Abstract
Removable pressure-sensitive adhesives (PSAs) are used in the production of self-adhesive materials such as protective films, masking tapes or biomedical electrodes. This work presents a new and environmentally friendly method of obtaining this type of adhesive materials, i.e., photochemically induced free radical telomerization. Adhesive binders to removable PSAs, i.e., the photoreactive acrylic telomer syrups (ATS) were prepared from n-butyl acrylate, acrylic acid, and 4-acrylooxybenzophenone. Tetrabromomethane (CBr4) or bromotrichloromethane (CBrCl3) were used as the telogens. ATS was modified with unsaturated polybutadiene resin and a radical photoinitiator. Adhesive compositions were coated onto a carrier and UV cross-linked. The effects of the chemical nature of telomers (i.e., terminal Br or Cl atoms) and their molecular weight (K-value), as well as the cross-linking degree on adhesive properties of PSAs, were studied. It was found that with the increase in telogen content in the system, the dynamic viscosity of ATS and K-value of acrylic telomers decrease, and the conversion of monomers increases. CBr4 turned out to be a more effective chain transfer agent than CBrCl3. Moreover, telomers with terminal Br-atoms (7.5 mmol of CBr4), due to slightly lower molecular weights and viscosity, showed a higher photocrosslinking ability (which was confirmed by high cohesion results at 20 and 70 °C, i.e., >72 h). Generally, higher values of the temperature at which adhesive failure occurred were noted for PSAs based on ATS with lower telogen content (7.5 mmol), both CBr4 and CBrCl3. The excellent result for removable PSA was obtained in the case of telomer syrup Br-7.5 crosslinked with a 5 J/cm2 dose of UV-radiation (adhesion ca.1.3 N/25 mm, and cohesion > 72 h).
1. Introduction
Pressure-sensitive adhesives (PSAs) are viscoelastic materials that remain permanently adhesive and can adhere even under light pressure [1]. Of the many base polymer materials, polyacrylates have enjoyed the fastest growth in commercial applications [2]. This is due to high resistance to oxidation and water, and lack of yellowing and transparency, which allows the use of acrylic PSAs in many industries, i.e., packaging tape, medical pads, protective films, optically clear adhesives, masking tapes, and hydrogels [3]. The most important properties of PSAs include tack (the adhesive’s ability to adhere quickly), peel adhesion (a force required to remove a coated flexible sheet material from a test panel at a specified angle and rate of removal), and cohesion (resistance to static shear load). These values result from the nature of adhesives (chemical composition and state of the adhesives) molecular weight of the base polymer, crosslinking yields, coating weight, temperature, time, as well as a test method and conditions and face stock materials [4]. Pressure-sensitive adhesives are usually classified as permanent or removable (e.g., masking tapes where a low release force is required so as not to damage the surface) [5]. Permanent PSAs should be characterized by high peel adhesion, high tack, and high cohesion. While a limited peel value and a high shear strength are required for removable adhesives. According to peel adhesion values, PSAs can be divided into excellent permanent (>14 N/25 mm), permanent (from 10 to 14 N/25 mm), semi-removable (from 6 up to 8 N/25 mm), removable (from 2 to 4 N/25 mm) and excellent removable (<1 N/25 mm) [6]. While I. Benedek points out that removable PSAs should exhibit a peel adhesion value of 1.5 N/25 mm and cohesion values of at least 100 min at room temperature [7].
To obtain removable PSAs with such properties, the following methods are used: physical modification of the polymer matrix using inorganic additives (e.g., glass spheres or calcium carbonate) [8], use of mixtures of sticky and non-sticky ingredients (resins) [9] and polymer mixtures, in which one has a glass transition temperature below room temperature and the other slightly above room temperature [10]. Other methods consist of obtaining PSAs with an appropriate cross-linking degree (high cohesion and low tack) [11,12], using a mixture of “hard” and “soft” monomers [13] or segments, mainly based on mixtures of polyurethanes and polyethylene glycols [14,15,16]. The method of the greatest industrial importance in the production of the adhesive binder for removable PSA is the emulsion polymerization of acrylic monomers. An interesting way is also the chemical modification of the polyacrylate emulsion with isobornyl methacrylate, thanks to which it is possible to obtain PSA with adhesion below 0.3 N/25 mm and cohesion above 100 h [17]. In contrast, the use of the UV technique in the removable PSAs preparation mainly concerns the step of cross-linking the polymer matrix (photoreactive or not) modified with unsaturated cross-linking monomers (multifunctional), i.e., ethylene glycol dimethylacrylate, bisphenol-A ethoxylate diacrylate, or trimethylolpropane triacrylate. Achieving dense polymer networks results in obtaining PSA with outstanding properties (peel adhesion <1 N/25 mm) [18]. Removable PSAs from UV technology is used as attachments of ultra-thin electrodes (1 to 2 μm) to human skin (peel adhesion below 0.01 N/25 mm and ultra-high optical transparency) [19]. This article describes, for the first time, a new method of preparing the polymer matrix to removable PSAs, i.e., photochemically induced free radical telomerization.
Telomerization is a method of obtaining macromolecules/oligomers characterized by low polydispersity. In telomerization, taxogen (also called monomer) reacts with telogen (chain transfer agent) according to the radical mechanism, but also ionic (anionic or cationic) [20,21]. The telomerization process can be initiated by thermal initiators (such as organic peroxides, hydroperoxides, azo compounds, etc.), UV radiation, γ radiation accompanying beta decay of 60Co to nonradioactive 60Ni, and redox processes involving metal ions with variable valence [22]. Particularly noteworthy is the process of photoinduced telomerization, in which it is possible to obtain permanent PSAs without the need to use organic solvents that have particular chemical structures, for example, terminal Si atoms [23,24].
The work aimed to present a new method of preparation of removable PSAs from acrylic telomer syrups (as a product of photoinduced telomerization) and polybutadiene resin. In addition, the influence of telogen content (CBr4 or CBrCl3) as well as the K-value of acrylic telomers on the thermal and mechanical properties of PSAs were determined. Potentially, the presented method could be used in the production of masking tapes or protective films because it is ecologically safe (processes without the use of organic solvents), fast (up to several dozen minutes), and enables obtaining adhesive binders as solutions of oligomers with a low content of volatile organic compounds.
2. Materials and Methods
2.1. Materials
The acrylic telomer syrups (ATS) were prepared using the following components:
- -
- monomers: n-butyl acrylate (BA), acrylic acid (AA BASF; Ludwigshafen, Germany), 4-acryloylooxybenzophenone (ABP, Chemitec, Scandicci, Italy)
- -
- telogens: tetrabromomethane (CBr4), bromotrichlomethane (CBrCl3), Merck, Warsaw, Poland)
- -
- radical photoinitiator: ethyl (2,4,6-trimethyl benzoyl)-phenyl-phosphinate (Omnirad TPOL, IGM Resins, Waalwijk, The Netherlands), i.e., acylphosphine-type (APO).
The adhesive compositions were prepared using the acrylic telomers syrups and:
- -
- hydroxyl-terminated polybutadiene resin Hypro 1200X90 HTB (HTB, CVC Thermoset Specialties, Emerald Kalama Chemical, Kalama, WA, USA)
- -
- radical photoinitiator: ethyl (2,4,6-trimethyl benzoyl)-phenyl-phosphinate (Omnirad TPOL, IGM Resins, Waalwijk, The Netherlands).
The components were used without additional purification. The chemical structures of the compounds are shown in Figure 1.
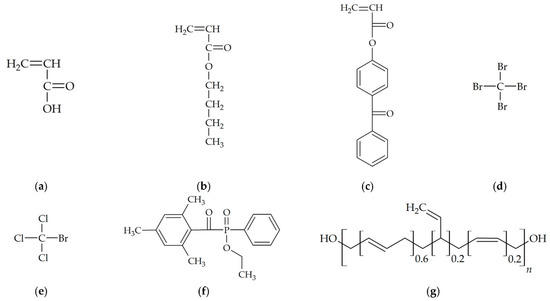
Figure 1.
Chemical structures of the compounds: (a) acrylic acid, (b) n-butyl acrylate, (c) 4-acrylooxybenzophenone, (d) tetrabromomethane, (e) bromotrichloromethane, (f) TPOL radical photoinitiator (APO-type), (g) polybutadiene resin HTB.
2.2. Synthesis and Characterization of Acrylic Telomer Syrups
Acrylic telomer syrups (ATSs) were obtained by photo-induced telomerization of BA, AA, and ABP initiated by a radical photoinitiator TPOL and CBr4 or CBrCl3 as a telogen (7.5 or 15 mmol/100 wt. parts of monomers mixture). The compositions of the reaction mixtures are shown in Table 1.

Table 1.
Compositions of monomers, photoinitiator, and telogen for acrylic telomer syrups.
The telomerization process was carried out in a glass reactor equipped with a mechanical stirrer (mixing speed 300 rpm) and a thermocouple under argon as inert gas at a temperature of 20 °C for 15 min. A mixture of monomers (50 g) was introduced into the reactor and purged with argon. The high-intensity UV lamp emitting UV-A radiation (UVAHAND 250, Dr. Hönle AG UV Technology, Gräfelting, Germany) as a UV light source was used. The UV irradiation inside the reactor (15 mW/cm2) was controlled with UV-radiometer SL2W (UV-Design, Brachttal, Germany). A schematic diagram of the photochemically induced telomerization process is shown in Figure 2.
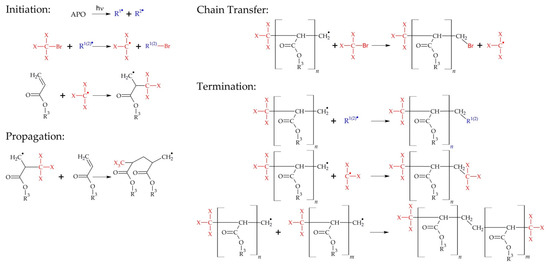
Figure 2.
Scheme of the photochemically induced telomerization process where: R1(2) is a radical formed from the decomposition of APO photoinitiator, X is a Br or Cl atom, R3 is H, C3H9 or C6H6COC6H6.
The solid content (SC) in ATS was determined using a thermobalance (MA 50/1.X2.IC.A; Radwag, Radom, Poland). In an aluminum pan, syrup samples (ca. 2 g) were heated at 105 °C for 4 h. Equation (1) was used to calculate the SC value:
where: m1 is the initial weight of a sample and m2 is the residual weight after an evaporation process.
K-values were determined for the dry acrylic telomers based on the EN ISO 1628-1:1998 standard and the Fikentscher Equation (2).
where: ηr = η/η0; η is the viscosity of a telomer/copolymer solution; η0 is the viscosity of a pure auxiliary diluent (i.e., tetrahydrofuran); c is the telomer concentration (g/cm3).
2.3. Preparation and Characterization of Pressure-Sensitive Adhesives
Pressure-sensitive adhesives were obtained from acrylic telomer syrups (100 wt. parts), polybutadiene resin HTB (7.5 wt. parts/100 wt. parts of ATS), and APO photoinitiator (2.5 wt. parts/100 wt. parts of ATS). Adhesive compositions were homogenized with a high-speed mechanical mixer (T10 Basic Ultra-Turrax, IKA, Königswinter, Germany), applied onto polyester foils, and UV-irradiated (UV-doses were 2, 3, 4, 5, or 6 J/cm2; UV-irradiation time was 32 s, 48 s, 64 s, and 96 s, respectively) using the medium-pressure mercury lamp (UV-ABC; Hönle UV-Technology, Gräfelfing, Germany). The base weight of the PSA layers was 15 g/m2. The UV exposure was controlled with the radiometer (Dynachem 500; Dynachem Corp., Westville, IL, USA). During UV irradiation of adhesive films, two independent processes take place. The first is the formation of a polymer network from telomeric chains with hanging benzophenone moieties (from the APB structure) that are capable of abstracting hydrogen (for example from the hanging acrylate chains of other telomeric chains) (Figure 3a). The second process consists of photocrosslinking with the participation of the radical photoinitiator APO, unsaturated HTB resin and unreacted monomers from ATS (Figure 3b).
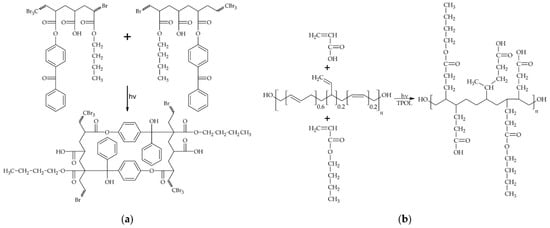
Figure 3.
Photocrosslinking of pressure-sensitive adhesives: (a) via copolymerizable photoinitiator ABP (hydrogen abstractor) and (b) via HTB and unreacted monomers [23].
Self-adhesive tests (i.e., adhesion to steel at 180°, tack, cohesion at 20 °C and 70 °C, and shear adhesion failure test) of UV-crosslinked PSAs were performed. Adhesion to steel at a 180° (also called peel adhesion) was determined at room temperature using the Zwick/Roell Z010 testing machine (Zwick/Roell, Ulm, Germany) according to the AFERA 5001 (standard developed by the European Association des Fabricants Europeens de Rubans Auto-Adhesifs—AFERA). The degreased steel plate was applied with a one-sided PSA film measuring 175 × 25 mm and pressed with a 2 kg rubber roller. The test was performed 20 min after the application of the film to the plate with a peeling speed of 300 mm/min. Tack values were determined using the Zwick/Roell Z010 testing machine (Zwick/Roell, Ulm, Germany) according to AFERA 5015 standard. PSA film with dimensions of 175 × 25 mm was mounted in the upper jaws to obtain loops with the adhesive layer on the outside. In the lower jaws, a degreased steel plate was placed perpendicularly to the sample, which was lowered perpendicularly at a speed of 100 mm/min. The contact area was about 6.25 cm2. The machine recorded the force needed to detach the adhesive film after a short contact with the steel surface, without external forces. The cohesion of PSAs (shear resistance) was measured according to the AFERA 5012 standard, with a device developed by the West Pomeranian University of Technology’s International Laboratory of Adhesives and Self-Adhesive Materials in Szczecin that measures the time when joint cracks occur automatically. A one-sided adhesive film was applied to the degreased steel plate to form a 25 × 25 mm (6.25 cm2) joint and pressed with a 2 kg rubber roller to improve wettability. A 1 kg weight was attached to the free end of the film. The setup was then placed in a tripod so that the force of gravity was exerted on the weld at an angle of 180°. The cohesion value was defined as the time needed for the weld to crack. The test was carried out at a temperature of 20 °C and 70 °C. The shear adhesion failure test (SAFT) was carried out to determine the resistance to increased temperature. For this purpose, the PSA samples were prepared analogously to the cohesion test, however, they were heated in the range of 20 ÷ 250 °C with a temperature increase of 0.5 °C/min. Three samples of each adhesive film were evaluated for each test. Material damage may occur in any of the previously mentioned tests, i.e., adhesive failure (af), when the adhesive layer remains on the carrier (cohesion forces > adhesion forces); cohesive failure (cf) when the adhesive remains on both the carrier and substrate (cohesion forces < adhesion forces) and mixed failure (mf) when both adherent and cohesive failures occur at the same time.
To determine the glass transition temperatures (Tg) of UV-crosslinked PSAs the differential scanning calorimeter method was used (DSC250, TA Instruments, New Castle, DE, USA). Samples (ca. 10 mg) were analyzed using hermetic aluminum pans at temperatures from −80 to 200 °C (heating rate of 10 °C/min).
3. Results and Discussion
3.1. The Physicochemical Properties of Acrylic Telomer Syrups and Dry Telomers
The physicochemical properties of acrylic telomer syrups (dynamic viscosity and solid content), as well as the K-value of dry acrylic telomers, are presented in Table 2.

Table 2.
The physicochemical properties of obtained acrylic telomer syrups and dry telomers.
In the beginning, it should be noted that carrying out the mass photopolymerization of BA, AA, and APB monomers (reference sample, Table 1) under the same reaction conditions as in phototelomerization, resulted in the gel formation in the entire volume of the reaction mixture. The use of chain transfer agents (i.e., CBr4 and CBrCl3) in the mass photopolymerization process allows for obtaining liquid reaction products (acrylic telomer syrups) in a relatively short time. As can be seen in Table 2, ATS with CBr4 is characterized by higher solid content (higher monomers conversion) than ATS with CBrCl3 telogen (2–4%). An increase in the telogen content in the reactive system caused a decrease in the solid content and the dynamic viscosity values of the ATS, as expected. A higher concentration of chain transfer agents (CBr4 or CBrCl3) causes the formation of more radicals, initiating the propagation step, as well as more frequent chain transfer and termination. This was similar to previously published results [25,26]. It is worth noting that telomer syrups with CBr4 were characterized by a much lower dynamic viscosity (<14 Pa·s) and are perfect for coating the carrier. In the case of ATS with CBrCl3, dynamic viscosity values were almost three times higher than their CBr4 counterparts. This is related to the molecular weights of the resulting telomeres. Based on the K-value results, which express the molecular weight of polymeric materials, it was proved that Br-telomeres (based on CBr4) was characterized by almost two times smaller molecular weights than Cl-telomeres (based on CBrCl3). The above results are due to the greater reactivity of CBr4 as a chain transfer agent. It is known that CBr4 has a high kinetic chain transfer constant [27].
3.2. Properties of UV-Crosslinked PSA
Adhesion to steal at 180° and tack of prepared PSAs based on Br- or Cl-telomer syrups and polybutadiene resin HTB, depending on the UV dose used in the UV-crosslinking step (in fact on the cross-linking degree) are shown in Figure 4.
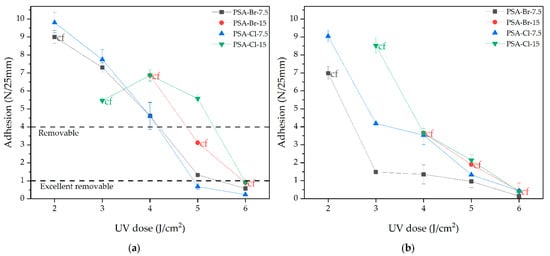
Figure 4.
Adhesion to steal at 180° (a) and tack (b) tack of PSAs based on acrylic telomer syrups.
As mentioned earlier, the 3D polymer network is formed in presented PSAs because of the formation of cross-links between the telomers’/oligomers’ chains and the polymer network made of polybutadiene resin and unreacted acrylate monomers (20–25 wt.% of unreacted BA or AA). The tests results showed that adhesion to steel and tack significantly depended on the UV dose (i.e., from the density of the polymer network), as well as the type and number of terminal groups in the polyacrylate chains, which interacted with the polar surface of the stainless steel. The adhesion values significantly decreased as the UV dose increased (from about 10 N/25 mm to even 0.2 N/25 mm for PSA-Cl-7.5). A similar dependence was shown for tack values (from 9.5 N to 0.1 N). Generally, cross-linking degree reduces chain mobility, thus decreasing adhesion and tack. Another factor affecting the adhesion (and tack) values is the chemical nature of the polymer forming the PSAs. This article presents new adhesive binders, i.e., acrylic telomers with terminal groups, namely: Br- (PSAs based on CBr4 telomers) or Br- and -Cl3 (samples from CBrCl3). In addition, the weight fraction of telomeres in the adhesive binder is significant. Based on SC measurements it was 75 to 81.5 wt.% (Table 2). The other additives are polybutadiene resin (but only in the amount of 7.5 wt. parts/100 wt. parts of telomer syrup). It can therefore be concluded that the PSAs were mainly based on telomers. Thus, their chemical nature/physicochemical properties (i.e., molecular weight characterize as K-value and special built-in terminal groups) determine the adhesive properties of PSAs. The molar content of telogens in the system was the same (7.5 or 15 mmol/100 g of monomers mixture). Therefore, the properties of PSAs are influenced by the chemical nature of telogens. The adhesion values can be partly explained by the electrostatic theory of adhesion. According to this theory, the presence of more electronegative groups in the PSAs (electronegativity of bromine is 2.8 and chlorine is 3.0 on the Pauling scale) [28] should cause higher adhesion and tack [29]. This theory explains the positive influence of halogen concentration on adhesion and tack (PSAs based on Br-15 and Cl-15 telomer syrups exhibited higher adhesion and tack, than those with lower content of telogens). It is worth noting that only at low doses of UV radiation (2 or 3 J/cm2) the adhesion values for PSA-Cl-7.5 (Cl atoms) were slightly higher than for PSA-Br-7.5 (only Br atoms). In the case of 4 J/cm2 of UV dose, the adhesion values for the discussed PSAs samples were equal (4.8 N/25mm). On the other hand, a further increase in the UV dose (increase in cross-linking density) caused the PSA-Br-7.5 films to be characterized by higher adhesion (1.5 and 0.8 N/25 mm) than the PSA-Cl-7.5 (<1 N/25 mm). This was related to the K-values of the telomeres (i.e., their molecular weights). Br-telomeres exhibited a lower K-value (26.3 a.u.) than Cl-telomeres (42.2 a.u.). Therefore, Br-telomers exhibited higher chain mobility and thus higher adhesion values. The same phenomenon explains the dependence of the tack values of the chemical nature of telomers. It is worth noting that only in the case of the PSA-Cl-15 films, no material damage was noted during the adhesion and tack tests. This was because the Cl-telomere had an appropriate K-value (ca. 42 a.u.). In the case of Cl-telomers, a higher dose of UV radiation was required to obtain properly cross-linked adhesive films (without cf) (>3 J/cm2). Based on ATS with a higher content of telogens (15 mmol), both CBr4 and CBrCl3, it was impossible to obtain PSAs at a low UV dose (2 or 3 J/cm2) (no results). In turn, all samples with Br-15 (the lowest K-value, 18 a.u.), regardless of the UV dose used, were insufficiently cross-linked (telomeric chains were too short). According to the previously indicated criteria and based on the tests carried out, it can be concluded that the PSA-Br-7.5 film, cross-linked by the UV dose of 5 J/cm2, showed the adhesion and tack appropriate for removable PSA, i.e., 1.3 N/25 mm and 1 N, respectively. In turn, cohesion tests at 20 and 70 °C (Figure 5) revealed that this sample also exhibited excellent shear resistance (>72 h). Cohesion tests confirmed that the values of this parameter increased with cross-linking density (with UV dose) for all tested PSAs. The weakest results were confirmed for the PSA-Br-15 (the lowest K-value for telomer), i.e., <5 h.
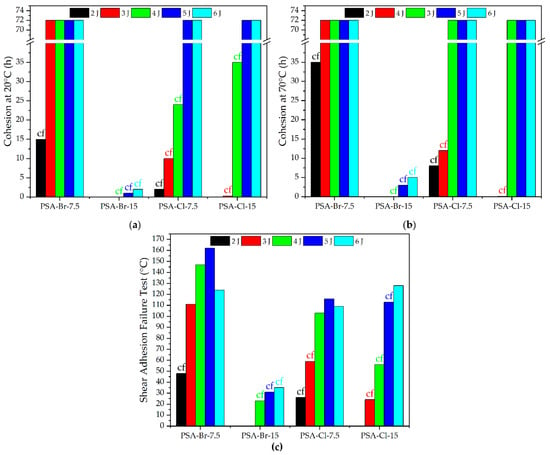
Figure 5.
Cohesion at 20 °C (a) and 70 °C (b) and the SAFT (c) of PSAs based on acrylic telomer syrup.
Interestingly, higher values of cohesion at 70 °C were noticed. This may be due to the better wettability of the steel substrate by PSAs. In particular, it concerned PSA-Cl samples and cross-linked with a UV dose of 4 J/cm2 (increase in cohesion at 70 °C to 72 h). It can also be stated that the best cohesion results (both at 20 and 70 °C) were recorded for the adhesive film based on Br-7.5 telomer syrup, as already at the UV dose of 3 J/cm2 the cohesion values were 72 h. However, the adhesion was too high for removable PSA products (7.3 N/25 mm; Figure 4a). This may be due to the lower viscosity of this telomer (13.8 Pa·s) and relatively high K-value (26 a.u.), thus facilitating the migration of radicals and formation of cross-linked structure. Considering the shear adhesion failure test (SAFT) results (Figure 3c) it can be concluded that the high thermal resistance was demonstrated for the PSA-Br-7.5 sample cross-linked by 5 J/cm2 (162 °C). Generally, higher values of the temperature at which adhesive failure occurred were noted for PSAs based on ATS with lower telogen content (7.5 mmol of CBr4 or CBrCl3). However, PSA-Br adhesive films (based on CBr4) turned out to be slightly better, even though Br-telomeres were characterized by slightly lower molecular weights than Cl-telomers (lower K-value, 26 a.u, and 42 a.u., respectively). It’s known that the shear measured as SAFT is directly dependent on the softening point (i.e., the molecular weight of the resin/polymer). In the case of PSAs based on ATS, it turns out that their photocrosslinking ability is also important. Systems with CBr4, due to slightly lower molecular weights and viscosity, show a higher photocrosslinking ability (already at 3 J/cm2, which was confirmed by cohesion tests at 20 and 70 °C).
The self-adhesive properties of PSAs are also affected by the glass transition temperature of the cross-linked system (resin/polymer). Figure 6 shows the DSC thermograms of the PSAs cross-linked using a UV dose of 6 J/cm2.
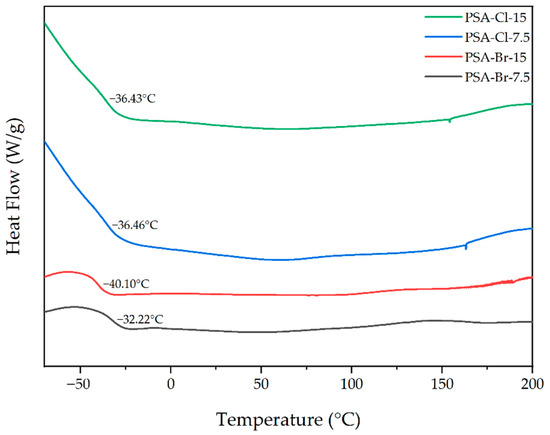
Figure 6.
DSC thermograms of the cross-linked PSAs.
The Tg values for all acrylic PSAs were below −20 °C. Additionally, the Tg values for the PSA-Br-7.5 and PSA-Cl-7.5 and PSA-Cl-15 samples were very similar (−32 °C and −36 °C, respectively). Only PSA-Br-15 exhibited a lower Tg (−40 °C). These results correspond very well with the results of cohesion at 20 and 70 °C for these adhesive films (cross-linked with a UV dose of 6 J/cm2). Namely, samples with higher Tg (of the order of −30 °C) were characterized by high cohesion, resulting from a high cross-linking degree with the UV dose, hence lower mobility of polymer chains and higher Tg values. The opposite is for the PSA-Br-15 sample with the lowest cross-linking degree and short, more mobile telomeric chains.
4. Conclusions
Acrylic telomer syrups (ATS) with terminal Br or Cl atoms (Br-telomers/Cl-telomers) were prepared via a UV-phototelomerization process using n-butyl acrylate, acrylic acid, 4-acrylooxybenzophenone. Two kinds of telogens, i.e., tetrabromomethane (CBr4) and bromotrichloromethane (CBrCl3) and radical photoinitiator (acylphosphine-type, APO) were tested as photoinitiating systems. Adhesive compositions (ATS compounded with the polybutadiene resin and APO photoinitiator) were used for the creation of removable pressure-sensitive adhesives (PSA) by UV cross-linking. The main conclusions are as follows:
- -
- CBr4 telogen allowed preparing of acrylic telomer syrup with significantly lower dynamic viscosity and slightly higher solids content than CBrCl3;
- -
- tBr-telomers are characterized by lower K-value compared to Cl-telomers.
- -
- A greeter concentration of telogens (both CBr4 and CBrCl3) results in the formation of much shorter telomer chains, which determines the use of a higher dose of UV radiation at the stage of cross-linking the adhesive films, but despite this, cohesive failure still occurs, and the cohesion values of such systems are low, especially in case of PSA based on Br-telomers.
- -
- The influence of terminal Br and Cl atoms and their amounts are consistent with the electrochemical theory of adhesion, but only in the range of low cross-linking densities of systems (small UV doses).
- -
- The excellent removable PSA was obtained using 7.5 mmol of CBr4/100 g of monomer mixtures, and after cross-linking of the adhesive film by 5 J/cm2 of UV dose, i.e., adhesion to steal was 1.3 N/25 mm, tack 1 N, cohesion at 20 and 70 °C >72 h and shear adhesion failure test ca. 160 °C.
Author Contributions
Conceptualization, M.W. and A.K.; methodology, validation, M.W. and A.K.; formal analysis, A.K.; investigation, M.W.; writing—original draft preparation, A.K. and M.W.; writing—review and editing, A.K.; visualization, M.W.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benedek, I.; Feldstein, M.M. Fundamentals of Pressure Sensitivity, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420059380. [Google Scholar]
- Taghizadeh, S.M.; Ghasemi, D. Rheological and Adhesion Properties of Acrylic Pressure-Sensitive Adhesives. J. Appl. Polym. Sci. 2011, 120, 411–418. [Google Scholar] [CrossRef]
- Park, H.W.; Seo, H.S.; Lee, J.H.; Shin, S. Adhesion Improvement of the Acrylic Pressure-Sensitive Adhesive to Low-Surface-Energy Substrates Using Silicone Urethane Dimethacrylates. Eur. Polym. J. 2020, 137, 109949. [Google Scholar] [CrossRef]
- Benedek, I.; Feldstein, M.M. Technology Pressure-Sensitive Adhesives and Products; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781420059397. [Google Scholar]
- Ortega-Iguña, M.; Chludzinski, M.; Sánchez-Amaya, J.M. Comparative Mechanical Study of Pressure Sensitive Adhesives over Aluminium Substrates for Industrial Applications. Polymers 2022, 14, 4783. [Google Scholar] [CrossRef] [PubMed]
- Czech, Z. Solvent-Based Pressure-Sensitive Adhesives for Removable Products. Int. J. Adhes. Adhes. 2006, 26, 414–418. [Google Scholar] [CrossRef]
- Benedek, I. Pressure-Sensitive Adhesives and Applications, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; ISBN 0-8247-5059-4. [Google Scholar]
- Graham, P.D.; Lu, Y.-Y.; Romsos, J.D. Adhesive Composition Having Non-Tacky Microspheres and Sheets Made Therefrom. US Patent 2009/024647, 1 October 2009. [Google Scholar]
- Müller-Buschbaum, P.; Bauer, E.; Wunnicke, O.; Stamm, M. The Control of Thin Film Morphology by the Interplay of Dewetting, Phase Separation and microphase Separation. J. Phys. Condens. Matter 2005, 17, S363. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P.; Ittner, T.; Maurer, E.; Körstgens, V.; Petry, W. Pressure-Sensitive Adhesive Blend Films for Low-Tack Applications. Macromol. Mater. Eng. 2007, 292, 825–834. [Google Scholar] [CrossRef]
- Chivers, R.A. Easy Removal of Pressure Sensitive Adhesives for Skin Applications. Int. J. Adhes. Adhes. 2001, 21, 381–388. [Google Scholar] [CrossRef]
- Czech, Z. Crosslinking of Pressure Sensitive Adhesive Based on Water-Borne Acrylate. Polym. Int. 2003, 52, 347–357. [Google Scholar] [CrossRef]
- Xue, J.; Wang, J.; Huang, H.; Wang, M.; Zhang, Y.; Zhang, L. Feasibility of Processing Hot-Melt Pressure-Sensitive Adhesive (HMPSA) with Solvent in the Lab. Processes 2021, 9, 1608. [Google Scholar] [CrossRef]
- Bae, J.H.; Won, J.C.; Lim, W.B.; Kim, B.J.; Lee, J.H.; Min, J.G.; Seo, M.J.; Mo, Y.H.; Huh, P. Tacky-Free Polyurethanes Pressure-Sensitive Adhesives by Molecular-Weight and HDI Trimer Design. Materials 2021, 14, 2164. [Google Scholar] [CrossRef]
- Lobo, S.; Sachdeva, S.; Goswami, T. Role of Pressure-Sensitive Adhesives in Transdermal Drug Delivery Systems. Ther. Deliv. 2015, 7, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, W.; Zhao, Y.; Jiang, L.; Xu, H.; Yang, X. Preparation and Characterization of PEG-Modified Polyurethane Pressure-Sensitive Adhesives for Transdermal Drug Delivery. Drug Dev. Ind. Pharm. 2009, 35, 704–711. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Wang, L.; Shao, L.; Bai, Y. Polyacrylate Emulsion Containing IBOMA for Removable Pressure Sensitive Adhesives. J. Appl. Polym. Sci. 2016, 133, 42886. [Google Scholar] [CrossRef]
- Chu, H.H.; Wang, C.K.; Sein, C.K.; Chang, C.Y. Removable Acrylic Pressure-Sensitive Adhesives Activated by UV-Radiation. J. Polym. Res. 2014, 21, 472. [Google Scholar] [CrossRef]
- Kim, S.W.; Ju, Y.H.; Han, S.; Kim, J.S.; Lee, H.J.; Han, C.J.; Lee, C.R.; Jung, S.B.; Kim, Y.; Kim, J.W. A UV-Responsive Pressure Sensitive Adhesive for Damage-Free Fabrication of an Ultrathin Imperceptible Mechanical Sensor with Ultrahigh Optical Transparency. J. Mater. Chem. A Mater. 2019, 7, 22588–22595. [Google Scholar] [CrossRef]
- Chen, J.; Chalamet, Y.; Taha, M. Telomerization of Butyl Methacrylate and 1-Octadecanethiol by Reactive Extrusion. Macromol. Mater. Eng. 2003, 288, 357–364. [Google Scholar] [CrossRef]
- Bolshakov, A.I.; Kuzina, S.I.; Kiryukhin, D.P. Tetrafluoroethylene Telomerization Initiated by Benzoyl Peroxide. Russ. J. Phys. Chem. A 2017, 91, 482–489. [Google Scholar] [CrossRef]
- Boutevin, B. From Telomerization to Living Radical Polymerization. J. Polym. Sci. A Polym. Chem. 2000, 38, 3235–3243. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef]
- Weisbrodt, M.; Kowalczyk, A. Self-Crosslinkable Pressure-Sensitive Adhesives from Silicone-(Meth)Acrylate Telomer Syrups. Materials 2022, 15, 8924. [Google Scholar] [CrossRef]
- Weisbrodt, M.; Kowalczyk, A.; Kowalczyk, K. Structural Adhesives Tapes Based on a Solid Epoxy Resin and Multifunctional Acrylic Telomers. Polymers 2021, 13, 3561. [Google Scholar] [CrossRef] [PubMed]
- Kraśkiewicz, A.; Kowalczyk, A.; Kowalczyk, K.; Schmidt, B. Novel Solvent-Free UV-Photocurable Varnish Coatings Based on Acrylic Telomers—Synthesis and Properties. Prog. Org. Coat. 2023, 175, 107365. [Google Scholar] [CrossRef]
- Starks, C.M. Free Radical Telomerization; Academic Press: Cambridge, MA, USA, 1974; ISBN 9780126636505. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. 1-Structure of Organic Compounds. In Principles in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–32. [Google Scholar]
- Chen, R.; Huang, Y.; Tang, Q.; Bai, L. Modelling and Analysis of the Electrostatic Adhesion Performance Considering a Rotary Disturbance between the Electrode Panel and the Attachment Substrate. J. Adhes. Sci. Technol. 2016, 30, 2301–2315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).