Abstract
Mycotoxins are toxic compounds produced as secondary metabolites by certain types of filamentous fungi under specific conditions. The contamination of nuts and nut-related products with mycotoxins is a significant global concern due to their severe consequences on human health, including carcinogenicity and immunosuppression. Aflatoxins, with a particular emphasis on aflatoxin B1, are the most common and toxic mycotoxins found in human food. Aflatoxin B1 (AFB1) is known to be highly toxic and carcinogenic. Consequently, global food regulatory organizations have established permissible levels for mycotoxins in nuts. Numerous methodologies have been developed for the detection of mycotoxins in nuts. However, high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC-QqQ-MS/MS) have shown clear benefits in terms of effectiveness and sensitivity. This review aims to provide a comprehensive overview of the major mycotoxins found in nuts, their physiological effects, and their worldwide prevalence. Additionally, the review will focus on nut sample pretreatment methods, analytical techniques employed for mycotoxin detection in nuts, and recent advancements in materials and solvents used for this purpose. Significant gaps exist in mycotoxin detection in nuts, including methodological variability and insufficient data from certain nut-producing countries that need further exploration in the future.
1. Introduction
Mycotoxins, a group of secondary metabolites produced by filamentous fungi, have attracted significant attention due to their various effects on human and animal health [1,2]. The term “mycotoxin” was used for the first time in the 1960s in the UK to characterize a toxin found in peanuts used for animal feed. It was linked to the sudden death of turkeys after consuming contaminated peanuts (Turkey-X disease) [1,3]. Mycotoxins are small molecules (molecular weight < 700) mainly produced by fungal genera such as Aspergillus, Fusarium, and Penicillium [2,4].
Over 400 mycotoxins have been identified, but only a few species pose food safety concerns as they have been reported to contaminate food, including nuts [2,5]. These include aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), aflatoxin M1 (AFM1), aflatoxin M2 (AFM2)), ochratoxin A (OTA), patulin, zearalenone (ZEA), fumonisins (including fumonisin 1 (FB1) and fumonisin B2 (FB2)), citrinin (CIT), fusarenon-X (FUS-X), diacetoxyscirpenol (DAS), trichothecenes (including T-2 toxin (T-2), HT-2 toxin (HT-2), nivalenol (NIV), and deoxynivalenol (DON) [2,5]. They can contaminate a variety of nuts [1,5]. Contamination can occur pre- or post-harvest, and many conditions enhance the production of mycotoxins by fungi, such as water activity, moisture, temperature, and storage conditions. Mycotoxins are usually thermostable, even at high temperatures (80–121 °C) during nut processing (roasting, drying) [6]. Approximately 25% of the world’s food crops, including nuts, are contaminated annually, resulting in significant agricultural and industrial losses [7,8].
Different species of fungi can coexist in the same nut product and produce various mycotoxins. These mycotoxins can act synergistically or have cumulative effects, making the overall toxicity more complex and posing additional risks to human and animal health. Additionally, nut products may contain masked mycotoxins. These modified derivatives are produced during the metabolism of mycotoxins by plants and animals through enzymatic processes and chemical reactions. They are referred to as “masked” because they cannot be detected by the analytical methods typically used for mycotoxin detection. However, they can be released into toxic-free forms during digestion and nut processing methods, which increases the risk to human and animal health [1,9].
Nuts are frequently consumed in the Mediterranean region due to their numerous benefits. They are rich in healthy fats (monounsaturated and polyunsaturated fats), proteins, fibers, vitamins (such as vitamin E and vitamin K), and minerals (like magnesium and potassium) [10]. The term “nut” is used to describe all types of nuts consumed by humans. Nuts are categorized into two main classes: tree nuts, which are one-seeded fruits growing on trees (including pistachios, hazelnuts, almonds, and cashew nuts), and groundnuts, which grow underground and belong to the Leguminosae family, such as peanuts [11,12].
The widespread presence of mycotoxins in nuts and their high toxicity has become a worldwide concern. Several national and international organizations, such as the US Food and Drug Administration (FDA), the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the European Food Safety Authority (EFSA), have addressed the problem of mycotoxins through various regulations, recommendations, and regulatory guidelines for major mycotoxins found in food and feedstuffs [1,2]. For instance, the EU limits the sum of aflatoxins (AFB1, AFB2, AFG1, AFG2) in pistachios, and almonds are set at 15 μg/kg, while the FDA sets the limits for total aflatoxins at 20 μg/kg [7]. The EU sets the maximum limits for OTA (Ochratoxin A) in nuts at 10 g/kg for dried nuts, and the maximum levels for fumonisins are 200–500 g/kg in foods, including nuts [7,13].
Although numerous studies have been published on the presence of mycotoxins in food and feed, however, to the best of our knowledge, a detailed overview of the occurrence of mycotoxins in nuts and the main developments in mycotoxin investigation in nuts has not fully been explored. The first section of the paper explores the presence of mycotoxins in nuts and their toxic effects, as well as the global prevalence of mycotoxins. It highlights the key mycotoxins commonly found, such as AFs, FBs, and OTA. The subsequent section examines common pretreatment methods, including solid-phase extraction (SPE)-based approaches, dispersive liquid–liquid microextraction (DLLME), and QuEChERS (Quick, easy, cheap, effective, rugged, and safe). It discusses their advantages and limitations, providing an update on pretreatment methods. Finally, a summary of the conventional techniques used for mycotoxin detection in nuts is presented. The detection techniques are divided into indirect techniques, such as high-performance liquid chromatography (HPLC), and direct techniques, such as enzyme-linked immunosorbent assay ELISA. The recent advances in these techniques are discussed, and the efficiency and sensitivity of each technique in the tested samples are compared.
2. Classification and Toxicity of Major Mycotoxins in Nuts
Numerous food crops, including nuts, are contaminated with various mycotoxins, which has raised global concerns about the effects of these mycotoxins when consumed by humans. The ingestion of mycotoxins can lead to the development of acute or chronic diseases resulting from long-term exposure to low doses of mycotoxins. These diseases include various types of cancer, hepatic diseases, as well as immunological and neurological disorders. Table 1 provides a list of the major mycotoxins found in nuts and their toxic effects on the health of mammals, including humans and different animals.
The International Agency for Research on Cancer (IARC) has classified mycotoxins into three groups based on their carcinogenicity to humans since 1987 [7,14,15]. Group 1 contains aflatoxins AFB1, AFB2, AFG1, AFG2, and AFM1, with AFB1 being the most toxic and potent carcinogen [3,5,14]. AFM1 was reclassified from Group 2B to Group 1 due to recent evidence of its carcinogenic potential [15]. Group 2B includes fumonisins and ochratoxin A, with FB1 being the most potent fumonisin. Group 3 comprises mycotoxins like trichothecenes, ZEA, and patulin, for which there is currently no evidence of carcinogenicity in humans [3,15].

Table 1.
Major mycotoxins contaminating the nuts and their impact on mammalian health.
Table 1.
Major mycotoxins contaminating the nuts and their impact on mammalian health.
| Mycotoxins | Genus | Major Effects | References |
|---|---|---|---|
| TA | Alternaria | High acute toxicity, precancerous changes in the mucosa of mice esophagus | [16] |
| OTA | Aspergillus, Penicillium | Nephrotoxic, carcinogenic, teratogenic, neurotoxic, embryotoxic, inhibition of DNA and RNA synthesis | [1,2,7] |
| DON | Fusarium | Human gastrointestinal illness, autoimmune disease, stimulates inflammation, inhibition of translation and protein synthesis, nausea, vomiting, diarrhea | [6,7] |
| FBs | Fusarium | Carcinogenic, hepatotoxic, nephrotoxic, liver cancer | [1,7,17] |
| Aflatoxins | Aspergillus flavus Aspergillus parasiticus | Immunosuppression, reduction of children growth, reduction of reproductivity, acute hepatitis, carcinogenic, hepatotoxic, mutagenic, teratogenic | [1,7,8,17] |
| T-2 toxin | Fusarium | Inhibition of protein synthesis and mitochondrial function, cytotoxic | [2,9,18] |
| ZEA | Fusarium | Estrogenic effects (fertility problems, breast augmentation) | [1,7,17] |
3. Contamination of Nuts
Nuts, including peanuts, almonds, cashew nuts, and pistachios, are exposed to contamination by mycotoxins due to their high fat content and various factors that can influence their contamination. These factors include pre- and post-harvest conditions, storage conditions, geographical location (warm and humid climates are favorable for mycotoxin production), and season (tropical and subtropical climates are more susceptible to contamination). Common mycotoxins found in nuts include Afs and OTA. Mycotoxins have become a leading concern, correlating with an increasing frequency of notifications in the “Rapid Alert System for Food and Feed” (RASFF). Mycotoxin contamination poses a significant challenge in the export of tree nuts and peanuts from the United States to European Union (E.U.) countries, leading to frequent rejections due to aflatoxin (AF) levels exceeding established limits. Almost 99% of U.S. mycotoxin notifications reported to the Rapid Alert System for Food and Feed (RASFF) from 2010 to 2019 were linked to AF contamination, notably affecting almonds, pistachios, and peanuts. A substantial majority of these notifications (57.9%) surpassed the FDA action level for food (≥20 ng g−1) [19]. The EU-RASFF recorded 4752 mycotoxin notifications for food products between 2011 and 2021, with 63% related to “Nuts, Nut products, and Seeds”. Groundnuts, highly susceptible to aflatoxins, accounted for the majority of incidents. The notifications for nuts and nut products increased from 8% in 2012 to a peak of 16% in 2018, reflecting an ongoing concern and emphasizing the need for enhanced control measures [20]. Table 2 provides information on mycotoxin levels in different nuts and countries, as well as the analytical techniques used for their detection.

Table 2.
Mycotoxin contamination in nuts: an international investigation on prevalence and levels across different countries.
4. Sampling of Nuts
The collection of nut samples for mycotoxin analysis plays a significant role in ensuring accurate and representative results. Therefore, it is important to establish approaches and standards to minimize sampling errors. These standards include the collection of samples from different locations and thorough mixing to ensure the creation of a homogeneous composite sample [4]. Another approach is to increase the number of samples to be analyzed in order to minimize analysis errors. To improve the accuracy of mycotoxin analysis and employ appropriate sampling, the FAO developed an online, free-access mycotoxin sampling tool in 2013 [14]. This tool enables a better assessment of mycotoxin contamination in nuts and different foodstuffs.
5. Sample Pretreatment Methods
The pretreatment of nut samples is considered an important and necessary step for mycotoxin detection in nuts to obtain accurate and reliable results. However, it is time-consuming and includes many steps, such as sample collection, sample homogenization, extraction, and clean-up [1]. Depending on the food matrix of the treated samples, clean-up and extraction steps could be combined into a single step or separated, as is the case with nut sample analysis [40]. The extraction step, followed by a clean-up step, helps to reduce the matrix effect that may occur in mycotoxin analysis, which increases the selectivity and accuracy [41,42]. The matrix effect refers to the influence of components other than mycotoxins on the analysis. This effect alters the detection of mycotoxins in nuts by causing a reduction in sensitivity, loss, or enhancement in response, ultimately leading to underestimated or overestimated results [43]. Figure 1 shows the common methods used for sample pretreatment in nuts.
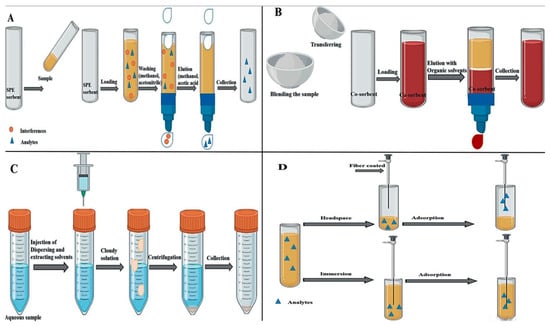
Figure 1.
Procedure of different pretreatment methods of nut samples ((A): SPE, (B): MSPD, (C) DLLME, (D) SPME).
In the following section, an overview of the sample pretreatment methods used for mycotoxin analysis in nuts over the years will be discussed. Table 3 shows examples of diverse pretreatment methods employed in global mycotoxin analysis of nuts.

Table 3.
Pretreatment methods used in mycotoxin analysis in nuts worldwide.
5.1. Liquid–Liquid Extraction (LLE)-Based Methods
5.1.1. Liquid–Liquid Extraction (LLE)
Liquid–liquid extraction (LLE) procedure is a common method used to separate mycotoxins in food samples, relying on the differences in solubilities between the liquid phase (usually aqueous) and an organic phase (such as hexane or cyclohexane, which are immiscible with water) [1]. However, this method is time-consuming and necessitates large volumes of solvents [14,58]. Moreover, its efficacy depends on the food matrix. As a result, several other environmentally friendly methods have been developed for mycotoxin analysis in nuts, such as dispersive liquid–liquid microextraction (DLLME) [14].
5.1.2. Dispersive Liquid–Liquid Microextraction (DLLME)
Dispersive liquid–liquid microextraction (DLLME) is a method for mycotoxin extraction in nuts using small volumes of organic solvents [14]. It involves injecting a solvent mixture into the aqueous sample to form a cloudy emulsion. After reaching equilibrium, centrifugation separates the extraction phase containing mycotoxins for quantification [14,58].
Only a few studies have utilized the DLLME method for detecting mycotoxins in nuts [14]. For example, in a study conducted by Arroyo-Manzanare et al. [54], the DLLME method was developed as an additional step for the determination of four types of aflatoxins in different nut samples using ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS). The recoveries of the developed method ranged between 60.7% and 104.3%. The limit of detection (LOD) values for the four aflatoxins were in the range of 0.18 and 0.29 μg/kg.
5.2. Solid-Phase Extraction (SPE)-Based Methods
5.2.1. Solid-Phase Extraction (SPE)
Solid-phase extraction (SPE) is a widely used method for pretreating nut samples to analyze mycotoxins [42]. It was introduced in the 1970s [2] and relies on chromatographic principles, utilizing a sorbent material to retain mycotoxins during sample loading [41]. The process involves washing to remove interfering substances, followed by elution of the analytes using a solvent for further analysis [59]. SPE method relies on various sorbents with different bonding phases for analyte retention [42]. These include C18 and C8 for nonpolar compounds, florisil for polar compounds, ion-exchange cartridges like propyl sulfonic acid (PRS), and additional adsorbents like immunoaffinity columns (IACs) and Oasis HLB [1,41,42]. In many studies, C18 cartridge was used for nut extraction prior to mycotoxin analysis using techniques such as high-performance liquid chromatography (HPLC) [35,50,55,60,61]. Immunoaffinity chromatography (IAC) uses antigen–antibody interaction to separate and purify mycotoxins from complex samples, including nuts [41,42]. It has been applied as a pretreatment method for mycotoxin analysis in nuts [51,62]. To address nut samples with multiple mycotoxins, researchers have developed multi-functional IACs with specific antibodies for each mycotoxin. In a study conducted by Vaclavikova et al. [63], a sensitive and fast method was developed for the simultaneous analysis of 12 mycotoxins in peanuts. The strategy combined a multi-functional IAC containing specific antibodies for the 12 mycotoxins with UHPLC-MS/MS. The developed method was optimized by optimizing the extraction and IAC clean-up processes. The recoveries for the mycotoxins in peanut samples ranged between 71% and 103%, and each sample run took only about 10 min.
5.2.2. Magnetic Solid-Phase Extraction (MSPE)
Magnetic solid-phase extraction (MSPE) merges SPE with magnetic separation using materials like Fe3O4 magnetic nanoparticles, graphene oxide, and metal–organic frameworks coated with a sorbent material, allowing an easy separation through an external magnetic field [42,64]. Karapinar et al. [64] established a simple MSPE method using Fe3O4@SiO2@TiO2 magnetic nanoparticles with APTMS-CP as an adsorbent, coupled with HPLC-FLD for detecting four aflatoxins (AFB1, AFB2, AFG1, and AFG2) in nut samples. The optimized extraction method involved adjustments in nanoparticle amount, pH, vortex time, and desorption conditions. The nut samples showed good aflatoxin recoveries (87.7% to 97.5%), and the LOD values ranged from 0.05 to 0.15 μg/kg.
5.2.3. Solid-Phase Microextraction (SPME)
SPME is a rapid extraction method using a fiber coated with a polymeric material like polydimethylsiloxane (PDMS) for the adsorption of analytes [42]. It can be applied by direct immersion in the liquid or headspace exposure of the food sample [59]. After exposure, the fiber is analyzed in an instrument, transporting desorbed analytes for further analysis. Initially used for volatile compounds with GC, SPME now includes semi- and non-volatile compounds, utilizing LC as well [14]. Although the SPME method is limited in its use for mycotoxin analysis [14], many researchers have employed SPME for the analysis of mycotoxins in nuts. Tsai et al. [65] developed an optimized SPME-CFI-MS method using a 2B pencil as the fiber, pH 8, and acetonitrile/ethanol/deionized water with butylamine for elution. The method was successfully applied to detect traces of AFB1 in peanut extract samples. Amde et al. [49] synthesized ZnO-NRs coupled to [HMIM][PF6] as an adsorbent, followed by HPLC for the analysis of four aflatoxins in groundnut samples. The optimized method included 10 mg of [HMIM][PF6] adsorbent, pH 7, and ultrasonication with acetonitrile for desorption. The recoveries of blank groundnut samples for the four aflatoxins ranged between 88.6% and 99.8%. The detection levels of AFB1 and AFB2 in groundnut samples were 1.08–8.71 μg/kg and 0.17–2.95 μg/kg, respectively. Saito et al. [48] utilized SPME-LC/MS to detect ochratoxins A and B in nuts, optimizing the method with Carboxen-1006 PLOT as the SPME capillary column and 20 draw/eject cycles of 40 mL at pH 3. The method achieved recoveries above 88% and detected ochratoxin levels between 0.7 ng/g and 8.8 ng/g in the nut samples analyzed.
5.2.4. Dispersive Micro-Solid-Phase Extraction (D-μ-SPE)
Dispersive micro-solid-phase extraction (D-μ-SPE) is a variation of SPE that uses smaller sorbent particles (micro- or nano-particles), enhancing extraction efficiency and improving recoveries due to their larger surface area. The method involves dispersing a solid sorbent material into the sample matrix [42,66]. Zhu et al. [67] developed a D-μ-SPE technique using (HMIM)(PF6) coupled to ZnO nanoflowers, followed by HPLC for detecting aflatoxins in peanuts. Optimal conditions included using 10 mg of (HMIM)(PF6) adsorbent, resulting in high recoveries of aflatoxins in peanuts (93.8–105.1%). The LOD values for the four aflatoxin types ranged from 0.024 to 0.067 μg/kg. Recently, a two-step extraction technique combining D-μ-SPE and DLLME has been widely used by researchers to improve mycotoxin recoveries from food samples while minimizing the use of toxic reagents [66]. Taherimaslak et al. [68] applied this approach to detect total aflatoxins in pistachio samples using surfactant-enhanced spectrofluorimetry. Optimal conditions were determined, utilizing 1-heptanol as the extracting solvent, 60 mg of Fe3O4 magnetic adsorbent, and 2 mL of acetonitrile as the desorbing solvent. The study achieved high recoveries for total aflatoxins in pistachio samples, ranging from 91.6% to 99.6%, with total aflatoxin levels in pistachio nuts between 1.98 and 3.55 μg/kg.
5.2.5. Nanoparticles-Based SPE
Various nanoparticles have emerged as promising adsorbents in the SPE technique due to their small size, large surface area, and effective interaction with mycotoxins, leading to increased adsorption capacity, enhanced extraction efficiency, and improved result quality. Various nanomaterials, including activated carbon-based nanomaterials and MNPs, have been studied for their application in SPE [69]. Activated carbon’s extensive surface area enables it to adsorb various compounds, but its selectivity is limited. To enhance selectivity, boron doping is an effective, low-cost, and non-toxic method, resulting in the activated carbon–boron (AC-B)–SPE technique. This method offers several advantages, including environmental friendliness, simplicity, rapidity, sensitivity, and ease of use [42]. Karapinar et al. [69] established an AC-B nanocomposite-based method followed by HPLC-FLD for the analysis of four aflatoxins in nuts. After optimizing the extraction conditions (5 mg AC-B sorbent, 3 min vortex time, pH 5) and the desorption conditions (3 mL acetonitrile, 3 min), it was applied to the analysis of aflatoxins in nut samples, yielding good recoveries (89.5–96.5%). Recent studies have shown that magnetic nanoparticles, especially Fe3O4, are a promising alternative sorbent for solid-phase extraction (SPE) due to their large surface area, high selectivity, and enhanced adsorption potential. They have been widely utilized in magnetic solid-phase extraction (MSPE) for analyzing mycotoxins in nut samples [57,64]. This extraction method will be further discussed later in the paper. A new nanocomposite-based sorbent for magnetic dispersive solid-phase extraction (MDSPE) was created by Karami-Osboo et al. [70] using magnetic nanoparticles from Spirulina algae with polydopamine surface modification. The adsorbent was characterized using Fourier transform infrared (FT-IR), X-ray Diffraction (XRD), and field emission scanning electron microscope (FE-SEM) methods and then applied with HPLC-FLD to analyze four aflatoxins in pistachio samples, achieving acceptable recoveries (72–95%).
5.3. Matrix Solid-Phase Dispersion (MSPD)
The matrix solid-phase dispersion (MSPD), known also as dispersive solid-phase extraction (DSPE), is considered as an expansion of the SPE. In MSPD, the nut sample is first prepared to obtain a homogeneous sample. Then, it is combined with a dispersing material such as C18 or silica. The resulting mixture is transferred into a cartridge or column, and the analytes are eluted from the solid matrix using extraction solvents. Finally, the eluate, which contains the mycotoxins, is collected for further analysis [14,42]. Bacaloni et al. [60] employed MSPD extraction with LC-MS/MS to analyze four aflatoxins in hazelnut samples. Using two sorbents (Carbograph-4 and Oasis HLB) spiked at 1 μg/kg, recoveries ranged from 70% to 83%, and at various concentration levels, recoveries ranged from 99% to 116%.
5.4. QuEChERS
The QuEChERS extraction method (quick, easy, cheap, effective, rugged, and safe) has recently become commonly used for the extraction of mycotoxins in food, including nuts, due to the excellent results it provides [41,42,54]. It was first used in a study by Anastassiades et al. [71] for the detection of pesticides in fruits and vegetables, and since then, it has been applied by many researchers for the detection of mycotoxins in foodstuffs [42,72]. An extraction method consists of two steps: liquid–liquid distribution and dispersive solid-phase extraction (d-SPE). In the first step, analytes (mycotoxins) are extracted from the sample matrix using an organic solvent like acetonitrile or ethyl acetate, with the aid of extraction salts (e.g., MgSO4, NaCl, NaOAc) to remove water and stabilize pH. The second step involves dispersive solid-phase extraction using sorbents like PSA or C18 to eliminate impurities like fatty acids and sugars. After centrifugation, the supernatant is collected for further analysis with different techniques [41,42,54,72,73].
In several studies, researchers have used the QuEChERS extraction method for the detection and analysis of different mycotoxins in nut samples, where satisfactory recoveries and results were obtained [23,44,45,46]. A modified QuEChERS technique has been developed to address challenges in analyzing high-lipid matrices like nuts. Traditional adsorbents like PSA and C18 face difficulties in mycotoxin extraction due to lipid interference, reducing efficiency. To overcome this, researchers introduced enhanced matrix removal-lipid (EMR-lipid) or Z-Sep sorbents, aiming to minimize matrix effects, enhance recoveries, and improve extraction efficiency by removing fats. Mateus et al. [73] used a modified QuEChERS method followed by UHPLC-ToF-MS to analyze mycotoxins in pistachio samples. They compared new sorbents (EMR-lipid and Z-Sep) with traditional ones (PSA and C18) in the d-SPE method. Z-Sep demonstrated superior efficiency with excellent recoveries (79–120%), while EMR-lipid showed better performance for fumonisin analysis. Z-Sep was preferred for its faster and easier performance, and both sorbents were used for specific mycotoxin analyses in pistachios. Alcántara-Durán et al. [74] developed a modified QuEChERS method using EMR-lipid as a sorbent for mycotoxin analysis in various nut samples. Compared to traditional sorbents like PSA and C18, EMR-lipid exhibited enhanced recoveries (75–98%), higher sensitivity, and significantly reduced matrix effects.
5.5. Green Solvents Extraction
5.5.1. Deep Eutectic Solvent (DES)
Researchers are searching for greener alternatives to toxic organic solvents to minimize environmental impact. Promising options include ionic liquids (ILs), switchable polarity solvents (SPSs), supramolecular solvents, and deep eutectic solvents (DESs) [53]. DESs have gained popularity due to their favorable properties, lower toxicity, cost-effectiveness, non-volatility, and biodegradability. DESs are created by combining a hydrogen bond acceptor (HBA), like choline chloride or tetrabutylammonium chloride, with a hydrogen bond donor (HBD), such as urea, amines, amides, or organic acids [75]. Deep eutectic solvent–MSPD (DES-MSPD) extraction combines MSPD principles with DES as the solvent for aflatoxin detection in nuts. Wu et al. [53] used this method, optimizing it with TBAC-hexyl alcohol as the DES solvent, diatomite as the dispersant, and a 1:1 sample-dispersant ratio. The method exhibited good linearity (R2 > 0.994) with low LOD values (0.03–0.10 μg/kg) for four aflatoxins in peanut samples.
5.5.2. Ionic Liquids (ILs)
Ionic liquids (ILs) are a new type of room-temperature liquid salts. They are formed from organic positively charged ions (cations) and organic or inorganic negatively charged ions (anions). They are being used as alternative solvents to organic toxic solvents because they are considered environmentally friendly. They offer several advantages: high thermal stability, non-flammability, high solubility, and low vapor pressure. Ionic liquids are currently being used in many extraction techniques (SPE, D-μSPE, SPME) for the detection of various mycotoxins in nut samples [42,76]. Fang et al. [50] developed an SPE method using a new IL-based silica, Sil@HIm-Im, for extracting AFB1 in peanut samples. Compared to another IL-based silica, Sil@BIm-Im, Sil@HIm-Im demonstrated the highest adsorption efficiency and was chosen as the sorbent. After optimizing the SPE method, HPLC analysis revealed a concentration of 0.023 μg/kg of AFB1 in the peanut samples.
Recently, the combination of ILs and nanomaterials has been widely explored in the extraction method for detecting mycotoxins in nuts. Their synergistic effect, when present together, enhances the extraction efficiency. Moreover, this combination also reduces the toxicity and environmental impact of the extraction process [49,67]. Zhu et al. [67] developed a D-μSPE method using an ionic liquid (HMIM)(PF6) and zinc oxide (ZnO) nanorods for aflatoxin analysis in peanuts. Excellent recoveries (93.8–105.1%) were achieved for the four aflatoxins. In another study, Amde et al. [49] utilized an SPME method with the same ionic liquid and ZnO-NRs as adsorbents, achieving recoveries ranging from 88.6% to 99.8% for the four aflatoxins in groundnuts when coupled with HPLC analysis.
5.6. Combination of Different Pretreatment Methods
The combination of different pretreatment methods when analyzing the sample can be an efficient approach for reducing matrix effects and enhancing recoveries and sensitivity. Many researchers have combined various pretreatment methods for the analysis of mycotoxins in nuts. Arroyo-Manzanares et al. [54] developed a method based on QuEChERS combined with DLLME, followed by UHPLC-MS/MS, for the determination of four aflatoxins in different nut samples. Good recoveries ranging from 60.7% to 104.3% were obtained. Another study conducted by Rezaee et al. [66] utilized SPE-DLLME, followed by HPLC-FLD, for the analysis of four aflatoxins in pistachios, and excellent recoveries (85–93%) were achieved. In a separate study by Taherimaslak et al. [68], DLLME extraction and d-SPE were combined for the determination of total aflatoxins using surfactant-enhanced spectrofluorimetry, with recoveries ranging from 91.6% to 99.6%. The advantages and disadvantages of different pretreatment methods are summarized in Table 4.

Table 4.
The advantages and disadvantages of different pretreatment methods.
6. Analytical Quantification of Mycotoxins in Nuts
The detection and quantification of mycotoxins in nut samples are critical to ensure food safety and prevent health hazards. Analytical techniques play an important role in identifying and quantifying mycotoxin levels in nuts. These techniques can be categorized into direct techniques like ELISA and TLC, as well as indirect techniques like HPLC and LC-MS/MS.
6.1. Indirect Techniques
6.1.1. High-Performance Liquid Chromatography (HPLC)
HPLC is widely used for mycotoxin detection in nuts due to its accuracy and sensitivity. It separates mycotoxins by passing samples through a chromatographic column using a high-pressure pump, utilizing the differences in their properties. Coupled with a UV or fluorescence detector, HPLC can be automated, offering increased throughput, precision, and accuracy compared to other methods like ELISA and TLC [1,41]. Moreover, it enables automation. However, it suffers from several drawbacks, including the high cost of equipment, the need for specialized expertise, and the requirement of derivatization for some mycotoxins [82,83]. Numerous researchers have extensively utilized HPLC to measure various mycotoxins found in nuts [17,24,27,30,34,38,47,49,50,53,57].
6.1.2. LC-MS
Since 1980, liquid chromatography–mass spectrometry (LC-MS) has become a widely used technique for mycotoxin analysis due to its many advantages [40]. It involves separating mycotoxins with reversed-phase LC, followed by ionization and mass analysis [40,84]. While thermospray (MS) was initially common for mycotoxin analysis, electrospray ionization (ESI) has largely replaced it due to higher sensitivity and selectivity, enabling the detection of lower analyte levels in complex matrices like nuts [40,85]. LC-MS is a highly sensitive method for mycotoxin detection in nuts, and it has the ability to detect multi-analytes simultaneously [25,40,86,87,88]. It can be used to analyze and quantify mycotoxins without the need for derivatization [4,25]. However, LC-MS is time-consuming with variable signal suppression/enhancement due to multiple steps involved [25,40]. Additionally, LS-MS instruments are usually expensive [40]. “Matrix effects” are a major challenge in mycotoxin analysis, impacting accuracy and precision. These effects arise from the co-elution of matrix components and their influence on analyte ionization efficiency, resulting in signal suppression/enhancement, particularly in complex matrices with diverse chemical properties [40,43,89]. Matrix effects appear especially when complex matrices are analyzed due to the presence of many interferences having different chemical properties [90]. LC-MS/MS has become a popular technique in mycotoxin analysis in nuts in recent years. Almost 80% of all published LC-MS studies on mycotoxins since 2012 have used LC-MS/MS [89].
Several methods aim to reduce matrix effects in mycotoxin analysis of nuts by LC-MS, with “dilute-and-shoot” being suitable if highly sensitive LC-MS is present. However, as many LC-MS instruments cannot detect aflatoxins below EU limits, a clean-up step might be necessary. The most effective solution could be using stable isotope dilution assay (SIDA) with a commercial C-aflatoxins internal standard for accurate quantification in food, including nuts [40,89].
Several assays were developed to increase the sensitivity of the LC-MS/MS technique and to reduce the matrix effects on mycotoxin analysis in nuts.
SIDA was used for the first time for the detection of aflatoxins in nuts by Cervino et al. [91], who utilized LC-MS/MS stable isotope dilution assay (SIDA) to detect aflatoxins in nuts. Deuterated aflatoxins B2 and G2 were synthesized using palladium nanoparticles and used for quantification. This technique effectively reduced matrix effects, yielding high recoveries (94–105%) for aflatoxins in almonds. SIDA proved to be a sensitive method for analyzing aflatoxins in various nuts, even below EU regulatory limits. Xavier et al. [92] developed an LC-MS technique for aflatoxins analysis in Brazil nuts using the“dilute-and-shoot” method and atmospheric pressure chemical ionization (APCI). Nut samples were diluted and injected into the LC-MS instrument after extraction. The technique demonstrated high sensitivity with low LOD (0.04–0.060 μg/kg) and LOQ (0.08–0.12 μg/kg) values, achieving accurate quantification of aflatoxins (recoveries: 92–100%). Despite the need for an expensive instrument and regular maintenance, the technique provided quick results (total run time was less than 5 min), increased confidence in the findings, and removed the necessity for a clean-up step. Huang et al. [25] developed a dilute-and-shoot method using an ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) to simultaneously detect six types of aflatoxins (B1, B2, G1, G2, M1, and M2) in peanuts and their derivative products. Peanut samples were extracted with 84% acetonitrile, diluted with an acetonitrile/water mixture (10:90, v/v), and then analyzed using the UHPLC-MS/MS instrument. The developed method demonstrated good recovery (74.7–86.8%) and excellent precision (RSD < 10.9%).
The sensitivity and selectivity of LC-MS/MS can be influenced by many factors, such as the choice of columns and the mobile phase. Huang et al. [25] improved LC-MS/MS sensitivity by using a UHPLC-MS/MS technique with a 1.8 µm column and a water/formic acid–acetonitrile/methanol mobile phase. Aflatoxin analysis in peanuts showed over 74.7% recovery, indicating accuracy. Their technique detected six types of aflatoxins in 75 peanut samples, including AFM1 and AFM2, for the first time.
6.1.3. LC Techniques–QqQ-MS/MS
Co-contamination of aflatoxins and other mycotoxins in nuts is common, leading to increased toxicity and amplified effects [93]. Monitoring and controlling their presence in food and feed is crucial due to potential synergistic or additive effects [94,95]. Liquid chromatography (LC) and gas chromatography–mass spectrometry (GC-MS) are widely used to detect the co-occurrence of mycotoxins [96]. LC-MS/MS, specifically using a triple quadrupole mass spectrometer (usually abbreviated as QqQ), allows simultaneous detection and quantitation of multiple mycotoxins, providing high specificity and sensitivity [40,84]. Multiple reaction monitoring (MRM) scans enable efficient analysis of complex mixtures, making it a powerful technique for mycotoxin analysis in nuts [97,98]. The development of LC-QqQ-MS-based multi-mycotoxin techniques in many studies has increased the ability to detect multiple mycotoxins in a single analysis, and they have seen significant advancements in terms of sensitivity and capability for quantitative analysis [40]. Cunha et al. [46] developed an effective LC-MS/MS technique for determining 16 mycotoxins in nuts using a modified QuEChERS procedure. The method showed good recovery (70–93%), repeatability (RSD ≤ 13%), and low LOQ values (1.5–5 μg/kg). The co-contamination was observed in 35% of the nut samples. Oyedele et al. [94] employed LC-QqQ-MS/MS to screen 84 groundnut samples from different agro-ecological zones in Nigeria, detecting 58 microbial metabolites (54 fungal, four bacterial) and quantifying 10 major mycotoxins. A similar LC-MS/MS multi-technique was developed by Spanjer et al. [99], in which 33 mycotoxins were analyzed in peanut and pistachio samples in a single 30-minute run time. The recoveries ranged between 80% and 110%, and the LOD values in peanuts were 0.15–10 μg/kg, while in pistachios, they were 0.5–200 μg/kg. Furthermore, a study conducted by Warth et al. [100] showed the power of the LC-MS/MS multi-toxin technique to detect 27 metabolites in groundnut samples. The LOD values ranged between 0.05 μg/kg and 250 μg/kg, and good recoveries were obtained (38–1155). In another study, Liao et al. [101] developed a similar LC-ESI-MS/MS technique for mycotoxin analysis in several nut samples (almonds, peanuts, pistachios). This innovative approach enabled the simultaneous detection of an impressive array of 26 mycotoxins. The results were good, with almond samples exhibiting a remarkable 87 ± 12% recovery rate, peanuts displaying an even more impressive 104 ± 16% recovery rate, and pistachios showing a 92 ± 18% recovery rate. Moreover, the technique had excellent sensitivity, as evidenced by the low LOQ values ranging from 0.2 to 12.4 μg/kg for almonds, 0.3 to 12.1 μg/kg for peanuts, and 0.3 to 12.8 μg/kg for pistachios.
Additionally, ultra-high-performance liquid chromatography (UHPLC) is a highly efficient and accurate technique for mycotoxin analysis in food. Its advantages include high resolution and retention-time reproducibility, as well as a high peak capacity when used with MS, enabling the separation of numerous components in a single run [61]. UHPLC’s use of submicron columns (<2.0 μm) allows for rapid detection of mycotoxins at extremely low levels [40]. Moreover, it enables fast and efficient analysis of multiple samples due to its high throughput [61]. UHPLC-MS also minimizes sample-matrix effects, making it a popular choice for multiclass mycotoxin analysis [61,102]. UHPLC-MS/MS has become a popular analytical technique for the multiclass analysis of mycotoxins in nuts because of its several advantages [54]. Therefore, in many studies, the UHPLC-QqQ-MS/MS technique allows for the simultaneous determination of several mycotoxins in nut samples.
Arroyo-Manzanares et al. [54] developed a sensitive UHPLC-MS/MS technique to detect 14 mycotoxins in nuts using QuEChERS and DLLME extraction. This technique showed low LOD values (0.17–45.1 μg/kg) and low LOQ values (0.57–150 μg/kg) with high recoveries (60.7–104.3%), indicating reliability in nut sample analysis. Furthermore, a UPLC-QqQ-MS/MS technique for the simultaneous determination of several mycotoxins in nut samples (peanuts, pistachios, almonds) was described by Kafouris et al. [103]. The technique was rapid, sensitive, and validated for 11 mycotoxins. LOD values ranged from 0.15 to 7.5 μg/Kg for pistachios, 0.15 to 15.3 μg/kg for almonds, and 0.08 to 15 μg/kg for peanuts. It showed mean recoveries of 74.4% to 131.7% in spiked samples. In the case of Hidalgo-Ruiz et al. [45], a UHPLC-QqQ-MS/MS technique was developed and validated for detecting six mycotoxins in nuts. Peanuts were chosen as the representative matrix after evaluating different types. The LOQ ranged from 0.5 to 1 µg/kg. Recoveries were 80–120%, with precision values < 20% for intra- and inter-day. Another multi-target UHPLC-QqQ-MS/MS technique was developed by Varga et al. [104] for mycotoxin analysis in nuts. It utilized sub-2-µm particle columns, enhancing resolution and accuracy. It successfully analyzed 191 fungal metabolites in 53 nut samples, quantifying 65 mycotoxins and semi-quantifying 126 others. Good recoveries were obtained and ranged from 80 to 120%.
6.2. Direct Techniques
6.2.1. Thin-Layer Chromatography (TLC)
TLC was used for aflatoxin analysis, with a significant impact on purification and identification in 1960 [42,43]. High-performance thin-layer chromatography (HPTLC) extended TLC, providing enhanced separation ability, higher resolution, and sensitivity and improving compound analysis like aflatoxins, was replaced by HPLC [42]. It employed different stationary phases like silica gel, F254 fluorescent silica gel, or organic acid-impregnated silica gel, with silica gel being the most common [1]. Visualization is achieved through fluoro densitometry or visual procedures (using UV-Vis spectroscopy) [1,105]. While TLC is simple, cost-effective, and high-throughput [1,43,106], it has a poor separation of closely related compounds, a low accuracy in determining the amounts of each component, and it is less sensitive for detection of small amounts of a substance [43,80]. Proper sample preparation varies depending on mycotoxin properties and type [1,43]. TLC is still a widely applied method for both quantitative and semi-quantitative measurements of mycotoxins in nut samples [1]. The TLC technique was used by many researchers for the first screening of mycotoxin presence in nut samples [21,22,56,107].
6.2.2. Enzyme-Linked Immunosorbent Assay ELISA
ELISA is widely used for detecting and quantifying mycotoxins [7,108]. This immunological technique relies on specific interactions between the mycotoxin and its corresponding antibodies coated onto a solid surface. If the mycotoxin is present in the nut sample, it binds to the antibodies. A secondary antibody, linked to an enzyme, is added, producing a signal upon reacting with a substrate [11,41]. ELISA can be qualitative or quantitative; visual color intensity provides semi-quantitative results, while quantitative results require careful calibration and are more time-consuming [14,108,109]. Direct competitive ELISA is a common technique to detect and quantify mycotoxins in nuts, relying on labeled and unlabeled antigens competing for binding to specific antibodies. The signal intensity is inversely proportional to the mycotoxin concentration [7,108,109,110]. This sensitive, inexpensive, and rapid technique serves as a primary screening tool for detecting contaminated nut samples [7,25,109,110,111,112]. However, ELISA has limitations like cross-reactivity and matrix effects, causing false results. Validated mycotoxin ELISA kits exist, but they are specific to certain mycotoxins, contamination levels, and food matrices, limiting their universal applicability. Additionally, ELISA lacks the ability to detect multiple mycotoxins simultaneously in nut samples, requiring separate tests, which can be time-consuming, and ELISA kits are usually one-time use [7,25,109,110,113]. Commercial ELISA kits are available for various mycotoxins, including aflatoxins AFs, ZEA, OTA, DON, T2/HT2, and FBs [1,41]. ELISA kit has become an indispensable tool for researchers studying the prevalence of mycotoxins in nuts [31,33,35,36].
Efforts to develop cost-effective monoclonal antibodies against aflatoxins for accessible immunochemical analysis were made. Therefore, several monoclonal antibodies were reported by Li et al. [112,114] and Oplatowska-Stachowiak et al. [115] and used for an accurate detection of aflatoxins in nut samples. Common steps included selecting the target antigen, immunizing mice, screening hybridomas, and purifying antibodies. The best monoclonal antibody was chosen based on sensitivity and cross-reactivity for ELISA development. The validated class-specific monoclonal antibody-based ELISA successfully detected specific aflatoxins in contaminated nut samples. Oplatowska-Stachowiak et al. [115] produced seven unique monoclonal antibodies with high sensitivity and cross-reactivity for aflatoxin detection. Among them, two antibodies (1 NP-D and 1 NP-C) exhibited impressive IC50 values for aflatoxin B1. The developed ELISA test demonstrated low LOD values for AFB1 and total AFs (0.4 and 0.3 μg/kg, respectively), and acceptable recoveries were high (97.1–107.5%). Researchers attempted to develop monoclonal antibodies with improved cross-reactivity for G-group aflatoxins. Li et al. [114] created three promising antibodies (2G6, 3A4, and 4G4) with good cross-reactivity to AFG1 and AFG2. Among them, 2G6 showed the highest sensitivity and specificity (IC50 of 17.18 ng/mL, 100% CR with AFG1, and 87% CR with AFG2). They utilized 2G6 to design a competitive indirect ELISA (CI-ELISA) with optimized parameters, achieving a LOD of 0.06 ng/mL and demonstrating high accuracy in detecting G-group aflatoxins in peanut samples, with recoveries of 94–103%. Li et al. [112] conducted a study using three class-specific monoclonal antibodies (8E11, 8F6, and 10C9) to detect aflatoxins. Among them, 10C9 displayed the most similar sensitivity for five aflatoxins and the highest cross-reactivity (CR = 65.2) to AFG2. With the 10C9 antibody, they developed an ELISA for peanut samples, achieving recoveries of 85.5% to 102%. The developed ELISA method also demonstrated low LOD values (0.06–0.09 ng/mL).
6.2.3. Lateral Flow Device (LFD)
Lateral flow tests are widely used for the rapid detection of mycotoxin prevalence in nut samples. These tests contain specific antibodies and mycotoxin–carrier protein conjugates to identify target mycotoxins [14,77,116]. The procedure involves applying a liquid sample to the test area. If the mycotoxin is present, a red color appears solely on the control line, indicating a positive result. In the absence of mycotoxins, both the test line and the control line turn red, showing a negative result. Lateral flow tests are rapid and simple techniques for mycotoxin screening in nut samples, providing visual results for the presence or absence of the target mycotoxin [14,116]. They are semi-quantitative, but accuracy can be improved with a lateral flow reader to measure color intensity [14]. Gold nanoparticles (AuNPs) can enhance sensing applications through metal enlargement, increasing optical or electrochemical signals by depositing silver or gold on their surface [117]. Colloidal gold, referring to suspended AuNPs, is widely used in mycotoxin detection due to its ease of preparation and visible signal production. Commonly, 40 nm particles are utilized in immunochromatographic strip tests for mycotoxin detection in nut samples [14,77,116,117].
Several studies have used the LFD that contains gold nanoparticles for the simultaneous detection of multiple mycotoxins in nuts. Li et al. [118] developed a multi-component immunochromatographic assay (ICA) for the simultaneous detection of three mycotoxins (AFB1, OTA, ZEA) in peanut samples. The assay utilized competitive immunoreactions between specific antibody–colloidal gold nanoparticle conjugate probes and mycotoxins or mycotoxin antigens. The ICA strip conditions were optimized, and visible results were obtained in 20 min. The visual detection limits of the ICA for AFB1, OTA, and ZEA were 0.25 ng/mL, 0.5 ng/mL, and 1 ng/mL, respectively. The prevalence of multiple mycotoxins in peanut samples was investigated using the developed ICA. In a study conducted by Chen et al. [119], a multiplex lateral flow immunoassay (LFA) was capable of detecting AFB1, ZEA, and OTA mycotoxins in peanut samples within 15 min. The optimized LFA employed 32 nm AuNPs, specific antibody amounts, and pH levels, resulting in visual detection limits of 10 µg/kg for AFB1, 50 µg/kg for ZEA, and 15 µg/kg for OTA in spiked samples. The quantitative analysis of peanut samples revealed LOD values of 0.13 µg/kg for AFB1, 0.46 µg/kg for ZEA, and 0.24 µg/kg for OTA, with good recovery rates ranging from 86.2 to 114.5%. In another study conducted by Zhang et al. [120], they developed an ultrasensitive immunochromatographic (IC) assay for detecting total aflatoxins in peanuts. They employed a competitive format using a monoclonal antibody (1C11) labeled with nanogold particles to enhance sensitivity. The optimized conditions resulted in lower visual detection limits (VDLs) compared to other studies: 0.03, 0.06, 0.12, and 0.25 ng/mL for AFB1, AFB2, AFG1, and AFG2, respectively. The validated IC assay was then used to analyze several peanut samples for aflatoxin detection.
While gold nanoparticle labels have been widely used in immunochromatographic assays due to their unique optical properties, they can be susceptible to interference from complex sample matrices, such as those found in food, which may lead to false positives or false negatives [121]. To address this issue, researchers have developed fluorescent lateral flow immunoassays based on lanthanide Eu3+ chelate labeling, which offers several advantages over gold nanoparticle labels. Wang et al. [121] developed fluorescent lateral flow immunoassays using lanthanide Eu3+ chelate labeling to overcome the limitations of gold nanoparticle labels in complex sample matrices, such as nuts. The optimized technique allowed simultaneous and quantitative detection of AFB1, ZEA, and CTN in peanut samples within 15 min. The limit of detection (LOD) for AFB1 and ZEA in peanut samples was 0.18 µg/kg and 0.57 µg/kg, respectively, with good recoveries (91.60–95.87% for AFB1 and 85.72–91.67% for ZEA).
6.3. Combination of Direct and Indirect Techniques
To achieve a comprehensive mycotoxin analysis in nuts, researchers and analysts often employ a hybrid approach that combines both direct and indirect methods. Initially, they use rapid screening techniques, like ELISA or TLC, to identify the presence or absence of specific mycotoxins. If contamination is detected during the screening, further confirmation and quantification can be performed using HPLC or LC-MS/MS to obtain more detailed information about the mycotoxin levels in the sample.
In TLC, the comparison of the migration distance ratio (Rf values) between nut samples and standards can provide preliminary or presumptive evidence of the presence of mycotoxins. However, this evidence is not conclusive, and further confirmation tests are necessary [40]. In many studies, TLC has been used for the detection of initial positive results. Subsequently, HPLC was employed for further confirmation and quantification of mycotoxins in nut samples. In a study conducted by Kujbida et al. [22], they investigated the aflatoxin occurrence in peanuts and cashew nuts using two chromatographic techniques (TLC and HPLC). TLC screening detected the contaminated samples, while HPLC was used for the quantification of four aflatoxins in positive samples. In many studies, TLC has been reported for assessing mycotoxigenic fungi in nuts, while HPLC was used for further confirmation and quantification of mycotoxins. A study by Amar et al. [17] aimed to detect aflatoxigenic fungi and aflatoxins in various nut samples (almonds, pistachios, hazelnuts, peanuts, walnuts) from the Algerian market. TLC was used for rapid screening of positive isolated fungi, and HPLC was employed for aflatoxin quantification in the nut samples, leading to good recoveries (72.6–91.8%). A similar approach has been reported in a study conducted by Ait Mimoune et al. [122] for mycotoxin analysis (Aflatoxins, CPA, OTA) in nut samples from various markets. TLC was used for qualitative analysis to detect aflatoxigenic strains in positive samples, followed by HPLC for quantification of mycotoxin levels. In a study conducted by Ozay et al. [107] in Turkey, hazelnut samples were analyzed for aflatoxin detection. The detection of aflatoxin-producing fungi involved qualitative tests using TLC plates, while quantitative analysis was conducted through HPLC. In another study in India conducted by Sharma et al. [27], TLC and HPLC were used to determine the presence and levels of AFs, OTA, and PAT in Chilgoza pine nuts. TLC was employed for the qualitative estimation of mycotoxins, while HPLC was used for their quantification. Overall, TLC is considered as a qualitative or semi-quantitative technique where positive results can be assessed visually without an accurate quantification of mycotoxin levels in nut samples.
The possibility of obtaining false-positive results in ELISA tests exists due to the cross-reactivity of antibodies. To address this issue, it is recommended to confirm the results using a suitable chromatographic technique, such as HPLC [7,77,113]. Therefore, several studies have used the ELISA test for rapid screening of mycotoxins in nut samples, and the results were then confirmed by chromatographic techniques such as HPLC and LC-MS. Chun et al. [35] conducted a study analyzing 85 nuts and nut products for aflatoxins, utilizing both ELISA and HPLC techniques. After ELISA screening, 31 samples were identified as positive for aflatoxins. Subsequently, HPLC quantification of total aflatoxins in these thirty-one samples revealed that nine of them were contaminated, and the results obtained were further confirmed by LC-MS. Two similar studies were reported by Shadbad et al. [36] and Leong et al. [31], in which nuts and nut products were first screened by ELISA to detect the contaminated samples. Subsequently, HPLC was employed for further confirmation and quantification of aflatoxins in the identified positive samples. Asis et al. [123] compared ELISA and HPLC techniques for AFB1 determination in peanut samples. The ELISA test demonstrated high sensitivity with an LOD of 0.5 µg/kg and 107% average recovery for peanut samples contaminated with only AFB1 due to its two-antibody system and lack of cross-reactivity with the peanut matrix. The results showed a strong correlation between the two techniques, with a correlation coefficient (r) of 0.977 and a p-value less than 0.0001 (p < 0.0001). In another study, Razzazi-Fazeli et al. [124] validated positive ELISA samples of peanut products using HPLC. The study confirmed thirty contaminated peanut samples detected by ELISA, then confirmed through HPLC. Comparing ELISA and HPLC, a stronger correlation was observed at lower levels of total aflatoxins (0–120 µg/kg) and AFB1 (0–80 µg/kg), with correlation coefficients (r) of 0.9244 and 0.8805, respectively. However, this correlation was not observed at higher levels of total aflatoxins or AFB1. In addition, researchers developing a class-specific monoclonal antibody ELISA for mycotoxin analysis in nuts aimed to validate its accuracy against more precise techniques like HPLC and UHPLC-MS/MS. Li et al. [112] compared the recoveries of the developed ELISA, using AFG2 as a competitor, with those of HPLC. The results indicated the suitability of the developed ELISA for nuts analysis, with recoveries ranging from 87.5% to 102% for ELISA and 87.7% to 97.6% for HPLC. A similar approach was observed by Li et al. [114], where they focused on an ELISA specific for AFG1 and AFG2 detection in peanuts, showing recoveries comparable to HPLC and demonstrating a good correlation between the two techniques. Furthermore, Oplatowska-Stachowiak [115] compared the levels of four aflatoxins in peanuts detected by a developed monoclonal antibody-based ELISA for AFB1 with those obtained using UHPLC-MS/MS.
The results obtained by lateral flow tests (LFTs) may not be as accurate or reliable as more conventional techniques. Therefore, it is generally recommended to confirm the results obtained from a lateral flow assay with a more accurate and quantitative technique, such as HPLC [77]. In a study conducted by Li et al. [118], the levels of mycotoxins (OTA, AFB1, ZEA) in peanut samples were compared using a multi-component immunoaffinity chromatography (ICA) and ELISA as the reference technique. In another study conducted by Wang et al. [121], the levels of mycotoxins (ZEA, AFB1) in contaminated peanut samples were verified using a developed lateral flow immunoassay (IA) and confirmed through HPLC-MS/MS, showing a strong correlation between the two techniques (R2 > 0.88). Zhang et al. [120] developed a nanogold-probe-based immunoassay for the detection of four aflatoxins in peanuts. The obtained results were then compared with HPLC results, revealing a good agreement between the two techniques. The advantages and limitations of each analytical technique are presented in Table 5.

Table 5.
Advantages and limitations of analytical techniques used in mycotoxin detection in nuts.
7. Conclusions
The prevalence of mycotoxins in nuts is an unavoidable and persistent worldwide problem, posing significant risks to human and animal health. To minimize human and animal exposure to mycotoxins, many sensitive and accurate analytical techniques have been developed, and they are classified as indirect techniques like HPLC, direct techniques such as ELISA, or a combination of direct and indirect techniques. Various pretreatment sample methods have also been employed, with IAC and QuEChERS being the most commonly used techniques. Recently, researchers have developed new materials and techniques to increase extraction efficiency, improve analytical methods’ efficiency, and reduce toxicity and processing time. Novel materials and solvents, such as nanomaterials used as sorbents in solid-phase extraction (SPE), ionic liquids (ILs), and deep eutectic solvents (DESs), have been explored in nut sample preparations. On the other hand, due to the increasing toxicology information regarding the co-contamination of mycotoxins in nuts, researchers have developed HPLC-QqQ-MS/MS for the simultaneous detection of multiple mycotoxins in nut samples. Many researchers have recently proposed new instrumental parameters and optimized the conditions of this technique to save time and cost. Other approaches gaining popularity include the use of stable isotope dilution analysis (SIDA) in HPLC-MS/MS to reduce matrix effects and improve result accuracy. Recent advances have also explored the development of monoclonal antibodies in ELISA to enhance specificity. The need for future research in mycotoxin detection emphasizes a global approach that combines sensitivity, accuracy, affordability, and efficiency. Future research should prioritize the development of analytical methods for detecting masked mycotoxins in nuts, which is crucial for ensuring food safety. It is highly important to conduct additional studies to create reliable and user-friendly detection methods. Collaborative efforts among researchers, the nut industry, and international organizations are recommended to establish a global surveillance and monitoring system for nut consumption, thereby enhancing quality control and the management of mycotoxins to safeguard public health on a worldwide scale.
Author Contributions
This work was a collaborative effort amongst all authors. Conceptualization and design: M.K. and J.C.A.; writing: H.H.; review and editing: M.K., J.C.A. and D.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data of this review are included within the edited manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AC-B | Activated carbon–boron |
| AFs | Aflatoxins |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| AFM2 | Aflatoxin M2 |
| AFPA | Aspergillus flavus and parasiticus agar |
| AME | Alternariol monomethyl ether |
| AOH | Alternariol |
| APCI | Atmospheric pressure chemical ionization |
| AuNPs | Gold nanoparticles |
| BEAU | Beauvericin |
| BSA | Bovine serum albumin |
| CIT | Citrinin |
| CPA | Cyclopiazonic acid |
| CTN | Chlorothalonil |
| DAS | Diacetoxyscirpenol |
| DESs | Deep eutectic solvents |
| DES-MSPD | Deep eutectic solvent–MSPD |
| D-μ-SPE | Dispersive micro-solid-phase extraction |
| DLLME | Dispersive liquid–liquid microextraction |
| DMRM | Dynamic MRM |
| DON | Deoxynivalenol |
| DSPE | Dispersive solid-phase extraction |
| ELISA | Enzyme-linked immunosorbent assay |
| EMR-lipid | Enhanced matrix removal-lipid |
| ENNs | Enniatins |
| ENA | Enniatin A |
| ENA1 | Enniatin A1 |
| ENB | Enniatin B |
| ENB1 | Enniatin B1 |
| ESI | Electrospray ionization |
| FBs | Fumonisins B |
| FB1 | Fumonisin-B1 |
| FB2 | Fumonisin-B2 |
| FE-SEM | Field emission scanning electron microscope |
| FT-IR | Fourier transform infrared |
| FUS-X | Fusarenon-X |
| GC | Gas chromatography |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| (HMIM)(PF6) | 1-hexAyl-3-methylimidazolium hexafluorophosphate |
| HPLC | High-performance liquid chromatography |
| HPLC-FLD | High-performance liquid chromatography with fluorescence detection |
| HPTLC | High-performance thin-layer chromatography |
| HT-2 | HT-2 toxin |
| IACs | Immunoaffinity columns |
| ICA | Immunochromatographic assay |
| ICS | Immunochromatographic strip |
| ILs | Ionic liquids |
| LC | Liquid chromatography |
| LC-MS | Liquid chromatography–mass spectrometry |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LFA | Lateral flow immunoassay |
| LFD | Lateral flow device |
| LFTs | Lateral flow tests |
| LLE | Liquid–liquid extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| Mab | Monoclonal antibody |
| MOFs | Metal–organic frameworks |
| MON | Moniliformin |
| MRM | Multiple reaction monitoring |
| MSPE | Magnetic solid-phase extraction |
| MSPD | Matrix solid-phase dispersion |
| NEO | Neosolaniol |
| NIV | Nivalenol |
| NMs | Nanoparticles materials |
| OTA | Ochratoxin A |
| OTB | Ochratoxin B |
| OVA | Ovalbumin |
| PAT | Patulin |
| PDMS | Polydimethylsiloxane |
| PRS | Propyl sulfonic acid |
| PSA | Primary secondary amine |
| QqQ | Triple quadrupole mass spectrometer |
| QuEChERS | Quick, easy, cheap, effective, rugged, and safe |
| RPLC | Reversed-phase LC |
| RSD | Relative standard deviation |
| SIDA | Stable isotope dilution assay |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
| SPSs | Switchable polarity solvents |
| STE | Sterigmatocystin |
| T-2 | T-2 toxin |
| TA | Tenuazonic acid |
| TCT | Trichothecenes |
| TLC | Thin-layer chromatography |
| UHPLC | Ultra-high-performance liquid chromatography |
| UHPLC-MS/MS | Ultra-high-performance liquid chromatography–tandem mass spectrometry |
| VDLs | Visual detection limits |
| XRD | X-Ray diffraction |
| ZEA | Zearalenone |
| ZnO-NRs | Zinc oxide nanorods |
| α-ZOL | α-Zearalenol |
| 3-AcDON | 3-acetyldeoxynivalenol |
| 15-AcDON | 15-acetyldeoxynivalenol |
References
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges; a review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- McMillan, A.; Renaud, J.B.; Burgess, K.M.; Orimadegun, A.E.; Akinyinka, O.O.; Allen, S.J.; Miller, J.D.; Reid, G.; Sumarah, M.W. Aflatoxin exposure in Nigerian children with severe acute malnutrition. Food Chem. Toxicol. 2018, 111, 356–362. [Google Scholar] [CrossRef]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- De Souza, R.G.M.; Schincaglia, R.M.; Pimentel, G.D.; Mota, J.F. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef]
- Dreher, M.L.; Maher, C.V.; Kearney, P. The Traditional and Emerging Role of Nuts in Healthful Diets. Nutr. Rev. 2009, 54, 241–245. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Mesas, A.E.; Garrido-Miguel, M.; Martínez-Ortega, I.A.; Jiménez-López, E.; Martínez-Vizcaíno, V. The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis. Nutrients 2021, 13, 2251. [Google Scholar] [CrossRef] [PubMed]
- Zaied, C.; Abid, S.; Bouaziz, C.; Chouchane, S.; Jomaa, M.; Bacha, H. Ochratoxin A levels in spices and dried nuts consumed in Tunisia. Food Addit. Contam. Part B 2010, 3, 52–57. [Google Scholar] [CrossRef]
- Schincaglia, A.; Aspromonte, J.; Franchina, F.A.; Chenet, T.; Pasti, L.; Cavazzini, A.; Purcaro, G.; Beccaria, M. Current Developments of Analytical Methodologies for Aflatoxins’ Determination in Food during the Last Decade (2013–2022), with a Particular Focus on Nuts and Nut Products. Foods 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yekeler, H.; Bitmiş, K.; Ozçelik, N.; Doymaz, M.Z.; Çalta, M. Analysis of Toxic Effects of Alternaria Toxins on Esophagus of Mice by Light and Electron Microscopy. Toxicol. Pathol. 2001, 29, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Amar, R.; Amina, M.; Salim, M.; Florence, M.; Nasserdine, S. Investigations on aflatoxigenic fungi and aflatoxins contamination in some nuts sampled in Algeria. Afr. J. Microbiol. Res. 2013, 7, 4974–4980. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.-H. Analysis of E.U. Rapid Alert System (RASFF) Notifications for Aflatoxins in Exported U.S. Food and Feed Products for 2010–2019. Toxins 2021, 13, 90. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Karoonuthaisiri, N.; Elliott, C.T.; Petchkongkaew, A. A 10-year analysis of RASFF notifications for mycotoxins in nuts. Trend in key mycotoxins and impacted countries. Food Res. Int. 2023, 172, 112915. [Google Scholar] [CrossRef]
- Fernández Pinto, V.; Patriarca, A.; Locani, O.; Vaamonde, G. Natural co-occurrence of aflatoxin and cyclopiazonic acid in peanuts grown in Argentina. Food Addit. Contam. 2001, 18, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Kujbida, P.; Maia, P.P.; de Araújo, A.N.; Mendes, L.D.; de Oliveira, M.L.; Silva-Rocha, W.P.; de Brito, G.Q.; Chaves, G.M.; Martins, I. Risk assessment of the occurrence of aflatoxin and fungi in peanuts and cashew nuts. Braz. J. Pharm. Sci. 2019, 55, e18135. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Chang, W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 2018, 88, 181–189. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.-M. Contamination of aflatoxins in different kinds of foods in China. Biomed. Environ. Sci. 2007, 20, 483–487. [Google Scholar] [PubMed]
- Huang, B.; Han, Z.; Cai, Z.; Wu, Y.; Ren, Y. Simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in peanuts and their derivative products by ultra-high-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2010, 662, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kamika, I.; Takoy, L.L. Natural occurrence of Aflatoxin B1 in peanut collected from Kinshasa, Democratic Republic of Congo. Food Control 2011, 22, 1760–1764. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.; Sharma, Y.P. Natural Incidence of Aflatoxins, Ochratoxin A, Patulin and Their Co-Occurrence in Chilgoza Pine Nuts Marketed in Jammu, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 45–50. [Google Scholar] [CrossRef]
- Singh, P.K.; Shukla, A. Survey of mycoflora counts, aflatoxin production and induced biochemical changes in walnut kernels. J. Stored Prod. Res. 2008, 44, 169–172. [Google Scholar] [CrossRef]
- Cheraghali, A.; Yazdanpanah, H.; Doraki, N.; Abouhossain, G.; Hassibi, M.; Ali-Abadi, S.; Aliakbarpoor, M.; Amirahmadi, M.; Askarian, A.; Fallah, N.; et al. Incidence of aflatoxins in Iran pistachio nuts. Food Chem. Toxicol. 2007, 45, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Diella, G.; Caggiano, G.; Ferrieri, F.; Ventrella, A.; Palma, M.; Napoli, C.; Rutigliano, S.; Lopuzzo, M.; Lovero, G.; Montagna, M.T. Aflatoxin contamination in nuts marketed in Italy: Preliminary results. Ann. Ig. Med. Prev. Comunità 2018, 30, 401–409. [Google Scholar] [CrossRef]
- Leong, Y.-H.; Ismail, N.; Latif, A.A.; Ahmad, R. Aflatoxin occurrence in nuts and commercial nutty products in Malaysia. Food Control 2010, 21, 334–338. [Google Scholar] [CrossRef]
- Juan, C.; Zinedine, A.; Moltó, J.; Idrissi, L.; Mañes, J. Aflatoxins levels in dried fruits and nuts from Rabat-Salé area, Morocco. Food Control 2008, 19, 849–853. [Google Scholar] [CrossRef]
- Adetunji, M.C.; Alika, O.P.; Awa, N.P.; Atanda, O.O.; Mwanza, M. Microbiological Quality and Risk Assessment for Aflatoxins in Groundnuts and Roasted Cashew Nuts Meant for Human Consumption. J. Toxicol. 2018, 2018, 1308748. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Shahid, M.; Sehar, M.; Malik, N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits, and nuty products. J. Food Saf. 2018, 38, e12462. [Google Scholar] [CrossRef]
- Chun, H.S.; Kim, H.J.; Ok, H.E.; Hwang, J.-B.; Chung, D.-H. Determination of aflatoxin levels in nuts and their products consumed in South Korea. Food Chem. 2007, 102, 385–391. [Google Scholar] [CrossRef]
- Siahi Shadbad, M.R.; Ansarin, M.; Tahavori, A.; Ghaderi, F.; Nemati, M. Determination of aflatoxins in nuts of Tabriz confectionaries by ELISA and HPLC methods. Adv. Pharm. Bull. 2012, 2, 123–126. [Google Scholar] [CrossRef]
- Hepsag, F.; Golge, O.; Kabak, B. Quantitation of aflatoxins in pistachios and groundnuts using HPLC-FLD method. Food Control 2014, 38, 75–81. [Google Scholar] [CrossRef]
- Kanik, T.; Kabak, B. Aflatoxins in almonds: Monitoring and exposure assessment. J. Food Saf. 2019, 39, e12646. [Google Scholar] [CrossRef]
- Bumbangi, N.F.; Muma, J.B.; Choongo, K.; Mukanga, M.; Velu, M.R.; Veldman, F.; Hatloy, A.; Mapatano, M.A. Occurrence and factors associated with aflatoxin contamination of raw peanuts from Lusaka district’s markets, Zambia. Food Control 2016, 68, 291–296. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Wei, B.; Feng, X. Recent Insights into Sample Pretreatment Methods for Mycotoxins in Different Food Matrices: A Critical Review on Novel Materials. Toxins 2023, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef]
- Ouakhssase, A.; Fatini, N.; Addi, E.A. A simple extraction method with no lipid removal for the determination of aflatoxins in almonds by liquid chromatography tandem-mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2021, 38, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruiz, J.L.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Determination of mycotoxins in nuts by ultra high-performance liquid chromatography-tandem mass spectrometry: Looking for a representative matrix. J. Food Compos. Anal. 2019, 82, 103228. [Google Scholar] [CrossRef]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S. Simple, Rapid, and Inexpensive Cleanup Method for Quantitation of Aflatoxins in Important Agricultural Products by HPLC. J. Agric. Food Chem. 2007, 55, 2136–2141. [Google Scholar] [CrossRef]
- Saito, K.; Ikeuchi, R.; Kataoka, H. Determination of ochratoxins in nuts and grain samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A 2012, 1220, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Amde, M.; Temsgen, A.; Dechassa, N. Ionic liquid functionalized zinc oxide nanorods for solid-phase microextraction of aflatoxins in food products. J. Food Compos. Anal. 2020, 91, 103528. [Google Scholar] [CrossRef]
- Fang, L.; Tian, M.; Yan, X.; Xiao, W. Isolation of Aflatoxin B1 from Moldy Foods by Solid-Phase Extraction Combined with Bifunctional Ionic Liquid-Based Silicas. J. Anal. Methods Chem. 2018, 2018, 8427580. [Google Scholar] [CrossRef] [PubMed]
- Rhemrev, R.; Pazdanska, M.; Marley, E.; Biselli, S.; Staiger, S. Automated Aflatoxin Analysis Using Inline Reusable Immunoaffinity Column Cleanup and LC-Fluorescence Detection. J. AOAC Int. 2015, 98, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Soriano, J.M.; Moltó, J.C.; Marín, R.; Mañes, J. Determination of aflatoxins in peanuts by matrix solid-phase dispersion and liquid chromatography. J. Chromatogr. A 2003, 1011, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Yang, Y.; Liu, Y.; Chen, X. Development of a deep eutectic solvent-based matrix solid phase dispersion methodology for the determination of aflatoxins in crops. Food Chem. 2019, 291, 239–244. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. A new approach in sample treatment combined with UHPLC-MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta 2013, 115, 61–67. [Google Scholar] [CrossRef]
- Vosough, M.; Bayat, M.; Salemi, A. Matrix-free analysis of aflatoxins in pistachio nuts using parallel factor modeling of liquid chromatography diode-array detection data. Anal. Chim. Acta 2010, 663, 11–18. [Google Scholar] [CrossRef]
- Stroka, J.; van Otterdijk, R.; Anklam, E. Immunoaffinity column clean-up prior to thin-layer chromatography for the determination of aflatoxins in various food matrices. J. Chromatogr. A 2000, 904, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Karami-Osboo, R.; Mirabolfathi, M. A Novel Dispersive Nanomagnetic Particle Solid-Phase Extraction Method to Determine Aflatoxins in Nut and Cereal Samples. Food Anal. Methods 2017, 10, 4086–4093. [Google Scholar] [CrossRef]
- Quigley, A.; Cummins, W.; Connolly, D. Dispersive Liquid-Liquid Microextraction in the Analysis of Milk and Dairy Products: A Review. J. Chem. 2016, 2016, 4040165. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Solid phase extraction as sample treatment for the determination of Ochratoxin A in foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3405–3420. [Google Scholar] [CrossRef]
- Bacaloni, A.; Cavaliere, C.; Cucci, F.; Foglia, P.; Samperi, R.; Laganà, A. Determination of aflatoxins in hazelnuts by various sample preparation methods and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1179, 182–189. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Yan, G.; Han, Y.; Sun, H. UHPLC-MS for the analytical characterization of traditional Chinese medicines. TrAC Trends Anal. Chem. 2014, 63, 180–187. [Google Scholar] [CrossRef]
- Dhanshetty, M.; Thorat, P.; Banerjee, K. High-Throughput Analysis of Aflatoxins in Cereals, Nuts, and Processed Products Involving Automated Immunoaffinity Cleanup and Inline HPLC–Fluorescence Detection. J. AOAC Int. 2021, 104, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Vaclavikova, M.; MacMahon, S.; Zhang, K.; Begley, T.H. Application of single immunoaffinity clean-up for simultaneous determination of regulated mycotoxins in cereals and nuts. Talanta 2013, 117, 345–351. [Google Scholar] [CrossRef]
- Karapınar, H.S.; Bilgiç, A. A new magnetic Fe3O4@SiO2@TiO2-APTMS-CPA adsorbent for simple, fast and effective extraction of aflatoxins from some nuts. J. Food Compos. Anal. 2022, 105, 104261. [Google Scholar] [CrossRef]
- Tsai, J.-J.; Lai, Y.-T.; Chen, Y.-C. Using Solid-Phase Microextraction Coupled with Reactive Carbon Fiber Ionization-Mass Spectrometry for the Detection of Aflatoxin B1 from Complex Samples. Separations 2022, 9, 199. [Google Scholar] [CrossRef]
- Rezaee, M.; Khalilian, F.; Mashayekhi, H.A.; Fattahi, N. A novel method for the high preconcentration of trace amounts of the aflatoxins in pistachios by dispersive liquid–liquid microextraction after solid-phase extraction. Anal. Methods 2014, 6, 3456–3461. [Google Scholar] [CrossRef]
- Zhu, A.; Jiao, T.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q. Dispersive micro solid phase extraction based ionic liquid functionalized ZnO nanoflowers couple with chromatographic methods for rapid determination of aflatoxins in wheat and peanut samples. Food Chem. 2022, 391, 133277. [Google Scholar] [CrossRef]
- Taherimaslak, Z.; Amoli-Diva, M.; Allahyari, M.; Pourghazi, K.; Manafi, M.H. Low density solvent based dispersive liquid–liquid microextraction followed by vortex-assisted magnetic nanoparticle based solid-phase extraction and surfactant enhanced spectrofluorimetric detection for the determination of aflatoxins in pistachio nuts. RSC Adv. 2015, 5, 12747–12754. [Google Scholar] [CrossRef]
- Karapınar, H.S.; Balıkçıoğlu, A. Boron-doped activated carbon nanocomposite as a selective adsorbent for rapid extraction of aflatoxins in nut samples. J. Food Compos. Anal. 2022, 112, 104680. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Ahmadpoor, F.; Nasrollahzadeh, M.; Maham, M. Polydopamine-coated magnetic Spirulina nanocomposite for efficient magnetic dispersive solid-phase extraction of aflatoxins in pistachio. Food Chem. 2022, 377, 131967. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Nez, O.; Gallart-Ayala, H.; Martins, C.P.; Lucci, P. New trends in fast liquid chromatography for food and environmental analysis. J. Chromatogr. A 2012, 1228, 298–323. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Barros, S.; Pena, A.; Silva, A.S. Development and Validation of QuEChERS Followed by UHPLC-ToF-MS Method for Determination of Multi-Mycotoxins in Pistachio Nuts. Molecules 2021, 26, 5754. [Google Scholar] [CrossRef]
- Alcántara-Durán, J.; Moreno-González, D.; García-Reyes, J.F.; Molina-Díaz, A. Use of a modified QuEChERS method for the determination of mycotoxin residues in edible nuts by nano flow liquid chromatography high resolution mass spectrometry. Food Chem. 2019, 279, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Zamora, C.; González-Sálamo, J.; Hernández-Borges, J. Deep Eutectic Solvents Application in Food Analysis. Molecules 2021, 26, 6846. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Zentek, J.; Razzazi, E. Review on sample preparation strategies and methods used for the analysis of aflatoxins in food and feed. Mol. Nutr. Food Res. 2009, 53, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A. Matrix solid phase dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [Google Scholar] [CrossRef]
- Boyacı, E.; Rodríguez-Lafuente, Á.; Gorynski, K.; Mirnaghi, F.; Souza-Silva, É.A.; Hein, D.; Pawliszyn, J. Sample preparation with solid phase microextraction and exhaustive extraction approaches: Comparison for challenging cases. Anal. Chim. Acta 2015, 873, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Marcinkowski, Ł.; De La Guardia, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13. [Google Scholar] [CrossRef]
- Pereira, V.; Fernandes, J.; Cunha, S. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Pascale, M. Detection methods for mycotoxins in cereal grains and cereal products. Zb Mat Srp Prir Nauk 2009, 117, 15–25. [Google Scholar] [CrossRef]
- Magoke, G.; Alders, R.; Krockenberger, M.; Bryden, W.L. Aflatoxin and Mycotoxin Analysis: An Overview Including Options for Resource-limited Settings. In Aflatoxins-Occurrence, Detection and Novel Detoxification Strategies; Claude Assaf, J., Ed.; IntechOpen: Dekouaneh, Lebanon, 2022. [Google Scholar]
- Malik, A.K.; Blasco, C.; Picó, Y. Liquid chromatography–mass spectrometry in food safety. J. Chromatogr. A 2010, 1217, 4018–4040. [Google Scholar] [CrossRef] [PubMed]
- Vestal, M.L. High-performance liquid chromatography-mass spectrometry. Science 1984, 226, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kos, G.; Sieger, M.; McMullin, D.; Zahradnik, C.; Sulyok, M.; Öner, T.; Mizaikoff, B.; Krska, R. A novel chemometric classification for FTIR spectra of mycotoxin-contaminated maize and peanuts at regulatory limits. Food Addit. Contam. Part A 2016, 33, 1596–1607. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Aflatoxin monitoring in Italian hazelnut products by LC-MS. Food Addit. Contam. Part B 2012, 5, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Malachová, A.; Stránská, M.; Václavíková, M.; Elliott, C.T.; Black, C.; Meneely, J.; Hajšlová, J.; Ezekiel, C.N.; Schuhmacher, R.; Krska, R. Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018, 410, 801–825. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Cervino, C.; Asam, S.; Knopp, D.; Rychlik, M.; Niessner, R. Use of Isotope-Labeled Aflatoxins for LC-MS/MS Stable Isotope Dilution Analysis of Foods. J. Agric. Food Chem. 2008, 56, 1873–1879. [Google Scholar] [CrossRef]
- Xavier, J.J.M.; Scussel, V.M. Development of an LC-MS/MS method for the determination of aflatoxins B1, B2, G1, and G2 in Brazil nut. Int. J. Environ. Anal. Chem. 2008, 88, 425–433. [Google Scholar] [CrossRef]
- Sataque Ono, E.Y.; Hirozawa, M.T.; Omori, A.M.; De Oliveira, A.J.; Ono, M.A. Mycotoxins in Nuts and Seeds. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 255–270. [Google Scholar]
- Oyedele, O.A.; Ezekiel, C.N.; Sulyok, M.; Adetunji, M.C.; Warth, B.; Atanda, O.O.; Krska, R. Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 2017, 251, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of liquid chromatography–mass spectrometry for food analysis. J. Chromatogr. A 2012, 1259, 74–85. [Google Scholar] [CrossRef]
- Righetti, L.; Paglia, G.; Galaverna, G.; Dall’Asta, C. Recent Advances and Future Challenges in Modified Mycotoxin Analysis: Why HRMS Has Become a Key Instrument in Food Contaminant Research. Toxins 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-D.; Wong, J.W.; Zhang, K.; Hayward, D.G.; Lee, N.S.; Trucksess, M.W. Multi-mycotoxin Analysis of Finished Grain and Nut Products Using High-Performance Liquid Chromatography–Triple-Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2013, 61, 4771–4782. [Google Scholar] [CrossRef]
- Frenich, A.G.; Romero-González, R.; Del Mar Aguilera-Luiz, M. Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. TrAC Trends Anal. Chem. 2014, 63, 158–169. [Google Scholar] [CrossRef]
- Kafouris, D.; Christofidou, M.; Christodoulou, M.; Christou, E.; Ioannou-Kakouri, E. A validated UPLC-MS/MS multi-mycotoxin method for nuts and cereals: Results of the official control in Cyprus within the EU requirements. Food Agric. Immunol. 2017, 28, 90–108. [Google Scholar] [CrossRef]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.S.; Rodriguez, J.A.; Galán-Vidal, C.A.; Cepeda, A.; Miranda, J.M. Magnetic Solid Phase Extraction Applied to Food Analysis. J. Chem. 2015, 2015, 919414. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.-S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632. [Google Scholar] [CrossRef]
- Ozay, G.; Seyhan, F.; Pembeci, C.; Saklar, S.; Yilmaz, A. Factors influencing fungal and aflatoxin levels in Turkish hazelnuts (Corylus avellana L.) during growth, harvest, drying and storage: A 3-year study. Food Addit. Contam. Part A 2008, 25, 209–218. [Google Scholar] [CrossRef]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of Mycotoxins in Food: A Review of Bioanalytical to Analytical Methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A Review of Rapid Methods for the Analysis of Mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Marrubini, G.; Brusotti, G.; Massolini, G.; Caccialanza, G. Development and integration of an immunoaffinity monolithic disk for the on-line solid-phase extraction and HPLC determination with fluorescence detection of aflatoxin B1 in aqueous solutions. J. Pharm. Biomed. Anal. 2007, 44, 396–403. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Zhang, W.; Zhang, J.; Chen, X.; Jiang, J.; Xie, L.; Zhang, D. Development of a class-specific monoclonal antibody-based ELISA for aflatoxins in peanut. Food Chem. 2009, 115, 313–317. [Google Scholar] [CrossRef]
- Yao, H.; Hruska, Z.; Di Mavungu, J.D. Developments in detection and determination of aflatoxins. World Mycotoxin J. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Q.; Wang, T.; Zhou, H.; Zhang, W.; Ding, X.; Zhang, Z.; Chang, P.-K.; Zhang, Q. Development of an Enzyme-Linked Immunosorbent Assay Method Specific for the Detection of G-Group Aflatoxins. Toxins 2015, 8, 5. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Sajic, N.; Xu, Y.; Haughey, S.A.; Mooney, M.H.; Gong, Y.Y.; Verheijen, R.; Elliott, C.T. Fast and sensitive aflatoxin B1 and total aflatoxins ELISAs for analysis of peanuts, maize and feed ingredients. Food Control 2016, 63, 239–245. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; De Saeger, S.; Eremin, S.A.; Van Peteghem, C. Immunochemical methods for rapid mycotoxin detection: Evolution from single to multiple analyte screening: A review. Food Addit. Contam. 2007, 24, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Zhang, Z.; Ding, X.; Tang, X. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013, 49, 426–432. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, P.; Zhang, Q.; Zhang, W. Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts. Biosens. Bioelectron. 2011, 26, 2877–2882. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Zhang, Z.; Zhang, Q.; Zhang, W.; Yu, L.; Jiang, J.; Chen, X.; Wang, X.; Li, P. Simultaneous Lateral Flow Immunoassay for Multi-Class Chemical Contaminants in Maize and Peanut with One-Stop Sample Preparation. Toxins 2019, 11, 56. [Google Scholar] [CrossRef]
- Aït Mimoune, N.; Riba, A.; Verheecke, C.; Sabaou, N. Fungal contamination and mycotoxin production by Aspergillus spp. in nuts and sesame seeds. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 301–305. [Google Scholar] [CrossRef]
- Asis, R.; Di Paola, R.D.; Aldao, M.A.J. Determination of Aflatoxin B 1 in Highly Contaminated Peanut Samples Using HPLC and ELISA. Food Agric. Immunol. 2002, 14, 201–208. [Google Scholar] [CrossRef]
- Razzazi-Fazeli, E.; Noviandi, C.T.; Porasuphatana, S.; Agus, A.; Böhm, J. A survey of aflatoxin B1 and total aflatoxin contamination in baby food, peanut and corn products sold at retail in Indonesia analysed by ELISA and HPLC. Mycotoxin Res. 2004, 20, 51–58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).