Abstract
In recent years, there has been an increasing need and demand for gas sensors to detect hazardous gases in the atmosphere, as they are indispensable for environmental monitoring. Typical hazardous gas sensors that have been widely put to practical use include conductometric gas sensors, such as semiconductor gas sensors that use the change in electrical resistance of metal oxide semiconductors, catalytic combustion gas sensors, and electrochemical gas sensors. However, there is a growing demand for gas sensors that perform better and more safely, while also being smaller, lighter, less energy-demanding, and less costly. Therefore, new gas sensor materials are being explored, as well as optical gas sensor technology that expresses gas detection not electrically but optically. Cadmium sulfide (CdS), cadmium selenide (CdSe), and cadmium telluride (CdTe) are typical group II-VI non-oxide semiconductors that have been used as, for example, electronic materials. Recently, they have attracted attention as new gas sensor materials. In this article, recent advances in conductometric and optical gas sensing technologies using CdS, CdSe, and CdTe are reviewed.
1. Introduction
The importance of gas sensors for detecting hazardous gases (toxic, flammable, explosive, corrosive, malodorous, etc.) in the atmosphere has been increasing in recent years as the demand for air quality monitoring to ensure the safety and security of the living environment and to improve the quality of life (QOL) increases [1,2,3,4,5,6,7,8,9,10,11]. Conductometric gas sensors (such as semiconductor gas sensors, catalytic combustion gas sensors, and electrochemical gas sensors) that express gas detection results by electrical signals [1,2,3,4,5,6] are the most common hazardous-gas sensors that have been widely put to practical use. Semiconductor gas sensors use the change in electrical resistance of transition metal oxide semiconductors, such as tin oxide (SnO2), when they come into contact with gas; while catalytic combustion gas sensors use the change in electrical resistance of the platinum wire embedded in metal oxide matrix that results from the heat generated by the catalytic combustion of gas on the surface of the metal oxide. However, in semiconductor and catalytic combustion gas sensors, the gas sensor element is often heated to high temperatures, resulting in high levels of power consumption and requiring attention to safety in high-temperature areas. Other typical conductometric gas sensors include electrochemical gas sensors that use electrolyte solutions or solid electrolytes and detect gases by the electric current that flows during the redox reaction of the gas. Gas sensors that use solid electrolytes often heat the gas sensor element to high temperatures, and thus have the same problems as the semiconductor and catalytic combustion sensor types. Given this background, the development of gas sensors with higher performance and better safety characteristics, while also being smaller, lighter, more energy efficient, and less costly than conventional sensors, is needed, and new gas sensor materials in semiconductor gas sensors are being explored. Furthermore, compared to conductometric gas sensors [1,2,3,4,5,6], which express the results of gas detection by electrical signals, optical gas sensors [1,2,3,4,7,8,9,10,11], which express the gas detection results by optical signals (absorption or emission intensity, refractive index, phase, polarization, etc.), have many advantages, such as operation at room temperature, no risk of electric sparks, low power consumption, high immunity to electromagnetic noise, and remote, non-contact signal readout. Therefore, various optical gas sensor technologies are being explored. Cadmium sulfide (CdS), cadmium selenide (CdSe), and cadmium telluride (CdTe) are typical group II-VI non-oxide semiconductors, which have been applied to electronic materials. Recently, they have attracted attention as candidates for new high-performance gas sensor materials, and the number of research papers on their use is increasing (Table 1). This is due to advances in the technology for fabricating nanomaterials (nanoparticles (quantum dots (QDs), nanowires, nanorods, nanoribbons, nanosheets, nanoflowers, etc.) in various shapes, from CdS, CdSe, and CdTe, which exhibit electronic and optical properties different from those of the bulk samples of the same composition due to quantum size effects. At the same time, the high ratio of surface atoms and the large specific surface area of these nanomaterials can be expected to bring about sharp changes in their electronic and optical properties due to gas adsorption and desorption. In this review, we summarize and review the reports published since 2003 on gas sensing technologies using CdS, CdSe, and CdTe [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].

Table 1.
Recently published papers on gas sensing technology using CdS, CdSe, and CdTe.
The conductometric gas sensors using CdS, CdSe, and CdTe [12,13,14,15,16,17,18,19,20,23,24,25,26,27,28,39,40,41] introduced in this review all take advantage of the fact that the electrical resistance of sensors made with these three compounds changes upon contact with gas (Table 2). Figure 1a shows a schematic diagram of the semiconductor gas sensor configuration. In air, oxygen (O2) accepts electrons from the semiconductor and chemisorbs onto the semiconductor surface in the form of anions, such as O2− and O−. When the test gas is added to the air, the test gas reacts with the adsorbed oxygen anions and donates electrons to the semiconductor, thus changing the electrical resistance of the semiconductor. Based on this principle of semiconductor gas sensors, gas sensor materials, including not only CdS, CdSe, and CdTe, but also composites of these materials with other inorganic and organic functional materials, have been investigated for their sensitivity to various gases. Furthermore, it has been reported that the gas response (electrical resistivity change ratio) and the gas sensitivity (gas response variation divided by the gas concentration variation) of gas sensor materials is greater under light irradiation than in the dark. This phenomenon is based on the effect of light irradiation on gas adsorption/desorption and the change in the electronic state of the semiconductor due to the excitation of electrons in the semiconductor by light irradiation.

Table 2.
Conductometric and optical gas sensors using CdS, CdSe, and CdTe.
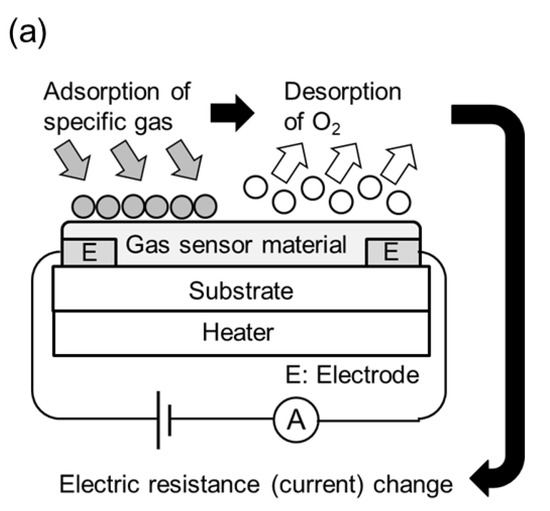
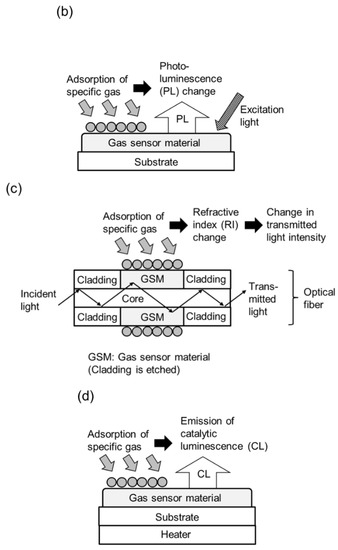
Figure 1.
Schematic diagrams of conductometric and optical gas sensor configurations using CdS, CdSe, and CdTe. (a) Semiconductor gas sensor, (b) Optical gas sensor using PL characteristic change, (c) Optical gas sensor using refractive index change, and (d) Optical gas sensor using catalytic luminescence.
Conversely, the optical gas sensors using CdS, CdSe, and CdTe [21,22,29,30,31,32,33,34,35,36,37,38,42,43,44] introduced in this review, are based on the use of the phenomenon in which the photoluminescence (PL) (fluorescence) properties and refractive index of the sensor material change upon contact with the gas, and the catalytic luminescence emitted when the sensor material and gas come into contact (Table 2). Figure 1b–d show schematic diagrams of optical gas sensor configurations using PL characteristic change, refractive index change, and catalytic luminescence, respectively. Optical gas sensors using photoluminescent QDs have attracted particular interest. The surface of QDs of CdS, CdSe, and CdTe is usually coated with an organic surfactant, or similar material, to passivate surface defects and thus emit bright PL in fresh air without test gas. When the test gas is added in air, the test gas adsorbs on the organic surfactant surface of the QDs, activating or passivating the surface defects of the QDs and causing electron transfer between the test gas and the QDs, which changes the PL intensity of the QDs. Furthermore, it has been reported that the surface of a QD is coated with functional organic molecules that selectively react with a specific test gas and emit PL at a different wavelength from that of the QD, and the gas is detected and discriminated by measuring the PL at the two wavelengths of the QD and the organic molecules. Various methods have been reported that utilize the phenomenon in which the PL properties and refractive index of the sensor material change upon contact with the gas. For example, the cladding of an optical fiber consisting of a core and cladding is partially etched and then coated with a semiconductor nanomaterial, and the refractive index of the semiconductor nanomaterial changes upon contact with the gas to be tested. This phenomenon changes the intensity of the evanescent wave from the optical fiber, which in turn changes the transmitted light intensity. Another method is to use the catalytic luminescence emitted by the QDs when they come into contact with the test gas.
2. Gas Sensing Technologies Using CdS, CdSe, and CdTe
2.1. Conductometric Gas Sensors Using CdS
Navale et al. reported a conductometric nitrogen dioxide (NO2) gas sensor based on CdS QD thin films [12]. The CdS QD thin films fabricated on glass substrates consisted of CdS QDs with an average particle size of 36 nm and had a porous structure; at 38 °C, the electrical resistance of the CdS QD films increased in response to oxidizing NO2 and chlorine (Cl2), reflecting the n-type semiconductor properties of CdS, and decreased in response to reducing hydrogen sulfide (H2S), ammonia (NH3), ethanol (C2H5OH), and methanol (CH3OH). The change in electrical resistance induced by each of these gases was reversible. The CdS QD thin film showed high selectivity and high sensitivity to NO2 with good reproducibility. The sensitivity of the sensor was still stable 20 days after sensor fabrication. The sensitivity was nitrogen dioxide >> hydrogen sulfide >> ammonia > chlorine > methanol > ethanol. The response to 200 ppm NO2 in air was as high as 61%, with a response time of 50 s and a lower limit of detection (LOD) of 5 ppm. The 100 ppm NO2 response/recovery time was 50 s/12 min. NO2 sensitivity was higher at low humidity and lower at high humidity.
Yang et al. reported a conductometric nitrogen dioxide gas sensor based on CdS/zinc oxide (ZnO) core-shell nanowires [13]. The CdS/ZnO nanowire thin films fabricated on alumina substrates consisted of nanowires with diameters of 20–60 nm and lengths of several μm, which, when irradiated with light at wavelengths of 367 nm or 468 nm, generated significantly larger photocurrents than CdS nanowires alone, at room temperature, under visible light irradiation at a wavelength of 468 nm. The nanowires exhibit a reversible electrical resistivity change in response to 5–1000 ppb NO2 in air. The response (change ratio in electrical resistance) to NO2, was dependent on the intensity of 468 nm light irradiation, reaching a maximum at a light intensity of 0.68 mW/cm2 (6.7% for 5 ppm NO2 and 337% for 1000 ppm NO2). Response/recovery times were fast (26 ms/2.1 ms). The sensor characteristics remained unchanged for five months, demonstrating excellent long-term stability.
Zhu et al. reported a conductometric ethanol gas sensor based on Zn1−xCdxS nanowires (NWs) (x = 0–1.0) [14]. The Zn1−xCdxS NW films fabricated on ceramic tubes consist of NWs with diameters of 20–80 nm and lengths of several hundred nm, which exhibit a reversible electrical resistance change in response to 2–1000 ppm ethanol in air and exhibit higher ethanol response (change ratio in electrical resistance) and faster response and recovery speeds than CdS-only and ZnS-only NWs. All NWs showed a linear relationship between concentration and sensitivity from 2–50 ppm, but at higher concentrations, the relationship deviated from linearity and the increase in sensitivity with increasing concentration gradually slowed down. In the 160–280 °C operating temperature range, all NWs showed the highest sensitivity at 206 °C. At 206 °C and 25% relative humidity (RH), the response (change ratio in electrical resistance) to 20 ppm ethanol was highest (12.8) for Zn1−xCdxS (x = 0.4) NWs with 90% response/recovery times of 2 s/1 s. Response/recovery was even faster (<1 s/<1 s) at x = 1.0. The sensitivity of all NWs tested at 206 °C was ethanol >> methanol >> (acetone ((CH3)2CO), formaldehyde (HCHO), benzene (C6H6), methylbenzene (toluene) (C6H5CH3), xylene (C6H4(CH3)2), carbon monoxide (CO), ammonia, nitrogen dioxide, and methane (CH4)), indicating high selectivity for ethanol. The sensitivity to 100 ppm ethanol was highest at 50% RH in the 15–90%RH relative humidity range, and the effect of humidity variation on response and recovery speeds was small.
Li et al. reported a conductometric ethanol gas sensor using CdS nanowires modified with cerium oxide (CeO2) nanoparticles [15]; CdS-only nanowires, 5 at.% CeO2/CdS nanowires, and 10 at.% CeO2/CdS nanowires were all ethanol-sensitive and showed a reversible change in electrical resistance in response to ethanol. The response (change ratio in electrical resistance) to various gases was ethanol >> formaldehyde > (acetone, toluene, and acetylene (C2H2)). The sensitivity to ethanol was investigated at 138–255 °C. The 5 at.% CeO2/CdS nanowires showed the highest sensitivity at the lowest operating temperature of 161 °C. The 90% response/recovery time to 5–40 ppm ethanol was <3 s/<3 s for the CdS-only nanowires and <12 s/<3 s for the 5 at.% CeO2/CdS nanowires.
Vanalakar et al. reported a conductometric nitrogen dioxide gas sensor based on CdS nanowires [16]. The CdS nanowire thin film consisted of CdS nanowires of about 30 nm width and had a porous structure. At 200 °C, the CdS nanowire thin film showed a reversible electrical resistance change when exposed to nitrogen dioxide, ammonia, or acetone. The response (change ratio in electrical resistance) to 100 ppm NO2 was 1850% and the LOD was 5 ppm. The response time (several seconds) was much shorter than the recovery time (>~100 s).
Zhu et al. reported a conductometric trimethylamine ((CH3)3N (TMA)) gas sensor based on a microrod of an n-type organic semiconductor perylene diimide derivative (1,6,7,12-tetra-chlorinated perylene-N-(2-hydroxyethyl)-N′-hexylamine-3,4,9,10-tetracarboxylic bisimide, TC-PDI) and a microbelt made from CdS [17]. The fabricated TC-PDI/CdS microbelt thin films (about 1 μm wide and 100–200 μm long) exhibit higher electrical conductivity than TC-PDI microrods due to the n-n heterojunction and show high sensitivity to TMA of 0.2–100 ppm in air at room temperature (25 °C), with response/recovery times of 325 s/510 s. The TC-PDI/CdS microbelt thin films showed good TMA selectivity, stability, and reproducibility in gas sensing.
Vishwakarma et al. reported a conductometric acetone gas sensor based on composite thin films consisting of nanocrystals (about tens of nm in size) of CdS (1–2 wt.%) added to titanium dioxide (TiO2) [18]. The TiO2-only, 1 wt.% CdS-TiO2 composite, and 2 wt.% CdS-TiO2 composite thin films fabricated on alumina substrates showed reversible electrical resistance changes at room temperature on exposure to 0–5000 ppm acetone, propanol (C3H7OH), and liquefied petroleum gas (LPG) in air. The response (change ratio in electrical resistivity) to 5000 ppm acetone, propanol, and LPG was 25%, 22%, and 10%, respectively, for the TiO2-only film, 44%, 30%, and 12% for the 1 wt.% CdS-TiO2 composite film, and 71%, 36%, and 14.5%, respectively, for the 2 wt.% CdS-TiO2 composite film. The response of each film to each gas increased with increasing gas concentration, and the increase in response with increasing gas concentration became slower in the higher concentration range. For all films, the sensitivity was acetone > propanol > LPG, with the 2 wt.% CdS-TiO2 composite film showing the highest acetone selectivity, with 90% response/recovery times of 55 s/115 s to 5000 ppm acetone.
Madlul et al. reported a conductometric ethanol gas sensor based on a composite thin film of CdS QDs and copper (Cu) [19]. The polycrystalline CdS thin film (CdS:Cu5%) with 5% Cu, fabricated on a silicon substrate, consisted of nanoparticles with a particle size of tens of nm and a porous structure; the CdS:Cu5% film showed an increase in electrical resistance on exposure to ethanol in air (1 mL ethanol vaporized in 0.023 m3 of air) at room temperature. The response (change ratio in electrical resistance) to ethanol was 26% and the response time was 160 s. However, after exposure to ethanol vapor in air containing no ethanol, the electrical resistance value became constant after decreasing to 183 s and did not recover to the value in air before ethanol exposure, indicating that reversibility was incomplete.
Guo et al. reported a conductometric acetone gas sensor based on CdS/cobalt oxide (Co3O4) composite fibers [20]. The CdS/Co3O4 composite fibers with CdS content of 0–70 wt.% had a porous structure and exhibited a reversible change in electrical resistance at room temperature in response to 0.5–100 ppm acetone in air. The response (change ratio in electrical resistivity) to acetone and the response/recovery time of the composite fibers were significantly improved by adding an optimal amount of CdS (50 wt.%) to the composite with Co3O4. The response/recovery times to 50 ppm acetone were accelerated from 43 s/44 s for Co3O4 alone to 11 s/29 s for the CdS/Co3O4 composite. When irradiated with green light of 520 nm wavelength, the response to 50 ppm acetone increased by 25%, and the response/recovery time was further accelerated to 5 s/4 s. The sensitivity to various gases was acetone >> ethanol >> methanol >> formaldehyde >> nitrogen dioxide >> hydrogen sulfide >> benzene >> carbon monoxide, indicating high selectivity for acetone.
2.2. Optical Gas Sensors Using CdS
Jiao et al. reported an optical methanol gas sensor based on CdS QDs [21]. The CdS QD film fabricated on a ceramic substrate showed strong catalytic luminescence (CL) at 330 °C, selectively sensitive to alcohol, with a gas sensitivity of methanol >> ethanol >> isopropanol ((CH3)2CHOH) > propanol (CH3(CH2)2OH) > isobutanol ((CH3)2CHCH2OH) > butanol (CH3(CH2)3OH) >> formic acid (HCOOH) >> carbon tetrachloride (CCl4). The CL intensity at a wavelength of 575 nm varied linearly with a LOD of 0.5 μg/mL for 1.2–76.1 μg/mL methanol in air. The CL intensity and signal-to-noise ratio depended on the gas distribution rate, reaching a maximum at 250 mL/min.
Narasimman et al. reported an optical ethanol gas sensor based on CdS nanoflowers assembled from CdS nanorods [22]. CdS nanoflowers (refractive index 2.68) were loaded on the surface of an optical fiber partially chemically etched from the cladding of an optical fiber consisting of a resin cladding (refractive index 1.398) and a silica core (refractive index 1.458). The CdS nanoflowers, assembled from CdS nanorods with an average length of 170 nm, exhibited a reversible change in transmitted light intensity around the wavelength range 590–663 nm at room temperature, sensitive to 10–300 ppm ethanol, methanol, isopropanol, and acetone in air. The refractive index of CdS changed in response to ethanol, methanol, isopropanol, and acetone, changing the intensity of evanescent waves within the light propagating in the optical fiber, resulting in a change in the transmitted light intensity of the optical fiber. The gas response (change ratio in transmitted light intensity) was ethanol >> methanol >> isopropanol >> acetone, showing high selectivity and sensitivity to ethanol, with a response of approximately 4.6% at 300 ppm ethanol and response/recovery times of 90 s/100 s.
2.3. Conductometric Gas Sensors Using CdSe
Joshi et al. reported a conductometric liquefied petroleum gas sensor based on a junction thin film of CdSe and p-type polyaniline (PANI) with n-type semiconductor properties [23]. The n-CdSe/p-PANI junction diode fabricated on a stainless-steel substrate, consisting of CdSe with a grain size of about 0.7 μm and PANI with a grain size of 1.5–1.6 μm, showed a reversible electrical resistance change at room temperature (300 K) on exposure to 0.02–0.10 vol.% LPG in air. The current-voltage (I–V) characteristics of this junction diode changed significantly with exposure to various concentrations of LPG. The gas response (change ratio in current value) reached a maximum of 70% for 0.08 vol.% LPG, and a further increase of LPG concentration to 0.10 vol.% resulted in a reverse decrease in sensitivity. Depending on the LPG concentration, the response time varied between 50–100 s, while the recovery time was 200 s.
Lin et al. reported a conductometric ethanol gas sensor based on CdSe nanoribbons [24]. The ethanol sensitivity of the CdSe nanoribbons was increased by visible light irradiation. CdSe nanoribbons (42 nm wide and 500 nm long) fabricated on ceramic tubes exhibited a reversible electrical resistance change under heating, sensitive to 50–1000 ppm ethanol in air, with an operating temperature range of 160–300 °C. The optimum operating temperature in terms of sensitivity and thermal stability was found to be 200 °C. At 200 °C, visible light irradiation on the surface of the CdSe nanoribbon increased the ethanol sensitivity (change ratio in electrical resistivity) compared to sensitivities displayed in the dark condition, and the higher the visible light irradiation intensity, the higher the ethanol sensitivity. Response and recovery times ranged from several seconds to several tens of seconds.
Wu et al. reported a conductometric ethanol gas sensor based on a three-dimensional ZnO/CdSe heterostructure [25]. The ZnO/CdSe heterostructure, consisting of ZnO nanorods (about 50 nm in diameter and about 1 μm in length) and CdSe nanoribbons (tens to hundreds of nm in width) fabricated on ceramic tubes, showed a reversible electrical resistance change at 160–260 °C on exposure to ethanol in air. Ethanol response (change ratio in electrical resistance) was highest at 160 °C, and this optimum operating temperature was reduced by about 100 °C compared to ZnO alone. Both the ZnO/CdSe heterostructure thin film and the ZnO nanorod thin film alone exhibited fast and reversible electrical resistance changes in response to 25–300 ppm ethanol in air. Response and recovery times ranged from a few seconds to several tens of seconds. Furthermore, the ethanol sensitivity of the ZnO/CdSe heterostructure was significantly increased under visible light irradiation compared to dark conditions; the ethanol sensitivity of the ZnO/CdSe heterostructure was 20 times higher in the dark and 3 times higher under visible light irradiation compared to the ZnO nanorods alone.
Laatar et al. reported a conductometric ethanol gas sensor based on porous anodic alumina (PAA) with CdSe nanorods (NRs) grown on it [26]. The size of the CdSe NRs in the CdSe NR/PAA composite thin films fabricated on aluminum foil wrapped around ceramic tubes ranged from 6–13 nm; the CdSe NR/PAA composite films exhibited reversible electrical resistance changes under heating on exposure to 25–300 ppm ethanol in air. In the operating temperature range of 160–260 °C, the ethanol response (change ratio in electrical resistivity) was highest at 200 °C. Ethanol sensitivity was higher under visible light irradiation than in the dark, and sensitivity increased with increasing visible light irradiation intensity. Visible light irradiation also improved the stability of the sensor output signal. Response and recovery times ranged from tens to hundreds of seconds.
Chizhov et al. reported a conductometric nitrogen dioxide gas sensor based on composite materials consisting of ZnO and CdSe QDs, CdSe/CdS core-shell QDs, and CdS/ZnSe core-shell QDs [27]. The ZnO/CdSe QD, ZnO/(CdSe/CdS QD), and ZnO/(CdS/ZnSe QD) nanocomposite films, consisting of ZnO of about 20 nm grain size and QDs of a few nm grain size, exhibited reversible electrical resistance changes at room temperature and in the dark on exposure to 0.12–2 ppm NO2 in air. Under cyclic green light (500–600 nm, peak wavelength 535 nm) irradiation, the reversible change ratio in electrical resistance (gas response) due to NO2 increased compared to in the dark, and the electrical resistance change due to light irradiation (photo response) changed with the NO2 concentration. Gas response under light irradiation was evaluated in two ways: the change ratio in electrical resistance due to NO2 and the change ratio in effective photoresponse due to NO2. Both the electrical resistance in the dark and under light irradiation increased with increasing NO2 concentration. The logarithm of NO2 concentration and the logarithm of response showed a linear relationship for ZnO/CdSe QDs and ZnO/(CdSe/CdS QDs), but for ZnO/(CdS/ZnSe QDs), the logarithm of NO2 concentration and the logarithm of response did not have a linear relationship, and NO2 response increased rapidly with increasing NO2 concentration. Under light irradiation, ZnO/(CdS/ZnSe QDs) showed the highest NO2 sensitivity. Conversely, ZnO/(CdSe/CdS QDs) and ZnO/(CdS/ZnSe QDs) showed slower recovery in NO2-free air after NO2 exposure compared to ZnO/CdSe QDs. The NO2 sensitivity of ZnO/CdSe QDs under light irradiation increased with increasing humidity in the 0–40% RH range, as the electrical resistance in air decreased with increasing humidity. The LOD was estimated to be around 50 ppb. Response and recovery times were around a few tens of minutes.
Geng et al. reported a conductometric nitrogen dioxide gas sensor based on PbxCd1−xSe QD gels containing atomically dispersed lead (Pb) ion sites [28]. The PbxCd1−xSe QD gels (x = 0–1.0) fabricated on alumina or silicon substrates consisted of QDs of a few nm crystal size and exhibited a reversible electrical resistance change at room temperature on exposure to 3 ppb–1.32 ppm NO2 in air. All PbxCd1−xSe QD gels (x = 0–1.0) had p-type semiconductor properties and showed a proportional decrease in electrical resistance with NO2 concentration; the addition of Pb increased the NO2 sensitivity up to 16-fold or more (from 0.004%/ppb for CdSe to 0.065%/ppb for Pb0.4Cd0.6Se. The LOD was estimated to be 3 ppb for PbxCd1−xSe QD gels (x ≥ 0.09). High sensitivity (0.06%/ppb), short response time (~28 s), and short recovery time (~60 s) were achieved in the Pb0.09Cd0.91Se QD gel. The sensor also showed excellent reproducibility and stability after repeated exposure to NO2. The sensitivity to various gases was nitrogen dioxide >> sulfur dioxide >> ammonia >> acetone >> carbon monoxide > (ethanol, formaldehyde, and methanol) > hydrogen (H2), indicating high NO2 selectivity. Furthermore, a portable NO2 sensor device was fabricated using this gas sensor that was able to detect down to 10 ppb.
2.4. Optical Gas Sensors Using CdSe
Nazzal et al. reported an optical alkylamine gas sensor based on CdSe QD-dispersed polymethylmethacrylate (PMMA) thin films [29]. PMMA thin films containing CdSe QDs coated with hexadecylamine (CH3(CH2)15NH2 (HDA)) were prepared on a fused silica substrate. The CdTe QD-dispersed PMMA thin films were sensitive to triethylamine ((C2H5)3NH2) (TEA)) in air or argon (Ar). Upon exposure to TEA, the PL intensity increased by about 120% in 2–3 min, and the peak wavelength shifted by about 3 nm, while the peak width increased by about 1 nm. Upon return to vacuum, the PL properties were reversibly restored, and this change occurred reproducibly. The changes in PL properties of CdSe QD-dispersed PMMA thin films induced by various alkylamine gases were very different from each other. Exposure to benzylamine (C6H5CH2NH2 (BZA)) for 5 min under light irradiation reduced the PL intensity by about 80%. This response to BZA was reversible and reproducible. Conversely, no change in PL intensity was observed upon exposure to butylamine (CH3(CH2)3NH2 (BTA)). Higher response (i.e., change ratio in PL intensity) to TEA was obtained when the excitation light (wavelength 514.5 nm) was continuously irradiated compared to when the CdSe QD-dispersed PMMA thin film was irradiated only during gas-sensor property measurements. (The concentrations of HDA, TEA, BZA, and BTA are not listed in the cited paper and are assumed to be saturated concentrations).
Potyrailo et al. reported an optical methanol and toluene gas sensor based on a composite thin film of CdSe QDs and polymethylmethacrylate (PMMA) [30]. The CdSe/PMMA composite thin films, consisting of green photoluminescent CdSe QDs (2.8 nm in particle size), red photoluminescent CdSe QDs (5.6 nm in particle size), and PMMA, were measured at room temperature (20 °C) and atmospheric pressure for polar methanol (46 Torr = 61,000 ppm) and non-polar toluene (11 Torr = 14,000 ppm) in air at room temperature (20 °C) and atmospheric pressure, respectively. The PL intensity of green-emitting CdSe QDs in the composite films was reversibly changed by methanol, but not by toluene, and the response and recovery times were short (<0.5 min). Conversely, the PL intensity of red-emitting CdSe QDs in the composite thin film was reversibly changed by methanol and the response/recovery time was slow (4 min/20 min). The PL intensity of red-emitting CdSe QDs was also reversibly changed by toluene, with a response/recovery time of 4 min/0.5 min. Thus, the difference in the response patterns of green- and red-emitting CdSe QDs in the composite thin film could be used to detect and discriminate polar methanol and non-polar toluene.
Yao et al. reported an optical triethylamine (TEA) gas sensor based on CdSe aerogels [31]. Porous CdSe aerogels prepared by supercritical drying of wet gels showed an increase in PL (peak wavelength of 538 nm) intensity at room temperature in response to TEA in argon, followed by a reversible decrease in PL intensity in TEA-free argon. Response and recovery times to TEA were several minutes. The PL intensity increased linearly with increasing TEA concentration (4.7 × 103–75 × 103 ppm). The sensor response characteristics were greatly affected by the surface condition of the aerogel. The increase in PL intensity due to TEA was suppressed for aerogels prepared after pyridine treatment of wet gels, and no change in PL intensity due to TEA was observed for aerogels heated under a vacuum. When the concentration of TEA in argon was varied in the range of 4.7 × 103–40 × 103 ppm (TEA vapor pressure: 0.005–0.04 atm), a nearly linear (proportional) relationship between TEA concentration and the change ratio in PL intensity (gas response) was observed; but when repeated exposure to argon with TEA and argon without TEA was performed, the relationship deviated from proportionality at higher concentrations, and response saturation was observed. Response and recovery times ranged from a few minutes to several minutes.
Saren et al. reported an optical ozone (O3) gas sensor using CdSe/ZnS core-shell QDs [32]. CdSe/ZnS QDs with different ZnS shell thicknesses, supported on a porous membrane filter, showed a decrease in PL (peak wavelength 528 nm) intensity in response to 1.1 ppm ozone; CdSe/ZnS QDs with thick ZnS shells showed response times of several seconds to tens of seconds and the change ratio in PL intensity (gas response) was about 60%. The response time for CdSe/ZnS QDs with a thin ZnS shell was only a few seconds, and the gas response was 100% (complete quenching of PL). In addition to the decrease in PL intensity, ozone adsorption-induced luminescence (AL) occurred only at the beginning of ozone exposure; all CdSe/ZnS QDs with different ZnS shell thicknesses showed a maximum AL intensity a few seconds after ozone exposure, followed by a rapid decay in a few seconds, and after several tens of seconds, the AL intensity was almost completely quenched. The rate of AL decay was faster, the thinner the ZnS shell of the CdSe/ZnS QDs. All three CdSe/ZnS QDs with PL wavelengths of 528, 592, and 626 nm showed a decrease in PL intensity on exposure to 1.1 ppm ozone. When CdSe/ZnS QDs with a PL wavelength of 592 nm were maintained in air at a constant flow rate and repeatedly pulsed with ozone, the PL and AL intensities decreased with increasing numbers of ozone treatments, and the PL spectrum shifted to shorter wavelengths. The degree of decrease in PL intensity and AL intensity was especially large in the initial stage of repeated ozone treatments. When CdSe/ZnS QDs with a PL wavelength of 592 nm were held under a vacuum and then in air containing ozone, the PL intensity decreased by about 45% (gas response of about 45%) in a few minutes, and subsequent vacuum degassing resulted in only about a 5% recovery of the PL intensity, which was almost irreversible, without recovery to the level before ozone exposure. (In addition to CdSe QDs, changes in PL and AL during ozone exposure have been reported for porous silicon, but are omitted from this review.)
Orlova et al. reported an optical ammonia gas sensor based on CdSe/ZnS QD-supported porous glass [33]. CdSe/ZnS core-shell QDs supported in porous borosilicate glass showed a decrease in PL intensity and emission decay time at room temperature on exposure to 1.7 × 10−3 mol/L ammonia in air, and these decreases became more pronounced with increasing ammonia exposure time. Subsequently, PL intensity and PL decay time partially recovered within a week in ammonia-free air at atmospheric pressure. This recovery process was accelerated and full recovery was achieved by vacuum degassing. As the ammonia gas was generated by the evaporation of aqueous ammonia, it was necessary to investigate the effect of water vapor on PL. Twenty minutes of exposure of CdSe/ZnS QD-supported porous glass to saturated water vapor (1.1 × 10−4 mol/L) at 20 °C resulted in a slight increase in PL intensity and a decrease in PL decay time.
Ando et al. reported optical ozone gas sensors using CdSe/CdZnS core-shell QDs, CdSe/ZnS core-shell QDs, and CdSeTe/ZnS core-shell QDs [34,35]. CdSe/CdZnS QD thin films (peak PL wavelength 609 nm), CdSe/ZnS QD thin films (peak PL wavelength 658 nm), and CdSeTe/ZnS QD thin films (peak PL wavelength 692 nm), all fabricated on glass substrates, showed a decrease in PL intensity at room temperature of 25 °C on exposure to ozone in the range 0.1–500 ppm in air. The PL intensity decreased in response to 0.1–500 ppm ozone in air at 25 °C and then recovered reversibly in ozone-free air. The response/recovery time was 10–20 min. The response to ozone (change ratio in PL intensity) increased with increasing ozone concentrations for both QD films: 5%/16%/60% for CdSe/CdZnS QD films (peak PL wavelength: 609 nm) at 1 ppm/10 ppm/500 ppm ozone. This red-emitting CdSe/CdZnS QD film could detect ozone in a wide concentration range from 0.5 to 500 ppm. Conversely, the green-emitting CdSe/ZnS QD thin film (peak PL wavelength 545 nm) showed a higher response for low concentrations of ozone, below 0.5 ppm, than the red-emitting CdSe/ZnS QD thin film, with 28%/55% response for ozone at 0.1 ppm/1 ppm. The green-emitting CdSe/ZnS QD thin film was suitable as a sensor material when limited to detecting low concentrations of ozone because when exposed to ozone above 50 ppm, the PL spectra blue-shifted, and the shift and the ozone-induced PL intensity change were irreversible in air without ozone, which reflected on poor resistance of small, green-emitting QDs against oxidation when compared with larger, red-emitting QDs. In addition, thin films consisting of red-emitting CdSe/ZnS QDs coated with polyethylene glycol inhibited ozone adsorption on the QDs, resulting in reduced ozone sensitivity and response time. All the CdSe-based core-shell QDs tested showed no change in PL intensity when exposed to 100% oxygen, nitrogen (N2), argon, carbon dioxide (CO2), or 1% hydrogen-containing air, indicating high ozone selectivity. Conversely, thin films of five CdTe core-type QDs with PL peak wavelengths of 551–750 nm showed only a 4–8% response to 10 ppm ozone. Thus, CdSe-based core-shell QDs showed particularly high sensitivity to ozone.
Optical alkylamine gas sensors using thin films of CdSe/ZnS QDs (PL peak wavelength 545–658 nm) and CdTeSe/ZnS QDs (PL peak wavelength 714 nm) fabricated on a glass substrate were also reported [36]. Thin films of CdSe/ZnS QDs (PL peak wavelength 658 nm) were sensitive to 0.2–25 vol.% of hexylamine (CH3(CH2)5NH2 (HA)), diethylamine ((C2H5)2NH (DEA)), and triethylamine (TEA) in air at room temperature (25 °C). The PL intensity decreased in all cases, and the PL intensity recovered reversibly in air without alkylamine. The PL peak wavelengths and spectra were identical before and after exposure to alkylamine. Response time was about 10 min and recovery time was about 30 min. Alkylamine gas response (change ratio in PL intensity) was hexylamine > diethylamine > triethylamine; and PL intensity decreased with increasing alkylamine concentration. Thin films of polyethylene glycol-coated CdSe/ZnS QDs (PL peak wavelength 663 nm) inhibited alkylamine adsorption on the QDs, resulting in a significant decrease in alkylamine sensitivity. Thin films of CdSe/ZnS QDs with PL peak wavelengths of 545, 569, and 658 nm, and CdSeTe/ZnS QDs with a PL peak wavelength of 714 nm, had responses to 1.0 vol.% HA in air of 40%, 30%, 22%, and 15%, respectively, and thin films of CdSe/ZnS QDs with a PL peak wavelength of 545 nm showed the highest sensitivity. In all cases, the change in PL intensity due to HA was reversible, and alkylamine had no effect on the PL peak wavelength and the shape of the PL spectrum. Conversely, the CdTe QD thin film with a PL peak wavelength of 594 nm showed a low response of 3.6% to 1.0 vol.% HA in air, indicating that CdSe-based core-shell QDs have a relatively high sensitivity to HA.
An optical ozone gas sensor using CdSe/ZnS QD thin films composited with noble metal nanoparticles (gold (Au), platinum (Pt), and palladium (Pd)) was also reported [37]. CdSe/ZnS QD thin films alone and CdSe/ZnS QD thin films composited with noble metal (Au, Pt, or Pt-Pd alloy (Pt/Pd = 85/15, w/w)) nanoparticles with PL peak wavelengths of 652, 657, 656, and 659 nm, respectively, all showed reversible PL intensity changes on exposure to 0.5–200 ppm ozone in air at room temperature (25 °C). The PL peak wavelengths of these films were similar to those of CdSe/ZnS QD thin films. Ozone exposure did not affect the peak PL wavelength or the shape of the PL spectrum. The response time to 0.5 ppm ozone, about 10 min, was not significantly different among the films, but the recovery time in air was different among the films (QD-only film, ~20 min; Pt-QD composite film. ~20 min; Au-QD composite film, ~12 min; and Pt-Pd-QD composite film ~10 min). The recovery time in air was longer when the QDs were exposed to higher concentrations of ozone, and the enhanced ozone sensitivity of QDs due to Pt and Au nanoparticle composites, and the enhanced recovery speed due to Pt-Pd alloy nanoparticle composites, were considered to reflect the microstructure of each thin film, the enhancement of ozone adsorption by Pt and Au, and the catalytic activity of Pd for ozone decomposition.
These QD-only and noble metal-QD composite thin films also showed reversible PL intensity changes on exposure to 1–100 ppm nitrogen dioxide (NO2) in air at room temperature (25 °C), and the response properties to nitrogen dioxide and ozone were different [38]. The response (change ratio in PL intensity) to NO2 increased with increasing NO2 concentration. The NO2 sensitivity was: Pt-Pd-QD composite film > Pt-QD composite film > Au-QD composite film > QD alone film, and the response to 100 ppm NO2 was 49% for Pt-Pd-QD film, 44% for Pt-QD film, 42% for Au-QD film, and 25% for QD-only film. The response time to 100 ppm NO2 (about 10 min) did not greatly vary between the films, but the recovery time in air did vary (112 min for Pt-Pd-QD film > 80 min for Au-QD film > 76 min for QD-only film > 66 min for Pt-QD film). The ratio of [100 ppm NO2 response]/[100 ppm ozone response] was significantly different for each film (0.66 for the QD-only film, 0.89 for the Au-QD film, 0.79 for the Pt-QD film, and 1.58 for the Pt-Pd-QD film). These differences in the sensitivity of each thin film to each gas suggest the possibility of discriminative detection of NO2 and ozone.
2.5. Conductometric Gas Sensors Using CdTe
Strobel et al. reported a conductometric ammonia gas sensor using clusters of cylindrical aerographite (AG) modified with CdTe [39]. Polycrystalline CdTe thin films supported on AG were sensitive to 100 ppm ammonia in air containing about 30% RH at room temperature and showed a reversible electrical resistance change. The polycrystalline CdTe thin films showed a reversible electrical resistance change in response to 100 ppm ammonia in air with about 30% RH. The ammonia response (change ratio in electrical resistivity) was 86%, 103%, 85%, and 52% at bias voltages of 0.01, 0.1, 0.5, and 1 V, respectively. Conversely, the response to 1000 ppm hydrogen was low at 7.5%, 10%, 8.6%, and 5.3% for bias voltages of 0.01, 0.1, 0.5, and 1 V, respectively, and less than 3% for 100 ppm ethanol, acetone, and n-butanol. Thus, high selectivity and sensitivity to ammonia were demonstrated. The sensitivity was examined by changing the ammonia concentration in the range of 20–200 ppm at a bias voltage of 0.1 V. The electrical resistivity changed reversibly in all cases, and the response to 20, 50, 100, and 200 ppm ammonia was 9%, 53%, 103%, and 153%, respectively. From these results, the LOD was estimated to be approximately 3 ppm. The dependence of the gas response on ammonia concentration was not linearly proportional, the rate of increase in gas response decreased with increasing gas concentration, and the ammonia sensitivity averaged over the concentration range of 20–200 ppm was estimated to be 0.8%/ppm. When response/recovery was examined by varying the bias voltage in the range of 0.01–1 V, all responses were reversible, and the 90% response/recovery times at bias voltages of 0.01, 0.1, 0.5, and 1 V were ~52 s/~48 s, ~21 s/~72 s, ~18 s/~44 s, and ~17 s/~26 s, respectively. Thus, the response/recovery speed was accelerated when the bias voltage was increased from 0.01 V to 1 V. Conversely, the sensitivity was reduced by about half.
Jaiswal et al. reported a conductometric nitrogen dioxide (NO2) gas sensor based on nanocomposite thin films (CdTe/ZnO@PSi) consisting of porous silicon (PSi) loaded with ZnO functionalized with CdTe [40]. In the working temperature range of 30–180 °C, both the CdTe/ZnO@PSi and ZnO@PSi thin films exhibited a reversible electrical resistance change on exposure to 1 ppm NO2. Compared to the ZnO@PSi thin film’s NO2 response (change ratio in electrical resistivity) of ~5.72% and response/recovery time of ~41 s/124 s at an operating temperature of 150 °C, the CdTe/ZnO@PSi thin film showed higher NO2 sensor characteristics at an operating temperature of 90 °C, with a NO2 response of ~19.82%, about 3.5 times higher, and a shorter response/recovery time of ~13 s/54 s. Reversibility and reproducibility after 16 repeated exposures and stability over 90 days were good. Thus, high NO2 selectivity, improved NO2 sensitivity, faster response/recovery times, and lower operating temperatures were achieved in CdTe/ZnO@PSi thin films. The response of CdTe/ZnO@PSi thin films to various gases (100 ppm) was NO2 >> ammonia > (hydrogen sulfide, carbon monoxide, ethanol, and hydrogen), indicating high selectivity for NO2. At 90 °C, the response of CdTe/ZnO@PSi films to 1 ppm NO2 was almost unaffected by RH in the 5–90% range.
Goyal et al. reported a conductometric ammonia gas sensor using CdTe thin films before and after gamma irradiation [41]. The CdTe thin films before gamma irradiation, prepared by vacuum evaporation, had a porous structure and exhibited a reversible electrical resistance change on exposure to 3.6–36 ppm ammonia in air at room temperature. Ammonia gas was obtained by the evaporation of aqueous ammonia. After gamma irradiation (gamma irradiation dose: 50, 100, and 150 kGy), the CdTe films became less porous and more conductive, but the ammonia response (change ratio in electrical resistivity) decreased; the response to 36 ppm ammonia was 14.59% and 10.28% for the CdTe films before and after gamma irradiation (150 kGy), respectively. The response/recovery time to 36 ppm ammonia was 15 s/129 s for the CdTe films before gamma irradiation and was shorter than that for the CdTe films after gamma irradiation at doses of 50, 100, and 150 kGy. The response to ammonia at 3.6 ppm, the detection limit concentration, was 3.55% before gamma irradiation, 2.99% after 50 kGy of gamma irradiation, and 3.17% after 150 kGy of gamma irradiation. Thus, gamma irradiation caused microstructural changes in the CdTe thin film, such as reduced porosity, which reduced the ammonia sensitivity, response speed, and recovery speed.
2.6. Optical Gas Sensors Using CdTe
Ma et al. reported an optical formaldehyde gas sensor based on a multilayer thin film composed of CdTe QDs and a polyelectrolyte [42]. The CdTe QD/polyelectrolyte multilayer thin film (QDMF) exhibited reversible PL intensity changes on exposure to formaldehyde in the air at room temperature. The PL intensity of QDMF decreased linearly as the concentration of formaldehyde in the air increased in the range of 5–500 ppb, with a LOD of 1 ppb for formaldehyde. PL intensity decreased with increasing formaldehyde exposure time. During the first 30 min of exposure, the PL intensity decreased significantly from 157.0 to 55.2 but remained constant after 30 min. PL quenching by formaldehyde in the presence of other gases (methanol, ethanol, acetone, benzene, toluene, ethoxyethane (diethyl ether) ((C2H5)2O), chloroform (CHCl3), and acetaldehyde) at various concentrations was investigated. The response (change ratio in PL intensity) to 50 ppb formaldehyde was only 0.61% in the presence of 20,000 ppb methanol. The response to formaldehyde was reduced by 13.01% when 500 ppb acetaldehyde was added to 50 ppb formaldehyde, but in QDMF modified with bovine serum albumin (BSA), the effect of the presence of acetaldehyde on the response to formaldehyde under the same conditions was not observed. The effect of acetaldehyde on formaldehyde response (interference effect) was reduced to 3.66% under the same conditions.
Bakar et al. reported an optical 2-propanol gas sensor in which the 45° inclined end face of an optical fiber was coated with a CdTe QD thin film [43]. The CdTe QD thin film formed on the end face of the optical fiber exhibited PL quenching at room temperature in response to the volatile organic compounds (VOCs) ethanol, 2-propanol, and acetone in nitrogen (the reversibility was not mentioned). The PL peak wavelength (ca. 595 nm) and PL spectrum shape of the CdTe QD were not changed by exposure to VOCs. The gas response (change ratio in PL intensity) was 2-propanol > acetone > ethanol (the concentration of each gas was not mentioned, so it is assumed to be the saturation concentration in nitrogen). The rate of the decrease in PL intensity (gas response speed) also differed depending on the type of VOC, but the decrease in PL intensity saturated at 30 min after exposure to the VOC and the PL intensity reached a stable value.
Ahmad et al. reported an optical formaldehyde gas sensor using an organic/inorganic composite material (CdTe/CdS QD-HBQP) obtained by bonding 3-(6-hydrazinyl-1,3-dioxo-benzoisoquinolinyl) propanoic acid (HBQP), an organic dye responsive to formaldehyde, to the surface of a CdTe/CdS core-shell QD [44]. First, the response to formaldehyde in water was investigated. At room temperature, when formaldehyde was added from 0 to 26 μmol/L to a pH 7.5 phosphate buffer in which CdTe/CdS QD-HBQP was dispersed, the PL intensity of the HBQP-coated CdTe/CdS QD at 666 nm under excitation light irradiation at a wavelength of 385 nm decreased with increasing formaldehyde concentration. The PL intensity at 540 nm, originating from HBQP molecules released from CdTe/CdS QD-HBQP in reaction with formaldehyde, increased with increasing formaldehyde concentration (the reversibility was not mentioned). The PL intensity at both 540 nm and 666 nm showed a linear relationship with the concentration of formaldehyde in the phosphate buffer solution, with a response time of 7.2 s for both wavelengths when 50 μmol/L formaldehyde was added. The LOD for formaldehyde in phosphate buffer was estimated to be 0.49 μmol/L. Next, the response to formaldehyde in air was examined; solid materials with CdTe/CdS QD-HBQPs loaded on cellulose paper showed a significant response to increasing concentrations of formaldehyde in air from 0 to 13.5 μmol/L at room temperature, as well as in phosphate buffer. Under light irradiation at a wavelength of 385 nm, the PL intensity of HBQP-coated CdTe/CdS QD at 666 nm decreased with increasing formaldehyde concentration, while the PL intensity at 540 nm of HBQP molecules released from CdTe/CdS QD-HBQP increased (the reversibility was not mentioned). The PL intensity at both 540 nm and 666 nm showed a linear relationship with the concentration of formaldehyde in air. The LOD for formaldehyde detection in air was estimated to be 0.1 μmol/L. The color change of this solid gas sensor was visually examined by changing the concentration of formaldehyde in the air in the range of 0–130 μmol/L. The color changed from red at 0 μM, to yellow at 40 μmol/L, and green at 130 μmol/L.
3. Conclusions
This review provides an overview of recent reports on conductometric and optical gas sensors using non-oxide II-VI semiconductors, CdS, CdSe, and CdTe. Compared to metal oxide semiconductors, which have long been used in semiconductor and catalytic combustion gas sensors, there is still a limited number of reports on the application of non-oxide II-VI semiconductors to gas sensors, but the number of reported cases showing high performance for these semiconductors as gas sensors is increasing for both conductometric and optical types. Semiconductor gas sensors, which are typical conductometric gas sensors, have had the problems of high power consumption and safety issues in high-temperature areas because heating was required in many cases. However, there were examples of semiconductor gas sensors using non-oxide II–VI semiconductors with nanostructures such as quantum dot, nanowire, nanorod, nanoribbon, microbelt, and fiber discussed in this review that achieved reduced operating temperatures and heater-less room-temperature operation. Furthermore, there have been examples of high gas sensitivity and selectivity achieved by not only a single type, but also by combining multiple types of non-oxide II-VI semiconductors, by combining non-oxide II-VI semiconductors with metal oxide semiconductors, metal ions, organic functional materials, etc., or by using activation with light irradiation. Optical gas sensors using non-oxide II-VI semiconductors have attracted attention because of their advantages over conductometric gas sensors, such as heater-less, room temperature operation, high electromagnetic noise immunity, and remote/non-contact signal readout. The reported examples of optical gas sensors that use PL or refractive index introduced in this paper have all achieved room-temperature operation, and a variety of promising results have been reported, including the method of expressing changes in PL characteristics, catalytic emission, or refractive index of nanomaterials, such as quantum dot, nanoflower, and aerogel made of non-oxide II-VI semiconductors. Non-oxide II-VI semiconductors have great potential as new high-performance conductometric and optical gas sensor materials, and their future development is expected.
Author Contributions
Conceptualization, M.A. and Y.S.; methodology, M.A.; validation, H.K., S.T., Y.H. and Y.S.; investigation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, H.K., S.T., Y.H. and Y.S.; supervision, Y.S.; funding acquisition, M.A., Y.S. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number JP17K05957 (to Masanori Ando and Yasushi Shigeri), and the Grant from the Steel Foundation for Environmental Protection Technology (#4: 38th and #1: 39th (to Yasushi Shigeri and Masanori Ando), and #2: 41st (to Masanori Ando, Hideya Kawasaki and Yasushi Shigeri)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Leslie Sargent Jones (Appalachian State University, Retired) for her careful reading of our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miura, N. (Ed.) Advanced Chemical Sensors—State of the Art in the Gas, Bio and Ion Sensing Technologies; Japan Association of Chemical Sensors, The Electrochemical Society of Japan, TIC: Kyoto, Japan, 2008. [Google Scholar]
- Ai, M.; Muro, H. (Eds.) Handbook of Next-Generation Sensors; Baifukan: Tokyo, Japan, 2008. [Google Scholar]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Fuśnik, Ł.; Szafraniak, B.; Paleczek, A.; Grochala, D.; Rydosz, A. A Review of Gas Measurement Set-Ups. Sensors 2020, 22, 2557. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Eguchi, K. Optical Gas Sensors. In Gas Sensors; Sberveglieri, G., Ed.; Kluwer: Dordrecht, The Netherlands, 1992; pp. 307–328. [Google Scholar]
- Shi, J.; Zhu, Y.; Zhang, X.; Baeyens, W.R.G.; García-Campaña, A.M. Recent developments in nanomaterial optical sensors. TrAC-Trend. Anal. Chem. 2004, 23, 351–360. [Google Scholar] [CrossRef]
- Ando, M. Recent advances in optochemical sensors for the detection of H2, O2, O3, CO, CO2 and H2O in air. TrAC-Trend. Anal. Chem. 2006, 25, 937–948. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef]
- Navale, S.T.; Mane, A.T.; Chougule, M.A.; Shinde, N.M.; Kim, J.-H.; Patil, V.B. Highly selective and sensitive CdS thin film sensors for detection of NO2 gas. RSC Adv. 2014, 4, 44547–44554. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, L.; Zu, B.; Guo, Y.; Xu, T.; Dou, X. CdS/ZnO Core/Shell Nanowire-Built Films for Enhanced Photodetecting and Optoelectronic Gas-Sensing Applications. Adv. Opt. Mater. 2014, 2, 738–745. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhang, D.; Li, C.; Sun, D.; Wen, S.; Chen, Y.; Ruan, S. Gas Sensors Based on Metal Sulfide Zn1−xCdxS Nanowires with Excellent Performance. ACS Appl. Mater. Interf. 2015, 7, 20793–20800. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, W.; Wu, R.; Zhang, M. CeO2 Enhanced Ethanol Sensing Performance in a CdS Gas Sensor. Sensors 2017, 17, 1577. [Google Scholar] [CrossRef] [PubMed]
- Vanalakar, S.A.; Patil, V.L.; Patil, P.S.; Kim, J.H. Rapid synthesis of CdS nanowire mesh via a simplistic wet chemical route and its NO2 gas sensing properties. New J. Chem. 2018, 42, 4232–4239. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Ma, P.; Li, S.; Fan, F.; Cui, K.; Ge, S.; Zhang, Y.; Yu, J. Low-Power and High-Performance Trimethylamine Gas Sensor Based on nn Heterojunction Microbelts of Perylene Diimide/CdS. Anal. Chem. 2019, 91, 5591–5598. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Sharma, A.K.; Yadav, N.K.; Yadava, L. Development of CdS-doped TiO2 nanocomposite as acetone gas sensor. Vacuum 2021, 191, 111363. [Google Scholar] [CrossRef]
- Madlul, S.F.; Mahan, N.K.; Ali, E.M.; Abd, A.N. Synthesis of CdS:Cu5% thin films by chemical method based on silicon for gas sensor applications. Mater. Today Proc. 2021, 45, 5800–5803. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Li, T.; Zhang, J.; Yu, L. Green light-driven acetone gas sensor based on electrospinned CdS nanospheres/Co3O4 nanofibers hybrid for the detection of exhaled diabetes biomarker. J. Colloid Interf. Sci. 2022, 606, 261–271. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, L.; Lv, Y.; Su, Y. A new alcohols sensor based on cataluminescence on nano-CdS. Sens. Actuators B Chem. 2013, 186, 750–754. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. Clad-modified fiber optic sensor utilizing CdS nanoflower as cladding for the detection of ethanol. J. Mater. Sci. Mater. Electron. 2021, 32, 23900–23910. [Google Scholar] [CrossRef]
- Joshi, S.S.; Lokhande, C.D.; Han, S.-H. A room temperature liquefied petroleum gas sensor based on all-electrodeposited n-CdSe/p-polyaniline junction. Sens. Actuators B Chem. 2007, 123, 240–245. [Google Scholar] [CrossRef]
- Lin, Z.; Liao, F.; Zhu, L.; Lu, S.; Sheng, M.; Gao, S.; Shao, M. Visible light enhanced gas sensing of CdSe nanoribbons of ethanol. CrystEngComm 2014, 16, 4231–4235. [Google Scholar] [CrossRef]
- Wu, B.; Lin, Z.; Sheng, M.; Hou, S.; Xu, J. Visible-light activated ZnO/CdSe heterostructure-based gas sensors with low operating temperature. Appl. Surf. Sci. 2016, 360, 652–657. [Google Scholar] [CrossRef]
- Laatar, F.; Harizi, A.; Zarroug, A.; Ghrib, M.; Hassen, M.; Gaidi, M.; Ezzaouia, H. Novel CdSe nanorods/porous anodic alumina nanocomposite-based ethanol sensor: Sensitivity enhancement by visible light illumination. J. Mater. Sci. Mater. Electron. 2017, 28, 12259–12267. [Google Scholar] [CrossRef]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-Activated Sub-ppm NO2 Detection by Hybrid ZnO/QD Nanomaterials vs. Charge Localization in Core-Shell QD. Front. Mater. 2019, 6, 231. [Google Scholar] [CrossRef]
- Geng, X.; Li, S.; Mawella-Vithanage, L.; Ma, T.; Kilani, M.; Wang, B.; Ma, L.; Hewa-Rahinduwage, C.C.; Shafikova, A.; Nikolla, E.; et al. Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing. Nat. Commun. 2021, 12, 4895. [Google Scholar] [CrossRef]
- Nazzal, A.Y.; Qu, L.; Peng, X.; Xiao, M. Photoactivated CdSe Nanocrystals as Nanosensors for Gases. Nano Lett. 2003, 3, 819–822. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Leach, A.M. Selective gas nanosensors with multisize CdSe nanocrystal/polymer composite films and dynamic pattern recognition. Appl. Phys. Lett. 2006, 88, 134110. [Google Scholar] [CrossRef]
- Yao, Q.; Brock, S.L. Optical sensing of triethylamine using CdSe aerogels. Nanotechnology 2010, 21, 115502. [Google Scholar] [CrossRef]
- Saren, A.A.; Kuznetsov, S.N.; Kuznetsov, A.S.; Gurtov, V.A. Excitonic Chemiluminescence in Si and CdSe Nanocrystals Induced by their Interaction with Ozone. ChemPhysChem 2011, 12, 846–853. [Google Scholar] [CrossRef]
- Orlova, A.O.; Gromova, Y.A.; Maslov, V.G.; Andreeva, O.V.; Baranov, A.V.; Fedorov, A.V.; Prudnikau, A.V.; Artemyev, M.V.; Berwick, K. Reversible photoluminescence quenching of CdSe/ZnS quantum dots embedded in porous glass by ammonia vapor. Nanotechnology 2013, 24, 335701. [Google Scholar] [CrossRef]
- Ando, M.; Kamimura, T.; Uegaki, K.; Biju, V.; Shigeri, Y. Sensing of ozone based on its quenching effect on the photoluminescence of CdSe-based core-shell quantum dots. Microchim. Acta 2016, 183, 3019–3024. [Google Scholar] [CrossRef]
- Ando, M.; Biju, V.; Shigeri, Y. Development of Technologies for Sensing Ozone in Ambient Air. Anal. Sci. 2018, 34, 263–271. [Google Scholar] [CrossRef]
- Ando, M.; Kamimura, T.; Uegaki, K.; Biju, V.; Damasco Ty, J.T.; Shigeri, Y. Reversible photoluminescence sensing of gaseous alkylamines using CdSe-based quantum dots. Sens. Actuators B Chem. 2017, 246, 1074–1079. [Google Scholar] [CrossRef]
- Ando, M.; Inagaki, K.; Kawasaki, H.; Biju, V.; Shigeri, Y. Photoluminescent Ozone Sensor with Enhanced Sensitivity by Using CdSe/ZnS Quantum Dots Modified with Gold and Platinum. Anal. Sci. 2020, 36, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Inagaki, K.; Kawasaki, H.; Shigeri, Y. Reversible sensing of nitrogen dioxide using photoluminescent CdSe/ZnS quantum dots and enhanced response by combination with noble metals. J. Ceram. Soc. Jpn 2022, 130, 180–186. [Google Scholar] [CrossRef]
- Strobel, J.; Ghimpu, L.; Postica, V.; Lupan, O.; Zapf, M.; Schönherr, S.; Röder, R.; Ronning, C.; Schütt, F.; Mishra, Y.K.; et al. Improving gas sensing by CdTe decoration of individual Aerographite microtubes. Nanotechnology 2019, 30, 065501. [Google Scholar] [CrossRef]
- Jaiswal, J.; Singh, P.; Chandra, R. Low-temperature highly selective and sensitive NO2 gas sensors using CdTe-functionalized ZnO filled porous Si hybrid hierarchical nanostructured thin films. Sens. Actuators B Chem. 2021, 327, 128862. [Google Scholar] [CrossRef]
- Goyal, S.; Chauhan, R.P. Ammonia gas sensing response of gamma-irradiated CdTe thin films. Mater. Sci. Semicond. Process. 2021, 121, 105394. [Google Scholar] [CrossRef]
- Ma, Q.; Cui, H.; Su, X. Highly sensitive gaseous formaldehyde sensor with CdTe quantum dots multilayer films. Biosens. Bioelectron. 2009, 25, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Bakar, N.A.; Rahmi, A.; Umar, A.A.; Salleh, M.M.; Yahaya, M. Fluorescence gas sensor using CdTe quantum dots film to detect volatile organic compounds. Mater. Sci. Forum. 2011, 663–665, 276–279. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhou, Z.; Li, H.-Y.; Zang, S.-Q. Crafting CdTe/CdS QDs surface for the selective recognition of formaldehyde gas via ratiometric contrivance. Sens. Actuators B Chem. 2020, 304, 127379. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).