Functionalization of Violet Phosphorus Quantum Dots with Azo-Containing Star-Shape Polymer for Optically Controllable Memory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
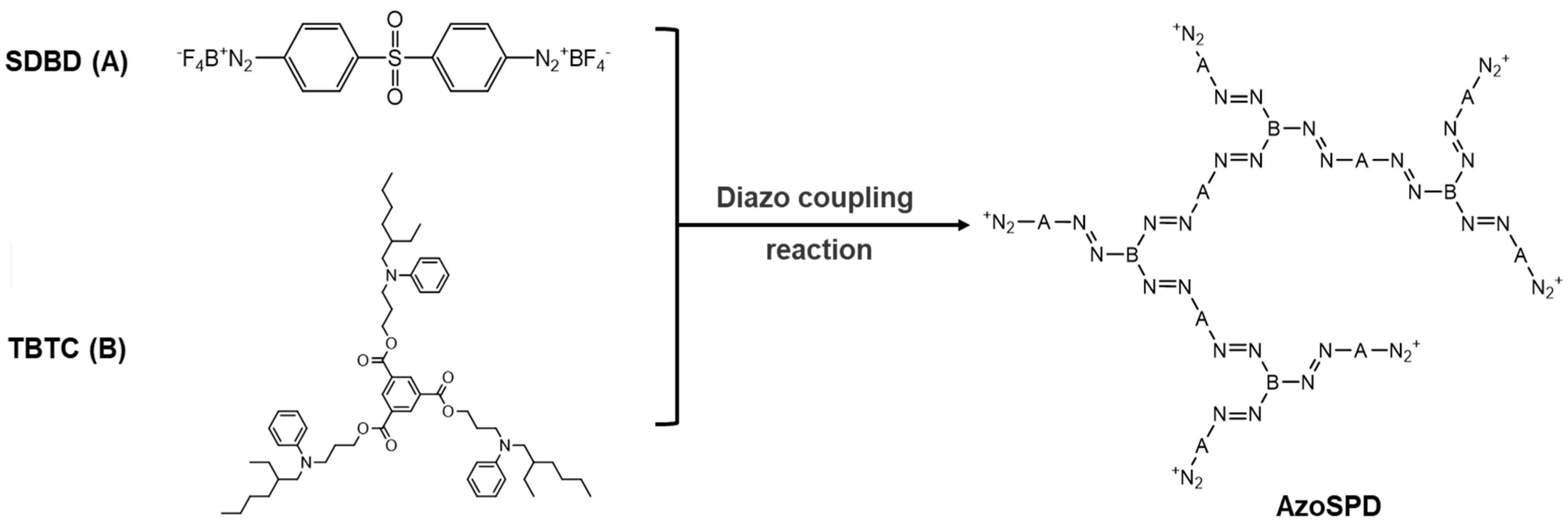
2.2. Synthesis of Modified Molecule AzoSPD
2.2.1. Synthesis of Monomer SDBD
2.2.2. Synthesis of Monomer TBTC
2.2.3. Synthesis of the Star-Shaped Polymer Diazonium Salt Rich in Azobenzene Structure (AzoSPD)
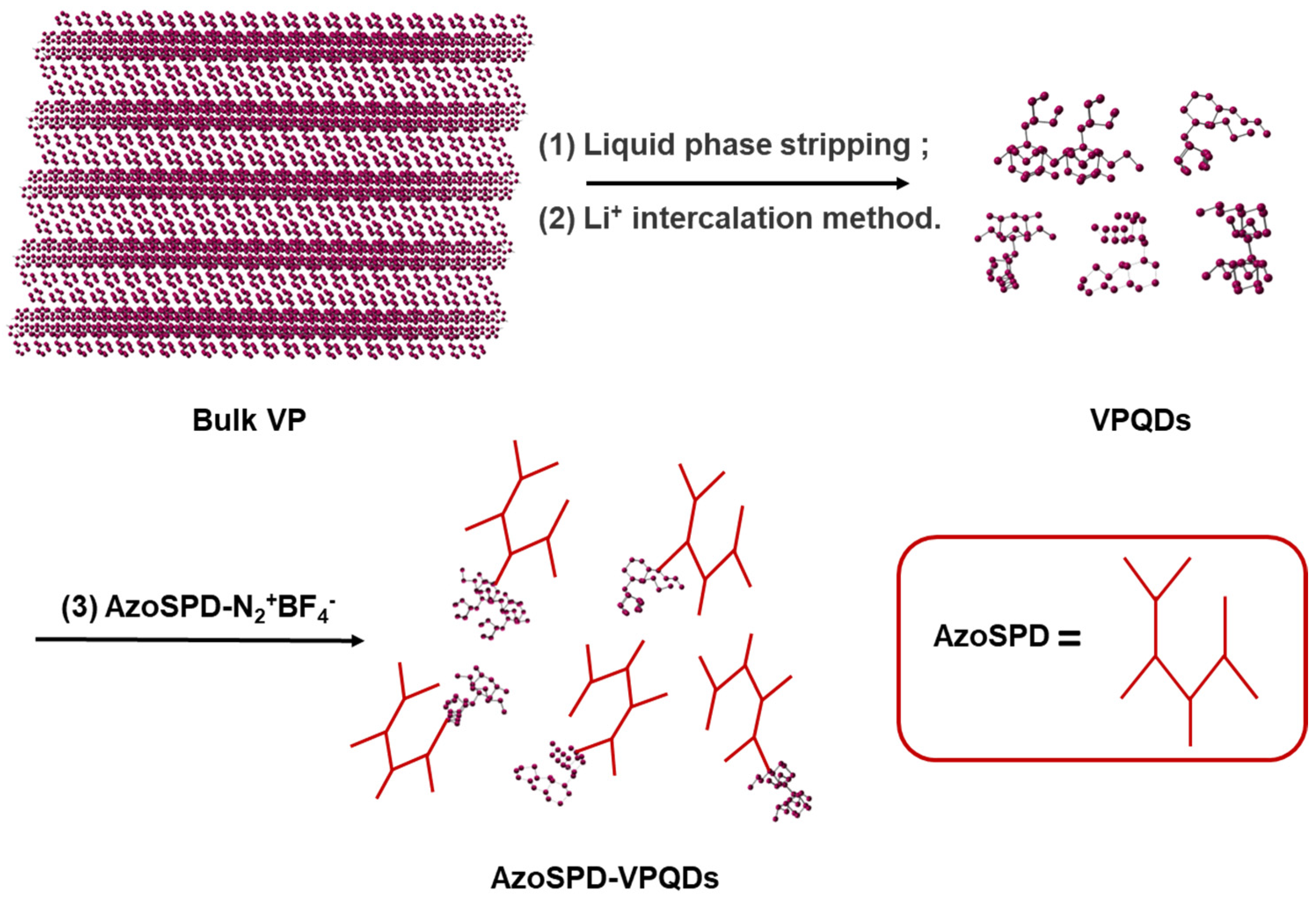
2.3. Preparation and Modification of Violet Phosphorus Quantum Dots (VPQDs)
2.3.1. Stripping of VPQDs
2.3.2. Modification of VPQDs
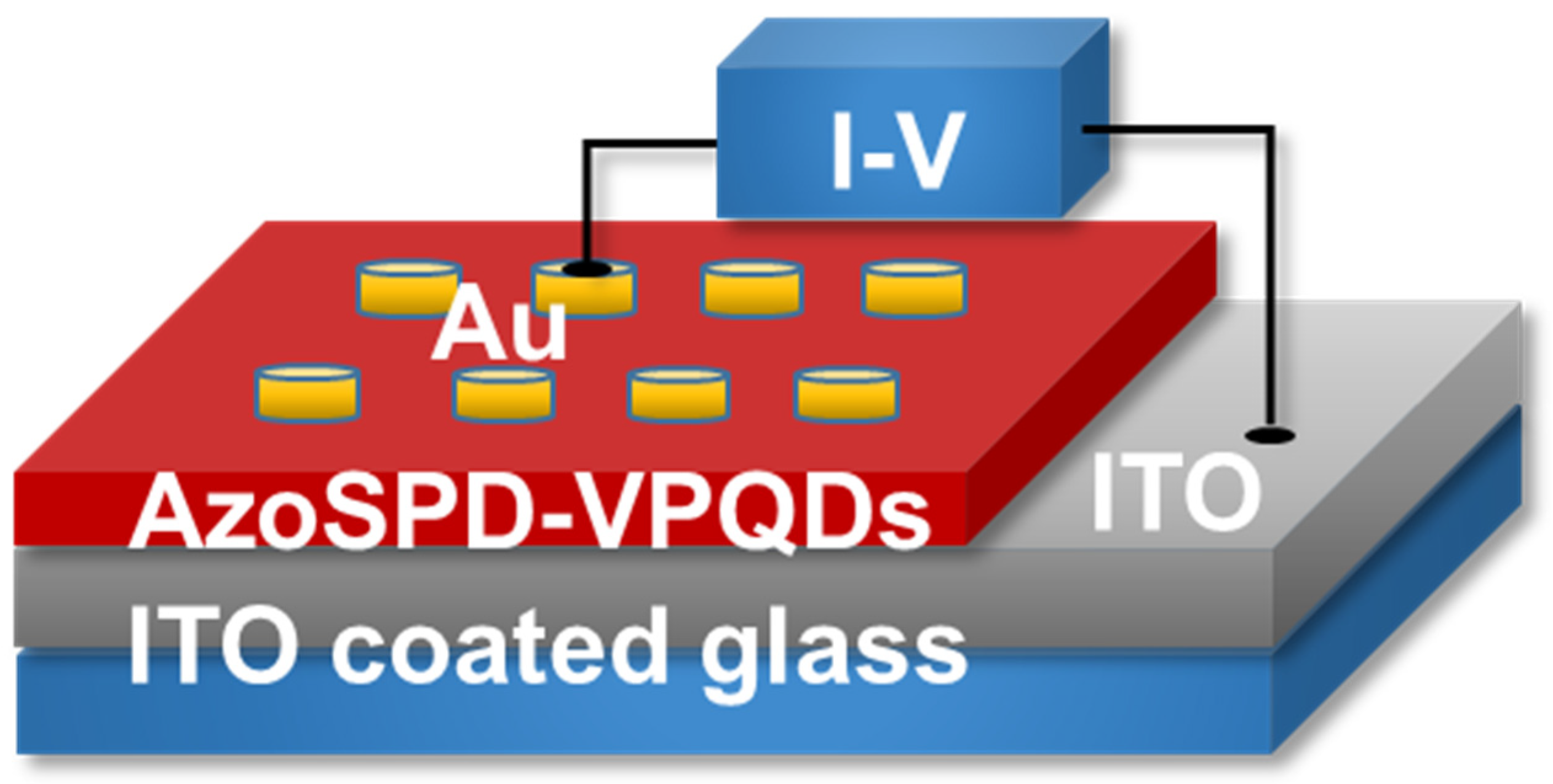
2.4. Device Structure and Fabrication
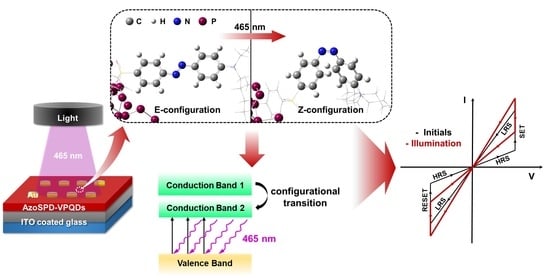
3. Results and Discussion
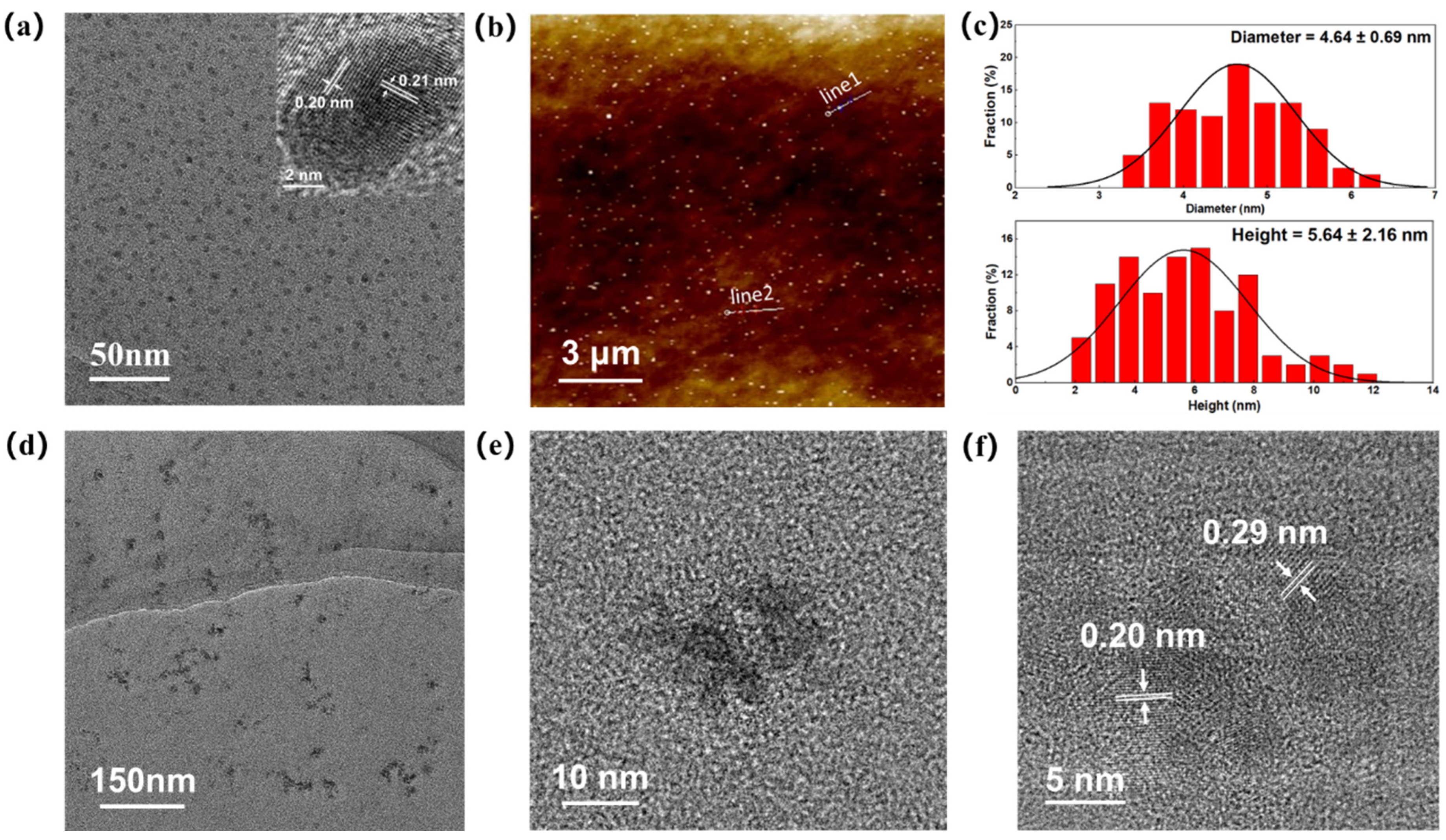
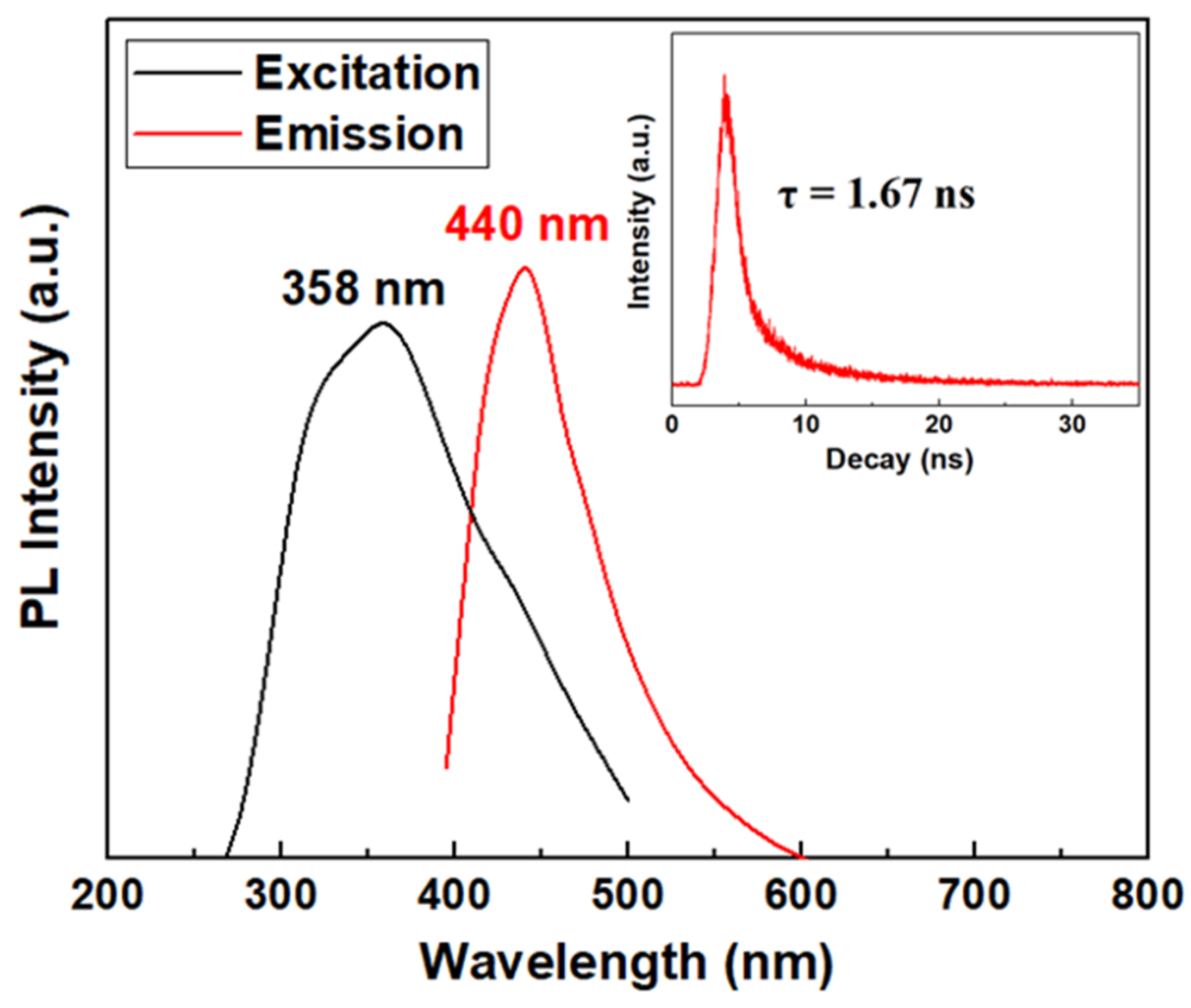
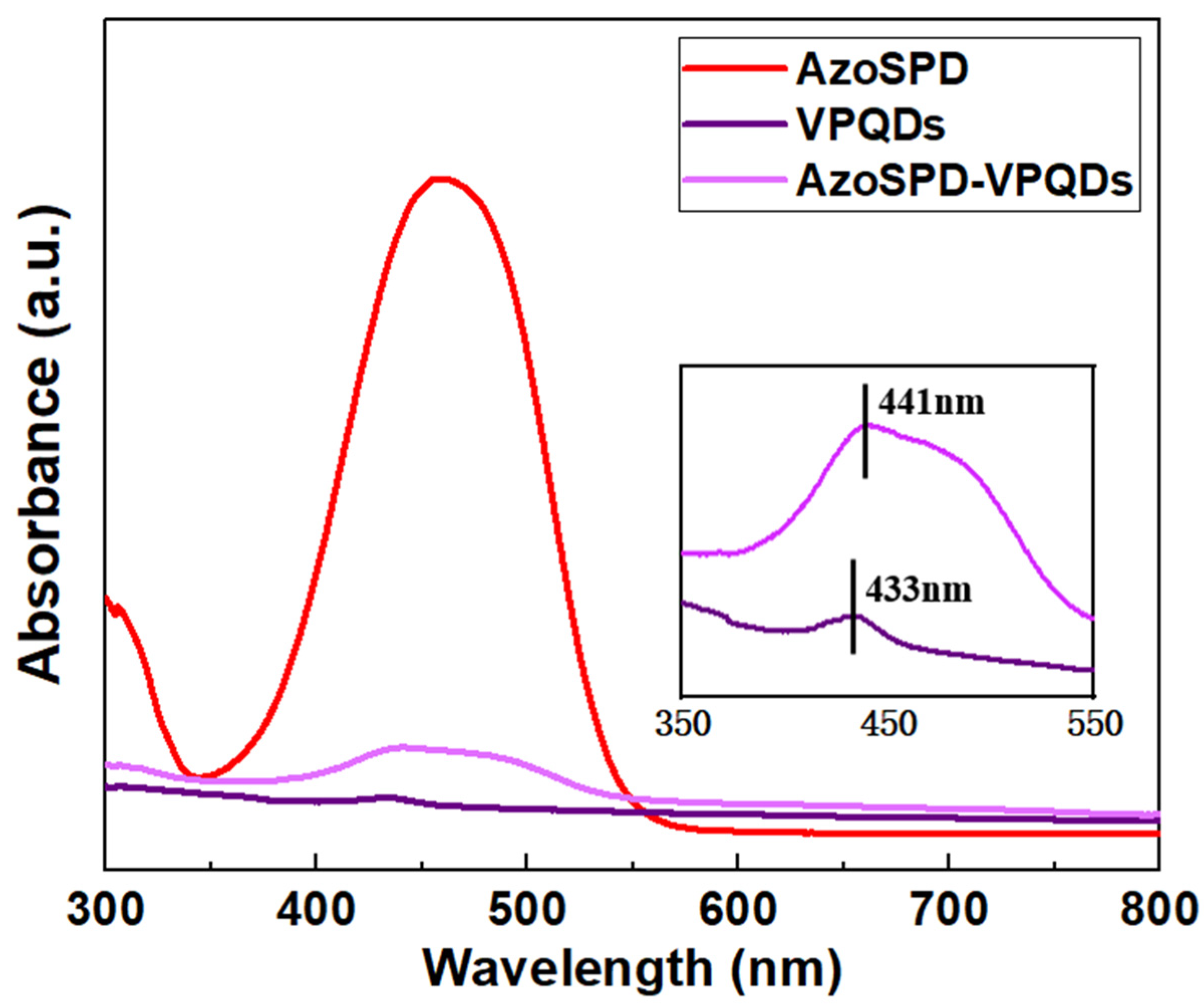
3.1. Characterization of the VPQDs and AzoSPD–VPQDs
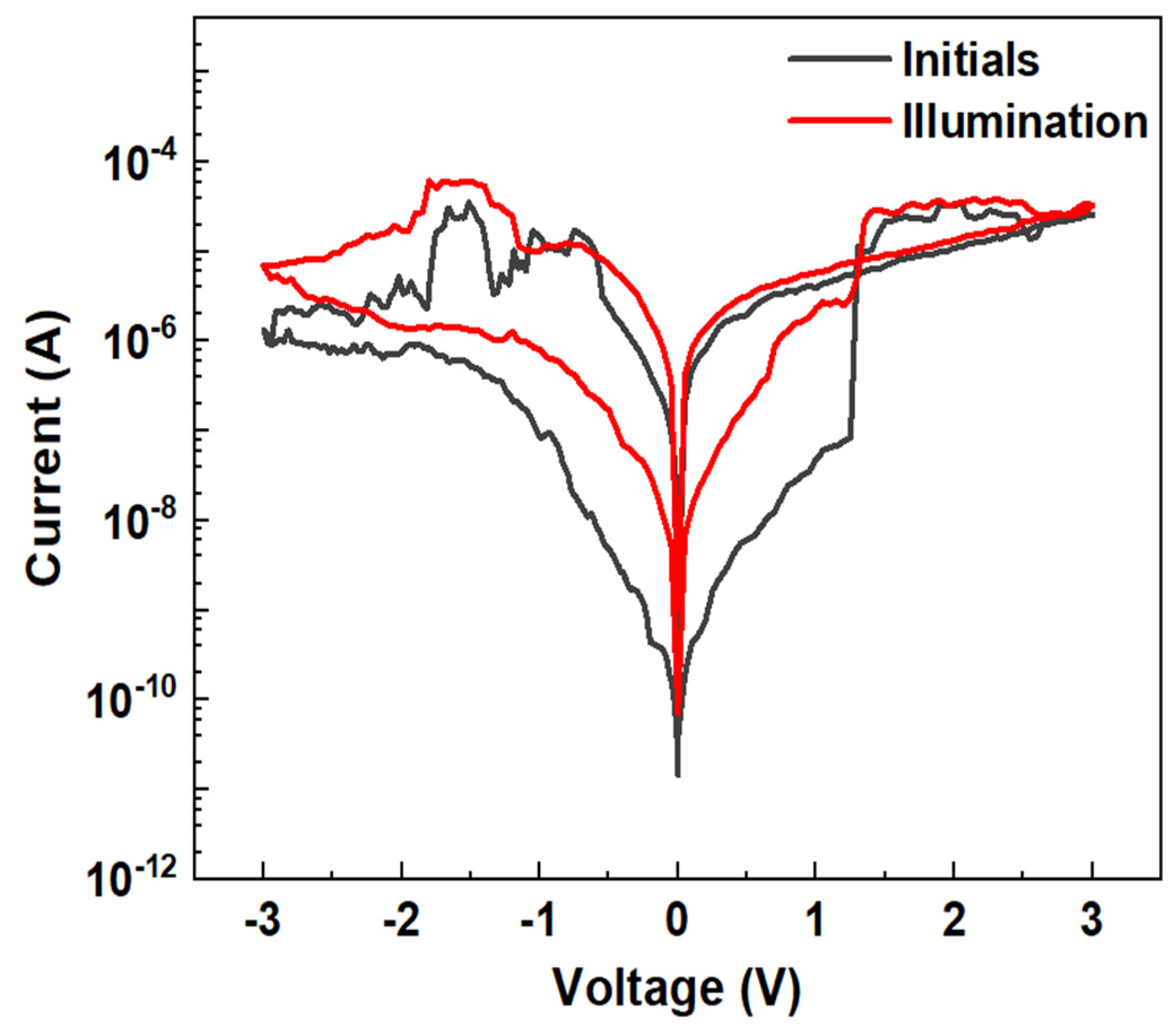
3.2. Current–Voltage Characteristics and Performance Parameters
3.3. Photo Response Characteristics
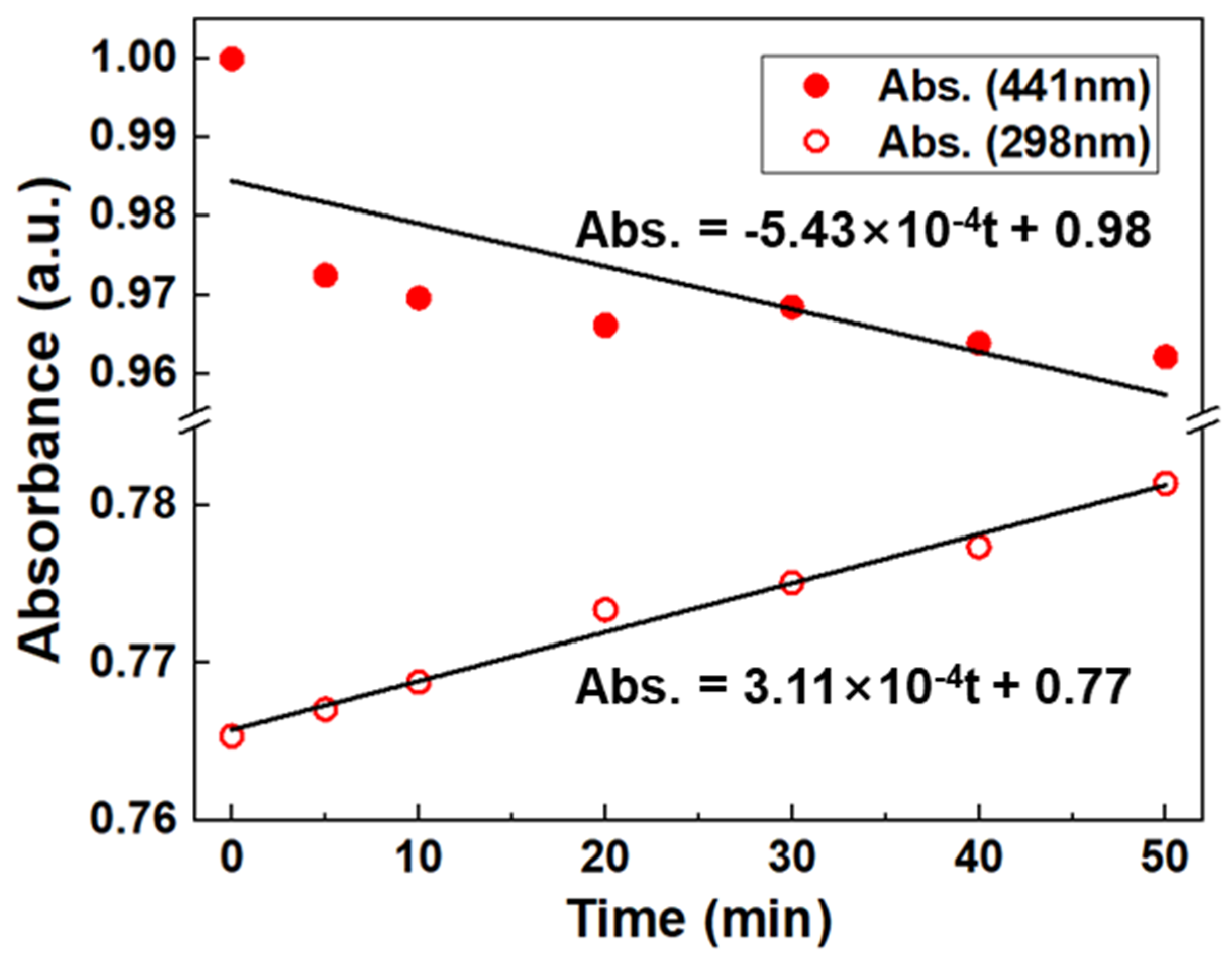
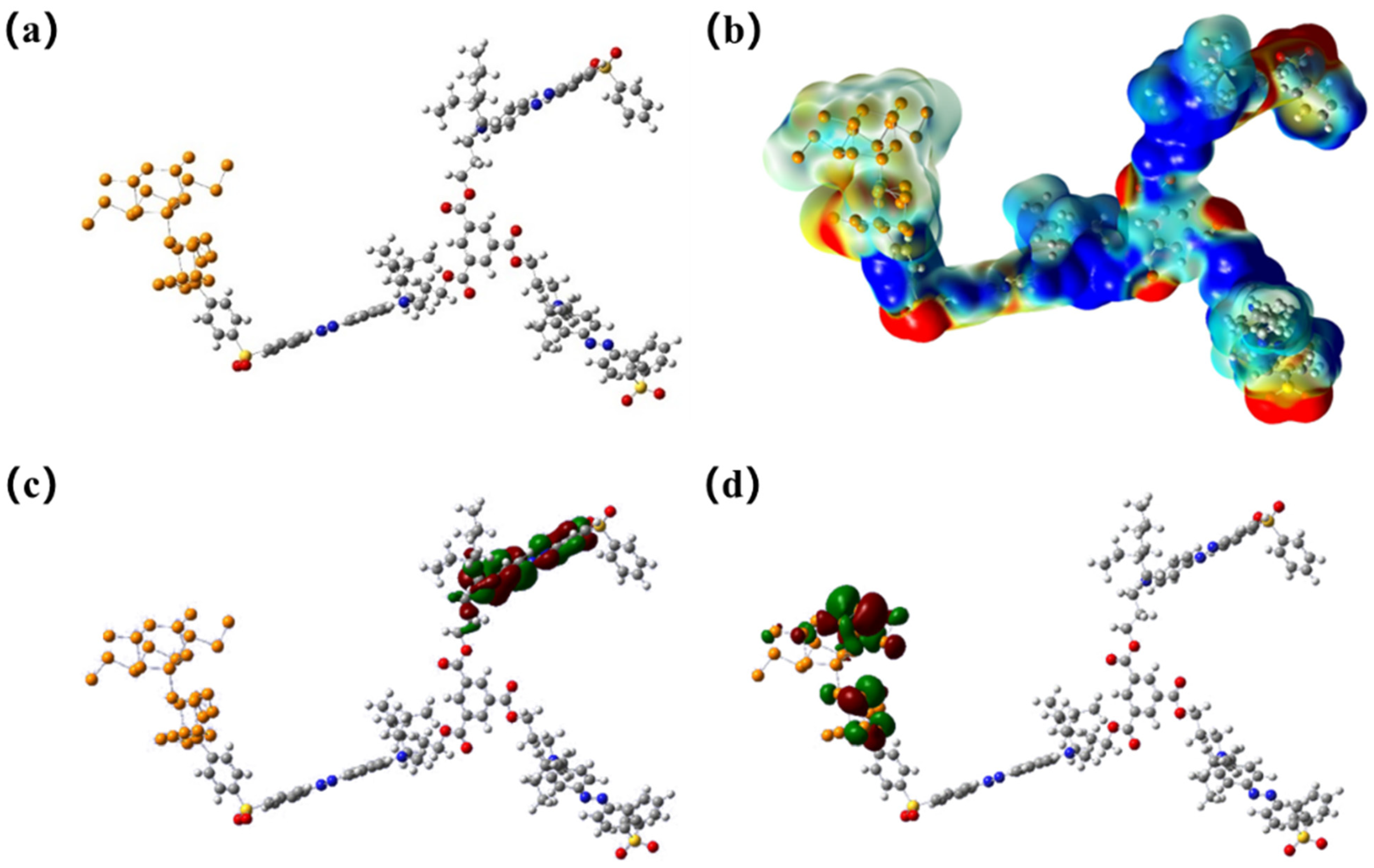
3.4. Mechanism Explanation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, K.; Shang, G.; Hsu, H.; Han, S.; Roy, V.A.L.; Zhou, Y. Emerging 2D Metal Oxides: From Synthesis to Device Integration. Adv. Mater. 2023, 35, 2207774. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Lee, H.-S.; Bergeron, H.; Balla, I.; Beck, M.E.; Chen, K.-S.; Hersam, M.C. Multi-Terminal Memtransistors from Polycrystalline Monolayer Molybdenum Disulfide. Nature 2018, 554, 500–504. [Google Scholar] [CrossRef] [PubMed]
- García De Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pei, Y.; Chen, H.; Zhao, J.; Zhou, Z.; Wang, H.; Zhang, L.; Wang, J.; Li, X.; Qin, C.; et al. Self-Assembled Networked PbS Distribution Quantum Dots for Resistive Switching and Artificial Synapse Performance Boost of Memristors. Adv. Mater. 2019, 31, 1805284. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, W.; Tong, Y.; Zhang, Y.; Huang, K.; Song, L.; Zhong, S.; Ganeshkumar, R.; Zhao, R. Memristive Devices with Highly Repeatable Analog States Boosted by Graphene Quantum Dots. Small 2017, 13, 1603435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Liu, X.; Lin, J.; Chen, J.-Y.; Wu, W.-W.; Wei, J.; Liu, Y.; Liu, Q.; Liao, L. High on/off Ratio Black Phosphorus Based Memristor with Ultra-Thin Phosphorus Oxide Layer. Appl. Phys. Lett. 2019, 115, 193503. [Google Scholar] [CrossRef]
- Han, S.-T.; Hu, L.; Wang, X.; Zhou, Y.; Zeng, Y.-J.; Ruan, S.; Pan, C.; Peng, Z. Black Phosphorus Quantum Dots with Tunable Memory Properties and Multilevel Resistive Switching Characteristics. Adv. Sci. 2017, 4, 1600435. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, B.; Tian, X.; Gu, M.; Chen, Y. Direct Covalent Modification of Black Phosphorus Quantum Dots with Conjugated Polymers for Information Storage. Nanoscale 2019, 11, 3527–3533. [Google Scholar] [CrossRef]
- He, C.L.; Zhuge, F.; Zhou, X.F.; Li, M.; Zhou, G.C.; Liu, Y.W.; Wang, J.Z.; Chen, B.; Su, W.J.; Liu, Z.P.; et al. Nonvolatile Resistive Switching in Graphene Oxide Thin Films. Appl. Phys. Lett. 2009, 95, 232101. [Google Scholar] [CrossRef]
- Rehman, M.M.; Siddiqui, G.U.; Gul, J.Z.; Kim, S.-W.; Lim, J.H.; Choi, K.H. Resistive Switching in All-Printed, Flexible and Hybrid MoS2-PVA Nanocomposite Based Memristive Device Fabricated by Reverse Offset. Sci. Rep. 2016, 6, 36195. [Google Scholar] [CrossRef]
- Qian, K.; Tay, R.Y.; Nguyen, V.C.; Wang, J.; Cai, G.; Chen, T.; Teo, E.H.T.; Lee, P.S. Hexagonal Boron Nitride Thin Film for Flexible Resistive Memory Applications. Adv. Funct. Mater. 2016, 26, 2176–2184. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, B.; Ge, Y.; Zhu, Y.; Nie, G.; Song, Y.; Lim, C.-K.; Zhang, H.; Prasad, P.N. Chemistry, Functionalization, and Applications of Recent Monoelemental Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2022, 122, 1127–1207. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H.; Zhang, B.; Gu, M.; Zhao, D.; Zhao, X.; Li, L.; Zhou, J.; Wu, K.; Cheng, Y.; et al. Structure and Properties of Violet Phosphorus and Its Phosphorene Exfoliation. Angew. Chem. Intl. Edit. 2020, 59, 1074–1080. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Li, H.; Xu, X.; Yang, J.; Huo, Z.; Wang, L.; Zhang, W.; Xiao, X.; Ma, Y. Recent Advances in Surface Modifications of Elemental Two-Dimensional Materials: Structures, Properties, and Applications. Molecules 2022, 28, 200. [Google Scholar] [CrossRef]
- Liao, K.; Lei, P.; Tu, M.; Luo, S.; Jiang, T.; Jie, W.; Hao, J. Memristor Based on Inorganic and Organic Two-Dimensional Materials: Mechanisms, Performance, and Synaptic Applications. ACS Appl. Mater. Interfaces 2021, 13, 32606–32623. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, X.; Gu, J.; Liu, B.; Zhang, B.; Song, S.; Fan, F.; Chen, Y. Covalent Functionalization of Black Phosphorus with Conjugated Polymer for Information Storage. Angew. Chem. Int. Ed. 2018, 57, 4543–4548. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, P.; Zhang, T.; Zhu, X.; Zhang, M.; Chen, M.; Du, P.; Wang, G.; Ji, H.; Yang, J.; et al. Azide Passivation of Black Phosphorus Nanosheets: Covalent Functionalization Affords Ambient Stability Enhancement. Angew. Chem. Int. Ed. 2019, 58, 1479–1483. [Google Scholar] [CrossRef]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent Functionalization and Passivation of Exfoliated Black Phosphorus via Aryl Diazonium Chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef]
- Hamon, F.; Djedaini-Pilard, F.; Barbot, F.; Len, C. Azobenzenes—Synthesis and Carbohydrate Applications. Tetrahedron 2009, 65, 10105–10123. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.; Fan, F.; Zhang, Z.; Han, T.; He, Z.; Wu, Z.; Yu, Z.; Gao, P.; Chen, D.; et al. A Dual-Mode Organic Memristor for Coordinated Visual Perceptive Computing. Fundam. Res. 2022, S266732582200303X. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.; Yang, J.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-Birds-One-Stone: Multifunctional Supercapacitors beyond Traditional Energy Storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, X.; Xu, X.; Zhang, Y.; Wang, Y.; Jin, M.; Liu, Z.; Cheng, Y.; Zheng, H.; Zhang, J. Wide Bandgap Tuning of Violet Phosphorus Quantum Dots by Functionalization. J. Phys. Chem. Lett. 2022, 13, 8236–8244. [Google Scholar] [CrossRef]
- Ali, M.; Sokolov, A.; Ko, M.J.; Choi, C. Optically Excited Threshold Switching Synapse Characteristics on Nitrogen-Doped Graphene Oxide Quantum Dots (N-GOQDs). J. Alloy Compd. 2021, 855, 157514. [Google Scholar] [CrossRef]
- Sokolov, A.; Ali, M.; Li, H.; Jeon, Y.; Ko, M.J.; Choi, C. Partially Oxidized MXene Ti3C2Tx Sheets for Memristor Having Synapse and Threshold Resistive Switching Characteristics. Adv. Elect. Mater. 2021, 7, 2000866. [Google Scholar] [CrossRef]
- Sokolov, A.S.; Ali, M.; Riaz, R.; Abbas, Y.; Ko, M.J.; Choi, C. Silver-Adapted Diffusive Memristor Based on Organic Nitrogen-Doped Graphene Oxide Quantum Dots (N-GOQDs) for Artificial Biosynapse Applications. Adv. Funct. Mater. 2019, 29, 1807504. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, S.; Zhao, X.; Cheng, Y.; Zhang, J. Violet Phosphorus Quantum Dots as Distinguishable Environmental Biosensors. Adv. Mater. Inter. 2022, 9, 2200705. [Google Scholar] [CrossRef]
- Huh, W.; Lee, D.; Lee, C. Memristors Based on 2D Materials as an Artificial Synapse for Neuromorphic Electronics. Adv. Mater. 2020, 32, 2002092. [Google Scholar] [CrossRef]
- Ge, R.; Wu, X.; Kim, M.; Shi, J.; Sonde, S.; Tao, L.; Zhang, Y.; Lee, J.C.; Akinwande, D. Atomristor: Nonvolatile Resistance Switching in Atomic Sheets of Transition Metal Dichalcogenides. Nano Lett. 2018, 18, 434–441. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, H.; Deng, C.; Lv, T.; Hu, S.; Wu, H.; Xue, S.; Tao, Y.; Deng, L.; Xiong, W. An Ultrathin Memristor Based on a Two-Dimensional WS2/MoS2 Heterojunction. Nanoscale 2021, 13, 11497–11504. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, S.; Xie, Z.; Lu, Y.; Gong, G.; Liu, G.; Shang, J.; Yao, C.; Li, R.-W. Anti-Oxidative Passivation and Electrochemical Activation of Black Phosphorus via Covalent Functionalization and Its Nonvolatile Memory Application. J. Mater. Chem. C 2020, 8, 7309–7313. [Google Scholar] [CrossRef]
- Ren, S.; Li, Z.; Liu, X.; Li, Y.; Cao, G.; Zhao, J. Oxygen Migration Induced Effective Magnetic and Resistive Switching Boosted by Graphene Quantum Dots. J. Alloys Compd. 2021, 863, 158339. [Google Scholar] [CrossRef]
- Bokka, N.; Adepu, V.; Selamneni, V.; Sahatiya, P. Non-Contact, Controlled and Moisture Triggered Black Phosphorus Quantum Dots/PVA Film for Transient Electronics Applications. Mater. Lett. 2021, 290, 129477. [Google Scholar] [CrossRef]
- Wang, J.; Lv, Z.; Xing, X.; Li, X.; Wang, Y.; Chen, M.; Pang, G.; Qian, F.; Zhou, Y.; Han, S.-T. Optically Modulated Threshold Switching in Core–Shell Quantum Dot Based Memristive Device. Adv. Funct. Mater. 2020, 30, 1909114. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, F.; Chen, W.; Liu, G. Functionalization of Violet Phosphorus Quantum Dots with Azo-Containing Star-Shape Polymer for Optically Controllable Memory. Processes 2023, 11, 3429. https://doi.org/10.3390/pr11123429
Shu F, Chen W, Liu G. Functionalization of Violet Phosphorus Quantum Dots with Azo-Containing Star-Shape Polymer for Optically Controllable Memory. Processes. 2023; 11(12):3429. https://doi.org/10.3390/pr11123429
Chicago/Turabian StyleShu, Fan, Weilin Chen, and Gang Liu. 2023. "Functionalization of Violet Phosphorus Quantum Dots with Azo-Containing Star-Shape Polymer for Optically Controllable Memory" Processes 11, no. 12: 3429. https://doi.org/10.3390/pr11123429
APA StyleShu, F., Chen, W., & Liu, G. (2023). Functionalization of Violet Phosphorus Quantum Dots with Azo-Containing Star-Shape Polymer for Optically Controllable Memory. Processes, 11(12), 3429. https://doi.org/10.3390/pr11123429