1. Introduction
Hydrocolloids are amphiphilic or hydrophilic biopolymers with a neutral taste, widely used in the food industry due to their various functional properties [
1,
2]. They are particularly valued as thickeners, dietary fibers, emulsifiers, gelling agents, stabilizers, clarifying agents, fat substitutes, flocculants, whipping agents, and opacifying agents. Additionally, they are applied in encapsulating flavors or bioactive compounds, creating edible films, and inhibiting crystallization [
3]. The physicochemical properties of food hydrocolloids enhance the demand for carbohydrate polymers in the food industry. Consequently, novel hydrocolloids—being inexpensive, natural, renewable, available, and biodegradable—have attracted significant attention as new food ingredients [
3,
4].
Food hydrocolloids present physicochemical properties of interest that increase the demand for carbohydrate polymers in the food industries; for this reason, novel hydrocolloids as new food ingredients that are inexpensive, natural, renewable, available, and biodegradable have received much attention [
5].
Several hydrocolloids are commercially available, including pectin, starch, gelatin, inulin, agar, guar gum, xanthan gum, carrageenan, alginate, carob bean gum, arabic gum, methylcellulose, carboxymethyl cellulose, hydroxypropyl methylcellulose, and hydroxyethyl methylcellulose, among others [
4]. However, the food industry is actively developing new products to promote the growth of sustainable and ecological foods, focusing on the nutritional benefits provided to consumers [
6].
The extraction of hydrocolloids is a crucial process for their application and development. Recent research has extensively investigated the extraction of polysaccharides from plants and fungi. For example, Zhang et al. [
7] obtained hydrocolloids from
Tremella spp., Keisandokoht et al. [
8] from
Ocimum Basilicum L. seeds, Karazhiyan et al. [
9] from
Lepidium sativum seeds, and Quintana-Martinez et al. [
10] from
Cucurbita moschata peel, all demonstrating potential for use in the food industry. Various methods have been employed for hydrocolloid extraction, with aqueous extraction being the most common. However, techniques such as ultrasound-assisted extraction [
11], sequential ultrasound-assisted extraction with heating treatment [
12], microwave-assisted extraction [
11,
13], and enzyme-assisted extraction [
14] have been applied to enhance yield and technological properties. Extraction conditions—such as acid type, particle size, temperature, water-to-seed ratio, pH, salt concentration, solvent nature, and extraction time [
14]—can significantly influence yield and purity. For instance, Lastra-Ripoll et al. [
15] found significant differences in extraction yield based on pH, with higher yields at pH 3 for hydrocolloids from sesame. Karazhiyan et al. [
9] achieved higher yields in acidic and alkaline media compared to neutral pH for hydrocolloids from cress seed, while Marsiglia-Fuentes et al. [
16] reported higher yields at pH 3 and 10 for mango hydrocolloids.
Hydrocolloids, mucilaginous substances, and high molecular weight gums exhibit strong shear-thinning behavior. These rheological characteristics suggest their potential application as novel thickeners [
17]. The viscosity of hydrocolloids primarily depends on their physical entanglements and conformation [
18]; consequently, the techno-functional properties of hydrocolloids are influenced by their solubility in the aqueous phase of food. To effectively increase the viscosity of solutions, form gels, or stabilize emulsions, good water solubility is essential [
10,
19,
20,
21]. Understanding the rheological behavior of new hydrocolloid sources is crucial, as they are used to modify textural attributes. The demand for food hydrocolloids with specific functionalities is an active area of study, and they are considered important additives in the food industry [
22]. Hydrocolloids derived from plants are a valuable source and play an important role in food formulation.
Dioscorea rotundata is a tropical crop from the genus
Dioscorea spp., originating from Africa and Asia [
23], and is an important source of carbohydrates [
24]. It has a high moisture content and consists primarily of starch (70–80% on a dry weight basis), as well as protein, sugars, and fiber [
25]. This composition makes it a promising source of hydrocolloids derived from plant polysaccharides. Various technological ingredients have been extracted from
Dioscorea spp.; for example, Juang et al. [
26], Shao et al. [
27], Zou et al. [
28], Ahmadu et al. [
29], Elmi-Sarlina et al. [
30], and Oliveira et al. [
31] obtained starch, while Ma et al. [
32] extracted mucilage. The aim of this research was to obtain hydrocolloids from
D. rotundata and evaluate their physicochemical, technological, rheological, morphological, and pasting properties.
2. Materials and Methods
2.1. Materials
The D. rotundata tubers used in this study were obtained from the local food market in Cartagena, Colombia. They were selected based on their shape and size, then cut, milled, and frozen until needed. Hexane and ethanol (99.5% purity) were sourced from Panreac (Barcelona, Spain). Acetic acid, sodium hydroxide, and phenolphthalein were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used were of analytical grade.
2.2. Hydrocolloids Extraction
Hydrocolloid extractions from
D. rotundata tubers were carried out following the procedures described by López-Barraza et al. [
33] under acid, neutral and alkali conditions (
Figure 1):
- -
Acid extraction: The fresh sample was suspended with 1% w/v citric acid (HDRpH3).
- -
Neutral extraction: The fresh sample was suspended in distilled water (HDRpH7).
- -
Alkali extraction: The fresh sample was suspended with 1% w/v NaOH (HDRpH10).
In all cases, a pulp-to-solvent ratio of 1:10 (acidic, neutral, or alkaline extraction) was used, and the mixture was continuously agitated at 80 °C for 4 h. The supernatant was then obtained by centrifugation for 15 min at 4000 rpm (1789 rcf) and mixed with ethanol to precipitate the biopolymer fraction. The mixture was centrifuged again, and the precipitate was dried at 45 °C for 12 h.
2.3. Proximal Composition
The proximal composition (moisture, ash, protein, fat, and carbohydrates) of the hydrocolloids was determined following the procedures described by the AOAC [
34].
2.4. FTIR Analysis
The characterization of hydrocolloids was performed using Fourier Transform Infrared Spectroscopy (FTIR) with a Spectrum 400 model apparatus (PerkinElmer, Shelton, CT, USA) operating in ATR mode. For the analysis, the dried hydrocolloids were mixed with KBr and ground into a fine powder, which was then pressed into slices for analysis. The spectra were recorded over a wavelength range of 5000 to 550 cm−1 with a resolution of 4 cm−1 in transmission mode.
2.5. Technological Properties
Solubility, water holding capacity (WHC), and oil holding capacity (OHC) were determined following the procedures described by Lastra-Ripoll et al. [
15]. Solubility (Equation (2)) was assessed by dispersing hydrocolloids in water (1%
w/
v) at 25, 45, and 65 °C; aliquots were centrifuged at 4000 rpm, and the supernatant was dried at 125 °C to a constant weight. WHC (Equation (3)) was measured by centrifuging a mixture of hydrocolloids in water (0.5%
w/
v), while OHC (Equation (4)) was evaluated by centrifuging a mixture of hydrocolloids in sunflower oil (0.5%
w/
v).
2.6. Rheological Properties
Hydrocolloids were dispersed in water (10% wt) to determine their rheological properties. A controlled stress rheometer (Modular Advanced Rheometer System Haake Mars 60, Thermo-Scientific, Dreieich, Germany), equipped with a parallel plate (diameter: 35 mm and gap: 1 mm), was used following the procedures described by Quintana-Martinez et al. [
10]. Steady-state behavior was evaluated through viscous flow tests over a range of shear rates from 0.001 to 1000 s
−1 at 25 °C.
Oscillatory behavior was evaluated by frequency sweeps over a range of 10−2 to 102 rad·s−1 to obtain the mechanical spectrum, using a stress value within the linear viscoelastic range. Stress sweeps were conducted by applying stress values from 0.001 to 1000 Pa at 1 Hz, at 25 °C.
2.7. Pasting Properties
Pasting properties were analyzed following the procedures described by Mieles-Gómez et al. [
35] using a controlled stress rheometer (Modular Advanced Rheometer System, Haake Mars 60, Thermo-Scientific, Dreieich, Germany). Hydrocolloid dispersions were heated from 40 °C to 95 °C at a rate of 5 °C/min, maintained at 95 °C for 2.5 min, then cooled back to 40 °C and held for 2 min. All analysis was done at 10 s
−1. Pasting parameters included peak viscosity (PV), pasting temperature (PT), trough viscosity (TR), setback viscosity (SB), breakdown viscosity (BD), and final viscosity (FV).
2.8. Statistical Analysis
All tests were performed in triplicate. A one-way ANOVA test was applied to determine statistically significant differences (p < 0.05) using Statgraphics Centurion XVI (Statgraphics, Rockville, MD, USA).
3. Results and Discussion
3.1. Evaluating Hydrocolloid Extraction Processes and Proximal Composition
Hydrocolloids from
D. rotundata extracted under acidic (HDRpH3), neutral (HDRpH7), and alkaline (HDRpH10) conditions yielded 24.70 ± 0.06%, 20.60 ± 0.40%, and 22.90 ± 0.12%, respectively (
Table 1). These results indicate that the extraction pH significantly influences the amount of hydrocolloids extracted (
p < 0.05). The highest yield was observed under acidic conditions, which was associated with increased viscosity of the samples. At pH values below 2.5 and 4, viscosity increased, suggesting that repulsive forces cause aggregation among polysaccharide molecules. The marginal increase or decrease in the pH value of the extraction is related to the addition of a neutral solvent, such as ethanol; the pH will attempt to balance the pH of the two components based on their ability to transfer H+ ions in the case of an acidic solution, or OH– ions when the solution is basic [
15]. However, the hydrocolloid obtained at pH 10 had a yield 7.56% lower than at pH 3 but 10.57% higher than at pH 7. The yields obtained were higher compared to those reported for other vegetable sources. For example, Aguirre and Encarnacion [
36] reported yields of 7.85% for hydrocolloids from sapote peel extracted at pH 2 and 60 °C, and 5.49% at pH 3 and 60 °C. Lastra-Ripoll et al. [
15] reported yields ranging from 4% to 18% for hydrocolloids from
Sesamum indicum, while López-Barraza et al. [
33] obtained a yield of 13.81% for hydrocolloids from
Pereskia bleo at pH 3. The temperature used during extraction facilitated the solubilization of the samples, as higher temperatures provide the necessary activation energy for the molecules, allowing them to move more freely and enhance the fluidity of the extract.
The proximate composition of
D. rotundata hydrocolloids (
Table 1) indicates that the hydrocolloids have high carbohydrate and moderate protein content. HDRpH7 was the sample with the higher carbohydrate content, with values of 87.45 ± 0.87%, followed by HDRpH3 (85.06 ± 0.85%) and HDRpH10 (80.50 ± 0.81%). However, the protein content shows the opposite behavior, where the sample with the highest value was HDRpH10 (11.87 ± 0.12%), followed by HDRpH3 (6.01 ± 0.06%) and HDRpH7 (4.47 ± 0.04%). Then the alkali condition led to the obtainment of hydrocolloids with higher protein and moderate carbohydrate content, while the neutral condition led to the obtainment of hydrocolloids with higher carbohydrate content and moderate protein content. This finding could be attributed to the co-precipitation of proteins and polysaccharides on mixing the supernatant with ethanol.
The high carbohydrate content makes the hydrocolloid a naturally hydrophilic product and indicates good product purity. The values obtained can be attributed to the nature of polysaccharide formation, including the species origin, soil quality, and maturity, and also the hydrocolloid extraction process. Then, in all cases the samples present lower moisture, fat and ash content. These results demonstrate that D. rotundata is a promising source for product design, with its high carbohydrate content making the hydrocolloid inherently hydrophilic.
3.2. Characterization of Hydrocolloids Using FTIR Spectroscopy
The FTIR spectrum of
D. rotundata hydrocolloids is shown in
Figure 2. All samples exhibit a broad peak around 1650 cm
−1, which is associated with the stretching vibrations of the HO-H bond in water. The moisture content was similar across the samples, suggesting that any observed variations may be attributed to differences in the molecular order of starch at the level of individual chemical bonds [
1,
2].
In this region, the broad band around 1650 cm
−1 can obscure the presence of other bands, such as those corresponding to proteins [
3]. The spectral region between 4000 and 1400 cm
−1 is particularly useful for identifying most functional groups in organic molecules, as absorptions in this area primarily result from stretching vibrations. The region between 1400 and 600 cm
−1 is generally more complex due to a combination of stretching and bending vibrations. Each compound exhibits distinct absorption features in this “fingerprint region,” which is crucial for the identification of specific substances [
3].
In carbohydrates, a prominent peak appears between 1100 and 1000 cm
−1, with its position varying depending on the specific type of carbohydrate. The CH stretching vibration is represented by a significant peak between 2700 and 2950 cm
−1, while C-O bonds are indicated by peak bands at 1157 and 1022 cm
−1. A carbohydrate profile is evident with multiple peaks between 1160 and 1130 cm
−1, and sugar components such as C-O-C and C-OH are associated with peaks in the range of 1100 to 980 cm
−1 [
4]. Additionally, the protein content in hydrocolloids can be identified by the amide band in this region. It is also common to observe the adsorption of carboxylic groups within these wave numbers [
6,
36].
The similarity observed among the three spectra presented in
Figure 2 suggests that the macromolecular populations do not exhibit significant structural differences, as indicated by the similar carbohydrate profiles of the samples. Comparable FTIR spectra have been reported for starches isolated from
Pisum sativum,
Dioscorea opposita,
Vicia faba, and
Eleocharis dulcis [
37], as well as for polysaccharides from edible brown and red seaweeds, where distinct bands are observed in the regions between 4000 and 2000 cm
−1, such as hydrogen-bonded O-H (3260 cm
−1), C-H (2926 cm
−1), and C-O (947 cm
−1). Similarly, FTIR spectra of sesame hydrocolloids show bands corresponding to -OH (3800–3100 cm
−1), -CH (3000–2800 cm
−1), and C-O (1022 cm
−1) [
15].
3.3. Technological Properties of Hydrocolloids
The technological properties of the hydrocolloids are summarized in
Table 2. Solubility, defined as the ability of the sample to dissolve in a liquid solvent [
38], shows significant differences (
p < 0.05) among the samples. Specifically, HDRpH3 exhibited the lowest solubility (2.41 ± 0.02%), while HDRpH7 (6.85 ± 0.07%) and HDRpH10 (6.55 ± 0.07%) demonstrated higher solubility values. This indicates that neutral and basic pH conditions result in better solubility. Similar findings were reported by Chiranthika et al. [
39], who observed solubility values of 2.55 ± 0.02% for starch from
Dioscorea alata and 3.72 ± 0.03% for starch from
Dioscorea esculenta. Increased solubility is typically associated with higher kinetic energy in the system, which disrupts the molecular arrangement of the granules and facilitates the entry of water molecules [
40].
The water holding capacity (WHC) of the hydrocolloids, defined as their ability to retain internal and added water when subjected to forces such as pressing, centrifuging, or heating [
41], varies significantly (
p < 0.05). HDRpH7 exhibited the highest WHC (475.97 ± 0.21%), followed by HDRpH10 (356.67 ± 20.70%) and HDRpH3 (294.95 ± 67.55%). This variation is attributed to the ability of the smaller pores in the samples to effectively trap and retain water. Water absorption is influenced by the moisture content of the surrounding medium, with the hydrocolloids absorbing water until equilibrium is reached—a reversible process. This absorption causes the granules to swell, increasing their diameter by up to 10%. The WHC values for the hydrocolloid samples are notably higher than those reported for starches from
Dioscorea alata (177%) and
Dioscorea esculenta (264%), as well as
Dioscorea rotundata flour (57%) [
42]. The WHC values are influenced by factors such as surface polarity, hydrophobicity, and amino acid composition [
43]. These properties are crucial for gelatinization and swelling, affecting the texture, sensory characteristics, nutritional aspects, and mouthfeel of food products, as well as contributing to improved food volume [
41]
Oil holding capacity (OHC), defined as the ability to retain oil and aggregate under force applications such as pressing, centrifuging, or heating [
38], did not show significant statistical differences (
p > 0.05). The OHC values were 275.55 ± 5.94% for HDRpH3, 268.90 ± 17.61% for HDRpH7, and 269.14 ± 47.82% for HDRpH10. This indicates that the extraction pH did not significantly affect OHC. Similar findings were reported for starches from
Dioscorea alata (202%) and
Dioscorea esculenta (186%) [
39]. The ability to retain oil is a valuable property in applications where flavor retention and improved mouthfeel are desired. Oil absorption involves the physical compression of oil by food components and the interaction between polar protein side chains and lipids [
44]. These properties can enhance the stability of food products, prevent syneresis or flavor loss, and modify texture during processing and storage.
3.4. Rheological Properties and Behavior of Hydrocolloids
3.4.1. Steady-Sate
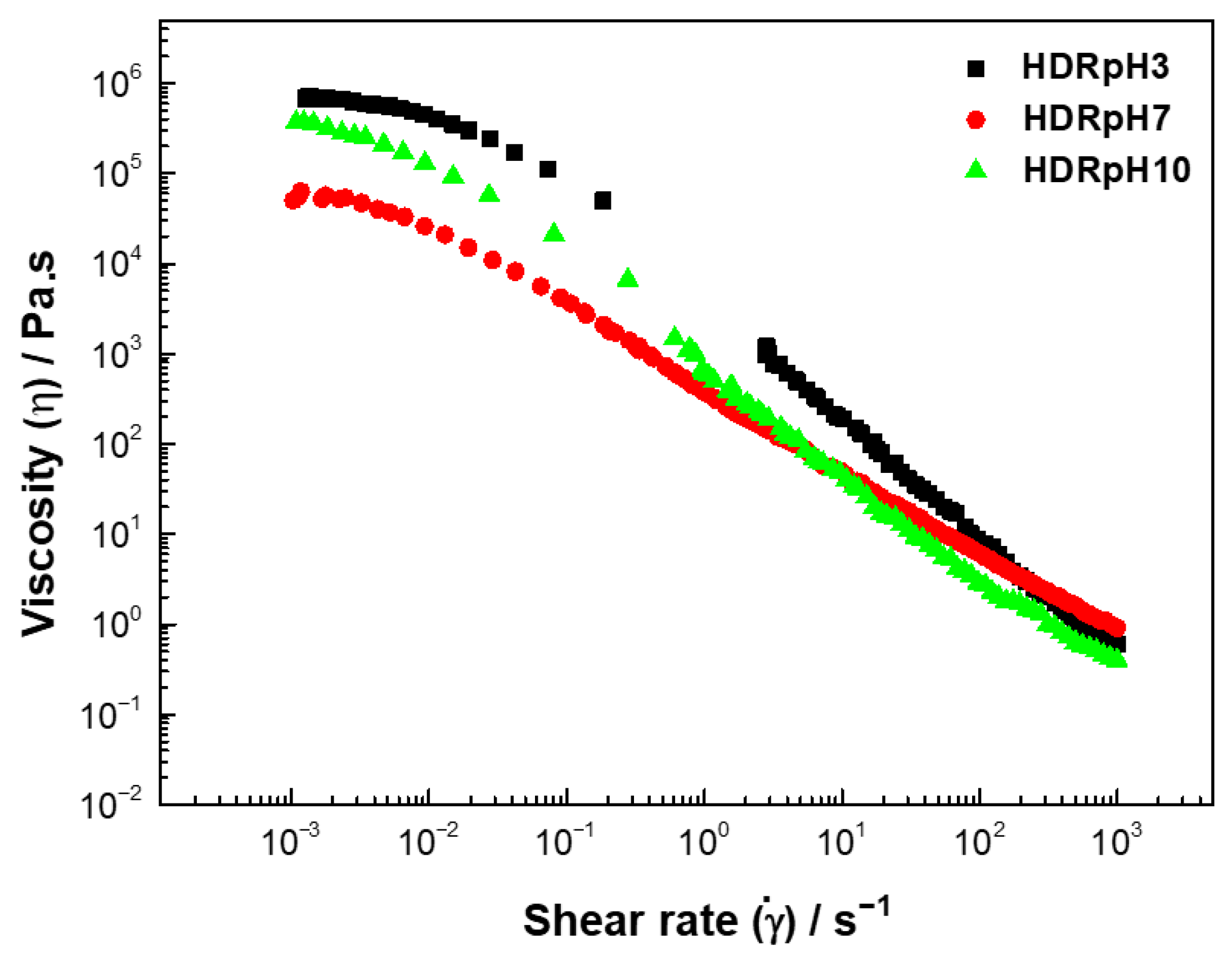
The viscous flow curves (
Figure 3) for the HDRpH3, HDRpH7, and HDRpH10 present a decrease in viscosity with the increase in shear rate indicative of non-Newtonian shear-thinning behavior [
45]. The steady shear flow behavior of the hydrocolloids was characterized using the Cross model (Equation (4)), as this model allows for the determination of material viscosity as a function of the physical conditions applied:
where
is the infinite shear viscosity,
is the zero-shear viscosity, K, in a constant parameter with the dimension of time, and m is the power law constant.
The adjustment parameters for the Cross model (
Table 3) indicate that the zero-shear viscosity
varies with the extraction pH (
p < 0.05). HDRpH3 exhibits the highest zero-shear viscosity at 751,000 Pa·s, followed by HDRpH10 and HDRpH7. This variation is attributed to the balance between the disruption of polymer entanglements at low shear rates and the formation of new entanglements, which results in a constant viscosity under these conditions. The infinite-shear viscosity
is relatively low for HDRpH3 and HDRpH10, with values of 0.051 and 0.038 Pa·s, respectively, while HDRpH7 shows a significantly higher of 0.673 Pa·s (
p < 0.05). This suggests a more viscous nature of the fluid at pH 7. In all cases, the flow behavior index (m) was less than one (0.80–0.90), confirming the shear-thinning nature of the hydrocolloids. This behavior is likely related to the disruption and fracture of the three-dimensional polymer network, with the predominance of disruption over new entanglements at higher shear rates. The constant time (K) increases with the pH. As the shear rate increases, the molecules align with the flow direction, leading to a decrease in apparent viscosity and the onset of shear-thinning behavior.
3.4.2. Analysis of Viscoelastic Properties
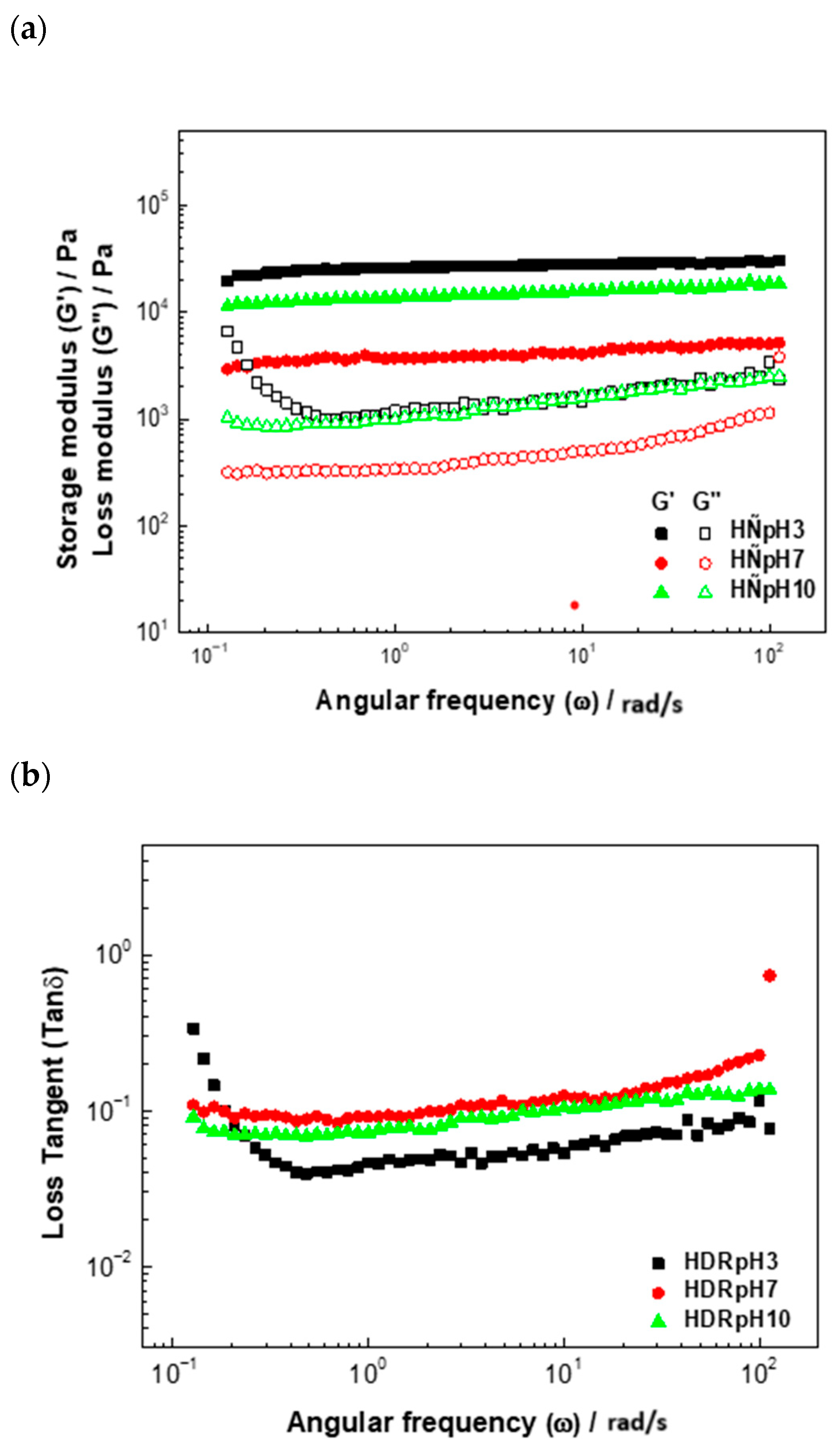
The viscoelastic properties were rigorously assessed within the linear viscoelastic region using a stress sweep set at 1 Pa. The storage modulus (
) and loss modulus (
) of
D. rotundata hydrocolloids as functions of angular frequency (
) are depicted in
Figure 4a. In all samples, G′ was consistently and significantly higher than G″ (G′ > G″) across the entire frequency range, clearly demonstrating a predominantly elastic, solid-like behavior consistent with the formation of a strong or ‘true’ gel network [
46,
47]. This elastic dominance is attributed to the strong, non-covalent association and cross-linking of the polymer chains within the network.
The condition of solubilization profoundly influenced the network’s final strength and structure. HDRpH3 exhibited the highest G′ values, indicating the strongest elastic network. Conversely, the lowest G′ values were found in HDRpH7. This suggests that acidic conditions promoted the most effective chain aggregation and network formation. Crucially, the G″ profile for the HDRpH3 sample was notably distinct (as observed in
Figure 4a). This lower and less steep G″ curve, combined with the highest G’, signifies a lower viscous energy dissipation and a higher degree of structural rigidity for the network formed at pH 3. This behavior is consistent with acidic environments promoting chain association through mechanisms like hydrogen bonding, yielding a more robust, structured gel [
40]. These findings are corroborated by the loss factor Tan
analysis (
Figure 4b). Although Tan
showed no statistically significant differences (
p > 0.05), its values increased consistently with the pH of extraction (HDRpH3 < HDRpH10 < HDRpH7). This progressive rise in Tan
reflects a shift toward a more liquid-like suspension, confirming that acidic conditions (pH 3) yield hydrocolloids with the highest intrinsic elastic properties, which directly impacts stability and texture in product development.
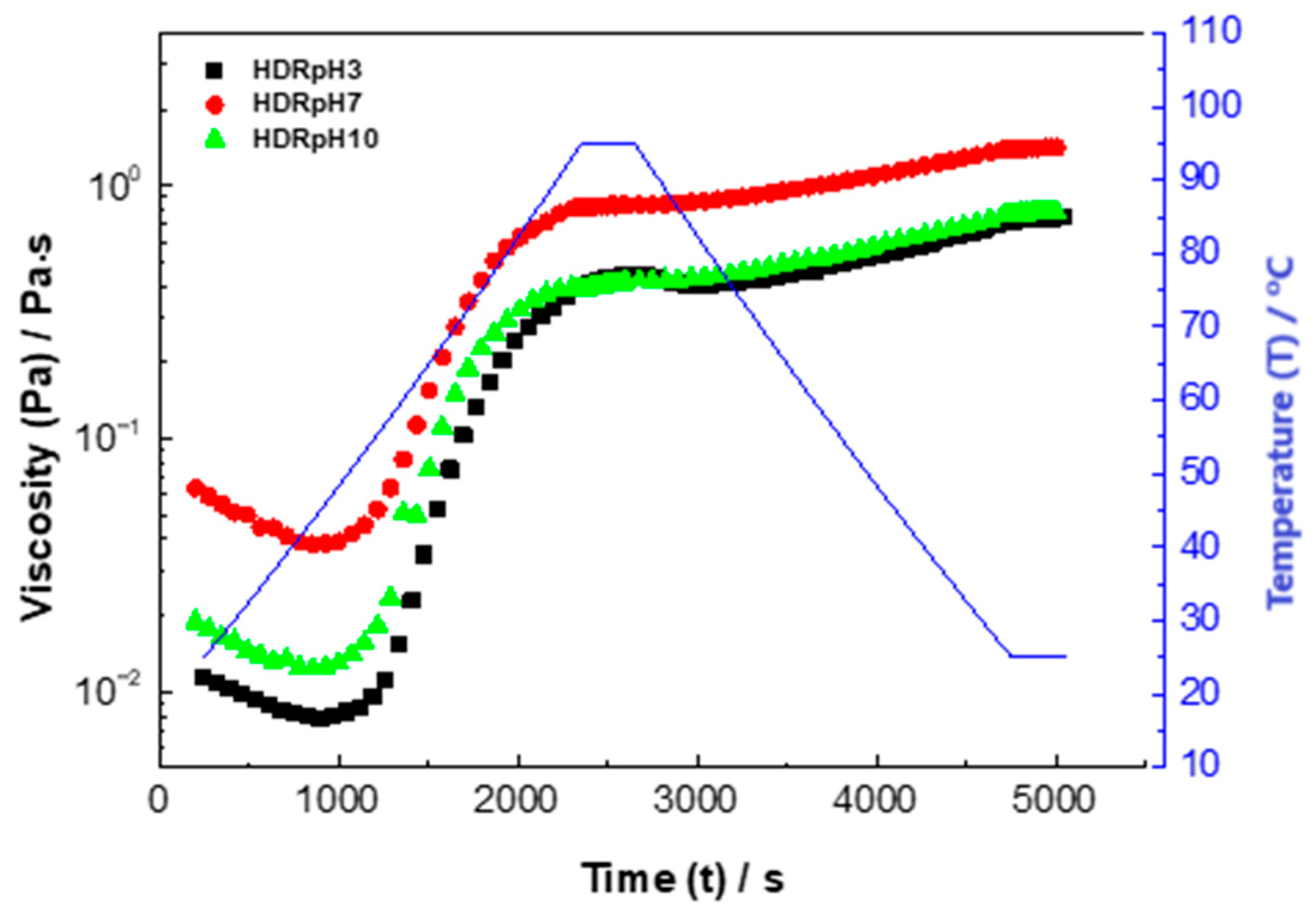
3.5. Pasting Properties of Hydrocolloids: Analysis and Characterization
The pasting characteristics of the
D. rotundata hydrocolloids are illustrated in
Figure 5, showing the pasting temperature (PT), peak viscosity (PV), trough viscosity (TR), breakdown (BD) viscosity, and final viscosity (FV), and summarized in
Table 4. The pasting characteristics of the hydrocolloids are summarized as follows: HDRpH3, HDRpH7, and HDRpH10 had pasting temperatures of 71.54 °C, 69.05 °C, and 71.56 °C, respectively, associated with the minimum temperature required to work with the hydrocolloid [
48]. Final viscosities were 0.32 Pa·s for HDRpH3, 0.58 Pa·s for HDRpH7, and 0.31 Pa·s for HDRpH10, indicating the ability of the material to form a viscous paste; then, it is the viscosity at which the sample reaches at the end of the test [
48].
PV peaks were 0.45 Pa·s for HDRpH3, 0.92 Pa·s for HDRpH7, and 0.55 Pa·s for HDRpH10, indicating the maximum viscosity reached during gelatinization. Breakdown viscosities were 0.43 Pa·s for HDRpH3, 0.83 Pa·s for HDRpH7, and 0.43 Pa·s for HDRpH10, measuring the ease with which swollen samples can be disintegrated, while final viscosities were 0.74 Pa·s for HDRpH3, 0.74 Pa·s for HDRpH7, and 1.41 Pa·s for HDRpH10, indicating the tendency for samples to retrograde or syneresis when cooling cooked flour pastas [
48].
The hydrocolloids began to gelatinize at similar temperatures, with viscosity increasing rapidly. The gelatinization curves for HDRpH3 and HDRpH10 were almost perpendicular to the x-axis, indicating a rapid increase in viscosity. Gelatinization for HDRpH3 and HDRpH10 occurred earlier compared to HDRpH7, which maintained a lower viscosity throughout the process. This suggests that hydrocolloids extracted at neutral pH require higher temperatures to reach their final viscosity, making it more difficult for water absorption and subsequent granule swelling, thereby hindering gelatinization.
The variation in apparent viscosity with temperature can be attributed to the increased thermal movement of molecules, which expands intermolecular distances and weakens molecular interactions as temperature rises. Among the samples, HDRpH7 reached its maximum viscosity peak first, although it required more energy to achieve this peak.
Similar results were observed for various starches:
Spirodela starch had a pasting temperature of 66.9 °C, with a trough viscosity of 0.761 Pa·s, a maximum viscosity of 2.664 Pa·s, a minimum viscosity of 1.536 Pa·s, a final viscosity of 2.298 Pa·s, and a breakdown viscosity of 1.128 Pa·s. Corn starch exhibited a pasting temperature of 74.6 °C, a trough viscosity of 0.775 Pa·s, a maximum viscosity of 1.696 Pa·s, a minimum viscosity of 1.332 Pa·s, a final viscosity of 2.107 Pa·s, and a breakdown viscosity of 0.365 Pa·s. Rice starch showed a pasting temperature of 71.5 °C, a trough viscosity of 1.233 Pa·s, a maximum viscosity of 0.1261 Pa·s, a minimum viscosity of 0.634 Pa·s, a final viscosity of 1.868 Pa·s, and a breakdown viscosity of 0.627 Pa·s [
49].
The distinctive behavior of the HDRpH7 sample stands out compared to the other two hydrocolloids. This difference is likely due to the neutral pH of HDRpH7, which enhances the stability of the molecules. At neutral pH, the reduced charge on the molecules leads to greater stability and less mobility, which inhibits the breaking of hydrogen bonds. As a result, water has less interaction with the granules, impeding their swelling and delaying the onset of gelatinization. This stability and the associated difficulty in achieving gelatinization are evident in the pasting properties of the HDRpH7 sample.
3.6. Microstructural Characteristics
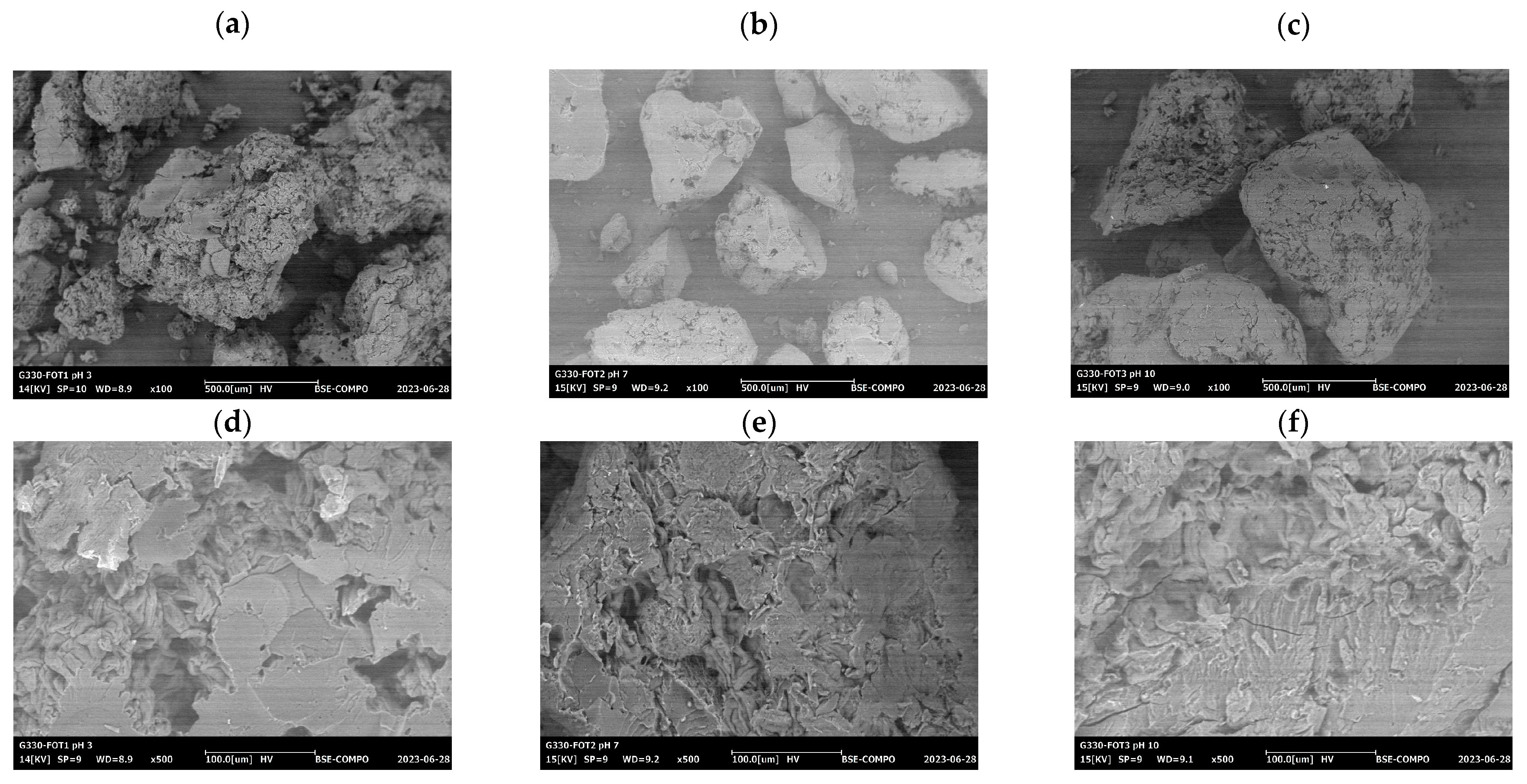
SEM images of
D. rotundata hydrocolloids, depicted in
Figure 6, reveal that the granules exhibit irregular shapes, including round, oval, and polygonal forms. The surfaces of these granules are generally smooth but show a wide distribution of sizes. Notably, the hydrocolloids display a high degree of irregularity, with many exhibiting uneven surfaces characterized by numerous small depressions or pores. This irregularity in shape and texture is consistent across all extraction conditions (acidic, neutral, and alkaline). The observed porosity is likely due to the evaporation of moisture during the solidification process. As the hydrocolloids dry, moisture loss creates voids and depressions on the surface, contributing to the overall porous structure [
50].
The variation in granule shape and surface texture could be attributed to differences in the extraction conditions. For instance, acidic and alkaline conditions may influence the granule’s surface morphology and porosity by affecting the rate of moisture evaporation and the extent of polymer chain interactions. The porosity and surface characteristics are crucial as they can impact the functional properties of the hydrocolloids, including their texture and ability to interact with other substances in food matrices [
51]. The irregular and porous nature of these hydrocolloids could enhance their ability to form gels or interact with other ingredients, which is beneficial for applications requiring texture modification or thickening [
51]. Understanding these microstructural features provides insights into how
D. rotundata hydrocolloids might perform in various applications, such as food texture modification or as stabilizers in different products.
The micrograph
Figure 6, of
D. rotundata hydrocolloids at 100× and 500× magnification, reveals granules with irregular, often rough surfaces. These granules are varied in shape, including round, oval, and polygonal forms, with visible small depressions. This rough texture and the variability in size could be attributed to increased moisture evaporation during the acid extraction process. Acidic conditions may disrupt the granule structure, leading to these pronounced surface features [
52]. The observed porosity aligns with findings that acidic environments can alter the structural integrity of polysaccharides, enhancing surface roughness due to moisture loss [
53]. The micrograph
Figure 6a,d, the granules from the acidic extraction (HDRpH3) display a pronounced roughness with numerous small depressions and pores. This detailed view emphasizes the impact of acidic conditions on the granule surface, highlighting the increased porosity and surface irregularities. The detailed texture observed supports the hypothesis that acidic conditions lead to greater disruption in the granule structure due to intensified moisture evaporation [
54]. The micrograph
Figure 6b,e, for the neutral pH condition (HDRpH7), shows granules with a somewhat smoother surface compared to HDRpH3. The granules still display irregular shapes but with fewer and less pronounced surface depressions. This suggests that neutral pH conditions lead to a more stable granule structure with less pronounced moisture loss compared to acidic conditions. This finding supports studies that indicate neutral pH can stabilize the hydrocolloid structure by minimizing disruption and porosity. The micrograph
Figure 6c,e, under alkaline conditions (HDRpH10), the granules are more uniform in size and shape, exhibiting smoother surfaces compared to the acidic and neutral conditions. The reduced porosity and more consistent texture observed suggest that alkaline conditions promote a more stable hydrocolloid structure. This result is consistent with reports indicating that alkaline environments can enhance the formation of more uniform granules by influencing moisture content and structural stability [
55]. This finding corroborates the idea that alkaline conditions promote a more homogeneous granule structure due to better control of moisture loss and structural integrity.
Alkaline conditions produce the smoothest and most uniform granules. These observations suggest that pH conditions during extraction play a crucial role in determining the final structure and texture of the hydrocolloids. The varying surface textures and porosities are indicative of how pH affects granule morphology, which could influence the functional properties of the hydrocolloids in various applications.
3.7. Potential Use of Hydrocolloids as Food Ingredients
Hydrocolloids are commonly used as dietary fibers, thickeners, gelling agents, emulsifiers, stabilizers, fat substitutes, clarifying agents, flocculants, opacifying agents, and whipping agents; in addition, they have applications in the areas of edible films, encapsulating flavors and inhibiting crystallization, and encapsulation of bioactive compounds. A high number of hydrocolloids are commercial, that is, starch, pectin, inulin, gelatin, agar, xanthan gum, guar gum, carrageenan, alginate, carob bean gum, gum arabic, gum gel, methylcellulose, carboxymethyl cellulose, hydroxypropyl methylcellulose, and hydroxyethyl methyl cellulose, among others, of which starch, gelatin, pectin, and carrageenan account the largest percentage of the hydrocolloid market. In addition, some hydrocolloids have nutritional and physiological effects, including β-glucan, pectin, inulin, arabic gum, psyllium, resistant starches, guar gum, chitosan, carrageenan, among others [
56]. However, the food industry is developing new products to promote the increase in sustainable and ecological foods, based on the nutritional benefits provided to consumers [
57].
Then, D. rotundata hydrocolloids present different properties, taking into account the solubilization condition, making them of interest for their applications in food products. HDRpH7 presents higher solubility. WHC and OHC are advantageous for utilizing hydrocolloids in the development of various food products, including emulsifiers, baked goods, fruit preparations, and dairy products. These properties enhance stability, modify textures, and prevent syneresis.
The viscous flow behavior of D. rotundata hydrocolloids demonstrates a decrease in viscosity with increasing shear rate. This shear-thinning behavior is significant for various food applications, as it influences the organoleptic properties, such as mouthfeel, which are closely related to the viscosity of hydrocolloids. The concentration of the hydrocolloids and the application temperature also affect these characteristics. Also, HDRpH7 present higher loss tangent showing a better balance between elastic and viscous modulus appropriate for their employee in emulsions, sauces, dressings and drinks, while HDRpH3 could employee as ingredient in jelly, cheese, bakery product due to the higher elastic properties; Also, HDRpH3 and HDRpH10 could be employee in products to modify texture as desserts, ice cream and yogurts.
4. Conclusions
In this study, hydrocolloids from Dioscorea rotundata were successfully extracted under varying pH conditions (pH 3, pH 7, and pH 10), yielding between 20 and 24%. The highest extraction yield was observed at pH 3. This outcome is attributed to the enhanced interaction between carboxyl groups in an acidic environment, where the lower pH increases the proportion of the non-dissociated form of the hydrocolloid. This reduction in electrostatic repulsion between molecules facilitates closer chain aggregation and decreases viscosity, which enhances the extraction efficiency. The extracted hydrocolloids demonstrated promising techno-functional properties suitable for various food applications. Significant variations were noted in their water holding capacity (WHC) and oil holding capacity (OHC), with WHC being notably higher in hydrocolloids extracted at neutral pH. This variation is due to the ability of smaller pores in the hydrocolloids to quickly trap and retain water. These properties are crucial for applications in food products where moisture and oil retention are important for texture and stability. The hydrocolloids are predominantly composed of carbohydrates and proteins, with a low ash content. This composition indicates a high proportion of functional carbohydrates, which contribute to their effectiveness as thickeners and stabilizers. The low ash content suggests minimal mineral content, which can be beneficial for specific food applications requiring controlled mineral levels. Rheological analysis revealed that the hydrocolloids exhibit pseudoplastic behavior, characterized by a decrease in viscosity with increasing shear rate. This non-Newtonian behavior is typical for many hydrocolloids and indicates that the material becomes less viscous under higher shear conditions. The onset of gelatinization occurred at approximately 70 °C for all samples, demonstrating their ability to form gels at relatively low temperatures. Overall, the D. rotundata hydrocolloids represent an excellent source of functional carbohydrates for product development. Their properties align well with the requirements for use as thickeners, stabilizers, or additives in the food industry. The hydrocolloids’ low levels of fat, protein, and ash are within the acceptable range for such products, further supporting their viability for commercial applications. These findings underscore the potential of D. rotundata hydrocolloids in enhancing the texture, stability, and overall quality of food products, when neutral condition extraction led to obtain samples with better technological, rheological and microstructural properties.