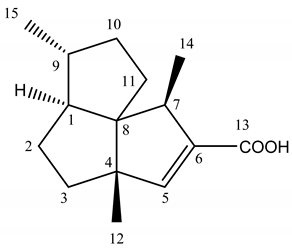
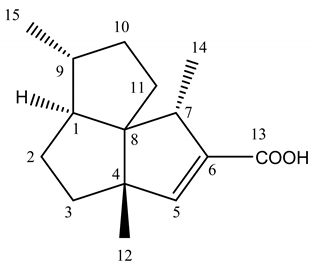
Abstract
The essential oils of six endemic Malagasy Helichrysum species were investigated by GC (RI), GC–MS and 13C NMR spectrometry. In total, 153 compounds were identified accounting for 90.8% to 99.9% of the total composition. The main constituents were α-pinene for H. benthamii, 1,8-cineole for H. dubardii, (E)-β-caryophyllene for H. indutum, and H. bojerianum. H. diotoides essential oil was characterized by the presence of two lilac alcohols and four lilac acetates whereas H. hirtum essential oil exhibited an atypical composition with 7β-H-silphiperfol-5-ene, 7-epi-subergorgiol, and 7-epi-silphiperfol-5-en-13-oic acid as major components.
1. Introduction
Madagascar, a big island in the Indian Ocean, is one of the countries in the world having particular hotspot biodiversity. Together with this biological richness, medicinal plants hold an important place in the everyday life of Malagasy people. The medicinal plants inventoried in Madagascar consist of 3245 species, of which 60% are endemic. Croton L. and Helichrysum Mill. are the most represented genera.
Helichrysum Mill. [1] is a large genus of the Asteraceae family and 112 species are known in Madagascar among which 46 are endemic [2]. Essential oils and extracts are obtained from the whole plant or from different parts of the plant and they are used in perfumery and aromatherapy. Biological activity or insecticidal activity have been reported [3]. In the literature, the chemical composition of essential oil (EO) from seven species has been studied [3,4,5,6,7,8,9,10,11]. H. faradifani and H. gymnocephalum are the most studied species and four papers reported their chemical compositions. Three different compositions are described for H. faradifani essential oil: (i) (E)-β-caryophyllene (34.6%) [6], (ii) β-himachalene (15–32.8%) associated with α-fenchene (13.1–27.3%) [5], and (iii) α-fenchene (32.3% and 35.5%) [3,10]. The chemical composition of H. gymnocephalum oil is homogeneous and characterized by the occurrence of 1,8-cineole (66.7% [7], 59.7% [6], 47.4% [4], and 17.4% [9]). monoterpene hydrocarbons such as β-pinene (38.2–40.5%) are dominant in H. selaginifolium oil [6,7]. Two chemical compositions are reported for H. hypnoides essential oil dominated by 1,8-cineole (51.5%) [11] or (E)-β-caryophyllene (34.0%) [6]. This last compound is also found as a major component in H. cordifolium oil (46.4–55.6%) [6,11] and in H. russillonii oil (29.5%) [11]. Finally, H. bracteiferum oil exhibited a chemical composition with β-pinene/1,8-cineole/α-humulene as major components [6,8,11]. These literature data reported an important chemical variability characterized by the presence of monoterpenes such as α-fenchene, β-pinene, 1,8-cineole or sesquiterpene hydrocarbons: (E)-β-caryophyllene, β-himachalene and α-humulene.
In this study, we were interested in six species: Helichrysum dubardii R. Vig. and Humbert, H. benthamii R. Vig. and Humbert, H. hirtum Humbert, H. indutum Humbert, H. bojerianum DC., H. diotoides DC [11]. Helichrysum dubardii and H. benthamii consist of subshrub plants with ericoid growth form, the leaves are deltoid, erect and applied on the twigs. H. dubardii leaves have only a single midrib vein, glabrous on the upper side, silvery white on the lower side. The bractal appendages of the inflorescences are yellowish white. In H. benthamii, the leaves have one to three veins arising from the base, covered with a dense gray tomentum above, loose underneath. The bractal appendages of the flowers are sulfur yellow [12]. H. hirtum and H. indutum are subshrub plants, leaves evenly distributed on the stem while the flowers have white bractal appendages. The first species has twigs covered with glandular hairs interspersed with fine cottony hairs and glands; the leaves are sessile, oblong. and with five veins arising from the base. The second species is covered with homogeneous fine cottony hairs sometimes dotted with sessile glands [12]. H. bojerianum and H. diotoides are also subshrub plants, with leaves evenly distributed on the stem. The bractal appendages of the inflorescences are sulfur yellow. H. bojerianum is covered with an ashy white aranose tomentum. The leaves are elliptical, acute sessile, with three veins arising from the base while H. diotoides is covered with a grayish aranose tomentum. The leaves are deltoid and sessile.
The aim of this work was to study for the first time, the chemical composition of the leaf essential oil extracted from these six endemic species growing wild in the center of Madagascar: H. dubardii, H. benthamii, H. hirtum, H. indutum, H. bojerianum, and H. diotoides.
2. Results
Twelve oil samples obtained by hydrodistillation (yields: 0.11–0.26%) of aerial parts of six Helichrysum species growing wild in Madagascar were analyzed by gas chromatography (GC) in combination with retention indices on two columns of different polarity, by gas chromatography coupled with mass spectroscopy (GC–MS) and by carbon-13 nuclear magnetic resonance (13C NMR). Due their complexity, H. hirtum and H. dubardii essential oils were also fractionated on silica gel column chromatography (CC). In total, 153 compounds were identified accounting for 90.8% to 99.9% of the total composition (Table 1).

Table 1.
Chemical composition of six Helichrysum essential oil samples.
2.1. Helichrysum Benthamii and H. dubardii Essential Oils
Two samples of H. benthamii (Hbe1 and Hbe2) produced a monoterpene hydrocarbon-rich oil characterized by the pre-eminence of α-pinene (50.8–51.9%), associated with sesquiterpene hydrocarbons: α-copaene (5.4–6.2%), α-humulene (3.1–4.4%) and (E)-β-caryophyllene (1.5–2.3%). The third sample of H. benthamii (Hbe3), also characterized by α-pinene (23.1%) as major compound, exhibited a slightly different chemical composition with percentages of sesquiterpene hydrocarbons more elevated: α-copaene (8.5%), α-humulene (6.4%) and (E)-β-caryophyllene (5.2%).
The main components of H. dubardii oil samples (Hd1-Hd3) were 1,8-cineole (26.9–35.7%), followed by α-pinene (5.8–6.6%), terpinen-4-ol (4.7–4.9%) and α-terpineol (4.0–4.3%). Sesquiterpene hydrocarbons were represented by α-muurolene (3.2–7.2%), γ-cadinene (1.6–3.5%), δ-cadinene (1.8–4.2%). It is noticeable that beyerene, a rare diterpene hydrocarbon was found in the three samples (0.2–0.5%).
2.2. Helichrysum Indutum, H. bojerianum and H. diotoides Essential Oils
Helichrysum indutum, H. bojerianum and H. diotoides (Hi, Hbo and Hdi samples) produced sesquiterpene-rich oils (47.9–70.2%): (E)-β-caryophyllene, ar- and γ-curcumenes, γ- and δ-cadinenes, aristolochene. Furthermore, among the monoterpenes, linalool was found in an appreciable amount in all samples (5.3–9.8%) whereas 1,8-cineole was identified only in H. indutum EO (13.4%). It could be pointed out that H. diotoides oil is characterized by the presence of several lilac derivatives: lilac alcohol A (2S,2′S,5′S) (1.7%), lilac alcohol B (2R,2′S,5′S) (3.6%) [13], lilac acetate A (0.6%), lilac acetate B (2.3%), lilac acetate C (0.3%), and lilac acetate D (8.7%).
2.3. Helichrysum Hirtum Essential Oil
The chromatographic profile of H. hirtum oil samples (Hh1, Hh2 and Hh3) varied drastically from the others and was characterized by the presence of many oxygenated sesquiterpenes. In the process of analyzing the chemical composition of the essential oils, we noticed that several compounds remained undetermined, providing very unsatisfactory matching with commercial or in- house MS libraries. Then, the EO was fractionated by silica gel column chromatography (CC), using a gradient of solvents. These compounds could however be identified from the fraction of CC by applying our in-house 13C NMR computerized methodology [14,15].
We highlighted the identification of presilperfolane and silphiperfolane derivatives as major components in the three samples by comparison of their carbon chemical shifts values with those reported in the literature [16,17,18,19,20,21,22]: 7-epi-silphiperfol-5-en-13-oic acid (4.0–20.8%) and silphiperfol-5-en-13-oic acid (4.0–10.6%) (Table 2), 7-epi-subergorgiol (7.6–14.8%), 7β-H-silphiperfol-5-ene (1.8–14.8%), presilphiperfolan-9-α-ol (6.9–8.0%), 7-epi-silphiperfolenal (1.4–2.5%), 13- hydroxysilphiperfol-6-ene (1.2–1.9%) and 7α-H-silphiperfol-5-ene (up to 0.3%). The presence of a compound including an acid group as major component is very unusual in essential oils.

Table 2.
13C NMR data (400 MHz, CDCl3) of compounds 152 and 153.
We detailed the identification by 13C NMR of 7-epi-silphiperfol-5-en-13-oic acid (152) and silphiperfol-5-en-13-oic acid (153) in the sample Hh1 (Table 2). For these two compounds:
- -
- all the expected signals were observed;
- -
- the chemical shift variations between the reference spectrum (Marco et al. [16]) and the recorded spectrum of the sample Hh1 were low. Indeed, they were less than or equal to 0.08 ppm for at least 12 signals out of 15. Only the carbons of the acid function or near the acid function (i.e., C5, C6, C13) exhibited a higher chemical shift variation;
- -
- a DEPT sequence confirmed the number of hydrogens linked to each carbon.
It should be point out that the chemical shift values of carbons, measured on spectra recorded using high field spectrometers, were given with two decimal places. Nevertheless, it occasionally arose that chemical shift values were given with only one decimal. In such a case, although it is not mathematically correct, comparison of data given with one decimal and those given with two decimals unambiguously allowed identification of compounds.
3. Discussion
In a recent review, Rafidison et al., highlighed the actual state of Malagasy medicinal plants and particularly the pharmacological and ethnobotanical investigations. Croton and Helichrysum are the most cited genera. Even more, H. faradafini is present in the top 20 most cited species. Concerning essential oils, H. faradafini, H. bracteiferum, and H. gymnocephalum are actually the most produced in Madagascar and used as expectorant and as a preventative or curative remedy for treating coughs, colds, and bronchitis [2].
The studied oils reported several major components previously described in Malagasy Helichrysum EOs: (i) H. dubardii oil exhibited a close composition reported from H. bracteiforum oil and characterized by 1,8-cineole as major component (26.9–35.7% vs. 27.3% respectively) [6,11]; (ii) H. indutum EO composition is dominated by (E)-β-caryophyllene which is also reported to be in similar amounts in H. faradifani [6], H. hypnoides [6] and H. russillonii [11] EOs (33.1% vs. 34.6%, 34.0% and 29.5% respectively). Thus, H. dubardii and H. indutum EOs, which exhibited a chemical profile close to H. bracteiferum and H. faradifani respectively, were the good candidates for domestication.
H. bojerianum and H. diotoides EOs exhibited a different chemical composition. Even if, the percentage of (E)-β-caryophyllene was low in H. bojerianum and H. diotoides EOs (16.1% and 15.0% respectively), both oils can be classified as sesquiterpene hydrocarbon-rich oil (76.8% and 58.6% respectively). However, the H. bojerianum EO appeared original by the presence of six lilac derivatives (two alcohols and four acetates) at an appreciable ratio around 15%.
The composition of H. benthamii EO exhibited α-pinene as major component (23.1–51.9%) while β-pinene was frequently reported as the major component [6,7,8,11]. However, percentages up to 20% have never been observed in the Helichrysum genus.
Finally, H. hirtum EO can be classified as sesquiterpene hydrocarbon-rich oil (91.0%) but the chemical composition differed drastically from the others by the (i) absence of monoterpene hydrocarbons (only traces of α-pinene and 0.1% of p-cymenene), (ii) a very low amount of oxygenated monoterpenes (1.3%), (iii) the presence of several sesquiterpenes exbibiting silphiperfolane and presilphiperfolane skeletons. To our knowledge, the presence of silphiperfolane and presilphiperfolane derivatives has never been reported in Helychrysum EOs but these skeletons were reported in asteraceae family (Petasites, Matricaria, Sphaeranthus, Otanthus) [23].
This original chemical composition can be an important feature for marketing. Taking into account that the wild populations of H. hirtum were distributed in a limited area (Tapia or Uapaca bojeri Forest, highlands of Madagascar—around Arivonimamo), over-exploitation has greatly increased the vulnerability of H. hirtum [24]. Therefore, the protection of H. hirtum populations should be a high priority now and domestication can be considered as an excellent alternative to supply the continuous market needs by producing high quality and stable raw material, and at the same time, alleviating the pressure on natural resources from overharvesting.
4. Materials and Methods
4.1. Plant Material
Aerial parts of five Helichrysum species were collected in September 2016 (dry season) at the region of Itasy, district of Arivonimamo (Figure 1): H. dubardii (Ambatobe, 19°14′42.05″ S, 47°00′25.09″ E), H. benthamii (Ambatobe, 19°14′42.05″ S, 047°00′25.09″ E), H. bojerianum (Near Mount of Tsiafakafokely, 2203 m above sea level, 19°16′42.7″ S, 047°12′52.7″ E), H. diotoides (South of Alakamisiy kely, 19°15′10.6″ S, 47°06′39.3″ E), H. hirtum (West of Arivonimamo, 19°01′85.09″ S, 47°16′90.9″ E). Aerial parts of H. indutum were collected in September 2019 (dry season) at region Alaotra Mangoro, District of Maromizaha (19°16′48.9′’ S, 047°02′05.2′’ E) (Figure 1). Voucher specimens were deposited at TAN and CNARP herbaria under the accession Rakotonandrasana 1501 for H. dubardii, 1502 for H. benthamii, ST 1518 for H. bojerianum, ST 1508 for H. diotoides, ST 1519 for H. hirtum and, ST 1520 for H. indutum.
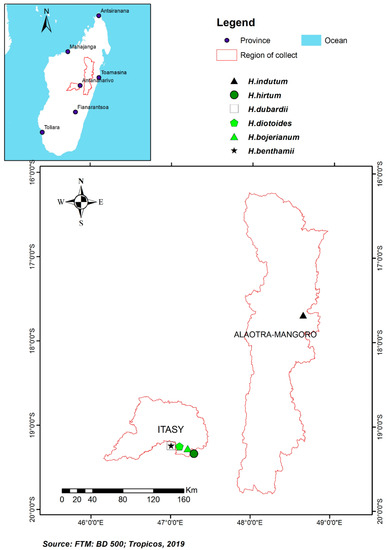
Figure 1.
Collection maps of the six Helichrysum species.
The essential oils were obtained by hydrodistillation of fresh aerial parts (around 500–1000 g) over 3 h. Yields were calculated from fresh material: H. dubardii, 0.13–0.26%; H. benthamii, 0.12–0.21%; H. bojerianum, 0.26%; H. diotoides, 0.21%; H. hirtum, 0.11–0.18% and H. indutum 0.19%.
4.2. Gas Chromatography (GC) Analysis
GC analyses were performed on a Clarus 500 FID gas chromatograph (PerkinElmer, Courtaboeuf, France) equipped with two fused silica gel capillary columns (50 m, 0.22 mm, film thickness 0.25 m), BP-1 (polydimethylsiloxane) and BP-20 (polyethylene glycol). The oven temperature was programmed from 60 to 220 °C at 2 °C/min and then held isothermal at 220 °C for 20 min, injector temperature: 250 °C; detector temperature: 250 °C; carrier gas: hydrogen (1.0 mL/min); split: 1/60. The relative proportions of the oil constituents were expressed as percentages obtained by peak area normalization, without using correcting factors. Retention indices (RIs) were determined relative to the retention times of a series of n-alkanes with linear interpolation (‘Target Compounds’ software of PerkinElmer).
4.3. Mass Spectrometry
The EOs were analyzed with a PerkinElmer TurboMass detector (quadrupole, PerkinElmer, Courtaboeuf, France), directly coupled to a PerkinElmer Autosystem XL (PerkinElmer), equipped with a fused silica gel capillary column (50 m × 0.22 mm i.d., film thickness 0.25 µm), BP-1 (polydimethylsiloxane). Carrier gas, helium at 0.8 mL/min; split: 1/75; injection volume: 0.5 µL; injector temperature: 250 °C; oven temperature programmed from 60 to 220 °C at 2 °C/min and then held isothermal (20 min); ion source temperature: 250 °C; energy ionization: 70 eV; electron ionization mass spectra were acquired over the mass range 40–400 Da.
4.4. NMR Analysis
13C NMR analyses were performed on an AVANCE 400 Fourier Transform spectrometer (Bruker, Wissembourg, France) operating at 100.623 MHz for 13C, equipped with a 5 mm probe, in CDCl3, with all shifts referred to internal tetramethylsilane (TMS). 13C NMR spectra were recorded with the following parameters: pulse width (PW): 4 µs (flip angle 45°); acquisition time: 2.73 s for 128 K data table with a spectral width (SW) of 220.000 Hz (220 ppm); CPD mode decoupling; digital resolution 0.183 Hz/pt. The number of accumulated scans ranged from 2000–3000 for each sample (around 40 mg of oil in 0.5 mL of CDCl3). Exponential line broadening multiplication (1.0 Hz) of the free induction decay was applied before Fourier Transformation.
4.5. Identification of Individual Components
Identification of the components was based: (i) on comparison of their GC retention indices (RIs) on polar and apolar columns, determined relative to the retention times of a series of n-alkanes with linear interpolation (“Target Compounds” software of PerkinElmer), with those of authentic compounds and (ii) on comparison of the signals in the 13C NMR spectra of EOs with those of reference spectra compiled in the laboratory spectral library, with the help of a laboratory-made software [13,14,15]. In the investigated samples, individual components were identified by NMR at contents as low as 0.5%. Several compounds were identified by comparison of 13C NMR chemical shifts with those reported in the literature, for instance 7-epi silphiperfol-5-en-13-oic acid and silphiperfol-5-en-13-oic acid [16], beyerene [17], δ-terpineol [18], 7-epi-silphiperfolenal and 7- episubergorgiol [19], 13-hydroxysilphiperfol-6-ene [20], pogostol [21], 14-hydroxy-α-humulene [22], and lilac alcohol B [13].
4.6. Essential Oil Fractionation
H. dubardii oil sample Hd1 (1.0 g) was submitted to flash chromatography (silica gel: 200–500 µm). Four fractions (FHd1-FHd4) were eluted with a mixture of solvents of increasing polarity with pentane:diethyl ether (P:E) 100:0 to 0:100: FHd1 (P:E 100:0; 231.4 mg), FHd2 (P:E 98:2; 289.1 mg), FHd3 (P:E 95:5; 163.2 mg), and FHd4 (P:E 0:100; 218.4 mg). All fractions of chromatography were analyzed by GC (RI), GC–MS and 13C NMR.
An H. hirtum oil sample Hh1 (1.0 g) was also submitted to flash chromatography (silica gel: 200–500 µm). Six fractions (FHh1-FHh6) were eluted with a mixture of solvents of increasing polarity P:E 100:0 to 0:100: FHh1 (P:E 100:0; 143.1 mg), FHh2 (P:E 98:2; 23.0 mg); FHh3 (P:E 95:5; 156.5 mg), FHh4 (P:E 90:10; 524.2 mg), FHh5 (P:E 80:20, 126.0 mg) and FHh6 (P:E 0:100, 18.0 mg). All fractions of chromatography were analyzed by GC (RI), GC–MS, and 13C NMR.
5. Conclusions
This study provides useful scientific data to promote in situ conservation and to select chemical profiles for eventual production. Our results confirmed that Malagasy Helichrysum EOs exhibited an important chemical variability and these data are useful for projects of biodiversity conservation.
Author Contributions
Conceptualization, D.J.R.R.; Botanical data and mapping S.R.R.; Chemical analysis, D.J.R.R., G.B., M.P., and A.B.; writing—original draft preparation, D.J.R.R. and F.T.; writing—review M.P. and A.B.; editing, M.P.; supervision, F.T.; project administration, C.A., P.A.R.R., and D.J.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Delphin J.R. Rabehaja thanks the University of Corsica for a financial support as Associated Professor, October–November 2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gautier, L.; Chatelain, C.; Callmander, M.W.; Phillipson, P.B. Richness, similarity and specificity of Madagascar flora compared with Sub-Saharan Africa. Plant Ecol. Evol. 2013, 145, 55–64. [Google Scholar] [CrossRef]
- Rafidison, V.; Ratsimandresy, F.; Rakotondrajaona, R.; Rasamison, V.; Rakotoarisoa, M.; Rakotondrafara, A.; Rakotonandrasana, S.R. Synthèse et analyse de données sur les inventaires de plantes médicinales de Madagascar. Ethnobot. Res. Appl. 2019, 18, 1–19. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Rakotosaona, R.; Randrianarivo, E.; Nicoletti, M.; Maggi, F. Chemical composition and insecticidal activity of the essential oil from Helichrysum faradifani endemic to Madagascar. Nat. Prod. Res. 2018, 32, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimicrobial and Antioxidant Activities, Attribution of the Activity Origin by Correlations. Molecules 2011, 16, 8273–8291. [Google Scholar] [CrossRef] [PubMed]
- Ralijerson, L.B.; Rabehaja, R.D.J.; Rajaonarison, J.F.; Urverg, R.S.; Hérent, M.-F.; Manga, H.M.; Tilquin, B. Comparison Between the Fresh and Dry Essential Oil of Helichrysum faradifani Scott Elliot from Madagascar. J. Essent. Oil Res. 2005, 17, 597–600. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Ranarivelo, L.; Ratsimbason, M.; Bernardini, A.-F.; Casanova, J. Constituents of the essential oil of six Helichrysum species from Madagascar. Flavour Fragr. J. 2001, 16, 253–256. [Google Scholar] [CrossRef]
- Möellenbeck, S.; König, T.; Schreier, P.; Schwab, W.; Rajaonarivony, J.; Ranarivelo, L. Chemical Composition and Analyses of Enantiomers of Essential Oils from Madagascar. Flavour Fragr. J. 1997, 12, 63–69. [Google Scholar] [CrossRef]
- Ramanoelina, P.A.R.; Bianchini, J.-P.; Gaydou, E.M. Chemical Composition of Essential Oil of Helichrysum bracteiferum. J. Essent. Oil Res. 1992, 4, 531–532. [Google Scholar] [CrossRef]
- De Medici, D.; Pieretti, S.; Salvatore, G.; Nicoletti, M.; Rasoanaivo, P. Chemical Analysis of Essential Oils of Malagasy Medicinal Plants by Gas Chromatography and NMR Spectroscopy. Flavour Fragr. J. 1992, 7, 275–281. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Tomi, F.; Bernardini, A.-F.; Casanova, J. Chemical variability of the essential oil of Helichrysum faradifani Sc. Ell. from Madagascar. Flavour Fragr. J. 2006, 21, 111–114. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Kirimer, N. Compositions of the Essential Oils of Four Helichrysum Species from Madagascar. J. Essent. Oil Res. 2012, 14, 53–55. [Google Scholar] [CrossRef]
- Humbert, H. Flore de Madagascar et des Comores, 189e. Famille: Composées, Tome II; Museum National d’Histoire Naturelle: Paris, France, 1962. [Google Scholar]
- Schneider, M.A.; Stefan Dötterl, S.; Seifert, K. Diastereoselective Synthesis of a Lilac Aldehyde Isomer and Its Electrophysiological Detection by a Moth. Chem. Biodivers. 2010, 13, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Tomi, F.; Casanova, J. 13C-NMR as a tool for identification of individual components of essential oils from Labiatae–A review. Acta Hortic. 2006, 723, 185–192. [Google Scholar] [CrossRef]
- Bighelli, A.; Casanova, J. Analytical Tools for Analyzing Cymbopogon Oils. In Essential Oil Bearing Grasses–Cymbopogons, 1st ed.; Taylor and Francis: Boca Raton, FL, USA, 2009; p. 195. [Google Scholar]
- Marco, J.A.; Sanz-Cervera, J.F.; Morante, M.D.; García-Lliso, V.; Vallès-Xirau, J.; Jakupovic, J. Tricyclic sesquiterpenes from Artemisia chamaemelifolia. Phytochemistry 1996, 41, 837–844. [Google Scholar] [CrossRef]
- Grande, M.; Morán, J.R.; Macías, M.J.; Mancheño, B. Carbon-13 nuclear magnetic resonance spectra of some tetracyclic diterpenoids isolated from Elaeoselinum species. Phytochem. Anal. 1993, 4, 19–24. [Google Scholar] [CrossRef]
- Bull, S.D.; Carman, R.M. δ-terpineol. Aust. J. Chem. 1992, 45, 2077–2081. [Google Scholar] [CrossRef]
- Verdan, M.H. Estudo Fitoquímico E Avaliação Da Atividade Citotóxica Das Rainhas-Do-Abismo: Sinningia Leucotricha E S. Canescens (Gesneriaceae). Ph.D. Thesis, Univerdade Federal do Parana, Curitiba, Brazil, 2015. [Google Scholar]
- Surup, F.; Kuhnert, E.; Liscinskij, E.; Stadler, M. Silphiperfolene-Type Terpenoids and Other Metabolites from Cultures of the Tropical Ascomycete Hypoxylon rickii (Xylariaceae). Nat. Prod. Bioprospect. 2015, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Marschall, H.; Splittgerber, U.; Wolf, D. Analysis of the polar fraction of Haitian vetiver oil. Flavour Fragr. J. 2000, 15, 153–173. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Altarejos, J.; Barragán, A.; Lara, A.; Laurent, R. Minor components in the essential oil of Juniperus oxycedrus L. wood. Flavour Fragr. J. 1993, 8, 185–189. [Google Scholar] [CrossRef]
- Joulain, D.; König, A.W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Rakotondrasoa, O.L.; Malaisse, F.; Rajoelison, G.L.; Razafimanantsoa, T.M.; Rabearisoa, M.R.; Ramamonjisoa, B.R.; Raminosoa, N.; Verheggen, F.J.; Poncelet, M.; Haubruge, E.; et al. La forêt de tapia, écosystème endémique de Madagascar: Écologie, fonctions, causes de dégradation et de transformation. Biotechnol. Agron. Soc. Environ. 2012, 16, 541–552. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).