Abstract
Small auxin-up-regulated RNA (SAUR) genes are involved in the regulation of dynamic and adaptive growth in higher plants. However, their function and mode of action in citrus root growth are still unknown. Here, we demonstrate that in Poncirus trifoliata, PtrSAUR32 acted downstream of the auxin response factor PtrARF8 to regulate root growth by interacting with PtrPP2C.Ds, subfamily type 2C protein phosphatases which interacted with H-ATPase and PtrHA. In this study, several members of SAUR family in Poncirus trifoliata are identified to be associated with the growth and development of the roots. Among them, PtrSAUR32 was found to be highly expressed in the RT (root tip), and the level of its expression was significantly positively corelated to the length of primary roots (p < 0.01). The overexpression of PtrSAUR32 in citrus significantly promoted the growth of primary roots. In PtrSAUR32 transgenic citrus plants, the expressions of several auxin biosynthesis and transport genes were altered in accordance with the expression of PtrSAUR32. Y1H and dual-luciferase reporter assays proved that the expression of PtrSUAR32 is regulated by PtrARF8. Y2H and BiFC assay results indicated that PtrSAUR32 interacted with PtrPP2C.Ds subfamily members PtrPP2C.D1, PtrPP2C.D6, and PtrPP2C.D7, of which PtrPP2C.D7 could interact with PtrHA in vivo.
1. Introduction
Plants are dependent on their roots to capture water and nutrients from the soil to maintain different kinds of complex life activities. Therefore, understanding the regulation of root system architecture (RSA) is of great significance to plant growth [1,2]. RSA, with a strong plasticity, is mainly determined by the morphological development of the roots, such as root depth, lateral root expansion, and root length densities [3]. RSA is regulated by both intrinsic (genetics) and environmental response pathways, and plant hormones such as auxin, cytokinin (CK), abscisic acid (ABA), brassinosteroid (BR), etc., play significant roles in the development of roots [4,5].
It is well known that auxin is a master regulator involved in almost every facet of plant root development [6,7,8]. In the auxin signaling mechanism, auxin response factors (ARFs) transcriptionally activate early auxin-responsive genes, such as auxin/indole-3-acetic acid (Aux/IAA), Gretchen Hagen3 (GH3), and small auxin-up RNA (SAUR) [9]. Normally, Aux/IAA proteins act as the negative regulators of auxin signaling by interacting with ARFs and GH3 genes convert active IAA into its inactive form, while SAURs are primary auxin response genes [10,11,12]. In Arabidopsis, multiple AUXIN/IAA-ARF complexes participate in root development. For example, AtIAA28-AtARFs and AtSHY2/IAA3-AtARF modules cooperatively regulate the development of lateral roots [13,14]. Additionally, AtGH3.3, AtGH3.5, and AtGH3.6 were required for adventitious root initiation [15]. All this evidence indicates that early auxin response genes play important roles in root development.
SAURs, the largest family of early auxin-responsive genes, are implicated in many biological processes, including leaf growth, senescence, and apical hook development, as well as root growth and development [11,16]. The best-known function of SAURs is to promote cell elongation [16,17]. For instance, a reduction in AtSAUR19-24 expression led to diminished cell expansion [18]. Transgenic plants that express AtSAUR63 promoted the elongation growth of hypocotyls, petals, and stamen filaments [19]. Root growth depends on spatially distinct cell division and elongation activities in the root meristem. The research shows that AtARF6/8 and AtARF7/19 can bind to the auxin response elements of AtSAUR15 to regulate auxin-mediated lateral root and adventitious root formation [20]. In Arabidopsis, overexpressing AtSAUR76 resulted in longer roots, indicating that AtSAUR76 positively regulates the root growth [21]. SAUR is a large gene family with diversified functions. Transgenic plants overexpressing OsSAUR39 had shorter primary roots and a significantly lower number of lateral roots, which means that some SAURs act as negative regulators in root development [22].
SAURs are a key factor in auxin-induced acid growth, in which auxin activates the plasma membrane (PM) H-ATPases, resulting in proton efflux, and finally changes the ability of cell wall expansion to drive cell expansion [17,23]. On the one hand, the expression of SAUR genes was rapidly up-regulated by auxin, and SAURs bonded to the proteins of phosphatase 2C D (PP2C.D) and inhibited their phosphatase activity [20,23,24,25]. On the other hand, PP2C.Ds physically interact with PM H-ATPases and negatively regulate their activity [17]. Together, SAUR interacts with PP2C.Ds to inhibit their activities, thereby stimulating the activities of plasma membrane H-ATPases, resulting in apoplastic acidification and PM hyperpolarization, and ultimately promoting an increase in cell size [17,23]. Meanwhile, some of SAURs like AAM1 (AtSAUR32) and AtSAUR36 seem to have an opposite role in cell expansion [26,27].
Citrus is one of the most important fruit crops worldwide. However, environmental stresses, such as drought, cold, and soil salinity, limit the productivity of citrus. The application of grafting techniques has largely enabled citrus to overcome these limitations, imparting desirable qualities on citrus such as yield improvement, enhanced growth, and stress resistance [28]. Therefore, the importance of rootstock in citrus production is self-evident. As the organs used by plants to perceive the soil environment, rootstock roots play crucial roles in responding to environmental stresses and directly affect the development and growth of a citrus plant. Even though the morphology of roots is important to the function of roots, the genetic regulation of formation of roots system is still largely unknown in citrus. The importance of SAUR genes in the regulation of dynamic and adaptive growth, and the molecular mechanisms of the action of SAUR proteins, have been revealed in recent years. However, most of these studies were conducted in Arabidopsis and other herbaceous plant species. The function and mode of action of SAUR genes in woody plants such as citrus plants still remain largely unknown. In our previous study, SAUR gene family members species were characterized in several citrus [29], and one SAUR gene was potentially related to the morphology of the root system by comparative transcriptomic analysis of the roots in different morphologies [30]. To understand the function of SAURs in citrus root growth and development, as well as the molecular mechanisms of root morphogenesis in citrus for improvement of the root system architecture and stress tolerance of citrus rootstocks, the function and mechanisms of PtrSAUR32 in the regulation of citrus root growth and development were explored in this study.
2. Results
2.1. PtrSAUR Genes Related to Root Growth in Trifoliate Orange
In this study, trifoliate orange (P. trifoliata), one of the most important citrus rootstocks, was employed to study the functions of SAUR genes in the root growth and development of citrus. There were 68 SAUR genes identified from P. trifoliata. In this study, we chose 20 of them, which were homologues of AtSAURs involved in Arabidopsis root growth, for investigation of the relationship of SAUR with the growth of citrus roots. Transcriptional analysis of the PtrSAUR genes in the root, stem, and leaf of trifoliate orange demonstrated that the expression of some PtrSAUR genes was tissue-specific, among which PtrSAUR32, PtrSAUR17, and PtrSAUR15 had significantly higher expressions in the root than in the stem and leaf, indicating that PtrSAUR32, PtrSAUR17, and PtrSAUR15 might be involved in root development and growth in trifoliate orange (Figure 1A). Both PtrSAUR32 and PtrSAUR17 showed a more predominant tissue-specific expression in the root and were selected for further study. The expressions of PtrSAUR32 and PtrSAUR17 in different zones of the root were analyzed (Figure 1B,C). The expression of PtrSAUR32 was higher in the meristematic/elongation zone (RT) and lower in the elongation/differentiation and lateral root initiation zone (RM) and lateral root growth zone (RC), while the expression of PtrSAUR17 in the RC was higher than in the RT and RM, suggesting that PtrSAUR32 and PtrSAUR17 may play different roles in root morphogenesis.
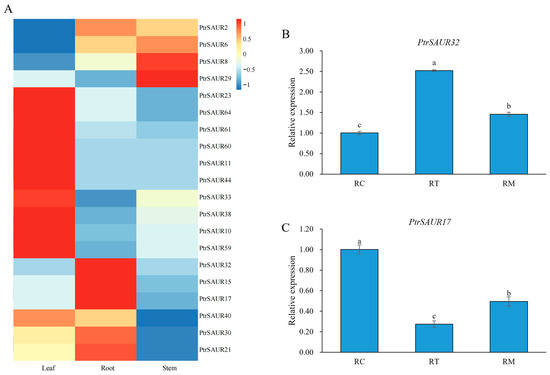
Figure 1.
Characteristics of PtrSAUR genes expressions in citrus. (A) A heat map of PtrSAURs expression in different tissues. (B) Expression patterns of PtrSAUR32 in different root zones. (C) Expression patterns of PtrSAUR17 in different root zones. RT (root tip): the meristematic/elongation zone of the root; RM (middle part of root): root elongation/differentiation and lateral root initiation zone; RC (root collar): lateral root growth zone. Different letters (a–c) indicate the level of significant difference at p < 0.05.
2.2. SAUR32 Involved in Regulating Root Growth in Citrus
Several species of citrus varieties are used as rootstocks in citrus production. The morphologies of their root systems are significantly different [30]. Here, relationship between SAUR17 and SAUR32 genes and root morphology was investigated with the seedlings of 12 citrus rootstock varieties differing in root morphology. The seedlings of P. trifoliata varieties had the longest primary roots at 30 days post sowing (dps), while the seedlings of C. reticulata varieties showed the shortest length of primary roots. Most of P. trifoliata varieties (except Donghu No. 1) and Volkamer produced more lateral roots than other varieties (Figure 2A, Supplementary Table S3). At 10–20 dps, the lateral roots rapidly generated from the primary root (Figure 2A, Table S3).
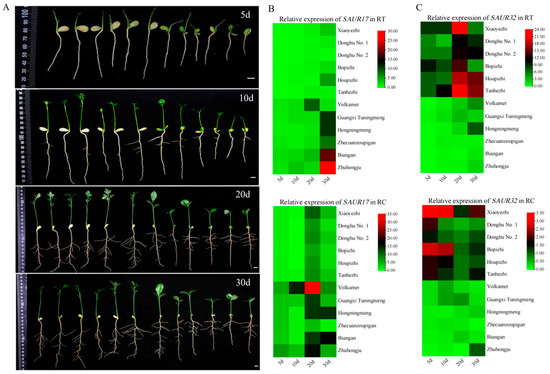
Figure 2.
Root indexes and expressions of SAUR17/32 in RT and RC. (A) Root morphology of citrus rootstocks at different days after sowing. From left to right are Poncirus trifoliata varieties (Xiaoyezhi, Donghu No. 1, Donghu No. 2, Bopizhi, Houpizhi, Tanhezhi), C. volkameriana variety (Volkamer), C. limonia variety (Guangxi Tuningmeng), C. limon variety (Hongningmeng), and C. reticulata varieties (Zhecuanzoupigan, Biangan, Zhuhongju). Bar = 1 cm. (B) Expression patterns of SAUR17 in RT and RCs. (C) Expression patterns of SAUR32 in RT and RCs.
The expressions of two SAUR genes demonstrated different associations with rootstock varieties and the development of roots. SAUR32 showed more distinct expression in the varieties with longer primary roots, such as P. trifoliata varieties, than that of SAUR17 (Figure 2B,C). High expression of SAUR32 was observed in the RC at 5–10 dps, and in the RT at 20–30 dps in P. trifoliata varieties. The high activity of SAUR17 was only observed in the RT at 30 dps of Zhuhongju, and the RC at 20 dps of Volkamer and Biangan. The results of the correlation analysis indicate that the expression level of SAUR32 in the RT was extremely significantly correlated to the length of primary roots (p < 0.01), and the expression of SAUR17 in the RC was significantly correlated to the number of lateral roots (p < 0.05) (Figure 3A,B). However, the level of SAUR17 expression did not show an obvious correlation with the length of primary roots. Therefore, we did not consider it as a candidate gene for the further investigation of its function in root growth. The expression of SAUR32 showed an extremely significant correlation with the growth of primary roots, and a positive association with the varieties with longer roots; therefore, it was selected as a major candidate gene for further functional study of its role in root growth.
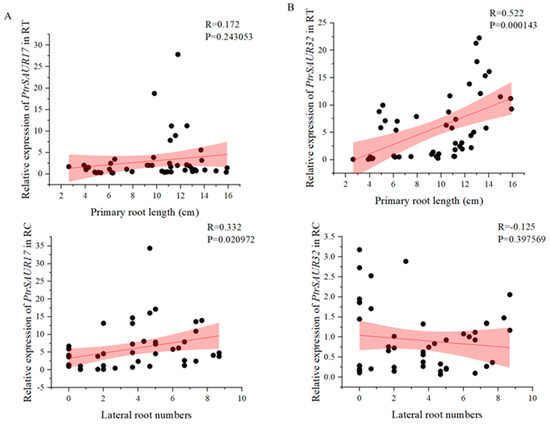
Figure 3.
Correlation analysis between root indexes and the expression of PtrSAUR17/32. (A) Relationship between root indexes and the expression of PtrSAUR17. (B) Relationship between root indexes and the expression of PtrSAUR32.
2.3. Molecular Characteristcs of PtrSAUR32
PtrSAUR32 encodes a protein of 111 amino acid residues with a molecular weight of 12.35 kDa and theoretical pI of 9.1. A phylogenetic tree constructed with PtrSAUR32 and its homologous genes from several species showed that PtrSAUR32 is homologous to PtSAUR14 of poplar, Solyc03g123550 of tomato, and AtSAUR40/41 and AtSAUR71/72 of A. thaliana (Figure 4A), but it only shares low similarities in its amino acid sequence: 23.53%, 20.13%, 24.78% and 24.63%, respectively. Multiple-sequence comparative analysis revealed that PtrSAUR32 contains a SAUR-specific domain SSD (Figure 4B), which is highly conserved [16,31,32]. Figure 4C shows that there are many predicted cis-acting elements located in the promoter of PtrSAUR32, such as light-responsive elements’ AE-box, ATC-motif, Box4, etc.; hormone response elements’ ABRE, CGTCA-motif, TGA-element, etc.; stress response elements’ MBS and TC-rich repeats; the element essential for anaerobic induction ARE; and the element involved in the differentiation of the palisade mesophyll cells HD-Zip1. All of this suggests that the expression of PtrSAUR32 may be regulated by various factors, such as light, plant hormones, and stresses.
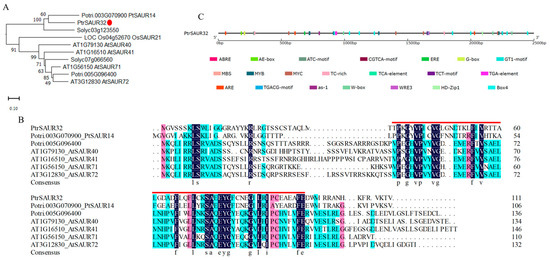
Figure 4.
Bioinformatic analysis of PtrSAUR32. (A) Phylogenic tree of PtrSAUR32 and its homologues. The homologues are from Arabidopsis thaliana, Oryza sativa, Solanum lycopersicum, and Populus L. PtrSAUR32 is indicated with a red dot. (B) Multiple-sequence alignment of PtrSAUR32 with Arabidopsis and poplar homologous. The SAUR-specific domain is highlighted with a red line. (C) The cis-acting elements in the promoter of PtrSAUR32.
2.4. Reponse of PtrSAUR32 to Auxin and Abiotic Stresses
To explore whether the expression of PtrSAUR32 can be induced by auxin and abiotic stresses, the relative expression of PtrSAUR32 under various treatments was analyzed with RT-qPCR. The results show that the level of PtrSAUR32 expression was first reduced and then increased under IAA treatment (Figure 5A). Under cold and salt treatments, the expression of PtrSAUR32 decreased (Figure 5B), while under PEG treatment, the expression of PtrSAUR32 increased (Figure 5C). Drought treatment slightly promoted the expression of PtrSAUR32 in the first 7 days and then significantly suppressed its expression in the rest period time of treatment (Figure 5D). These results indicate that the transcription of PtrSAUR32 is affected by auxin and abiotic stresses.
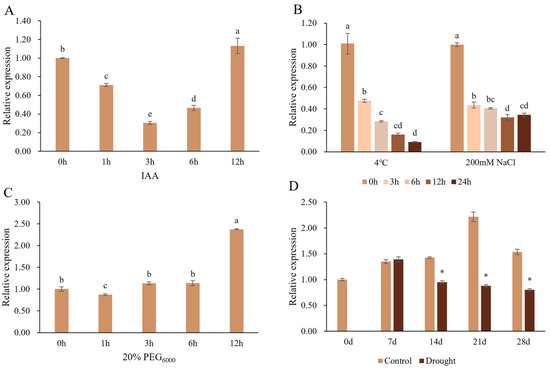
Figure 5.
The expression analysis of PtrSAUR32 under abiotic stress and IAA treatments. (A) IAA treatment. (B) Cold and NaCl treatments. (C) PEG treatment. (D) Drought treatment. Different letters (a–e) and asterisks (*) mean significant difference at p < 0.05.
2.5. PtrSAUR32 Promotes the Growth and Development of Root
To further analyze the expression patterns of PtrSAUR32, we obtained the ProPtrSAUR32::GUS transgenic plants. PtrSAUR32 was mainly expressed in the roots, and a strong GUS activity was observed in the root tips (Figure 6A,B). The function of PtrSAUR32 in regulating the root growth and development of citrus root was verified with transgenic trifoliate orange plants. The plants with self roots were generated from the cuttings of two PtrSAUR32 overexpression plants (PtrOE-1, PtrOE-3) and one silenced plant (PtrRNAi) (Figure 6C). The length of primary roots was significantly increased and decreased by the overexpression and silencing of PtrSAUR32, respectively, which shows a significant correlation with the expression level of PtrSAUR32 in the roots of transgenic plants (Figure 6D–F). These results suggest that PtrSAUR32 plays an important role in regulating the growth and development of roots in citrus.
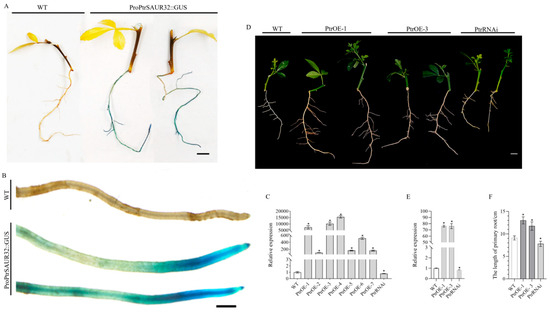
Figure 6.
Functional analysis of PtrSAUR32 in citrus. (A) GUS staining of PtrSAUR32::GUS transgenic citrus. Bar = 1 cm. (B) GUS staining of root tip. Bar = 1 mm. (C) The expression of PtrSAUR32 in the leaves of transgenic citrus. (D) Morphological characteristics of transgenic citrus roots. Bar = 1 cm. (E) The expression of PtrSAUR32 in the roots of transgenic citrus. (F) Statistical analysis of primary roots length. * means significant difference at p < 0.05.
2.6. PtrSAUR32 Interacts with the Genes Related to the Growth and Development of Root
To explore the mechanism of PtrSAUR32 regulation in root growth, the association between PtrSAUR32 and genes involved in auxin and cytokinin signal pathways related to root growth and root development was investigated. The results demonstrate that in PtrSAUR32 transgenic plants, the expression of response regulator (RR) PtrRR14 significantly corresponded with the expression of PtrSAUR32 (Figure 7B,C). The expression of both PtrYUCs and PtrPINs were influenced by PtrSAUR32 (Figure 7A). Compared to WT, the expressions of PtrPINs and PtrYUCs were higher in both the overexpressing and silenced plants. Among them, PtrPIN1 and PtrPIN3 showed significant positive correlations with PtrSAUR32, while the negative correlation of PtrPIN4, PtrPIN3-1, and PtrYUC3 with PtrSAUR32, and the positive correlation of PtrYUC5 and PtrYUC3-1 with PtrSAUR32 were not significant (Figure 7C).
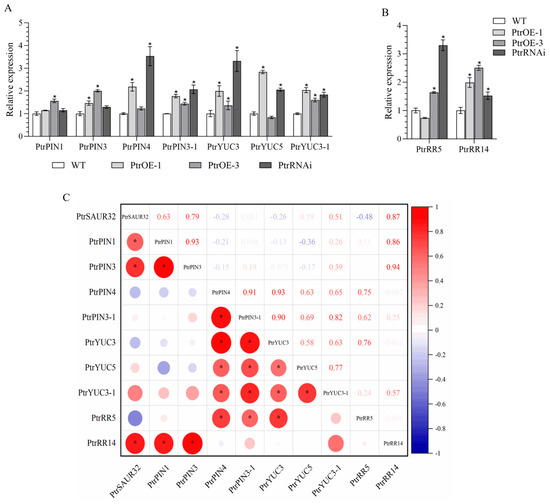
Figure 7.
The expression levels of PtrYUCs, PtrPINs, and PtrRRs in transgenic citrus plants roots. (A) The expression of PtrYUCs and PtrPINs in roots. (B) The expression of PtrRRs in roots. (C) The correlation between expressions of PtrSAUR32 and related genes in the roots. * means significant difference at p < 0.05.
In P. trifoliata, six members of A-ARFs, termed as PtrARF1, PtrARF5, PtrARF6, PtrARF7, PtrARF8, and PtrARF19 in this study, were identified to be homologous to A-ARFs of Arabidopsis (Figure S1), which are closely related to root organogenesis in Arabidopsis [33]. In PtrSAUR32 transgenic plants, the expressions of PtrARF6 and PtrARF8 were significantly correlated to the expression of PtrSAUR32 (Figure 8A). Root growth is the consequence of cell divisions and cell expansion, of which SAURs bond to the PP2C.D to intervene in the interaction of PP2C.D with AHA to drive cell expansion [17,23]. In this study, the expression of eight PtrPP2C.D genes (termed as PtrPP2C.D1, PtrPP2C.D2, PtrPP2C.D3, PtrPP2C.D4, PtrPP2C.D5, PtrPP2C.D6, PtrPP2C.D7 and PtrPP2C.D8 for PtrPP2C30, PtrPP2C41, PtrPP2C35, PtrPP2C18, PtrPP2C38, PtrPP2C36, PtrPP2C37, and PtrPP2C27, as reported by Yang et al. [34], respectively) were analyzed in transgenic plants. Overall, the expressions of PtrPP2C.Ds were down-regulated in the PtrSAUR32 overexpression transgenic lines, and up-regulated in the PtrSAUR32 silenced transgenic plant (Figure 8B). This indicates that PtrSAUR32 negatively affects the expression of PtrPP2C.Ds. Taken together, the above results suggest that PtrSAUR32 may regulate root growth and development in citrus through interaction with PtrARFs and PtrPP2C.Ds.
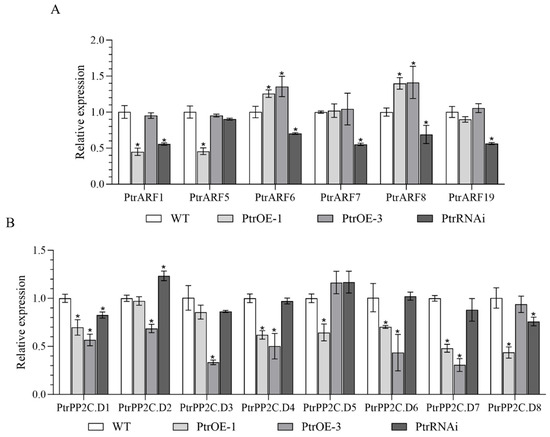
Figure 8.
The expression levels of PtrARFs and PtrPP2C.Ds in transgenic citrus plants roots. (A) The expression of PtrARFs in roots. (B) The expression of PtrPP2C.Ds in roots. * means significant difference at p < 0.05.
2.7. PtrSAUR32 Interacts with PtrPP2C.Ds
The above results reveal the positive relationship between PtrSAUR32 and PtrPP2C.Ds in citrus. It can be presumed that PtrSAUR32 may interact with PtrPP2C.Ds as in Arabidopsis [25]. To this end, we first analyzed the subcellular localization of PtrSAUR32. A transient expression assay in N. benthamiana leaves showed that a strong GFP fluorescent signal tagged to PtrSAUR32 was observed at the PM (plasma membrane) (Figure 9A), suggesting that PtrSAUR32 is located at the PM and can interact with PtrPP2C.Ds. The yeast two-hybrid assay (Y2H) showed that the yeast cells could grow normally and the color of colonies turned to blue on SD/-Leu-Trp-His-Ade (SD/-L-T-H-A) medium supplemented with X-α-gal when PtrSAUR32 was co-transformed with PtrPP2C.D1, PtrPP2C.D6, and PtrPP2C.D7, respectively (Figure 9B), indicating that PtrSAUR32 could interact with PtrPP2C.D1, PtrPP2C.D6, and PtrPP2C.D7. A further bimolecular fluorescence complementation experiment (BiFC) confirmed the interactions of PtrSAUR32 with PtrPP2C.D1, PtrPP2C.D6, and PtrPP2C.D7 at the PM (Figure 9C). These results suggest that PtrSAUR32 may be a member of the SAURs in citrus that interacts with PP2C.D to regulate the activity of H-ATPase (HA) and affect the cell expansion.
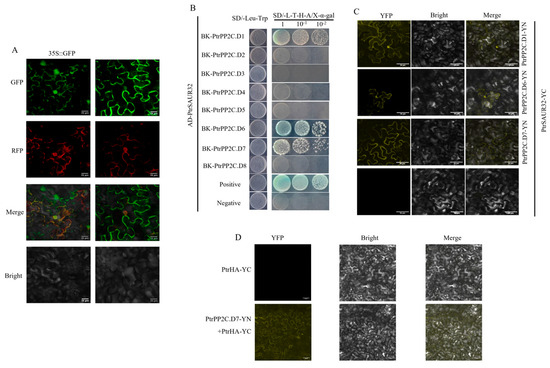
Figure 9.
PtrSAUR32 interacted with PtrPP2C.Ds. (A) Subcellular localization of PtrSAUR32. Bars = 20 μm. (B) Interaction assay between PtrSAUR32 and PtrPP2CDs via yeast two-hybrid system. SD/-Leu-Trp, SD medium without Leu and Trp; SD/-L-T-H-A/X-α-gal, SD medium without Leu, Trp, His and Ade but containing X-α-gal (40 µg/mL). Positive: pGADT7-T and pGBKT7-53. Negative: pGADT7-T and pGBKT7-Lam. (C) BiFC analysis of the interaction between PtrSAUR32 and PtrPP2C.Ds. Bars = 50 μm. (D) The physical interaction between PtrPP2C.D7 and PtrHA. Bars = 50 μm.
Multiple comparative analyses revealed that the amino acids sequence of Ptrif.0001s2325 (termed as PtrHA) has high similarities of 87% and 85% with AHA1 and AHA2, respectively (Figure S2), suggesting that PtrHA may also play the same role as AHA1 and AHA2. The BiFC assay results show that a strong YFP fluorescent signal was observed at the plasma membrane when PtrHA and PtrPP2C.D7 were co-injected into tobacco leaves, indicating that PtrPP2C.D7 interacted with PtrHA (Figure 9D).
2.8. PtrSAUR32 Is the Direct Target of PtrARF8
In auxin-mediated acid growth theory, the expression of SAUR genes is regulated through the canonical SCFTIR1/AFB signaling pathway, in which the auxin response factors, ARFs, play an important role; additionally, AtSAUR15 acts downstream of AtARFs to regulate auxin signaling-mediated root formation [20,23]. In PtrSAUR32 transgenic citrus plants, the expression of PtrARFs demonstrated a strong correlation with PtrSAUR32, suggesting that PtrARFs possibly interact with PtrSAUR32. To verify this assumption, the CDS of PtrARF1, PtrARF5, PtrARF6, PtrARF7, PtrARF8, and PtrARF19 were transiently expressed, respectively, in citrus leaves for investigation of the expression of PtrSAUR32. The results show that compared to the control, the expression of PtrSAUR32 was significantly up-regulated by the transient overexpression of PtrARF1, PtrARF5, PtrARF6, PtrARF7, and PtrARF8, but not by PtrARF19 (Figure 10A). The above results suggest that the expression of PtrSAUR32 might be regulated by PtrARFs.
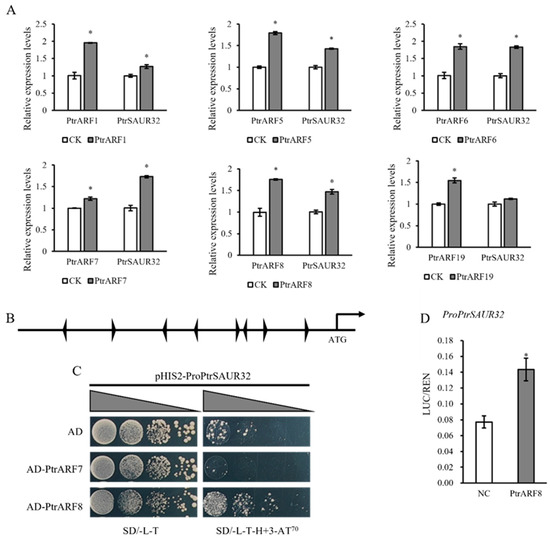
Figure 10.
The expression of PtrSAUR32 was activated by PtrARF8. (A) Expression analysis of PtrSAUR32 in citrus leaves transiently expressing PtrAFRs. (B) Identification of auxin response elements (AuxREs) in PtrSAUR32 promoter. (C) Yeast one-hybrid (Y1H) assay showed the interaction between PtrSAUR32 promoter and PtrARFs. The yeast cells were diluted with 0.9% NaCl into four concentration gradients (10−1, 10−2, 10−3 and 10−4) and then cultured in SD/-L/-T medium and SD/-L-T-H medium containing 70 mM 3-AT. SD/-L-T, SD medium without Leu and Trp; SD/-L-T-H, SD medium without Leu, Trp, and His. (D) Dual-luciferase reporter assay showing that PtrARF8 activates the transcription level of PtrSAUR32. NC: Negative Control. * means significant difference at p < 0.05.
The bioinformatic analysis indicates that eight auxin-responsive elements (AuxREs) are located in the promoter region of PtrSAUR32 (Figure 10B). According to the results of the qPCR analysis, PtrARF6, PtrARF7, and PtrARF8 were selected for determining their interaction with PtrSAUR32. The coding sequences of PtrARF6, PtrARF7, and PtrARF8 were constructed into the empty vector PGADT7, and the PtrSAUR32 promoter was fused to the empty vector pHIS2 to perform a yeast-one-hybrid (Y1H) assay to detect the bindings between PtrARFs and the PtrSAUR32 promoter. PtrARF6 was not considered in the subsequent experiments due to yeast cells containing both the transcription factor PtrARF6 and the promoter of PtrSAUR32. The Y1H assay showed that the yeast cells grew better on the SD/-L-T-H medium containing 70 mM 3-AT when ProPtrSAUR32 co-transformed with PtrARF8, indicating that PtrARF8 was able to bind to the promoter of PtrSAUR32 in vitro (Figure 10C). This interaction was further confirmed by the dual-luciferase reporter assay. A higher ration of LUC/REN was detected in the leaves of tobacco plants transiently expressing PtrARF8 and ProPtrSAUR32 constructs than those of NC (Figure 10D). These results demonstrate that PtrARF8 is the transcription factor of PtrSAUR32.
3. Discussion
3.1. PtrSAUR32 Is an Important Gene in Regulating Citrus Root Growth
Auxin plays a critical role in root morphogenesis [8]. The early auxin-responsive gene family SAUR is involved in multiple processes mediated by auxin, such as hypocotyl growth, apical hook development, and cotyledon open [18,25,35], and it participates in the regulation of root growth and development [20,21]. This study reveals that the transcription of PtrSAUR32 was significantly related to the growth of primary roots in P. trifoliata. The overexpression and silencing of PtrSAUR32 confirmed that PtrSAUR32 is an important gene regulating the growth and development of roots in citrus, which is in agreement with the findings of Qiu et al. in Arabidopsis [36]. Additionally, the homologues of PtrSAUR32 in Arabidopsis (AtSAUR40/41/71/72) could modulate ion homeostasis and salt tolerance [36]. It is worth to carrying out further experiments to verify whether PtrSAUR32 can also improve the abiotic stress tolerance of citrus. Root development is a fairly complex process regulated by the interaction of auxin with other plant hormones. It is well known that cytokinin crosstalk with auxin controls root meristem size and ensures root growth [37,38,39]. Further, 10−8 to 10−4 M BA affected the elongation of Arabidopsis roots [40]. In this study, the expression of the cytokinin response regulator PtrRR14 significantly corresponded to PtrASUR32 in transgenic citrus plants, providing further evidence to prove that PtrSAUR32 may be involved in the cytokinin signaling-mediated root growth and development.
3.2. The Mechanism of PtrSAUR32 Regulation in Root Growth and Development in Citrus
In Arabidopsis, AtSAUR15 interacts with AtPP2C.Ds to inhibit their activities, thereby stimulating plasma membrane H-ATPases, which drive cell expansion and facilitate LR and AR formation [20]. In P. trifoliata, PtrSAUR32 also interacts with three PtrPP2C.D proteins (PtrPP2C.D1, PtrPP2C.D6, and PtrPP2C.D7), of which PtrPP2C.D7 can bind with PtrHA in the plasma membrane in vivo, indicating that PtrPP2C.Ds could regulate the activity of PM H-ATPase in citrus. In Arabidopsis, three AUTOINHIBITED PLASMA MEMBRANE H+-ATPases AHA1, AHA2, and AHA7 are predominant in root epidermal cells, in which AHA2 drives root cell expansion during growth [41]. PtrHA is highly homologous to AHA2, and it interacts with PtrPP2C.D7; therefore, it can be assumed that PtrHA plays a similar role in citrus root development to AHA2 in Arabidopsis.
It had been proved that the promotion of root organogenesis in Arabidopsis by SAUR15 is regulated by ARF6 and ARF7 [20]. Our study demonstrates that PtrSAUR32 is regulated by PtrARF8. As an important hormone, auxin biosynthesis and transport and auxin-dependent signaling processes all affect root development [42,43]. YUCCA (YUC) genes are known as the key rate-limiting enzymes that function in tryptophan-dependent auxin biosynthesis [44]. In Arabidopsis, AtYUC3, AtYUC5, AtYUC7, AtYUC8, and AtYUC9 were highly expressed in the root to promote root development [45]. PtrYUC3, PtrYUC5, and PtrYUC3-1 were homologues of AtYUC3, AtYUC5, AtYUC7, AtYUC8, and AtYUC9. However, the overexpression and silence of PtrSAUR32 did not significantly affect the expressions of 3 PtrYUCs in this study. Further work is required to clarify whether the PtrSAUR32 is directly involved in the regulation of auxin synthesis or if it interacts with other members of PtrYUCs. Auxin transport is essential for the establishment and maintenance of local auxin concentrations, and the auxin efflux carrier PIN proteins are the most crucial components of auxin transport [46,47]. In roots, AtPIN1 was predominantly expressed in the stele and endodermis cells and was regulated the flow of auxin to the quiescent center (QC) in the root meristem, while AtPIN3 and AtPIN4 mediated the redistribution of auxin in the roots as well [48,49]. We detected the expression levels of homologous genes (PtrPIN1, PtrPIN3, PtrPIN4, and PtrPIN3-1) of AtPIN1, AtPIN3, and AtPIN4. The expressions of PtrPIN1 and PtrPIN3 in PtrSAUR32 transgenic plants were significantly increased, implying that PtrSAUR32 might mediate the auxin transport in the roots of citrus.
3.3. Function of PtrSAUR32 in Response to Abiotic Stress
Bioinformatic analysis revealed a large number of stress-responsive cis-regulatory elements existing in the promoter of PtrSAUR32. The expression of PtrSAUR32 under various abiotic stress treatments showed that PtrSAUR32 significantly responded to the abiotic stresses, suggesting that PtrSAUR32 may play a role in abiotic stress tolerance in citrus. The overexpression of PavSAUR55 in Arabidopsis improved root elongation and showed higher tolerances to NaCl and mannitol treatments [50]. In this study, the expression of PtrSAUR32 was up-regulated by osmatic and drought stresses, implying that PtrSAUR32 may promote the adaptive growth of citrus roots in response to abiotic stress. This would possibly improve the tolerance of citrus to various abiotic stress such as drought. However, further experiments are required to clarify this presumption.
4. Materials and Methods
4.1. Plant Materials
Seven Poncirus trifoliata varieties (Donghu No. 1, Donghu No. 2, Houpizhi, Bopizhi, Tanhezhi, Xiaoyezhi, Donghaizhi), three Citrus reticulata varieties (Biangan, Zhecuanzoupigan, Zhuhongju), one C. limon variety (Hongningmeng), one C. volkameriana variety (Volkamer), and one C. limonia variety (Guangxi Tuningmeng) were used as plant materials in this study. All citrus varieties were provided by the National Citrus Germplasm Repository (Chongqing, China). Donghaizhi was used for the RT–qPCR analysis of gene expressions in different tissues and organs, under different hormone treatments and stresses, and under stable genetic transformation. Other varieties were used for analyzing the correlation between root indexes and gene expression in the roots of seedlings at different developmental stages. Further, 30-day-old tobacco (Nicotiana benthamiana) plants were used for the transient expression of candidate genes. All citrus and tobacco plants were grown in a greenhouse under 26 °C and a 16 h light/8 h dark cycle.
4.2. Treatments
Citrus seeds were germinated at 28 °C on wet filter paper in a Petri dish after removal of the seed coat and then cultured in pots filled with vermiculite under 26 °C and a 16 h light/8 h dark cycle, 12000LX for 30 days; roots, stems, and leaves were subsequently collected for analysis of the expression of PtrSAURs. Additionally, the root was divided into different zones for gene expression analysis, termed according to Zhang et al. [51] as the RT (root tip): the meristematic/elongation zone; RM (middle part of root): the root elongation/differentiation and lateral root initiation zone; and RC (Root collar): lateral root growth zone. In order to analyze the correlation between gene expression and root development, different zones of the root were sampled at 5, 10, 20, and 30 days after sowing; at the same time, the number of lateral roots in the RC and the length of the primary root were determined.
After citrus seedlings were grown under 26 °C and a 16 h light/8 h dark cycle for 30 days, abiotic stress and hormone treatments were carried out. Root samples were collected for qPCR analysis after treatment with 1µM IAA and 20% PEG6000 for 0, 1, 3, 6, 12 h, respectively. Root samples were collected at 0, 3, 6, 12, and 24 h after salt stress (200 mM NaCl) and cold treatments (4 °C). For drought treatment, the water supply was withheld after 30 days of normal conditions, and root samples were taken at 7, 14, 21, and 28 days, respectively.
4.3. DNA, RNA Extraction, and Gene Expression Analysis
Genomic DNA was extracted according CTAB Plant DNA Extraction Kit (Biomed, Beijing, China). Total RNA was extracted using the EASYspin Plus Plant RNA Kit (Aidlab, Beijing, China), and first-strand cDNA synthesis was performed using RevertAid™ Master Mix (Thermo Scientific, Waltham, MA, USA). First-strand cDNA was used as a template for RT–qPCR to analyze gene expression, as described in previous study [51]. The primer sequences used in this study are listed in Supplementary Data Table S1.
4.4. Bioinformatic Analysis
The website Expasy (https://www.expasy.org/resources/protparam) (accessed on 29 March 2023) was used to compute the physical and chemical parameters of PtrSAUR32 (Ptrif.0004s1277). Homologous genes of PtrSAUR32 in other species were obtained from the website Phytozome (https://phytozome-next.jgi.doe.gov/) (accessed on 17 January 2025). The software MEGA11 was used to construct a phylogenetic tree by the neighbor-joining method, with a bootstrap of 1000 replicates, and other parameters were in default values. Multiple comparisons of PtrSAUR32 and its homologous genes were performed using DNAMAN9 software. The website PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 12 May 2023) was used to analysis the cis-acting elements of PtrSAUR32 promoter (2.5 kb DNA sequence region upstream of ATG).
4.5. Subcellular Localization of PtrSAUR32
The PtrSAUR32 coding sequence, without a termination codon, was fused to the Cam35S-GFP vector. Agrobacterium cells containing PtrSAUR32::GFP or Cam35S::GFP were suspended in the infection solution (10 mM MgCl2, 10 mM MES, 150 μM acetosyringone, pH 5.6) until the OD600 was 1.0. The above suspension was co-injected with Agrobacterium cells carrying a red membrane marker into the leaves of a 4-week-old N. benthamiana. Fluorescence signals were observed under an FV3000 confocal microscope (Olympus Tokyo, Japan) after infiltration for two days. The primer sequences used for vector construction in this study are listed in Supplementary Data Table S2.
4.6. Generation of Transgenic Plants
The full-length CDS of PtrSAUR32 was subcloned into the pFGC5941MDB3F-GN vector to generate overexpression lines, and a specific DNA fragment of PtrSAUR32 was subcloned into the pFGC5941MDB3F-GN vector to generate RNA interference (RNAi) lines. The promoter of PtrSAUR32 was cloned into a p1300GNGM-GUS vector to drive the expression of the GUS reporter gene. The above vectors were transformed into Agrobacterium tumefaciens EHA105. Transgenic citrus plants were generated as previously described [52]. Transgenic citrus plants were identified by GFP and genomic PCR.
4.7. GUS Staining
The seedlings of WT and the PtrSAUR32 promoter transgenic plants were used for β-glucuronidase (GUS) staining, and were submerged in GUS dye solution at 37 °C for 12 h and decolorized with 70% ethanol.
4.8. Y2H and BiFC Assay
The GAL4 yeast two-hybrid (Y2H) system was used to detect the interactions between PtrSAUR32 and PtrPP2C.Ds, according to Yu et al. [53]. The coding sequence of PtrSAUR32 was cloned into the GAL4 activation domain vector pGADT7, and coding sequences of PtrPP2C.Ds were cloned into the DNA-binding domain vector pGBKT7. Both vectors were co-transformed into the yeast strain Y2H, growing on SD/-Leu/-Trp-deficient medium. The positive colonies were used for selecting interaction proteins on selective SD/-Leu/-Trp/-His/-Ade medium with X-α-gal.
Bimolecular fluorescence complementation (BiFC) assays were performed, as previously described [52]. Full-length coding sequences of PtrSAUR32 and its candidate interactive proteins were constructed into N-terminal or C-terminal yellow fluorescence protein fragments, respectively, and then co-injected into tobacco leaves. The CDS of PtrPP2C.D7 and PtrHA was constructed into N-terminal and C-terminal yellow fluorescence protein fragments and co-injected into tobacco leaves. The fluorescence signal was observed under a FV3000 confocal microscope.
4.9. Transient Expression of PtrARFs
The CDS of PtrARF1, PtrARF5, PtrARF6, PtrARF7, PtrARF8, and PtrARF19 were fused into the pFGC5941MDB3F-GN vector, respectively. The fusion vector was introduced into EHA105. Agrobacterium cells containing CDS of the target gene were suspended in the infection solution, and citrus leaves were soaked in this solution and vacuumed for 10 min. After three days of infection, the total RNA was extracted for expression analysis.
4.10. Y1H and Dual-Luciferase Reporter Assay
For the yeast one-hybrid (Y1H) assay, the PtrSAUR32 promoter sequence (proPtrSAUR32), 2.5 kb upstream region of ATG, was inserted into the vector pHIS2, while the coding sequences of PtrARF6, PtrARF7, and PtrARF8 were inserted into pGADT7, respectively. The fused vector pHIS2-PtrSAUR32 was co-transformed into the yeast strain Y187 cell with AD-PtrARF6, AD-PtrARF7, AD-PtrARF8, and pGADT7. The growth of yeast cells on SD/-L-T-H (containing 3-AT) medium was used to determine the interactions between proPtrSAUR32 and PtrARF6, PtrARF7, and PtrARF8. Yeast cells containing proPtrSAUR32 and pGADT7 were used as the control.
For the dual-luciferase reporter assay, the promoter of PtrSAUR32 was cloned into the vector pGreenII 0800-LUC to drive the reporter gene. The CDS of PtrARF8 was inserted into the vector pGreen II 62-SK to generate the effector construct. The constructed vectors were introduced into GV3101 (pSoup), respectively, and co-injected into N. benthamiana. The Dual Luciferase Reporter Gene Assay Kit (Yeasen, Shanghai, China) was used to assess Firefly and Renilla luminescent signals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14111579/s1, Figure S1: Phylogenetic relationship and conserved motifs; Figure S2: Multiple sequence alignment of PtrHA with AHA1 and AHA2 of Arabidopsis; Table S1: Primers used for qRT-PCR analysis; Table S2: Primers used for vector construction; Table S3: Root indexes of twelve citrus rootstock varieties at different days after sowing.
Author Contributions
X.W., S.Z. (Shiping Zhu) and X.Z. conceived and designed the experiments. X.W., X.L., S.Z. (Saihang Zheng), and F.W. performed the experiments. X.W., X.Z., and S.Z. (Shiping Zhu) contributed to paper writing and paper revising. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2023YFD1200103-05, 2023YFD2300604, 2021YFD1400801), China Agriculture Research System (CARS-Citrus), Fundamental Research Funds for the Central Universities (SWU-XDJH202308), Shuangcheng cooperative agreement research grant of Yibin (Grant No. XNDX2022020008).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, 45. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, J.; Goh, T.; Roberts, I.; Guyomarc’H, S.; Lucas, M.; De Smet, I.; Fukaki, H.; Beeckman, T.; Bennett, M.; Laplaze, L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013, 18, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef]
- Agrawal, R.; Singh, A.; Giri, J.; Magyar, Z.; Thakur, J.K. MEDIATOR SUBUNIT17 is required for transcriptional optimization of root system architecture in Arabidopsis. Plant Physiol. 2023, 192, 1548–1568. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harbor Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harbor Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Qi, Y. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Vassileva, V.; Parizot, B.; Demeulenaere, M.; Grunewald, W.; Audenaert, D.; Van Campenhout, J.; Overvoorde, P.; Jansen, L.; Vanneste, S.; et al. A novel AUX/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 2010, 20, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA-ARF modules regulate lateral root formation: The role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mongelard, G.; Flokova, K.; Pacurar, D.I.; Novak, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inze, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012, 71, 684–697. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Lv, M.; Hepworth, S.R.; Li, D.; Ma, C.; Li, J.; Wang, S.M. SAUR15 promotes lateral and adventitious root development via activating H+-ATPases and auxin biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef]
- Markakis, M.N.; Boron, A.K.; Van Loock, B.; Saini, K.; Cirera, S.; Verbelen, J.P.; Vissenberg, K. Characterization of a small auxin-up RNA (SAUR)-like gene involved in Arabidopsis thaliana development. PLoS ONE 2013, 8, e82596. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, N.; Zhang, F.; Yu, R.; Chen, H.; Deng, X.W.; Wei, N. SAUR17 and SAUR50 differentially regulate PP2C-D1 during apical hook development and cotyledon opening in Arabidopsis. Plant Cell 2020, 32, 3792–3811. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.; Yoon, H.; Park, C. Functional characterization of a small auxin-up RNA gene in apical hook development in Arabidopsis. Plant Sci. 2007, 172, 150–157. [Google Scholar] [CrossRef]
- Hou, K.; Wu, W.; Gan, S.S. SAUR36, a SMALL AUXIN UP RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013, 161, 1002–1009. [Google Scholar] [CrossRef]
- Simpson, C.R.; Nelson, S.D.; Melgar, J.C.; Jifon, J.; King, S.R.; Schuster, G.; Volder, A. Growth response of grafted and ungrafted citrus trees to saline irrigation. Sci. Hortic. 2014, 169, 199–205. [Google Scholar] [CrossRef]
- Wang, F.; Yu, H.; Hu, Z.; Guan, D.; Zhang, P.; Zhu, S.; Zhao, X. Genome-wide analysis of SAUR gene family in citrus. Acta Hortic. Sin. 2020, 47, 23–40. [Google Scholar]
- Luo, G. Identification of Genes Related to Root Development in Citrus Rootstock and Its Functional Study. Master’s Thesis, Southwest University, Chongqing, China, 2020. [Google Scholar]
- Marchler-Bauer, A.; Zheng, C.; Chitsaz, F.; Derbyshire, M.K.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Lanczycki, C.J.; et al. CDD: Conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013, 41, D348–D352. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ge, Y.; Cai, G.; Pan, X.; Xu, L. WOX-ARF modules initiate different types of roots. Cell Rep. 2023, 42, 112966. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, R.; Hu, W.; Wu, Q.; Tong, X.; Li, X. Identification and expression analysis of PP2C gene family in Poncirus trifoliata. J. Fruit Sci. 2022, 39, 532–547. [Google Scholar]
- Nagpal, P.; Reeves, P.H.; Wong, J.H.; Armengot, L.; Chae, K.; Rieveschl, N.B.; Trinidad, B.; Davidsdottir, V.; Jain, P.; Gray, W.M.; et al. SAUR63 stimulates cell growth at the plasma membrane. PLoS Genet. 2022, 18, e1010375. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]
- Dello, I.R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Dello, I.R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar]
- Moubayidin, L.; Perilli, S.; Dello, I.R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef]
- Hoffmann, R.D.; Olsen, L.I.; Ezike, C.V.; Pedersen, J.T.; Manstretta, R.; Lopez-Marques, R.L.; Palmgren, M. Roles of plasma membrane proton ATPases AHA2 and AHA7 in normal growth of roots and root hairs in Arabidopsis thaliana. Physiol. Plant. 2019, 166, 848–861. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodriguez, L.; Li, L.; Zhang, X.; Friml, J. Functional innovations of PIN auxin transporters mark crucial evolutionary transitions during rise of flowering plants. Sci. Adv. 2020, 6, eabc8895. [Google Scholar] [CrossRef]
- Tan, S.; Luschnig, C.; Friml, J. Pho-view of auxin: Reversible protein phosphorylation in auxin biosynthesis, transport and signaling. Mol. Plant. 2021, 14, 151–165. [Google Scholar] [CrossRef]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazimalova, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef]
- Omelyanchuk, N.A.; Kovrizhnykh, V.V.; Oshchepkova, E.A.; Pasternak, T.; Palme, K.; Mironova, V.V. A detailed expression map of the PIN1 auxin transporter in Arabidopsis thaliana root. BMC Plant Biol. 2016, 16 (Suppl. 1), 5. [Google Scholar] [CrossRef]
- Hou, Q.; Hong, Y.; Wen, Z.; Shang, C.; Li, Z.; Cai, X.; Qiao, G.; Wen, X. Molecular characterization of the SAUR gene family in sweet cherry and functional analysis of PavSAUR55 in the process of abscission. J. Integr. Agric. 2023, 22, 1720–1739. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Wang, X.; Feng, J.; Yi, Q.; Zhu, S.; Zhao, X. Mining key genes related to root morphogenesis through genome-wide identification and expression analysis of RR gene family in citrus. Front. Plant Sci. 2022, 13, 1068961. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Hu, Z.; Wang, X.; Yi, Q.; Feng, J.; Zhao, X.; Zhu, S. CcRR5 interacts with CcRR14 and CcSnRK2s to regulate the root development in citrus. Front. Plant Sci. 2023, 14, 1170825. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, J.; Zhang, M.; Li, X.; Sun, Y.; Zhang, M.; Ding, Z. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in Arabidopsis. Sci. Adv. 2023, 9, eade2493. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).