Porphyrin Hetero-Trimer Involving a Hydrophilic and a Hydrophobic Structure with Application in the Fluorescent Detection of Toluidine Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
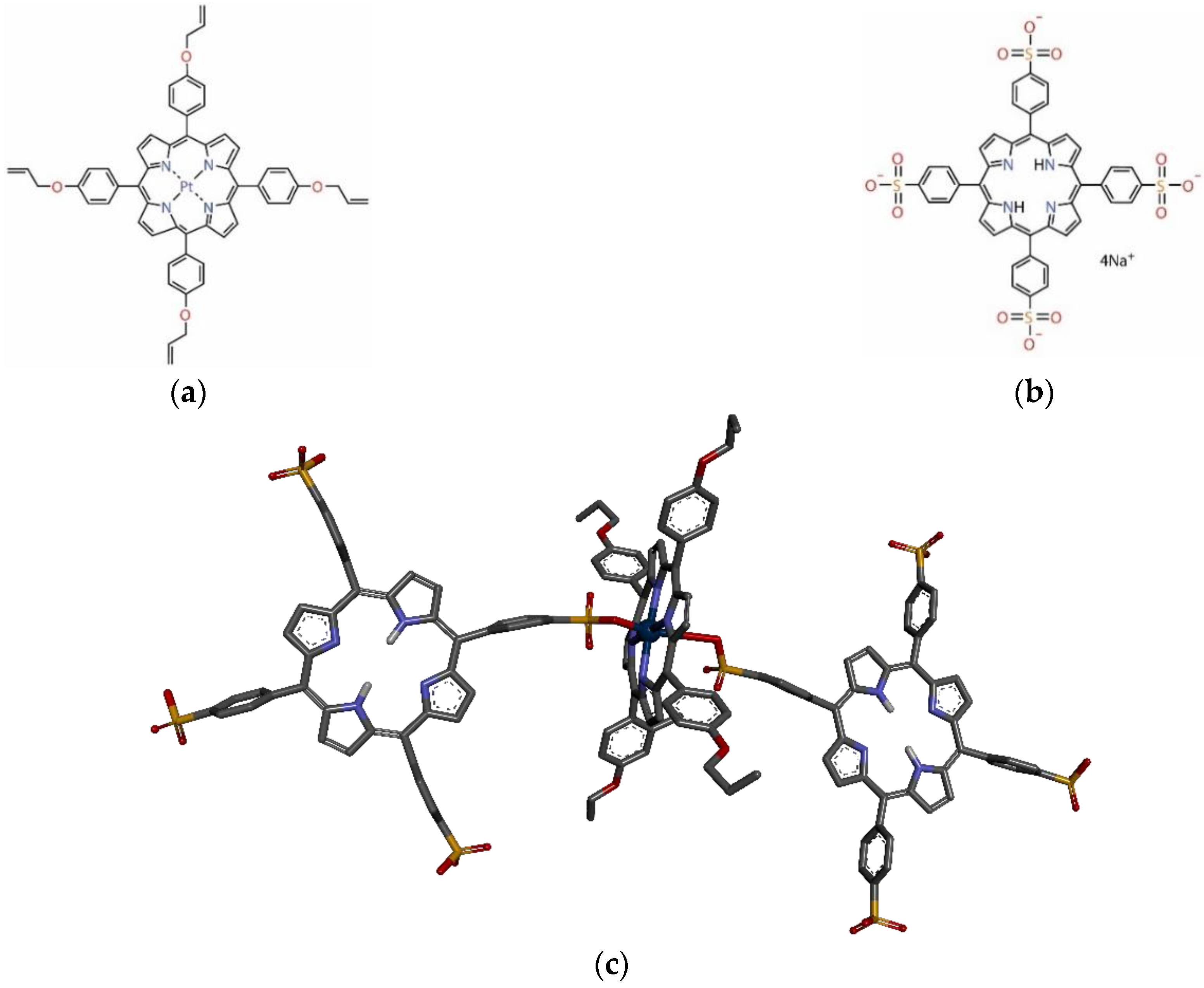
2.1.1. Obtaining of 5,10,15,20-Tetra-(4-allyloxy-phenyl)porphyrin
2.1.2. Obtaning of Pt(II)-5,10,15,20-Tetra-(4-allyloxy-phenyl)porphyrin
2.1.3. Obtaining of Benzenesulfonic Acid, 4,4′,4″,4‴-(21H,23H-Porphine-5,10,15,20-tetrayl)tetrakis-tetrasodium Salt, Commonly Named as 5,10,15,20-Tetrakis(4-sulfonatophenyl)-porphyrin (TSPP)
2.2. Apparatus
2.3. Formation of the Pt-allyloxyPP-TSPP Hetero-Trimer
3. Results and Discussions
3.1. UV–Vis Spectroscopy
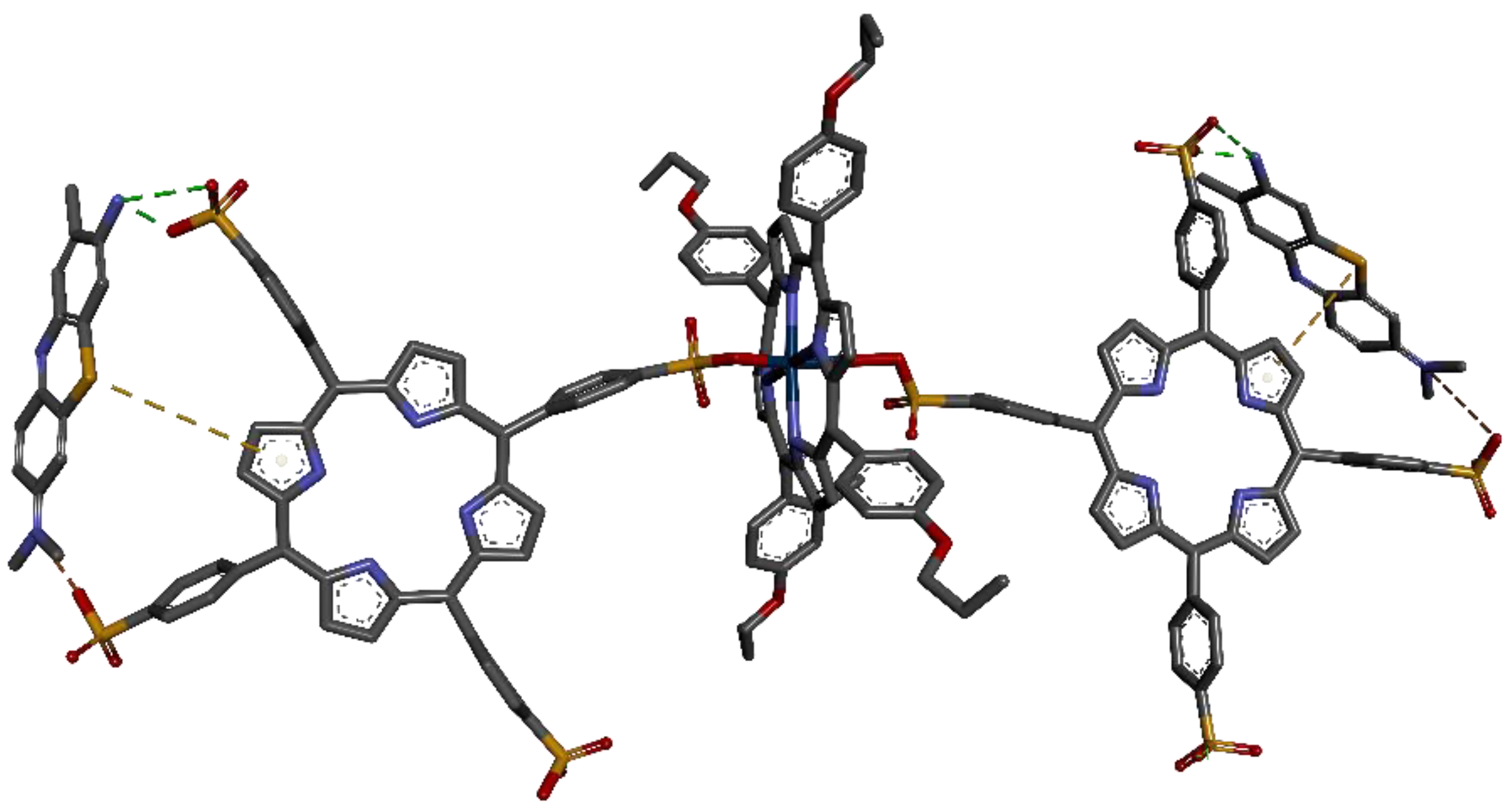
3.2. Characterization of the Pt-allyloxyPP-TSPP Hetero-Trimer
3.2.1. 1H-NMR Analysis
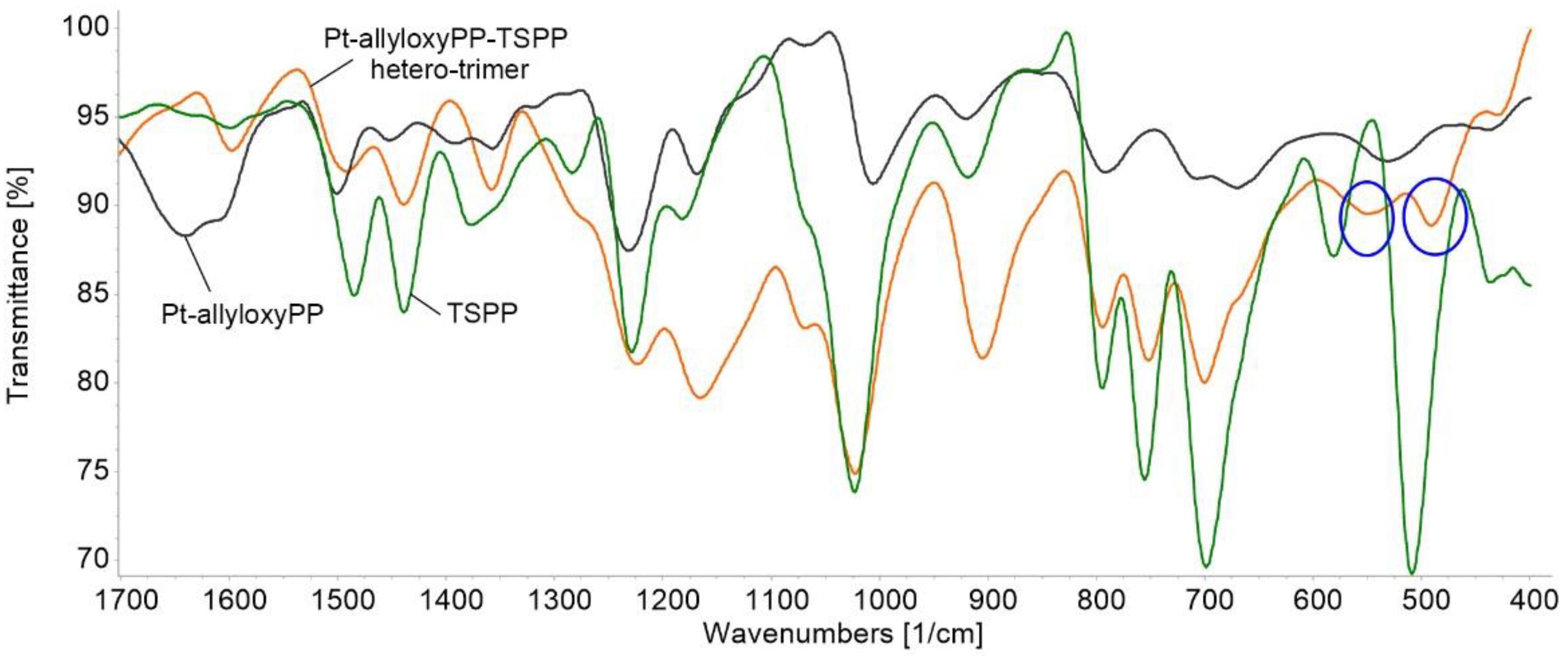
3.2.2. FT-IR Characterization
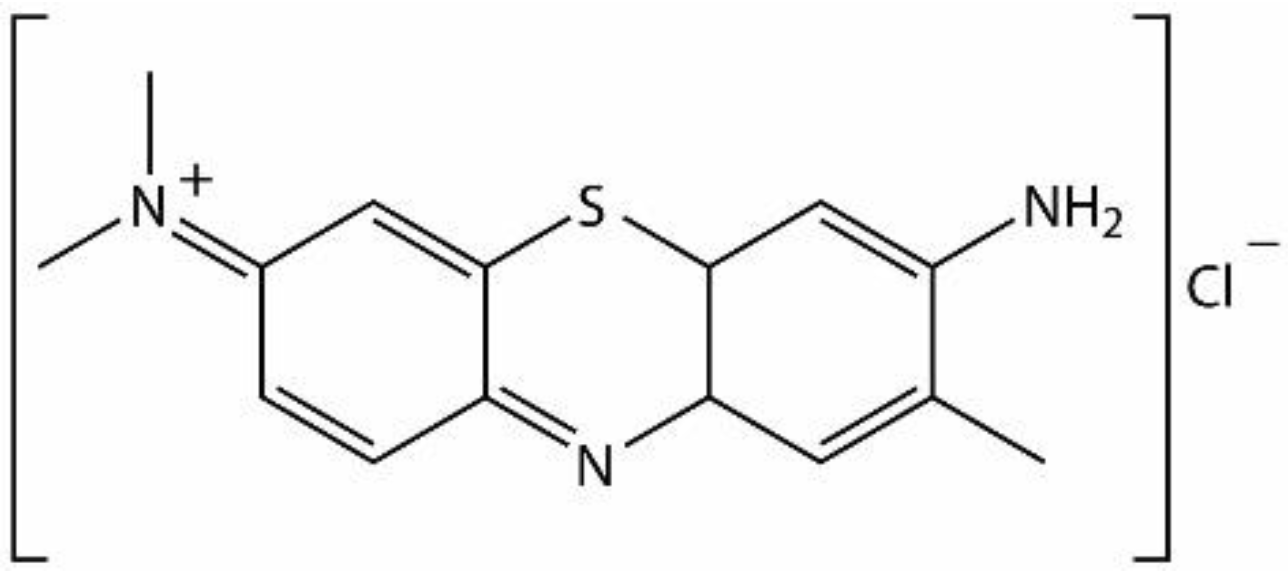
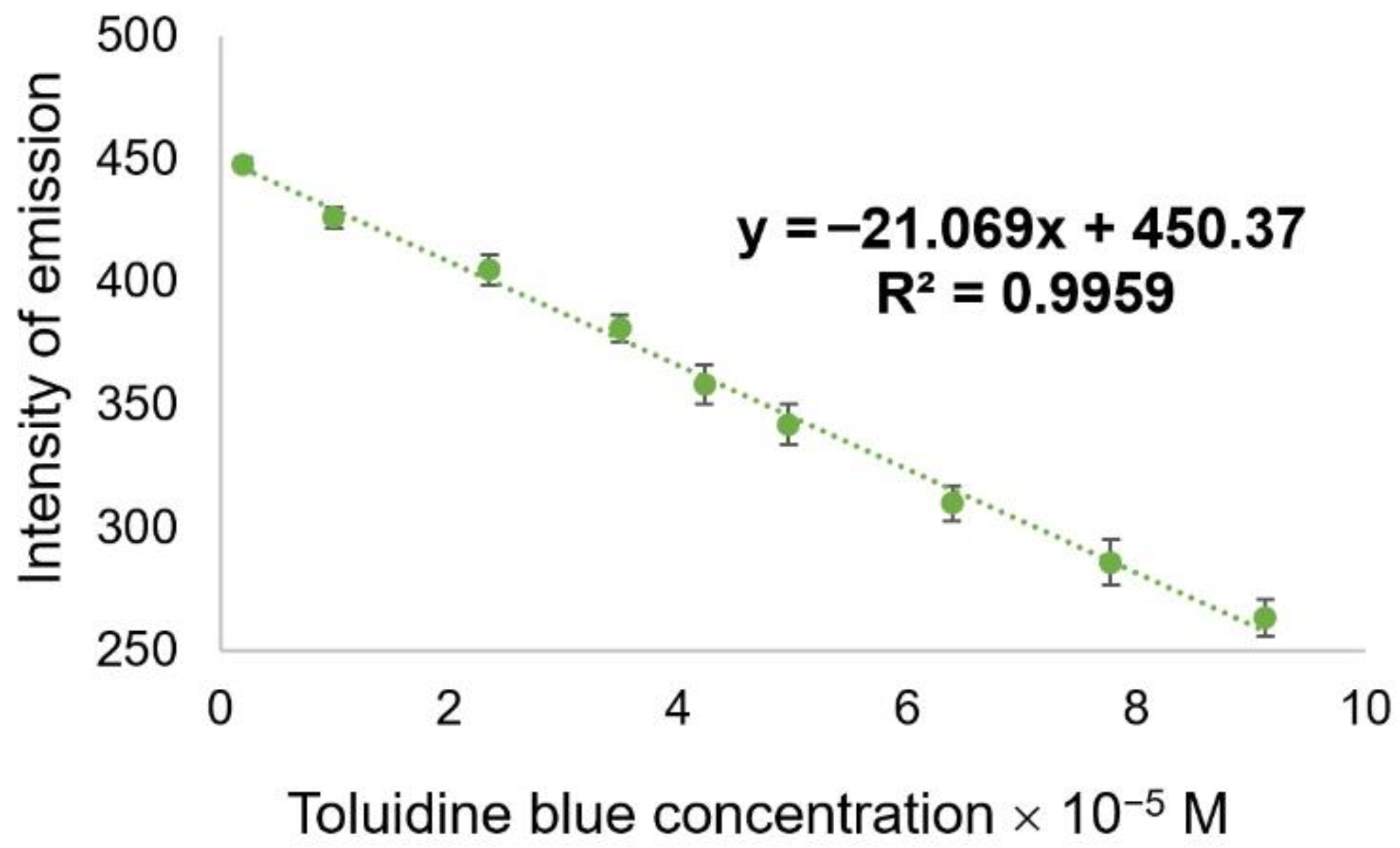
3.3. Fluorimetric Detection of Toluidine Blue by the Pt-allyloxyPP-TSPP Hetero-Trimer
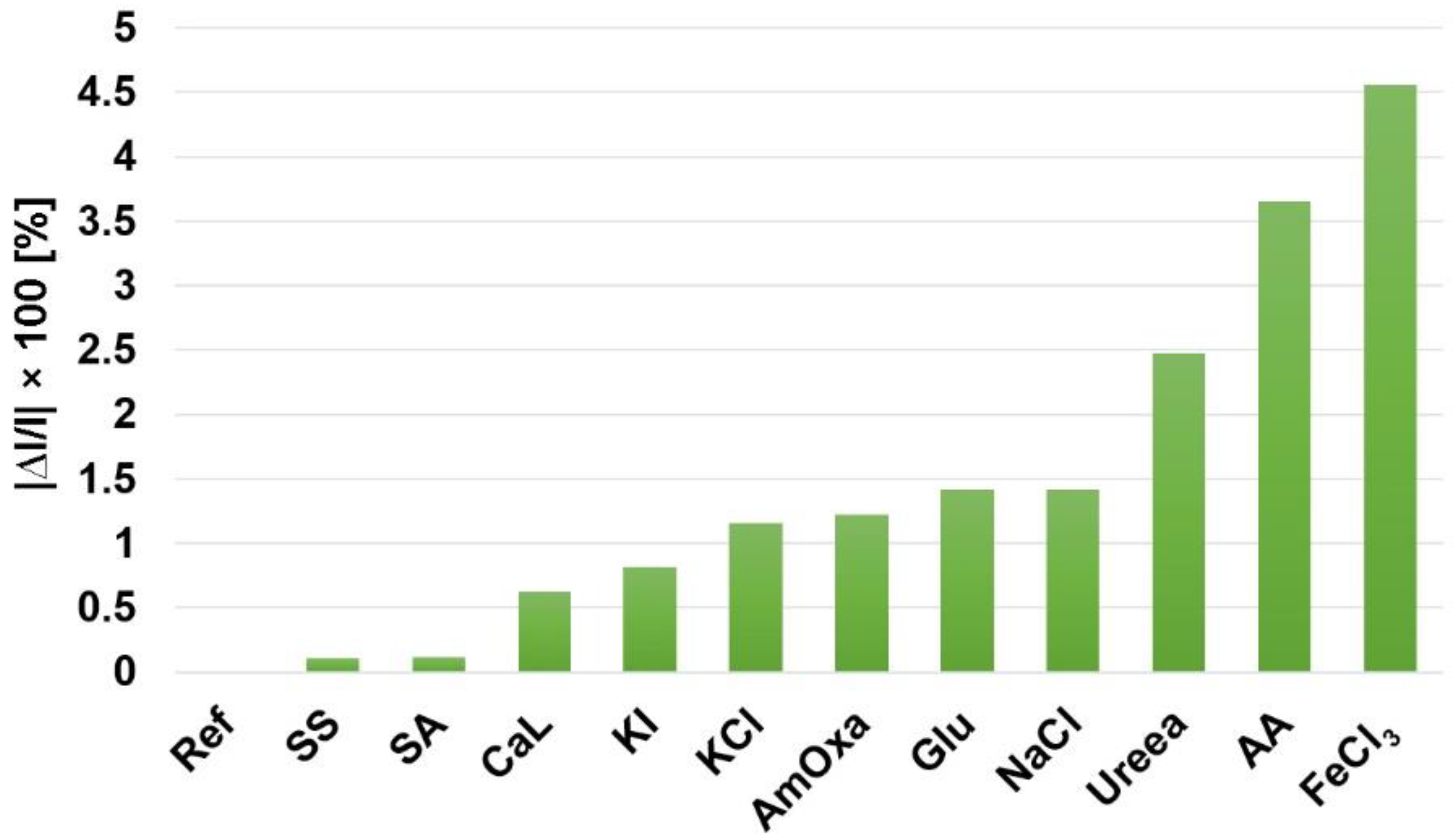
Interference Test for Fluorimetric Detection of Toluidine Blue Using as Sensitive Material Pt-allyloxyPP-TSPP Hetero-Trimer
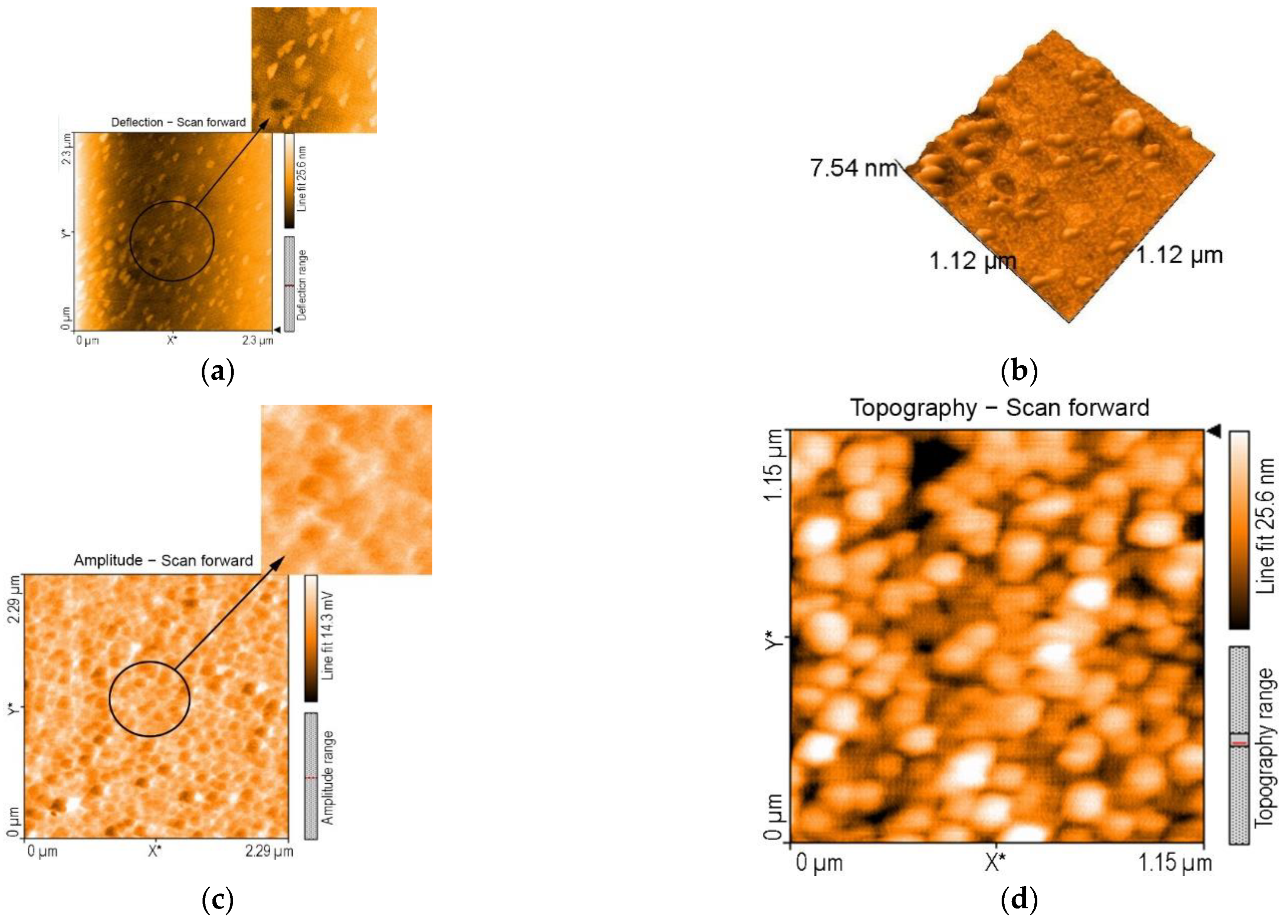
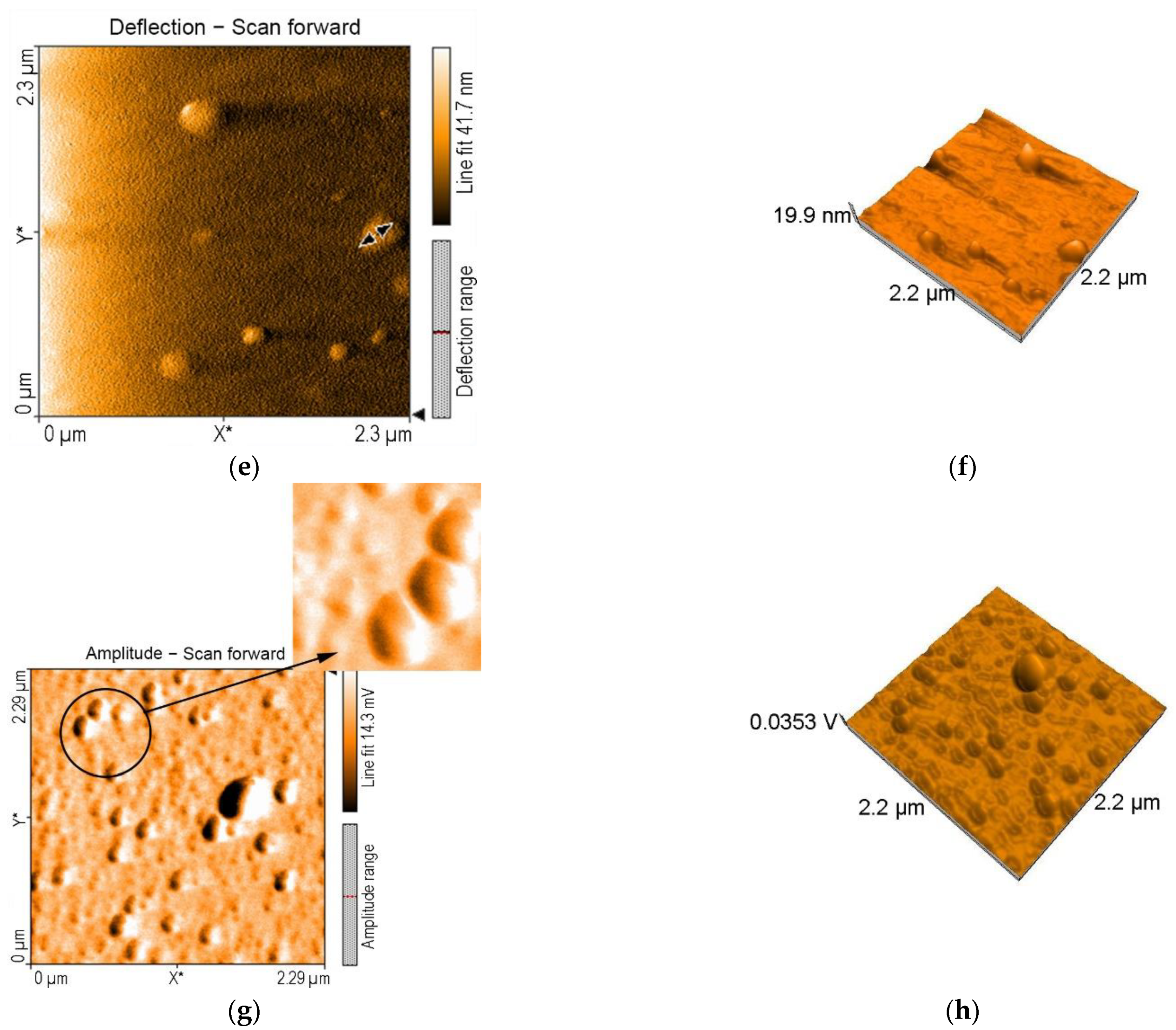
3.4. AFM Investigations
3.5. Infrared Spectra Comparison before and after the Detection of Toluidine Blue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nomoto, A.; Mitsuoka, H.; Ozeki, H.; Kobuke, Y. Porphyrin hetero-dimer as charge separating system for photocurrent generation. Chem. Commun. 2003, 9, 1074–1075. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, H.; Li, J.; Dy, J.T.; Tamaki, K.; Nakazaki, J.; Nakayama, D.; Nishiyama, C.; Uchida, S.; Kubo, T.; et al. Ethynyl-linked push–pull porphyrin hetero-dimers for near-IR dye-sensitized solar cells: Photovoltaic performances versus excited-state dynamics. Phys. Chem. Chem. Phys. 2012, 14, 16703. [Google Scholar] [CrossRef]
- Mazur, L.M.; Roland, T.J.; Leroy-Lhez, S.; Sol, V.; Samoc, M.; Samuel, I.D.W.; Matczyszyn, K. Efficient Singlet Oxygen Photogeneration by Zinc Porphyrin-Dimers Upon One- and Two-Photon Excitation. J. Phys. Chem. B 2019, 123, 4271–4277. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Baryshnikov, G.; Ding, Y.; Zhao, C.; Gu, T.; Sha, F.; Liang, X.; Zhu, W.; Wu, X.; et al. Twisted-Planar-Twisted expanded porphyrinoid dimer as a rudimentary reaction-based methanol indicator. Nat. Commun. 2020, 11, 5289. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Badea, V.; Fagadar-Cosma, G.; Palade, A.; Lascu, A.; Fringu, I.; Birdeanu, M. Trace oxygen sensitive material based on two porphyrin derivatives heterodimer complex. Molecules 2017, 22, 1787. [Google Scholar] [CrossRef]
- Manera, M.G.; Ferreiro-Vila, E.; García-Martín, J.M.; Cebollada, A.; García-Martín, A.; Giancane, G.; Valli, L.; Rella, R. Enhanced magneto-optical SPR platform for amine sensing based on Zn porphyrin dimers. Sens. Actuators B Chem. 2013, 182, 232–238. [Google Scholar] [CrossRef]
- Schmitt, J.; Jenni, S.; Sour, A.; Heitz, V.; Bolze, F.; Pallier, A.; Bonnet, C.; Toth, E.; Ventura, V. A Porphyrin Dimer–GdDOTA Conjugate as a Theranostic Agent for One- and Two-Photon Photodynamic Therapy and MRI. Am. Chem. Soc. 2018, 29, 3726–3738. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Chou, H.H.; Cheng, W.T.; Li, Y.R.; Yeh, C.Y.; Chen, P. Porphyrin dimers as hole-transporting layers for high-efficiency and stable perovskite solar cells. ACS Energy Lett. 2018, 3, 1620–1626. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Ding, D.; Qurashi, M.; Boczarow, I.; Balaz, M.; Anderson, H.; Kuimova, M. Tuning the sensitivity of fluorescent porphyrin dimers to viscosity and temperature. Chem.-Eur. J. 2017, 23, 11001–11010. [Google Scholar] [CrossRef]
- Lamare, R.; Ruppert, R.; Elhabiri, M.; Ulrich, G.; Ruhlmann, L.; Weiss, J. Design and Synthesis of Charged Porphyrin Dimers for Polyoxometalate Recognition. C. R. Chim. 2021, 24, pp. 115–126. Available online: https://comptes-rendus.academie-sciences.fr/chimie/articles/10.5802/crchim.105/ (accessed on 8 November 2022).
- Habets, T.; Lensen, D.; Speller, S.; Elemans, J.A. Self-assembly of covalently linked porphyrin dimers at the solid–liquid interface. Molecules 2019, 24, 3018. [Google Scholar] [CrossRef]
- Schissler, C.; Schneider, E.K.; Felker, B.; Weis, P.; Nieger, M.; Kappes, M.M.; Bräse, S. A Synthetic Strategy for Cofacial Porphyrin-Based Homo- and Heterobimetallic Complexes. Chem.-Eur. J. 2021, 27, 3047–3054. [Google Scholar] [CrossRef]
- Gil-Ramiírez, G.; Karlen, S.D.; Shundo, A.; Porfyrakis, K.; Ito, Y.; Briggs, G.A.D.; Morton, J.J.L.; Anderson, H.L. A Cyclic Porphyrin Trimer as a Receptor for Fullerenes. Org. Lett. 2010, 12, 3544–3547. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, Y.-Z.; Zheng, J.-Y. Clawlike Tripodal Porphyrin Trimer: Ion-Controlled On−Off Fullerene Binding. J. Org. Chem. 2014, 79, 1184–1191. [Google Scholar] [CrossRef]
- Kato, K.; Osuka, A. Propeller-shaped Semi-fused Porphyrin Trimers: Molecular symmetry-dependent Chiroptical Response. Chem. Eur. J. 2020, 26, 10217–10221. [Google Scholar] [CrossRef]
- Tyurin, V.S.; Mikhalitsyna, E.A.; Semeikin, A.S.; Beletskaya, I.P. Synthesis of New Porphyrin Trimers via Buchwald-Hartwig Amination Reaction. Macroheterocycles 2015, 8, 358–365. [Google Scholar] [CrossRef][Green Version]
- Polevaya, Y.P.; Tyurin, V.S.; Beletskaya, I.P. Linear conjuncted porphyrin trimer synthesis via “click” reaction. J. Porphyr. Phthalocyanines 2013, 17, 1–15. [Google Scholar] [CrossRef]
- Hamamura, T.; Nakazaki, J.; Uchida, S.; Kubo, T.; Segawa, H. Controlling the Rotation of Porphyrin Units in Ethynyl-linked Porphyrin Trimers for Dye-sensitized Solar Cells by Anchoring onto TiO2 Surface. Chem. Lett. 2014, 43, 796–798. [Google Scholar] [CrossRef]
- Bhuse, D.V.; Bhuse, V.M.; Bhagat, P.R. Star-type melamine based conjugated carboxy functionalized porphyrin trimer for DSSCs: An efficient approach to clean, aggregation free and true energy generation. Mater. Chem. Phys. 2022, 287, 126312. [Google Scholar] [CrossRef]
- Sridharan, G.; Shankar, A.A. Toluidine blue: A review of its chemistry and clinical utility. J. Oral Maxillofac. Pathol. 2012, 16, 251–255. [Google Scholar] [CrossRef]
- Singh, S.; Halder, A.; Sinha, O.; Chakrabarty, N.; Chatterjee, T.; Adhikari, A.; Singh, P.; Shikha, D.; Ghosh, R.; Banerjee, A.; et al. Spectroscopic Studies on the Biomolecular Recognition of Toluidine Blue: Key Information Towards Development of a Non-Contact, Non-Invasive Device for Oral Cancer Detection. Front. Oncol. 2020, 10, 529132. [Google Scholar] [CrossRef]
- Mills, S. How effective is toluidine blue for screening and diagnosis of oral cancer and premalignant lesions? Evid. Based Dent. 2022, 23, 34–35. [Google Scholar] [CrossRef]
- Raja, N.; Naikodi, S.; Govindarajan, A.; Palanisamy, K. Toluidine blue staining of murine mast cells and quantitation by a novel, automated image analysis method using whole slide skin images. J. Histotechnol. 2021, 44, 190–195. [Google Scholar] [CrossRef]
- Wong, A.L.; Hricz, N.; Malapati, H.; von Guionneau, N.; Wong, M.; Harris, T.; Boudreau, M.; Cohen-Adad, J.; Tuffaha, S. A simple and robust method for automating analysis of naïve and regenerating peripheral nerves. PLoS ONE 2021, 16, e0248323. [Google Scholar] [CrossRef]
- Redman, R.S.; Krasnow, S.H.; Sniffen, R.A. Evaluation of the carcinogenic potential of toluidine blue O in the hamster cheek pouch. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 473–480. [Google Scholar] [CrossRef]
- Dunipace, A.J.; Beaven, R.; Noblitt, T.; Yiming, L.; Zunt, S.; Stookey, G. Mutagenic potential of toluidine blue evaluated in the Ames test. Mutat Res. 1992, 279, 255–259. [Google Scholar] [CrossRef]
- Bekka, E.; Hopfer, C.; Eyer, F.; Muckter, H.; Gudermann, T. Mitochondrial toxicity and oxidative stress induced by methylene blue and toluidine blue in mammalian cells. Clin. Toxicol. 2018, 56, 453–608. [Google Scholar] [CrossRef]
- Ali, F.F. Adsorption of Toluidine blue dye from industrial waste water on the remnants of tea leaf. Int. J. Chemtech. Res. 2017, 10, 497–507. [Google Scholar]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid silica-porphyrin materials with tailored pore sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Plesu, N.; Lascu, A.; Anghel, D.; Cazacu, M.; Ianasi, C.; Fagadar-Cosma, G.; Fratilescu, I.; Epuran, C. Novel Platinum-Porphyrin as Sensing Compound for Efficient Fluorescent and Electrochemical Detection of H2O2. Chemosensors 2020, 8, 29. [Google Scholar] [CrossRef]
- Song, R.; Robert, A.; Bernadou, J.; Meunier, B. Sulfonated and acetamidosulfonylated tetraarylporphyrins as biomimetic oxidation catalysts under aqueous conditions. Inorg. Chim. Acta 1998, 272, 228–234. [Google Scholar] [CrossRef]
- Busby, C.; DiNello, R.K.; Dolphin, D. A Convenient Preparation of meso-Tetra(4-sulfonatopheny1)porphyrin. Can. J. Chem. 1975, 55, 1554–1555. [Google Scholar] [CrossRef]
- Hambright, P. Chemistry of water soluble porphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 129–210. [Google Scholar]
- Tsolekile, N.; Ncapayi, V.; Obiyanwa, G.K.; Songca, S.; Oluwafemi, O.S. Synthesis of meso-tetra-(4-sulfonatophenyl) porphyrin (TPPS4)—CuInS/ZnS quantum dots conjugate as an improved photosensitizer. Int. J. Nanomed. 2019, 14, 7065–7078. [Google Scholar] [CrossRef]
- Sobczynski, J.; Tonnesen, H.H.; Kristensen, S. Influence of aqueous media properties on aggregation and solubility of four structurally related meso-porphyrin photosensitizers evaluated by spectrophotometric measurements. Pharmazie 2013, 68, 100–109. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, M.; Li, A.; An, H.; Ye, H.; Zhang, Y. Aggregation and supramolecular chirality of 5,10,15,20-tetrakis-(4- sulfonatophenyl)-porphyrin on achiral poly(2-(dimethylamino)ethyl methylacrylate)-grafted ethylene-vinyl alcohol membrane. J. Mater. Chem. C. 2015, 3, 3650–3658. [Google Scholar] [CrossRef]
- Wurthner, F.; Keiser, T.E.; Saha-Moller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]
- Hanyz, I.; Wrobel, D. The influence of pH on charged porphyrins studied by fluorescence and photoacoustic spectroscopy. Photochem. Photobiol. Sci. 2002, 1, 126–132. [Google Scholar] [CrossRef]
- Marcioni, M.; Alongi, J.; Ranucci, E.; Malinconico, M.; Laurienzo, P.; Ferruti, P.; Manfredi, A. Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials? Polymers 2021, 13, 1018. [Google Scholar] [CrossRef]
- Kathiravan, A.; Kumar, P.S.; Renganathan, R.; Anandan, S. Photoinduced electron transfer reactions between meso-tetrakis(4-sulfonatophenyl)porphyrin and colloidal metal-semiconductor nanoparticles Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 175–181. [Google Scholar] [CrossRef]
- Anto, P.L.; Anto, R.J.; Varghese, H.T.; Panicker, C.Y.; Philipe, D.; Brolo, A.G. FT-IR, FT-Raman and SERS spectra of anilinium sulfate. J. Raman Spectrosc. 2009, 40, 1810–1815. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, G. Novel fluorescent pH sensor based on 5-(4-carboxyphenyl)-10,15,20-tris(phenyl)-porphyrin. Arab. J. Chem. 2019, 12, 1587–1594. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Tudose, R.; Armeanu, I.; Mosoarca, E.; Vlasici, D.; Costisor, O. UV-VIS and Fluorescence Spectra of Meso-Tetraphenylporphirin and Meso-Tetrakis-(4-Metoxyphenil) Porphyrin in the THF and THF Water Systems The influence of pH. Rev. Chim. Buc. 2007, 58, 451–455. [Google Scholar]
- Gjuroski, I.; Furrer, J.; Vermathen, M. Probing the Interactions of Porphyrins with Macromolecules Using NMR Spectroscopy Techniques. Molecules 2021, 26, 1942. [Google Scholar] [CrossRef]
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Protonenresonanzspektroskopie. In Tabellen zur Strukturaufklärung Organischer Verbindungen Mit Spektroskopischen Methoden; Fresenius, W., Huber, J.F.K., Pungor, E., Rechnitz, G.A., Tölg, G., West, T.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; Volume 15, pp. 108–181. [Google Scholar]
- Hunter, C.A.; Sanders, J.K.M. The nature of.pi.-.pi. interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Abraham, R.J.; Smith, K.M. NMR spectra of porphyrins. 21. Applications of the ring-current model to porphyrin and chlorophyll aggregation. J. Am. Chem. Soc. 1983, 105, 5734–5741. [Google Scholar] [CrossRef]
- Vermathen, M.; Marzorati, M.; Bigler, P. Self-Assembling Properties of Porphyrinic Photosensitizers and Their Effect on Membrane Interactions Probed by NMR Spectroscopy. J. Phys. Chem. B 2013, 117, 6990–7001. [Google Scholar] [CrossRef]
- Ryan, A.; Gehrold, A.; Perusitti, R.; Pintea, M.; Fazekas, M.; Locos, O.B.; Blaikie, F.; Senge, M.O. Porphyrin Dimers and Arrays. Eur. J. Org. 2011, 29, 5817–5844. [Google Scholar] [CrossRef]
- Norvaiša, K.; O’Brien, J.E.; Gibbons, D.J.; Senge, M.O. Elucidating Atropisomerism in Nonplanar Porphyrins with Tunable Supramolecular Complexes. Eur. J. Chem. 2020, 27, 331–339. [Google Scholar] [CrossRef]
- Webb, M.J.; Deroo, S.; Robinson, C.V.; Bampos, N. Host–guest interactions in acid–porphyrin complexes. Chem. Commun. 2012, 48, 9358–9360. [Google Scholar] [CrossRef]
- Chandran, M.; Sankara, S.P.; Bhagatsingh, R.; Shunmuganarayanan, A.; Navaneethakrishnan, S.; Rajaputi, V. FT-IR and FT-Raman Spectroscopic Analyzes of Indeno Quinoxaline Derivative Crystal. Asian J. Appl. Sci. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Jones, J.R.; Hench, L.L. Biomedical materials, fourier transform infrared spectroscopy. In Encyclopedia of Condensed Matter Physics; Bassani, F., Liedl, G.L., Wyder, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 108–116. ISBN 9780123694010. [Google Scholar] [CrossRef]
- Yamasaki, H.; Komoto, S. Mechanism of Histamine Release by Toluidine Blue from Rat Mast Cells. Int. Arch. Allergy Immunol. 1971, 41, 854–867. [Google Scholar] [CrossRef]
- Mei, Q.; Shi, Y.; Hua, Q.; Tong, B. Phosphorescent chemosensor for Hg2+ based on iridium(III) complex coordinated with 4-phenylquinazoline and sodium carbazole dithiocarbamate. RSC Adv. 2015, 5, 74924–74931. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712–724. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Tarabukina, E.; Fagadar-Cosma, E.; Enache, C.; Zakharova, N.; Birdeanu, M. Molecular Properties and Aggregation of Porphyrin Modified Polysiloxane in Solutions. J. Macromol. Sci. B Phys. 2013, 52, 1077–1091. [Google Scholar] [CrossRef]
- Shi, Y.; Sanchez-Molina, I.; Cao, C.; Stang, P.J. Synthesis and photophysical studies of self-assembled multicomponent supramolecular coordination prisms bearing porphyrin faces. Proc. Natl. Acad. Sci. USA 2014, 111, 9390–9395. [Google Scholar] [CrossRef]
- Balachandran, V.; Murali, M.K. FT-IR and FT-Raman spectral analysis of 3-(trifluromethyl) phenyl isothiocyanate. Elixir Vib. Spec. 2011, 40, 5105–5107. [Google Scholar]
- Muthuselvi, C.; Pandiarajan, S.S.; Ravikumar, B.; Athimoolam, S.; Krishnakumar, R.V. Spectroscopic Investigation of 7′-Nitro-6′-phenyl-1′,6′,7′,7a′- tetrahydro-spiro[indeno[1,2-b]quinoxaline-11,5′-pyrrolo[1,2-c] [1,3]thiazole] Crystal. Asian J. Appl. Sci. 2018, 11, 9–19. [Google Scholar] [CrossRef][Green Version]
- Adar, F. FT-IR and Raman: A Synergism, Not Competing Technologies. Spectroscopy 2009, 24. Available online: https://www.spectroscopyonline.com/view/ft-ir-and-raman-synergism-not-competing-technologies-0 (accessed on 14 November 2022).
- Hadjamar, Z.; Chabira, S.f.; Sebaa, M. FTIR-ATR Analysis, Curve Fitting of Rigid PVC Tube Used in Industrial Areas. In Proceedings of the 2nd International Conference on Industry 4.0 and Artificial Intelligence (ICIAI 2021), Sousse, Tunisia, 28–30 November 2021. [Google Scholar] [CrossRef]
- Kubicki, J.; Lorenc, M.; Cochelin, P.; Mongin, O.; Amar, A.; Boucekkine, A.; Gaje, A.; Humphrey, M.G.; Morshedi, M.; Lorenzen, S.; et al. Nitro End Groups Remarkable Vibrational Reporters for Charge Transfer in the Excited States of Oligo(p-phenyleneethynylene)- Bridged Donor-Acceptor Dyads. J. Phys. Chem. C 2020, 124, 9755–9764. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR study on cellulose with the varied moisture contents: Insight into the supramolecular structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Mora, A.S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 2020, 123, 109460. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascu, A.; Epuran, C.; Fratilescu, I.; Birdeanu, M.; Halip, L.; Fagadar-Cosma, E. Porphyrin Hetero-Trimer Involving a Hydrophilic and a Hydrophobic Structure with Application in the Fluorescent Detection of Toluidine Blue. Chemosensors 2022, 10, 481. https://doi.org/10.3390/chemosensors10110481
Lascu A, Epuran C, Fratilescu I, Birdeanu M, Halip L, Fagadar-Cosma E. Porphyrin Hetero-Trimer Involving a Hydrophilic and a Hydrophobic Structure with Application in the Fluorescent Detection of Toluidine Blue. Chemosensors. 2022; 10(11):481. https://doi.org/10.3390/chemosensors10110481
Chicago/Turabian StyleLascu, Anca, Camelia Epuran, Ion Fratilescu, Mihaela Birdeanu, Liliana Halip, and Eugenia Fagadar-Cosma. 2022. "Porphyrin Hetero-Trimer Involving a Hydrophilic and a Hydrophobic Structure with Application in the Fluorescent Detection of Toluidine Blue" Chemosensors 10, no. 11: 481. https://doi.org/10.3390/chemosensors10110481
APA StyleLascu, A., Epuran, C., Fratilescu, I., Birdeanu, M., Halip, L., & Fagadar-Cosma, E. (2022). Porphyrin Hetero-Trimer Involving a Hydrophilic and a Hydrophobic Structure with Application in the Fluorescent Detection of Toluidine Blue. Chemosensors, 10(11), 481. https://doi.org/10.3390/chemosensors10110481







