Abstract
This review article provides an overview of the bioactive compounds of clove, their health benefits, and their potential application in food and beverages. Cloves are rich in phenolic compounds, mainly eugenol, which exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, and wound-healing properties. Traditional methods of clove extraction, such as Soxhlet and maceration, have limitations. Green extraction methods, such as ultrasound-assisted extraction, pressurised liquid extraction, and microwave-assisted extraction, have shown promising results. The potential application of clove extract in various food and beverage products are also discussed. Finally, future perspectives and challenges for clove extraction are highlighted. Overall, the review highlights the potential of clove extract as a natural source of bioactive compounds for various applications in the food and beverage industry.
1. Introduction
Clove (Syzygium aromaticum) is an evergreen tree native to Indonesia, but it is also grown in other countries, such as Madagascar, Tanzania, and Sri Lanka [1]. Clove trees can grow up to 12 m tall and have dark green leaves and small white flowers [2]. Clove production begins with planting clove tree seedlings, usually grown in nurseries for up to a year before being transplanted into the field [3]. Clove trees require a warm, humid climate and well-draining soil. The trees are usually spaced 8 to 10 m apart to achieve adequate sunlight and air circulation [4]. Clove trees take approximately 5 to 7 years to reach maturity and produce their first crop of flowers [5]. The flowers are typically harvested by hand between July and September when they are still blossoming. The blossoms are then exposed to the sun for several days until they attain a dark-brown hue. Once the cloves have been thoroughly dried, they are separated from the stems and other debris before being placed in bags for storage and transportation. The quantity of cloves produced yearly can vary depending on weather conditions and other factors, as clove production is seasonal [6]. Indonesia is the world’s most significant clove producer, accounting for roughly 70% of global production. Madagascar, Tanzania, and Sri Lanka are three other significant producers [7]. Cloves are a significant export commodity for many of these nations and are utilised in numerous industries, including the food, cosmetic, and pharmaceutical sectors [8].
Clove is a spice with a distinct flavour and various health advantages widely utilised in traditional medical and culinary activities [9]. In recent years, there has been a growing interest in clove valorisation, or the process of creating higher-value products from clove, in addition to its traditional applications. Up to 85% of clove essential oil comprises eugenol, the primary bioactive component [10]. Numerous health benefits have been identified, including anti-inflammatory, analgesic, and antioxidant properties. Eugenol has been shown to have antibacterial and antifungal properties, making it effective against a broad range of microorganisms [11]. Acetyl eugenol is another important bioactive compound in clove, accounting for up to 15% of its essential oil [12]. Similar health benefits to eugenol have been discovered, including anti-inflammatory and analgesic properties [13]. It has also been discovered that acetyl eugenol has anticancer properties, making it a potential cancer treatment agent [14]. Clove and other plants, including black pepper and cinnamon, contain the bioactive compound caryophyllene. It has been found to have anti-inflammatory and analgesic properties, making it effective in treating pain and inflammation [15].
Current extraction methods for clove bioactive compounds include steam distillation, solvent extraction, and supercritical fluid extraction [16,17]. These methods have advantages and limitations, but recent advances in extraction techniques have shown promise in increasing the yield and purity of bioactive compounds [18]. Potential applications of clove in food and beverage products include its use as a natural flavouring agent, natural preservative, and antioxidant [19]. Due to its bioactive compounds, clove can also be used to develop functional foods and nutraceuticals. In addition to its potential applications, the valorisation of clove waste can provide an opportunity to create value-added products and reduce environmental impact [20]. Clove stems and leaves can produce biofuels, and clove oil production waste can produce biodegradable plastics [21].
This review will focus on the valorisation of clove, specifically its bioactivities, current extraction methods, potential applications, and future perspectives in food and beverage products. The bioactive compounds found in clove, such as eugenol, acetyl eugenol, and caryophyllene, have been found to have numerous health benefits, including anti-inflammatory, analgesic, and antibacterial properties. These bioactive compounds have led to the development of new pharmaceuticals and nutraceuticals.
2. Bioactive Compounds of Clove Extract
Clove is a spice that is rich in bioactive compounds, which are responsible for its numerous health benefits. The main bioactive compounds found in cloves are eugenol, acetyl eugenol, and caryophyllene. The bioactive compounds found in cloves have led to the development of novel pharmaceuticals and nutraceuticals. The composition of clove extracts is shown in Table 1. The explanation and application of bioactive compounds from clove extract are shown below.

Table 1.
Composition of active compounds from clove extract adapted from Haro-González et al. [22].
2.1. Eugenol
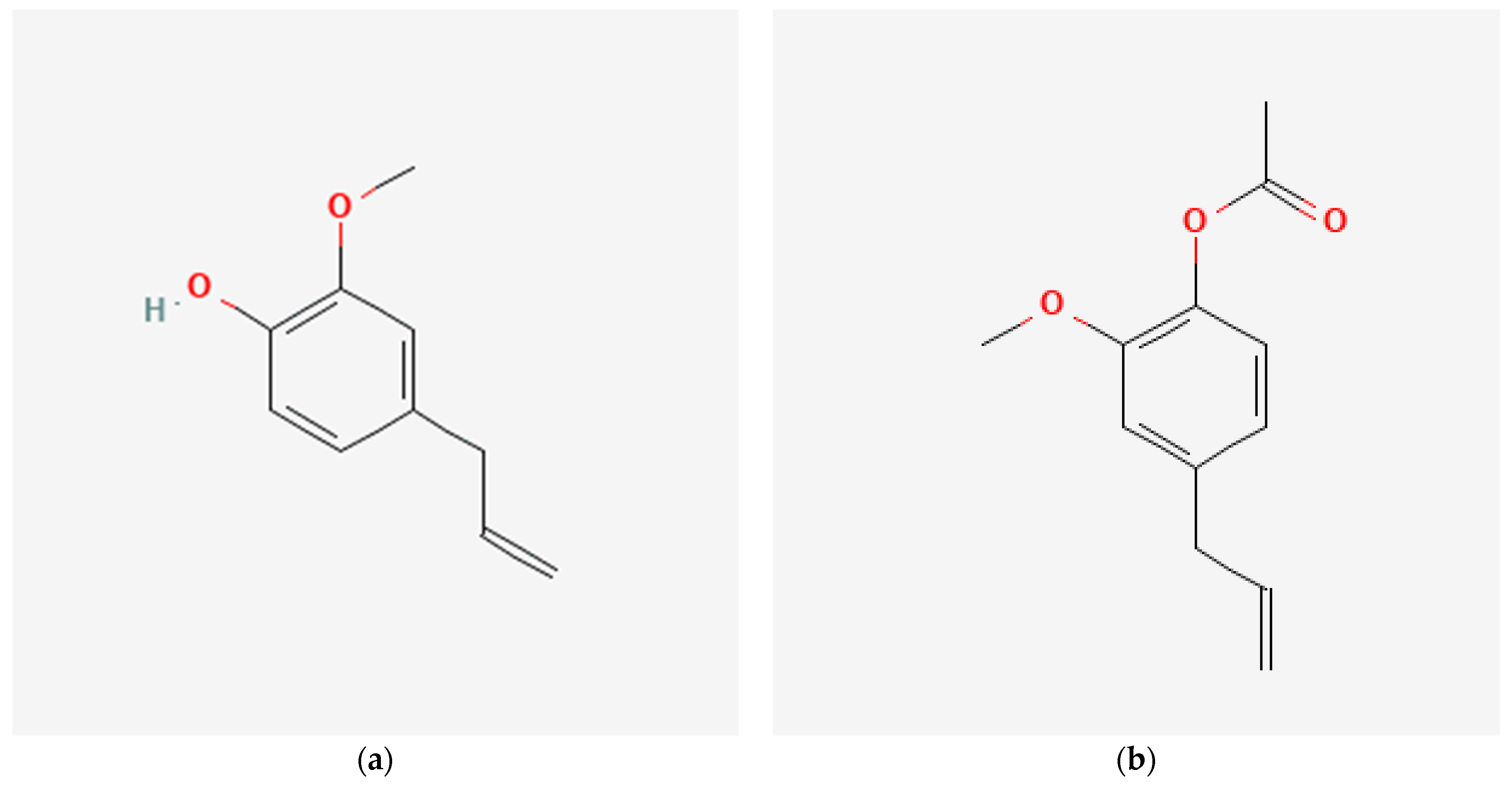
Eugenol, as shown in Figure 1a, is the main bioactive compound found in clove extract, and it is responsible for many of its beneficial properties [23]. Eugenol has been comprehensively studied for its various biological activities, which include antioxidant, anti-inflammatory, analgesic, antimicrobial, and anticancer properties [24]. One of the most well-known properties of eugenol is its analgesic influence, which makes it effective in relieving pain. Eugenol has been shown to inhibit the activity of certain enzymes that play a role in pain perception, such as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) [25]. It makes eugenol a potential natural alternative to synthetic analgesics. Eugenol is also known for its potent antioxidant activity, which makes it effective in preventing oxidative damage induced by free radicals [26]. Free radicals are unstable molecules that can cause damage to cells and tissues, resulting in various diseases and ageing [27]. Its antioxidant activity is attributed to its ability to scavenge free radicals and protect cells against oxidative stress [28].
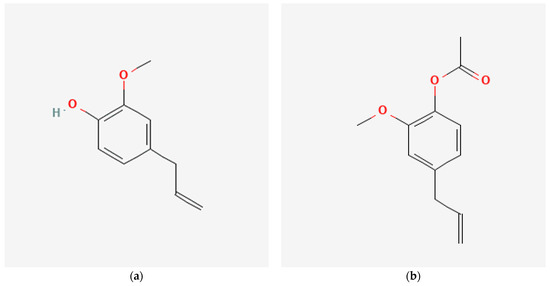
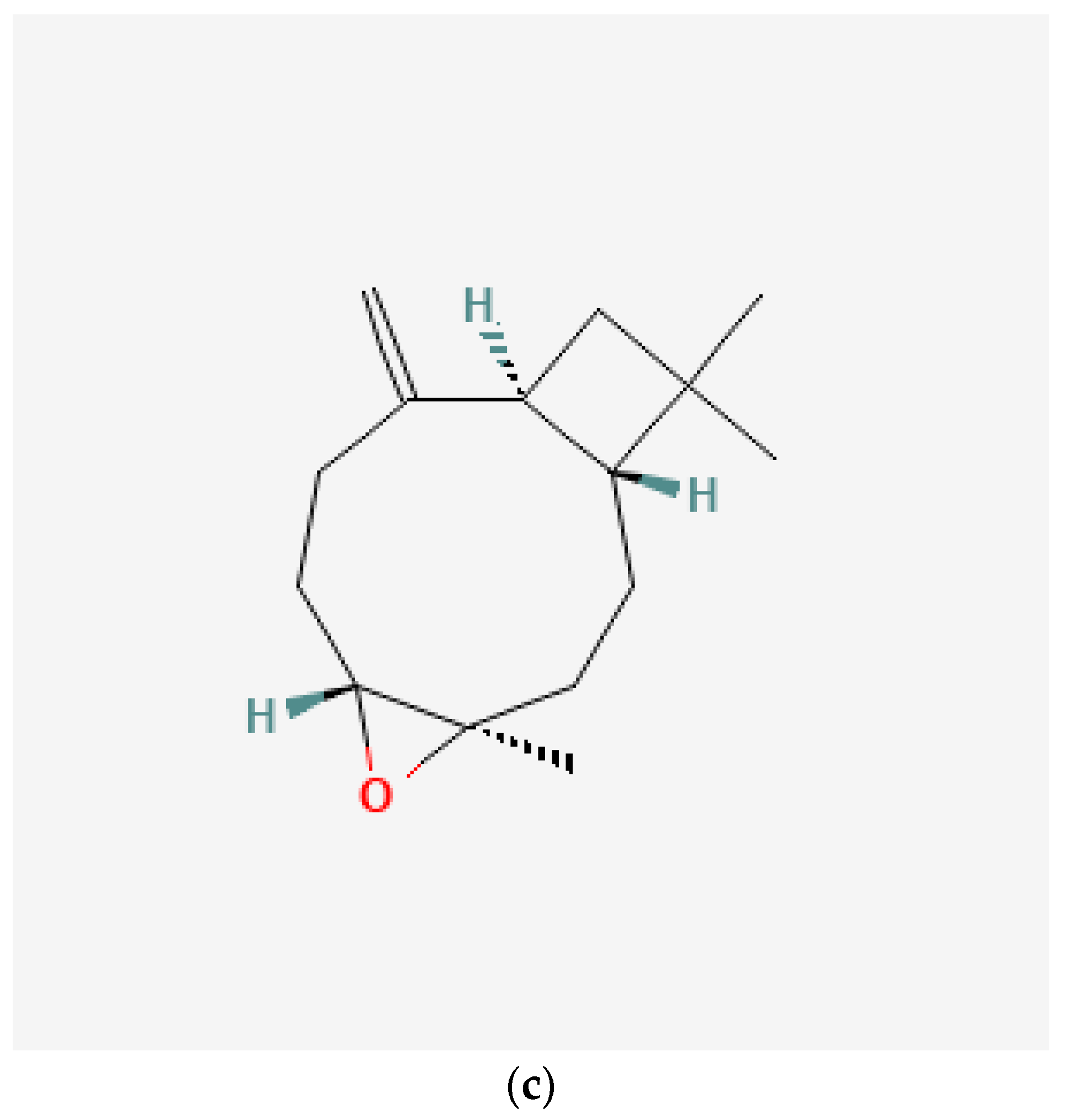
Figure 1.
Chemical structure of (a) eugenol, (b) acetyl eugenol, and (c) caryophyllene obtained from PubChem.
In addition, eugenol has been found to have anti-inflammatory properties that make it effective in reducing inflammation. Inflammation is a normal immune system response to injury and infection, but chronic inflammation can contribute to the development of various diseases. Eugenol’s anti-inflammatory properties are attributed to its capacity to inhibit the production of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α) [29]. It has been investigated for its potential as a drug delivery agent. Due to its lipophilic nature, eugenol has been found to enhance the solubility and bioavailability of certain pharmaceuticals [30]. One example of eugenol’s use as a drug delivery agent is in the treatment of cancer. Eugenol has been shown to enhance the solubility and bioavailability of certain anticancer drugs, such as paclitaxel and curcumin, which can increase their therapeutic efficacy [31]. In addition, eugenol has been found to have anticancer properties, making it a potential adjuvant therapy for cancer treatment [32]. Eugenol has also been studied for its potential in transdermal drug delivery. Transdermal drug delivery is a non-invasive technique for delivering drugs through the skin and into the bloodstream [33]. Eugenol’s lipophilic nature allows it to penetrate the epidermis and enhance the permeation of certain drugs [34].
2.2. Acetyl Eugenol
The natural compound acetyl eugenol is found in clove extract and is structurally related to eugenol. The chemical structure of acetyl eugenol is shown in Figure 1b. Similar to eugenol, acetyl eugenol has been investigated for its diverse biological activities, such as antioxidant, anti-inflammatory, and antimicrobial properties [35]. One of acetyl eugenol’s most well-known properties is its analgesic effect, which makes it an effective pain reliever [36]. It has been demonstrated that acetyl eugenol inhibits the activity of pain-related enzymes, such as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) [14]. It causes acetyl eugenol to be a possible natural substitute for synthetic analgesics.
Additionally, acetyl eugenol possesses antioxidant properties, effectively preventing oxidative damage induced by free radicals [37]. In addition, acetyl eugenol has anti-inflammatory properties, making it an effective anti-inflammatory agent. Inflammation is a normal immune response to injury and infection; however, chronic inflammation can contribute to the development of various diseases. Due to its capacity to inhibit the production of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1 β) and tumour necrosis factor-alpha (TNF-α), acetyl eugenol possesses anti-inflammatory properties [38]. It also has been discovered that acetyl eugenol has antimicrobial properties that make it effective against infections. It has been demonstrated that acetyl eugenol is effective against various bacteria and fungi, making it a potential alternative to synthetic antimicrobial agents [39].
2.3. Caryophyllene
Caryophyllene is a natural compound present in the essential oil of clove extract. It belongs to the class of compounds known as sesquiterpenes, which occur naturally in plants and have diverse biological activities [40]. Caryophyllene has been found to have a range of biological activities, including anti-inflammatory, antioxidant, and anticancer properties [41]. One of the most remarkable properties of caryophyllene is its ability to operate as a selective agonist of cannabinoid receptor type 2 (CB2) [42]. CB2 receptors are predominantly expressed in immune cells and have been implicated in regulating inflammation and immune response. Studies have shown that caryophyllene can reduce inflammation and pain by activating CB2 receptors, which modulate the immune response. It makes caryophyllene a prospective therapeutic agent for treating various inflammatory diseases, such as arthritis, asthma, and inflammatory bowel disease.
Moreover, caryophyllene has been found to have antioxidant properties that make it effective in preventing oxidative damage induced by free radicals [43]. Free radicals are unstable molecules that can damage cells and tissues, leading to diseases and ageing. Caryophyllene’s antioxidant activity is attributed to its ability to scavenge free radicals and protect cells against oxidative stress. Additionally, caryophyllene has been found to have anticancer properties that make it effective in inhibiting the growth and proliferation of cancer cells [44]. Studies have shown that caryophyllene can induce apoptosis (cell death) in cancer cells and inhibit the formation of new blood vessels that supply nutrients to tumours.
3. Bioactivity of Clove Extract
Clove extract has many bioactivities, including antioxidant, anti-inflammatory, antimicrobial, anticancer, and neuroprotective effects [45]. The antioxidant activity of clove extract is attributed to the presence of phenolic compounds, which scavenge free radicals and reduce oxidative stress [27]. Its anti-inflammatory activity is also due to these phenolic compounds, which reduce the production of inflammatory cytokines and enzymes. Clove extract exhibits antimicrobial activity against a wide range of microorganisms, which is predominantly attributed to the presence of eugenol [46]. Studies have also shown that clove extract has anticancer activity, inducing cell demise in cancer cells and inhibiting their proliferation [47]. Furthermore, clove extract has neuroprotective effects, which is ascribed to eugenol and has been shown to reduce oxidative stress and inflammation in the brain [48]. These bioactivities are attributed to clove extract’s abundant bioactive compounds, making it a potential therapeutic agent for preventing and treating various diseases.
3.1. Antioxidant Activity
Clove extract has also been discovered to have a synergistic antioxidant effect when combined with other natural antioxidants, such as vitamins C and E, and carotenoids [49]. In one study, clove extract and vitamin C produced a more significant antioxidant effect than either compound independently [50]. Clove extract’s antioxidant activity has also been studied for its potential in food preservation [51]. It has demonstrated efficacy in inhibiting lipid oxidation and preventing food deterioration due to its potent antioxidant properties. It is known that the liver plays a crucial role in the metabolism of various compounds, including those from natural sources, such as herbs and spices. In the case of clove extracts, liver enzymes such as cytochrome P450 are likely to be involved in their metabolism. These enzymes help to break down the compounds in clove extracts into smaller molecules that can be excreted from the body. The antioxidant compounds in clove extracts, such as phenolic acids and flavonoids, may also exert their effects by interacting with liver enzymes and reducing oxidative stress [52,53].
Furthermore, the caryophyllene present in clove extract has been shown to have a regulatory effect on various genes involved in antioxidant defence, apoptosis, and energy metabolism. Specifically, it has been shown to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, which is a major regulator of antioxidant genes, such as heme oxygenase-1 (HO-1) [54]. This pathway is responsible for the production of endogenous antioxidants, which help to protect against oxidative stress [55]. In addition to its effects on antioxidant defence and apoptosis, clove extract has also been shown to regulate genes involved in energy metabolism. For example, it has been shown to activate the sirtuin 1 (SIRT1) pathway, which is involved in regulating cellular energy metabolism and maintaining cellular homeostasis [56]. Clove extract has also been shown to increase the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a), which is involved in regulating mitochondrial biogenesis and energy metabolism.
Clove extract’s antioxidant properties have prospective applications in cosmetics [39,57]. Clove extract has been demonstrated to have anti-ageing properties as it inhibits the formation of reactive oxygen species and prevents UV-induced damage. Additionally, it has been incorporated into anti-wrinkle lotions and other cosmetics [58]. In conclusion, clove extract’s antioxidant activity is an essential bioactivity with potential health benefits in preventing and treating chronic diseases. Its potent antioxidant activity, synergistic effects with other natural antioxidants, and potential applications in the culinary and cosmetic industries make it a promising natural antioxidant for further investigation and development.
3.2. Anti-Inflammatory
Clove extract has exhibited significant anti-inflammatory effects [59]. These effects are attributable to eugenol, the primary bioactive compound found in clove extract. Eugenol is known to inhibit the production of pro-inflammatory mediators and enzymes, including cyclooxygenase (COX) and lipoxygenase (LOX) [60]. Clove extract effectively reduced inflammation in the paws of rats with induced inflammation. The study demonstrated that the extract inhibited the production of inflammatory cytokines, including tumour necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β), and decreased the activity of COX-2 and LOX enzymes [61]. When given to mice with induced inflammation, eugenol reduced the expression of genes implicated in the inflammatory response. According to another study, it decreased inflammatory cytokine levels in the blood. Furthermore, clove extract has been shown to modulate the expression of apoptotic genes, such as Bcl-2 and Bax. Bcl-2 is an anti-apoptotic gene that helps to prevent cell death, while Bax is a pro-apoptotic gene that promotes cell death [62]. By regulating the expression of these genes, clove extract can help to maintain cellular homeostasis and prevent cell damage.
Additionally, clove extract has been studied for its potential for treating inflammatory bowel disease (IBD) [63]. Clove extract reduced inflammation and damage to the colon in rodents with induced colitis. The study demonstrated that clove extract reduced the activity of inflammatory enzymes such as myeloperoxidase (MPO) and the levels of inflammatory cytokines [64]. The study investigated the potential anti-aging effects of clove extract and eugenol on UVB-irradiated normal human dermal fibroblasts and hairless mice [65]. The results showed that extract and eugenol suppressed matrix metalloproteinase secretion and activator protein 1 phosphorylation while activating nuclear erythroid 2-related factor/antioxidant-response element signalling and inhibiting nuclear factor-κB and interleukin-6 expression. In vivo, the extract improved procollagen type I and elastin levels via TGF/Smad signalling, reduced wrinkles, and increased filament aggregating protein. The study concluded that the clove extract effectively ameliorated UVB-induced photoaging and has potential as a natural anti-aging product.
Moreover, clove extract has anti-inflammatory effects on the skin [66]. It has been applied topically in creams and ointments to treat acne, eczema, and psoriasis, which induce inflammation and redness.
3.3. Antimicrobial
It has been discovered that clove extract has substantial antimicrobial activity against a broad spectrum of microorganisms, including bacteria, fungi, and viruses [67]. This activity can be ascribed to eugenol, the primary bioactive compound in clove extract. Eugenol is known to disrupt the cell membranes of microorganisms and inhibit their growth and reproduction [68]. Oulkheir et al. [69] demonstrated that clove extract possesses potent antibacterial activity against various bacteria, including Gram-positive and Gram-negative bacteria. Clove extract was also found to inhibit the growth of methicillin-resistant Staphylococcus aureus (MRSA), an antibiotic-resistant strain of bacteria [70]. El-Shouny et al. [71] also demonstrated that clove extract is effective against Helicobacter pylori, a bacterium that is a leading cause of stomach ulcers and stomach cancer.
3.4. Antifungal Activity
Clove extract has been found to have significant antifungal activity against a wide range of fungi. Eugenol is known to disrupt the cell membranes and inhibit the growth and reproduction of fungi [68]. Several studies have investigated the antifungal activity of clove extract against Candida albicans [72]. The study showed that when applied topically, eugenol reduced the severity of skin infections caused by Candida albicans. Moreover, clove extract has been investigated for its potential to treat other fungal infections. Studies have shown that clove extract has antifungal activity against other pathogenic fungi, such as Aspergillus flavus, a common cause of food spoilage and respiratory infections [73]. In addition, clove extract has been studied for its potential to prevent viral infections. Several varieties of viruses, such as herpes simplex virus (HSV), influenza virus, and human immunodeficiency virus (HIV), can be inhibited by clove extract, according to scientific studies [74].
3.5. Wound Healing
Clove extract has been found to have potential wound-healing properties due to its antimicrobial and anti-inflammatory effects [62]. Clove extract has anti-inflammatory properties that can help to reduce swelling and pain associated with wounds. Inflammation is an essential part of the wound-healing process. However, excessive inflammation can delay healing and cause tissue damage. Eugenol, the main bioactive compound in clove extract, has been shown to accelerate wound healing by promoting angiogenesis, increasing collagen production, and reducing inflammation [75]. In addition, eugenol has been found to increase the expression of growth factors that are involved in the wound-healing process, such as vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β) [76]. This promotes angiogenesis, which is the formation of new blood vessels, and stimulates the growth of fibroblasts, which produce collagen and help repair damaged tissue. Eugenol has been shown to inhibit the production of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α), which can contribute to excessive inflammation [29].
4. Application of Clove Extract
As a result of its diverse bioactive compounds and advantageous properties, clove extract has a vast array of potential applications. Clove extract is a versatile natural substance with numerous potential applications in various industries. It is a valuable ingredient for promoting health and well-being due to its abundant bioactive compounds and beneficial properties. The following are prevalent applications of clove extract:
- Food and beverage industry: Due to its robust flavour and antimicrobial properties, clove extract can be used as a natural food flavouring agent and preservative. It is a prevalent ingredient in baked goods, chewing gum, and beverages [77].
- Oral care products: Clove extract is a natural treatment for various oral health conditions, including toothache, bad door, and gum disease. It is a ubiquitous component of toothpaste, mouthwash, and dental floss [78].
- Skincare products: Clove extract’s antimicrobial and antioxidant properties make it an effective treatment for acne, wrinkles, and other skin conditions. Creams, lotions, and cleansers typically contain this ingredient [79].
- Nutraceuticals: Clove extract is a rich source of potentially beneficial bioactive compounds. It is frequently used as a dietary supplement to enhance digestion, reduce inflammation, and strengthen the immune system [80].
- Pharmaceuticals: Clove extract and its bioactive compounds have diverse therapeutic properties that make them effective in the treatment of a variety of diseases, including arthritis, cancer, and diabetes. It is a common ingredient in herbal remedies and dietary supplements [81].
5. Traditional Extraction for the Valorisation of Cloves
Bioactive compounds can be extracted from cloves using Soxhlet and maceration, two traditional extraction methods [82]. The Soxhlet extraction method entails placing ground cloves in a thimble and then placing the thimble in a Soxhlet apparatus. The apparatus is coupled to a solvent reservoir and condenser, and the solvent is heated until it boils. As the solvent boils, it vaporises and ascends the apparatus, condensing and dripping onto the ground cloves in the thimble. The clove bioactive compounds are dissolved by the solvent, which then drips back into the solvent reservoir, where it is heated and proceeds. This cyclical process is repeated until the desired quantity of bioactive compounds is achieved.
Maceration is a more straightforward extraction technique that entails soaking ground cloves in a solvent, such as water or alcohol [78,83]. The solvent dissolves the clove bioactive compounds, and the solution is filtered to remove solid particles. The solution that has been filtered can then be concentrated to produce a rudimentary extract. The Soxhlet and maceration techniques are straightforward and efficient for extracting bioactive compounds from cloves. However, they may be less effective than green extraction methods, reducing yields and bioactive compound concentrations. Therefore, these methods may be best adapted for small-scale extractions or use in traditional medicine.
5.1. Soxhlet Extraction
Soxhlet extraction is a widely used method for extracting bioactive compounds from plant materials, including cloves. Clove is a spice that has been used for centuries for its flavour and medicinal properties. Soxhlet extraction is a well-established method for obtaining high-quality extracts from plant materials, as it efficiently extracts a wide range of bioactive compounds. This method involves using a specialised glassware setup consisting of a heating mantle, a boiling flask, a reflux condenser, and a collection flask. A suitable solvent, such as ethanol or methanol, is used to dissolve the bioactive compounds from cloves, and the extraction process continues until all the desired compounds have been extracted. The extracted solution is then concentrated to obtain a more concentrated crude extract, which can be used for various food, pharmaceutical, and cosmetic applications [84]. While Soxhlet extraction is a time-consuming process, it is an effective method for obtaining high yields of bioactive compounds from cloves, making it an essential method for valorising this valuable natural resource [85].
Guan et al. [86] examined the optimal conditions for the Soxhlet extraction of essential oil from clove stems utilising a response surface methodology (RSM) with a central composite design (CCD). The effects of extraction time, solvent-to-sample ratio, and extraction temperature on oil yield were evaluated. The optimal conditions were determined to be 3.3 h extraction time, a solvent-to-sample ratio of 27.9:1, and an extraction temperature of 79.8 °C, yielding a maximal oil yield of 16.6%. The study also found that the extracted oil contained high levels of bioactive compounds with antioxidant and antimicrobial properties, such as eugenol and eugenol acetate. The study determined that the extraction time and sample-to-solvent ratio were the most influential variables influencing the yield of clove oil during Soxhlet extraction. Increasing extraction time and decreasing the sample-to-solvent ratio increased clove oil yields. The study found that the type of solvent used significantly affected yield, with ethanol producing the maximum yield compared with other solvents tested, such as hexane and acetone.
5.2. Maceration Extraction
Maceration is a common technique for extracting bioactive compounds from plant materials, such as cloves. This technique involves soaking cloves in an appropriate solvent, such as ethanol, for an extended time to dissolve the bioactive compounds. Depending on the solvent employed and the desired outcome, the extraction process may be conducted at room or higher temperatures [87]. During maceration extraction, the solvent permeates the clove material and dissolves the bioactive compounds of interest, such as eugenol, acetyl eugenol, and caryophyllene. Depending on the desired yield and the type of solvent used, the extraction time can range from several hours to several days [88]. After the clove material has been extracted, the solvent is separated from it using filtration or centrifugation, and the resulting extract is concentrated using evaporation to yield a more concentrated, unrefined extract. Maceration extraction is a straightforward, cost-effective, and equipment-free technique for extracting bioactive compounds from cloves [89]. In contrast to other procedures, such as Soxhlet extraction, maceration extraction typically yields a smaller quantity of bioactive compounds. Therefore, optimising the extraction duration, temperature, and the solvent-to-sample ratio is essential to obtain a high-quality clove extract with a high bioactive compound yield.
Ferioli et al. [90] optimised clove maceration extraction using response surface methodology (RSM) with three variables: ethanol concentration, extraction time, and extraction temperature. Using maceration, the optimal conditions for extracting phenolic compounds from cloves were 50 °C, a solvent-to-sample ratio of 10:1, and an extraction time of 120 h. In addition, the study revealed that the extract exhibited potent antioxidant and antimicrobial properties with applications in the food industry. Because ethanol is a polar solvent, it can dissolve polar compounds such as eugenol and acetyl eugenol, the primary bioactive compounds in clove. Ethanol concentration and solvent ratio can affect the efficacy of clove extraction using maceration. The greater the concentration of ethanol is, the more excellent the solubility of these polar compounds is, resulting in a greater extraction yield. In addition, the solvent-to-plant-material ratio can influence extraction efficiency. A higher solvent-to-plant-material ratio can enhance the contact between the solvent and the plant material, allowing greater solvent penetration into the plant tissue and more efficient extraction of the target compounds to be achieved. However, a solvent ratio that is too high can result in the dilution of the target compounds and diminished extraction efficiency.
6. Green Extraction for the Valorisation of Clove
Clove is a famous spice that has been utilised for its medicinal properties for centuries. Clove bioactive compounds have been extracted using traditional extraction methods such as Soxhlet and maceration. However, these methods have several drawbacks, such as lengthy extraction periods, toxic solvents, and low yields. Green extraction techniques have been devised to overcome these limitations, such as supercritical fluid extraction, microwave-assisted extraction, ultrasound-assisted extraction, and pressurised liquid extraction. These techniques use non-toxic solvents, reduce extraction duration, and produce greater yields than conventional procedures. Green extraction techniques have transformed the extraction of bioactive compounds from clove, making it more sustainable and efficient. These benefits make green extraction techniques ideal for producing natural products with high added value for use in the food, pharmaceutical, and cosmetic industries.
6.1. Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) is a green extraction technique that uses ultrasonic waves to enhance the extraction of bioactive compounds from plant materials. In the case of clove, UAE is an effective method for extracting high yields of bioactive compounds, including eugenol, caryophyllene, and acetyl eugenol [91]. The UAE process involves immersing clove powder or whole cloves in a solvent and subjecting them to high-frequency ultrasonic waves. These waves create cavitation bubbles that implode and generate high temperatures and pressures, leading to enhanced mass transfer and faster extraction. UAE offers several advantages over traditional extraction methods, including shorter extraction times, lower solvent consumption, and higher yields [92]. Moreover, UAE uses non-toxic solvents, making it an eco-friendly and sustainable extraction method. Additionally, using ultrasound waves has been shown to improve the functional properties of the extracted compounds, such as antioxidant activity and antimicrobial properties [88].
The yield of cloves derived using ultrasound-assisted extraction varied depending on the operating conditions, according to Tekin et al. [93]. At a frequency of 20 kHz, a temperature of 50 °C, a solvent-to-material ratio of 30 mL/g, and extraction duration of 30 min, the highest yield (6.98%) was obtained. Increases in temperature, solvent-to-material ratio, and extraction time were observed to increase yield, while a decrease in frequency was observed to increase yield. The increase in yield with the increase in temperature, solvent-to-material ratio, and extraction time can be attributed to the increased solubility of the active compounds in the solvent, enhanced diffusion of the solvent into the plant matrix, and increased release of compounds from the plant material [94]. On the other hand, the relationship between frequency and yield can be explained by the fact that high-frequency ultrasound waves can induce cavitation, forming bubbles that can rupture the cell walls and release bioactive compounds [95]. However, excessive cavitation can also degrade the compounds, resulting in a reduction in yield.
6.2. Supercritical Carbon Dioxide (SC-CO2)
SC-CO2 extraction is a modern technique for extracting bioactive compounds from natural sources, including clove, that has recently gained significant attention. SC-CO2 extraction is a green method that uses carbon dioxide in its supercritical state as the solvent. It offers several advantages over traditional extraction methods, such as higher extraction efficiency, shorter extraction times, and better selectivity for the target compounds [96]. In SC-CO2 extraction, carbon dioxide is pressurised to a supercritical state, exhibiting both liquid-like and gas-like properties, making it an excellent solvent for extracting hydrophobic and hydrophilic compounds [97]. The extraction process involves passing supercritical carbon dioxide through the clove matrix, where it dissolves and carries the target compounds.
Additionally, the short extraction times in SC-CO2 extraction can reduce the risk of the degradation and alteration of the extracted compounds. Finally, the selectivity of SC-CO2 extraction can be optimised by adjusting the pressure and temperature conditions, making it possible to target specific compounds of interest. Overall, SC-CO2 extraction has significant potential as a modern extraction technique for extracting bioactive compounds from cloves.
Chatterjee and Bhattacharjee [98] found that supercritical carbon dioxide extraction at a temperature of 45 °C, a pressure of 30 MPa, and a CO2 flow rate of 2.5 L/min resulted in the highest yield of essential oil from cloves (16.6%). The extracted essential oil was found to contain a high concentration of eugenol (81.6%) and caryophyllene (8.5%), the major bioactive compounds responsible for the pharmacological properties of the oil. Pressure, temperature, flow rate, and extraction time are the process parameters that affect clove supercritical carbon dioxide extraction. As they influence the solubility of the target compounds in the supercritical fluid, pressure and temperature are regarded as the most significant parameters. High pressure and temperature increase the density of the supercritical fluid, resulting in enhanced compound penetration and extraction.
The flow rate of the supercritical fluid is also crucial, because it impacts the residence time of the solvent in the extraction vessel, which can impact extraction efficiency [99]. A slower flow rate permits a prolonged residence time and improved extraction efficiency. The extraction time is also crucial, because it affects the extract’s overall yield and quality. Generally, longer extraction times result in greater yields, but they may also contribute to the extraction of undesirable compounds.
6.3. Pressurised Liquid Extraction
Pressurised liquid extraction (PLE) is a green extraction method that has recently garnered popularity due to its ability to efficiently and effectively extract bioactive compounds [100]. PLE employs solvents at high pressure to extract the desired compounds from plant matter. In the case of cloves, PLE has been utilised to extract bioactive compounds, including eugenol, acetyl eugenol, and caryophyllene, which have become known for their antioxidant, anti-inflammatory, and antimicrobial properties [101]. It has been demonstrated that PLE is a more effective and quicker extraction method than conventional techniques such as maceration and Soxhlet extraction [86].
Multiple process parameters influence the efficacy of PLE in clove extraction. The type of solvent employed is a crucial factor in determining the extraction yield. In a study, water, methanol, and ethanol were more effective solvents for clove oil extraction than acetone, ethyl acetate, and n-hexane [102]. Temperature and pressure within the PLE system also influence clove extraction efficiency. An increase in temperature and pressure results in an increase in extraction yield. However, elevated temperatures and pressures can degrade some bioactive compounds [103]. The duration of extraction is another variable that affects PLE yield. Longer extraction times result in greater yields and increase the risk of bioactive compound degradation. Therefore, it is essential to optimise the extraction time to acquire the highest possible yield without sacrificing extract quality [104]. The particle size of the plant material is also a significant factor in PLE production. The smaller the particulate size is, the greater the surface area is, facilitating the extraction process and increasing the yield [105].
6.4. Microwave-Assisted Extraction
Microwave-assisted extraction (MAE) is a green extraction technique that has recently gained attention due to its efficiency, speed, and potential for reducing solvent consumption. MAE uses microwave radiation to heat the solvent and extract the desired compounds from the plant material [106]. MAE has been used to extract bioactive compounds such as eugenol, acetyl eugenol, and caryophyllene from cloves [107]. MAE is a more efficient and faster extraction technique than traditional maceration and Soxhlet extraction methods.
In clove extraction, the efficiency of MAE can be influenced by several parameters. The type of solvent used can affect the solubility and selectivity of the target compounds. Ethanol, methanol, and water are commonly used solvents in clove extraction using MAE [108]. The power and frequency of microwave radiation also play a crucial role in extraction efficiency. Kapadiya et al. [109] found that higher power and lower frequency can result in better extraction yields, but excessive heating can lead to the degradation of the target compounds. The extraction time is another critical parameter affecting the extracted compounds’ yield and quality. Longer extraction times generally result in higher yields, but there is a risk of degradation due to prolonged exposure to microwave radiation [110]. Finally, the plant material’s particle size can also affect MAE efficiency. Finer particle sizes have a higher surface area, which can facilitate the extraction process but can also lead to clogging of the extraction system [111].
7. Future Perspective and Challenge for Green Clove Extraction
The extraction of bioactive compounds from cloves has been the subject of intensive study, with several traditional and green extraction techniques being developed and optimised. Nevertheless, problems must be solved before clove extract can reach its full potential. The standardisation of extraction methods and the quantification of bioactive compounds are among the challenges. Clove extract quality and efficacy can be affected by the varying quantities of bioactive compounds produced by distinct extraction techniques. To assure the consistency and quality of clove extract, the standardisation of extraction methods and the development of reliable methods for quantifying bioactive compounds are required.
Identifying and isolating novel bioactive compounds from cloves is a further obstacle. Despite the fact that numerous bioactive compounds in cloves have been identified and characterised, it is still possible to discover additional compounds with potential health benefits. Developing efficient techniques for identifying and isolating novel compounds can result in the discovery of new bioactive compounds and the creation of new applications for clove extract. In addition, the scalability of extraction methods is an essential factor in the commercial production of clove extract. Some extraction techniques, such as supercritical fluid extraction and pressurised liquid extraction, necessitate costly apparatuses and high operating expenses. For clove extract to be widely used in the food, pharmaceutical, and cosmetic industries, developing more cost-effective and scalable extraction techniques will be crucial. Conclusion: The future of clove extraction is bright, with numerous potential industrial applications. For the development and commercialisation of clove extract, it will be essential to address the obstacles of standardisation, identification of novel compounds, and scalability.
8. Summary
Clove (Syzygium aromaticum) is a spice widely used in cooking and traditional medicine due to its unique flavour and numerous health benefits. However, in recent years, there has been growing interest in the valorisation of clove, or the process of creating higher-value products from clove beyond its traditional uses. The valorisation of clove can involve various processes, such as extraction and purification of its active compounds, development of new applications in food and beverage industries, and conversion of clove waste into valuable products. One major area of focus in the valorisation of clove is the extraction and purification of its active compounds, such as eugenol, acetyl eugenol, and caryophyllene. These compounds have been found to have numerous health benefits, including anti-inflammatory, analgesic, and antibacterial properties, making them valuable in the development of new pharmaceuticals and nutraceuticals. Another area of interest in the valorisation of cloves is the development of new applications in the food and beverage industries. Clove can be used as a natural flavouring agent in various products, such as candies, baked goods, and beverages. It can also be used as a natural preservative due to its antimicrobial properties. This review article provides an overview of the principles and mechanisms of green extraction and compares the advantages and disadvantages of each method. The article also discusses the essential active compounds in cloves and presents the appropriate green extraction conditions for obtaining high-quality extracts.
The potential applications and future perspectives of clove extracts in the food and beverage industry are vast and promising. However, some limitations or challenges may arise in the production and utilisation of clove extracts, such as variability in the chemical composition of the extracts, possible toxicity at high doses, and limited knowledge of the mechanism of action of specific bioactive compounds. To address these challenges, further research is needed to better understand the composition and activity of clove extracts, optimise extraction methods, and develop innovative applications in food and beverages. Additionally, regulatory measures and quality control standards should be established to ensure the safety and efficacy of clove extracts in the industry. Future prospects for the use of different extraction methods are also discussed. Overall, the valorisation of clove has the potential to create new economic opportunities, promote sustainable practices, and expand the use of this versatile and beneficial spice beyond its traditional applications.
Author Contributions
Conceptualisation, A.H.A.A., N.R.P. and D.N.R.; methodology, L.Q.; software, D.N.R.; validation, D.N.R.; writing—original draft preparation, L.Q. and N.R.P.; writing—review and editing, I.I. and L.Q.; visualisation, N.R.P. and L.Q.; supervision, M.A.C.Y.; funding, A.H.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia and Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Acknowledgments
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia, Skim pensyarah lantikan baru Universiti Malaysia Sabah (SLB2242) and Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mbaveng, A.; Kuete, V. Syzygium aromaticum. In Medicinal Spices and Vegetables from Africa; Elsevier: London, UK, 2017; pp. 611–625. [Google Scholar]
- Kumar, K.S.; Yadav, A.; Srivastava, S.; Paswan, S.; sankar Dutta, A. Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 13–22. [Google Scholar]
- Chomchalow, N. Spice production in Asia-An overview. AU J. Technol. 2001, 5. [Google Scholar]
- Arung, E.T.; Matsubara, E.; Kusuma, I.W.; Sukaton, E.; Shimizu, K.; Kondo, R. Inhibitory components from the buds of clove (Syzygium aromaticum) on melanin formation in B16 melanoma cells. Fitoterapia 2011, 82, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Milind, P.; Deepa, K. Clove: A champion spice. Int. J. Res. Ayurveda Pharm. 2011, 2, 47–54. [Google Scholar]
- Mahulette, A.; Yahya, S.; Wachjar, A. Measuring the potential of biomass, carbon storage, and carbon sink of forest cloves. In Proceedings of the IOP Conference Series: Earth and Environmental Science, The 1st International Seminar on Natural Resources and Environmental Management 2019, IPB International Convention Center (IICC), Bogor, Indonesia, 15 August 2019; Volume 399, p. 012063. [Google Scholar]
- Ozdal, T.; Tomas, M.; Toydemir, G.; Kamiloglu, S.; Capanoglu, E. Introduction to nutraceuticals, medicinal foods, and herbs. In Aromatic Herbs in Food; Elsevier: London, UK, 2021; pp. 1–34. [Google Scholar]
- Blakeney, M.; Mengistie, G. Zanzibar: Cloves. In Extending the Protection of Geographical Indications; Routledge: London, UK, 2013; pp. 342–356. [Google Scholar]
- Khanal, A.; Devkota, H.P.; Kaundinnyayana, S.; Gyawali, P.; Ananda, R.; Adhikari, R. Culinary herbs and spices in Nepal: A review of their traditional uses, chemical constituents, and pharmacological activities. Ethnobot. Res. Appl. 2021, 21, 1–18. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Mittal, M.; Gupta, N.; Parashar, P.; Mehra, V.; Khatri, M. Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: A comprehensive review. Int. J. Pharm. Pharm. Sci. 2014, 6, 67–72. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.; Santo-Buelga, C.; Ferreira, I.C.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Jarupan, L.; Nampitch, T.; Chonhenchob, V.; Harnkarnsujarit, N. Renovation of Agro-Waste for Sustainable Food Packaging: A Review. Polymers 2023, 15, 648. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Murugesh Babu, K.; Ravindra, K. Bioactive antimicrobial agents for finishing of textiles for health care products. J. Text. Inst. 2015, 106, 706–717. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Gunawardena, D.; Govindaraghavan, S.; Münch, G. Anti-inflammatory properties of cinnamon polyphenols and their monomeric precursors. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 409–425. [Google Scholar]
- Srinivasan, K. Antioxidant Potential of Spices and Their Active Constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Mastelic, J.; Jerkovic, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivančić-Baće, I.; Smrečki, V.; Žarković, N.; Brčić-Kostic, K.; Vikić-Topić, D. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Hung, S.-L.; Pai, S.-F.; Lee, Y.-H.; Yang, S.-F. Eugenol Suppressed the Expression of Lipopolysaccharide-induced Proinflammatory Mediators in Human Macrophages. J. Endod. 2007, 33, 698–702. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar]
- Bhadoriya, S.S.; Mangal, A.; Madoriya, N.; Dixit, P. Bioavailability and bioactivity enhancement of herbal drugs by “Nanotechnology”: A review. J. Curr. Pharm. Res. 2011, 8, 1–7. [Google Scholar]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Smith, N.B. Applications of ultrasonic skin permeation in transdermal drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1107–1120. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Ali, A. The application of anethole, menthone, and eugenol in transdermal penetration of valsartan: Enhancement and mechanistic investigation. Pharm. Biol. 2015, 54, 1042–1051. [Google Scholar] [CrossRef]
- Abdou, A.; Elmakssoudi, A.; El Amrani, A.; JamalEddine, J.; Dakir, M. Recent advances in chemical reactivity and biological activities of eugenol derivatives. Med. Chem. Res. 2021, 30, 1011–1030. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Santos, M.V.; Nascimento, L.E.; Praxedes, É.A.; Borges, A.A.; Silva, A.R.; Bertini, L.M.; Pereira, A.F. Syzygium aromaticum essential oil supplementation during in vitro bovine oocyte maturation improves parthenogenetic embryonic development. Theriogenology 2019, 128, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Singh, S.K.; Verma, P.; Gahtori, R.; Sibuh, B.Z.; Kesari, K.K.; Jha, N.K.; Dhanasekaran, S.; Thakur, V.K.; Wong, L.S. Polyester nanomedicines targeting inflammatory signaling pathways for cancer therapy. Biomed. Pharmacother. 2022, 154, 113654. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Mukhija, M.; Sundriyal, A.; Loshali, A. Essential Oils and Their Biological Applications: Extraction methods, Types, Biological Activities, Antimicrobial Fumes. In Handbook of Research on Advanced Phytochemicals and Plant-Based Drug Discovery; IGI Global: Beijing, China, 2022; pp. 395–412. [Google Scholar]
- Meeran, M.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB2) receptors in rats. Chem. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Al-Taee, H.; Azimullah, S.; Meeran, M.N.; Almheiri, M.K.A.; Al Jasmi, R.A.; Tariq, S.; Khan, M.A.; Adeghate, E.; Ojha, S. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef]
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Comparative anticancer potential of clove (Syzygium aromaticum)—An Indian spice—Against cancer cell lines of various anatomical origin. Asian Pac. J. Cancer Prev. 2011, 12, 1989–1993. [Google Scholar]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.-A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45–63. [Google Scholar] [CrossRef]
- Bhagat, N.; Chaturvedi, A. Spices as an Alternative Therapy for Cancer Treatment. Syst. Rev. Pharm. 2016, 7, 46–56. [Google Scholar] [CrossRef][Green Version]
- Mesole, S.B.; Alfred, O.O.; Yusuf, U.A.; Lukubi, L.; Ndhlovu, D. Apoptotic Inducement of Neuronal Cells by Aluminium Chloride and the Neuroprotective Effect of Eugenol in Wistar Rats. Oxidative Med. Cell. Longev. 2020, 2020, 8425643. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Naveena, B.; Muthukumar, M.; Sen, A.; Babji, Y.; Murthy, T. Improvement of shelf-life of buffalo meat using lactic acid, clove oil and vitamin C during retail display. Meat Sci. 2006, 74, 409–415. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2018, 276, 180–186. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Gelboin, H.V. Role of Human Cytochromes P450 in the Metabolic Activation of Chemical Carcinogens and Toxins. Drug Metab. Rev. 1994, 26, 165–183. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Attia, M.S. Potential Impacts of Clove Essential Oil Nanoemulsion as Bio Fungicides against Neoscytalidium Blight Disease of Carum carvi L. Agronomy 2023, 13, 1114. [Google Scholar] [CrossRef]
- Rahimi, V.; Askari, V.R. A mechanistic review on immunomodulatory effects of selective type two cannabinoid receptor β-caryophyllene. Biofactors 2022, 48, 857–882. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T.A. Syzygium aromaticum L. (Clove) Extract Regulates Energy Metabolism in Myocytes. J. Med. Food 2014, 17, 1003–1010. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Ramos, G.P.; Apel, M.A.; De Morais, C.B.; Ceolato, P.C.; Schapoval, E.E.; Dall’Agnol, M.; Zuanazzi, J.A. In vivo and in vitro anti-inflammatory activity of red clover Trifolium pratense dry extract. Rev. Bras. Farm. 2012, 22, 176–180. [Google Scholar] [CrossRef]
- Andrade, F.; Mendes, A.N. Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef]
- Joshi, V.; Umashankara, M.; Ramakrishnan, C.; Urs, A.N.N.; Suvilesh, K.N.; Velmurugan, D.; Rangappa, K.S.; Vishwanath, B.S. Dimethyl ester of bilirubin exhibits anti-inflammatory activity through inhibition of secretory phospholipase A2, lipoxygenase and cyclooxygenase. Arch. Biochem. Biophys. 2016, 598, 28–39. [Google Scholar] [CrossRef]
- Banerjee, K.; Madhyastha, H.; Sandur, R.; Manikandanath, N.; Thiagarajan, N.; Thiagarajan, P. Anti-inflammatory and wound healing potential of a clove oil emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef]
- Debnath, T.; Kim, D.H.; Lim, B.O. Natural Products as a Source of Anti-Inflammatory Agents Associated with Inflammatory Bowel Disease. Molecules 2013, 18, 7253–7270. [Google Scholar] [CrossRef]
- Chniguir, A.; Zioud, F.; Marzaioli, V.; El-Benna, J.; Bachoual, R. Syzygium aromaticum aqueous extract inhibits human neutrophils myeloperoxidase and protects mice from LPS-induced lung inflammation. Pharm. Biol. 2019, 57, 55–63. [Google Scholar] [CrossRef]
- Hwang, E.; Lin, P.; Ngo, H.T.; Yi, T.-H. Clove attenuates UVB-induced photodamage and repairs skin barrier function in hairless mice. Food Funct. 2018, 9, 4936–4947. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Oulkheir, S.; Aghrouch, M.; El Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and geranium against a gram negative and gram positive pathogenic bacteria. J. Dis. Med. Plants 2017, 3, 1–5. [Google Scholar]
- Alanazi, A.K.; Alqasmi, M.H.; Alrouji, M.; Kuriri, F.A.; Almuhanna, Y.; Joseph, B.; Asad, M. Antibacterial Activity of Syzygium aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 8551. [Google Scholar] [CrossRef] [PubMed]
- El-Shouny, W.A.; Ali, S.S.; Hegazy, H.M.; Elnabi, M.K.A.; Ali, A.; Sun, J. Syzygium aromaticum L.: Traditional herbal medicine against cagA and vacA toxin genes-producing drug resistant Helicobacter pylori. J. Tradit. Complement. Med. 2019, 10, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Nzeako, B.C.; Al-Kharousi, Z.S.N.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activities of thyme, clove and oregano essential oils. J. Food Saf. 2007, 27, 91–101. [Google Scholar] [CrossRef]
- Schnitzler, P.; Astani, A.; Reichling, J. Antiviral Effects of Plant-Derived Essential Oils and Pure Oil Components. Lipids Essent. Oils Antimicrob. Agents 2011, 239–254. [Google Scholar]
- Unalan, I.; Boccaccini, A.R. Essential oils in biomedical applications: Recent progress and future opportunities. Curr. Opin. Biomed. Eng. 2021, 17, 100261. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Kaur, D.; Chandrul, K.K. Syzygium aromaticum L.(Clove): A vital herbal drug used in periodontal disease. Indian J. Pharm. Biol. Res. 2017, 5, 45–51. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2020, 61, 3740–3755. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crop. Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Alamgir, A.; Alamgir, A. Herbal drugs: Their collection, preservation, and preparation; evaluation, quality control, and standardization of herbal drugs. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacognosy; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 453–495. [Google Scholar]
- Rizkiyah, D.N.; Putra, N.R.; Yunus, M.A.C.; Veza, I.; Irianto, I.; Aziz, A.H.A.; Rahayuningsih, S.; Yuniarti, E.; Ikhwani, I. Insight into Green Extraction for Roselle as a Source of Natural Red Pigments: A Review. Molecules 2023, 28, 1336. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Bruna-Maynou, F.J.; Castro, R.; Rodríguez-Dodero, M.C.; Barroso, C.G.; Guerrero, E.D. Flavored Sherry vinegar with citric notes: Characterization and effect of ultrasound in the maceration of orange peels. Food Res. Int. 2020, 133, 109165. [Google Scholar] [CrossRef]
- Nurhadi, B.; Saputra, R.; Setiawati, T.; Husein, S.; Faressi, F.; Utari, C.; Sukri, N.; Kayaputri, I.; Setiasih, I. Comparison of Curcuma domestica and Curcuma xanthorrhiza oleoresins extracted using maceration, Soxhlet, and ultrasound-assisted extraction (UAE). In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Food and Bio-Industry 2019, Bandung, Indonesia, 29–30 July 2019; Volume 443, p. 012074. [Google Scholar]
- Boyko, N.N.; Zhilyakova, E.T.; Pisarev, D.I.; Novikov, O.O.; Sahaidak-Nikitiuk, R.; Kuznietsova, V.Y.; Shpychak, O.S.; Tkachev, A.; Kovalenko, A.M.; Sushchuk, N.A. A novel method for the extraction of the main compounds from the essential oil of clove buds. FARMACIA 2020, 68, 1. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; D’Alessandro, V.; D’Antuono, L.F. Comparison of two extraction methods (high pressure extraction vs. maceration) for the total and relative amount of hydrophilic and lipophilic organosulfur compounds in garlic cloves and stems. An application to the Italian ecotype “Aglio Rosso di Sulmona” (Sulmona Red Garlic). Food Chem. 2020, 312, 126086. [Google Scholar] [CrossRef] [PubMed]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Yunus, M.A.C.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Tekin, K.; Akalın, M.K.; Şeker, M.G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crop. Prod. 2015, 77, 954–960. [Google Scholar] [CrossRef]
- Alexandru, L.; Cravotto, G.; Giordana, L.; Binello, A.; Chemat, F. Ultrasound-assisted extraction of clove buds using batch- and flow-reactors: A comparative study on a pilot scale. Innov. Food Sci. Emerg. Technol. 2013, 20, 167–172. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experimental and Modelling. J. Food Process. Preserv. 2022, e16670. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. CO2 Util. 2021, 56, 101839. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P. Supercritical carbon dioxide extraction of eugenol from clove buds: Process optimization and packed bed characterization. Food Bioprocess Technol. 2013, 6, 2587–2599. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Petenate, A.J.; Meireles, M.A.A. Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2. J. Supercrit. Fluids 2014, 93, 56–66. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rovio, S.; Hartonen, K.; Holm, Y.; Hiltunen, R.; Riekkola, M.L. Extraction of clove using pressurized hot water. Flavour Fragr. J. 1999, 14, 399–404. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Jaski, J.M.; Abrantes, K.K.B.; Zanqui, A.B.; Stevanato, N.; da Silva, C.; Barão, C.E.; Bonfim-Rocha, L.; Cardozo-Filho, L. Simultaneous extraction of sunflower oil and active compounds from olive leaves using pressurized propane. Curr. Res. Food Sci. 2022, 5, 531–544. [Google Scholar] [CrossRef]
- Idham, Z.; Zaini, A.S.; Putra, N.R.; Rusli, N.M.; Mahat, N.S.; Yian, L.N.; Che Yunus, M.A. Effect of flow rate, particle size and modifier ratio on the supercritical fluid extraction of anthocyanins from Hibiscus sabdariffa (L.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012031. [Google Scholar] [CrossRef]
- Kusuma, H.; Mahfud, M. Comparison of kinetic models of oil extraction from sandalwood by microwave-assisted hydrodistillation. Int. Food Res. J. 2017, 24, 1–6. [Google Scholar]
- Kennouche, A.; Benkaci-Ali, F.; Scholl, G.; Eppe, G. Chemical Composition and Antimicrobial Activity of the Essential Oil of Eugenia caryophyllata Cloves Extracted by Conventional and Microwave Techniques. J. Biol. Act. Prod. Nat. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef]
- Kapadiya, S.M.; Parikh, J.; Desai, M.A. A greener approach towards isolating clove oil from buds of Syzygium aromaticum using microwave radiation. Ind. Crop. Prod. 2018, 112, 626–632. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Zare, M.; Razzaghi, S. Eugenol Enrichment of Clove Bud Essential Oil Using Different Microwave-assisted Distillation Methods. Food Sci. Technol. Res. 2017, 23, 385–394. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the ‘Green’ Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).