Abstract
The inhibitory activities of the leachates and volatiles from 53 plant species (spices and herbs) were evaluated against lettuce (Lactuca sativa “Great Lakes 366”) seedling growth using the sandwich and dish pack methods, respectively. With the sandwich method, parsley (Petroselinum sativum) showed the strongest inhibitory effect on lettuce radicle growth (77%), followed by tarragon (Artemisia dracunculus) (72%). However, caraway (Carum carvi), dill (Anethum graveolens) (seed), laurel (Laurus nobilis), rosemary (Rosmarinus officinalis), and sage (Salvia officinalis) were the most inhibitory species (100% inhibition of lettuce radicle and hypocotyl growth inhibition at all distance wells) in the dish pack method. Cardamom (Elettaria cardamomum) and thyme (Thymus vulgaris) also showed strong inhibitory activity (100% for radicle and hypocotyl growth inhibition at all 41 and 58 mm distance wells). The headspace sampling and gas chromatography-mass spectrometry (GC-MS) analysis identified the main inhibitory active compounds as carvone in caraway and dill (seeds), 1,8-cineole in laurel and cardamom, and borneol in thyme. Both camphor and 1,8-cineole were detected in rosemary and sage, and the total activity evaluation showed that camphor was the major inhibitory compound in rosemary, although both compounds played equal roles in sage.
1. Introduction
A range of secondary metabolites is synthesized by plants, with the exact composition varying among species. Application of these compounds as agricultural chemicals has been well investigated, with many of the insecticides and fungicides that are used in recent years originating from natural plants []. The inhibitory effects of specific volatiles or essential oils of aromatic plants, including spices and herbs on plant growth have also been investigated in both field and laboratory assays [,,,]. As a result, many plant growth inhibitory substances (allelochemicals) have been identified. However, only a small number of these allelochemicals are currently being used in commercial herbicide production because their effects are not as strong or long-lasting as synthetic herbicides []. Nonetheless, there have been some reports on the direct use of allelopathic plants in agriculture. For example, some plants in the Brassicaceae family are used as bio-fumigation materials to reduce the incidence of soil-borne diseases, nematodes, or weeds [,,,,].
Allelochemicals are released from plants into the environment through several routes, including volatilization from the leaf tissues, leaching of non-volatiles from the foliage by rainfall, exudation from living roots, or the decomposition of residues by soil microorganisms [,,]. Several bioassay methods that correspond to each of these routes of allelochemical release have been developed to evaluate the activities of allelochemicals [,,,,,,]. Among these bioassays, the sandwich method is used to evaluate the allelopathic activities of leachates by placing plant samples between two layers of agar [,,]. The dish pack method is however used to evaluate the growth inhibitory activities of plant volatiles by placing samples in a six-well multi-dish to determine the relationship between the degree of growth inhibition on receptor seedlings and their distance from the donor samples. The speed of diffusion and intensity of activity of the volatiles can be estimated using this bioassay [,,]. Indeed, it was through this method that Sekine et al. [] found that cuminaldehyde was the main antifungal compound in black Zira (Elwendia persica).
Therefore, in this study, we evaluated the growth inhibitory activities of the leachates and volatiles of 53 plant species (spices and herbs) using the sandwich and dish pack methods, respectively, and determined the active component(s) responsible for any such inhibitory effects.
2. Results and Discussion
2.1. Screening of Allelopathic Activity
In this study, we examined the plant growth inhibitory activities of 53 species of plants (spices and herbs) against the growth of lettuce seedlings using the sandwich and dish pack methods. The volatile compounds responsible for the activities of the most inhibitory species were also identified. Lettuce was chosen as the test plant because it germinates quickly with high uniformity and has a high sensitivity to allelochemicals []. In addition to this, lettuce had previously been used by many researchers to investigate plant allelopathy [,,,,]. The inhibitory effects of the leachates and volatiles released by the spices and herbs on the radicle and hypocotyl growth of lettuce seedlings are shown in Table 1. In both the sandwich (36 species) and dish pack (23 species) methods, lettuce radicle growth was inhibited more than hypocotyl growth (Table 1). The stronger inhibition of the radicle that was observed could be the result of the radicle emerging before the hypocotyl, the nutrients stored in the seed being supplied to the hypocotyl, or differences in the actions of the allelopathic substances []. The allelopathic activity, in this study, was mainly discussed in terms of the lettuce radicle inhibition because the radicle is likely to be directly affected by the available leachates or volatiles, whereas hypocotyl growth is likely to be influenced by several complex factors.

Table 1.
Allelopathic activities of 53 spices and herbs based on the sandwich and dish pack methods.
Itani et al. [,] found that Oxalis corniculata, Rumex acetosella, and Begonia spp., showed 90% to 95% inhibition when lettuce was treated with 10 mg of plant samples in the sandwich method. Using the same amount of sample, we found that none of the species tested exhibited > 90% growth inhibition with the sandwich method (Table 1). However, all of the species showed some degree of radicle growth inhibition, with parsley (Petroselinum sativum) showing the strongest inhibition (77%), followed by tarragon (Artemisia dracunculus) (72%). It has previously been reported that parsley contains myristicin and apiole both of which have shown inhibitory activities against the seedling growth of rice [,]. In addition, some members of the genus Artemisia, including tarragon, have been reported to be allelopathic [,,]. However, the plant growth-inhibitory substance(s) that are specific to tarragon remain unknown. Furthermore, most of the samples inhibited hypocotyl growth, with clove (Eugenia aromatica) showing the strongest inhibition (72%), followed by oriental mustard (Brassica juncea) (69%). However, seven of the evaluated samples showed a stimulatory growth effect, with seri roots (Oenanthe javanica) and red shiso (Perilla frutescens), in particular, promoting growth by >20%. Sekine et al. [] previously showed that 500 mg of the test sample was adequate for assessing the allelopathic activity of black Zira against the mycelial growth of Fusarium oxysporum using the dish pack method. Using the same amount of sample, we found that caraway (Carum carvi), dill seeds (Anethum graveolens), laurel (Laurus nobilis), rosemary (Rosmarinus officinalis), and sage (Salvia officinalis) caused complete inhibition of lettuce seed growth in all five wells (Table 1).
Other species including cardamom (Elettaria cardamomum) and thyme (Thymus vulgaris), also caused 100% inhibition in three of the five wells. Additionally, the pepper tree (Schinus molle) and mace (Myristica fragrans) caused strong inhibition in the two 41 mm distance wells. However, their activity sharply decreased with increasing distance (58, 82, and 92 mm) from the source well, indicating low volatility of the allelopathic compounds produced by these species. By contrast, the green and red varieties of shiso (Perilla frutescens) stimulated the radicle growth of lettuce by two-fold as compared with the control. It has been reported that shiso leaves contain the aromatic compound pellylaldehyde [], and we observed that 50 μL of authentic pellylaldehyde had a similar promotional effect when tested using the dish pack method (Sekine, unpublished report). However, since this type of effect was not the focus of this study, the presence of pellylaldehyde in shiso was not further investigated in this study. The five species that showed 100% inhibition in all of the wells at a sample weight of 500 mg (excluding laurel) also showed the same level of inhibition at a reduced sample weight of 250 mg. Laurel showed less than 100% inhibition at the two furthest wells from the source well (82 and 92 mm distance wells). Furthermore, caraway, dill (seed), rosemary, and sage also showed 100% inhibition in all of the wells, even 100 mg.
2.2. The GC-MS Analysis of Volatiles Constituents
The volatiles that were produced by the seven most inhibitory species (i.e., caraway, dill (seed), laurel, rosemary, sage, cardamom, and thyme) were determined through headspace sampling and GC-MS analysis. The GC-MS analysis resulted in the detection of 12 compounds (Table 2). Among these species, sage contained the most considerable number of compounds (n = 9). Among the detected compounds, limonene was present in most species (n = 6) in varying amounts, while borneol was only detected in thyme.

Table 2.
Volatile compounds identified from the spices and herbs with high growth inhibitory activities.
2.3. Evaluation of Allelopathic Activities of the Detected Volatiles
Evaluation of allelopathic activities of the authentic samples of the 12 detected compounds showed that borneol, camphor, carvone, and 1,8-cineole resulted in 100% growth inhibition in all of the wells. In addition, 3-carene and β-pinene exhibited high levels of inhibition when 50 μL of the compounds were used (Table 3). Furthermore, growth inhibition remained high for borneol, camphor, and carvone (>50%) when the sample volume was reduced to <50 μL or 50 mg, which equated to a vapour concentration of <1 ppm after 24 h (Table 3). The compound 1,8-cineole also showed high growth inhibition but at a higher vapour concentration than the other three compounds (10.3 ppm in the 41 mm distance well and 9.43 ppm in the 92 mm distance well). There have been several reports on the plant growth inhibitory activities of these four monoterpenoids [,,,,,,,]. Abraham et al. [] estimated that these compounds inhibited the germination of maize (Zea mays) in the order of camphor > 1,8-cineole > α-pinene > limonene.

Table 3.
Plant growth inhibitory activities and vapour phase concentrations of authentic compounds.
In another study, Nishida et al. [] reported that the root growth of Brassica campestris was inhibited by these compounds and β-pinene in the order of camphor > 1,8-cineole > β-pinene > α-pinene > camphene. The inhibition trends reported in both studies are similar to our findings despite the use of different types of receptor plants. Contrary to the results of this study where borneol showed higher activity than carvone, Vokou et al. [] reported a different inhibition trend (carvone > camphor > 1,8-cineole = borneol) of some of these monoterpenoids against lettuce. This variation could be due to differences in the type of bioassay and method of vapour concentration measurement that was used. Limonene of these top four compounds was detected in six of the seven most inhibitory species, whereas both camphor and 1,8-cineole were found in rosemary and sage (Table 2). To determine which of these two compounds played a significant role in the activity of each of these species, they were further evaluated based on their specific activity (EC50) and total activity. Evaluation of the specific activity (i.e., biological activity per unit weight of the compound) expressed as the EC50 and essential for the development of pesticides, as a compound that exhibits a small EC50 value has a high specific activity. By contrast, the evaluation by total activity (i.e., biological activity per unit weight of the sample containing the bioactive compound) is important for biological use [,]. Hiradate et al. [] isolated novel plant growth inhibitory compounds from Spiraea thunbergii through the concept of total activity. The EC50 values of authentic camphor and 1,8-cineole were 0.0633 ppm and 7.21 ppm, respectively. The total activity, based on these values and the concentration of the compounds, was calculated to be almost 10 times higher for camphor than for 1,8-cineole (23.9 and 2.58, respectively) in rosemary. Almost the same total activity for both compounds in sage (7.93 for 1,8-cineole and 7.49 for camphor) indicates that they play equal roles in the inhibitory activity of this herb.
3. Materials and Methods
3.1. Screening of Spices and Herbs
Dried samples of 53 species of spices and herbs were tested for their potential allelopathy through leachates and volatiles (Table 1). Thirty-eight of the species were donated by YASUMA Co. Ltd. (Tokyo, Japan), 14 were cultivated in the fields of the Miyagi Prefectural Agriculture and Horticulture Research Centre (Natori, Japan) or the National Institute for Agro-Environment Science (Tsukuba, Japan), and one species was donated by the Ferdowsi University of Misshad (Iran). Each sample was dried in a hot air circulation oven at 60 °C for 4 h and, then, ground finely with a Japanese traditional grinder, “Yagen”, just before the experiment.
3.2. Sandwich Method
The activity of the leachates produced by each plant sample was evaluated following the principles of the sandwich method using six-well multi-dishes (Nunc, external dimensions 128 × 86 mm, 35 mm diameter wells) []. Each well was filled with 10 mg of ground sample to which 5 mL of 0.5% agar (w/v) was added. The sample was, then, wholly integrated with the first layer of agar. As soon as the agar had hardened, a second agar layer (5 mL) was added and again allowed to gelatinise. Five lettuce (Lactuca sativa “Great Lakes 366”, Takii Seed, Japan) seeds were placed on top of the gelatinized agar in the well. Three wells of a multi-dish were used as three replications of a single species. In addition, a control multi-dish was set up in the same manner only without the addition of any samples to the wells. The multi-dishes were incubated in a dark growth chamber at 25 °C for three days. The growth rate of the lettuce seedlings relative to the control was then measured to calculate the inhibition rate (control = 100% growth).
3.3. Dish Pack Method
The activity of the volatiles released by each plant sample was evaluated following the dish pack method procedure [,] using six-well multi-dishes (Figure 1). A ground sample (500 mg) was placed in the lower-left well (denoted as the 0 mm distance or source well) of a multi-dish, and a filter paper (Advantec, No.1, 33 mm; Toyo) that had been wetted with distilled water (0.75 mL).
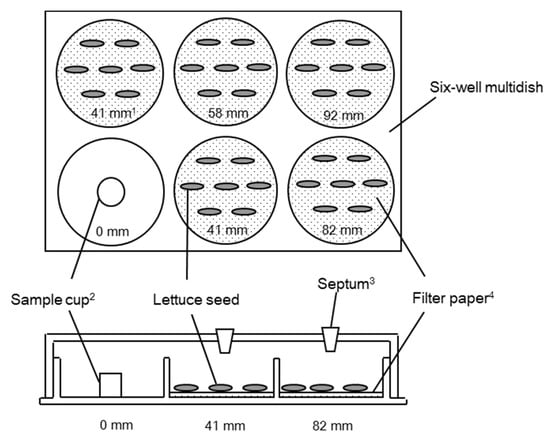
Figure 1.
Top and side views of the multi-dish that was used for testing the plant growth inhibitory activities of volatile compounds with the dish pack method. (1) Distance from the sample or compound; (2) The source well (or cup in the case of authentic compounds); (3) Septa were attached for headspace vapour sampling, which was undertaken using a Hamilton gas-tight syringe and analyzed by gas chromatography-mass spectrometry (GC-MS) analysis; (4) Each filter paper was wetted with 0.7 mL of distilled water.
Seven lettuce seeds were placed on the surface of each of the remaining wells. The multi-dish was then covered, and the sides were sealed with adhesive tape to prevent volatile losses due to volatilization. In addition, a control dish was set up in the same manner only without the addition of any sample to the source well. The multi-dishes were incubated in a dark growth chamber at 25 °C for three days. The radicle and hypocotyl lengths of the lettuce seedlings in each well were measured. The inhibition rate was, then, calculated in the same manner as for the sandwich method (see Section 3.2). There was a 1 mm headspace between the multi-dish cover and the wells to allow any volatiles produced by the sample to spread throughout the multi-dish. The levels of vapour diffusion and inhibition were estimated by the relationship between the level of seedling growth inhibition and the distance of the seedling from the source well. In this experiment, there were no replications for each species. However, those species that showed strong inhibition were assayed for a second time using a reduced amount of sample (250 mg) and this process was, then, repeated for a third time using a further reduced weight (100 mg).
3.4. Identification and Evaluation of Plant Growth Inhibitory Volatiles
The volatiles that were released by the species showing the highest activities were determined using the headspace method. A finely ground sample (200 mg) of each species was put into a glass vial equipped with aluminium crimp seal cap with a polytetrafluoroethylene (PTFE)/silicone septum and was kept under laboratory conditions for 30 min. A headspace vapour sample (1 mL) was, then, taken from the vial through the septum using a Hamilton gas-tight syringe. The collected gas was then immediately injected into a QP-5050A gas spectrometer (SHIMAZU, Kyoto, Japan) using EQUITY-5 gas chromatography (GC) column (Supelco, 30 m × 0.25 mm, i.d. 0.25 μm). Each compound was identified by comparing its mass spectrum with values recorded in the NIST Mass Spectral Library and the retention time of an authentic sample on the GC. The quantity of each compound from a given plant sample was determined by comparing the peak area of the compound with that of its authentic sample. The operating conditions of the gas chromatographer-mass spectrometer were as follows: Inlet 200 °C and column oven 40 °C for 30 s and, then, programmed to increase by 8 °C/min to 160 °C, which was maintained for 5 min. The split-less injection was applied using 1.0 mL of vapour-phase sample or 2.0 μL of the liquid sample. Headspace sampling and analysis were not replicated.
To evaluate the growth inhibitory activities of authentic samples of compounds that corresponded to the detected compounds, the same procedure was applied for testing the dried samples. The only change was a cup containing 50 μL of the authentic compound (or 50 mg for solid compounds) placed in the source well instead of the dried sample (Figure 1). Authentic samples were purchased from Wako Pure Chemicals Industries, Ltd. (borneol, camphene, camphor, carvone, 1,8-cineole, limonene, α-pinene, β-pinene, and γ-terpinene), Sigma Chemical Co. Ltd. (3-carene and p-cymene), and Tokyo Chemical Industry Co. Ltd. (β-myrcene). The compounds that showed strong inhibition of lettuce seedling growth were re-assayed using a reduced amount of sample (5 μL or 5 mg until amounts of 0.5 μL or 0.05 mg were reached). Since the reduced quantities of the compounds were difficult to measure, the compounds were added to 50 μL of dimethyl sulfoxide (DMSO), which has been shown to have no effect on the germination or elongation of lettuce seeds at concentrations of 50 μL and below (Fujii, unpublished report).
To measure the vapour concentration of the authentic compound in each well, separate multi-dishes were set out in the same manner as above but without lettuce seeds. One multi-dish corresponded to a well, and the vapour was collected from the multi-dish only once to avoid any unusual diffusion of vapour during the collection process. The vapour was collected after 24 h using a Hamilton gas-tight syringe (1.0 mL) through a septum located on top of the wells. The gas chromatography-mass spectrometry (GC-MS) analysis was undertaken using the same procedure as in Section 3.4. In addition, the biological activity of the most active commonly detected authentic compound among the tested species was evaluated based on its specific activity and total activity. Specific activity (expressed as EC50), is the effective concentration of the compound that induces half-maximum inhibition. In our study, the EC50 value was calculated using a probit analysis [], and the total activity was calculated as:
Total activity = (1/EC50) × concentration in the sample (μg/g plant)
4. Conclusions
The leachates from parsley, followed by tarragon showed the strongest inhibitory activity against lettuce seedling growth using the sandwich method. On the other hand, the volatile constituents from caraway, dill seed, laurel, rosemary, and sage followed by cardamom and thyme showed the highest inhibition using the dish pack method. Headspace sampling and GC-MS analysis identified the main inhibitory compounds as carvone in caraway and dill seed, 1,8-cineole in laurel and cardamom, and borneol in thyme. Both camphor and 1,8-cineole were detected in rosemary and sage. Although both compounds played potentially equal roles in the inhibitory activity of sage, the total activity evaluation demonstrated that camphor was the major inhibitory compound in rosemary.
Author Contributions
Conceptualization, T.S. and Y.F.; methodology, T.S.; validation, T.S. and M.S.; formal analysis, T.S.; investigation, T.S.; resources, T.S., M.A., and Y.F.; data curation, T.S. and M.S..; writing—original draft preparation, T.S., K.S.A., and M.S.; writing—review and editing, T.S., M.A., K.S.A., and Y.F.; supervision; Y.F.; funding acquisition, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant-in-aid for research on Agriculture and Food Science (25029AB) from the Ministry of Agriculture, Forestry, and Fisheries of Japan. JSPS KAKENHI grant number 26304024, MEXT scholarship number 120573, and JST CREST grant number JPMJCR17O2 also supported this work.
Acknowledgments
We would like to show great appreciation to Mami Sugano for her great assistance in the operation and analysis of samples by GC-MS.
Conflicts of Interest
The authors have not declared any conflict of interest.
References
- Benner, J.P. Pesticides from nature. Part I: Crop protection agents from higher plants—An overview. In Crop Protection Agents from Nature; Copping, L.G., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1996; pp. 217–229. [Google Scholar]
- Abraham, D.; Braguini, W.L.; Kelmer–Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes of germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Barney, J.; Hays, A.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, S.; Anaya, A.L. Effect of some sesquiterpene lactones on the growth of certain secondary tropical species. J. Chem. Ecol. 1978, 4, 305–313. [Google Scholar] [CrossRef]
- Dudai, N.; Mayer, A.M.; Poljakoff-Mayber, A.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Libbey, C.; Boydston, R. Weed suppression with Brassica green manure crops in green pea. Weed Sci. 1997, 45, 439–445. [Google Scholar] [CrossRef]
- Mayton, H.S.; Olivier, C.; Vaughn, S.F.; Loria, R. Correlation of fungicidal activity of Brassica species with allyl isothiocyanate production in macerated leaf tissue. Phytopathology 1996, 86, 267–271. [Google Scholar] [CrossRef]
- Olivier, C.; Vaughn, S.F.; Mizubuti, E.S.G.; Loria, R. Variation in allyl isothiocyanate production within Brassica species and correlation with fungicidal activity. J. Chem. Ecol. 1998, 25, 2687–2701. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Boydston, R.A. Volatile allelochemicals released by crucifer green manures. J. Chem. Ecol. 1997, 23, 2107–2116. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Isbell, T.A.; Weisleder, D.; Berhow, M.A. Biofumigant compounds released by field pennycress (Thlaspi arvense) seed meal. J. Chem. Ecol. 2005, 31, 167–177. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Tukey, H.B., Jr. Implications of allelopathy in agricultural plant science. Bot. Rev. 1969, 35, 1–16. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Alrubeaa, A.J. Allelopathic effects of Citrus aurantium L. I. Vegetational patterning. J. Chem. Ecol. 1985, 11, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Bradow, J.M.; Connick, W.J., Jr. Allelochemicals from Palmer amaranth, Amaranthus palmeri S. Wats. J. Chem. Ecol. 1987, 13, 185–202. [Google Scholar] [CrossRef]
- Connick, W.J., Jr.; Bradow, J.M.; Legendre, M.G.; Vail, S.L.; Menges, R.M. Identification of volatile allelochemicals from Amaranthus palmeri S. Wats. J. Chem. Ecol. 1987, 13, 463–472. [Google Scholar] [CrossRef]
- Fujii, Y. Screening of allelopathic candidates by new specific discrimination, and assessment methods for allelopathy, and the identification of L-DOPA as the allelopathic substance from the most promising velvet beans (Mucuna pruriens). Bull. Natl. Inst. Agro-Environ. Sci. 1994, 10, 115–218, (In Japanese with English summary). [Google Scholar]
- Haig, T. Application of hyphenated chromatography-mass spectrometry techniques to plant allelopathy research. J. Chem. Ecol. 2001, 27, 2363–2396. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Fujii, Y.; Parvez, S.S.; Parves, M.M.; Ohmae, Y.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Morikawa, C.I.O.; Miyaura, R.; Tapia, Y.; Figueroa, M.D.L.; Rengifo Salgado, E.L.; Fujii, Y. Screening of 170 Peruvian plant species for allelopathic activity by using the Sandwich Method. Weed Biol. Manag. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Shiraishi, S.; Watanabe, I.; Kuno, K.; Fujii, Y. Allelopathic activity of leaching from dry leaves and exudates from roots of groundcover plants assayed on agar. Weed Biol. Manag. 2002, 2, 133–142. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsuyama, M.; Hiradate, S.; Shimozawa, H. Dish Pack Method: A New Bioassay for volatile allelopathy. In Proceedings of the Fourth World Congress on Allelopathy, New South Wales, Australia, 21–26 August 2005; pp. 493–497. [Google Scholar]
- Sunohara, Y.; Baba, Y.; Matsuyama, S.; Fujimura, K.; Matsumoto, H. Screening and identification of phytotoxic volatile compounds in medicinal plants and characterizations of a selected compound, eucarvone. Protoplasma 2015, 252, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Sugano, M.; Azizi, M.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium percisum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Itani, T.; Hirai, K.; Fujii, Y.; Kohda, H.; Tamaki, M. Screening for allelopathic activity among weeds and medical plants using the “sandwich method”. J. Weed Sci. Tech. 1998, 43, 258–266, (In Japanese with English summary). [Google Scholar] [CrossRef]
- Hirai, N.; Sakashita, S.; Sano, T.; Inoue, T.; Ohigashi, H.; Premasthira, C.; Asakawa, Y.; Harada, J.; Fujii, Y. Allelochemicals of the tropical weed Sphenoclea zeylanica. Phytochemistry 2000, 55, 131–140. [Google Scholar] [CrossRef]
- Takemura, T.; Sakuno, E.; Kamo, T.; Hiradate, S.; Fujii, Y. Screening of the growth-inhibitory effects of 168 plant species against lettuce seedlings. Am. J. Plant. Sci. 2013, 4, 1095–1104. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.T.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Macias, F.A.; Fischer, N.H.; Williamson, G.B. Just how insoluble are monoterpenes? J. Chem. Ecol. 1993, 19, 1799–1807. [Google Scholar] [CrossRef]
- Itani, T.; Fujita, T.; Tamaki, M.; Kuroyanagi, M.; Fujii, Y. Allelopathic activity and oxalate content in oxalate-rich plants. J. Weed Sci. Technol. 1999, 44, 316–323, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Harada, J. Plant growth-inhibiting substance contained in parsley plants. Jpn. J. Crop. Sci. 1984, 53, 128–129. (In Japanese) [Google Scholar]
- Harada, J. Plant growth-inhibiting substances contained in parsley seed oil. Jpn. J. Crop. Sci. 1984, 53, 130–131. (In Japanese) [Google Scholar]
- Muller, C.H.; Muller, W.H.; Haines, B.L. Volatile growth inhibitors produced by aromatic shrubs. Science 1964, 143, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.W.; Kil, B.-S. Assessment of allelopathic potential in Artemisia princeps var. Orientalis Residues. J. Chem. Ecol. 1992, 18, 1933–1940. [Google Scholar] [CrossRef]
- Morinaka, Y.; Fukuda, N.; Takayanagi, K. Evaluation of perilla (Perilla frutescens) aroma–Analysis of volatile aromatic components in fresh perilla leaves by adsorptive column method. J. Jpn. Soc. Hort. Sci. 2002, 71, 411–418, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Asplund, R.O. Monoterpenes: Relationship between structure and inhibition of germination. Phytochemistry 1968, 7, 1995–1997. [Google Scholar] [CrossRef]
- Fischer, N.H.; Williamson, G.B.; Weidenhamer, J.D.; Richardson, D.R. In search of allelopathy in the Florida scrub: The role of terpenoids. J. Chem. Ecol. 1994, 20, 1355–1380. [Google Scholar] [CrossRef]
- Halligan, J.P. Toxic terpenes from Artemisia californica. Ecology 1975, 56, 999–1003. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plants species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Kang, G.; Mishyna, M.; Appiah, K.S.; Yamada, M.; Takano, A.; Prokhorov, V.; Fujii, Y. Screening for plant volatile emissions with allelopathic activity and the identification of L-Fenchone and 1,8-cineole from anise (Illicium verum) leaves. Plants 2019, 8, 457. [Google Scholar] [CrossRef]
- Fujii, Y.; Hiradate, S. A critical survey of allelochemicals in action—The importance of total activity and the weed suppression equation. In Proceedings of the Fourth World Congress on Allelopathy, New South Wales, Australia, 21–26 August 2005; pp. 73–76. [Google Scholar]
- Hiradate, S. Isolation strategies for finding bioactive compounds: Specific activity vs. total activity. In Natural Products for Pest Management in ACS Symposium Series 927; Rimando, A.M., Duke, S.O., Eds.; ACS Publications: Washington, DC, USA, 2006; pp. 113–126. [Google Scholar]
- Hiradate, S.; Morita, S.; Sugie, H.; Fujii, Y.; Harada, J. Phytotoxic cis-cinnamoyl glucosides from Spiraea thunbergii. Phytochemistry 2004, 65, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).