Abstract
Garlic (Allium Sativum L.) is a source of organosulphur compounds with well-known sensorial and biological activity. Organosulphur precursors of garlic aroma are also detected in the plant leaves, but limited literature on this subject is available. This study is aimed at the characterization of the volatile profile of the floral scapes of Sulmona red garlic (aglio rosso di Sulmona) cultivated in the Abruzzo region (Italy). Floral scapes are manually removed from the plant before flowering and used as an ingredient of local gastronomy. The organosulphur volatile profile of the scapes is investigated by HS-SPME-GC-MS and compared to that provided by the clove. The GC-MS chromatogram of garlic clove, which is characterized by the predominant contribution of a few organosulphur organic compounds, is significantly more intense than that of the scapes. Almost all the organosulphur compounds contributing to the clove aroma were detected in the scape volatile profile, which, however, exhibits a more balanced contribution of major and minor organo sulphur compounds. Moreover, a significantly higher relative abundance of terpenes and aldehydes is observed in the scape aroma. The geographical/varietal origin of clove seeds (Sulmona versus Spain or France) and cultivation area interactively influence the aroma profile of Sulmona red garlic scapes.
1. Introduction
Garlic (Allium sativum L., Liliaceae) has been cultivated since ancient times for its flavoring properties and used worldwide as a culinary spice and herbal remedy for the treatment of many common ailments [1,2]. Apart from its long history in traditional medicine, the beneficial therapeutic properties of garlic have been demonstrated by several in vivo and in vitro as well as clinical studies [3,4,5,6]. The broad range of biological and medical functions attributed to garlic, including cancer- and cardio-protective effects and antimicrobial, anti-inflammatory, antithrombotic, and antiatherosclerotic activities [7,8,9], is associated with the presence of a high content of organosulphur compounds, which are also responsible for its characteristic pungent flavor and taste. Major organosulphur compounds in intact garlic are S-allyl-L-cysteine sulfoxide (alliin) and γ-glutamyl-S-allyl-L-cysteine [10]. When garlic clove tissues are disrupted by cutting or crushing, which promotes the hydrolytic action of the vacuolar enzyme alliinase, alliin and other alk(en)yl cysteine sulfoxides, located in the cytoplasm, are transformed into sulfenic acids and successively by condensation reactions into thiosulfinates [10,11]. Allicin (diallyl thiosulfinate) is the predominant thiosulfinate, accounting for 70–80% of the total thiosulfinates [11]. Thiosulfinates, because of their instability, undergo further rearrangements, leading to a wide variety of derived sulfur compounds, including mono-, di- tri- and polysulfides and vinyldithiins. Sulfides and second generation polysulfides are considered the principal flavour compounds of garlic and other Allium species.
Despite the bulb being the part of garlic mainly used as seasoning in cuisine and traditional medicine, organosulphur precursors are also present in the aerial part of Allium species [12]. For this reason, leaves of Allium plants, generally regarded as waste materials, are potential sources of phytochemicals [13,14,15,16]. In this regard, it is known that the concentration of organosulphur volatile precursors in the garlic leaves is maximum in the early stages of vegetation, but when the leaves begin to wither, they are transferred from the leafy part to the clove [12,17].
Sulmona red garlic (“aglio rosso di Sulmona”) is a typical red Italian garlic ecotype, named for the reddish color of the tunic surrounding the cloves, cultivated in the Peligna Valley (Abruzzo region, Central Italy) [18]. This landrace has been included in the regional list of traditional agri-food products (TAPs), recognised by the Italian legislature as those products “whose methods of processing, storage and maturing are consolidated over time” [19]. Moreover, Slow Food Foundation for Biodiversity included Sulmona red garlic among the small-scale and family-based quality food productions to be protected and valorized [20]. Previous studies [21,22,23,24] demonstrated that Sulmona red garlic exhibits specific composition characteristics that are distinctive of its varietal and geographical origin, making this landrace well distinguishable from other red garlic varieties cultivated in neighboring regions of Central Italy and Sicily.
In garlic cultivation, including Sulmona red garlic, the plants are grown from seed cloves. To encourage bulb development, the floral scapes—the plant’s reproductive structures—are manually removed from the leaf apparatus at a tender stage, well before flowering. Traditionally, these stems are also used as ingredients in local cuisine. For simplicity, garlic floral scapes will be referred to hereafter as scapes. Besides their culinary use, scapes represent a potential source of organosulfur compounds and other beneficial substances [25,26]. Hydroalcoholic extracts from Sulmona red garlic scapes have been previously characterized using multi-analytical approaches to evaluate how different extraction systems affect the recovery of key organosulfur compounds and other phytochemicals [27,28]. However, the volatile organosulfur profile of fresh scapes—which could be highly relevant to the sensory properties of this food specialty—has not yet been investigated.
In the present work, the volatile profile of the floral scape of Sulmona red garlic is determined by Head Space-Solid Phase Micro Extraction-Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS). Preliminarily, the volatile profile acquired from different parts of the scape (base, center, and tip) was compared with that of the garlic bulb. Additionally, the influence of the growing area within the territory designed for cultivating Sulmona red garlic on the volatile profile of the scapes is investigated. Additionally, the volatile profiles of scapes extracted from plants grown in the same field from autochthonous Sulmona red garlic cloves and commercial cloves of different geographical origin (Spain or France) are compared.
2. Materials and Methods
2.1. Samples
Sulmona red garlic scape samples were kindly provided by garlic producers operating in different areas within the territory designed for the cultivation of this variety, namely Sulmona, Prezza, Campo di Fano, Pacentro, and Raiano. The scapes were extracted in May 2021 from garlic plants grown from either Sulmona red garlic cloves or cloves of commercial red garlic varieties coming from non-Italian countries (Spain or France).
Due to the impossibility of analyzing all the samples soon after harvesting, the scapes were maintained under frozen conditions and thawed immediately before analysis. The analyses were conducted on five biological replicates for each group. To investigate the distribution of the organosulphur volatiles within the scape, 600 mg of sample was extracted from the base, centre, or tip of scapes of Sulmona red garlic plants cultivated near Campo di Fano. For comparative purposes, the volatile profiles of six biological replicates of Sulmona Red Garlic bulbs coming from Campo di Fano were determined using an equivalent amount (600 mg) of bulb tissue subjected to the same freezing and thawing procedure. To evaluate the influence of the cultivation area and clove seed origin on the volatile profile, the central part of the scape was analyzed.
2.2. Headspace Solid-Phase Micro-Extraction Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) Analysis
An HS-SPME-GC-MS procedure previously applied for geographical tracing of Italian red garlic varieties [24] was adapted to this study. A polydimethylsiloxane (PDMS)-coated SPME fiber (100 μm thickness; Supelco, Bellefonte, PA, USA) was used for volatile compound extraction. For headspace sampling, frozen floral scapes were allowed to thaw at room temperature for a few min, then cut into small pieces and gently blotted with filter paper to remove excess surface moisture. Approximately 600 mg of the cut sample was transferred into a 4 mL glass vial sealed with a Mininert® cap for SPME. The vial was placed in an oil bath maintained at 45 °C under continuous magnetic stirring. HS-SPME was performed by exposing the PDMS fiber to the sample headspace for 15 min. After extraction, the fiber was retracted into the needle, removed from the vial, and immediately inserted into the GC injection port for thermal desorption at 250 °C for 3 min. After each run, the fiber was reconditioned in the GC injection port at 280 °C for 15 min, followed by a blank run to ensure the absence of carryover. All analyses were conducted using a Trace 1300 ISQ LT GC–MS system (Thermo Fisher Scientific, Waltham, MA, USA). The GC was equipped with a split/splitless injector and a dedicated SPME glass liner. Injections were performed in split mode with a split ratio of 5:1. Separation was achieved using a J&W HP-5MS capillary column (5% phenyl–methylpolysiloxane, 30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Helium (99.9995% purity from Nippon Gases, Madrid, Spain) was used as the carrier gas at a constant flow rate of 1.1 mL/min. The oven temperature program was as follows: initial temperature 40 °C (held for 3 min), ramped to 150 °C at 6 °C/min (held for 0.5 min), then to 270 °C at 15 °C/min (held for 1.17 min). Volatile compounds were identified by comparing their mass spectra with those available in the NIST 14 library [29] and by matching the calculated values of their linear retention indices (determined using a C7–C40 n-alkane mixture injected under the same chromatographic conditions), with those reported in the literature.
2.3. Statistical Analysis
To establish the statistical differences between the samples, one-way analysis of variance (ANOVA) combined with the post hoc Tukey HSD (honestly significant difference) test was carried out using the freely available online web calculator Astasa [30]. A t-test was applied to compare the mean total areas of GC/MS chromatograms collected from different samples using Microsoft Excel routines. Statistical significance was established at p < 0.05. In addition, Principal Component Analysis (PCA) [31] was carried out for explorative purposes on the autoscaled data. In this study, visualization of the data distribution by considering the scores and loadings plots was limited to the first two components. This approach allows a clear representation of the major sources of variation in the dataset, while higher-order components, which typically capture less variance, were not considered for graphical analysis. PCA was performed using in-house routines in the MATLAB environment (R2019b; The Mathworks, Natick, MA, USA).
3. Results and Discussion
3.1. Volatile Profile of Sulmona Red Garlic Scapes and Cloves
To characterize the aroma profile of Sulmona red garlic, the volatile compounds of both scapes and cloves were analyzed using HS-SPME-GC-MS. This analytical approach was chosen for its effectiveness in profiling sulfur-containing volatiles, which are key contributors to garlic aroma. The HS-SPME-GC-MS conditions adopted in this study were selected to ensure reliable extraction of organosulfur compounds while minimizing thermal degradation and matrix effects. The extraction temperature of 45 °C and exposure time of 15 min were chosen based on prior experience [24]. The PDMS-coated fiber was selected for its known affinity toward sulfur-containing volatiles and its robustness under repeated thermal desorption.
The list of the 36 volatiles detected in Sulmona red garlic scapes and cloves is reported in Table 1, including 26 organosulphur compounds, while monoterpenes (5) and aldehydes (3) are the most represented among the non-sulphur ones. Typical chromatograms obtained from Sulmona Red Garlic cloves and scapes, collected under identical experimental conditions, are shown in Figure 1. Despite a general similarity in the chromatographic profiles between scapes and cloves, the cumulative peak area (TIC: Total Ion Current, reported in the figure caption) was six times greater in the clove samples compared to the scapes.

Table 1.
Retention time (RT), experimental and literature retention index (RI (exp) and RI (lit), respectively) of the volatile compounds detected in garlic cloves and scapes.
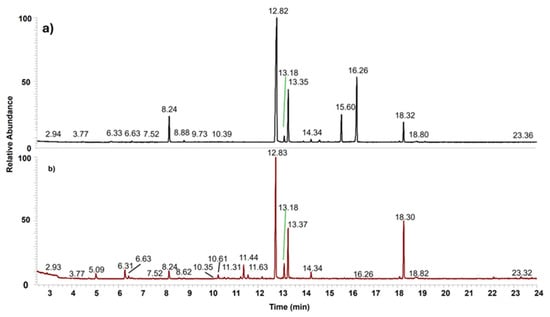
Figure 1.
Typical GC-MS chromatogram obtained from Sulmona red garlic clove (a) and scape (b). TIC = 6.35 × 1010 (a) and 1.11 × 1010 (b).
The contents of the volatile compounds, expressed as relative (%) peak areas in the chromatograms, collected from Sulmona red garlic clove (C) and from the base, centre and tip of the scapes (ST, SB and SC, respectively) are reported in Table 2. Both bulb and scape samples are from Campo di Fano. It can be observed that diallyl disulfide and 2-vinyl-4H-1,3-dithiine alone account for about 66% of the volatile profile of Sulmona red garlic clove, while the sum of the relative peak areas of these two compounds in the scape is significantly lower, about 36 (ST), 19 (SB) and 27% (SC). In the scape, the maximum relative abundance of diallyl disulfide is observed in the tip (about 36%), but the relative contribution of this compound is significantly lower than that exerted in the clove volatile profile (about 52%). The contribution of 2-vinyl-4H-1,3-dithiine, about 14% in the clove volatile profile, is close to 0.3% for the various parts of the scape. A second dithiin (3-vinyl-4H-1,2-dithiin), contributing for about 5% to the aroma of the garlic clove, was not detected in any of the scape samples. Regarding allyl (Z)-1-propenyl disulfide, which is the third most abundant organosulphur volatile of Sulmona red garlic clove, a significantly higher relative abundance can be detected in the scape base (32.5 vs. 12.2%). Significantly higher abundances of its isomer allyl (E)-1-propenyl disulfide were also detected in the scape (2.8–3.9%) compared to the clove (ca 1.5%). Regarding other organosulphur compounds detected with relative abundances above 1%, it must be noted a comparable or significantly higher abundance of diallyl trisulfide and allyl methyl trisulfide in the scape compared to the clove. The contribution of methyl allyl disulfide to the volatile profile of the scape centre and tip is comparable to that of the clove, but a significantly lower abundance is detected at the scape base.

Table 2.
Mean peak areas (%) and relative standard errors of volatile compounds detected in the tip, base and centre of the garlic scape (ST, SB and SC, respectively) and in the clove (C) from Campo di Fano; n = 5 for scapes and n = 6 for cloves.
Five monoterpenes, namely β-myrcene, limonene, o-cymene, β-ocimene and γ-terpinene, were detected in the aroma profile of Sulmona red garlic scape at relative abundances significantly higher than those observed in the clove. Terpenes are ubiquitous vegetal components playing the role of mediating interactions between the plant and the external environment, which include antimicrobial and antifungal action, repellence towards herbivores, and attraction of pollinators [34,35]. Moreover, terpenes have been associated with a wide range of biological activities, including antioxidant, antifungal, antimicrobial, anti-inflammatory, antiallergic, and antimetastatic activities [36,37]. Hexenal and (E)-2-hexenal, whose relative contributions to the scape aroma are significantly higher compared to that observed in the garlic clove, belong to the class of C6-aldehydes which, like terpenoids, are volatile compounds emitted by vegetal systems to communicate with neighboring plants, insects, and microbes [34]. Limonene, the most abundant terpenoid found in the scapes, is responsible for citrus-like sensorial attributes [38]. β-myrcene is associated with citrusy and sweet-balsamic-herbaceous notes at low concentration, while contributing with pungent and bitter notes at high concentration. γ-terpinene has herbaceous, citrus sensorial attributes [39]. o-cymene and β-ocymene are responsible for gasoline and sweet, herbal scents, respectively [40]. Hexanal and trans-2-hexenal can contribute to the sensorial attributes of the scapes with grassy and green odors [41]. β-ionone, formed by degradation of β-carotene, is a volatile compound with violet odour [42]. The ester ethyl hexanoate is responsible for fruity sensorial notes [39].
PCA was applied to the volatile profiles of the group of SB, SC, and ST samples to better visualize possible differences in the distribution of the volatile compounds along the scape. Figure 2 displays the samples (score plot) and the variables (loading plot) projected in the plane of the first two PCs accounting for about 53% of the total data variance. The score plot (Figure 2a) reveals a clear separation of SC and ST from SB samples along PC1, while ST and SC datapoints are grouped into two separated clusters along PC2. It follows that the loading plot displayed in Figure 2b allows identifying at negative loading values on PC1 the volatile compounds present at higher relative abundance in the scape base compared to the scape centre and tip (hexanal, allyl methyl sulfide, γ-terpinene, ethyl hexanoate, allyl (Z)-1-propenyl disulfide, for instance). Conversely, the volatile compounds with positive PC1 loadings (allyl methyl trisulfide, dimethyl trisulfide, diallyl trisulfide, methyl allyl disulfide, in particular) exhibit a lower contribution to the volatile profile of the scape base compared to the scape center and tip. Regarding the difference in the aroma profile between the scape tip and center, which is described by PC2, the loading plot suggests a higher relative abundance of mono-sulfides and non sulphur compounds (terpenes and aldehydes, in particular) in the scape apex compared to the center, accompanied by a decrease in the content of almost all the other organosulphur volatile compounds. The comparison of the total intensity of the chromatograms collected from the various parts of the scape revealed that, while SB exhibits a significantly more intense chromatogram than ST, no significant differences between CS and BS and between CS and TS samples were detected.
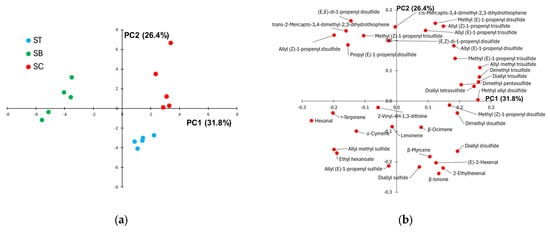
Figure 2.
(a) Score plot showing the samples collected from the tip (ST), base (SB) and centre (SC) of the Sulmona Red Garlic scape, projected on the plane of the first two principal components; (b) corresponding loading plot.
3.2. Influence of Garlic Glove Origin and Cultivation Site on the Volatile Profile of Floral Scape
We acquired the volatile profiles from the central part of scapes coming from plants cultivated in different localities within the territory designated for the cultivation of Sulmona red garlic, using both autochthone clove seeds and commercial clove seeds of red garlic varieties of different origin. To classify the scape samples according to the cultivation area, three subgroups were identified: the plants cultivated in the municipality of Sulmona (S), those grown in the sites of Prezza, Campo di Fano and Raiano, located west of Sulmona (WS), and the samples coming from Pacentro, which is east of Sulmona (ES). The geoclimatic differences between the above growing sites are relatively small. In fact, the two furthest locations (Pacentro and Raiano) are about 15 kilometers apart as the crow flies, with Sulmona in between. In addition, the samples were classified according to the geographical origin of the seed clove, coming from Sulmona, France and Spain.
The contents of the volatile compounds, expressed as relative (%) peak areas in the chromatograms, within the groups defined as above, are reported in Table 3.

Table 3.
Mean peak areas (%) and relative standard errors of volatile compounds detected in scapes coming from different sites (S, ES or WS) and from clove seeds of different origin (Sulmona, Spain or France).
PCA was applied to provide a graphical visualization of the data (Figure 3). The score plot (Figure 3a) reveals a good separation of Sulmona Red Garlic samples (full symbols) according to the cultivation site. In particular, the samples cultivated within Sulmona municipality are located around the origin of PC1-PC2 graph, the scapes coming from WS sites can be found at negative PC1 scores and small PC2 scores, while the scapes from ES sites exhibit positive PC1 scores and negative PC2 scores. Based on the related loading plot (Figure 3b), moving from west to east cultivation sites results in a progressive decrease in the content of non sulphur volatile compounds (terpenes and aldehydes, in particular) and a progressive increase in the contribution of organosulphur volatiles to the aroma of scapes. As highlighted by Tukey test (Table A1), the difference in relative abundances between these two groups is also significant for several volatiles.
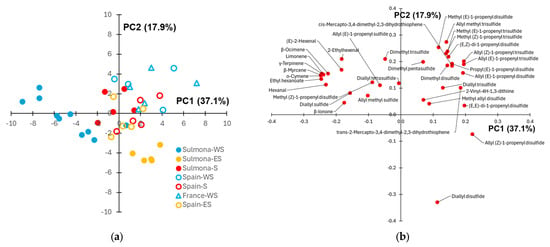
Figure 3.
(a) Score plot showing the samples collected from Sulmona Red Garlic scapes cultivated in different sites and using clove seeds of different origin; (b) corresponding loading plot. In the legend (a), the first part of the group name refers to the origin of the clove seed (Sulmona, Spain or France) and the second part refers to the cultivation site (S = Sulmona, WS = west of Sulmona, ES = east of Sulmona).
The score plot further indicates that the volatile profiles of scapes from plants cultivated in the Sulmona municipality show minimal distinction between those grown from autochthonous and commercial Spanish cloves. In contrast, scapes derived from commercial Spanish cloves cultivated in the ES territory are clearly separated from the autochthonous samples grown in the same area. However, a significant difference in the abundance between these two groups is observed only in the case of allyl (Z)-1-propenyl disulfide (Table A1). Looking at the scape samples coming from the WS area, a better separation between plants grown from Sulmona and foreign (Spain or French) clove seeds is observed. Moreover, the separation according to the origin of the clove seed occurs in a direction approximately perpendicular to that observed for the samples grown in the ES territory. A significative difference in the abundance of some disulfides and trisulfides between the above sample groups was detected by Tukey test (Table A1). With respect to the overall intensity of the GC-MS chromatograms, it is noteworthy that no significant differences were observed between the mean total areas of scapes from plants grown from Sulmona and Spanish clove seeds within the same area (Pacentro or Sulmona). However, scapes cultivated from autochthonous clove seeds at Campo di Fano exhibited a significantly higher GC-MS signal compared to those grown from French clove seeds in the same locality.
4. Conclusions
This study highlighted that the GC-MS chromatogram of Sulmona red garlic scapes, despite being less intense than that of the clove, is characterized by almost all the organosulphur volatile compounds which contribute to the volatile profile of the clove. Nevertheless, while the three major organosulphur compounds of clove aroma (diallyl disulfide, 2-vinyl-4H-1,3-dithiine and allyl (Z)-1-propenyl disulfide, in the decreasing order of relative abundance) contribute to more than 75% of the total chromatogram area, the aroma profile of the scapes is characterized by a more balanced abundance of major and minor organosulphur volatile compounds and a significantly greater relative contribution of non sulphur volatiles. It follows that the typical pungency of a garlic clove is attenuated in the scape. Furthermore, some non-sulphur volatile organic compounds (terpenes and aldehydes), widely distributed in fruits and vegetables, could contribute to the aroma of scapes with pleasant sensory attributes. The aroma profile of Sulmona red garlic scapes grown from authentic clove seeds is moderately influenced by the geographic origin within the territory designed for the cultivation of this variety. The real possibility of discriminating against the scapes of plants grown from autochthonous and non-autochthonous cloves has not been fully demonstrated. This point should be investigated in more detail by increasing the number of scape samples to better represent the combined effect of the cultivation area and the varietal/geographical origin of the clove seeds.
Author Contributions
Conceptualization, A.A.D. and L.D.M.; methodology, S.R.; software, A.B.; validation, S.R., A.B. and V.D.C.; formal analysis, A.B., M.D.S. and R.F.; investigation, S.R. and R.F.; resources, V.D.C.; data curation, S.R., M.D.S. and V.D.C.; writing—original draft preparation, A.A.D., M.D.S. and L.D.M.; writing—review and editing, A.A.D.; visualization, A.B.; supervision, L.D.M.; project administration, M.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request.
Acknowledgments
The authors are grateful to the growers of Sulmona Red Garlic who kindly provided the scape samples analysed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Statistically significant differences (p < 0.05) according to the Tukey HSD test in the mean pairs for scapes coming from different sites (S, ES or WS) and from clove seeds of different origin (Sulmona, Spain or France).
Table A1.
Statistically significant differences (p < 0.05) according to the Tukey HSD test in the mean pairs for scapes coming from different sites (S, ES or WS) and from clove seeds of different origin (Sulmona, Spain or France).
| Compound | Pairs of Groups with Significantly Different Means # |
|---|---|
| Allyl methyl sulfide | - |
| Dimethyl disulfide | BC, BG |
| Hexanal | AB, AC, AD, AE, AF, AG |
| (E)-2-Hexenal | AB, AC, AF, BE, BG |
| Diallyl sulfide | AB |
| Allyl (E)-1-propenyl sulfide | - |
| Methyl allyl disulfide | - |
| Methyl (Z)-1-propenyl disulfide | BC |
| Methyl (E)-1-propenyl disulfide | AG, BE, BG, CG, DG, FG |
| Dimethyl trisulfide | AB, BC, BG |
| β-Myrcene | AB, AF, AG, BC |
| Ethyl hexanoate | AB, AD, AF, AG |
| 2-Ethylhexenal | AB, AC, AF, BE, BG |
| o-Cymene | AB, AD, AF, AG, BC |
| Limonene | AB, AG, BC |
| β-Ocimene | AB |
| γ-Terpinene | AB, AD, AF, AG, BC |
| Diallyl disulfide | AB, BC, BE, BG |
| Allyl (E)-1-propenyl disulfide | AE, AG |
| Allyl (Z)-1-propenyl disulfide | AB, AE, AG, BC, BD, BF |
| Propyl (E)-1-propenyl disulfide | - |
| (E,Z)-di-1-propenyl disulfide | - |
| (E,E)-di-1-propenyl disulfide | - |
| cis-Mercapto-3,4-dimethyl-2,3-dihydrothiophene | BE |
| Allyl methyl trisulfide | AE, AG, BE, BG, CE, CG, DG, EG, FG |
| Methyl (Z)-1-propenyl trisulfide | BC, CD, CF, DE |
| Methyl (E)-1-propenyl trisulfide | - |
| trans-2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene | - |
| 2-Vinyl-4H-1,3-dithiine | - |
| Diallyl trisulfide | CE CF CG |
| Allyl (Z)-1-propenyl trisulfide | AE, AF, AG, BG |
| Allyl (E)-1-propenyl trisulfide | AE, AF, AG |
| Dimethyl pentasulfide | AF |
| β-Ionone | AB, AC, AD, AF, AG |
| Diallyl tetrasulfide | - |
# A = Sulmona-WS; B = Sulmona-ES; C = Sulmona-S; D = Spain-ES; E = Spain-WS; F = Spain-S; G = France-WS.
References
- Sahidur, M.R.; Islam, S.; Jahurul, M.H.A. Garlic (Allium Sativum) as a Natural Antidote or a Protective Agent against Diseases and Toxicities: A Critical Review. Food Chem. Adv. 2023, 3, 100353. [Google Scholar] [CrossRef]
- Dhall, R.K.; Cavagnaro, P.F.; Singh, H.; Mandal, S. History, Evolution and Domestication of Garlic: A Review. Plant Syst. Evol. 2023, 309, 33. [Google Scholar] [CrossRef]
- Rakshit, D.; Nayak, S.; Kundu, S.; Angelopoulou, E.; Pyrgelis, E.-S.; Piperi, C.; Mishra, A. The Pharmacological Activity of Garlic (Allium sativum) in Parkinson’s Disease: From Molecular Mechanisms to the Therapeutic Potential. ACS Chem. Neurosci. 2023, 14, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A Systematic Review of the Effects on Cardiovascular Diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 6797–6819. [Google Scholar] [CrossRef] [PubMed]
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of Garlic and Their Anticancer and Antioxidant Activity: A Review of the Epidemiologic and Experimental Evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Chermut, T.R.; Sequeira, J.; de Souza Gouveia Moreira, L.; Teixeira, K.T.R.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. From the Distinctive Smell to Therapeutic Effects: Garlic for Cardiovascular, Hepatic, Gut, Diabetes and Chronic Kidney Disease. Clin. Nutr. 2021, 40, 4807–4819. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological Properties and Therapeutic Applications of Garlic and Its Components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Iwar, K.; Ochar, K.; Seo, Y.A.; Ha, B.K.; Kim, S.H. Alliums as Potential Antioxidants and Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 8079. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological Properties of Onions and Garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Lanzotti, V. The Analysis of Onion and Garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical Methods for Bioactive Sulfur Compounds in Allium: An Integrated Review and Future Directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Horníčková, J.; Velíšek, J.; Ovesná, J.; Stavělíková, H. Distribution of S-Alk(En)Yl-L-Cysteine Sulfoxides in Garlic (Allium sativum L.). Czech J. Food Sci. 2009, 27, S232–S235. [Google Scholar] [CrossRef]
- Baky, M.H.; Shamma, S.N.; Khalifa, M.R.; Farag, M.A. How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics. Metabolites 2023, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Fadel, H.M. Investigation of the Volatile Aroma Components of Garlic Leaves Essential Oil. Possibility of Utilization to Enrich Garlic Bulb Oil. Eur. Food Res. Technol. 2002, 214, 105–107. [Google Scholar] [CrossRef]
- Nasim, S.A.; Dhir, B.; Samar, F.; Rashmi, K.; Mahmooduzzafar; Mujib, A. Sulphur Treatment Alters the Therapeutic Potency of Alliin Obtained from Garlic Leaf Extract. Food Chem. Toxicol. 2009, 47, 888–892. [Google Scholar] [CrossRef]
- Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Potential of Flavonol and Sulfur Compounds from Allium Leaves as an Antioxidant and Xanthine Oxidase Inhibition in Silico Study. Food Chem. Adv. 2023, 3, 100383. [Google Scholar] [CrossRef]
- Liu, P.; Weng, R.; Xu, Y.; Feng, Y.; He, L.; Qian, Y.; Qiu, J. Metabolic Changes in Different Tissues of Garlic Plant during Growth. J. Agric. Food Chem. 2020, 68, 12467–12475. [Google Scholar] [CrossRef]
- Lasalvia, A.; Cairone, F.; Cesa, S.; Maccelli, A.; Crestoni, M.E.; Menghini, L.; Carradori, S.; Marinacci, B.; Gallorini, M.; Elsallabi, O.; et al. Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs. Antioxidants 2022, 11, 2088. [Google Scholar] [CrossRef]
- Didonna, A.; Renna, M.; Santamaria, P. Traditional Italian Agri-Food Products: A Unique Tool with Untapped Potential. Agriculture 2023, 13, 1313. [Google Scholar] [CrossRef]
- Slow Food Foundation for Biodiversity. Available online: https://www.fondazioneslowfood.com/en/ark-of-taste-slow-food/sulmona-red-garlic/ (accessed on 25 June 2025).
- Pianezze, S.; Perini, M.; Bontempo, L.; Ziller, L.; D’Archivio, A.A. Geographical Discrimination of Garlic (Allium sativum L.) Based on Stable Isotope Ratio Analysis Coupled with Statistical Methods: The Italian Case Study. Food Chem. Toxicol. 2019, 134, 110862. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Foschi, M.; Aloia, R.; Maggi, M.A.; Rossi, L.; Ruggieri, F. Geographical Discrimination of Red Garlic (Allium sativum L.) Produced in Italy by Means of Multivariate Statistical Analysis of ICP-OES Data. Food Chem. 2019, 275, 333–338. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F.; D’Archivio, A.A. Geographical Discrimination of Red Garlic (Allium sativum L.) Using Fast and Non-Invasive Attenuated Total Reflectance-Fourier Transformed Infrared (ATR-FTIR) Spectroscopy Combined with Chemometrics. J. Food Compos. Anal. 2020, 86, 103351. [Google Scholar] [CrossRef]
- Biancolillo, A.; Aloia, R.; Rossi, L.; D’Archivio, A.A. Organosulfur Volatile Profiles in Italian Red Garlic (Allium sativum L.) Varieties Investigated by HS-SPME/GC-MS and Chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- Tocmo, R.; Wang, C.; Liang, D.; Huang, D. Organosulphide Profile and Hydrogen Sulphide-Releasing Capacity of Garlic (Allium sativum L.) Scape Oil: Effects of PH and Cooking. J. Funct. Foods 2015, 17, 410–421. [Google Scholar] [CrossRef]
- Naheed, Z.; Cheng, Z.; Wu, C.; Wen, Y.; Ding, H. Total Polyphenols, Total Flavonoids, Allicin and Antioxidant Capacities in Garlic Scape Cultivars during Controlled Atmosphere Storage. Postharvest Biol. Technol. 2017, 131, 39–45. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; D’Alessandro, V.; D’Antuono, L.F. Comparison of Two Extraction Methods (High Pressure Extraction vs. Maceration) for the Total and Relative Amount of Hydrophilic and Lipophilic Organosulfur Compounds in Garlic Cloves and Stems. An Application to the Italian Ecotype “Aglio Rosso Di Sulmona” (Sulmona Red Garlic). Food Chem. 2020, 312, 125086. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Masciulli, F.; Ingallina, C.; Mannina, L.; Maria Loreta, L.; Di Simone, S.C.; Acquaviva, A.; Nilofar; Recinella, L.; Leone, S.; et al. Comprehensive Metabolite and Biological Profile of “Sulmona Red Garlic” Ecotype’s Aerial Bulbils. Food Res Int. 2024, 175, 113654. [Google Scholar] [CrossRef]
- NIST14: Mass Spectral Database; NIST: Gaithersburg, MD, USA. Available online: https://www.nist.gov/news-events/news/2014/07/latest-nist-mass-spectral-library-expanded-coverage-features/ (accessed on 7 January 2025).
- Vasavada, N. Online Web Statistical Calculators. Available online: http://astatsa.com/ (accessed on 12 January 2025).
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the Key Aroma Compounds in Mulberry Fruits by Application of Gas Chromatography–Olfactometry (GC-O), Odor Activity Value (OAV), Gas Chromatography-Mass Spectrometry (GC–MS) and Flame Photometric Detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, Y.; Wu, Z.; Wang, C.; Zhang, J. Dynamic Characterization of Volatile and Non-Volatile Profiles during Toona Sinensis Microgreens Growth in Combination with Chemometrics. Food Res Int. 2025, 206, 116013. [Google Scholar] [CrossRef]
- Das, A.; Lee, S.H.; Hyun, T.K.; Kim, S.W.; Kim, J.Y. Plant Volatiles as Method of Communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—an Integrative Approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bi, S.; Lao, F.; Wu, J. Factors Affecting Aroma Compounds in Orange Juice and Their Sensory Perception: A Review. Food Res Int. 2023, 169, 112835. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Coll, L.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Volatile Composition of Prickly Pear Fruit Pulp from Six Spanish Cultivars. J. Food Sci. 2020, 85, 358–363. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhang, Y.; Liu, Y.; Yang, R.; Dai, X.; Wang, T.; Lv, C.; Zuo, L.; Liu, Y.; et al. Effect of Drying Methods on Aroma Profiling of Large-Leaf Green Tea (Camellia sinensis var. Assamica) Determined by HS-SPME-GC-MS. Foods 2025, 14, 1275. [Google Scholar] [CrossRef]
- Shan, Q.; Wan, Y.; Liang, J.; He, W.; Zeng, J.; Liang, W.; Xiong, S.; Zhang, M.; Wang, B.; Zou, X.; et al. HS–SPME Combined with GC–MS and GC–O for Characterization of Key Aroma-Active Compounds in Fruity and Grassy Peppers (Capsicum chinense Jacq.). Food Chem.: X 2024, 24, 101944. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-harpaza, L.; Ibdah, M. Β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).