Abstract
The complexity of the milk matrix, driven by its lipid-rich composition, complicates pesticide residue analysis. This study developed a simplified and robust analytical procedure for the quantification of 250 pesticides in cow’s milk. Sample preparation involved acidified ethyl acetate extraction followed by centrifugation at 0 °C. A subsequent clean-up step was performed using micro solid-phase extraction (μSPE) in a 96-well format with the enhanced matrix removal-lipid (EMR-lipid) sorbent. Final extracts were analyzed by gas chromatography coupled to high-resolution mass spectrometry (GC-Q-Orbitrap-MS). Method validation demonstrated satisfactory linearity within the 5–100 µg/L range, recoveries between 70.6% and 119.8%, and precision, expressed as relative standard deviation (RSD), was acceptable for both intraday (1.8–19.2%) and interday (1.6–18.5%) conditions. The limit of quantification (LOQ) was set at 10 µg/kg for all compounds. The method was applied to 23 commercial cow’s milk samples, and no pesticide residues were detected above the current European Union (EU) maximum residue limits (MRLs).
1. Introduction
Milk is a globally produced and consumed food commodity, essential in a balanced diet []. Beyond its direct consumption, milk serves as a key ingredient in important products, such as formulated milk for infants. However, fatty food products such as milk can contain relatively high levels of pesticide residues, which can enter the food chain through contaminated animal feed and water, posing a risk of exposure to these toxic substances for both animals and humans [,,]. To ensure food safety, MRLs have been set by various countries and organizations. The EU has established MRLs for most pesticides in milk and dairy products, with limits as low as 0.8 µg/kg for endrin and 2 µg/kg for chlordane []. Therefore, effective analytical methods are necessary to monitor pesticide residues in milk to confirm compliance with these safety limits. Although gas chromatography coupled with mass spectrometry (GC-MS) is highly sensitive and capable of accurately detecting target compounds in complex matrices, it is now less frequently used due to its sensitivity to matrix effects []. However, GC coupled to high-resolution mass spectrometry (HRMS), compared to traditional GC-MS, offers full-scan capabilities, high accuracy, and selective screening of target compounds. This allows low levels of pesticides to be detected and quantified, even in complex matrices such as milk. Milk contains organic acids, sugars, fats, proteins, and other impurities that can interfere with chromatographic analysis. Furthermore, the use of full scan HRMS is becoming increasingly popular in the residue analysis of food samples, especially when a large number of analytes must be monitored []. Compared to other high-resolution mass analyzers, such as time-of-flight (TOF), the Orbitrap offers superior mass accuracy, resolving power, and dynamic range, along with the ability to operate at different resolution settings depending on analytical needs. These features make it particularly suitable for detecting trace-level compounds in complex matrices and allow for more confident identification of both target analytes and non-target analytes in full-scan acquisition modes.
The currently available methods for the extraction of multiple pesticide residues from fatty milk are still restricted by the laborious and time-consuming sample pretreatment process, limited sensitivity, and selective clean-up []. These methods involve the use of solvents such as n-hexane [] and dichloromethane [], sonication times of 20–30 min, laborious cleaning steps using solid phase extraction (SPE), or combinations of various salts [], followed by evaporation under nitrogen flow [].
The extraction of multiple compounds with diverse chemical properties and polarities represents a significant challenge. The QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) method offers advantages, such as rapid processing, high throughput, efficiency, and low cost. However, milk is a complex matrix and has a high matrix effect, characterized by high concentrations of organic acids, sugars, lipids, and proteins. For instance, pesticide residues and other contaminants were analyzed in milk samples [,] using QuEChERS extraction. PAHs and pesticide residues were also evaluated in milk samples [,]. The clean-up step can be carried out as using dSPE clean-up [,], and both methods required manual weighing of small quantities of “enhanced matrix removal-lipid” (EMR-lipid) and other sorbents such as PSA and Z-Sep+ (zirconia-based sorbent) to remove fatty matrix components, whereas other authors used Z-Sep, C18, and PSA during the dSPE clean-up [,]. For this work, various clean-up approaches after the extraction of QuEChERS have been proposed, with the aim of reducing the number of co-extractive interferences during pesticide residue analysis, using many steps and sorbents, which are difficult to handle [,,,,]. An interesting option is the use of the clean-up step with the EMR-lipid material that has been proposed to remove fat from fat-rich food products [,]. In most published articles, EMR-lipid has been used as a cleaning sorbent in dispersive SPE (dSPE) in matrices with high fat content, such as pork, fish, smoked foods [], olive oil or avocado fruit [], biological matrices such as human plasma [], or even in milk formulas for analysis of mycotoxins, polycyclic aromatic hydrocarbons or neonicotinoids []. The use of well-plate format (µSPE) minimizes weighing errors, improves reproducibility, and facilitates high-throughput workflows, which are key issues for routine laboratories. However, the use of EMR-lipid in a 96-well plate format for µSPE is rather limited, although it has demonstrated clinical applicability in blood [] and even in infant milk formulas []. The present study builds upon the well-established QuEChERS extraction and GC-HRMS principles, but its novelty lies in the first application, to the best of our knowledge, of EMR-lipid in a ready-to-use 96-well plate format for the determination of 250 pesticide residues in whole milk, combining broad analyte coverage with reduced solvent use and simplified sample handling.
In summary, the aim of the study was to develop a simple method that reduces extraction time by using a small amount of salts and solvents for the analysis of pesticide residues in whole milk. The final extracts were analyzed using the analytical methodology already developed [], based on GC-HRMS, using a single quad-Orbitrap as an analyzer, which was utilized to perform selective target screening for the detection and quantification of low levels of pesticides.
2. Materials and Methods
2.1. Reagents and Chemicals
Standards for 275 pesticides were purchased from Dr. Ehrenstorfer (Augsburg, Germany) or Sigma Aldrich (St. Louis, MO, USA). One isotopically labelled compound, atrazine-d5 from Sigma Aldrich, was used as the surrogate standard. All standards had a purity greater than 95%.
HPLC gradient grade acetonitrile and HPLC grade acetic acid were acquired from Honeywell (Seelze, Germany) and Panreac AppliChem (Barcelona, Spain), respectively. PAR grade ethyl acetate was purchased from Fluka (St. Louis, MO, USA). Formic acid (FA) (Optima LC–MS) was supplied by Fisher Scientific (Geel, Belgium). Magnesium sulfate and sodium acetate were obtained from Fluka, while tri-sodium citrate dehydrate, sodium chloride, and sodium citrate dibasic sesquihydrate were sourced from Panreac AppliChem, Scharlab (Barcelona, Spain), and Sigma Aldrich, respectively. Primary secondary amine (PSA) was obtained from Scharlab, C18 (octadecyl) bonded phase from Supelco (Bellefonte, PA, USA), and Agilent Captiva EMR-lipid in a 96-well plate format from Agilent Technologies (Santa Clara, CA, USA).
Stock standard solutions of the target pesticides were prepared in a suitable solvent (methanol or ethyl acetate) at a concentration of 1000 mg/L. The stock solutions were stored in amber screw-capped glass vials. After that, working solutions containing all pesticides were then prepared at 4.5 mg/L in acetonitrile and at 0.5, 0.2, and 0.1 mg/L in ethyl acetate. The surrogate standard (atrazine-d5) was added at a final concentration of 25 µg/kg prior to extraction. This surrogate was used to check whether sample preparation and analysis were performed correctly. All standard solutions were stored at −20 °C.
2.2. GC-Orbitrap-MS Parameters
The GC system consisted of a TriPlus RSH autosampler, a TRACE 1300 gas chromatograph, and a Q-Exactive Orbitrap mass analyzer (Thermo Fisher Scientific, Bremen, Germany). The injector was equipped with a single taper liner (78.5 mm × 4 mm internal diameter (ID)). Sample introduction was performed using hot splitless injection (1 μL, 280 °C, splitless time 1 min). High-purity helium (99.999%) served as a carrier gas at a constant flow rate of 1 mL/min. Separation was achieved on a VF-5 MS capillary column (30 m × 0.25 mm i.d. and 0.25 µm film thickness from Agilent, Santa Clara, CA, USA). The oven temperature program lasted 29.00 min and was as follows: initial temperature of 50 °C held for 1 min, then ramped to 170 °C at 20 °C/min (6 min), followed by an increase to 310 °C at 10 °C/min (14 min). The final temperature of 310 °C was maintained for 8 min. The Q-Exactive Orbitrap mass analyzer was operated in electron ionization (EI) mode at 70 eV with an emission current of 50 µA. Mass calibration was performed weekly using perflurotributylamine (PFTBA) from Thermo Fisher Scientific.
The filament delay was set for 5 min, and data acquisition was performed from 5 to 29 min. The ion source and transfer line temperatures were maintained at 250 °C. Full-scan (FS) MS acquisition was carried out in profile mode over an m/z range of 40 to 500. The maximum injection time was set to 1 μscan, with a resolution power of 60,000 full width at half maximum (FWHM). The automatic gain control (AGC) target was set to 1 × 106 ions. Instrument control was performed using Xcalibur 4.1 and chromatographic data were processed with TraceFinder 4.1 software (Thermo Fisher Scientific, Les Ulis, France). Identification criteria included: (i) at least two fragment ions with mass accuracy ≤ 5 ppm, and (ii) ion ratio within (±30% (relative) of the average calibration standard from the same sequence. Detailed GC-MS parameters are provided in Table S1.
2.3. Sample Extraction
Cow’s milk samples were processed using a modified version of AOAC QuEChERS, a typical multiresidue extraction method commonly applied in routine laboratories. Prior to extraction, milk samples were homogenized by vortex mixing to ensure a representative distribution of both aqueous and lipid phases. A 5 g aliquot of the sample was weighed into a 50 mL polypropylene centrifuge tube. Ethyl acetate (10 mL) containing 1% acetic acid and 10 µL of the surrogate standard (atrazine-d5) was added, and the mixture was vortexed for 2 min. Subsequently, 4 g of MgSO4 and 1.5 g of sodium acetate were added, followed by another 2 min of vortex mixing. The tube was then centrifuged at 7500 rpm (6200× g) at 0 °C for 8 min.
A 1 mL aliquot of the supernatant was loaded onto a well Agilent Captiva EMR-lipid. After the liquid was passed through the cartridge by gravity, vacuum was applied to remove any remaining liquid. The resulting extract was then transferred to sample vials for GC-Orbitrap-MS analysis, and 1 µL was injected into the system.
2.4. Validation
The performance of the analysis was evaluated in accordance with the analytical quality control and validation guidelines for the analysis of pesticide residues in food and feed, as described in SANTE 11312/2021v2 []. The parameters assessed included linearity, limit of quantification (LOQ), recovery, and precision. Additionally, the expanded measurement uncertainty (U) was calculated using intra-laboratory validation data, according to the SANTE guidelines. The whole cow’s milk used for the development and validation of the method was also sourced directly from local grocery stores in Almeria (Spain). This represents typical market samples and provides realistic matrix conditions for pesticide residue analysis.
According to the SANTE guideline, an extension of the scope of the method was carried out to other matrices within the milk subgroup (milk and dairy products group). This verification procedure was applied to various types of milk, including semi-skimmed, lactose-free semi-skimmed, skimmed, and evaporated milk, ensuring the reliability of the method in various matrices. This was achieved by analyzing three spiked samples (spiked at the target LOQ, 25 µg/kg, and at 50 μg/kg for all pesticides) and one blank matrix. Quantification was carried out using a matrix-matched calibration prepared in their specific matrix. The method performance criteria remained consistent with the validation parameters for recovery (70–120%) and repeatability (≤20%).
2.5. Applicability of the Method
A total of 23 cow’s milk samples with different compositions (whole, semi-skimmed, skimmed, lactose-free, calcium-enriched, and omega-3-enriched) were purchased from local grocery stores in Almería (Spain). The samples, representing different commercial brands, were stored in their original containers under the recommended conditions until analysis. For the development and validation of the method, whole cow’s milk was selected as the most challenging matrix in terms of sample preparation. An overview of the samples included in this study is provided in Table S2 (see Supplementary Materials).
3. Results
3.1. Optimization of the Extraction Procedure
3.1.1. Optimization of Extraction Salts
The QuEChERS method was modified and optimized considering the high protein and lipid content of milk, with lipids being one of the most important and significant sources of interferences. As an extraction solvent, acetonitrile was tested because it must meet specific requirements, including the ability to precipitate fats and proteins while efficiently extracting analytes from milk samples. This solvent not only enables the extraction of a wide range of compounds, but also serves as an effective protein precipitant [], a process that can be enhanced by adding FA []. In this study, whole cow’s milk was used and three different versions of QuEChERS were compared. Using acetonitrile with 1% FA as the extraction solvent, a comparison of extraction salts was made by spiking whole cow’s milk (5 g) with a standard mixture at 100 μg/kg. This spiking level for the preliminary evaluation of extraction salts was selected to ensure that the target compounds could be detected with sufficient sensitivity and to evaluate whether their absence in the chromatogram, if any, was due to poor extraction efficiency rather than limitations of instrumental sensitivity. Three different extraction salts were tested: (A) Original QuEChERS salts [] containing 4 g of MgSO4 and 1 g of NaCl, (B) the AOAC version [] containing 6 g of MgSO4 and 1.5 g of sodium acetate, and (C) the EN version [] consisting of 4 g of MgSO4, 1 g of NaCl, 3.5 g of disodium hydrogen citrate sesquihydrate, and 1 g of trisodium citrate dihydrate. The clean-up step was based on the addition of 150 mg of MgSO4 combined with 25 mg of PSA and C18 as sorbents [], followed by filtration through a nylon filter. In the last step, an aliquot of extract was evaporated to dryness under a N2 stream and redissolved with 1 mL of ethyl acetate before chromatographic analysis.
The extraction efficiency was evaluated on recovery values obtained from triplicate experiments, with acceptable recovery defined as 70 to 120%. As shown in Figure 1, a total of 122 compounds achieved suitable recovery using method (A), while 196 and 74 compounds met the recovery criteria with methods (B) and (C), respectively. The improved recoveries obtained using the AOAC QuEChERS version may be attributed to the slightly lower pH provided by the sodium acetate buffering system compared to the citrate-based European version. Based on these results, AOAC salts (B) were selected as the optimal approach for extracting pesticides from whole cow’s milk.
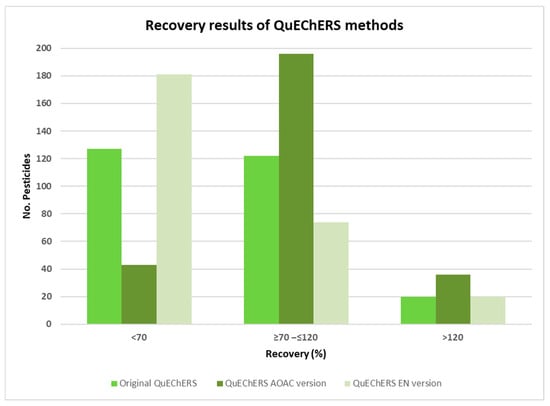
Figure 1.
Recovery results of the Original QuEChERS, QuEChERS AOAC, and QuEChERS EN methods.
3.1.2. Optimization of the Extraction Solvent
Although acetonitrile is compatible with both liquid chromatography coupled to MS (LC-MS) and GC-MS, the potential benefits of using ethyl acetate as the extraction solvent were also tested, taking into account that the evaporation step could be omitted. Having already decided on the use of AOAC salts, a comparison was made using acetonitrile with 1% FA and ethyl acetate with 1% acetic acid as extraction solvents, spiking whole cow’s milk (5 g) with a standard mixture at 100 μg/kg.
Since fatty substances have lower melting points than solvents, frozen lipids can be removed by centrifugation, while pesticides remain dissolved in the solvent []. To take advantage of this property for the removal of lipids, an additional centrifugation step was introduced at 7500 rpm (6200× g) at 0 °C for 8 min. The use of ethyl acetate increased the number of compounds with acceptable recovery (205) compared to acetonitrile (186), while also eliminating the need for a drying step. Consequently, ethyl acetate with 1% acetic acid was used as the extraction solvent.
3.1.3. Optimization of the Clean-Up Step
An efficient clean-up step is critical for removing the maximum possible amount of matrix co-extracts and improving the quality of analytical results. For this purpose, dSPE and μSPE (EMR-lipid 96-well plate) were compared for the cleaning step, evaluating the cleanliness of the extract, the workflow, and the recovery of the pesticides under study. Although EMR-lipid tubes have been used in some previous publications [,,], their application as a 96-well plate format or μSPE is less frequent [,]. This format offers the advantage of a streamlined, single-step process, where 1 mL of sample extract is simply passed through the well. Although automation is possible, manual processing is also feasible with the use of a vacuum pump, requiring only a few seconds per sample.
For dSPE clean-up, 1.5 mL of sample extract was transferred to a 15 mL centrifuge tube containing 50 mg of PSA and 25 mg of C18, combined with 150 mg of MgSO4 to remove any moisture traces from the previous step, and then the sample was vortexed for 1 min. The tube was subjected to a centrifugal process for a duration of 5 min at 3700 rpm (3061× g). In contrast, the μSPE cleaning involved directly loading 1 mL of sample extract into a well and placing it under vacuum. Both methods produced comparable pesticide recoveries, with 238 compounds showing acceptable recoveries using dSPE and 242 compounds with μSPE. Despite a similar performance, the μSPE approach aligns with the study’s goal of developing a simplified method. The use of EMR-lipid significantly reduced the complexity of the workflow by eliminating the need to weigh multiple salts and minimized the use of disposable materials, such as plasticware. Additionally, μSPE offers a higher sample throughput, which is a key advantage for routine analysis, allowing for simultaneous cleaning of a large number of samples (up to 96). For these reasons, EMR-lipid was selected as the preferred clean-up sorbent over dSPE. However, to demonstrate matrix removal by μSPE in future studies, a comparison of Total Ion Current (TIC) profiles before and after the μSPE process was carried out.
3.2. Method Validation
For method validation, whole cow’s milk was selected as the representative matrix according to the SANTE/11312/2021 v2 guidelines [], which designate it as the standard dairy matrix. Thus, given that it has the highest fat content, it also represents the most challenging matrix, making it appropriate to evaluate the performance of the method in other types of milk.
3.2.1. Linearity and Matrix Effect (ME)
In this study, a calibration curve was prepared using a blank sample extract of whole cow’s milk. Linearity was initially evaluated for 275 pesticides within the concentration range of 5 to 100 μg/L, covering five concentration levels (5, 10, 25, 50, and 100 μg/L). A solvent-based calibration curve was also prepared using ethyl acetate at the same concentration levels. The determination coefficients (R2) were calculated for 265 of the 275 compounds studied, and the R2 values were consistently above 0.99. Table S3 (see Supplementary Materials) lists the 275 pesticides that were included in the analysis and highlights with an asterisk those that did not meet the established validation criteria. Only compounds with R2 values greater than 0.99 were considered for the validation study, establishing the linear range of the method between 5–100 µg/L.
The ME was assessed in whole cow’s milk using Equation (1):
As shown in Figure 2, a strong ME (>50%) was observed for 248 compounds. A moderate ME (20% < ME < 50%) was detected in 17 compounds. In particular, none of the compounds exhibited a low or negligible ME (<20%). To mitigate for the pronounced matrix effects, matrix-matched calibration was employed to ensure accurate quantification. The EMR-Lipid clean-up was primarily aimed at enhancing extract cleanliness and improving instrument stability, rather than completely eliminating the matrix effects.
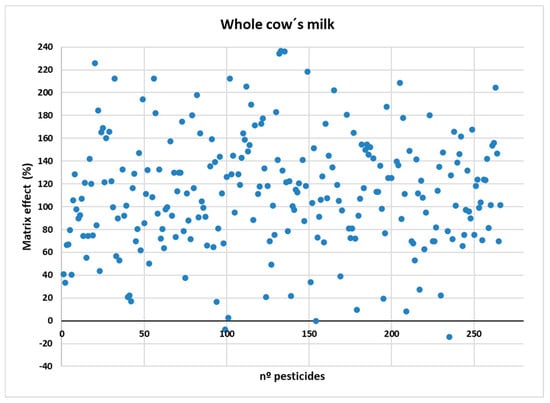
Figure 2.
Matrix effect (%) comparing the slope of the curve in solvent and in whole cow’s milk.
3.2.2. Recovery, Precision, and Uncertainty
Recovery tests were carried out at 10 μg/kg (MRL), 25 μg/kg (2.5× MRL), and 50 μg/kg (5× MRL), using five replicates at each level. Mean recoveries ranged from 71.1 to 119.7%, with an associated repeatability, expressed as RSD, below 20%, as can be observed in Table 1.

Table 1.
Summary of method performance for 250 pesticides in cow’s milk.
Intraday precision, expressed as relative standard deviation (% RSD), was calculated from recovery data at these concentrations and ranged from 1.8% to 19.2%. Interday precision, determined over five different days, ranged from 1.6% to 18.5%, all within acceptable limits. The complete dataset is provided in Table S3. Regarding the recovery values, no significant losses were observed during the µSPE step, confirming the efficiency of the extraction procedure.
For most of the 265 compounds that met the linearity criteria, the results were acceptable. However, fifteen compounds showed extraction difficulties and did not achieve acceptable recovery or precision values: 1,2,3,6-tetrahydrophthalimide cis, bifenox, chlordane cis, chlordane trans, difenoconazole, endrin, fenamiphos, fenamiphos sulfone, fenamiphos sulfoxide, fipronil, fipronil sulfone, heptachlor, heptachlor-epoxide-cis, heptachlor-epoxide-trans, and methamidophos. In summary, 250 compounds were successfully validated, meeting the established performance criteria for recovery and precision.
The standard uncertainty was determined using Equation (2). Uncertainty due to bias, u(bias), was estimated from the recovery experiment, while % RSD of inter-day precision was used to estimate the uncertainty arising from precision, u(precision). The expanded uncertainty was calculated for each pesticide by multiplying the standard uncertainty by a coverage factor (k) of 2, which approximately gives a confidence level of 95%. Further details on the calculation procedure of the uncertainty are provided in the Supplementary Materials (Section S1).
As can be observed in Table 1, for the 250 validated pesticides, the expanded uncertainty at 10 µg/kg was found to be below 50% in all cases, but they were still in compliance with the requirement (50%) of the SANTE/11312/2021 v2 guidelines [].
Unlike most previous studies, which involve more complex procedures in terms of sample preparation and time requirements, with lower recovery rates, and face reproducibility challenges, this study achieved broader and more reliable pesticide detection. Although previous research has often focused on specific pesticide families, such as organochlorine pesticides [,] or organophosphorus pesticides in milk samples [,], this study covered a comprehensive range of 250 compounds, including both long-banned substances, like organochlorine compounds, and, more recently, introduced pesticides for crop protection. Table 1 summarizes the validation results for these compounds.
Finally, the suitability of the method was confirmed by testing other types of milk. Estimated mean recovery and RSD values at 10 and 50 µg/kg for semi-skimmed, lactose-free semi-skimmed, skimmed, and evaporated milk are shown in Table S4. For all pesticides, recoveries ranged from 70.1–119.8%, with RSD values between 0.1% and 19.7%, confirming the applicability of the proposed method to different milk types commonly available on the market.
3.2.3. Limits of Quantification
LOQs were assessed by determining the lowest concentration of spiked samples that produced satisfactory recovery (70–120%) and precision (RSD ˂ 20%). The LOQs were established at 10 μg/kg for 250 pesticides, as these compounds met the established criteria.
The validated LOQ value of 10 μg/kg aligns with the EU MRL for the target pesticides. Analytes that did not meet the validation criteria and were not included in the validation list may still be detected through screening analysis, indicating their potential presence.
3.3. Sample Analysis
The performance of the developed methodologies were evaluated through the analysis of 23 cow’s milk samples. An internal quality control procedure was implemented to ensure the reliability of the analytical results. This procedure included: (i) the analysis of one blank matrix sample prior to each batch to confirm the absence of the target pesticides; (ii) the use of matrix-matched calibration, and (iii) the inclusion of two spiked blank samples at 10 and 25 μg/kg with the pesticide mixture within each batch. This approach allowed for the assessment of recovery rates and the verification of potential cross-contamination. In all cases, recoveries at both spiking levels were within the range of 70–120%. Additionally, a matrix-matched calibration curve was used to quantify any detected compound. None of the 250 target pesticides were detected in the analyzed samples. Furthermore, the analysis confirmed that none of the 250 pesticides exceeded the established LOQ of 10 μg/kg in any of the samples.
4. Conclusions
The implementation of μSPE clean-up contributed to reduced reagents and materials, streamlined laboratory workflow, and enhanced sample throughput, key advantages for routine sample analysis. Moreover, this study presents the first GC-HRMS method capable of simultaneously detecting a broad spectrum of 250 pesticides in milk. Nevertheless, a limited number of compounds did not meet the validation criteria, primarily due to low recovery or poor precision values. For these analytes, alternative extraction conditions would be necessary to achieve reliable quantification. Additionally, to further mitigate ME, several strategies could be considered in future studies. These include the use of additional lipid-targeting sorbents during the clean-up step to enhance the removal of co-extracted fats, sample dilution prior to instrumental analysis to reduce the impact of matrix components, and the implementation of advanced instrumental configurations such as online GC–APCI interfaces, which offer improved selectivity and reduced susceptibility to matrix interference. However, given the wide range of physicochemical properties among the 250 pesticides analyzed, the effectiveness of additional sorbents may vary depending on the compound, and thus their use should be carefully optimized to avoid compromising recovery or selectivity.
In summary, this study developed and successfully validated a new QuEChERS extraction method for the analysis of pesticides in milk, which incorporated acidified ethyl acetate extraction and centrifugation at 0 °C followed by a clean-up step with EMR-lipid. This method is both simplified and efficient in workflow. In conclusion, 250 pesticides provided suitable validation parameters with LOQ values lower than the established MRLs for the target compounds, after GC-HRMS analysis. Additionally, the applicability of the method was tested for other types of milk that differed mainly in fat and lactose content. Automating μSPE cleaning could further improve the efficiency, although this would require significant investment in automation equipment. Furthermore, the high sensitivity of the method (LOQ of 10 µg/kg) and the advanced analytical performance through GC-Q-Orbitrap make it a valuable approach for the complete analysis of pesticide residues in complex matrices. The proposed workflow also shows potential for future automation and digital integration, as the 96-well µSPE format could be adapted to LIMS-based environments and batch processing systems, improving efficiency in routine pesticide monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13120405/s1, Table S1: GC-MS parameters of the targeted pesticides, Table S2: Milk types and sample numbers included in this study, Table S3: Summary of data obtained for validation tests performed to whole milk matrix, Section S1: Measurement Uncertainty Estimation, Table S4: Summary of data obtained for average recovery and estimated RSD values calculated by quantifying 3 spiked samples of semi-skimmed, lactose-free semi-skimmed, skimmed, and evaporated milk.
Author Contributions
M.V.-P.: conceptualization, formal analysis, methodology, writing—original draft preparation, data curation, validation; O.D.P.: methodology, supervision, formal analysis, writing—review and editing; R.R.-G.: methodology, investigation, data curation, writing—reviewing & editing; A.G.F.: conceptualization, supervision, resources, funding acquisition, methodology, writing—reviewing & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the financial support to PPIT-UAL, Junta de Andalucía-ERDF 2021–2027. Program: 54.A.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linehan, K.; Patangia, D.V.; Ross, R.P.; Stanton, C. Production, Composition and Nutritional Properties of Organic Milk: A Critical Review. Foods 2024, 13, 550. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Rodríguez-Cañás, I.; Alvariño, R.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Occurrence of mycotoxins in total mixed ration of dairy farms in Portugal and carry-over to milk. Food Control 2024, 165, 110682. [Google Scholar] [CrossRef]
- Butovskaya, E.; Caprai, E.; Peloso, M.; Gasparini, M.; Borgia, M.; Abdul, M.E.; Candotti, P.; Menotta, S. Plant-based milk alternatives: Assessing the occurrence of chemical and microbiological contaminants in soy, oat, rice and almond beverages from Italian market. Food Control 2025, 169, 111005. [Google Scholar] [CrossRef]
- Madej, K.; Kalenik, T.K.; Piekoszewski, W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 2018, 269, 527–541. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 396/2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin, and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, L70, 1–16. [Google Scholar]
- Wei, Y.; Huang, C.; Chen, L.; Chen, Q.; Hou, J.; Wu, H.; Han, C.; Shen, Y. Determination of chlordimeform and its metabolite residue in milk by gas chromatography–tandem mass spectrometry. Food Res. Int. 2024, 192, 114754. [Google Scholar] [CrossRef] [PubMed]
- Mol, H.G.J.; Tienstra, M.; Zomer, P. Evaluation of gas chromatography–electron ionization–full scan high resolution Orbitrap mass spectrometry for pesticide residue analysis. Anal. Chim. Acta 2016, 935, 161–172. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Li, X.; Wei, B.; Du, Z.; Wei, G.; Wang, S. Determination of multi-residue pesticides in dairy products using single-step emulsification/demulsification clean-up strategy combined with low-pressure gas chromatography-tandem mass spectrometry. Food Chem. 2024, 458, 140246. [Google Scholar] [CrossRef]
- Chen, X.; Panuwet, P.; Hunter, R.E.; Riederer, A.M.; Bernoudy, G.C.; Barr, D.B.; Ryan, P.B. Method for the quantification of current use and persistent pesticides in cow milk, human milk and baby formula using gas chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 970, 121–130. [Google Scholar] [CrossRef]
- Gomes Martins, J.; Amaya Chávez, A.; Waliszewski, S.M.; Colín Cruz, A.; García Fabila, M.M. Extraction and clean-up methods for organochlorine pesticides determination in milk. Chemosphere 2013, 92, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Ganji, S.K.; Kommu, M.; Tiruveedula, R.R.; Upadhyayula, V. Quantitative determination of targeted and untargeted pesticide residues in coconut milk by liquid chromatography—Atmospheric pressure chemical ionization–high energy collisional dissociation tandem high-resolution mass spectrometry. J. Chromatogr. A 2021, 1659, 462649. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Shao, B.; Yin, J.; Yang, Y.; Yang, Y. Rapid Screening for Hazardous Substances with Regulatory Differences in Milk Between Countries Using Ultra-High Performance Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Foods 2025, 14, 967. [Google Scholar] [CrossRef]
- Mokhtar, H.I.; Salama, G.M.; El Gindy, A.; Abdel Hameed, E.A. Optimization of the Combined Use of Z-Sep Plus and EMR-Lipid in QuEChERS Procedure for the Analysis of Eight Pesticides in Real Milk Samples. Food Anal. Methods 2024, 18, 245–263. [Google Scholar] [CrossRef]
- Qin, J.; Tong, K.; Chang, Q.; Xie, Y.; Wu, X.; Fan, C.; Zhang, H.; Shi, Z.; Chen, H. Simultaneous determination of PAHs and pesticides in milk based on improved QuEChERS combined with GC-MS/MS. J. Food Compos. Anal. 2025, 141, 107336. [Google Scholar] [CrossRef]
- Galindo, M.V.; Hantao, L.W.; Sampaio, N.M.F.M.; Pessoto, M.A.; Oliveira, W.d.S.; Godoy, H.T. Analysis of polycyclic aromatic hydrocarbons in Brazilian human milk: A simple and effective approach. Food Control 2025, 167, 110796. [Google Scholar] [CrossRef]
- Oyekunle, J.A.O.; Adekunle, A.S.; Adewole, A.M.; Elugoke, S.E.; Durodola, S.S.; Oyebode, B.A. Determination of organochlorine pesticide residues in some evaporated milk samples in Nigeria using gas chromatography-mass spectrometry. Chem. Africa 2021, 4, 349–366. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Vaclavik, L.; Mastovska, K. Method development and validation for total haloxyfop analysis in infant formulas and related ingredient matrices using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 5521–5528. [Google Scholar] [CrossRef]
- Theurillat, X.; Dubois, M.; Huertas-Pérez, J.F. A multi-residue pesticide determination in fatty food commodities by modified QuEChERS approach and gas chromatography-tandem mass spectrometry. Food Chem. 2021, 353, 129039. [Google Scholar] [CrossRef]
- Sereshti, H.; Jazani, S.S.; Nouri, N.; AliAbadi, M.H.S. Development of a green miniaturized quick, easy, cheap, effective, rugged, and safe approach in tandem with temperature-assisted solidification of floating menthol droplet for analysis of multiclass pesticide residues in milk. J. Sep. Sci. 2022, 45, 1106–1115. [Google Scholar] [CrossRef]
- Castilla-Fernández, D.; Moreno-González, D.; Beneito-Cambra, M.; Molina-Díaz, A. Critical assessment of two sample treatment methods for multiresidue determination of veterinary drugs in milk by UHPLC-MS/MS. Anal. Bioanal. Chem. 2019, 411, 1433–1442. [Google Scholar] [CrossRef]
- Slámová, T.; Sadowska-rociek, A.; Fraňková, A.; Surma, M.; Banout, J. Application of QuEChERS-EMR-Lipid-DLLME method for the determination of polycyclic aromatic hydrocarbons in smoked food of animal origin. J. Food Compos. Anal. 2020, 87, 103420. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Flores-Flores, M.; Irigoyen, Á.; González-Peñas, E. Development and validation of a methodology based on Captiva EMR-lipid clean-up and LC-MS / MS analysis for the simultaneous determination of mycotoxins in human plasma. Talanta 2020, 206, 120193. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K.; Liu, J.; So, P.; Leung, T.; Wong, M.; Zhao, D. A High-Throughput Integrated Nontargeted Metabolomics and Lipidomics Workflow Using Microelution Enhanced Matrix Removal- Lipid for Comparative Analysis of Human Maternal and Umbilical Cord Blood Metabolomes. Anal. Chem. 2025, 97, 2629–2638. [Google Scholar] [CrossRef]
- Galindo, M.V.; Vargas-Pérez, M.; Lopez-Ruíz, R.; Oliveira, W.d.S.; Godoy, H.T.; Garrido-Frenich, A.; Romero-González, R. Monitoring of pesticide residues in Brazilian infant formulas using the suspect screening methodology by GC-HRMS. J. Food Compos. Anal. 2025, 139, 107166. [Google Scholar] [CrossRef]
- Vargas-Pérez, M.; Domínguez, I.; González, F.J.E.; Frenich, A.G. Application of full scan gas chromatography high resolution mass spectrometry data to quantify targeted-pesticide residues and to screen for additional substances of concern in fresh-food commodities. J. Chromatogr. A 2020, 1622, 461118. [Google Scholar] [CrossRef]
- Document No. SANTE. 11312/2021. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed, 1–51. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 19 November 2025).
- Zhang, L.Q.; Zhang, X.M.; Zhang, H.W.; Wang, H.; Xu, H.; Wang, F.M.; Lin, C.; Xiao, J.; Xu, W.Y. Multiclass and multiresidue screening of veterinary drugs and pesticides in infant formula using Quadrupole-Orbitrap MS with PRM scan mode. J. Mass Spectrom. 2020, 55, e4497. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, G.; Viera, M.S.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Determination of organochlorine pesticides (OCPs) in breast milk from Rio Grande do Sul, Brazil, using a modified QuEChERS method and gas chromatography-negative chemical ionisation-mass spectrometry. Int. J. Environ. Anal. Chem. 2018, 98, 1005–1016. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Anastassiades, M.; Scherbaum, E.; Taşdelen, B.; Štajnbaher, D. Recent Developments in QuEChERS Methodology for Pesticide Multiresidue Analysis. Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany; Berlin, Germany, 2007; pp. 439–458. [Google Scholar] [CrossRef]
- Lehotay, S.J.; O’Neil, M.; Tully, J.; Valverde, A.; Contreras, M.; Mol, H.; Heinke, V.; Anspach, T.; Lach, G.; Fussell, R.; et al. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Tienstra, M.; Mol, H.G.J. Application of Gas Chromatography Coupled to Quadrupole-Orbitrap Mass Spectrometry for Pesticide Residue Analysis in Cereals and Feed Ingredients. J. AOAC Int. 2018, 101, 342–351. [Google Scholar] [CrossRef]
- Sanchez Costa, L.; Rodríguez Martínez, P.; Medina Sala, M. Determination of 23 organochlorine pesticides in animal feeds by GC-MS/MS after QuEChERS with EMR-lipid clean-up. Anal. Methods 2018, 10, 5171–5180. [Google Scholar] [CrossRef]
- Manzano Sánchez, L.; Jesús, F.; Ferrer, C.; Gómez-Ramos, M.M.; Fernández-Alba, A. Evaluation of automated clean-up for large scope pesticide multiresidue analysis by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2023, 1694, 463906. [Google Scholar] [CrossRef] [PubMed]
- Lobato, A.; Fernandes, V.C.; Pacheco, J.G.; Delerue-Matos, C.; Gonçalves, L.M. Organochlorine pesticide analysis in milk by gas-diffusion microextraction with gas chromatography-electron capture detection and confirmation by mass spectrometry. J. Chromatogr. A 2021, 1636, 461797. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.L.; Smilowitz, J.T.; Winter, C.K.; Emami, S.; Schmidt, R.J.; Bennett, D.H.; Hertz-Picciotto, I.; Taha, A.Y. Quantification of Nonpersistent Pesticides in Small Volumes of Human Breast Milk with Ultrahigh Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 6676–6689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).