Abstract
Carbohydrates are a primary nutrient for plant growth, and sugar transporter proteins play a crucial role in sugar allocation. In this study, hexose transporter genes encoding in the genome of colored calla lily ‘Jingcai Yangguang’ (Zantedeschia elliottiana cv. Jingcai Yangguang) were identified, and their expression patterns following infection by Pectobacterium carotovora subsp. Carotovora were investigated. Additionally, the transport characteristics of three hexose transporters, ZeSTP7, ZeSTP15, and ZeSTP17, were determined. The results showed that the sugar transporter protein family in Z. elliottiana comprises 18 members, most of which possess 12 transmembrane domains. Phylogenetic analysis revealed that the ZeSTP gene family was divided into five subgroups. Tandem gene duplication events were identified on the 16 chromosomes of Z. elliottiana, with multiple tandemly duplicated genes detected. Comparative analysis of synteny between species identified ZeSTP8 and OsSTP22 as homologous gene pairs, while OsSTP6 (OsMST6) was identified as a homologous gene pair with both ZeSTP14 and ZeSTP17. Following infection by P. carotovora subsp. carotovora, the transcript levels of ZeSTP7, ZeSTP15, and ZeST17 were all significantly elevated. Yeast mutant hexose complementation tests indicated that ZeSTP7 could transport glucose and galactose, whereas ZeSTP15 and ZeSTP17 exhibited limited transport capacity in this respect. This study provides a systematic identification and analysis of hexose transporter genes at the genome-wide level, highlighting the role of ZeSTP genes in the response of colored calla lily to soft rot and laying a theoretical foundation for further understanding the functions of sugar transporter genes.
1. Introduction
Sugars are primary nutrients and products of photosynthesis in plants, primarily stored as sucrose and transported through the phloem to various plant tissues, facilitating sugar translocation from source to sink organs [1,2]. The intercellular transmembrane transport of sugars relies on specific carriers known as sugar transport proteins [3]. Currently, three main types of sugar transport proteins have been identified in plants: monosaccharide transporters (MSTs), sucrose transporters (SUC/SUTs), and sugar will eventually be exported transporters (SWEETs) [4]. Both SUTs and MSTs belong to the major facilitator superfamily (MFS) [5].
In most plants, sucrose synthesized in mesophyll cells through photosynthesis is transported from the source to sink organs. A portion of the sucrose is stored, while the rest is hydrolyzed back into monosaccharides and further absorbed by sink organs with the assistance of monosaccharide transporters [6]. Sugar transport protein/hexose transporters (STPs), as the primary members of the MST family, typically possess 12 α-helices forming transmembrane domains and function as H+/sugar cotransporters, primarily responsible for transporting hexoses from the apoplast into cells [7]. Additionally, hexose transporters are involved in plant growth and development, pollen germination, and the transport of sugars during biotic and abiotic stress responses in plants.
STPs are important regulators of plant growth and development. In Arabidopsis, the expression level of AtSTP1 is high during seed germination (particularly during seed imbibition) and in the early stages of seedling growth (1–7 days after germination). After germination, AtSTP1 is highly active in the roots and may play a crucial role in seedling growth by regulating the uptake of monosaccharides in the roots [8]. AtSTP1 exhibits circadian expression in guard cells; it mediates the uptake of glucose and other monosaccharides via osmotic adjustment to modulate stomatal movement and reallocate carbon skeletons [9,10]. AtSTP13 and AtSTP14 are subject to similar circadian control, being strongly induced at night and repressed under continuous light [7]. AtSTP7 supplies pentoses and arabinose to the cell wall [11]; AtSTP13 participates in programmed cell death [12]. Thus, STPs are essential for plant growth. Furthermore, during pollen and pollen-tube development, AtSTP9 (early phase) [13] and AtSTP6 (late phase) [14] act sequentially to supply a sustained carbon flux to the pollen grain and elongating tube, and AtSTP4 continuously imports apoplastic monosaccharides into sink cells of the root apex and anther, ensuring a steady carbon supply [15]. In fruits and storage organs, VvHT1 drives hexose import in young grape berries, thereby setting the final sugar-to-acid ratio [16], and fifteen MeSTPs in cassava coordinately promote early tuberous expansion [17]. Under environmental stress, STPs contribute to plant defense: AtSTP3 and AtSTP4 transcripts are rapidly and transiently elevated after mechanical wounding [18], and OsSTP14 is strongly upregulated in rice subjected to flooding and high-temperature stress [19].
In higher plants, sugars are the main nutrients that pathogens extract from host plants. Therefore, competition for sugar allocation occurs between plants and pathogens, with sugar transport proteins playing a key role in this process [20]. Several hexose transporters have been confirmed to participate in sugar transport during plant–pathogen interactions. For example, in Arabidopsis thaliana, AtSTP4 is expressed in mature leaves infected by fungi [21]. AtSTP13 is induced by Botrytis cinerea and actively absorbs hexoses to support host plant defense against gray mold invasion [12]. The phosphorylation-dependent regulation of AtSTP13 is crucial for A. thaliana defense, and the double mutant Atstp13 and Atstp1 weakens A. thaliana resistance to Pseudomonas syringae [22]. In maize, ZmSTP2 and ZmSTP20 respond to Fusarium graminearum, and the double mutant zmstp2 and zmstp20 enhances maize susceptibility [23]. However, STPs play a negative regulatory role in resistance to powdery mildew in A. thaliana and stripe rust in wheat. For example, AtSTP8 is localized on the endoplasmic reticulum in A. thaliana and is recruited to the extrahaustorial membrane during powdery mildew infection, thereby enhancing powdery mildew colonization on A. thaliana [24]. In wheat, TaSTP6 is induced by abscisic acid, and overexpression of TaSTP6 and TaSTP13 promotes host plant susceptibility to wheat stripe rust and powdery mildew [25,26]. In summary, hexose transporters are involved in plant immune processes and have different roles in different plants.
Previous studies have reported genome-wide identification of the STP gene family in A. thaliana, rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays L.). The earliest identification of 14 STP genes was in A. thaliana [7], with most AtSTPs exhibiting broad substrate specificity. In rice, 28 OsSTP genes were identified [19]; 81 TaSTP genes were identified in wheat [27]; and 24 ZmSTP genes were identified in maize [23].
Calla lily (Zantedeschia spp.) is a perennial herbaceous flower belonging to Araceae, of which colored calla lily holds significant economic value [28]. However, bacterial soft rot caused by Pectobacterium carotovora subsp. carotovora significantly reduces the quality of colored calla lily [29,30]. Currently, there is no cure for calla lily soft rot, and its impact is primarily controlled through cultivation management, physical, and chemical means [31]. STP genes have been confirmed to play important roles in the interactions between multiple plants and pathogens. Despite in-depth research on the STP gene family in A. thaliana and wheat, a bioinformatics analysis of the ZeSTP gene family in colored calla lily is still lacking. Therefore, this study identified and analyzed the ZeSTP gene family in Z. elliottiana cv. Jingcai Yangguang, revealing the responsiveness and expression patterns of ZeSTP genes to P. carotovora subsp. carotovora. This work lays a theoretical foundation for further exploring the potential role of the STP family in the molecular breeding of colored calla lilies.
2. Results
2.1. Identification of ZeSTP Gene Family Members in Z. elliottiana
A total of 18 ZeSTP genes were identified through the analysis of the annotation information from the genome sequencing results of Z. elliottiana. These genes were sequentially named ZeSTP1 to ZeSTP18 based on their chromosomal locations in the genome. The molecular weights and isoelectric points of the STP family proteins in Z. elliottiana were analyzed. The results showed that the molecular weight ranged from 9.1 kDa (ZeSTP6) to 91.9 kDa (ZeSTP13), and the isoelectric points ranged from 5.1 (ZeSTP4) to 10.86 (ZeSTP1). Among them, ZeSTP2, ZeSTP4, and ZeSTP6 were acidic proteins, while the others were basic proteins. In addition, the transmembrane domains of the ZeSTP protein family were predicted, revealing that most proteins possess 12 transmembrane domains (TMD) (Table 1).

Table 1.
Physicochemical properties of STP gene family members in Z. elliottiana 1.
2.2. Phylogenetic Analysis of the ZeSTP Family in Z. elliottiana
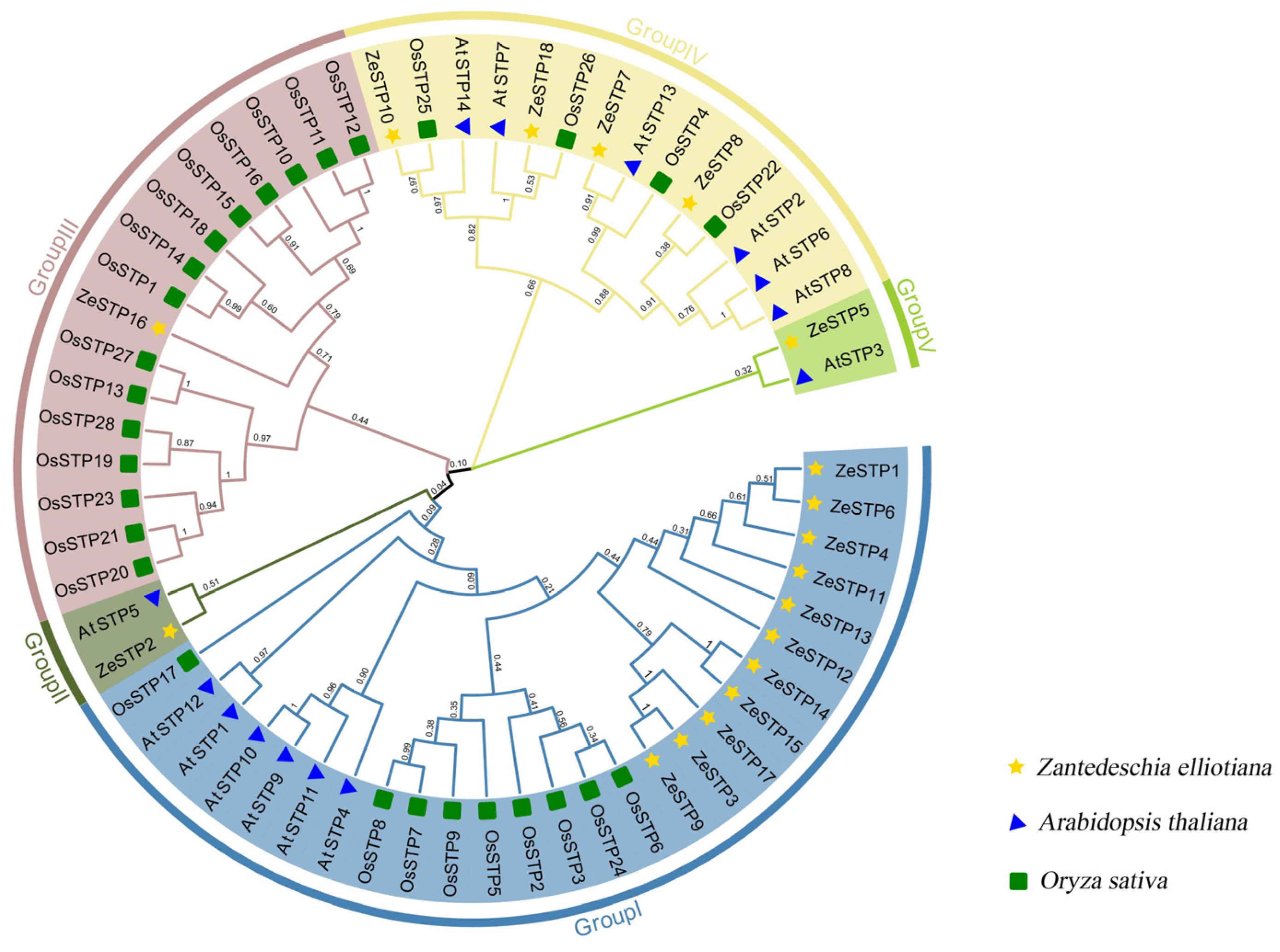
The differences in STP genes among species may arise from the expansion of gene families in different species. To explore this, a multiple sequence alignment of ZeSTP family protein sequences with O. sativa, A. thaliana was performed, and the phylogenetic tree was constructed.
Based on the branches of the phylogenetic tree, the ZeSTP family can be divided into five subgroups: group I, group II, group III, group IV, and group V. Each subgroup contains members from multiple species, indicating that the STP genes in these subgroups likely share a common evolutionary ancestor. The root node of the STP genes splits into two major branches, one leading to the ancestral gene of group V and the other to the common ancestral gene of groups I, II, III, and IV.
Gene expansion events may have occurred in Z. elliottiana. For example, in group I, the evolutionary branch of ZeSTP12 further diverged into five ZeSTP genes (Figure 1). Additionally, in groups II and V, the ZeSTPs of Z. elliottiana are more closely related to the AtSTPs of A. thaliana, while in group III, the ZeSTPs of Z. elliottiana are more closely related to the OsSTPs of rice. In contrast, the distribution of STP members from O. sativa, A. thaliana, and Z. elliottiana is relatively even in group IV.
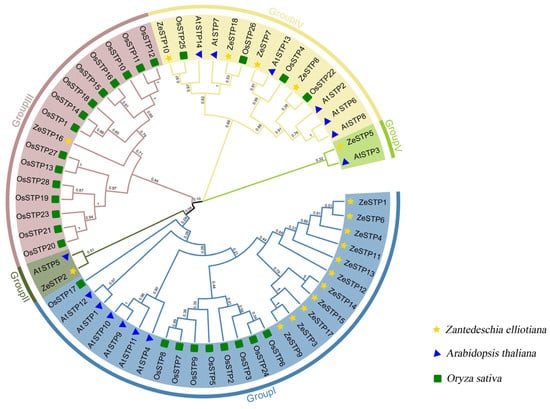
Figure 1.
Phylogenetic tree of STP proteins in O. sativa, A. thaliana, and Z. elliottiana. Different-colored branches represent different groupings, while different-shaped patterns represent different species.
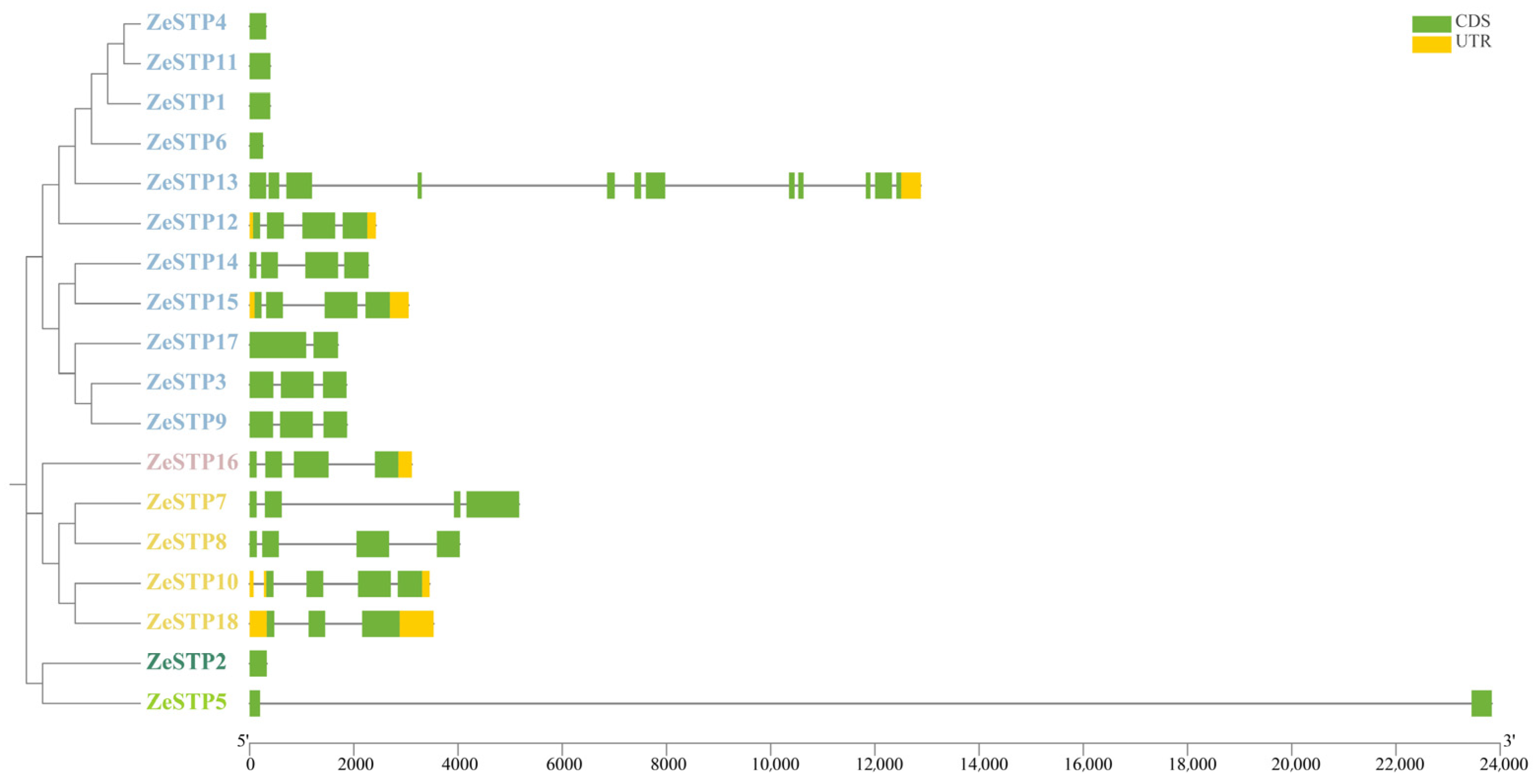
2.3. Analysis of Sequence Structural Characteristics of the ZeSTP Gene Family in Z. elliottiana
Gene structure is crucial for the study of protein function. Therefore, in this study, the visual analysis of the sequence structure of the ZeSTP gene family in Z. elliottiana was performed. The results showed significant differences in the number of exons among the 18 ZeSTP genes (Figure 2). Among them, ZeSTP13 has the highest number of exons with 12, while some ZeSTP genes (such as ZeSTP4 and ZeSTP11) contain only one exon. The multiple exons in ZeSTP13 may be the reason for its increased gene length. Additionally, an unusually long intron, exceeding 22 kb in length, was inserted into ZeSTP5, which is also a cause for its increased gene length. The length of most ZeSTP genes is within 6 kb. Among the 18 ZeSTP genes, some genes contain untranslated regions (UTRs) at both the 5′ and 3′ ends, such as ZeSTP10, ZeSTP12, ZeSTP15, and ZeSTP18; while some genes (such as ZeSTP13 and ZeSTP16) have only one UTR region at the 3′ end, and most genes do not contain UTR regions (Figure 2).
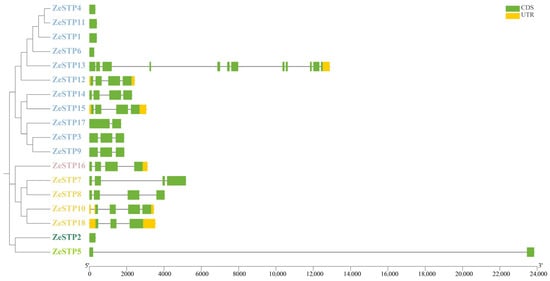
Figure 2.
Gene structure of the ZeSTP family in Z. elliottiana. The green boxes, yellow boxes, and black lines represent coding sequences, non-coding regions, and gene lengths, respectively. Different-colored branches represent different groupings within the phylogenetic tree of the ZeSTP gene family.
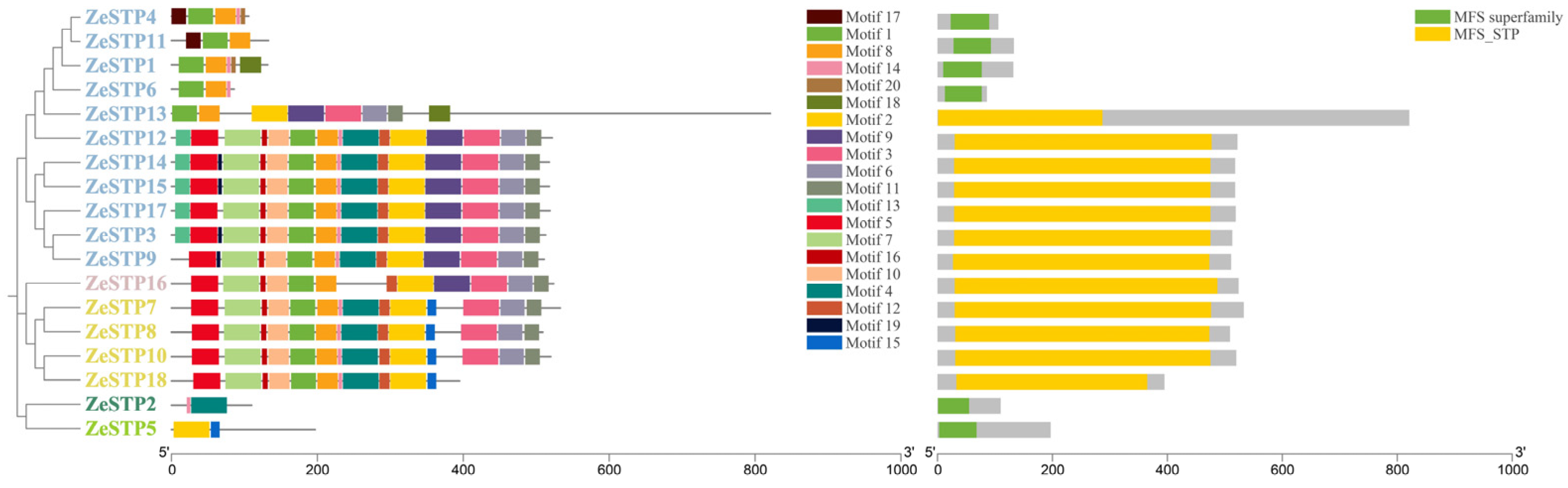
Conserved motif analysis revealed differences in conserved motifs among different evolutionary branches of the ZeSTP gene family. In the phylogenetic tree, ZeSTP genes within the same subgroup show similar conserved motifs, indicating sequence variation within conserved regions (Figure 3). However, even if the conserved motifs of a gene are low, the structural conservation of the protein may still be high. The conserved domains of the gene family can be divided into two categories: MFS superfamily and MFS_STP. For example, genes containing the MFS superfamily conserved domain, such as ZeSTP4, ZeSTP11, ZeSTP1, ZeSTP6, ZeSTP2, and ZeSTP5, have only one conserved domain in their coding sequence (CDS) region (Figure 2), and both the length of the conserved motifs and domains are less than 200 bp. In contrast, genes containing the MFS_STP conserved domain have more than one conserved domain in their CDS region (Figure 2), and both the length of the conserved motifs and domains are greater than 200 bp (Figure 3).
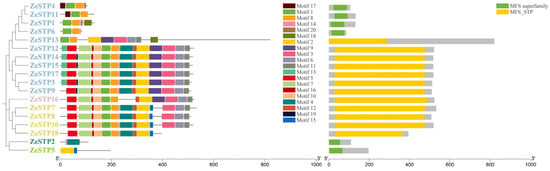
Figure 3.
Distribution of conserved motifs and domains in the ZeSTP family of Z. elliottiana.
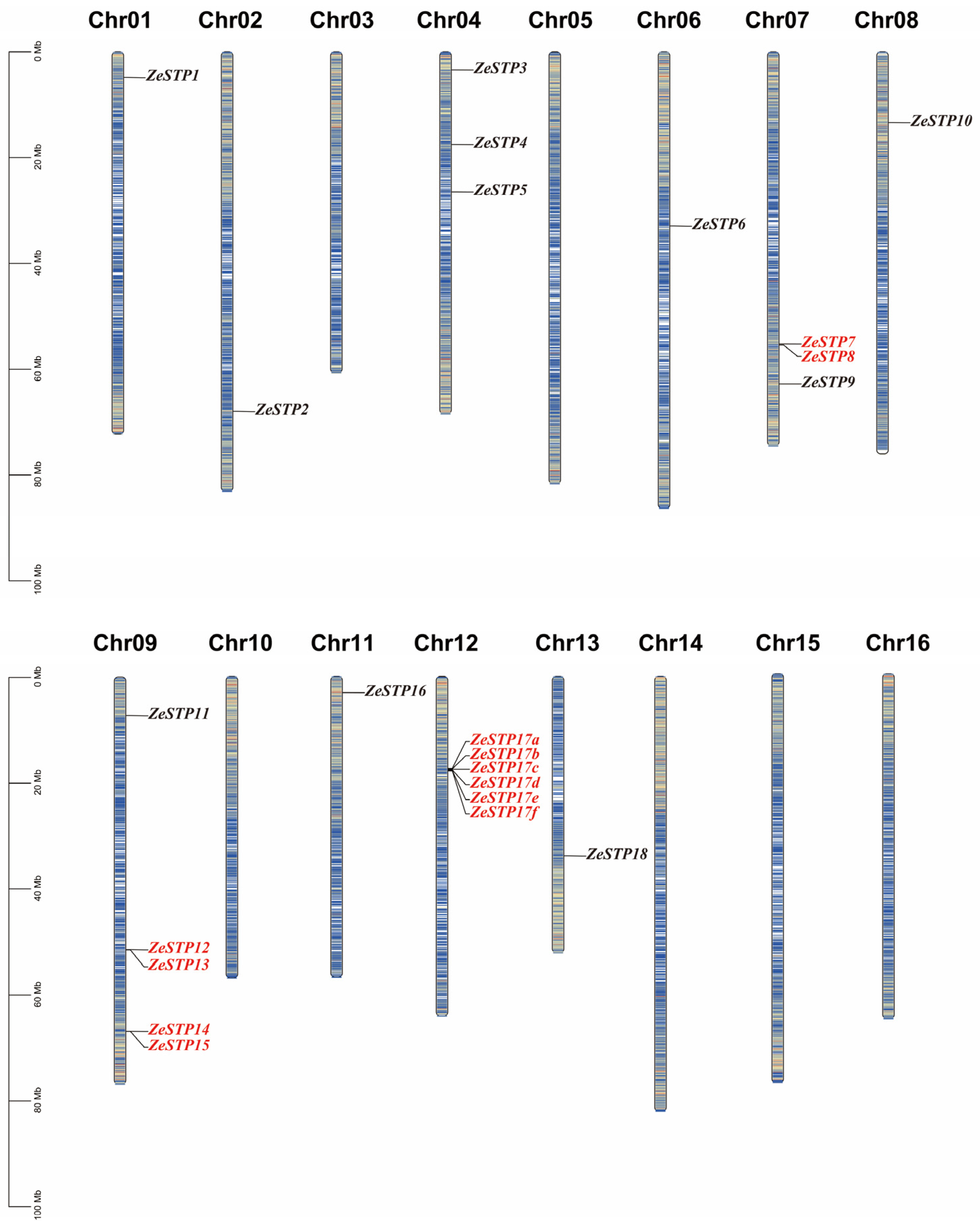
2.4. Chromosomal Localization Analysis of the ZeSTP Gene Family in Z. elliottiana
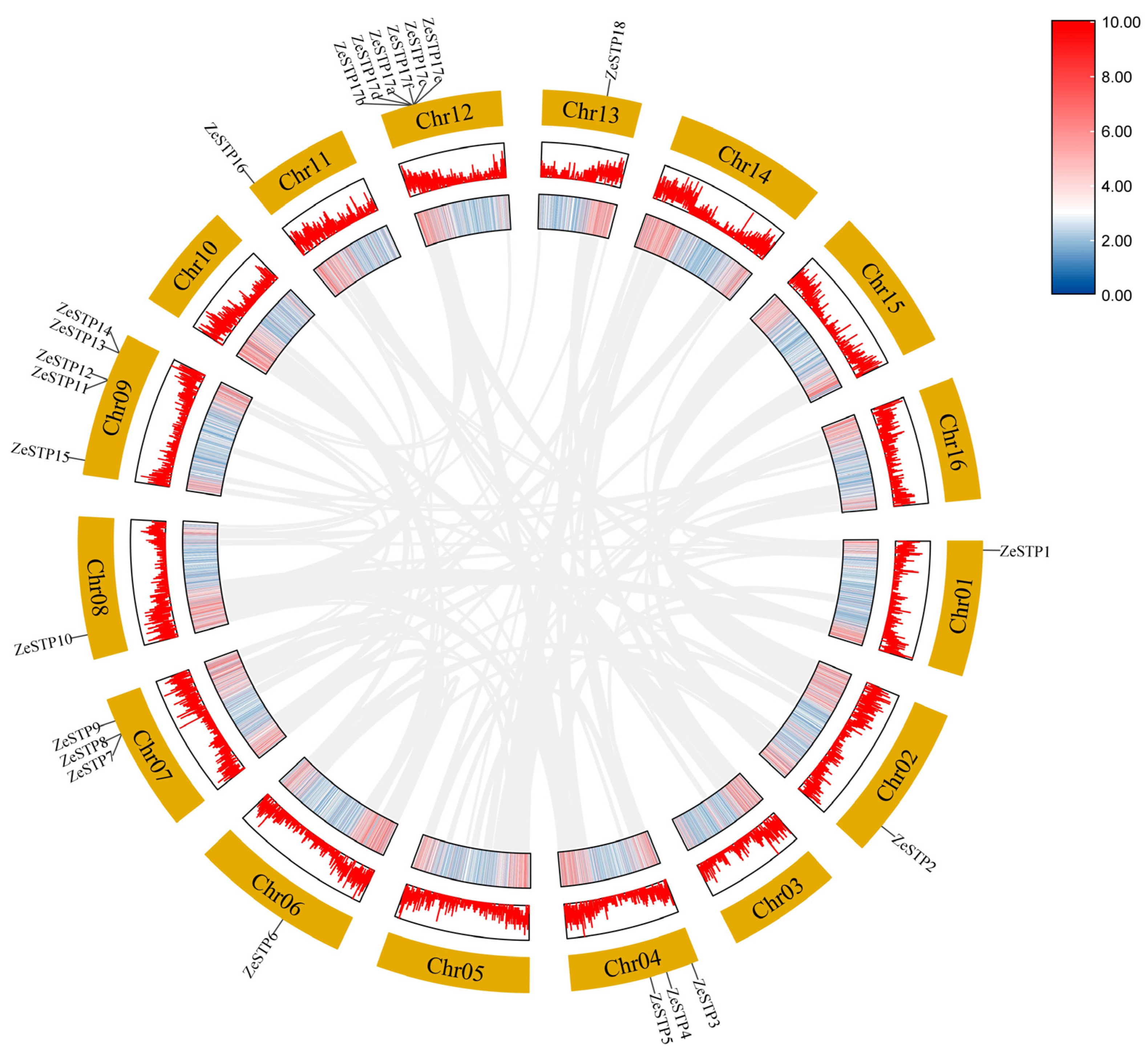
To investigate the replication of the STP gene family in the genome of Z. elliottiana, a chromosomal distribution map of the STP gene family was created based on the chromosomal positions of the genes provided in the annotation file. The results showed that the distribution of ZeSTP genes across the 16 chromosomes is not uniform (Figure 4). ZeSTP genes are mainly concentrated on chromosomes 1, 2, 3, 4, 6, 7, 8, 9, 11, 12, and 13 (Figure 4), while the remaining six chromosomes do not contain any ZeSTP genes. Additionally, ZeSTP genes on chromosomes 1, 4, 6, 8, 11, and 12 are predominantly located at the upper ends of the chromosomes, whereas those on chromosomes 2, 7, 9, and 13 are mostly found at the lower ends.
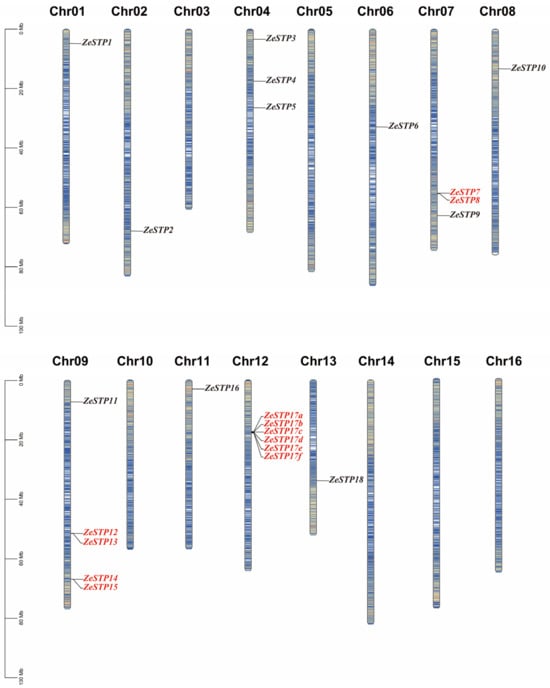
Figure 4.
Chromosomal distribution of STP family genes in Z. elliottiana. The 18 STP family members of colored calla lily are labeled. The lines on the chromosomes represent gene density in the calla lily genome. Red lines indicate higher gene density, while blue lines indicate lower gene density.
It is shown that ZeSTP7 and ZeSTP8 on chromosome 7 may have undergone tandem gene duplication events. Similarly, chromosome 9 contains five ZeSTP genes, among which ZeSTP12 and ZeSTP13 have experienced tandem duplication events, as well as ZeSTP14 and ZeSTP15. Furthermore, the ZeSTP17 gene on chromosome 12 may have undergone multiple tandem duplication events, resulting in six ZeSTP17 genes encoding the same CDS region (Figure 4). Tandemly duplicated genes play an important role in the evolutionary process of species.
2.5. Intraspecific Collinearity Analysis of STP Genes in Z. elliottiana and Interspecific Collinearity Analysis with O. sativa
To investigate the expansion of the ZeSTP gene family, the gene duplication events were examined. In Z. elliottiana, multiple pairs of tandemly duplicated genes, ZeSTP7/ZeSTP8, ZeSTP12/ZeSTP13, ZeSTP14/ZeSTP15, and ZeSTP17a/b/c/d/e/f, were identified (Figure 4). However, intraspecific collinearity analysis of the ZeSTP gene family in Z. elliottiana did not reveal any collinear blocks (Figure 5).
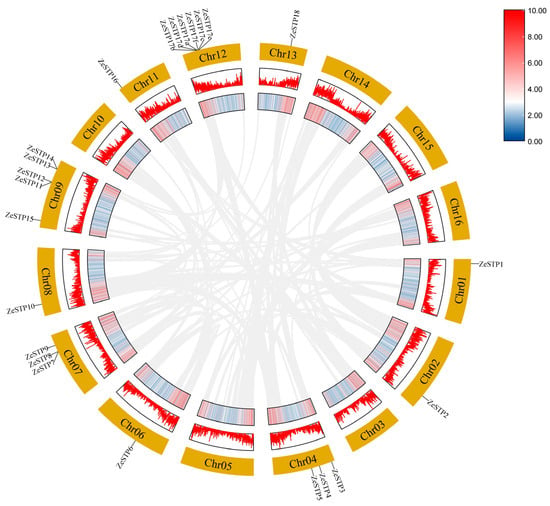
Figure 5.
Homology analysis of ZeSTP genes in Z. elliottiana (chromosomes 1–16). Chromosome-scale localization (outer ring); genomic gene density distribution (middle rings); genome-wide synteny blocks (gray connectors).
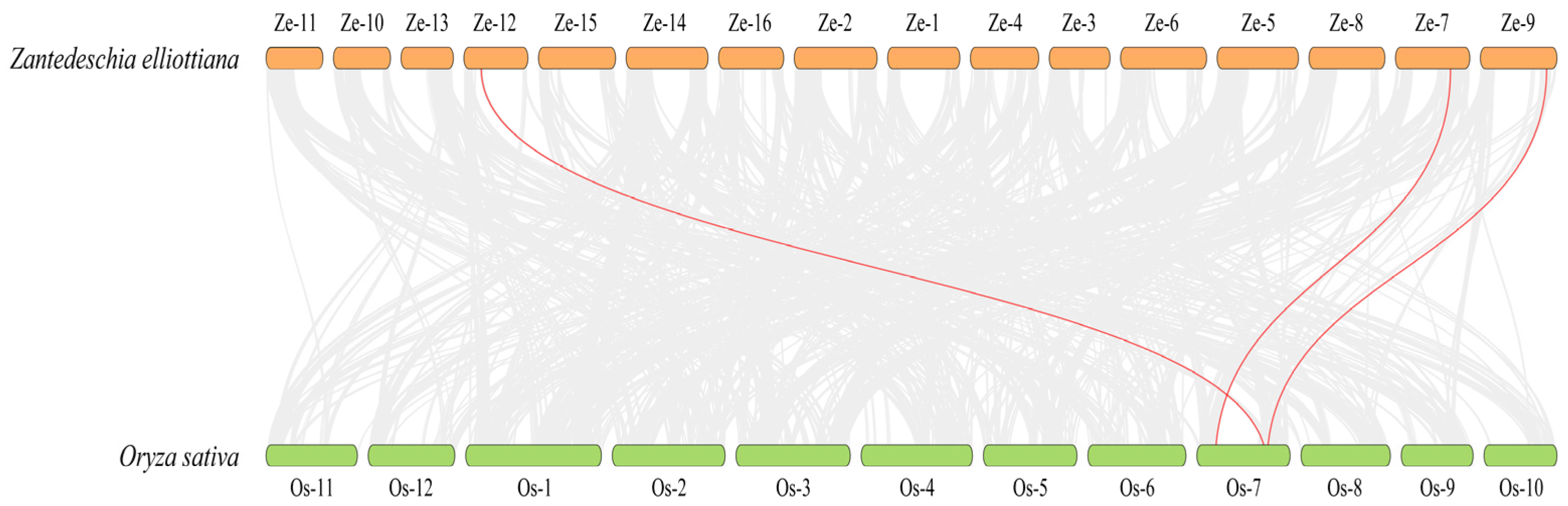
Nevertheless, the collinearity analysis between O. sativa and Z. elliottiana, which are both monocotyledonous plants, was further conducted. The results showed there are three pairs of homologous genes between the two species (Figure 6). Specifically, ZeSTP8 on chromosome 7 of Z. elliottiana and OsSTP22 on chromosome 7 of O. sativa form a significant collinear gene pair. Additionally, ZeSTP14 on chromosome 9 and ZeSTP17 on chromosome 12 of Z. elliottiana are homologous to OsSTP6 (OsMST6) on chromosome 7 of O. sativa. This suggests that the ancestral genes of ZeSTP14 and ZeSTP17 likely originated from the same gene. Interestingly, all ZeSTP genes in Z. elliottiana that showed collinearity with O. sativa have undergone duplication events, and the homologous rice genes are all located on chromosome 7 (Figure 6).
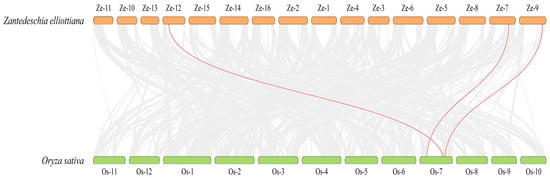
Figure 6.
Interspecific collinearity analysis of STP genes between Z. elliottiana and O. sativa. Gray lines indicate collinear blocks between the two species, while red lines represent collinear gene pairs between STP genes of Z. elliottiana and O. sativa.
2.6. Expression Analysis of ZeSTP Genes in Z. elliottiana After Inoculation with P. carotovora subsp. carotovora
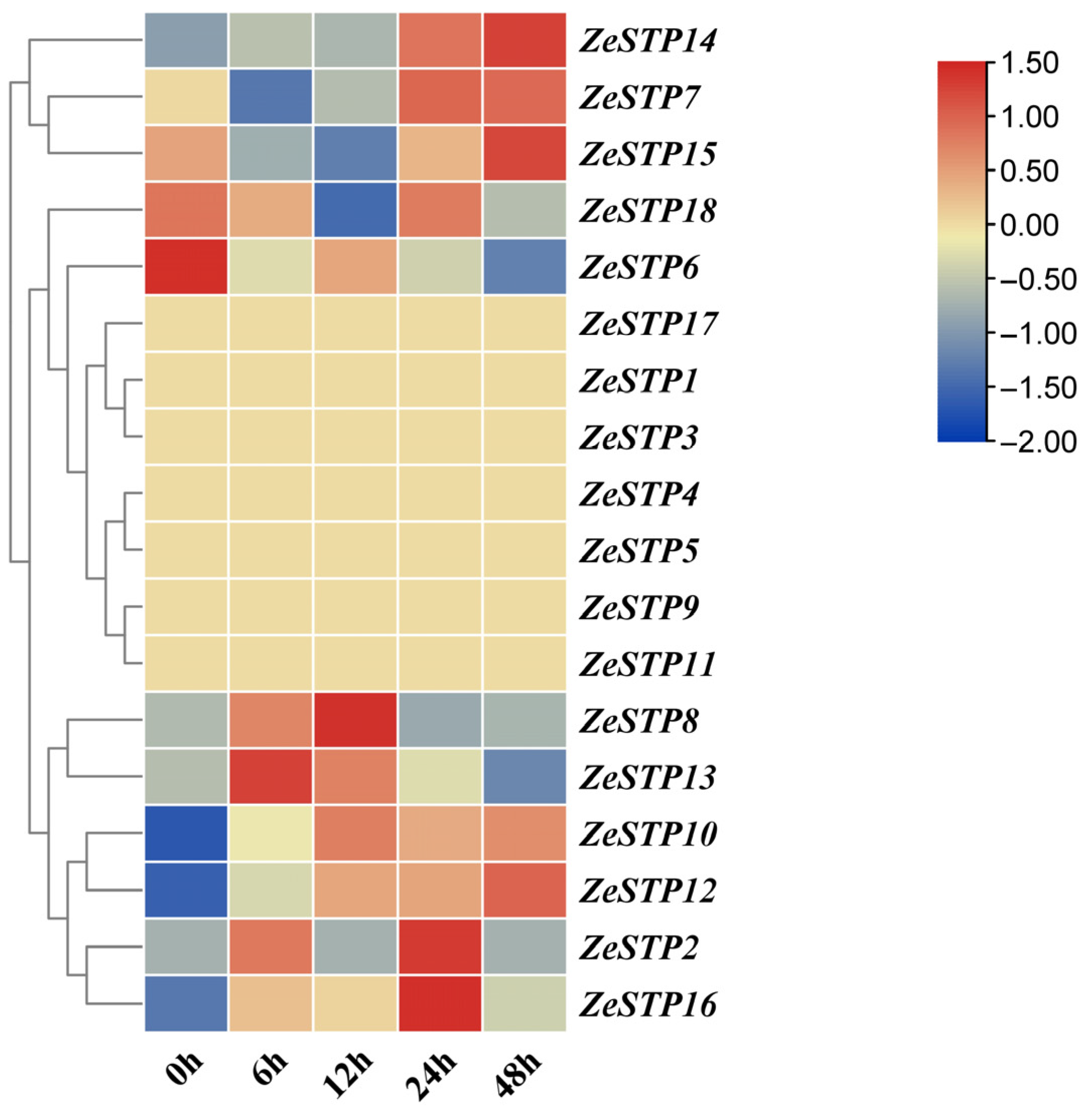
In previous studies, the transcriptome data of Z. elliottiana cv. Jingcai Yangguang inoculated with P. carotovora subsp. carotovora was analyzed [32]. To investigate the response of ZeSTP genes in Z. elliottiana to P. carotovora subsp. carotovora, the expression levels of ZeSTP genes in the transcriptome at 0, 6, 12, 24, and 48 h post-inoculation were analyzed (Figure 7).
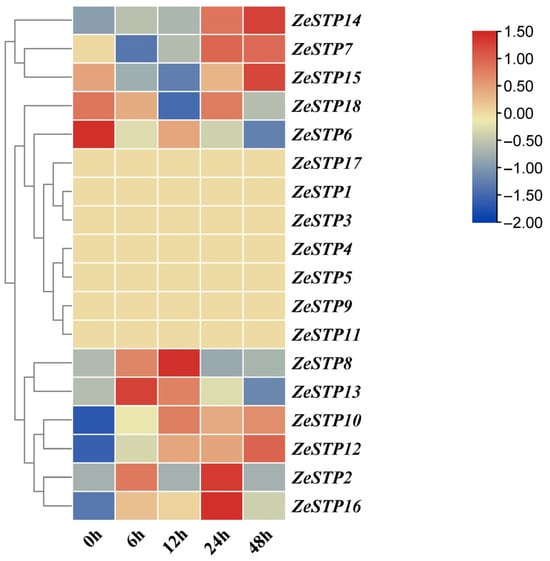
Figure 7.
Heatmap of ZeSTP gene family expression in the transcriptome data after inoculation with P. carotovora subsp. carotovora. The gene expression values are log-transformed with base 2. Color marks indicate changes in gene expression: red shows high expression, and blue shows low expression.
The transcriptome heatmap showed that ZeSTP7, ZeSTP14, and ZeSTP15 transcript levels increased from 12 to 48 h (Figure 7). ZeSTP15 exhibited sustained high expression, with 48 h levels 2.76-fold higher than at 12 h. ZeSTP10 and ZeSTP12 were also progressively upregulated. These genes, especially ZeSTP7 and ZeSTP15, are likely associated with calla lily soft rot disease infection.
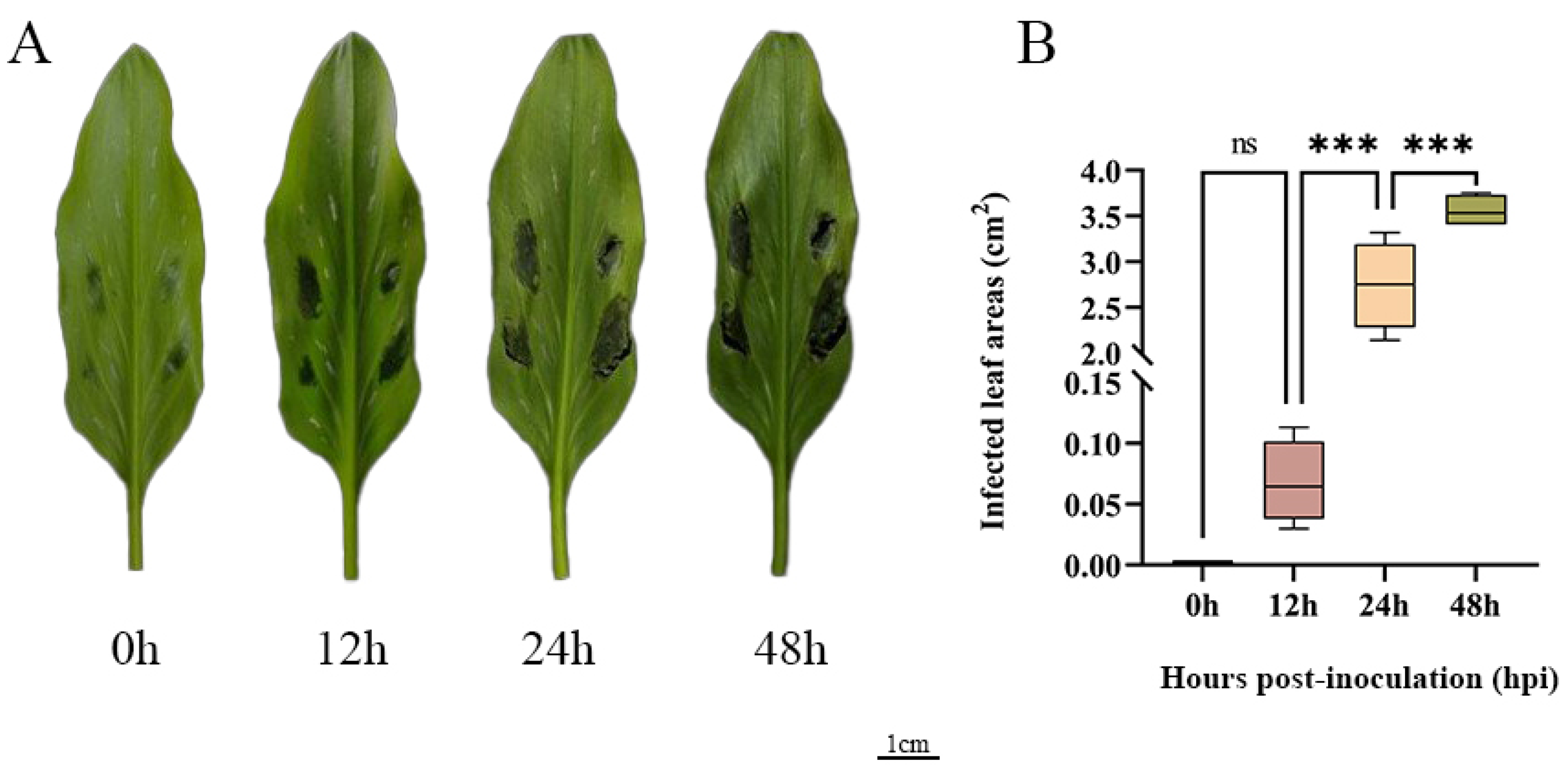
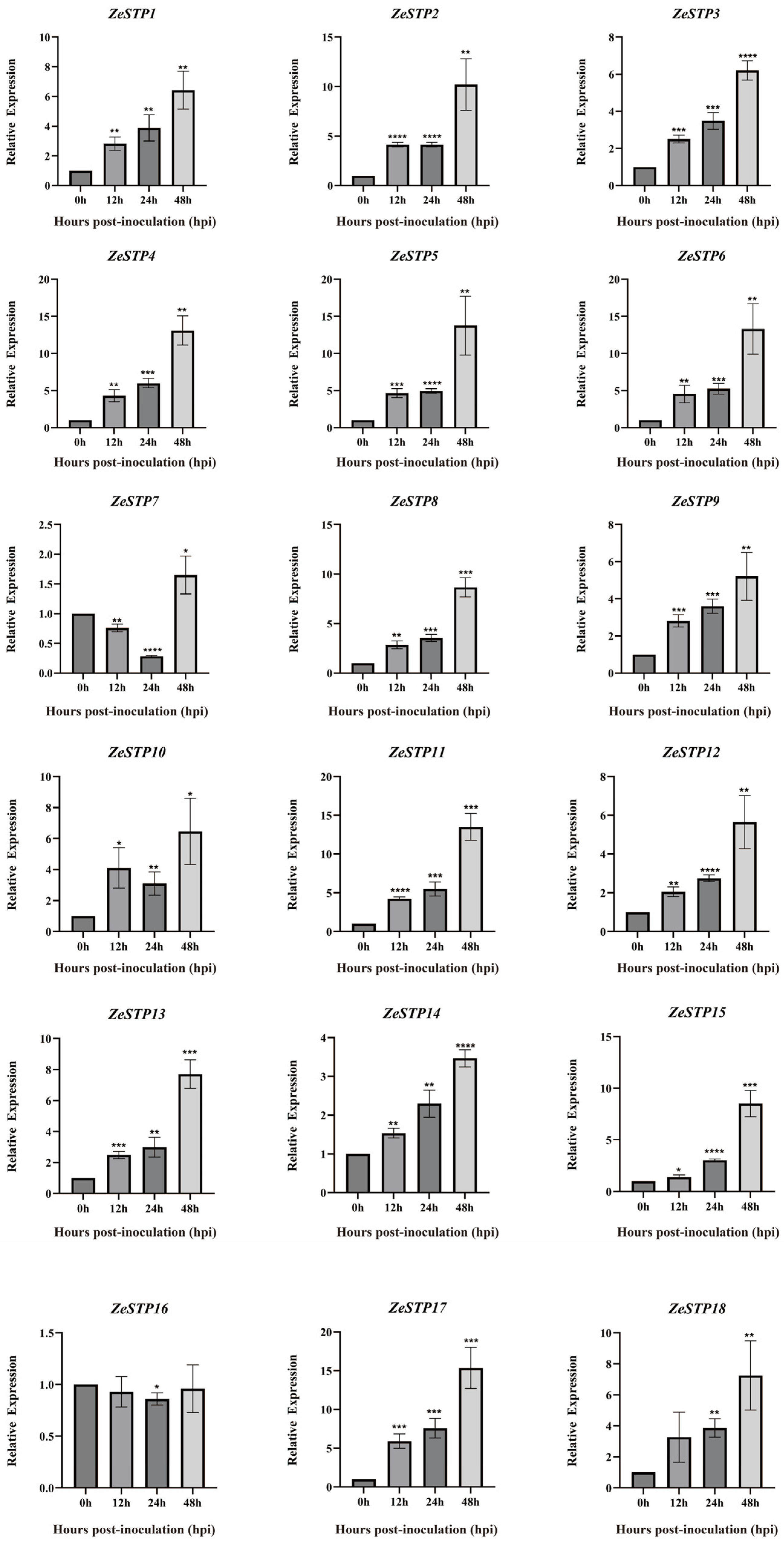
To further explore how ZeSTP genes in Z. elliottiana respond to P. carotovora subsp. carotovora, colored calla lily leaves were inoculated with P. carotovora subsp. carotovora (Figure 8A). Following inoculation, both the symptomatic area and the severity of disease intensified progressively over time (Figure 8B). Symptomatic leaf tissue was sampled as 2 cm diameter discs centered on the inoculation site at 0, 12, 24, and 48 h post-inoculation, and the expression levels of ZeSTP genes were measured (Figure 9). Gene expression levels of ZeSTP15 and ZeSTP17 rise significantly over time, with ZeSTP17 expression increasing 5.91-, 7.57-, and 15.35-fold at 12, 24, and 48 h, respectively, compared to 0 h. Conversely, ZeSTP7 and ZeSTP16 expression levels dropped 3.57- and 1.17-fold at 24 h but rebounded by 48 h (Figure 9). These results suggest that the ZeSTP gene family is involved in the interaction with P. carotovora subsp. carotovora, with ZeSTP15 and ZeSTP17 showing robust responses.
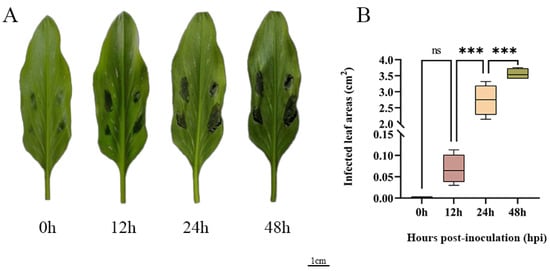
Figure 8.
(A) Disease phenotypes at different time points after inoculation of P. carotovora subsp. carotovora on the leaves of Z. elliottiana cv. Jingcai Yangguang. (B) The lesion areas of the leaves after inoculation at different time points (one-way ANOVA: ns: non-significant; *** p < 0.001).
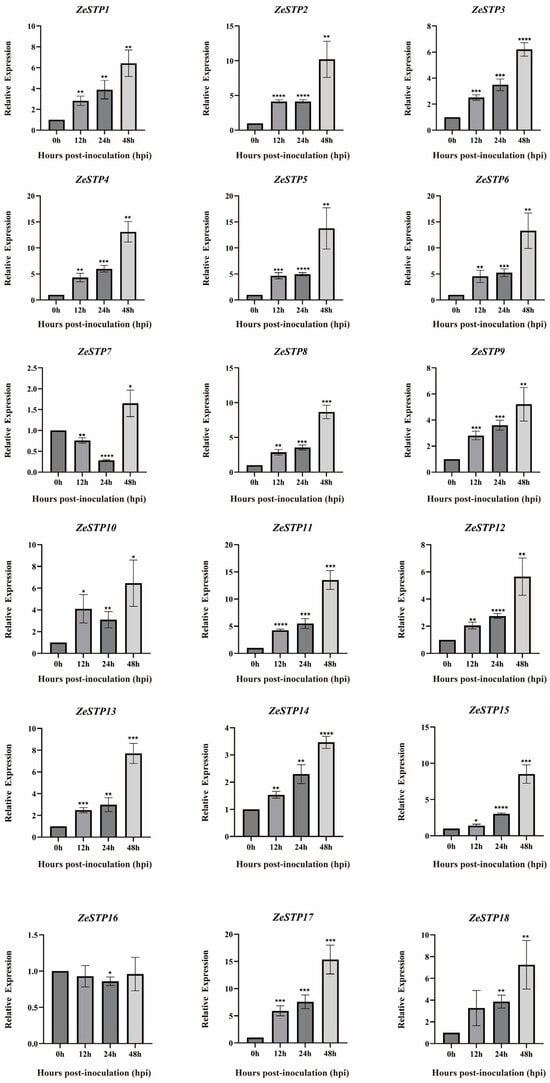
Figure 9.
Expression levels of ZeSTP family genes in Z. elliottiana leaves after P. carotovora subsp. carotovora inoculation. Expression dynamics (0–48 h post-inoculation) were quantified by RT-qPCR with gene-specific primers. Data represent the mean ± SE (n = 3); asterisks denote significant differences (Student’s t-test: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
2.7. Sugar Transport Properties of ZeSTP7, ZeSTP15, and ZeSTP17
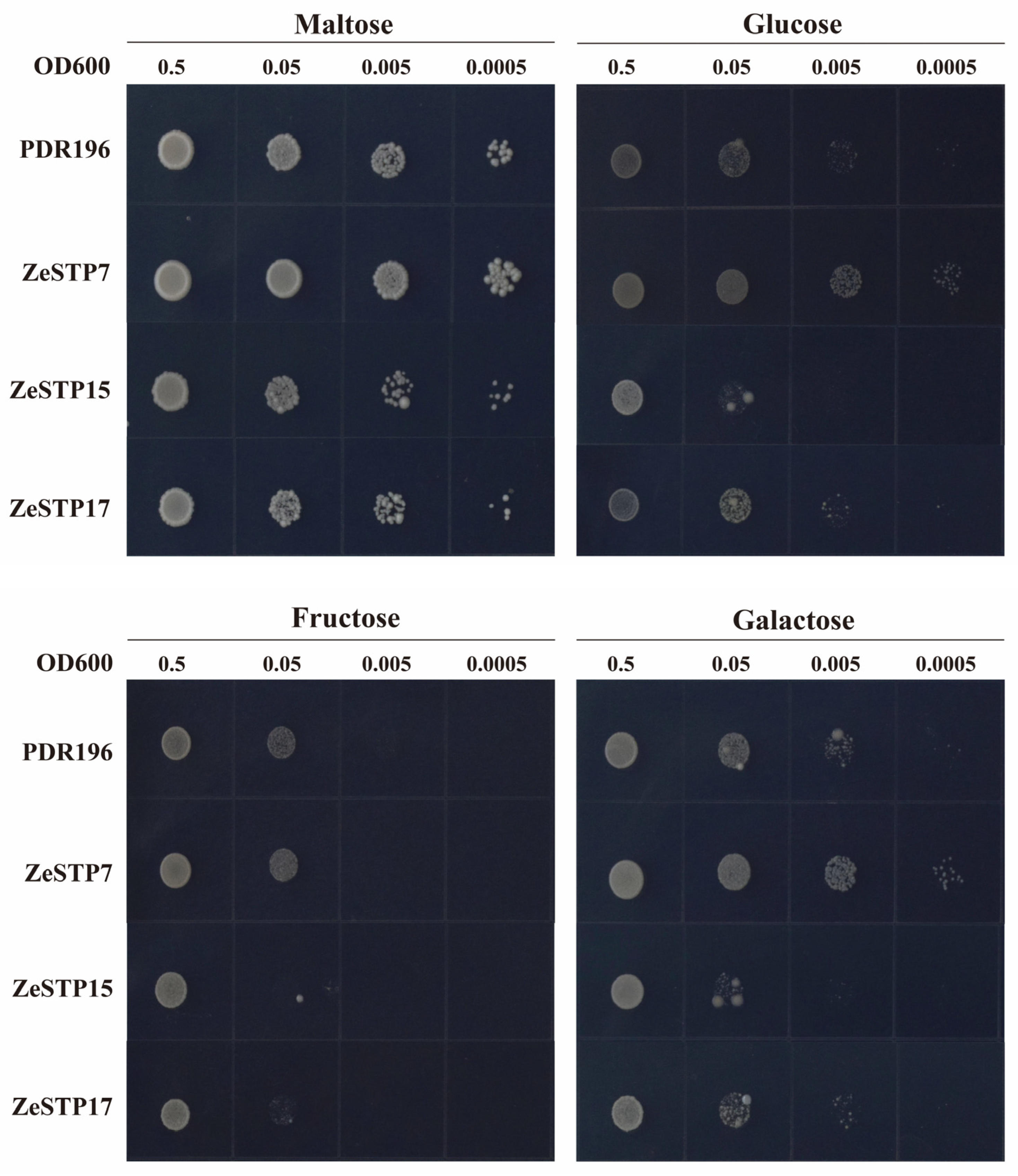
The hexose transport-deficient yeast strain EBY.VW4000, which lacks over 20 endogenous hexose transporter genes and cannot grow on SD/-Ura medium with hexoses as the carbon source was used to assess the sugar transport activity of ZeSTP proteins in calla lily. Based on genomic and transcriptomic analyses and tandem repeat detection, ZeSTP7, ZeSTP15, and ZeSTP17 were selected for functional characterization. All three proteins enabled growth on SD/-Ura medium with 2% maltose. ZeSTP7 restored growth on media containing glucose and galactose (Figure 10), indicating its capacity to transport these sugars. In contrast, ZeSTP15 and ZeSTP17 showed no growth on media with 2% fructose and only faint growth on media with 2% glucose and 2% galactose, suggesting limited transport ability for these sugars.
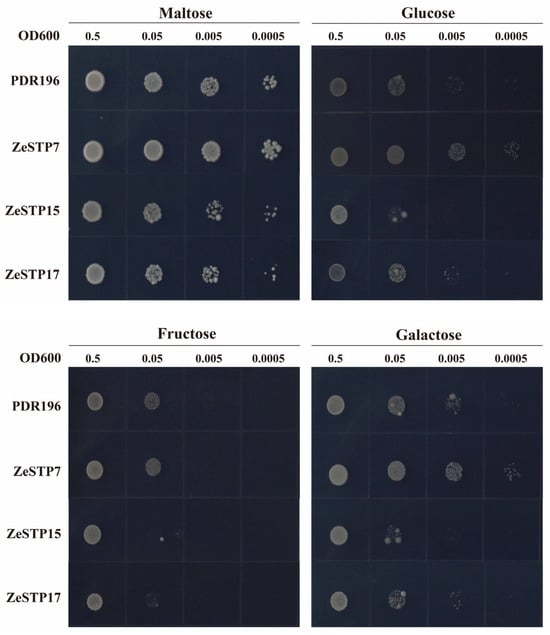
Figure 10.
Functional complementation assay of ZeSTP in hexose transporter-null yeast EBY.VW.4000. Transformants expressing PDR196 (empty vector), ZeSTP7, ZeSTP15, or ZeSTP17 were cultured on SD/-Ura medium with 2% maltose, glucose, fructose, or galactose at 30 °C for 72 h.
3. Discussion
The ZeSTP gene family is divided into five subgroups, with group III comprising only monocots. This implies that group III members were retained in monocots but lost in dicots following whole-genome duplication. In the collinearity analysis between O. sativa and Z. elliottiana, ZeSTP8 and OsSTP22 were identified as a significant collinear gene pair, while OsSTP6 (OsMST6) is homologous to ZeSTP14 and ZeSTP17. Significant collinearity indicates conserved chromosomal positions and order across species, suggesting a common ancestral origin [33,34,35]. Thus, ZeSTP8 and OsSTP22, as well as ZeSTP14 and ZeSTP17 with OsSTP6, share evolutionary relatedness. Differential selective pressures have led to motif variations among ZeSTP subgroups, while conserved domains remain stable. This suggests that despite sequence divergence, the three-dimensional structures and functional domains of ZeSTP proteins are likely conserved.
The ZeSTP gene family is unevenly distributed across the 16 chromosomes of Z. elliottiana, likely reflecting gene function, genomic evolution, and intergenic interactions. Tandem duplication events have produced gene pairs such as ZeSTP7/ZeSTP8, ZeSTP14/ZeSTP15, and ZeSTP17a-f, with ZeSTP17 retaining six tandem paralogs harboring conserved coding regions. Similar extensive tandem duplications in the ZePER gene family were reported by Wang, significantly increasing ZePER gene numbers [36]. These tandem repeats are evolutionarily stable, maintaining gene expression patterns and functions [37,38,39]. The multiple tandem repeats likely explain the high expression of ZeSTP7, ZeSTP15, and ZeSTP17 in Z. elliottiana leaves inoculated with P. carotovora subsp. carotovora, especially for ZeSTP17, suggesting their potential roles in responding to P. carotovora subsp. carotovora. Wang identified two whole-genome duplication events in Z. elliottiana, with paralogous gene pairs of PER genes on chromosomes Chr1, Chr2, Chr4, Chr5, Chr8, Chr12, Chr15, and Chr16 [36]. However, intraspecific collinearity analysis revealed no collinear blocks for ZeSTPs. Therefore, it is speculated that the ZeSTP gene family has undergone purifying selection during evolution, as evidenced by its remarkably conserved sequence architecture, suggesting their critical roles in maintaining essential biological processes within the species.
Sugar transporters in plants are primarily divided into two major categories: SWEETs and STPs. SWEETs mediate photoassimilate partitioning, seed development, and floral nectar secretion. STP proteins mediate monosaccharide transport and are key in intracellular sugar allocation and utilization. These plasma membrane H+/monosaccharide symporters transport a broad range of substrates, including glucose, galactose, fructose, and pentoses [40].
AtSTP13 is crucial for A. thaliana disease resistance, with expression upregulated upon infection by necrotrophic pathogens like B. cinerea and P. syringae. Overexpression of AtSTP13 can enhance the plant’s ability to uptake glucose from the apoplast and increase resistance to B. cinerea [12,22,41]. Similarly, VvHT5, an AtSTP13 ortholog, restricts fungal sugar utilization by promoting extracellular monosaccharide reabsorption, enhancing grapevine resistance to gray mold [42]. In this study, hexose mutant yeast assays showed that ZeSTP7 transports glucose and galactose, while ZeSTP15 and ZeSTP17 exhibit limited capacity for these sugars. Interestingly, ZeSTP7 clusters with AtSTP13 in the phylogenetic tree, and it is highly expressed following inoculation with P. carotovora subsp. carotovora, suggesting it participates in colored calla lily–P. carotovora subsp. carotovora interactions by transporting glucose and galactose. Conversely, TaSTP13, an AtSTP13 ortholog, increases wheat susceptibility to stripe rust and powdery mildew by elevating cytoplasmic hexose levels [25]. The glucose-transport-deficient mutant HvSTP13GR enhances barley resistance to leaf rust and powdery mildew [43]. Thus, STPs can enhance plant resistance by promoting defense responses or depriving pathogens of sugars, or increase susceptibility by facilitating pathogen sugar acquisition [44,45].
During plant–pathogen interactions, STP genes are activated by defense signaling components, hormonal pathways, and metabolic regulators. OsMST6 expression, upregulated by salt stress and sugar, is high during early and mid-grain formation in rice and declines thereafter [46]. Activated by the transcription factor OsERF120, OsMST6 enhances cold tolerance in rice seedlings by regulating sugar and ABA signaling pathways during cold stress [47]. Collinearity analysis between Z. elliottiana and O. sativa identified OsSTP6 (OsMST6) as homologous to ZeSTP14 and ZeSTP17. Collinear genes often share biological functions and participate in common metabolic or signaling pathways. In this study, ZeSTP17 and ZeSTP15 expression levels significantly increased following inoculation, suggesting their functional analogy to OsMST6, potentially mediating sugar transport during host–pathogen interactions through transcriptional or signaling regulation, while possibly responding to abiotic stresses.
The defense responses of plants to P. carotovora subsp. carotovora are associated with changes in gene expression and the metabolome level. These changes also can be consider as specific responses of plants to biotic stress [48]. Transcriptome analysis of Pinellia ternata shows that 20 h after infection by P. carotovora, the expression levels of genes encoding cell wall proteins and enzymes and proteins involved in defense mechanisms during biotic stress increase, while the expression levels of genes for pectin lyase, expansin, and PR1 decrease [49]. When tomato plants are infected with P. carotovora subsp. carotovora for 24 h, the expression levels of MYB, EREBP, PR3, and PR6 genes significantly increase [50]. Similarly, 24 h after inoculation of pepper plants with P. carotovora subsp. carotovora, 13 key induced defense genes (such as PR1 and PAL) are significantly upregulated [51]. In the transcriptome analysis of this study, some ZeSTPs genes, such as ZeSTP6 and ZeSTP18, responded during the 0–6 h post-inoculation period. During the 12–48 h post-inoculation period, the primary responses may trigger a series of secondary reactions, leading to the upregulation of ZeSTP7, ZeSTP15, and ZeSTP17. Alternatively, changes in ZeSTPs may induce alterations in other pathways or genes, and in the later stages of infection, ZeSTPs may act in synergy or antagonism with other pathways.
Previous studies have shown that P. carotovorum invasion activates jasmonic acid (JA), ethylene (ET), and salicylic acid (SA) signaling pathways. In transgenic Arabidopsis overexpressing the bacterial expI gene, SA-dependent defense genes PR1, NPR1, and NPR4 were significantly induced [52]. In Chinese cabbage, JA/ET-responsive transcription factors of the ERF and WRKY families, including WRKY33, were upregulated upon P. carotovora infection [53], suggesting these pathways are likely conserved in our system. Additionally, STPs may cooperate with defense genes post pathogen challenge: PDF1.2 and PAD3 were strongly induced within 48 h after Botrytis cinerea inoculation, with concurrent AtSTP13 expression [12]. The wild-type MtSTP13.1 restricts apoplastic hexose availability, thereby activating pathogenesis-related (PR) and flavonoid biosynthesis genes to enhance basal resistance against Erysiphe pisi in pea [54]. Here, it shows that ZeSTPs likely participate in Z. ellioltiana–P. carotovora subsp. carotovora interactions. This study is not an exhaustive investigation of the defense mechanisms of Z. ellioltiana against P. carotovora subsp. carotovora but rather a preliminary assessment of the response of ZeSTPs to P. carotovora subsp. carotovora. The mechanisms involved will be addressed in subsequent studies.
In this study, although our gene-expression profiling uncovered the overall regulatory trend of the ZeSTP family during soft rot infection, the pooled samples unavoidably contained both pathogen-attacked cells and adjacent, non-infected cells, as well as different tissue layers (epidermis, cortex, vasculature). Such bulk analysis masks cell-type-specific or local expression patterns and ignores the spatiotemporal dynamics of these genes. To fully elucidate the function of the ZeSTP family, future work should apply single-cell RNA-seq and/or in situ hybridization to resolve expression at the cellular scale.
4. Materials and Methods
4.1. Plant Materials and Treatments
The experimental material used was Z. elliottiana cultivar Jingcai Yangguang, grown in the greenhouse of the Beijing Academy of Agriculture and Forestry Sciences, with ambient conditions of 25 °C, 60% relative humidity, and a photoperiod of 16 h light/8 h dark.
Fully expanded leaves were cut from the base of the petiole and soaked in 0.5% sodium hypochlorite for 20 min, then rinsed with sterile distilled water and air-dried. The inoculation of P. carotovora subsp. carotovora was performed according to the method described by Luzzatto [55]. The soft rot bacteria were streaked on LB agar and cultured at 28 °C for 16 h. A single colony was selected and cultured in 4 mL of LB liquid medium at 28 °C with shaking at 150 rpm for 10 h. The bacterial culture (2 mL) was centrifuged at room temperature at 12,000 rpm for 3 min. The supernatant was discarded, and 1 mL of fresh LB liquid medium was added (C1). After vortexing, 0.1 mL was transferred to 0.9 mL of fresh LB liquid medium (C2) and vortexed again. The OD600 value was measured using a spectrophotometer. Based on the formula OD600 = 1.0 (109 CFU) [56], the concentration of C1 was calculated. C1 was diluted to a working concentration of 107 CFU/mL with double-distilled water for injection. The bacterial suspension (0.1 mL) was inoculated at four symmetrical points along the midrib on the underside of the leaf. The inoculated plant material was cultured at 28 °C, and symptoms were recorded at 0, 12, 24, and 48 h. Leaf discs (2 cm in diameter, centered on the inoculation site) were harvested; all samples were frozen in liquid nitrogen and stored at −80 °C. Lesion areas post-inoculation were quantified using ImageJ 1.54 software https://imagej.nih.gov/ij/ (accessed on 30 June 2025).
4.2. Identification and Physicochemical Property Analysis of Sugar Transporter Protein-Encoding Genes in Calla Lily
Based on the annotation file of the genome data of Z. elliottiana cv. Jingcai Yangguang [32], the protein sequences of 45 AtSTPs were used as references for BLASTp https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 May 2025) analysis [57]. These sequences were compared with the entire proteome of the colored calla lily to identify potential ZeSTP genes. The bioinformatics tool ExPASy-ProtParam http://web.expasy.org/protparam/ (accessed on 20 May 2025) was employed to analyze the coding sequence length, amino acid sequence length, molecular weight, and isoelectric point of the STP gene family in colored calla lily. The transmembrane domains of ZeSTP proteins were predicted using the online tool DeepTMHMM https://dtu.biolib.com/DeepTMHMM (accessed on 25 June 2025).
4.3. Construction of the Phylogenetic Tree and Structural Analysis of the ZeSTP Gene Family
The complete amino acid sequences of rice and A. thaliana were downloaded from the rice genome database http://plants.ensembl.org/Oryza_sativa/Info/Index (accessed on 24 June 2025) and A. thaliana database https://www.arabidopsis.org/ (accessed on 20 May 2025), respectively. The protein sequences of OsSTPs and AtSTPs were compared with those of ZeSTPs. A multiple sequence alignment of STP proteins was performed using MUSCLE, and a phylogenetic tree was constructed using the neighbor-joining method in MEGA 11 with 1000 bootstrap replicates. The tree was beautified using the online tool Evolview v4 https://evolgenius.info/evolview/#/treeview (accessed on 25 June 2025).
Based on the annotation file of the Jingcai Yangguang genome data, the gene annotation files of ZeSTPs were obtained. The gene structure patterns were visualized using TBtools-II v2.310 software [58] and combined with the phylogenetic tree of the ZeSTP gene family for presentation.
4.4. Conserved Motif and Domain Analysis of ZeSTP Proteins
The MEME Suite version 5.5.7 https://meme-suite.org/meme/ (accessed on 25 June 2025) was used to search for conserved motifs, with the maximum number of motifs set to 20. Based on the results generated by MEME, the conserved motif models were redrawn using TBtools-II. The conserved domain results of ZeSTP proteins were obtained through NCBI https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi (accessed on 20 May 2025), visualized using TBtools-II, and combined with the phylogenetic tree of the ZeSTP gene family for presentation.
4.5. Chromosomal Localization Analysis of the ZeSTP Gene Family
The chromosomal positions of ZeSTPs were obtained based on the chromosome annotation file and visualized using TBtools-II software. The ZeSTP gene family was named according to the order of their appearance on the chromosomes. The tandem gene pairs within the ZeSTP gene family were identified using the One Step MCScanX module in TBtools-II, with CPU for BlastP set to 2, E-value set to 1 × 10−3, and Number of Blast hits set to 10.
4.6. Intraspecific and Interspecific Collinearity Analysis
The whole-genome data file and gene annotation file of colored calla lily were obtained. The whole-genome data file and gene annotation file of rice were retrieved from the rice genome database (https://plants.ensembl.org/Oryza_sativa/Info/Index) (accessed on 24 June 2025). One Step MCScanX in TBtools-II was used for intraspecific collinearity analysis of colored calla lily and interspecific collinearity analysis between colored calla lily and rice, with CPU for BlastP set to 2, E-value set to 1 × 10−3, and Number of Blast hits set to 10.
4.7. Quantitative Analysis
Total RNA was extracted from the leaves of colored calla lily at 0 h, 12 h, 24 h, and 48 h post-inoculation using the EASYspin Plus Plant RNA Kit (Aidlab, Beijing, China). cDNA was synthesized according to the manufacturer’s instructions using the HiScript II First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). RT-qPCR was performed using the ChamQ SYBR qPCR Master Mix (Vazyme, China). The expression levels of ZeSTP genes were calculated using the 2−ΔΔCT method [59]. ZeActin was used as a reference gene to normalize the expression levels of ZeSTP genes. Primers were designed using Primer Premier 5 (Table A1).
4.8. Expression Analysis of ZeSTP Genes in Colored Calla Lily After P. carotovora subsp. carotovora Inoculation
Based on the transcriptome data of colored calla lily ‘Jingcai Yangguang’ [32], the expression heatmap of ZeSTP genes in the transcriptome after inoculation was created using TBtools-II software. The expression levels of ZeSTP genes after P. carotovora subsp. carotovora inoculation were measured by RT-qPCR, with three independent replicates for each gene at each time point. ZeSTP genes from 0 to 48 h post-inoculation were plotted using GraphPad Prism 9.5, and significant differences were marked at * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 using independent t-tests.
4.9. Sugar Transport Activity of ZeSTP7, ZeSTP14, and ZeSTP17 in Hexose-Mutant Yeast
To investigate the heterologous expression of ZeSTPs in yeast, pDR196, pDR196-ZeSTP7, pDR196-ZeSTP15, and pDR196-ZeSTP17 were transformed into the hexose-mutant yeast strain EBY.VW.4000, respectively [60]. The transformed yeast cultures were adjusted to an OD600 of 0.5 and diluted 10, 100, and 1000 times. Then, the yeast cultures were spotted on SD/-Ura media containing 2% maltose, 2% glucose, 2% fructose, and 2% galactose, respectively. The media were incubated at 30 °C for 3 days, and the growth of yeast was observed.
5. Conclusions
In summary, 18 ZeSTP genes were identified from colored calla lily and divided into five subgroups, all predicted to encode membrane-localized transporters. Genomic analyses revealed nine tandem duplication events within the ZeSTP family and three collinear orthologs with OsSTPs. Temporal expression profiling under the P. carotovora subsp. carotovora inoculation demonstrated significant upregulation of ZeSTP7, ZeSTP15, and ZeSTP17. Functional complementation in hexose transporter-deficient yeast confirmed ZeSTP7-mediated glucose/galactose transport, while ZeSTP15/17 exhibited limited substrate specificity. Integrated characterization—including physicochemical profiling, phylogenetic reconstruction, and synteny analysis—provides evolutionary insights into ZeSTP family expansion. These findings establish a foundation for functional dissection of ZeSTP genes in colored calla lily–pathogen interactions.
Author Contributions
Z.Z. and X.H. conceived the study. X.H. and Z.Z. analyzed the data. X.H., Z.Z., Y.L., Y.W., M.Z., L.W., W.T., D.C. and G.Z. performed the experiments. X.H. and Z.Z. wrote the paper. X.H., Z.Z. and Z.W. edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Special Project for the Construction of Industrial Technology Research Platforms and the Enhancement of Technological Capabilities (CYJS202502), the National Natural Science Foundation of China (32071812), and Beijing Academy of Agriculture and Forestry Sciences Specific Projects for Building Technology Innovation Capacity (KJCX20230108/KJCX20230203/KJCX20251312).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Beijing Academy of Agriculture and Forestry Sciences for providing a platform for our experiment and all those who contributed to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
The primer sequences for RT-qPCR used in this study.
Table A1.
The primer sequences for RT-qPCR used in this study.
| Primer | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| ZeSTP1-qRT | TGCCACCTCTGCCATCAT | GCCAGTTGGGCTTGCTAT |
| ZeSTP2-qRT | CTGCTTCCTCGCCTACCA | GCGTCCCACCTGTTGTTA |
| ZeSTP3-qRT | CGTTGGCATCGGTTTCGC | TCCGCCCAGGATCTTGTT |
| ZeSTP4-qRT | GGGGAGGTCAGGGAAGTA | CAGTTGGAAGCCGATGTT |
| ZeSTP5-qRT | CTAGTGCTATGTGCGCTCTTT | GCTCTTGCTCTGACCCTGT |
| ZeSTP6-qRT | GGGGAGGTCGGGAAAGTA | AAGATGGAGCCAAGGATGA |
| ZeSTP7-qRT | CATCGCACAAGCCTTCCTC | GATGGGCACGTTCTTGGTCT |
| ZeSTP8-qRT | GGTCTTCCAGCAGTTCACG | GACGCAGGCTTCTAGCAAT |
| ZeSTP9-qRT | GCTTCGCAAACCAGTCGG | TCTTGTTGGCAGCGTAGTTCA |
| ZeSTP10-qRT | GAGATGGCAAGGCAGGTCC | CACGGGCGAGTAGAACAGG |
| ZeSTP11-qRT | GCATAGTAGCGTCCAGTCG | CTTGGCGGTGTTGTAGTTC |
| ZeSTP12-qRT | TAACGTCTTCGCCACCTTCG | ATTTGCCCTCGCCGCTCA |
| ZeSTP13-qRT | TGGAGCCCGCTGAAAGGT | TGGCGTGTCAATGTATCG |
| ZeSTP14-qRT | CCAACATCCTGAAGCGAAAG | CGGTGATAACGGCGGACA |
| ZeSTP15-qRT | CGACATCCACGCCGAGTT | GGAAGAAGGGAATGAGCACC |
| ZeSTP16-qRT | ACTGGCGGTGCTACTGCTGA | AGGAACACGCCGTACTTGAGG |
| ZeSTP17-qRT | CATCGGGCGTATCCTTCTG | ATCTTGTTGGCTGCGTAGTTC |
| ZeSTP18-qRT | GGCTACGACATCGGCATTT | TCTTGGACTGGGAGTTCTTCTT |
| ZeActin-qRT | ATTTATGAGGGTTATGCTCTTCC | GGAGGAACTGCTCTTGGCTGTCT |
| pDR196 | CCCAGTCACGACGTTGTAAAACG | |
| pDR196-STP7 | tcccccgggctgcaggaattcATGCCGGCCGGCGGGTTC | ggtaccgggccccccctcgagGTTGGCACCGTTGTGGCC |
| pDR196-STP15 | tcccccgggctgcaggaattcATGGCGGGCGGGGCGTTC | ggtaccgggccccccctcgagGACCACATAGGCGGTCTTCTTG |
| pDR196-STP17 | tcccccgggctgcaggaattcATGGCCGGGGGAGCCTTC | ggtaccgggccccccctcgagTACATGGCCGTGGGCCCC |
References
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of soluble sugars in metabolism and sensing under abiotic stress. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 305–334. ISBN 978-3-030-61152-1. [Google Scholar]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Yu, F.; Tang, J.; Yu, L.; Wang, H.; Li, J. Genome-wide identification and expression profiling of sugar transporter protein (STP) family genes in cabbage (Brassica oleracea var. capitata L.) reveals their involvement in clubroot disease responses. Genes 2019, 10, 71. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Chen, C.; Ma, F.; Li, M. The Malus domestica sugar transporter gene family: Identifications based on genome and expression profiling related to the accumulation of fruit sugars. Front. Plant Sci. 2014, 5, 569. [Google Scholar] [CrossRef]
- Büttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12, 35–41. [Google Scholar] [CrossRef]
- Sherson, S.M.; Hemmann, G.; Wallace, G.; Forbes, S.; Germain, V.; Stadler, R.; Bechtold, N.; Sauer, N.; Smith, S.M. Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J. 2000, 24, 849–857. [Google Scholar] [CrossRef]
- Stadler, R.; Büttner, M.; Ache, P.; Hedrich, R.; Ivashikina, N.; Melzer, M.; Shearson, S.M.; Smith, S.M.; Sauer, N. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol. 2003, 133, 528–537. [Google Scholar] [CrossRef]
- Otori, K.; Tanabe, N.; Tamoi, M.; Shigeoka, S. Sugar transporter protein 1 (STP1) contributes to regulation of the genes involved in shoot branching via carbon partitioning in Arabidopsis. Biosci. Biotechnol. Biochem. 2019, 83, 472–481. [Google Scholar] [CrossRef]
- Rottmann, T.; Klebl, F.; Schneider, S.; Kischka, D.; Rüscher, D.; Sauer, N.; Stadler, R. Sugar transporter STP7 specificity for L-Arabinose and d-Xylose contrasts with the typical hexose transporters STP8 and STP12. Plant Physiol. 2018, 176, 2330–2350. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, P.; Gaillard, C.; Veillet, F.; Verbeke, J.; Lemoine, R.; Coutos-Thévenot, P.; La Camera, S. Expression of arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea. Plant Mol. Biol. 2014, 85, 473–484. [Google Scholar] [CrossRef]
- Schneidereit, A.; Scholz-Starke, J.; Büttner, M. Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis. Plant Physiol. 2003, 133, 182–190. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Büttner, M.; Sauer, N. AtSTP6, a new pollen-specific H+-Monosaccharide symporter from Arabidopsis. Plant Physiol. 2003, 131, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Truernit, E.; Schmid, J.; Epple, P.; Illig, J.; Sauer, N. The sink-specific and stress-regulated Arabidopsis STP4 gene: Enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 1996, 8, 2169–2182. [Google Scholar] [CrossRef] [PubMed]
- Vignault, C.; Vachaud, M.; Cakir, B.; Glissant, D.; Dédaldéchamp, F.; Büttner, M.; Atanassova, R.; Fleurat-Lessard, P.; Lemoine, R.; Delrot, S. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J. Exp. Bot. 2005, 56, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dang, H.; Chen, Z.; Wu, J.; Chen, Y.; Chen, S.; Luo, L. Genome-wide identification, expression, and functional analysis of the sugar transporter gene family in cassava (Manihot esculenta). Int. J. Mol. Sci. 2018, 19, 987. [Google Scholar] [CrossRef]
- Büttner, M.; Sauer, N. Monosaccharide transporters in plants: Structure, function and physiology. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1465, 263–274. [Google Scholar] [CrossRef]
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar transporter proteins (STPs) in gramineae crops: Comparative analysis, phylogeny, evolution, and expression profiling. Cells 2019, 8, 560. [Google Scholar] [CrossRef]
- Doidy, J.; Grace, E.; Kühn, C.; Simon-Plas, F.; Casieri, L.; Wipf, D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012, 17, 413–422. [Google Scholar] [CrossRef]
- Endler, A.; Meyer, S.; Schelbert, S.; Schneider, T.; Weschke, W.; Peters, S.W.; Keller, F.; Baginsky, S.; Martinoia, E.; Schmidt, U.G. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006, 141, 196–207. [Google Scholar] [CrossRef]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 354, 1427–1430. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Z.; Cao, H.; Zhou, F.; Si, H.; Zang, J.; Xing, J.; Zhang, K.; Dong, J. Identification and expression analysis of sugar transporter family genes reveal the role of ZmSTP2 and ZmSTP20 in maize disease resistance. J. Integr. Agric. 2023, 22, 3458–3473. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Tan, L.; Huai, B.; Ma, X.; Pan, Q.; Zheng, P.; Wen, Y.; Zhang, Q.; Zhao, Q.; et al. AtSTP8, an endoplasmic reticulum-localised monosaccharide transporter from Arabidopsis, is recruited to the extrahaustorial membrane during powdery mildew infection. New Phytol. 2021, 230, 2404–2419. [Google Scholar] [CrossRef]
- Huai, B.; Yang, Q.; Wei, X.; Pan, Q.; Kang, Z.; Liu, J. TaSTP13 contributes to wheat susceptibility to stripe rust possibly by increasing cytoplasmic hexose concentration. BMC Plant Biol. 2020, 20, 49. [Google Scholar] [CrossRef]
- Huai, B.; Yang, Q.; Qian, Y.; Qian, W.; Kang, Z.; Liu, J. ABA-induced sugar transporter TaSTP6 promotes wheat susceptibility to stripe rust. Plant Physiol. 2019, 181, 1328–1343. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qiao, L.; Hu, L.; Wang, X.; Wang, J.; Ruan, X.; Yang, G.; Yin, G.; Wang, C.; et al. The sugar transporter family in wheat (Triticum aestivum. L): Genome-wide identification, classification, and expression profiling during stress in seedlings. PeerJ 2021, 9, e11371. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; Thakur, M.; Rakwal, A.; Chauhan, S.; Bhargava, B. Exogenous applications of gibberellic acid modulate the growth, flowering and longevity of calla lily. Heliyon 2023, 9, e16319. [Google Scholar] [CrossRef]
- Niyokuri, A.N.; Nyalala, S. Calla lily soft rot causal agents, symptoms, virulence and management: A review. Int. J. Hortic. Sci. 2023, 29, 60–68. [Google Scholar] [CrossRef]
- He, P.; Cui, W.; He, P.; Munir, S.; Li, X.; Wu, Y.; Li, Y.; Asad, S.; He, P.; He, Y. Bacillus amyloliquefaciens subsp. plantarum KC-1 inhibits Zantedeschia hybrida soft rot and promote plant growth. Biol. Control 2021, 154, 104500. [Google Scholar] [CrossRef]
- Wright, P.J.; Triggs, C.M. Factors affecting bacterial soft rot of Zantedeschia tubers. N. Z. J. Crop Hortic. Sci. 2009, 37, 345–350. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Wang, D.; Gou, R.; Jiang, Y.; Zhang, G.; Zheng, Y.; Gao, D.; Chen, L.; Zhang, X.; et al. Chromosome level genome assembly of colored calla lily (Zantedeschia elliottiana). Sci. Data 2023, 10, 605. [Google Scholar] [CrossRef]
- Elemento, O.; Gascuel, O.; Lefranc, M.-P. Reconstructing the duplication history of tandemly repeated genes. Mol. Biol. Evol. 2002, 19, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, W.; Zhang, L.; Li, D.-Z.; Qi, J.; Ma, H. Phylogenomic profiles of whole-genome duplications in poaceae and landscape of differential duplicate retention and losses among major poaceae lineages. Nat. Commun. 2024, 15, 3305. [Google Scholar] [CrossRef]
- Ma, P.-F.; Liu, Y.-L.; Jin, G.-H.; Liu, J.-X.; Wu, H.; He, J.; Guo, Z.-H.; Li, D.-Z. The pharus latifolius genome bridges the gap of early grass evolution. Plant Cell 2021, 33, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Yang, T.; Gou, R.; Jiang, Y.; Zeng, Z.; Zhang, G.; Wei, Z. Genome-wide identification of class III peroxidases in colored calla lily and enhanced resistance to soft rot bacteria. Physiol. Mol. Plant Pathol. 2024, 130, 102236. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Guo, L.; Lu, S.; Liu, T.; Nai, G.; Ren, J.; Gou, H.; Chen, B.; Mao, J. Genome-wide identification and abiotic stress response analysis of PP2C gene family in woodland and pineapple strawberries. Int. J. Mol. Sci. 2023, 24, 4049. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, W.; Zhou, J.; Li, H.; Xu, X.; Zhang, B.; Gao, X. Repetitive DNA sequence detection and its role in the human genome. Commun. Biol. 2023, 6, 954. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; An, B.; Zhong, H.; Yang, J.; Kong, W.; Li, Y. A novel insight into functional divergence of the MST gene family in rice based on comprehensive expression patterns. Genes 2019, 10, 239. [Google Scholar] [CrossRef]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Monnereau, B.; Gaillard, C.; Maslard, C.; Noceto, P.-A.; Lebeurre, V.; Cantereau, A.; Coutos-Thévenot, P.; La Camera, S. Ectopic expression of the grape hexose transporter VvHT5 restores STP13-deficiency in Arabidopsis and promotes fungal resistance to Botrytis cinerea. Plant Pathol. 2025, 74, 493–506. [Google Scholar] [CrossRef]
- Skoppek, C.I.; Punt, W.; Heinrichs, M.; Ordon, F.; Wehner, G.; Boch, J.; Streubel, J. The barley HvSTP13GR mutant triggers resistance against biotrophic fungi. Mol. Plant Pathol. 2022, 23, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miller, A.J.; Qiu, B.; Huang, Y.; Zhang, K.; Fan, G.; Liu, X. The role of sugar transporters in the battle for carbon between plants and pathogens. Plant Biotechnol. J. 2024, 22, 2844–2858. [Google Scholar] [CrossRef]
- Voegele, R.T.; Mendgen, K.W. Nutrient uptake in rust fungi: How Sweet is parasitic life? Euphytica 2011, 179, 41–55. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Zhang, Y.; Chai, C.; Wei, G.; Wei, X.; Xu, H.; Wang, M.; Ouwerkerk, P.B.F.; Zhu, Z. Molecular cloning, functional characterization and expression analysis of a novel monosaccharide transporter gene OsMST6 from rice (Oryza sativa L.). Planta 2008, 228, 525–535. [Google Scholar] [CrossRef]
- Luo, S.; Zheng, S.; Li, Z.; Cao, J.; Wang, B.; Xu, Y.; Chong, K. Monosaccharide transporter OsMST6 is activated by transcription factor OsERF120 to enhance chilling tolerance in rice seedlings. J. Exp. Bot. 2024, 75, 4038–4051. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Strekalovskaya, E.I.; Klushina, N.V.; Gorbenko, I.V.; Krutovsky, K.V. The causative agent of soft rot in plants, the phytopathogenic bacterium Pectobacterium carotovorum subsp. carotovorum: A brief description and an overview of methods to control it. Agronomy 2025, 15, 1578. [Google Scholar] [CrossRef]
- Shu, F.; Han, J.; Ndayambaje, J.P.; Jia, Q.; Sarsaiya, S.; Jain, A.; Huang, M.; Liu, M.; Chen, J. Transcriptomic analysis of Pinellia ternata (Thunb.) Breit T2 plus line provides insights in host responses resist Pectobacterium carotovorum infection. Bioengineered 2021, 12, 1173–1188. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Matsaunyane, L.B.T.; Ntushelo, K. Gene Expression Responses of tomato inoculated with Pectobacterium carotovorum subsp. carotovorum. MicrobiologyOpen 2019, 8, e911. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Matsaunyane, L.B.T.; Kalu, C.M.; Ntushelo, K. Gene expression and evidence of coregulation of the production of some metabolites of chilli pepper inoculated with Pectobacterium carotovorum ssp. carotovorum. Funct. Plant Biol. 2019, 46, 1114–1122. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hong, J.-B.; Hong, S.-B.; Choi, M.-S.; Jeong, K.-Y.; Park, H.-J.; Hwang, D.-J.; Lee, S.-D.; Ra, D.-S.; Heu, S.-G. Disease-resistant transgenic Arabidopsis carrying the expI gene from Pectobacterium carotovorum subsp. carotovorum SL940. Plant Pathol. J. 2008, 24, 183–190. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, F.; Li, X.; Ma, R.; Xie, H. Jasmonic scid and ethylene signaling pathways participate in the defense response of Chinese cabbage to Pectobacterium carotovorum infection. J. Integr. Agric. 2021, 20, 1314–1326. [Google Scholar] [CrossRef]
- Gupta, M.; Dubey, S.; Jain, D.; Chandran, D. The Medicago truncatula sugar transport protein 13 and its Lr67res-like variant confer powdery mildew resistance in legumes via defense modulation. Plant Cell Physiol. 2021, 62, 650–667. [Google Scholar] [CrossRef]
- Luzzatto, T.; Yishay, M.; Lipsky, A.; Ion, A.; Belausov, E.; Yedidia, I. Efficient, long-lasting resistance against the soft rot bacterium Pectobacterium carotovorum in calla lily provided by the plant activator methyl jasmonate. Plant Pathol. 2007, 56, 692–701. [Google Scholar] [CrossRef]
- Yishay, M.; Burdman, S.; Valverde, A.; Luzzatto, T.; Ophir, R.; Yedidia, I. Differential pathogenicity and genetic diversity among Pectobacterium carotovorum ssp. carotovorum isolates from monocot and dicot hosts support early genomic divergence within this taxon. Environ. Microbiol. 2008, 10, 2746–2759. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.W. Using the basic local alignment search tool (BLAST). Cold Spring Harb. Protoc. 2007, 2007, pdb.top17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).