Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Electrodes
2.3. Reagents and Solutions
2.4. Methodology
3. Results and Discussions
3.1. Electrochemical Responses of SPEs in 10−1 M KCl Solution
3.2. Electrochemical Responses of SPCEs in 10−3 M K4[Fe(CN)6]–10−1 M KCl Solution
3.3. Electrochemical Responses of SPCEs Modified in 10−3 M Phe–10−1 M KCl Solution
- Γ—surface coverage, mol × cm−2;
- Ipa—the current of the peak, A;
- A—electrode surface, cm2;
- n—the number of electrons transferred during redox processes, 4 for peak pair I;
- F—Faraday’s constant, 96,485 C × mol−1;
- R—universal gas constant, 8.314 J × mol−1 × K−1;
- T—absolute temperature, K;
3.4. Influence of Phe Concentration on the Voltammetric Response of PB-SPCE
3.5. Reproducibility, Stability, and Interference Studies
3.6. Quantitative Determination of Phe in Pharmaceuticals Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Carmona, L.; González, M.C.; Escarpa, A. Nanomaterial-based electrochemical (bio)-sensing: One step ahead in diagnostic and monitoring of metabolic rare diseases. TrAC Trends Anal. Chem. 2019, 118, 29–42. [Google Scholar] [CrossRef]
- Pavan, S.; Rommel, K.; Mateo Marquina, M.E.; Höhn, S.; Lanneau, V.; Rath, A. Clinical practice guidelines for rare diseases: The orphanet database. PLoS ONE 2017, 12, e0170365. [Google Scholar] [CrossRef] [PubMed]
- Jumbo-Lucioni, P.P.; Garber, K.; Kiel, J.; Baric, I.; Berry, G.T.; Bosch, A.; Burlina, A.; Chiesa, A.; Pico, M.L.C.; Estrada, S.C. Diversity of approaches to classic galactosemia around the world: A comparison of diagnosis, intervention, and outcomes. J. Inherit. Metab. Dis. 2012, 35, 1037–1049. [Google Scholar] [CrossRef]
- Burrage, L.C.; Nagamani, S.C.; Campeau, P.M.; Lee, B.H. Branched-chain amino acid metabolism: From rare Mendelian diseases to more common disorders. Hum. Mol. Genet. 2014, 23, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors 2020, 20, 2496. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; Hennermann, J.B.; Langenbeck, U.; Lichter-Konecki, U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol. Genet. Metab. 2011, 104, S2–S9. [Google Scholar] [CrossRef]
- Fujimoto, A.; Okano, Y.; Miyagi, T.; Isshiki, G.; Oura, T. Quantitative Beutler test for newborn mass screening of galactosemia using a fluorometric microplate reader. Clin. Chem. 2000, 46, 806–810. [Google Scholar] [CrossRef]
- Nishimura, Y.; Tajima, G.; Bahagia, A.D.; Sakamoto, A.; Ono, H.; Sakura, N.; Naito, K.; Hamakawa, M.; Yoshii, C.; Kubota, M. Differential diagnosis of neonatal mild hypergalactosaemia detected by mass screening: Clinical significance of portal vein imaging. J. Inherit. Metab. Dis. 2004, 27, 11–18. [Google Scholar] [CrossRef]
- Dick, L.; Dooley, N.; Elliott, M.A.; Ford, S.J.; Gordon, M.R.; Halbert, G.W.; Kerr, W.J. Boron phenylalanine and related impurities: HPLC analysis, stability profile and degradation pathways. J. Pharm. Biomed. Anal. 2011, 56, 633–636. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Liu, Y.; Yang, X.; Luo, L.; Yao, S. Preparation of L-phenylalanine imprinted polymer based on monodisperse hybrid silica microsphere and its application on chiral separation of phenylalanine racemates as HPLC stationary phase. Sep. Purif. Technol. 2012, 87, 142–148. [Google Scholar] [CrossRef]
- Mo, X.; Li, Y.; Tang, A.; Ren, Y. Simultaneous determination of phenylalanine and tyrosine in peripheral capillary blood by HPLC with ultraviolet detection. Clin. Biochem. 2013, 46, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Neurauter, G.; Scholl-Bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef]
- Pecce, R.; Scolamiero, E.; Ingenito, L.; Parenti, G.; Ruoppolo, M. Optimization of an HPLC method for phenylalanine and tyrosine quantization in dried blood spot. Clin. Biochem. 2013, 46, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, F.L.; Ardelean, A.; Țurcuș, V.; Mândruțiu, I.; Belengeanu, A.; Bechet, D.; Frențescu, L. A simple, sensitive and highly accurate procedure for plasma phenylalanine determination by HPLC. Acta Endocrinol. Buc 2015, 11, 143–146. [Google Scholar]
- Peat, J.; Garg, U. Determination of phenylalanine and tyrosine by high performance liquid chromatography-tandem mass spectrometry. In Clinical Applications of Mass Spectrometry in Biomolecular Analysis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 219–225. [Google Scholar]
- Hroboňová, K.; Lomenova, A. Molecularly imprinted polymer as stationary phase for HPLC separation of phenylalanine enantiomers. Mon. Chem. Chem. Mon. 2018, 149, 939–946. [Google Scholar] [CrossRef]
- Lomenova, A.; Hroboňová, K. Comparison of HPLC separation of phenylalanine enantiomers on different types of chiral stationary phases. Food Anal. Methods 2018, 11, 3314–3323. [Google Scholar] [CrossRef]
- Kazan, R.M.; Seddik, H.A.; Marstani, Z.M.; Elsutohy, M.M.; Yasri, N.G. Determination of amino acids content in tea species using liquid chromatography via pre-column fluorescence derivatization. Microchem. J. 2019, 150, 104103. [Google Scholar] [CrossRef]
- Xu, X.; Ji, D.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Liu, C.-C.; Wen, W. Detection of Phenylketonuria Markers Using a ZIF-67 Encapsulated PtPd Alloy Nanoparticle (PtPd@ ZIF-67)-Based Disposable Electrochemical Microsensor. ACS Appl. Mater. Interfaces 2019, 11, 20734–20742. [Google Scholar] [CrossRef]
- Harms, E.; Olgemöller, B. Neonatal screening for metabolic and endocrine disorders. Dtsch. Ärztebl. Int. 2011, 108, 11. [Google Scholar] [CrossRef]
- Rhode, H.; Elei, E.; Taube, I.; Podskarbi, T.; Horn, A. Newborn screening for galactosemia: Ultramicro assay for galactose-1-phosphate-uridyltransferase activity. Clin. Chim. Acta 1998, 274, 71–87. [Google Scholar] [CrossRef]
- Jeong, J.-S.; Yoon, H.-R.; Hong, S.-P. Development of a new diagnostic method for galactosemia by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 2007, 1140, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Easley, C.J.; Jin, L.J.; Elgstoen, K.B.P.; Jellum, E.; Landers, J.P.; Ferrance, J.P. Capillary electrophoresis with laser-induced fluorescence detection for laboratory diagnosis of galactosemia. J. Chromatogr. A 2003, 1004, 29–37. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Chen, S.-T.; Sheikhzadeh, P. MCM-41-NH2 as an advanced nanocatalyst for electrooxidation and determination of amino acids. Catal. Commun. 2012, 19, 21–27. [Google Scholar] [CrossRef]
- Blau, N.; Shen, N.; Carducci, C. Molecular genetics and diagnosis of phenylketonuria: State of the art. Expert Rev. Mol. Diagn. 2014, 14, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed point-of-care testing–xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Bietenbeck, A.; Beaudoin, C.; Giannetti, A. Clinically relevant analytical techniques, organizational concepts for application and future perspectives of point-of-care testing. Biotechnol. Adv. 2016, 34, 139–160. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, S.; Carvalho, W.S.P.; Jiang, Y.; Serpe, M.J. Portable point-of-care diagnostic devices. Anal. Methods 2016, 8, 7847–7867. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sheibani, S. Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: A review. Microchim. Acta 2018, 185, 276. [Google Scholar] [CrossRef]
- Pundir, C.; Lata, S.; Narwal, V. Biosensors for determination of D and L-amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef]
- Ermiş, N.; Uzun, L.; Denizli, A. Preparation of molecularly imprinted electrochemical sensor for L-phenylalanine detection and its application. J. Electroanal. Chem. 2017, 807, 244–252. [Google Scholar] [CrossRef]
- Ou, S.-H.; Pan, L.-S.; Jow, J.-J.; Chen, H.-R.; Ling, T.-R. Molecularly imprinted electrochemical sensor, formed on Ag screen-printed electrodes, for the enantioselective recognition of d and l phenylalanine. Biosens. Bioelectron. 2018, 105, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.; Apetrei, C. A modified nanostructured graphene-gold nanoparticle carbon screen-printed electrode for the sensitive voltammetric detection of rutin. Measurement 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Study of different carbonaceous materials as modifiers of screen-printed electrodes for detection of catecholamines. IEEE Sens. J. 2014, 15, 3094–3101. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecular imprinting of macromolecules for sensor applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Dong, S.; Sun, Y.; Lu, X.; Zhao, L. An electrochemical sensor based on cellulose nanocrystal for the enantioselective discrimination of chiral amino acids. Anal. Biochem. 2016, 508, 50–57. [Google Scholar] [CrossRef]
- Kubota, L.T.; Gouvea, F.; Andrade, A.N.; Milagres, B.G.; Neto, G.D.O. Electrochemical sensor for NADH based on Meldola’s Blue immobilized on silica gel modified with titanium phosphate. Electrochim. Acta 1996, 41, 1465–1469. [Google Scholar] [CrossRef]
- Adamski, J.; Kochana, J. Meldola’s Blue—Doped titania sol-gel sensor for NADH determination. Open Chem. 2011, 9, 185–191. [Google Scholar] [CrossRef]
- Hope-Roberts, M.; Horobin, R. Biomedical applications and chemical nature of three dyes first synthesized by Raphael Meldola: Isamine blue, Meldola’s blue and naphthol green B. Biotech. Histochem. 2012, 87, 295–299. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Dias, S.L.; Benvenutti, E.V.; Lima, E.C.; Pavan, F.A.; Rodrigues, J.R.; Scotti, R.; Ribeiro, E.S.; Gushikem, Y. Cationic dyes immobilized on cellulose acetate surface modified with titanium dioxide: Factorial design and an application as sensor for NADH. J. Braz. Chem. Soc. 2007, 18, 1462–1472. [Google Scholar] [CrossRef]
- Njagi, J.; Erlichman, J.S.; Aston, J.W.; Leiter, J.; Andreescu, S. A sensitive electrochemical sensor based on chitosan and electropolymerized Meldola blue for monitoring NO in brain slices. Sens. Actuators B Chem. 2010, 143, 673–680. [Google Scholar] [CrossRef]
- Piano, M.; Serban, S.; Pittson, R.; Drago, G.; Hart, J.P. Amperometric lactate biosensor for flow injection analysis based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt, coated with lactate dehydrogenase and NAD+. Talanta 2010, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Titoiu, A.M.; Lapauw, M.; Necula-Petrareanu, G.; Purcarea, C.; Fanjul-Bolado, P.; Marty, J.; Vasilescu, A. Carbon Nanofiber and Meldola Blue Based Electrochemical Sensor for NADH: Application to the Detection of Benzaldehyde. Electroanalysis 2018, 30, 2676–2688. [Google Scholar] [CrossRef]
- Apetrei, I.; Rodriguez-Mendez, M.; Apetrei, C.; De Saja, J. Enzyme sensor based on carbon nanotubes/cobalt (II) phthalocyanine and tyrosinase used in pharmaceutical analysis. Sens. Actuators B Chem. 2013, 177, 138–144. [Google Scholar] [CrossRef]
- Georgescu, R.; van Staden, J.F.; Stefan-van Staden, R.-I.; Boscornea, C. Evaluation of amperometric dot microsensors for the analysis of folic acid in pharmaceutical tablets and urine samples. J. Porphyr. Phthalocyanines 2015, 19, 679–687. [Google Scholar] [CrossRef]
- Foster, C.W.; Metters, J.P.; Kampouris, D.K.; Banks, C.E. Ultraflexible screen-printed graphitic electroanalytical sensing platforms. Electroanalysis 2014, 26, 262–274. [Google Scholar] [CrossRef]
- Wahab, F.; Sayyad, M.; Tahir, M.; Aziz, F.; Khan, R.; Karimov, K.S. Sensing properties of cobalt-phthalocyanine-based multipurpose sensor. J. Electron. Mater. 2017, 46, 2045–2052. [Google Scholar] [CrossRef]
- Aller-Pellitero, M.; Fremeau, J.; Villa, R.; Guirado, G.; Lakard, B.; Hihn, J.-Y.; Del Campo, F.J. Electrochromic biosensors based on screen-printed Prussian Blue electrodes. Sens. Actuators B Chem. 2019, 290, 591–597. [Google Scholar] [CrossRef]
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Katic, V.; dos Santos, P.L.; dos Santos, M.F.; Pires, B.M.; Loureiro, H.C.; Lima, A.P.; Queiroz, J.C.; Landers, R.; Muñoz, R.A.; Bonacin, J.A. 3D printed graphene electrodes modified with Prussian blue: Emerging electrochemical sensing platform for peroxide detection. ACS Appl. Mater. Interfaces 2019, 11, 35068–35078. [Google Scholar] [CrossRef]
- Hutanu, F.; Ocnaru, E.; Arsene, M.; Badea-Doni, M. Characterization of surfactant-modified prussian blue screen-printed carbon electrodes for H2O2 detection. Bull. Transilv. Univ. Brasov Ser. Eng. Sci. 2013, 6, 95–102. [Google Scholar]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a hydrogen peroxide sensor based on screen-printed electrodes modified with inkjet-printed Prussian blue nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, S.; Xu, H.; Zhao, Q.; Zhang, Z.; Yao, Y. Preparation of poly (N-acetylaniline)–Prussian blue hybrid composite film and its application to hydrogen peroxide sensing. Anal. Methods 2014, 6, 8003–8010. [Google Scholar] [CrossRef]
- Bonacin, J.A.; Dos Santos, P.L.; Katic, V.; Foster, C.W.; Banks, C.E. Use of screen-printed electrodes modified by prussian blue and analogues in sensing of cysteine. Electroanalysis 2018, 30, 170–179. [Google Scholar] [CrossRef]
- Hutanu, F.; Gheorghe, G. Modified prussian blue screen printed electrodes for H2O2 detection. Food Environ. Saf. J. 2016, 12, 78–83. [Google Scholar]
- O’Halloran, M.P.; Pravda, M.; Guilbault, G.G. Prussian Blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta 2001, 55, 605–611. [Google Scholar]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Jäntschi, L.; Naşcu, H.I. Chimie Analitică şi Instrumentală; Academic Press: Cambridge, MA, USA, 2009; ISBN 973-744-191-5. [Google Scholar]
- Salazar, P.; Martín, M.; O’Neill, R.; Roche, R.; González-Mora, J. Surfactant-promoted Prussian Blue-modified carbon electrodes: Enhancement of electro-deposition step, stabilization, electrochemical properties and application to lactate microbiosensors for the neurosciences. Colloids Surf. B Biointerfaces 2012, 92, 180–189. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Vacchino, J.F.; Beeson, C.; McConnell, H.M. Potentiometric measurement of intracellular redox activity. J. Am. Chem. Soc. 1998, 120, 2464–2473. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M.; Ojani, R. Electrochemistry and electrocatalytic activity of polypyrrole/ferrocyanide films on a glassy carbon electrode. J. Solid State Electrochem. 2000, 4, 75–79. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. Application of voltammetric e-tongue for the detection of ammonia and putrescine in beef products. Sens. Actuators B Chem. 2016, 234, 371–379. [Google Scholar] [CrossRef]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical behavior and voltammetric determination of sulfaguanidine at a glassy carbon electrode modified with a multi-walled carbon nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Brubaker, J.P. A Diffusion Model for Cyclic Voltammetry with Nanostructured Electrode Surfaces. Master’s Thesis, University of Dayton, Dayton, OH, USA, 2014. [Google Scholar]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49. [Google Scholar] [PubMed]
- Yi, Y.; Zhang, D.; Ma, Y.; Wu, X.; Zhu, G. Dual-signal electrochemical enantiospecific recognition system via competitive supramolecular host–guest interactions: The case of phenylalanine. Anal. Chem. 2019, 91, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z. Perylene-functionalized graphene sheets modified with β-cyclodextrin for the voltammetric discrimination of phenylalanine enantiomers. Bioelectrochemistry 2019, 129, 189–198. [Google Scholar] [CrossRef]
- Aghaei, F.; Seifati, S.M.; Nasirizadeh, N. Development of a DNA biosensor for the detection of phenylketonuria based on a screen-printed gold electrode and hematoxylin. Anal. Methods 2017, 9, 966–973. [Google Scholar] [CrossRef]
- Sajini, T.; John, S.; Mathew, B. Tailoring of photo-responsive molecularly imprinted polymers on multiwalled carbon nanotube as an enantioselective sensor and sorbent for L-PABE. Compos. Sci. Technol. 2019, 181, 107676. [Google Scholar] [CrossRef]
- Wu, T.; Wei, X.; Ma, X.; Li, J. Amperometric sensing of L-phenylalanine using a gold electrode modified with a metal organic framework, a molecularly imprinted polymer, and β-cyclodextrin-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 2901–2907. [Google Scholar] [CrossRef]
- Seifati, S.M.; Nasirizadeh, N.; Azimzadeh, M. Nano-biosensor based on reduced graphene oxide and gold nanoparticles, for detection of phenylketonuria-associated DNA mutation. IET Nanobiotechnol. 2017, 12, 417–422. [Google Scholar] [CrossRef]
- European Pharmacopoeia 7.0, 7th ed.; Council of Europe: Strasbourg, France, 2011; Volume 1, ISBN 92-871-6700-1.

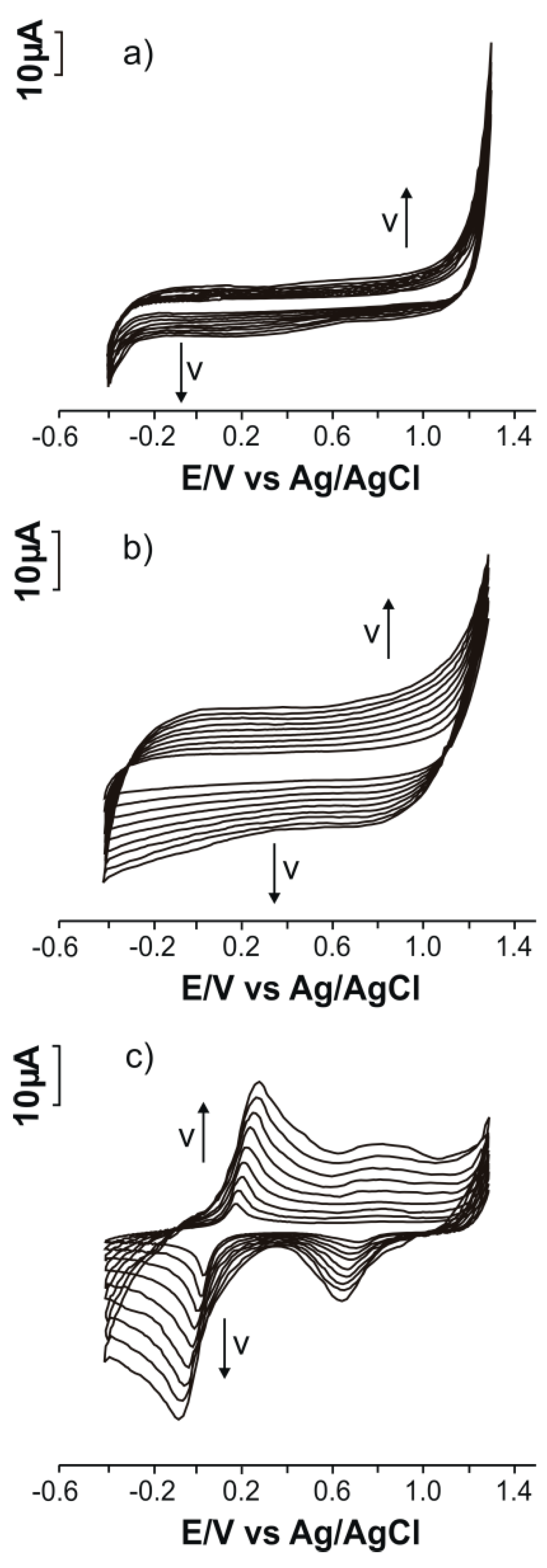
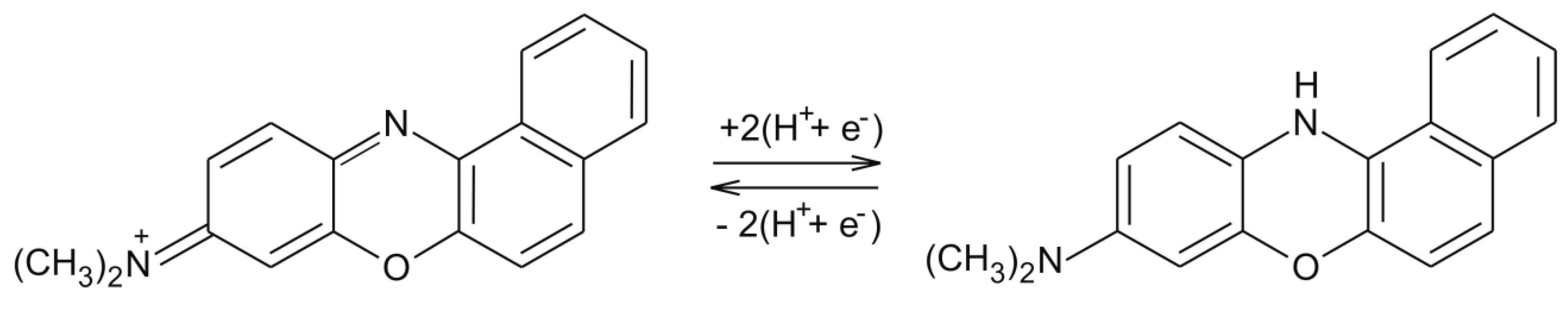

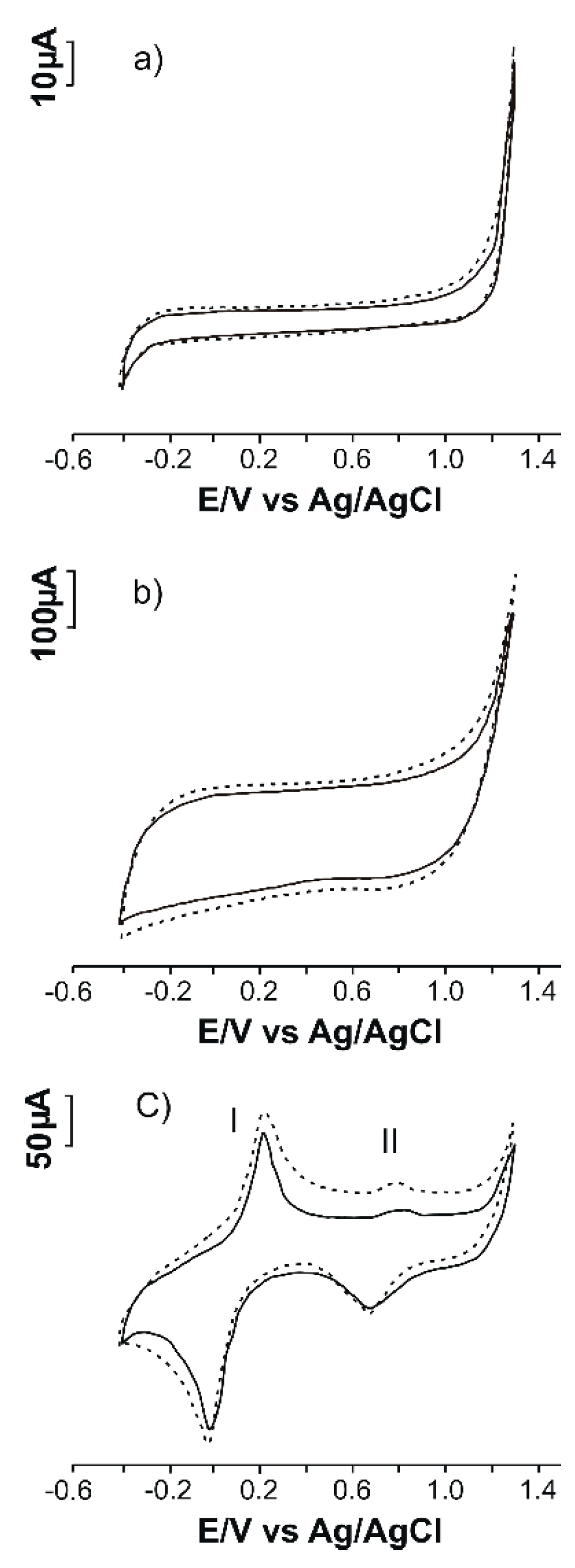



| Sensor | Slope | Active Area (cm2) | Geometric Area (cm2) | Roughness Factor |
|---|---|---|---|---|
| CoPc-SPCE | 0.00061675 | 0.8509 | 0.1256 | 6.77 |
| MB-SPCE | 0.00218020 | 3.0080 | 23.95 | |
| PB-SPCE | 0.00471540 | 6.5058 | 51.80 |
| Sensor | Detection Technique | LOD (M) | Reference |
|---|---|---|---|
| β-CD/CNTs@rGO | CV, DPV | 0.08 × 10−6 | [67] |
| Perylene-functionalized graphene/β-CD | CV, DPV | 0.08 × 10−9 for L-Phe 0.2 × 10−9 for D-Phe | [68] |
| DNA (thiol modified oligonucleotide probe)/hematoxylin | CV | 8.5 × 10−12 | [69] |
| MIP (4-[ (4-methacryloyloxy) phenylazo] benzoic acid)/MWCNT | CV, DPV | 0.2086 × 10−6 | [70] |
| ZIF-67 Encapsulated PtPd Alloy Nanoparticle (PtPd@ZIF-67) | CV, CA | 20 × 10−9 | [19] |
| MIP/thiolated β-CD/L-cysteine | CV, DPV | 0.33 × 10−12 | [71] |
| gold nanoparticles/rGO/alkanethiol single-stranded DNA/Oracet blue | CV | 21.3 × 10−15 | [72] |
| Drug | The Amount of Phe Reported by the Producer/mg | The Amount of Phe | |
|---|---|---|---|
| CV Method/mg | FTIR Method/mg | ||
| Amino 75 | 75 | 75 ± 2 | 75 ± 3 |
| L-Phenylalanine 500 | 500 | 500 ± 15 | 498 ± 20 |
| DLPA 500 | 500 | 500 ± 14 | 503 ± 22 |
| Phe Concentration Taken/M | Phe Concentration Found/M | Precision/% RSD | Recovery (%) | |
|---|---|---|---|---|
| Intra-day | 4 × 10−6 | 3.99 × 10−6 | 2.28 | 99.75 |
| Inter-day | 5 × 10−5 | 5.014 × 10−5 | 3.02 | 100.28 |
| Sample | Phe | ||
|---|---|---|---|
| Phe Added (×106 M) | Phe Found (×106 M) | Recovery (%) ± RSD (n = 5) | |
| L-Phenylalanine (Solaray) | 3 | 3.03 | 100.5 ± 0.9 |
| 5 | 4.97 | 99.4 ± 0.8 | |
| 7 | 7.06 | 100.9 ± 0.9 | |
| DLPA (Solgar) | 2 | 1.97 | 98.5 ± 0.5 |
| 4 | 3.96 | 99.0 ± 0.7 | |
| 6 | 6.06 | 101 ± 0.5 | |
| Amino 75 (Solgar) | 3 | 3.04 | 101.33 ± 0.5 |
| 6 | 6.15 | 102.5 ± 1.0 | |
| 9 | 9.14 | 101.56 ± 0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, A.; Apetrei, C. Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors. Chemosensors 2020, 8, 113. https://doi.org/10.3390/chemosensors8040113
Dinu A, Apetrei C. Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors. Chemosensors. 2020; 8(4):113. https://doi.org/10.3390/chemosensors8040113
Chicago/Turabian StyleDinu, Ancuta, and Constantin Apetrei. 2020. "Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors" Chemosensors 8, no. 4: 113. https://doi.org/10.3390/chemosensors8040113
APA StyleDinu, A., & Apetrei, C. (2020). Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors. Chemosensors, 8(4), 113. https://doi.org/10.3390/chemosensors8040113






