Potassium-Mediated Variations in the Photosynthetic Induction Characteristics of Phaseolus vulgaris L.
Abstract
1. Introduction
2. Results
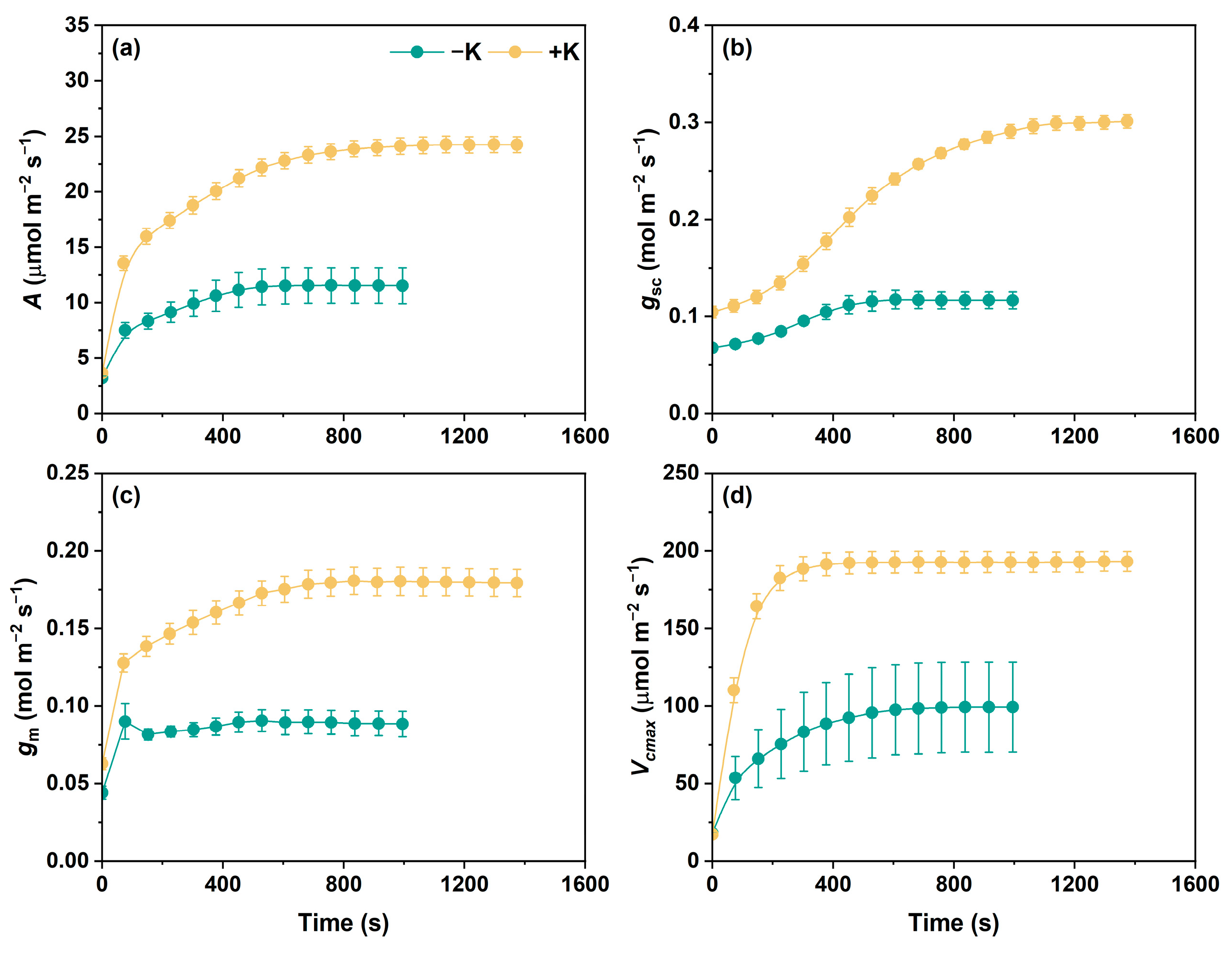
2.1. Photosynthetic Parameter Variations During Photosynthetic Induction
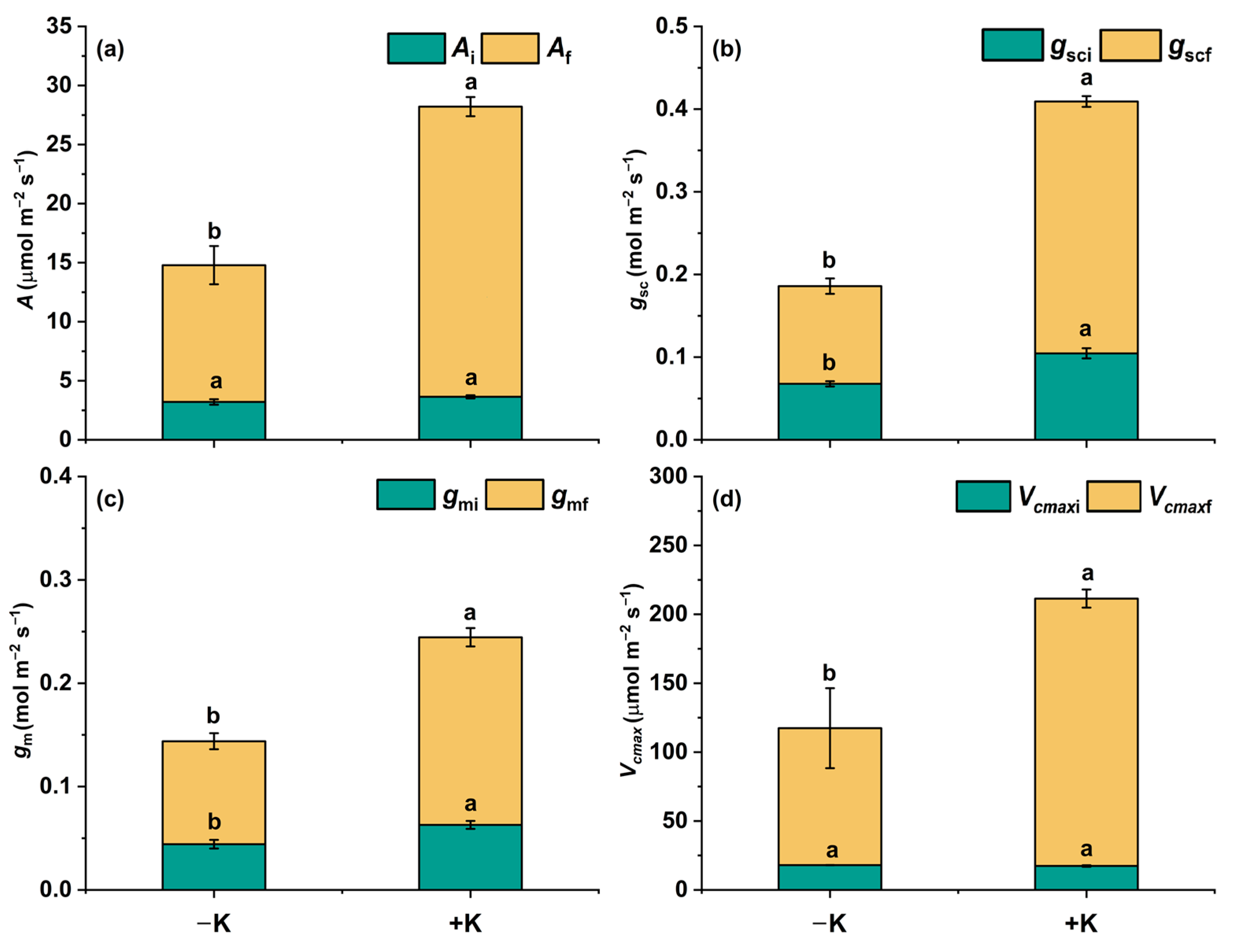
2.2. Steady Changes in Photosynthetic Traits During Photosynthetic Induction
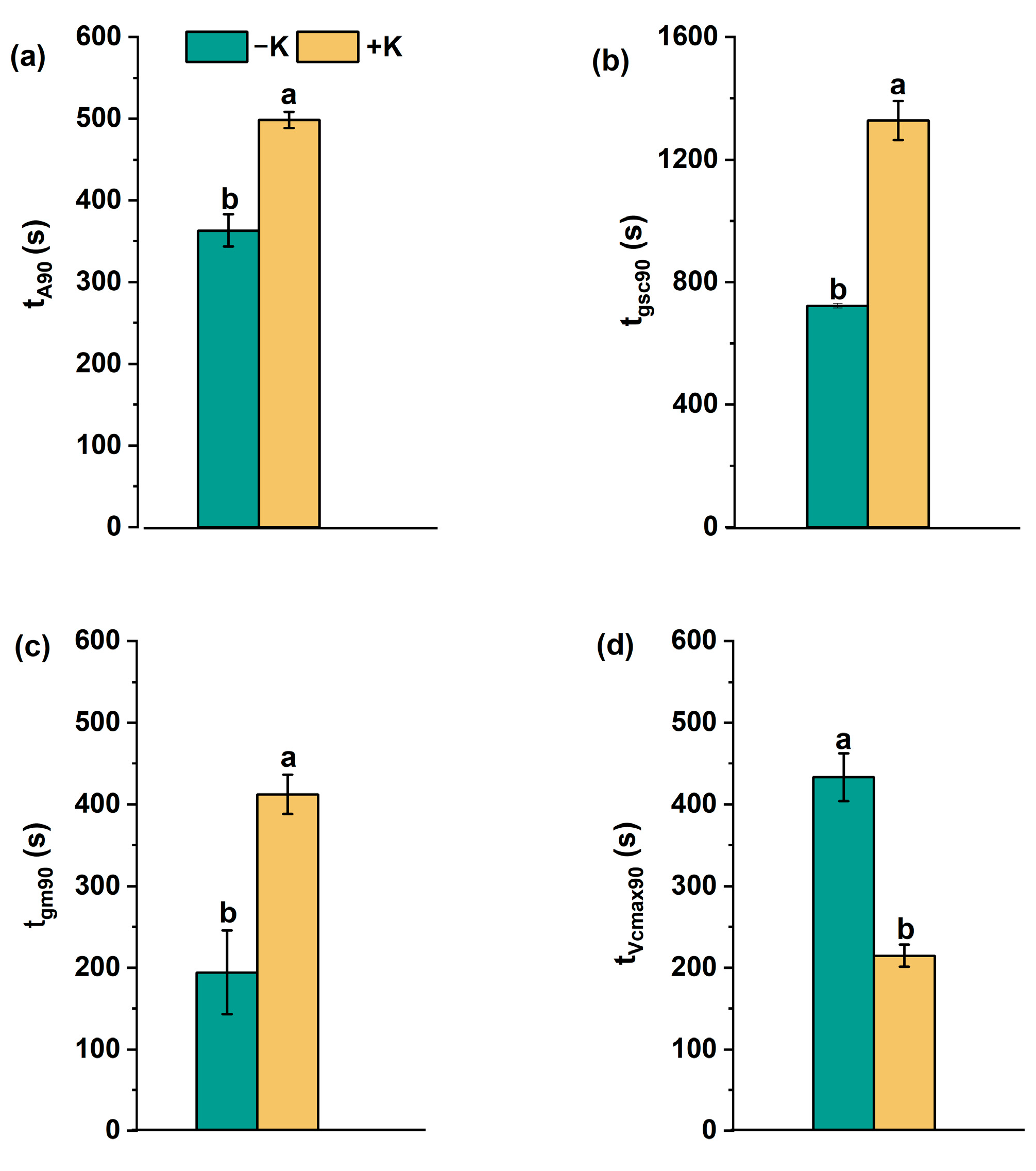
2.3. Dynamic Changes in Photosynthetic Traits During Photosynthetic Induction
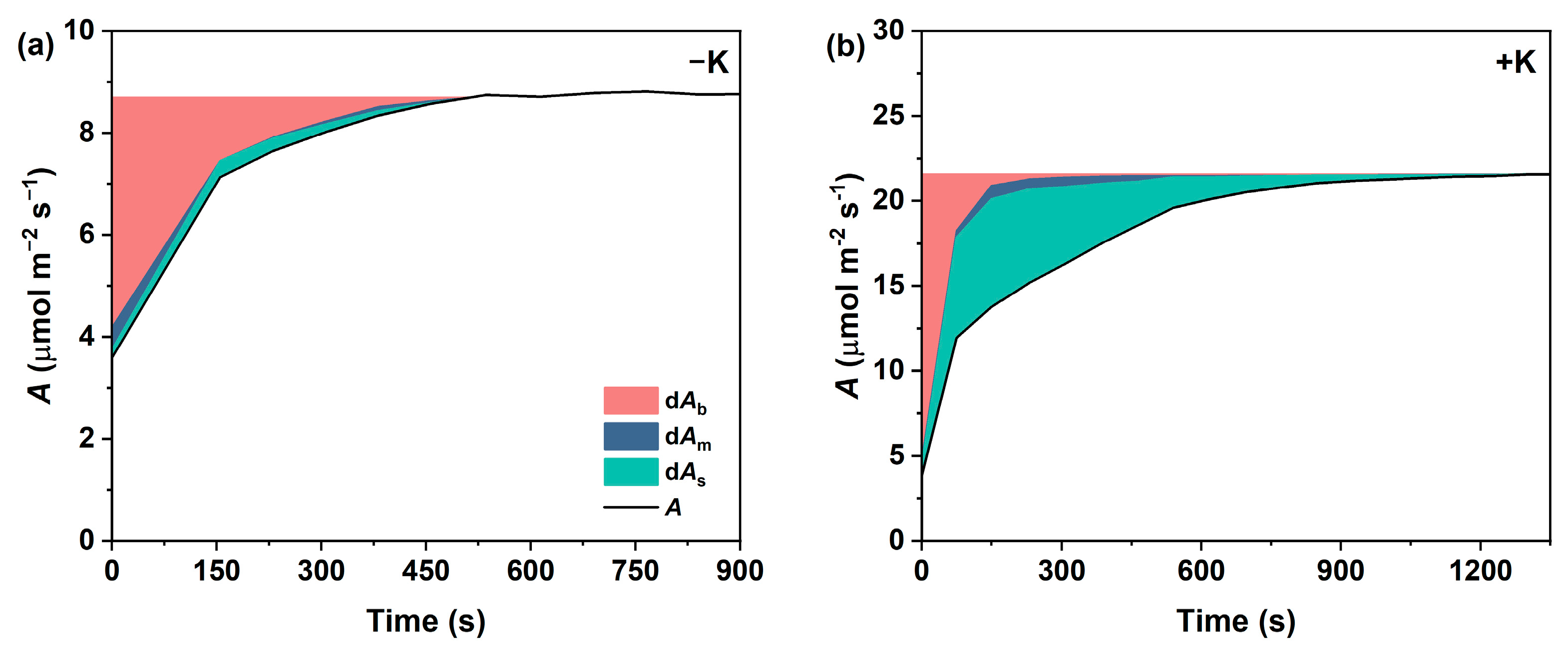
2.4. Temporal Responses of Photosynthetic Limitations During Photosynthetic Induction
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Measurement of Gas Exchange and Fluorescence
4.3. Estimation of Mesophyll Conductance (gm)
4.4. Calculations of the Maximum Carboxylation Rate of Rubisco (Vcmax)
4.5. Determination of Photosynthetic Induction Parameters
4.6. Limitation Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | net photosynthetic rate |
| gsc | stomatal conductance to CO2 |
| gm | mesophyll conductance to CO2 |
| Vcmax | maximum carboxylation rate |
| ΦPSII | actual phytochemical efficiency of PSII |
| Ai | steady-state A during the last 1 min of low-light induction period |
| gsi | steady-state gsc during the last 1 min of low-light induction period |
| gmi | steady-state gm during the last 1 min of low-light induction period |
| Vcmaxi | steady-state Vcmax during the last 1 min of low-light induction period |
| Af | steady-state A during the last 1 min of high-light induction period |
| gsf | steady-state gsc during the last 1 min of high-light induction period |
| gmf | steady-state gm during the last 1 min of high-light induction period |
| Vcmaxf | steady-state Vcmax during the last 1 min of high-light induction period |
| tA90 | time for A to reach 90% of photosynthetic induction |
| tgs90 | time for gsc to reach 90% of photosynthetic induction |
| tgm90 | time for gm to reach 90% of photosynthetic induction |
| tVcmax90 | time for Vcmax to reach 90% of photosynthetic induction |
| dAb | transient biochemical limitation during photosynthetic induction |
| dAm | transient mesophyll conductance limitation during photosynthetic induction |
| dAs | transient stomatal conductance limitation during photosynthetic induction |
| σb | time-integrated biochemical limitation during photosynthetic induction |
| σm | time-integrated mesophyll conductance limitation during photosynthetic induction |
| σs | time-integrated transient stomatal conductance limitation during photosynthetic induction |
References
- Rawat, J.; Pandey, N.; Saxena, J. Role of potassium in plant photosynthesis, transport, growth and yield. In Role of Potassium in Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Gu, H.; Ye, X.; Li, X.; Cong, R.; Ren, T.; Lu, J. Potassium availability influences the mesophyll structure to coordinate the conductance of CO2 and H2O during leaf expansion. Plant. Cell Environ. 2022, 45, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ren, T.; Pan, Y.; Li, X.; Cong, R.; Lu, J. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci. Rep. 2016, 6, 21725. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef]
- Sawhney, B.; Zelitch, I. Direct determination of potassium ion accumulation in guard cells in relation to stomatal opening in light. Plant Physiol. 1969, 44, 1350. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, K.; Pan, Y.; Ren, T.; Lu, J.; Wang, M.; Shen, Q.; Guo, S. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 2019, 42, 2231–2244. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, Y.; Chai, Y.; Meng, X.; Wang, M.; Wang, G.; Guo, S. Identification of photosynthetic parameters for superior yield of two super hybrid rice varieties: A cross-scale study from leaf to canopy. Front. Plant Sci. 2023, 14, 1110257. [Google Scholar] [CrossRef]
- Xiong, D.; Huang, J.; Peng, S.; Li, Y. A few enlarged chloroplasts are less efficient in photosynthesis than a large population of small chloroplasts in Arabidopsis thaliana. Sci. Rep. 2017, 7, 5782. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, J.; Pan, Y.; Lu, P.; Li, X.; Cong, R.; Ren, T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016, 39, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ye, X.; Song, Y.; Ren, T.; Wang, C.; Li, X.; Lu, J. Sufficient potassium improves inorganic phosphate-limited photosynthesis in Brassica napus by enhancing metabolic phosphorus fractions and Rubisco activity. Plant J. 2023, 113, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pan, Y.; Hu, W.; Cong, R.; Ren, T.; Guo, S.; Lu, J. The photosynthetic and structural differences between leaves and siliques of Brassica napus exposed to potassium deficiency. BMC Plant Biol. 2017, 17, 240. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Chen, X.; Han, H.; Shen, B.; Diao, M.; Liu, H. Exogenous selenium promotes the growth of salt-stressed tomato seedlings by regulating ionic homeostasis, activation energy allocation and CO2 assimilation. Front. Plant Sci. 2023, 14, 1206246. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Liang, Y.; Han, Y.; Han, Y.; Tan, J. High potassium application rate increased grain yield of shading-stressed winter wheat by improving photosynthesis and photosynthate translocation. Front. Plant Sci. 2020, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 2019, 19, 302. [Google Scholar] [CrossRef]
- Qi, D.; Zhao, X.; Le, X.; Jiang, C.; Wang, X.; Yi, H.; Yu, H. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agr. 2019, 18, 395–406. [Google Scholar]
- Qi, D.U.; Zhao, X.H.; Le, X.I.A.; Jiang, C.J.; Wang, X.G.; Yi, H.A.N.; Jing, W.A.N.G.; Yu, H.Q. A canopy conundrum: Can wind-induced movement help to increase crop productivity by relieving photosynthetic limitations? J. Exp. Bot. 2019, 70, 2371–2380. [Google Scholar]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Groszmann, M.; Evans, J. Stomatal, mesophyll conductance, and biochemical limitations to photosynthesis during induction. Plant Physiol. 2021, 185, 146–160. [Google Scholar] [CrossRef]
- Liu, T.; Barbour, M.; Yu, D.; Rao, S.; Song, X. Mesophyll conductance exerts a significant limitation on photosynthesis during light induction. N. Phytol. 2021, 233, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Z.; Shi, Z.; Wang, J.; Huang, W. Variation in photosynthetic efficiency under fluctuating light between rose cultivars and its potential for improving dynamic photosynthesis. Plants 2023, 12, 1186. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhao, X.H.; Jiang, C.J.; Li, C.H.; Cong, S.; Wu, D.; Chen, Y.Q.; Yu, H.Q.; Wang, C.Y. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J. Integr. Agric. 2015, 14, 856–863. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.; Bednarz, C. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Sakoda, K.; Taniyoshi, K.; Yamori, W.; Tanaka, Y. Drought stress reduces crop carbon gain due to delayed photosynthetic induction under fluctuating light conditions. Physiol. Plant. 2022, 174, e13603. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G.; Mikona, C. Photosynthetic Induction and leaf carbon gain in the Tropical understorey Epiphyte, Aspasia principissa. Ann. Bot. 2003, 91, 353–359. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Zhang, S.; Huang, W. Photosynthetic induction under fluctuating light is affected by leaf nitrogen content in tomato. Front. Plant Sci. 2022, 13, 835571. [Google Scholar] [CrossRef]
- Chen, J.W.; Kuang, S.; Long, G.; Meng, Z.; Li, L.; Chen, Z.; Zhang, G.; Yang, S. Steady-state and dynamic photosynthetic performance and nitrogen partitioning in the shade-demanding plant Panax notoginseng under different levels of growth irradiance. Acta Physiol. Plant. 2014, 36, 2409–2420. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Marcelis, L.; Yang, Q.; Li, T. Salt stress and fluctuating light have separate effects on photosynthetic acclimation, but interactively affect biomass. Plant Cell Environ. 2020, 43, 2192–2206. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. N. Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, W.; Huang, L.; Wang, D.; Xu, K.; Wei, Y. Photosynthetic Induction Characteristics in Saplings of Four Sun-Demanding Trees and Shrubs. Plants 2025, 14, 144. [Google Scholar] [CrossRef]
- Imtiaz, H.; Mir, A.; Corpas, F.; Hayat, S. Impact of potassium starvation on the uptake, transportation, photosynthesis, and abiotic stress tolerance. Plant Growth Regul. 2023, 99, 429–448. [Google Scholar] [CrossRef]
- Humble, G.; Hsiao, T. Light-dependent influx and efflux of potassium of guard cells during stomatal opening and closing. Plant Physiol. 1970, 46, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Nagarajah, S. The effect of potassium deficiency on stomatal and cuticular resistance in tea (Camellia sinensis). Physiol. Plant. 1979, 47, 91–94. [Google Scholar] [CrossRef]
- Christina, M.; Le Maire, G.; Battie-Laclau, P.; Nouvellon, Y.; Bouillet, J.P.; Jourdan, C.; de Moraes Gonçalves, J.L.; Laclau, J.P. Measured and modeled interactive effects of potassium deficiency and water deficit on gross primary productivity and light-use efficiency in Eucalyptus grandis plantations. Global Chang. Biol. 2015, 21, 2022–2039. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, Y.; Xiao, K.; Wei, Y.; Zhu, Y.; Cai, Q.; Chen, L.; Xie, H.; Zhang, J. OsPRX2 contributes to stomatal closure and improves potassium deficiency tolerance in rice. Biochem. Biophy. Res. Commun. 2018, 495, 461–467. [Google Scholar] [CrossRef]
- Arquero, O.; Barranco, D.; Benlloch, M. Potassium starvation increases stomatal conductance in olive trees. HortScience 2006, 41, 433–436. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 181–188. [Google Scholar]
- Cui, J.; Tcherkez, G. Potassium dependency of enzymes in plant primary metabolism. Plant Physiol. Biochem. 2021, 166, 522–530. [Google Scholar] [CrossRef]
- Hu, W.; Ren, T.; Meng, F.; Cong, R.; Li, X.; White, P.; Lu, J. Leaf photosynthetic capacity is regulated by the interaction of nitrogen and potassium through coordination of CO2 diffusion and carboxylation. Physiol. Plant. 2019, 167, 418–432. [Google Scholar] [CrossRef]
- Deans, R.; Farquhar, G.; Busch, F. Estimating stomatal and biochemical limitations during photosynthetic induction. Plant Cell Environ. 2019, 42, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, Q.; Wei, X.; Peng, S.; Li, Y. Nitrogen can alleviate the inhibition of photosynthesis caused by high temperature stress under both steady-state and flecked irradiance. Front. Plant Sci. 2017, 8, 945. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, Y.; Li, Y.N.; Liang, Y.; Sun, Q.; Li, G.; Liu, P.; Zhang, Z.S.; Gao, H.Y. Dynamic light caused less photosynthetic suppression, rather than more, under nitrogen deficit conditions than under sufficient nitrogen supply conditions in soybean. BMC Plant Biol. 2020, 20, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Berman, S.; Berg, T.; Chen, Y.; Marcelis, L.; Kaiser, E. Biochemical versus stomatal acclimation of dynamic photosynthetic gas exchange to elevated CO2 in three horticultural species with contrasting stomatal morphology. Plant Cell Environ. 2024, 47, 4516–4529. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.; Portis, A.; Nakano, H.; Von Caemmerer, S.; Long, S. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Perdomo, J.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Harley, P.; Loreto, F.; Di Marco, G.; Sharke, T. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef]
- Mott, K.; Woodrow, I. Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J. Exp. Bot. 2000, 51, 399–406. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Jin, W.; Li, L.; Xu, K.; Wei, Y. Potassium-Mediated Variations in the Photosynthetic Induction Characteristics of Phaseolus vulgaris L. Plants 2025, 14, 1623. https://doi.org/10.3390/plants14111623
Luo Q, Jin W, Li L, Xu K, Wei Y. Potassium-Mediated Variations in the Photosynthetic Induction Characteristics of Phaseolus vulgaris L. Plants. 2025; 14(11):1623. https://doi.org/10.3390/plants14111623
Chicago/Turabian StyleLuo, Qi, Wei Jin, Lili Li, Kedong Xu, and Yunmin Wei. 2025. "Potassium-Mediated Variations in the Photosynthetic Induction Characteristics of Phaseolus vulgaris L." Plants 14, no. 11: 1623. https://doi.org/10.3390/plants14111623
APA StyleLuo, Q., Jin, W., Li, L., Xu, K., & Wei, Y. (2025). Potassium-Mediated Variations in the Photosynthetic Induction Characteristics of Phaseolus vulgaris L. Plants, 14(11), 1623. https://doi.org/10.3390/plants14111623




