Abstract
In the processing of cotton and neem seeds to obtain oil for diverse uses, enormous quantities of seed husk are generated as waste, which when not properly disposed of, poses environmental problems. One way of reducing this waste is to use it for the production of activated carbon (AC) for its multiple applications. In this work, activated carbon was produced from cotton and neem seed husks by carbonization followed by acid activation. The prepared ACs were characterized for its porosity and surface properties as well as for its ability to bleach neutral cotton seed oil. The prepared ACs are very efficient in the decoloration process, as they removed about 96–98% of the pigments compared to 98.4% removal with commercial bleaching earth. Temperature had a pronounced effect on the bleaching of neutral cotton seed oil. Maximum adsorption was observed at 60 °C for a contact time of 45 min. The adsorption kinetics were modelled by the intra-particle and the pseudo-second order equations while the adsorption isotherms followed the Langmuir and Freundlich equations. It is concluded that the organic ACs are efficient in pigment removal from neutral cotton seed oil and therefore are potential bleaching agents for the vegetable oil industry.
1. Introduction
The demand for activated carbon (AC) for air and water treatment as well as in food processing has been on the increase the world over because of its very effective adsorbent properties [1,2,3,4]. Considering their multiple applications, activated carbons can be prepared from several animal or vegetable organic matter. Activated carbon has enormous potentials in the removal of dyes, odors, tastes and contaminants, in water purification and other decontamination processes [5]. It is widely used on an industrial scale as an adsorbent mainly in the purification/separation of liquids and gases, oil decoloration, the purification of foodstuffs and pharmaceutical produce and also as a catalyst and catalyst support in several reactions [6].
The use of the activated carbon in industry is very important and requires a solid having a very developed porous structure. Researchers have demonstrated that organic matter is effective for the preparation of such activated carbon with highly porous structure [1,7]. Synthetic activated carbons usually prepared from petroleum sources are expensive and non-biodegradable and therefore pose problems of environmental pollution after use.
Bleaching—also called decoloration—is one of the key processes in fats and oils refining, designed to remove not only pigments but also a wide range of other impurities. Most crude fats and oils contain impurities that have to be removed for both commercial and health reasons [8]. Bleaching requires the use of adsorbents singly or in combination for the removal of colored pigments and impurities or contaminants from oil to give it a desirable quality. The use of activated carbon in the bleaching process will, in addition to the decolorization of the oil, also remove contaminants such as polycyclic aromatic hydrocarbons (PAH) and other contaminants in this group from contaminated oils. This is because crude vegetable oils and marine oils can be polluted by carcinogenic PAH and/or persistent organic pollutants (POP) such as dioxins, furans, polychlorinated biphenyls (PCB), pesticides and herbicides [9]. Activated carbons can therefore be used either singly or in combination with other bleaching earths for the bleaching/decontamination process. Activated carbons of organic origin are biodegradable and more porous compared to those of petroleum origin and can therefore be more efficient in the bleaching process when used either singly or in combination with other bleaching earths [9].
Meanwhile in the production of cotton and neem seed oils, large quantities of organic matter are generated from the husk as waste. This waste can be used in the preparation of activated carbon that can eventually be used as a replacer or in combination with imported bleaching earth in the bleaching of vegetal oils.
Accordingly, the objectives of this work were therefore to optimize the conditions for the production of activated carbon from cotton and neem seed husks and to evaluate its potential in the decolorization of neutral cotton seed oil.
2. Material and Methods
2.1. Preparation of the Activated Carbon
Activated carbon was prepared from neem (Azadirachta indica juss.) and cotton (Gossypium hirsutum) seed husks. Neem trees grow mostly in the wild without any formal care from the population. Cotton farming is done by small holder farmers who sell their products to processing companies. The cotton farming season runs from June to December every year. No irrigation is carried out as the farmers depend only on natural rainfall. Fertilizers, pesticides and herbicides are supplied by the cotton processing companies to small holder famers who are the main stakeholders in cotton farming. Neem seed husks were supplied by women from Gazawa village, who have a long tradition of processing neem seeds to oil for diverse uses. Gazawa is located on longitude 10°31′51″ N and latitude 14°08′26″ E. Cotton seed husks were supplied by Société de Development du Coton du Cameroon (SODECOTON); a cotton processing company situated in the Extreme North Region of Cameroon. The husks were packaged in polyethylene plastic bags and transported to the laboratory for experiments. The husks were then cleaned of dirt and stones and then pounded in a mortar and filtered through a 50 µm polyethylene sieve. The treated raw material had a moisture content of about 13% d.b.
2.1.1. Carbonization of the Seed Husks
A 30g aliquot of the treated sample was carbonized in a VOLCA, MUFFLE furnace, (PROLABO) at 550 °C for 10 min. Experiments were then repeated at 20, 30, 40, 50, 60, 70 and 80 min. All experiments were carried out in triplicates. The carbonized sample was activated by the method described by Julien et al., 2010 [10]. Briefly, 15 g of the carbonized sample was mixed with 20 mL of 15 N H2SO4 in a 150 mL beaker and the reaction mixture was heated at 90 °C for one hour with continuous stirring [6]. The mixture was then allowed to cool at room temperature and then filtered through a Whatman No. 40 filter paper. The filtered sample was dried at 105 °C for 6 h and then heated in the furnace at 450 °C for 30 min.
The quantity of Burn-off which is the value derived from the reduction of the initial mass of raw material to the final mass of activated carbon was evaluated from the equation [11].
Y1 is the quantity of raw material carbonized and Y2 is the quantity of AC obtained.
2.1.2. Physicochemical Characterization of the Prepared Activated Carbons
Liquid Phase Adsorption Tests
Methylene blue and iodine indices were used to characterize the ACs.
The cationic dye, methylene blue, (MB) is an organic molecule that has been used by researchers as an average size representative model of organic pollutants to characterize the behavior of potential adsorbents. That is, it has for a long time been used to evaluate the performances of activated carbon before its use in water treatment plants, bleaching of vegetable oils and other uses [12]. To determine the MB index, a solution of MB was prepared by dissolving 600 g of the powder in a 2 L container and bringing it up to the mark. The mixture was agitated at 1500 revs/min for 12 h and then filtered to remove the undissolved particles. A 10 g aliquot of the prepared AC was weighed into a bottle and 100 mL of the MB solution was added to the bottle and agitated for 4 h. A control was treated in a similar manner but for the fact that it did not contain AC. After agitation, the mixture was filtered; an aliquot of 1 mL was measured and diluted with 100 mL of distilled water. The absorbance was then measured using a UV-Visible spectrophotometer (Jenway 7310, Jenway©, Staffordshire, UK).
Qads is Quantity of MB adsorbed per unit mass of activated carbon (in mg/g), C0 is initial concentration of MB (mg/L), Cr is residual concentration of MB (mg/L), V is the Volume of the solution (l) and m is the mass of the adsorbent (g).
The iodine index gives an indication on the micro-porosity of the material. It is the quantity of iodine adsorbed by the adsorbent in mg/g. It translates the affinity of adsorption of a material for small molecules. To determine iodine index, an iodine solution was prepared from a mixture of 0.50 g of I2 and 1.2 g of KI which was ground to fine particles in a porcelain mortar. The mixture was then dissolved in distilled water, quantitatively transferred to a 2 L volumetric flask and brought to the mark. A 0.4 M solution of Na2S2O3 5H2O was prepared by dissolving 2.48 g of the powder in distilled water and making it up to the mark in a 2 L volumetric flask. An aliquot of 25 mL of this solution was then diluted in 500 mL of distilled water. To determine the iodine index, an aliquot of 10 mg of activated carbon were placed in a bottle and 100 mL of 0.1 M iodine solution added to it. The mixture was agitated for four hours, filtered and then titrated against the 0.4 M sodium thiosulphate solution. A control experiment without the addition of AC, was conducted in a similar manner. All experiments were carried out in duplicates.
Adsorbed iodine (mg/g) was calculated from
where V1 is the volume of sodium thiosulphate (mL), V2 is the volume of iodine solution (mL), N is the normality of sodium thiosulphate, 12.69 is the amount of iodine needed for a 1 mL of 0.1 N sodium thiosulphate solution.
The surface area of the activated carbon was then evaluated from the equation [13].
Ic = 0.6366 × surface area + 174.34
Identification of functional groups at the surface of the ACs was done by FT-IR spectroscopy. The FTIR spectra of the samples were recorded between 4000 and 450 cm−1 in a Perkin-Elmer 1720 spectrometer. Pellets were prepared by thoroughly mixing carbon and KBr at the 1:400 carbon/KBr weight ratio in a small size agate mortar. The resulting mixture was compacted in a Perkin-Elmer manual hydraulic press at 10 ton for 3 min [1].
The Boehm titration method was also used for the analysis of some functional groups at the surface of the AC [14]. To do this, 0.1 g of AC was added to separate solutions of 40 mL of 0.1 M NaHCO3, 0.1 M Na2CO3 and 0.1 M NaOH for the identification of acidic groups and to 0.1 M HCl for the identification of basic groups. The mixtures were agitated at ambient temperature for 48 h. The mixtures were filtered and the aqueous solutions titrated against 0.1 M solutions of HCl and NaOH to determine the basic and acidic groups respectively. The number and types of acidic groups were estimated based on the fact that NaOH neutralizes carboxylique, lactonique and phenolique groups, Na2CO3 neutralizes carboxylique and lactonique groups while NaHCO3 neutralizes only carboxylique groups. Carboxylique groups were quantified by titration against NaHCO3. The difference between the groups quantified by titration with NaHCO3 and Na2CO3 was considered to be lactones while the difference between the groups titrated with NaOH and Na2CO3 were the phenols. Basic groups were identified by titration with HCl. In other to determine the remaining basic groups back titration of the HCl was carried out with 0.1 M NaOH.
2.2. Evaluation of the Use of Activated Carbons by the Bleaching of Neutral Cotton Seed Oil
The efficiency of the produced ACs was tested in the bleaching of neutral cotton seed oil. To do this, the adsorption kinetics and the adsorption isotherms for the bleaching process was studied. Preliminary experiments showed that AC produced after 80 min of carbonization at 450 °C had the best adsorption properties. This treatment was therefore used in the following parts of the work.
Bleaching kinetics of the neutral cotton seed oil was carried out in a batch system at 60°, 70° and 80 °C. For the bleaching experiments, 5% of AC was added to the appropriate quantity of the oil. The oil was then heated to the reaction temperature and maintained at this temperature for a given length of time. The mixture was filtered through a Whatman filter paper and the optical density of the oil was determined at a wavelength of 664 nm using UV-Visible spectrophotometer (Jenway 7310, Jenway©, OSA, UK). All experiments were carried out in triplicates. The absorbance of the oil was determined at 664 nm before and after bleaching of the oil using the activated hydrocarbon which gave an indication of the efficiency of the ACs. Another experiment was carried out in the same manner using commercial bleaching earth obtained from SODECOTON. Measurements at 664 nm translate the optimum absorption of the majority of the pigments (chlorophyll a and b, carotenes, xanthophyll and some trace metals).
The pseudo-second order model (Equation (5)) and intra-particle diffusion model (Equation (6)) were used to model the kinetics of the decolorization process [3].
where t is the adsorption time, qe is the adsorption capacity (mg/g) at equilibrium, qt is the amount (mg/g) of material adsorbed at time t and kp is the rate constant (g/mgmin) of the pseudo-second-order model. k is the intraparticle diffusion coefficient and C is a constant.
Determination of Adsorption Isotherms of the Oil
Adsorption isotherms of cotton seed oil on the prepared AC were studied at 60, 70 and 80 °C. Contact time as derived from kinetic studies was 45 min. The relative quantity of pigments adsorbed per gram of adsorbent (X/m) and the quantity of residual pigments (Xe) were estimated from Equations (7) and (8) respectively:
where A0 is the absorbance of the neutral cotton seed oil, A is the Absorbance of the bleached cotton seed oil and m is the mass of activated carbon used in the process.
Two models; the Langmuir and Freundlich were used to describe the adsorption isotherms for the removal of pigments from the oil.
The linearized forms of the Langmuir Equation (9) and Freundlich Equation (10) were used to determine the constants of the respective equations.
Xe is the residual relative amount of pigments at equilibrium (mg/g); a is the quantity of pigment adsorbed at monolayer or the maximum coverage (mg/g), KL the Langmuir adsorption equilibrium constant (g/mg) is related to direct measurement of the intensity of adsorption process.
3. Results and Discussion
3.1. Quantity of Burn-Off
According to Reference [15], the quantity of burn off can be used to characterize the porosity of an adsorbent. If the burn-off is less than 50%, then the AC will contain a majority of micropores, if it is greater than 75%, the AC will contain mostly macropores and for a quantity of burn-off between 50 and 75%, the adsorbent will have a mixed porous structure (mixture of micro, meso and macro pores). In this work, the quantity of burn off for both neem and cotton husks were respectively 67.63% and 71.33% indicating that they both possess a mixture of micro, meso and macropores.
3.2. Liquid Phase Adsorption Tests
Iodine and MB values are important parameters used to characterize ACs. The former gives information on the micro-porosity while the latter gives information on the meso-porosity of the adsorbents respectively. Average iodine values of the ACs were 472.44 and 505.46 mg/g while the corresponding surface areas of the ACs calculated from Equation (4) were respectively 468.27 and 520 m2/g for neem and cotton suggesting that these ACs could have a high ability to adsorb small molecules. That is, the adsorbents are micro-porous in nature. Micro-porosity of activated carbons can be linked to the method of preparation as well as the nature of the raw material from which it is prepared. AC prepared from cotton seed husk has a higher micro-porosity compared to that prepared from neem. MB values for ACs from neem and cotton were respectively 52.29 and 46.56 mg g−1 indicating the presence of macro-pores in the adsorbent and their abilities to adsorb macromolecules [14]. The presence of both micro and macropores in the ACs prepared from the two sources corroborates the results on the quantity of burn-off in which it was asserted that the two ACs were of mixed porosity because the burn–off was between 50 and 75 mg/g.
3.3. Identification of Functional Groups at the Surface of the ACs
Results from Boehm titration show that the structure of AC consists of a number of functional groups, which can be used to categorize the ACs for various uses. Identifying these functional groups can also be helpful in describing the mechanisms for the removal of different substrates to the adsorbent. For example, one could be able to qualify the type of bonding between the adsorbates and adsorbent as physical or chemical etc. In this work, the Boehm’s Titration Method was used to demonstrate that the prepared ACs consisted mostly of carboxylique, lactones, phenolic, basic and acidic groups (Table 1).

Table 1.
Some properties of the ACs.
From Table 1, it can be observed that AC from cotton was more basic than the one from neem. This could be linked probably to the nature of the raw material since all the ACs were prepared by the same method. It therefore seems that in addition to the porosity and specific surface area of activated carbons the surface composition should not be neglected in characterizing these activated carbons.
Figure 1a,b Show FTIR spectra for the activated carbon produced from neem. Absorption peaks were observed at 1600 and 1675 cm−1, at 900 and 400 cm−1 and at 1101 cm−1 which indicate the presence of C-C, C-H and C-O respectively. The identification of functional groups at the surface of the ACs is important because it can also help in describing the type and extent of sorption occurring at the surface of the hydrocarbon with a specific substrate.
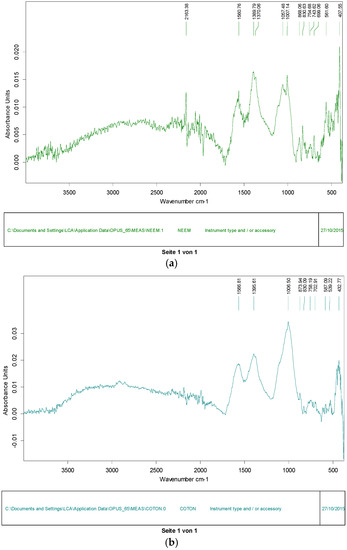
Figure 1.
(a) FTIR spectra for AC from neem; (b) FTIR spectra for AC from cotton.
3.4. Adsorption Kinetics of Neutral Cottonseed on the ACs
Results showed that the average efficiency of pigment uptake by the prepared adsorbents were 96.5 ± 1.3% for AC prepared from cotton and 97.8 ± 1.45% for AC from neem compared to 98.4% for the commercial bleaching earth. This indicates that the prepared ACs are quite good for the bleaching of neutral cotton seed oil.
Adsorption kinetics of neutral cotton seed oil were carried out at 60, 70 and 80 °C to evaluate the efficiency of the activated carbons as a potential adsorbent in the oil refining industry. After preliminary experiments the oil/activated carbon ratio was fixed 5%.
Figure 2 presents the adsorption kinetics of neutral Cottonseed oil (NCO) on the produced hydrocarbons. The adsorption ability of the ACs is linked to the combined effect of carbonization and acid activation. These two processes have the ability to destroy the organic matter contained in the matrices thereby liberating pores for adsorption of molecules. It can be observed that as expected, the quantity of pigment adsorbed increased with adsorption time and then stabilized afterwards when certainly the pores of the ACs are saturated. Time necessary to attain equilibrium decreased with an increase in temperature. Equilibrium time was more than 100 min at 60 °C and was reduced to 80 and 70 min at 70 °C and 80 °C respectively. These observations could be attributed to the fact that, at low temperatures, the high viscosity of the oil reduces the ability of the pigment to diffuse towards the adsorbent thereby increasing the adsorption time. At a given temperature and adsorption time pigment uptake was higher on AC prepared from neem compared to that from cotton seed husks. Activated carbon for neem therefore possesses better properties in oil refining compared to that from cotton.
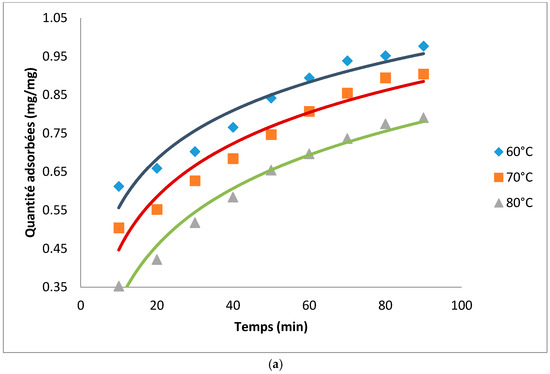
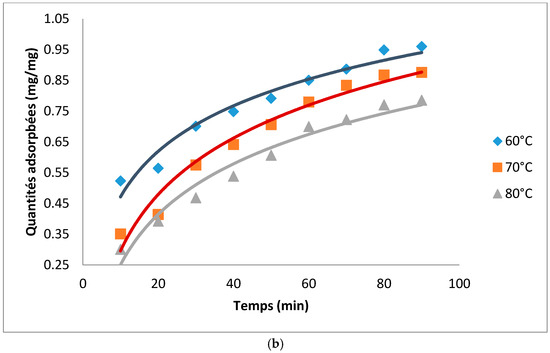
Figure 2.
(a) Adsorption kinetics for the decolorization of cotton seed oil using AC from cotton; (b) Adsorption kinetics for the decolorization of cotton seed oil using AC from neem.
To better appreciate the effect of temperature on the adsorption capacities of the adsorbents, the results of the kinetics were modelled by two equations; the intra-particle diffusion model and the pseudo-second order model. Table 2 shows the model constants as well as the R2 values of the models. R2 values for both models were greater than 0.94 at all temperatures. Figure 3 and Figure 4 show a close agreement of the experimental with the results predicted from the models, indicating that the models chosen could adequately be used to describe the adsorption kinetics. The adequacy of the second order pseudo model in describing the kinetic process shows that the kinetics of the decolorization of neutral cotton seed oil on the prepared adsorbents follows a two-phase adsorption mechanism: first there is the diffusion of the pigments to the surface of the adsorbent which is then followed the pigment-adsorbate interaction [3].

Table 2.
Constants and R2 values for equations used in modeling the adsorption kinetics.
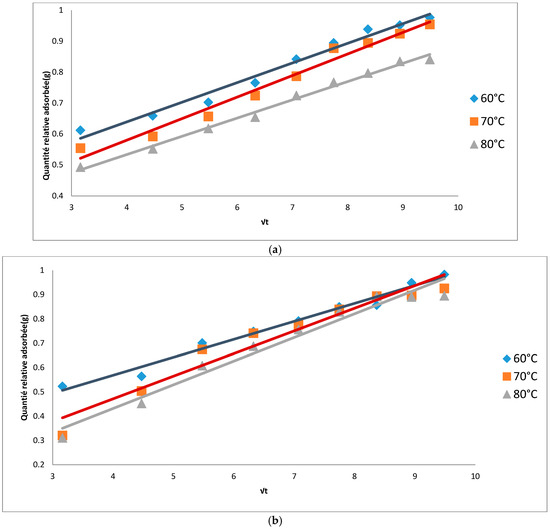
Figure 3.
(a) Intra particle diffusion kinetic model for the decolorization process using AC from cotton; (b) Intra particle diffusion kinetic model for the decolorization process using AC from neem.
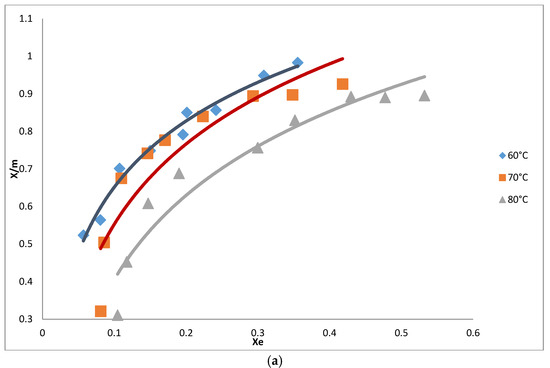
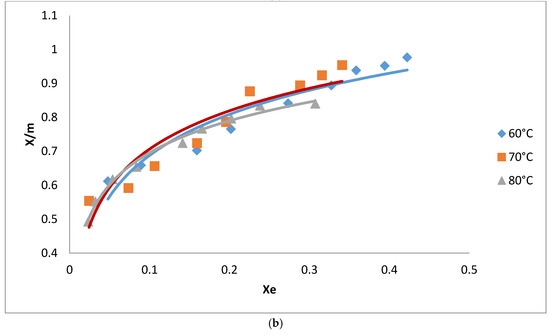
Figure 4.
(a) Adsorption isotherm for the removal of pigments from oil using AC from cotton; (b) Adsorption isotherm for the removal of pigments from oil using AC from neem.
Adsorption Isotherms
Adsorption isotherms for the decolorization of neutral cotton seed oil were carried out on both ACs at 60, 70 and 80 °C. Contact time was 45 min and the mass of the adsorbent varied from 0.8 to 1.2% of the mass of the oil used in the analysis. Figure 4 shows the influence of temperature on the adsorption isotherms using AC from cotton. As expected, the amounts of pigment adsorbed increased with an increase in the quantity of the adsorbent used. Maximum adsorption was observed at a low temperature of 60 °C compared to higher temperatures. Adsorption at low temperatures has a positive impact on the reduction of the energy cost required for the decolorization process. Temperature did not have a significant effect on the adsorption isotherms using AC from neem as opposed to that from cotton where quantities of pigments removed from the oil varied significantly with a change in temperature.
Two models Langmuir and Freundlich were used to study the adsorption isotherms of the decolorization of neutral cotton seed oil using the activated carbons prepared from neem and cotton seed husks. Table 3 gives the model constants as well as the R2 values for the models. R2 values as well as Figure 5 and Figure 6 indicate that the Langmuir isotherm is most suitable for modeling the adsorption of pigments from cotton seed oil using the prepared ACs. The Langmuir adsorption isotherm is established on the basis that the adsorbent has a homogenous adsorption surface. The description of the isotherms by this model therefore suggests that these adsorbents may have homogenous adsorption surfaces. The values of the constant a determined from the Langmuir adsorption isotherms were close to 1 indicating a good affinity between the activated carbon and the pigments of the cotton seed oil. It can therefore be deduced that adsorption was carried out on specific sites and that there is weak and physical interaction between the pigment and the activated carbon.

Table 3.
Model Constants and R2 values for the Langmuir and Freundlich equations.
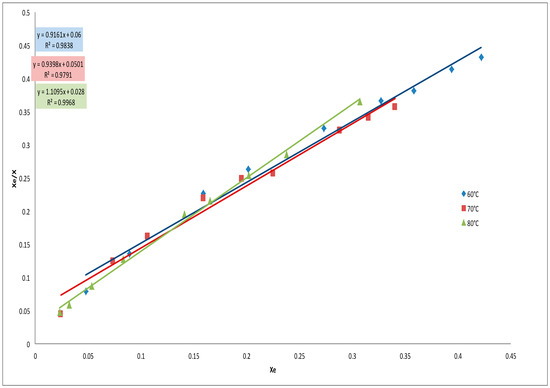
Figure 5.
Modeling of the adsorption isotherms for the decolorization process with AC from neem using the Langmuir isotherm.
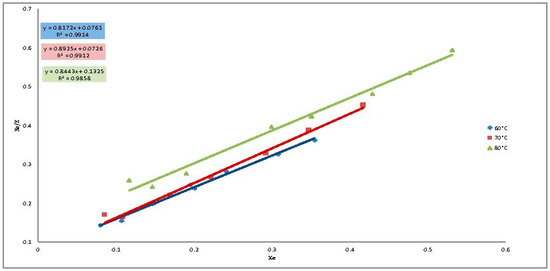
Figure 6.
Modeling of the adsorption isotherms decolorization process with ACC using the Langmuir isotherm.
It is generally accepted that, lower values of n (0.1 < n < 0.5) from the Freundlich isotherm indicate a good adsorption, values of n in the range (0.5 < n < 1) are characteristic of a moderate adsorption while n values greater than 1 characterize a weak adsorption process. In this work values of n were all lower than 0.5 indicating that all the prepared ACs were good for the oil bleaching process. The n values obtained were lower for CAN compared to ACC indicating that ACN is a better adsorbent compared to ACC [14].
4. Conclusions
Activated carbon prepared from neem and cotton seed husks is effective in the adsorption of pigments from neutral cotton seed oil. The ACs are mostly microporous in nature. The adsorption kinetics were modelled by the intra-particle and the pseudo-second order equations while the adsorption isotherms followed the Langmuir and Freundlich equations satisfactorily. Spectrophotometric analysis of the color of the oil indicates that there is no significant difference between the bleached oil using the prepared ACs and that using commercial bleaching earth. It is concluded that the prepared ACs are efficient in pigment removal from neutral cotton seed oil and therefore could be a potential bleaching agent for the vegetable oil industry. The use of these activated carbons in combination with conventional bleaching earths for oil decolorization as well as studies on the use of these ACs in water treatment and in medicine may reveal interesting results.
Acknowledgments
The authors would like to thank the National School of Agro-Industrial Sciences of the University of Ngaoundere where most of the experiments were carried.
Author Contributions
Abba Chetima contributed in the sourcing of the raw material, production of activated carbon, characterisation of the activated carbon as well as the determination of the adsorption kinetics and adsorption isotherms; Gaston Zomegni contributed in the production of the activated carbons and in the determination of the adsorption isotherms; Abdoul Ntieche Rahman carried out the FTIR analysis and contributed in the analysis of the kinetic data; Abdoul Wahabou and Divine Bup Nde did the planning of the work, writing, correction and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A | The Absorbance of the bleached cotton seed oil |
| a | The quantity of pigment adsorbed at monolayer or the maximum coverage (mg/g) |
| A0 | The absorbance of the neutral cotton seed oil |
| AC | Activated Carbon |
| C0 | Initial concentration of MB (mg/L) |
| Cr | Residual concentration of MB (mg/L) |
| IC | Iodine value |
| k | The intraparticle diffusion coefficient and C |
| KF | The Freundlich adsorption equilibrium constant (g/mg) |
| KL | The Langmuir adsorption equilibrium constant (g/mg) |
| kp | The rate constant (g/mg min) of the pseudo-second-order model |
| m | The mass of the adsorbent (g) |
| MB | Methylene Blue |
| Qads | Quantity of MB adsorbed per unit mass of activated carbon (in mg/g) |
| qe | The adsorption capacity (mg/g) at equilibrium |
| qt | The amount (mg/g) of material adsorbed at time t |
| t | The adsorption time |
| V | The Volume of the solution (l) |
| X/m | The relative quantity of pigments adsorbed per gram of adsorbent |
| Xe | The quantity of residual pigments |
| Y1 | The quantity of raw material carbonized and |
| Y2 | The quantity of AC obtained |
References
- Yakout, S.M.; Sharaf, E.G. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, X.; Cui, H.; Zhu, Z.; Liang, J.; Zhou, J. Equilibrium, Kinetic, and Thermodynamic Studies on the Adsorption of Cadmium from Aqueous Solution by Modified Biomass Ash. Bioinorg. Chem. Appl. 2017. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, G. Preparation and Characterization of High Surface Area Activated Carbon Fibers from Lignin. Polymers 2016, 8, 369. [Google Scholar] [CrossRef]
- Wafa, J. Décoloration des Huiles Végétales sur des Argiles Étude de la Stabilité Physicochimique des Huiles Décolorées, Diplôme D’études Approfondies en Chimie Organique; Faculté des Sciences de Sfax: Sfax, Tunisie, 2002. [Google Scholar]
- Chennouf-Abdellatif, Z.; Cheknane, B.; Zermane, F.; Gaigneaux, E.M.; Sadok, A.H.; Mohammedi, O.; Bouchenafa-Saib, N. Preparation of activated carbon based on synthetic and agricultural wastes: Application to the adsorption of methyl orange. Rev. Energ. Renouv. 2015, 18, 575–586. [Google Scholar]
- Moving beyond Colour. Alfa Laval Bleaching Processes for Fats and Oils. Available online: https://www.scribd.com/document/343094446/bleaching-processes-pdf (accessed on 17 July 2017).
- Norit Activated Carbon for Purification of Edible Oils, Document No. Info21-03/Edible Oils. Available online: http://www.vulcascot.co.at/media/content/downloads/norit_edible_oils.pdf (accessed on 17 July 2017).
- Julien, O.; Lydie, C. Evaluation de Charbons Actifs en Poudre (CAP) Pour L’élimination des Micropolluants Dans Eaux Résiduaires Urbaines, Ecole Polytechnique de Lausanne. 2010. Available online: https://www.grese.ch/Doc/Micropolluants/Evaluation%20de%20charbons%20actifs%20en%20poudre.pdf (accessed on 10 August 2017).
- Yuliusman, N.; Sanal, A.; Bernama, A.; Haris, F.; Ramadhan, I.T. Preparation of activated carbon from waste plastics polyethylene terephthalate as adsorbent in natural gas. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 176, p. 012055. [Google Scholar]
- Tsai, W.T.; Change, C.Y.; Ing, C.H.; Change, C.F. Adsorption of acid dyes from aqueous solution on activated bleaching earth. J. Colloid Interface Sci. 2004, 275, 72–78. [Google Scholar] [CrossRef] [PubMed]
- ASTM D-4607-94 (Reapproved 2006). Standard Test Method for Determination of Iodine Number of Activated Carbon. Available online: https://www.researchgate.net/file.PostFileLoader.html?id...assetKey (accessed on 3 August 2017).
- Bamba, D. Elimination du Diuron des Eaux par des Techniques Utilisant les Ressources Naturelles de la côte D’ivoire: Photocatalyse Solaire et Charbon Actif de Coques de Noix de Coco. Ph.D. Thesis, Universités de Cocody-Abidjan, Abidjan, Cote D’Ivoire, 2007. [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D. Surface and sorption properties of active-carbons. Russ. Chem. Bull. 1955, 4, 531–538. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).