Abstract
Hydrogen bonding, a prevalent molecular interaction in nature, is crucial in biological and chemical processes. The emergence of single-molecule techniques has enhanced our microscopic understanding of hydrogen bonding. However, it is still challenging to track the dynamic behaviour of hydrogen bonding in solution, particularly under physiological conditions where interactions are significantly weakened. Here, we present a nanoscale-confined, functionalised quantum mechanical tunnelling (QMT) probe that enables continuous monitoring of electrical fingerprints of single-molecule hydrogen bonding interactions for over tens of minutes in diverse solvents, including polar physiological solutions, which reveal reproducible multi-level conductance distributions. Moreover, the functionalised QMT probes have successfully discriminated between L(+)- and D(−)-tartaric acid enantiomers by resolving the conductance difference. This work uncovers dynamic single-molecule hydrogen bonding processes within confined nanoscale spaces under physiological conditions, establishing a new paradigm for probing molecular hydrogen-bonding networks in supramolecular chemistry and biology.
1. Introduction
Hydrogen bonding is a fundamental noncovalent interaction that represents a central focus across chemistry, material science, and biology [1,2,3,4,5]. Elucidating the hydrogen bonds at the single-molecule scale provides critical insights for clarifying biomolecular functions, including complementary base pairing in the DNA double helix, the intramolecular scaffolding of protein α-helices and β-sheets, and the dynamic water networks within cells [6,7]. Moreover, hydrogen-bond networks play pivotal roles in biological processes involving electron and proton transfer, such as long-range charge transfer in DNA [8], enzymatic catalysis [9,10], and photosynthesis [11,12]. Current structural biology techniques (e.g., X-ray diffraction and cryogenic electron microscopy) can resolve static hydrogen-bond maps [13,14] but fail to capture their dynamic and reversible nature. Although scanning probe microscopy (SPM) under cryogenic vacuum conditions enables single-atom characterisation of static hydrogen bonds through revealing intermolecular interactions and hydrogen-atom transfer [15,16], resolving dynamic hydrogen-bond structures in solution remains challenging.
Advances in single-molecule techniques have achieved the characterisation of hydrogen bonds in solution through optical [17], electrical [18], and mechanical [19] approaches. Among these techniques, quantum tunnelling combined with supramolecular junctions mediated by intermolecular hydrogen bonds provides both a platform for studying single-molecule electron transport and molecular recognition assisted by hydrogen bonds [20,21]. Two principal strategies are employed: (i) dynamic break-junction techniques, which extract conductance distribution information by statistically analysing the dynamic formation of hydrogen bonds; and (ii) chemical functionalisation of electrodes (e.g., STM tip and substrate) with molecules containing hydrogen-bond donors or acceptors, where bonding is controlled by servo-feedback regulation of electrode spacing. In the break-junction method, target molecules are anchored to the electrodes via linker groups, and hydrogen bonds mediate electron transport. Repeated stretching–reclosing cycles, which often number in the thousands, build supramolecular junctions that reveal statistical electrical features such as tunnelling decay constants and resonant transport [18,22]. However, this method suffers from electrode surface reconstruction, which broadens conductance distributions and perturbs hydrogen-bonded systems. Moreover, because hydrogen bonds are weaker than covalent bonds, the probability and stability of forming metal–hydrogen-bond–metal junctions are markedly reduced [23]. In contrast, chemical functionalisation fixes molecules onto electrode pairs, enabling quasi-static or static studies of hydrogen-bond dynamics [24] while expanding applications in biosensing [25,26]. Nevertheless, tunnelling-current detection across stable nanogaps remains ineffective for tracking single-molecule hydrogen-bond dynamics in physiological solutions, primarily due to their flexibility and dynamic reorganisation, which complicate electron transport mechanisms. These mechanisms probably involve the synergistic contributions of tunnelling-driven electron transfer and proton transfer process, which still lack understanding. Therefore, developing platforms that combine long-term stability of hydrogen bonds with high temporal resolution is critical for elucidating the hydrogen-bond dynamic processes.
We recently developed a solution-compatible quantum mechanical tunnelling (QMT) probe, featuring a gold nanogap junction with a fixed spacing of sub-5 nm, enabling single-molecule analysis through tunnelling current monitoring [27,28]. Herein, we demonstrate the long-term monitoring (>10 min) of dynamic intermolecular hydrogen-bond interactions employing the QMT probes in solution. Especially, 4-Mercaptobenzoic acid (4-MBA) molecules are immobilised on the probe surface via thiol–gold coordination, and dynamically form intermolecular hydrogen-bond networks. The hydrogen-bonded supramolecular junctions exhibit distinct multilevel conductance distributions across nonpolar, weakly polar, and polar physiological solutions. Furthermore, the functionalised QMT probes have been successfully extended to tunnelling-based hydrogen-bond recognition of D(−)/L(+)-tartaric acid molecules.
2. Materials and Methods
2.1. Materials
Gold-plating solution was obtained from Shenzhen Tianyue New Material Technology Co., Ltd. (Shenzhen, China) Potassium hydroxide (KOH), anhydrous ethanol, potassium chloride (KCl), 1,2,4-trichlorobenzene (TCB), and L(+)-tartaric acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) D(−)-tartaric acid was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) Phosphate-buffered saline (PBS) and 4-mercaptobenzoic acid (4-MBA) were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. (Shanghai, China) Trichloromethane (TCM) was obtained from Yonghua Chemical Co., Ltd. (Suzhou, China) Dual-barrel quartz capillaries (outer diameter: 1.2 mm; inner diameter: 0.9 mm; length: 120 mm) were purchased from Friedrich & Dimmock, Inc. (Millville, NJ, USA) Single-barrel quartz capillaries (outer diameter: 2.5 mm; inner diameter: 1.5 mm; length: 100 mm) were purchased from Donghai Donghua Quartz Products Co., Ltd. (Lianyungang, China) Copper wire (diameter: 0.5 mm), silver wire (diameter: 0.25 mm), and platinum wire (diameter: 0.25 mm) were purchased from Beijing Zhongjinyan New Material Technology Co., Ltd. (Beijing, China)
2.2. Fabrication and Characterisation of the QMT Probe
The detailed fabrication of the QMT probes has been reported in our prior work [29]. Briefly, a dual-barrel quartz capillary was pulled using a laser puller (P-2000, Sutter Instrument, Novato, CA, USA) to yield a dual-channel probe with a tip diameter of ~100 nm. Under argon protection, butane gas was introduced into the capillary, enabling pyrolytic deposition of a carbon electrode layer at the probe tip. Copper wires were inserted as external leads. An electrochemical etching process (the electrolyte: mixed solution of 0.2 M KCl and 0.2 M KOH, 1:1, v/v; working electrodes: two external Cu leads; quasi-reference electrode: Ag/AgCl; counter electrode: platinum wire) was then employed to remove the carbon layer between the two barrels, breaking the conductive connection, followed by feedback-controlled electrochemical deposition of Au to establish a sub-5 nm tunnelling gap. The probe was rinsed with 18.2 MΩ·cm deionised water and soaked for over 24 h to achieve surface reshaping, yielding a stable nanoscale gap structure.
Electrical characterisation was conducted under voltage-clamp mode using a MultiClamp 700B amplifier and Axon Digidata 1550B digitiser (Molecular Devices, San Jose, CA, USA), with a sampling rate of 100 kHz and a 10 kHz low-pass filter. The recorded current–voltage curves were fitted with the Simmons tunnelling model [30] (Figure S1) to extract the nanoscale gap distance. Raman characterisation was performed using a custom-built optical system, with spectra collected on a Raman spectrometer (TriVista, Teledyne Princeton Instruments, Trenton, NJ, USA).
2.3. Preparation of Flow Cell
A flow-through testing cell was constructed using a capillary glass tube with an inner diameter of 1.5 mm. The tube was placed at an incline, with the QMT probe (outer diameter 1.2 mm) inserted from the upper inlet. Upon injection of 20 μL solution, capillary action retained the liquid inside the tube to establish the testing environment. During continuous injection, the solution flowed along the tube wall, immersing the probe tip, and was refreshed as gravity drove the liquid out through the lower outlet.
2.4. Preparation of 4-MBA-Functionalised QMT Probes
The QMT probes were rinsed three times alternately with deionised water and ethanol, and subsequently immersed in 1 mM 4-MBA ethanol solution for at least 4 h to ensure molecular immobilisation on the gold nanoelectrode pair. After modification, the QMT probes were sequentially soaked in deionised water and ethanol for 30 s each to remove physically adsorbed residues, and then immediately transferred into the testing solution for electrical characterisation.
2.5. Hydrogen-Bond Recognition Tunnelling Measurements
The 4-MBA–functionalised probe was fixed in the flow cell, into which 20 μL of PBS solution (1 mM, pH 7.4) was first introduced, and tunnelling current was recorded for 30 min. Subsequently, PBS containing 50 μM D(−)-tartaric acid was injected, and electrical measurements were performed for an additional 30 min. After rinsing the system three times with fresh PBS to remove residual tartaric acid, PBS containing 50 μM L(+)-tartaric acid was introduced, and signals were collected for the same duration. Throughout the process, the nanoelectrode pair at the probe tip was kept fully immersed in solution.
3. Results and Discussion
3.1. Functionalisation and Characterisation of QMT Probe
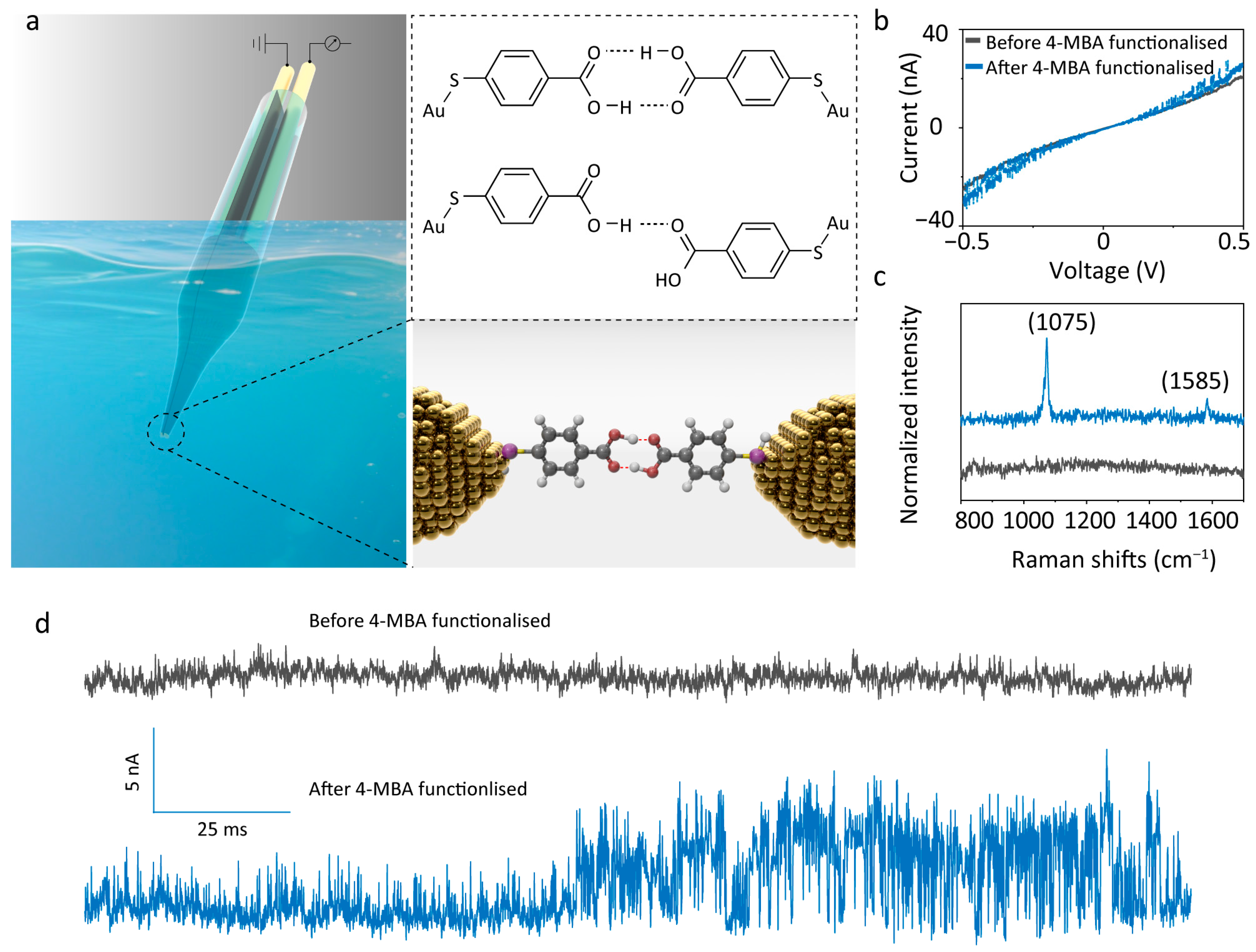
4-MBA is an aromatic molecule that contains a para-positioned thiol group (–SH) and a carboxyl group (–COOH) on the benzene ring: the strong affinity between the thiol and gold electrodes enables covalent anchoring, while the carboxyl group, serving as both a hydrogen-bond donor and acceptor, facilitates intermolecular dimerisation through hydrogen bonding (Figure 1a). In addition, the central benzene backbone enhances conductance across the molecular junction, thereby improving the signal-to-noise ratio of the tunnelling current. Figure 1b presents the current–voltage (I–V) characterisations of the QMT probe before and after 4-MBA modification. Prior to modification, the curve displayed pronounced nonlinearity, confirming excellent typical tunnelling current performance. The fluctuation-free profile indicated that the electrode gap was free of contaminants. After modification, a slight shift in the curve was observed, indicating the analyte attached to the electrode surface [27]. In addition, distinct current fluctuations were detected, confirming the presence of target analyte molecules within the nanogap. At a fixed bias of 100 mV, the post-modification tunnelling current–time curve (Figure 1d) exhibited pronounced switching peaks. This “telegraph noise” feature arises from modulation of the tunnelling barrier height as analyte molecules enter the tunnelling sensing region, thereby inducing current fluctuations [31]. To further verify molecular modification, Raman measurements were performed in air. A 785 nm excitation laser was focused on the gold nanogap junction, where localised surface plasmon resonance (LSPR) produced signal enhancement of up to three orders of magnitude, enabling effective detection of molecules confined within the nanogap [32,33]. The Raman spectrum in Figure 1c shows no characteristic peaks before modification, whereas two prominent peaks appear after modification: the band at 1075 cm−1 is assigned to the in-plane C–H deformation mode of the benzene ring (ν12), and the band at 1585 cm−1 corresponds to the symmetric C=C stretching mode of the benzene backbone (ν8a), corresponding to 4-MBA [34,35]. Collectively, these results confirm the successful functionalisation of 4-MBA molecules onto the gold nanoelectrode pair.
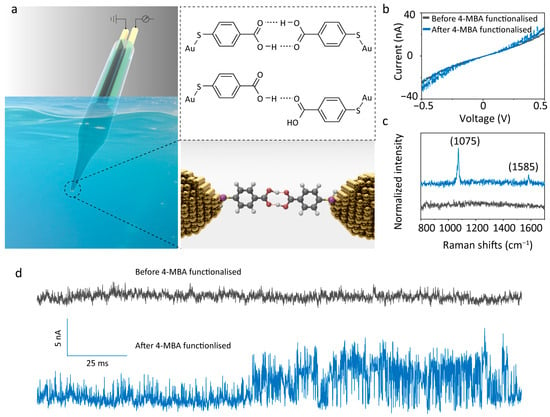
Figure 1.
Characterisation of the 4-MBA-functionalised QMT probes. (a) Schematic illustration of the 4-MBA-functionalised QMT probe and the chemical structure of the 4-MBA dimer formed by hydrogen bonds. (b) I–V traces, (c) Raman spectrum, and (d) current–time traces of the QMT probes recorded pre- and post-functionalisation with 4-MBA. The typical root-mean-square (RMS) noise level of the probe before functionalisation was approximately 0.43 nA (with a mean tunnelling current of 17.46 ± 0.43 nA).
3.2. Conductance Measurement of 4-MBA Supramolecular Junctions
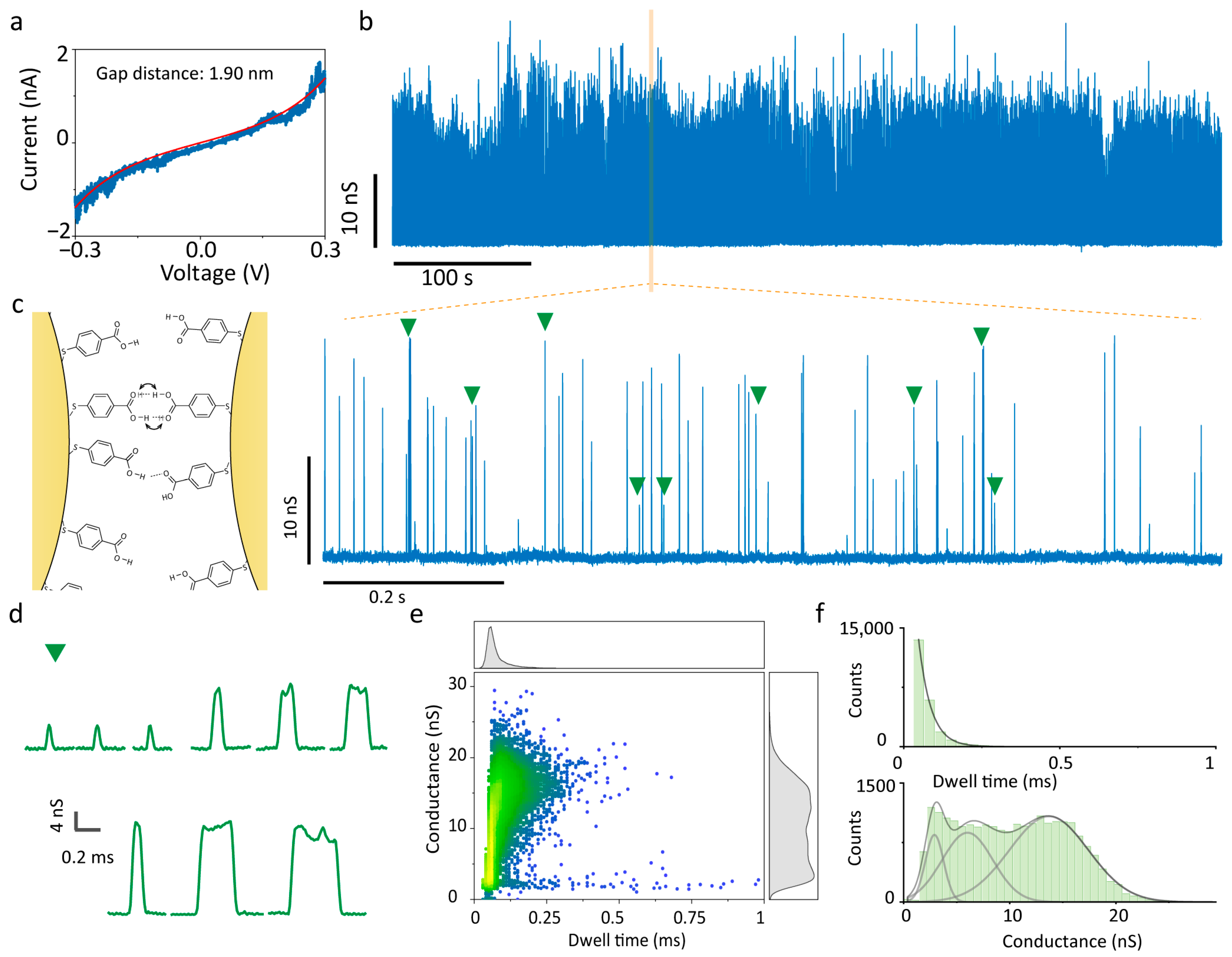
Subsequently, the 4-MBA-functionalised QMT probe was transferred into a fresh test solution for electrical measurements. Figure 2a presents the results of the 4-MBA-modified QMT probe with a gap distance of 1.90 nm measured in the nonpolar solvent TCB. In this medium, 4-MBA remains in a neutral state, with intact hydrogen-bond donors and acceptors, allowing for facile intermolecular association through hydrogen bonding. Owing to the high stability of the nanogap structure, tunnelling current fluctuations induced by hydrogen bonding could be continuously monitored over thirty minutes, during which the current trace exhibited intermittent spiking signals, and the voltage-independent conductance trace was extracted by calculating I/V (Figure 2b, Figures S2 and S3). A magnified view (Figure 2c) reveals that the conductance spikes are sparse and display multistate features, with representative events shown in Figure 2d. Statistical analysis of 23,069 conductance spikes in terms of amplitude and duration (Figure 2e) demonstrates that amplitudes span 1 nS to 30 nS: peaks with amplitudes <5 nS are short-lived and narrowly distributed (~0.1 ms), while those >5 nS exhibit broader durations (~0.3 ms), consistent with the representative states in Figure 2d. Kernel density estimation (KDE) analysis (Figure 2e, right panel), which substitutes continuous density curves for histograms to avoid bin-size dependence while retaining fine features, indicates a three-state distribution of current amplitudes. The histograms of dwell time (Figure 2f) show that durations follow a single-exponential decay with average lifetimes of τ = 39.14 ± 0.39 µs, confirming the characteristic of single-molecule events [36,37]. The histogram of amplitude was Gaussian-fitted with centres at 2.90 ± 1.73 nS, 6.02 ± 4.78 nS, and 13.69 ± 7.45 nS. Given that 4-MBA molecules are covalently anchored to the electrode surfaces and thus cannot diffuse freely, no external hydrogen-bond donors or acceptors were provided by TCB. It is preliminarily deduced that the observed conductance peaks are attributable to dynamic intermolecular hydrogen-bond bridges formed between surface-bound 4-MBA molecules. The fitted tunnelling gap (1.90 nm) is slightly larger than the theoretical length of a 4-MBA dimer (1.80 nm) [38], enabling head-to-head carboxyl group alignment via weak hydrogen bonds, whose repeated rupture and reformation give rise to transient conductance spikes. Otherwise, if the terminally tethered molecules are non-contacting, no telegraphic spikes arise unless analytes bridge the gap via hydrogen bonding [39]. Moreover, since the carboxyl group can serve as both a donor and acceptor, alternative misaligned single-hydrogen-bond interactions may also contribute to the observed multimodal conductance signals.
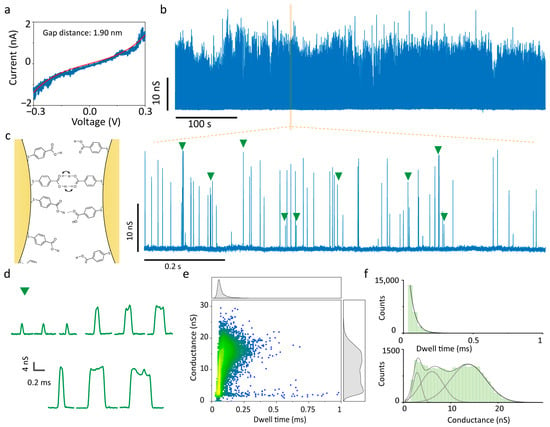
Figure 2.
Electrical measurements of 4-MBA-functionalised QMT probes with a wide tunnelling gap in TCB. (a) I–V trace of the QMT probe with Simmons tunnelling model fitting, indicating a gap distance of 1.90 nm. (b) Representative 10 min conductance trace, (c) zoomed-in conductance trace, and (d) typical events recorded in TCB. The typical events corresponding to the green areas marked in (c). The left panel of (c) shows a schematic of a proposed hydrogen-bonded configuration of the 4-MBA dimer junction. The arrows denote the intermolecular proton transfer process, which is a proposed mechanism for the observed conductance states. (e) Scatter plots and accompanying kernel density estimation of conductance versus dwell time; conductance spikes. (f) Histograms of dwell time and peak conductance with single-exponential decay and multiple Gaussian distributions, respectively.
3.3. Solvent-Dependent Measurements of 4-MBA Supramolecular Junctions
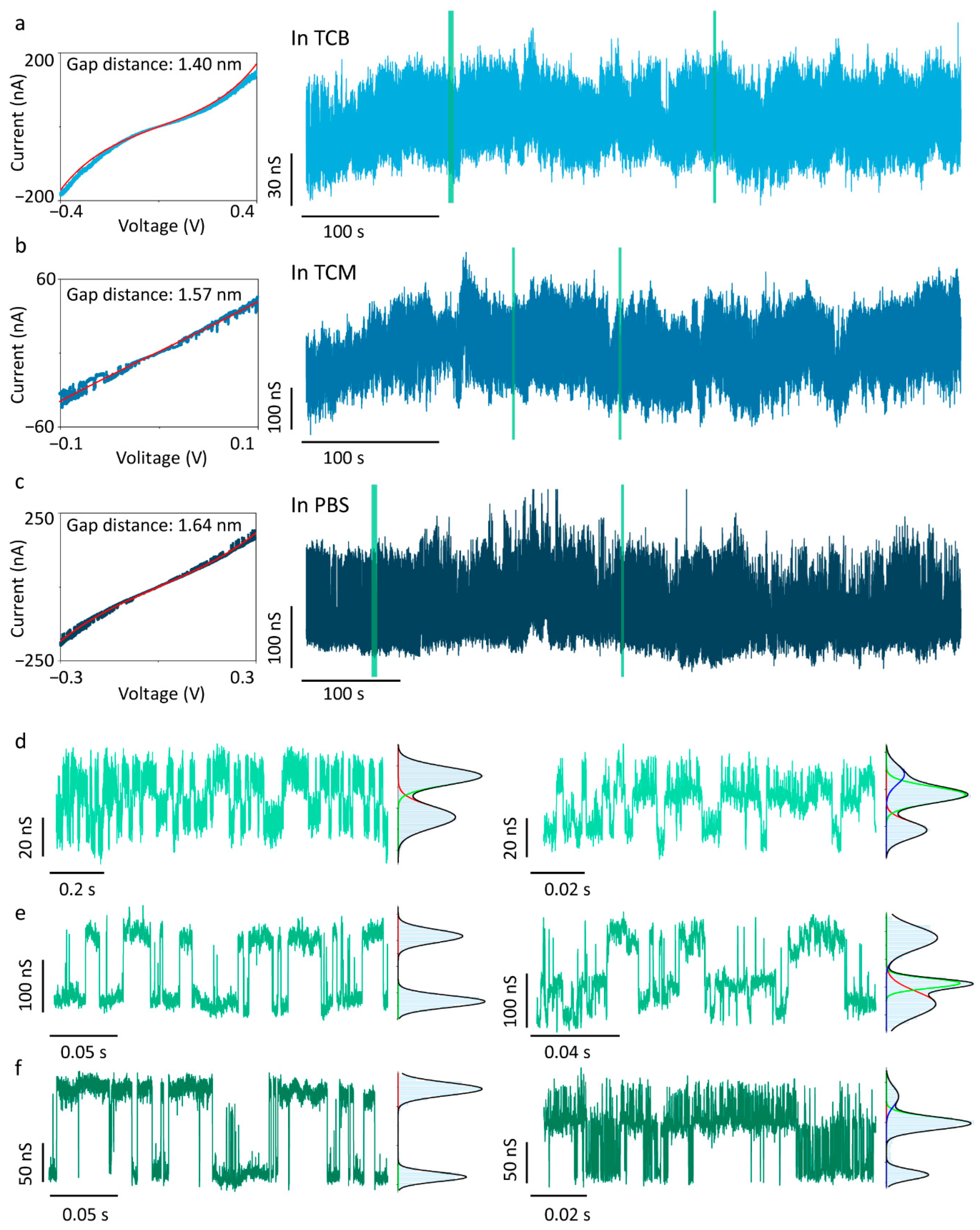
To further validate the intermolecular hydrogen bonding between 4-MBA molecules, a 4-MBA-functionalised QMT probe with a fitted nanogap of 1.40 nm was investigated in TCB (Figure 3a). This gap distance is smaller than the theoretical 4-MBA dimer length but larger than the monomer size, forcing surface-anchored 4-MBA molecules into close contact and thereby strengthening hydrogen-bond interactions [40]. The conductance trace exhibits continuous multimodal distributions, with each state’s duration extended by three to four orders of magnitude compared to the 4-MBA-functionalised QMT probe with a wider gap (Figure 2c). Figure 3d shows two segments of the conductance trace: one showing a bimodal distribution, the other a trimodal distribution. The high-state conductance and low-state conductance in the two-state Gaussian-fitted conductance are 379.35 ± 8.15 nS (GBH) and 363.11 ± 10.10 nS (GBL), whereas the three-state values are 365.11 ± 9.03 nS (GTL), 380.90 ± 8.50 nS (GTM), and 390.11 ± 10.60 nS (GTH), respectively. In addition to such stochastic transitions, multi-level conductance traces have also been reported in previous studies and are attributed to intermolecular proton transfer processes within supramolecular junctions mediated by hydrogen-bond interactions [24]. These results confirm that 4-MBA hydrogen-bond interactions in the tunnelling junction exhibit multistate conductance features, which are more pronounced in compact nanogap devices.
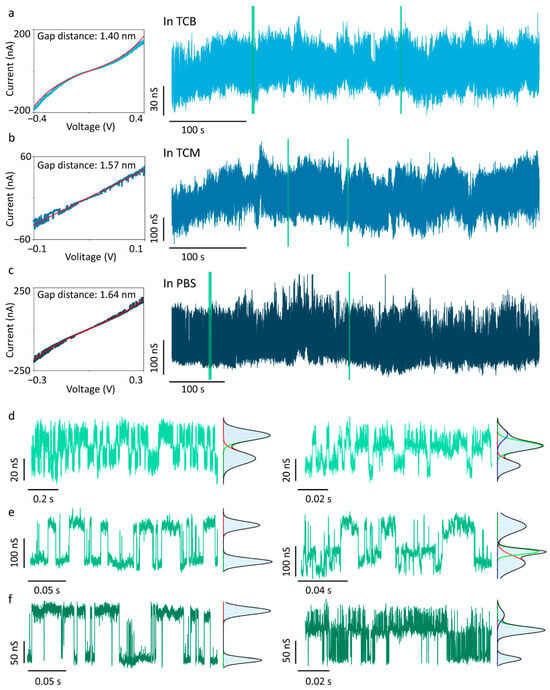
Figure 3.
Solvent-independent measurements of the 4-MBA-functionalised QMT probes with a compact nanogap. I–V traces with Simmons tunnelling model fitting and representative conductance trace of QMT probes measured in (a) TCB, (b) TCM, and (c) PBS. (d–f) The enlarged views of conductance traces and corresponding histograms from the green-marked regions in (a–c). The histograms show bimodal or trimodal distributions for selected segments.
Intermolecular hydrogen-bond interactions in solution are strongly modulated by solvent effects: nonpolar solvents enhance these interactions, whereas polar solvents weaken them [41]. In bulk solution, polar solvents diminish hydrogen-bond strength through competition for hydrogen-bonding sites and dielectric screening; however, the effect becomes far more complex under nanoconfinement, and its fundamental mechanism remains a topic of ongoing research. Traditional ensemble approaches, combining nuclear magnetic resonance (NMR) with the Hunter model, can analyse solvent effects on hydrogen-bond networks energetically [42], but these methods cannot be extended to the single-molecule scale. Consequently, studies on solvent effects at the single-molecule level remain scarce. To investigate solvent influence on 4-MBA intermolecular hydrogen bonding, the electrical measurement of functionalised QMT probes was conducted in TCM (Figure 3b,e) and PBS (Figure 3c,f). TCM is a weakly polar, aprotic organic solvent that provides minimal hydrogen-bond-accepting capability but no donor ability, exerting only slight perturbation on 4-MBA interactions. PBS is a polar solvent, exhibiting strong hydrogen-bond competition, which significantly weakens intermolecular hydrogen-bond interactions under macroscopic chemical conditions, making measurements highly challenging [43]. From a biological perspective, however, most hydrogen-bond-containing biomacromolecules reside in environments analogous to PBS, where hydrogen-bond networks are stable and maintain molecular function [7]. Single-molecule hydrogen-bond measurements in PBS are therefore critical for investigating molecular hydrogen-bond structures in vivo. As shown in Figure 3, the long-term conductance measurements in different solvents, including TCB, TCM, and PBS, consistently display both bimodal and trimodal features. The conductance values of different states in different solvents are listed in Table 1 for comparison. Owing to baseline drift caused by environmental noise, the relative populations of conductance states in long-term conductance traces cannot be reliably quantified, limiting direct kinetic analysis of 4-MBA intermolecular hydrogen bonds. However, we observe that each conductance state exhibits both spike- and stage-like features, with lifetimes spanning tens of microseconds to hundreds of milliseconds. Signals at the μs scale are constrained by the 100 kHz sampling rate, suggesting the possibility of even shorter lifetimes. This finding is in agreement with the previous reports that hydrogen-bond lifetimes can range from nanoseconds to seconds [44,45,46]. Nonetheless, our results indicate that 4-MBA molecules anchored at both ends of the nanogap can still form molecular bridges via intermolecular hydrogen bonding, even in highly polar PBS solution.

Table 1.
Comparisons of the conductance states of 4-MBA hydrogen bonds in different solvents.
The configuration of the tunnelling junction generates a nanoconfined region, resulting in low ion concentration near the nanogap and further creating low-polarity internal microenvironments that stabilise hydrogen-bond networks even in polar physiological conditions [47,48,49,50,51]. Moreover, the gap size matches the dimer length, forcing confined 4-MBA molecules into a head-to-head orientation that promotes carboxyl–carboxyl hydrogen-bond formation [40]. The nanoscale confinement mimics the low-polarity microenvironment inside biomolecules, stabilising the hydrogen-bond network and enabling the formation and characterisation of 4-MBA supramolecular junctions via hydrogen bonds in polar PBS solution.
3.4. Recognition Tunnelling via 4-MBA-Functionalised QMT Probe
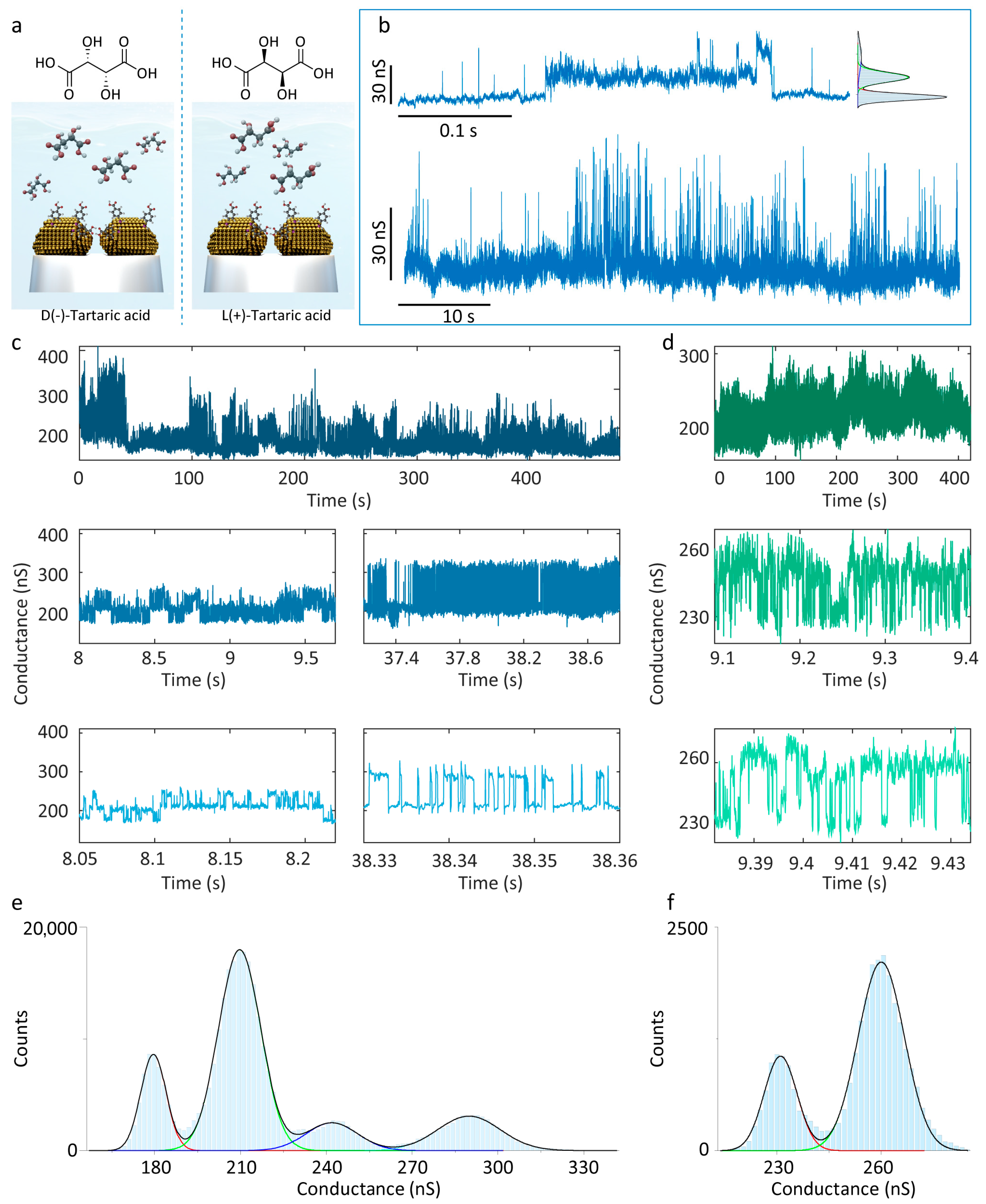
Recognition tunnelling has been established on STM as an effective biomolecule recognition strategy, encompassing targets such as nucleobases [52,53], amino acids [54], and proteins [55]. Here, we confirmed the flexibility of the 4-MBA-functionalised QMT probe for the application of biomolecular recognition, using a panel of D(−)/L(+)-tartaric acid (Figure S4). The molecular structures (Figure 4a) contain two carboxyl groups (-COOH), two secondary hydroxyl groups (-CHOH-), and two hydroxyl groups (-OH), providing multiple hydrogen-bond donor and acceptor sites. Firstly, careful control experiments performed by detecting the D(−)/L(+)-tartaric acid were carried out utilising bare QMT probes. The conductance distributions showed minor differences between D(−)/L(+)-tartaric acid, which is insufficient for discrimination (Figures S5 and S6). Subsequently, the 4-MBA-functionalised QMT probe was employed for hydrogen-bond recognition tunnelling tests on D(−)- and L(+)-tartaric acid solutions. In the PBS solution, the conductance trace exhibits a three-state distribution (Figure 4b) with centres at 131.28 ± 6.11 nS, 147.64 ± 9.26 nS, and 164.10 ± 26.25 nS, and a maximum-to-minimum conductance difference of 32.82 nS. In D(−)-tartaric acid solution, higher conductance states emerged (Figure 4c), while magnified views reveal characteristic distributions reflecting 4-MBA intermolecular hydrogen-bond interactions (Figure 4c, bottom left). Histogram analysis yields central values at 179.58 ± 10.46 nS, 209.68 ± 17.41 nS, 241.90 ± 21.21 nS, and 289.85 ± 25.98 nS. Obviously, the newly emerged high-conductance state arises from hydrogen bonding between D(−)-tartaric acid and 4-MBA. By contrast, measurements in L(+)-tartaric acid solution over extended periods show no comparable high-conductance states, and the distribution is narrower; analysis of stable segments reveals only a two-state distribution (centres at 231.06 ± 11.12 and 260.01 ± 15.62) with a difference of 28.95 nS. At this stage, the signal origin is ambiguous: it may arise solely from 4-MBA hydrogen bonding, or the L(+)-tartaric acid–4-MBA interaction may be masked. Nonetheless, these results indicate that the 4-MBA-functionalised QMT probe can effectively identify D(−)-tartaric acid through the emergence of new conductance states, enabling single-molecule discrimination of the two enantiomers. Compared with conventional STM platforms, the QMT probe offers advantages in portability, economy, and operational flexibility, enabling the extension of hydrogen-bond recognition tunnelling to broader biomolecular detection and facilitating in situ cellular characterisation.
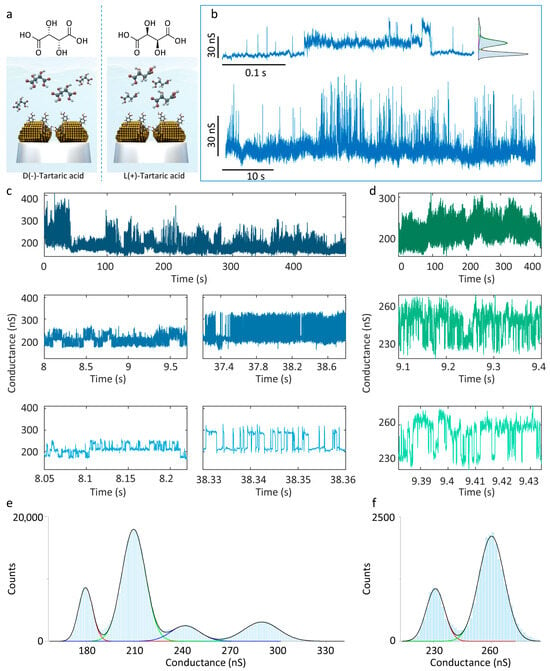
Figure 4.
Conductance measurements of D(−)/L(+)-tartaric acid. (a) Schematic diagram of 4-MBA-functionalised QMT probe recognises tartaric acid and the chemical structure of D(−)/L(+)-tartaric acid. (b) Representative conductance traces and corresponding histogram recorded in pure PBS solution. (c) Representative conductance traces measured in D(−)-tartaric acid solution. The zoomed-in section depicts the three-state features as a background signal for 4-MBA hydrogen-bond interaction, and the emergence of a high conductance state. (d) Representative conductance traces and zoomed-in section measured in L(+)-tartaric acid solution. The conductance histograms of (e) D(−)-tartaric and (f) L(+)-tartaric acid with Gaussian fitting, showing four states and two state distributions, respectively.
4. Conclusions
In summary, we have employed 4-MBA-functionalised QMT probes to achieve long-term single-molecule carboxyl–carboxyl hydrogen bonding in TCB, TCM, and PBS solutions, consistently observing multi-level conductance distributions. This persistence of intermolecular hydrogen bonding indicates that solvent competition, prevalent in bulk experiments, is markedly suppressed within the nanoconfined tunnelling junction, thereby preserving hydrogen-bond stability, which is an effect reminiscent of the protective hydrophobic interiors in biomolecules. In recognition tunnelling, D(−)-tartaric acid produced an additional high-conductance state, corresponding to hydrogen-bonding with surface-anchored 4-MBA, whereas L(+)-tartaric acid exhibited only the intrinsic bimodal distribution covered by 4-MBA dimer interactions. These results demonstrate the unique advantage of quantum mechanical tunnelling probes in resolving intermolecular interactions and establish a versatile platform for supramolecular chemistry, biosensing, and molecular electronics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors13100360/s1, Figure S1: The typical I–V curve of a bare tunnelling probe recorded in water. The red solid line represents a fit to the Simmons model. Figure S2: I–V curve of a 4-MBA-functionalised probe. Discrete current jumps show the voltage dependency. Figure S3: Representative current–time curves and conductance-time of a 4-MBA-functionalised probe at 100 mV and 400 mV. Figure S4: Circular dichroism spectra of D(−) and L(+) tartaric acid; Figure S5: Representative conductance traces, zoomed-in section, and corresponding histogram measured in D(−)-tartaric acid solution using non-functionalised QMT probes; Figure S6: Representative conductance traces, zoomed-in section, and corresponding histogram measured in D(−)-tartaric acid solution using non-functionalised QMT probes.
Author Contributions
Conceptualisation, L.T. and B.-F.Z.; methodology, B.-F.Z.; software, Y.Y.; validation, B.-F.Z., C.Y., L.Y. and L.T.; formal analysis, B.-F.Z., L.Y. and Y.Y.; Investigation, B.-F.Z., C.Y., Y.T., Y.Y. and S.F.; Resources, B.-F.Z., Y.T., Y.Y. and S.F.; data curation, B.-F.Z. and Y.Y.; writing—original draft preparation, B.-F.Z. and L.T.; writing—review and editing, B.-F.Z., L.Y., L.T., X.L. and C.K.; supervision, L.T., X.L. and C.K.; project administration, B.-F.Z. and L.T.; funding acquisition, L.T., X.L. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant nos. 62127818, 22374129, 62125504), the Natural Science Foundation of Zhejiang Province (grant no. LR22F050003), and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2024R0100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any additional data in support of the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Wang, L.; Zhao, L.; Zhang, Y.; Li, Z.-T.; Huang, F. Multiple hydrogen bonding driven supramolecular architectures and their biomedical applications. Chem. Soc. Rev. 2024, 53, 1592–1623. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Ren, X.; Tao, Z. Hydrogen-bond chemistry in rechargeable batteries. Joule 2023, 7, 2700–2731. [Google Scholar] [CrossRef]
- Lin, R.-B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef]
- Reek, J.N.H.; de Bruin, B.; Pullen, S.; Mooibroek, T.J.; Kluwer, A.M.; Caumes, X. Transition metal catalysis controlled by hydrogen bonding in the second coordination sphere. Chem. Rev. 2022, 122, 12308–12369. [Google Scholar] [CrossRef] [PubMed]
- Bondar, A.-N. Graphs of hydrogen-bond networks to dissect protein conformational dynamics. J. Phys. Chem. B 2022, 126, 3973–3984. [Google Scholar] [CrossRef] [PubMed]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water determines the structure and dynamics of proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Livshits, G.I.; Stern, A.; Rotem, D.; Borovok, N.; Eidelshtein, G.; Migliore, A.; Penzo, E.; Wind, S.J.; Di Felice, R.; Skourtis, S.S.; et al. Long-range charge transport in single G-quadruplex DNA molecules. Nat. Nanotechnol. 2014, 9, 1040–1046. [Google Scholar] [CrossRef]
- Cleland, W.W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef]
- Ishikita, H.; Saito, K. Proton transfer reactions and hydrogen-bond networks in protein environments. J. R. Soc. Interface 2014, 11, 20130518. [Google Scholar] [CrossRef]
- Saito, K.; Rutherford, A.W.; Ishikita, H. Mechanism of proton-coupled quinone reduction in Photosystem II. Proc. Natl. Acad. Sci. USA 2012, 110, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zeng, B.-F.; Zhang, B.; Tang, L. Single-molecular protein-based bioelectronics via electronic transport: Fundamentals, devices and applications. Chem. Soc. Rev. 2023, 52, 5968–6002. [Google Scholar] [CrossRef]
- Kretsch, R.C.; Li, S.; Pintilie, G.; Palo, M.Z.; Case, D.A.; Das, R.; Zhang, K.; Chiu, W. Complex water networks visualized by cryogenic electron microscopy of RNA. Nature 2025, 642, 250–259. [Google Scholar] [CrossRef]
- Wang, C.; Geng, X.; Chen, J.; Wang, H.; Wei, Z.; Huang, B.; Liu, W.; Wu, X.; Hu, L.; Su, G.; et al. Multiple H-bonding cross-linked supramolecular solid–solid phase change materials for thermal energy storage and management. Adv. Mater. 2024, 36, e2309723. [Google Scholar] [CrossRef]
- Ladenthin, J.N.; Frederiksen, T.; Persson, M.; Sharp, J.C.; Gawinkowski, S.; Waluk, J.; Kumagai, T. Force-induced tautomerization in a single molecule. Nat. Chem. 2016, 8, 935–940. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, P.; Yuan, B.; Ji, W.; Cheng, Z.; Qiu, X. Real-space identification of intermolecular bonding with atomic force microscopy. Science 2013, 342, 611–614. [Google Scholar] [CrossRef]
- Pullanchery, S.; Kulik, S.; Rehl, B.; Hassanali, A.; Roke, S. Charge transfer across C–H⋅⋅⋅O hydrogen bonds stabilizes oil droplets in water. Science 2021, 374, 1366–1370. [Google Scholar] [CrossRef]
- Nishino, T.; Hayashi, N.; Bui, P.T. Direct measurement of electron transfer through a hydrogen bond between single molecules. J. Am. Chem. Soc. 2013, 135, 4592–4595. [Google Scholar] [CrossRef] [PubMed]
- Pirrotta, A.; De Vico, L.; Solomon, G.C.; Franco, I. Single-molecule force-conductance spectroscopy of hydrogen-bonded complexes. J. Chem. Phys. 2017, 146, 092329. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhao, Z.H.; Li, A.X.; Wang, L. Electron transport through hydrogen bonded single-molecule junctions. Chin. J. Chem. 2023, 41, 3433–3446. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yao, C.; Xu, J.; Wang, D.; Zhao, X.; Li, X.; Liu, J.; Hong, W. Interface phenomena in molecular junctions through noncovalent interactions. Langmuir 2025, 41, 5705–5735. [Google Scholar] [CrossRef]
- Wimmer, M.; Palma, J.L.; Tarakeshwar, P.; Mujica, V. Single-molecule conductance through hydrogen bonds: The role of resonances. J. Phys. Chem. Lett. 2016, 7, 2977–2980. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.L.; Li, S.Y.; Hong, W.; Zhong, Y.W.; Wang, D.; Wan, L.J. Molecular conductance through a quadruple-hydrogen-bond-bridged supramolecular junction. Angew. Chem. Int. Ed. 2016, 55, 12393–12397. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.X.; Gong, Z.L.; Jia, C.C.; Lin, Y.W.; Gu, C.H.; He, G.; Zhong, Y.W.; Yang, J.L.; Guo, X.F. Direct observation of single-molecule hydrogen-bond dynamics with single-bond resolution. Nat. Commun. 2018, 9, 807. [Google Scholar] [CrossRef]
- Chang, S.; Huang, S.; He, J.; Liang, F.; Zhang, P.; Li, S.; Chen, X.; Sankey, O.; Lindsay, S. Electronic signatures of all four DNA nucleosides in a tunneling gap. Nano Lett. 2010, 10, 1070–1075. [Google Scholar] [CrossRef]
- Chang, S.; He, J.; Kibel, A.; Lee, M.; Sankey, O.; Zhang, P.; Lindsay, S. Tunnelling readout of hydrogen-bonding-based recognition. Nat. Nanotechnol. 2009, 4, 297–301. [Google Scholar] [CrossRef]
- Tang, L.; Yi, L.; Jiang, T.; Ren, R.; Paulose Nadappuram, B.; Zhang, B.; Wu, J.; Liu, X.; Lindsay, S.; Edel, J.B.; et al. Measuring conductance switching in single proteins using quantum tunneling. Sci. Adv. 2022, 8, eabm8149. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Nadappuram, B.P.; Cadinu, P.; Zhao, Z.; Xue, L.; Yi, L.; Ren, R.; Wang, J.; Ivanov, A.P.; Edel, J.B. Combined quantum tunnelling and dielectrophoretic trapping for molecular analysis at ultra-low analyte concentrations. Nat. Commun. 2021, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yi, L.; Liu, X.; Ivanov, A.P.; Edel, J.B.; Tang, L. Fabrication of electron tunneling probes for measuring single-protein conductance. Nat. Protoc. 2023, 18, 2579–2599. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized formula for the electric tunnel effect between similar electrodes separated by a thin insulating film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef]
- Albrecht, T. Electrochemical tunnelling sensors and their potential applications. Nat. Commun. 2012, 3, 829. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Käll, M. Surface-plasmon-enhanced optical forces in silver nanoaggregates. Phys. Rev. Lett. 2002, 89, 246802. [Google Scholar] [CrossRef]
- Zeng, B.-F.; Deng, R.; Zou, Y.-L.; Huo, C.-A.; Wang, J.-Y.; Yang, W.-M.; Liang, Q.-M.; Qiu, S.-J.; Feng, A.; Shi, J.; et al. Optical trapping of a single Molecule of length sub-1 nm in solution. CCS Chem. 2023, 5, 830–840. [Google Scholar] [CrossRef]
- Huang, S.C.; Wang, X.; Zhao, Q.Q.; Zhu, J.F.; Li, C.W.; He, Y.H.; Hu, S.; Sartin, M.M.; Yan, S.; Ren, B. Probing nanoscale spatial distribution of plasmonically excited hot carriers. Nat. Commun. 2020, 11, 4211. [Google Scholar] [CrossRef]
- Huh, H.; Trinh, H.D.; Lee, D.; Yoon, S. How does a plasmon-induced hot charge carrier break a C-C bond? ACS Appl. Mater. Interfaces 2019, 11, 24715–24724. [Google Scholar] [CrossRef]
- Needham, L.M.; Saavedra, C.; Rasch, J.K.; Sole-Barber, D.; Schweitzer, B.S.; Fairhall, A.J.; Vollbrecht, C.H.; Wan, S.S.; Podorova, Y.; Bergsten, A.J.; et al. Label-free detection and profiling of individual solution-phase molecules. Nature 2024, 629, 1062–1068. [Google Scholar] [CrossRef]
- Dief, E.M.; Low, P.J.; Díez-Pérez, I.; Darwish, N. Advances in single-molecule junctions as tools for chemical and biochemical analysis. Nat. Chem. 2023, 15, 600–614. [Google Scholar] [CrossRef]
- Bui, P.T.; Nishino, T. Electron transfer through coordination bond interaction between single molecules: Conductance switching by a metal ion. Phys. Chem. Chem. Phys. 2014, 16, 5490–5494. [Google Scholar] [CrossRef]
- Lindsay, S.; He, J.; Sankey, O.; Hapala, P.; Jelinek, P.; Zhang, P.; Chang, S.; Huang, S. Recognition tunneling. Nanotechnology 2010, 21, 262001. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Guo, J.; Chang, S.; Wang, X.; Zhou, J.; Liang, F.; He, J. Modulating and probing the dynamic intermolecular interactions in plasmonic molecule-pair junctions. Phys. Chem. Chem. Phys. 2019, 21, 15940–15948. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Solvent effects on hydrogen bonding. Angew. Chem. Int. Ed. 2007, 46, 3706–3709. [Google Scholar] [CrossRef]
- Meredith, N.Y.; Borsley, S.; Smolyar, I.V.; Nichol, G.S.; Baker, C.M.; Ling, K.B.; Cockroft, S.L. Dissecting solvent effects on hydrogen bonding. Angew. Chem. Int. Ed. 2022, 61, e202206604. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.T.J.; Wales, S.M.; Tilly, D.P.; Farrar, E.H.E.; Grayson, M.N.; Ward, J.W.; Clayden, J. A molecular communication channel consisting of a single reversible chain of hydrogen bonds in a conformationally flexible oligomer. Chem 2021, 7, 2460–2472. [Google Scholar] [CrossRef]
- Peng, C.S.; Baiz, C.R.; Tokmakoff, A. Direct observation of ground-state lactam–lactim tautomerization using temperature-jump transient 2D IR spectroscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 9243–9248. [Google Scholar] [CrossRef]
- Kumagai, T.; Hanke, F.; Gawinkowski, S.; Sharp, J.; Kotsis, K.; Waluk, J.; Persson, M.; Grill, L. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nat. Chem. 2013, 6, 41–46. [Google Scholar] [CrossRef]
- Nibbering, E.T.J.; Elsaesser, T. Ultrafast vibrational dynamics of hydrogen bonds in the condensed phase. Chem. Rev. 2004, 104, 1887–1914. [Google Scholar] [CrossRef]
- Yi, L.; Jiang, T.; Ren, R.; Cao, J.; Edel, J.B.; Ivanov, A.P.; Tang, L. Quantum mechanical tunnelling probes with redox cycling for ultra-sensitive detection of biomolecules. Angew. Chem. Int. Ed. 2025, 64, e202501941. [Google Scholar] [CrossRef]
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under confinement. Chem. Soc. Rev. 2022, 51, 2491–2543. [Google Scholar] [CrossRef] [PubMed]
- Trushin, M.; Andreeva, D.V.; Peeters, F.M.; Novoselov, K.S. Structure and flow of low-dimensional water. Nat. Rev. Phys. 2025, 7, 502–513. [Google Scholar] [CrossRef]
- Xiao, S.; Wollman, Z.; Xie, Q.; Duan, C. Current monitoring in nanochannels. Microfluid. Nanofluid. 2022, 26, 86. [Google Scholar] [CrossRef]
- Schuster, R.; Kirchner, V.; Xia, X.H.; Bittner, A.M.; Ertl, G. Nanoscale electrochemistry. Phys. Rev. Lett. 1998, 80, 5599–5602. [Google Scholar] [CrossRef]
- Tsutsui, M.; Taniguchi, M.; Yokota, K.; Kawai, T. Identifying single nucleotides by tunnelling current. Nat. Nanotechnol. 2010, 5, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, J.; Chang, S.; Zhang, P.; Liang, F.; Li, S.; Tuchband, M.; Fuhrmann, A.; Ros, R.; Lindsay, S. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010, 5, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ashcroft, B.; Zhang, P.; Liu, H.; Sen, S.; Song, W.; Im, J.; Gyarfas, B.; Manna, S.; Biswas, S.; et al. Single-molecule spectroscopy of amino acids and peptides by recognition tunnelling. Nat. Nanotechnol. 2014, 9, 466–473. [Google Scholar] [CrossRef]
- Zhang, B.; Ryan, E.; Wang, X.; Song, W.; Lindsay, S. Electronic transport in molecular wires of precisely controlled length built from modular proteins. ACS Nano 2022, 16, 1671–1680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).