Phosphonium-Based Carboxylate Ionic Liquids: Density, Heat Capacity, Ionic Conductivity, Thermogravimetric Analysis, Thermal Conductivity and Viscosity. J. Chem. Thermodyn. 2018, 121, 97–111. [Google Scholar] [CrossRef]
- Saikat, M.S.H.; Islam, M.M.; Mollah, M.Y.A.; Susan, M.A.B.H.; Miran, M.S. Thermal and Electrochemical Properties of Protic Ionic Liquids and Their Binary Mixtures with Water. Mater. Today Proc. 2019, 15, 498–503. [Google Scholar] [CrossRef]
- Yamaki, N.; Shiota, K.; Izato, Y.; Hoang, D.K.; Miyake, A. Thermal Hazard Analysis of a Biomass Pretreatment Process Using Ionic Liquids. J. Therm. Anal. Calorim. 2019, 138, 2945–2953. [Google Scholar] [CrossRef]
- Muhammad, A. Thermal and Kinetic Analysis of Pure and Contaminated Ionic Liquid: 1-Butyl-2.3-Dimethylimidazolium Chloride (BDMIMCl). Pol. J. Chem. Technol. 2016, 18, 122–125. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the Integral Isoconversional Method to Account for Variation in the Activation Energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Volli, V.; Lin, W.-C.; Krishna, G.V.S.; Bhardwaj, H.; Shu, C.-M. Oxidative Stability, Thermal Hazard Analysis, and Decomposition Kinetics of 1-Methylimidazolium Nitrate via DSC, TGA, and GC/MS. J. Therm. Anal. Calorim. 2019, 138, 3403–3413. [Google Scholar] [CrossRef]
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. Phys. Chem. 1966, 70A. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. BCSJ 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of Determining Activation Deterioration Constant of Electrical Insulating Materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Starink, M.J. A New Method for the Derivation of Activation Energies from Experiments Performed at Constant Heating Rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Vyazovkin, S. A Unified Approach to Kinetic Processing of Nonisothermal Data. Int. J. Chem. Kinet. 1996, 28, 95–101. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and Nonlinear Procedures in Isoconversional Computations of the Activation Energy of Nonisothermal Reactions in Solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of Activation Energy of Thermally Stimulated Solid-State Reactions under Arbitrary Variation of Temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Heym, F.; Korth, W.; Thiessen, J.; Kern, C.; Jess, A. Evaporation and Decomposition Behavior of Pure and Supported Ionic Liquids under Thermal Stress. Chem. Ing. Tech. 2015, 87, 791–802. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Shi, Y.; Sun, H.; Zhou, Z.; Mu, T. Investigations on the Thermal Stability and Decomposition Mechanism of an Amine-Functionalized Ionic Liquid by TGA, NMR, TG-MS Experiments and DFT Calculations. J. Mol. Liq. 2015, 206, 95–102. [Google Scholar] [CrossRef]
- Criado, J.M.; Málek, J.; Ortega, A. Applicability of the Master Plots in Kinetic Analysis of Non-Isothermal Data. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Málek, J. The Kinetic Analysis of Non-Isothermal Data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Thomas, E.; Vijayalakshmi, K.P.; George, B.K. Kinetic Stability of Imidazolium Cations and Ionic Liquids: A Frontier Molecular Orbital Approach. J. Mol. Liq. 2019, 276, 721–727. [Google Scholar] [CrossRef]
- Daily, L.A.; Miller, K.M. Correlating Structure with Thermal Properties for a Series of 1-Alkyl-4-Methyl-1,2,4-Triazolium Ionic Liquids. J. Org. Chem. 2013, 78, 4196–4201. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Bakshi, P.S.; Panda, S.; Sharma, O.P.; Gardas, R.; Khatri, O.P. Physicochemical and Tribophysical Properties of Trioctylalkylammonium Bis(Salicylato)Borate (N888n-BScB) Ionic Liquids: Effect of Alkyl Chain Length. Phys. Chem. Chem. Phys. 2017, 19, 6433–6442. [Google Scholar] [CrossRef] [PubMed]
- Montanino, M.; Carewska, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. The Role of the Cation Aliphatic Side Chain Length in Piperidinium Bis(Trifluoromethansulfonyl)Imide Ionic Liquids. Electrochim. Acta 2011, 57, 153–159. [Google Scholar] [CrossRef]
- Özdemir, M.C.; Özgün, B. Phenyl/Alkyl-Substituted-3,5-Dimethylpyrazolium Ionic Liquids. J. Mol. Liq. 2014, 200, 129–135. [Google Scholar] [CrossRef]
- Greeson, K.T.; Hall, N.G.; Redeker, N.D.; Marcischak, J.C.; Gilmore, L.V.; Boatz, J.A.; Le, T.C.; Alston, J.R.; Guenthner, A.J.; Ghiassi, K.B. Synthesis and Properties of Symmetrical N,N′-Bis(Alkyl)Imidazolium Bromotrichloroferrate(III) Paramagnetic, Room Temperature Ionic Liquids with High Short-Term Thermal Stability. J. Mol. Liq. 2018, 265, 701–710. [Google Scholar] [CrossRef]
- Tao, R.; Tamas, G.; Xue, L.; Simon, S.L.; Quitevis, E.L. Thermophysical Properties of Imidazolium-Based Ionic Liquids: The Effect of Aliphatic versus Aromatic Functionality. J. Chem. Eng. Data 2014, 59, 2717–2724. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Chernikova, E.A.; Glukhov, L.M.; Kapustin, G.I.; Koroteev, A.A. Effect of Hydroxyl Groups in a Cation Structure on the Properties of Ionic Liquids. Russ. J. Phys. Chem. A 2018, 92, 2379–2385. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Araujo, J.M.M.; Vega, L.F.; Llovell, F.; Pereiro, A.B. Functionalization of Fluorinated Ionic Liquids: A Combined Experimental-Theoretical Study. J. Mol. Liq. 2020, 302, 112489. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Zhou, X.; Cao, Y.; Mu, T. Thermal Stabilities and Decomposition Mechanism of Amino- and Hydroxyl-Functionalized Ionic Liquids. Thermochim. Acta 2014, 578, 59–67. [Google Scholar] [CrossRef]
- Coadou, E.; Goodrich, P.; Neale, A.R.; Timperman, L.; Hardacre, C.; Jacquemin, J.; Anouti, M. Synthesis and Thermophysical Properties of Ether-Functionalized Sulfonium Ionic Liquids as Potential Electrolytes for Electrochemical Applications. Chemphyschem 2016, 17, 3992–4002. [Google Scholar] [CrossRef]
- Mahrova, M.; Conte, M.; Roman, E.; Nevshupa, R. Critical Insight into Mechanochemical and Thermal Degradation of Imidazolium-Based Ionic Liquids with Alkyl and Monomethoxypoly(Ethylene Glycol) Side Chains. J. Phys. Chem. C 2014, 118, 22544–22552. [Google Scholar] [CrossRef]
- de Miguel, I.; Morales, E.; Herradón, B.; del Río, C.; Mann, E. Synthesis and Characterization of Oligo(Oxyethylene)-Functionalized Thiazolium Based Room Temperature Ionic Liquids. Tetrahedron Lett. 2016, 57, 3291–3293. [Google Scholar] [CrossRef]
- Nokami, T.; Yamashita, T.; Komura, T.; Handa, N.; Shimizu, M.; Yamaguchi, K.; Domi, Y.; Usui, H.; Sakaguchi, H.; Itoh, T. Effects of the Ether Oxygen Atom in Alkyl Side Chains on the Physical Properties of Piperidinium Ionic Liquids. Faraday Discuss. 2017, 206, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal Properties of Imidazolium Ionic Liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal Decomposition of Carboxylate Ionic Liquids: Trends and Mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef] [PubMed]
- Rotrekl, J.; Storch, J.; Kloužek, J.; Vrbka, P.; Husson, P.; Andresová, A.; Bendová, M.; Wagner, Z. Thermal Properties of 1-Alkyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids with Linear, Branched and Cyclic Alkyl Substituents. Fluid Phase Equilibria 2017, 443, 32–43. [Google Scholar] [CrossRef]
- Gusain, R.; Khatri, O.P. Fatty Acid Ionic Liquids as Environmentally Friendly Lubricants for Low Friction and Wear. RSC Adv. 2016, 6, 3462–3469. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, Electrochemical and Radiolytic Stabilities of Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 53. [Google Scholar] [CrossRef] [PubMed]
- Lethesh, K.C.; Shah, S.N.; Ayodele, O.B.; Mutalib, M.I.A.; Uemura, Y. Nitrile-Functionalized Azepanium Ionic Liquids: Synthesis Characterization and Thermophysical Properties. J. Mol. Liq. 2016, 221, 1140–1144. [Google Scholar] [CrossRef]
- Huang, G.; Lin, W.-C.; He, P.; Pan, Y.; Shu, C.-M. Thermal Decomposition of Imidazolium-Based Ionic Liquid Binary Mixture: Processes and Mechanisms. J. Mol. Liq. 2018, 272, 37–42. [Google Scholar] [CrossRef]
- Clough, M.T.; Crick, C.R.; Gräsvik, J.; Hunt, P.A.; Niedermeyer, H.; Welton, T.; Whitaker, O.P. A Physicochemical Investigation of Ionic Liquid Mixtures. Chem. Sci. 2015, 6, 1101–1114. [Google Scholar] [CrossRef]
- Navarro, P.; Larriba, M.; García, J.; Rodríguez, F. Thermal Stability, Specific Heats, and Surface Tensions of ([Emim][DCA]+[4empy][Tf2N]) Ionic Liquid Mixtures. J. Chem. Thermodyn. 2014, 76, 152–160. [Google Scholar] [CrossRef]
- Navarro, P.; Larriba, M.; Beigbeder, J.-B.; García, J.; Rodríguez, F. Thermal Stability and Specific Heats of {[Bpy][BF4] + [Bpy][Tf2N]} and {[Bpy][BF4] + [4bmpy][Tf2N]} Mixed Ionic Liquid Solvents. J. Therm. Anal. Calorim. 2015, 119, 1235–1243. [Google Scholar] [CrossRef]
- Larriba, M.; Navarro, P.; Beigbeder, J.-B.; García, J.; Rodríguez, F. Mixing and Decomposition Behavior of {[4bmpy][Tf2N]+[Emim][EtSO4]} and {[4bmpy][Tf2N]+[Emim][TFES]} Ionic Liquid Mixtures. J. Chem. Thermodyn. 2015, 82, 58–75. [Google Scholar] [CrossRef]
- Liu, J.; Yang, W.; Li, Z.; Ren, F.; Hao, H. Experimental Investigation of Thermo-Physical Properties of Geminal Dicationic Ionic Compounds for Latent Thermal Energy Storage. J. Mol. Liq. 2020, 307, 112994. [Google Scholar] [CrossRef]
- Javaherian, M.; Saghanezhad, S.J. Synthesis, Characterization and Applications of Dicationic Ionic Liquids in Organic Synthesis. MROC 2020, 17, 450–464. [Google Scholar] [CrossRef]
- Zaky, M.T.; Nessim, M.I.; Deyab, M.A. Synthesis of New Ionic Liquids Based on Dicationic Imidazolium and Their Anti-Corrosion Performances. J. Mol. Liq. 2019, 290, 111230. [Google Scholar] [CrossRef]
- Cognigni, A.; Kampichler, S.; Bica, K. Surface-Active Ionic Liquids in Catalysis: Impact of Structure and Concentration on the Aerobic Oxidation of Octanol in Water. J. Colloid Interface Sci. 2017, 492, 136–145. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Víllora, G.; Licence, P. Ecotoxicity Assessment of Dicationic versus Monocationic Ionic Liquids as a More Environmentally Friendly Alternative. Ecotoxicol. Environ. Saf. 2018, 150, 129–135. [Google Scholar] [CrossRef]
- Bender, C.; Kuhn, B.; Farias, C.; Ziembowicz, F.; Beck, T.; Frizzo, C. Thermal Stability and Kinetic of Decomposition of Mono- and Dicationic Imidazolium-Based Ionic Liquids. J. Braz. Chem. Soc. 2019. [Google Scholar] [CrossRef]
- Mezzetta, A.; Perillo, V.; Guazzelli, L.; Chiappe, C. Thermal Behavior Analysis as a Valuable Tool for Comparing Ionic Liquids of Different Classes. J. Therm. Anal. Calorim. 2019, 138, 3335–3345. [Google Scholar] [CrossRef]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Hatefipour, R. Low Viscosity Azide-Containing Mono and Dicationic Ionic Liquids with Unsaturated Side Chain. J. Mol. Liq. 2017, 225, 793–799. [Google Scholar] [CrossRef]
- Payagala, T.; Huang, J.; Breitbach, Z.S.; Sharma, P.S.; Armstrong, D.W. Unsymmetrical Dicationic Ionic Liquids: Manipulation of Physicochemical Properties Using Specific Structural Architectures. Chem. Mater. 2007, 19, 5848–5850. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, B. Design, Synthesis, and Analysis of Thermophysical Properties for Imidazolium-Based Geminal Dicationic Ionic Liquids. J. Phys. Chem. C 2018, 122, 2467–2474. [Google Scholar] [CrossRef]
- Boumediene, M.; Haddad, B.; Paolone, A.; Drai, M.; Villemin, D.; Rahmouni, M.; Bresson, S.; Abbas, O. Synthesis, Thermal Stability, Vibrational Spectra and Conformational Studies of Novel Dicationic Meta-Xylyl Linked Bis-1-Methylimidazolium Ionic Liquids. J. Mol. Struct. 2019, 1186, 68–79. [Google Scholar] [CrossRef]
- Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally-Stable Imidazolium Dicationic Ionic Liquids with Pyridine Functional Groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Kapustin, G.I.; Gorbatsevich, O.B.; Glukhov, L.M.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Properties of Dicationic Disiloxane Ionic Liquids. Molecules 2020, 25, 2949. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).