Characteristics of Soil Dissolved Organic Matter Structure in Albi-Boric Argosols Profiles Through Straw Incorporation: A Fluorescence Spectroscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurement Methods
2.3.1. Determination of Soil Physicochemical Indicators
2.3.2. Fluorescent Sample Handling and Measurement Methods
2.4. Statistical Analysis
3. Results
3.1. Soybean Yield Factors and Soil Chemical Properties
3.2. Variation in Soil DOC Content
3.3. Analysis of Soil DOC Component Fluorescence Index
3.4. The Fluorescent Spectral Characteristics of Soil DOC Components
3.5. Fmax Analysis of Soil DOC
3.6. The Correlation Analysis of Soil SOC and DOC Content, as Well as Their Components
4. Discussion
4.1. Influence of Different Treatments on Soil DOC Content
4.2. Influence of Different Treatments on Soil DOC Component Fluorescence Indexes
4.3. The Influence of Different Treatments on the DOC Components
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, N.N.; Sheng, M.Y.; Wang, L.J.; Shi, Q.L. Effects of long-term vegetation restoration on soil active organic carbon fractions content and enzyme activities in Karst rocky desertification ecosystem of southwest China. Chin. J. Plant Ecol. 2023, 47, 867–881. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zheng, Y.X.; Yang, Z.J.; Huang, S.C.; Wang, H.Z.; Yu, H.; Zhong, X.F. Differences of dissolved organic carbon in the rhizosphere and bulk soils of Chinese fir plantations at different stand ages. J. Subtrop. Resour. Environ. 2023, 18, 48–55. [Google Scholar] [CrossRef]
- Solomon, C.T.; Jones, S.E.; Weidel, B.C.; Buffam, I.; Fork, M.L.; Karlsson, J.; Larsen, S.; Lennon, J.T.; Read, J.S.; Sadro, S. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 2015, 18, 376–389. [Google Scholar] [CrossRef]
- Wei, D.; Cai, S.; Li, Y.; Jin, L.; Wang, W.; Li, Y.; Bai, Y.; Hu, Y. The Response of water-soluble organic carbon to organic material applications in black soil. Sci. Agric. Sin. 2020, 53, 1180–1188. [Google Scholar] [CrossRef]
- Liu, M.; Yu, W.; Jiang, Z.; Ma, Q. A research review on soil active organic carbon. Chin. J. Ecol. 2006, 25, 1412–1417. [Google Scholar] [CrossRef]
- Heilongjiang Soil Management Bureau. Heilongjiang Soils; Agriculture Press: Beijing, China, 1992; pp. 124–148. [Google Scholar]
- Zeng, Z.S.; Zhuang, J.P.; Li, M.P. On the genesis and classification of batching soil. Acta Pedol. Sin. 1963, 11, 111–129. [Google Scholar]
- Feng, H.Y.; Zhang, C.F. Research progress and prospects on improvement effect of albic soil. Heilongjiang Agric. Sci. 2022, 11, 104–109. [Google Scholar] [CrossRef]
- Zeng, Z.S.; Xu, Q.; Gao, Z.Q.; Zhang, Z.Y. Chinese Albic Soil; Science Press: Beijing, China, 1992; pp. 124–148. [Google Scholar]
- Zhao, D.L.; Liu, F.; Jia, H.B.; Hong, F.Y. The effect of transforming planosol by using a subsoil mixed plough. Sci. Agric. Sin. 1994, 27, 37–44. [Google Scholar]
- Wu, Z.J.; Ding, Q.T.; Yu, D.Q.; Yin, Y.L.; Yu, Z.F. Study on the effect of applying organic materials and subsoiling on improving the white slurry layer of good public soil. Chin. J. Soil. Sci. 1995, 26, 250–252. [Google Scholar] [CrossRef]
- Wang, Q.J.; Liu, F.; Chi, F.; Jiao, F.; Zhang, C.; Jiang, H.; Li, P.; Zhu, B. Effect of straw returning and nitrogen fertilizer regulation on nitrogen and rice yield in albic soil with different fertilities. Trans. Chin. Soc. Agric. Eng. 2019, 35, 105–111. [Google Scholar] [CrossRef]
- Zhu, B.G.; Kuang, E.J.; Zhang, C.L.; Meng, Q.Y. The Configuration of a New Three-Stage Subsoil Interval Mixing Four-Gang Plough for Improving Albi-Boric Argosols. In Advances in Machinery, Materials Science and Engineering Application IX; IOS Press: Amsterdam, The Netherlands, 2023; Volume 40, pp. 1019–1026. [Google Scholar] [CrossRef]
- Song, X.Y.; Yue, X.; Kong, X.P.; Liu, X.W.; Liu, L.; Li, Y.; Zhao, T.T.; Zeng, L.S.; Guo, X.D.; Li, X.L.; et al. Effects of application of organic material on composition and structural characteristics of humic substances in saline soil. Acta Pedol. Sin. 2020, 57, 414–424. [Google Scholar] [CrossRef]
- Sun, Z.A.; Chen, Q.; Han, X.; Wu, W.L.; Meng, F.Q. Estimation of winter wheat photosynthesized carbon distribution and allocation belowground via 13C pulse-labeling. Environ. Sci. 2018, 39, 2837–2844. [Google Scholar] [CrossRef]
- Che, Y.J.; Gu, S.Y.; Wang, Z.Y.; Bai, X.Y.; Feng, J.Y.; Zhang, W.J.; Li, Y.H. Analysis of DOC fluorescence spectral structure of black soil in different regions under conservation tillage. Chin. J. Eco Agric. 2024, 32, 1–12. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, K.; Pan, G. Impact of biochar amendment on deep soil organic carbon pool. J. Nanjing Agric. Univ. 2020, 43, 589–593. [Google Scholar] [CrossRef]
- Araya, K.; Kudoh, M.; Zhao, D.; Liu, F.; Jia, H. Improvement of planosol solum: Part 6: Field experiments with a three-stage subsoil mixing plough. J. Agric. Eng. Res. 1996, 65, 151–158. [Google Scholar] [CrossRef]
- Liu, F.; Jia, H.; Zhang, C.F.; Araya, K.; Kudoh, M.; Kawabe, H. Improvement of planosol solum. Part 9: Fertilizer distributor for subsoil. J. Agric. Eng. Res. 1998, 71, 213–219. [Google Scholar] [CrossRef]
- Jia, H.B.; Liu, F.; Zhang, H.; Zhang, C.; Araya, K.; Kudoh, M.; Kawabe, H. Improvement of planosol solum. Part10: Mixing of wheat straw and cron stalk into subsoil. J. Agric. Eng. Res. 1998, 71, 221–226. [Google Scholar] [CrossRef]
- Liu, F.; Gao, P.; Wang, Q.J.; Gao, Z.C.; Chang, B.C.; Sun, B. Research progress in improvement of subsoil. Soil. Fertil. Sci. China 2015, 1, 7–11. [Google Scholar]
- Zhu, B.; Zhang, C.; Jia, H.; Meng, Q.; Wang, N.; Zhang, L.; Kuang, E.; Wang, Q.; Liu, J.; Gao, X. Improving effect of Albi-Boric Argosols by deep tillage combined with subsoil mixed with ameliorative materials. Trans. Chin. Soc. Agric. Eng. 2018, 34, 107–114. [Google Scholar] [CrossRef]
- Lu, R.K. Analytic Technique of Soil Agricultural Chemistry; China Agriculture Science and Technique Press: Beijing, China, 2000. [Google Scholar]
- Li, T.K.; Kou, C.L.; Lv, J.L.; Zhang, X.L.; Yang, X.L. Effects of extraction conditions on the test results of soil dissolved organic carbon. Ecol. Environ. Sci. 2017, 26, 1878–1883. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Ao, J.; Wang, T.; Chang, R. Application of the Three-dimensional Excitation-emission Matrix Fluorescence Spectroscopy in the Analysis of Soil Dissolved Organic Matter Components. Chin. J. Soil. Sci. 2022, 53, 738–746. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals the ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.H.; Jiang, X.; Huang, X.F. Identification of WSOM fluorescence spectral components in suspended solids and correlation analysis with nitrogen forms of Lake Wuli, Lake Taihu. J. Lake Sci. 2018, 30, 102–111. [Google Scholar] [CrossRef]
- Huang, M.F.; Song, Q.J.; Xing, X.F.; Jian, W.J.; Liu, Y.; Zhao, Z.L. Analysis of fluorescence spectrum characteristics of petroleum-polluted water. Spectrosc. Spectral Anal. 2014, 34, 2466–2471. [Google Scholar] [CrossRef]
- Ni, J.Z.; Xu, J.M.; Xie, Z.M.; Wang, D.J. Study on the content and composition characteristics of soil water-soluble organic carbon under different fertilizer treatments. Acta Pedol. Sin. 2003, 40, 724–730. [Google Scholar] [CrossRef]
- Jia, H.B.; Liu, F.; Zhao, D.L. Gley white clay silicate clay mineral formation. Chin. J. Soil. Sci. 1995, 26, 162–164. [Google Scholar] [CrossRef]
- Li, B.B.; Wu, L.F. Concentration and components of dissolved organic carbon in soil profiles after crop residues were incorporated into the topsoil. J. Agro Environ. Sci. 2019, 38, 1567–1577. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Simpson, M.J. Shifts in microbial community and water extractable organic matter composition with biochar amendment in a temperate forest soil. Soil. Biol. Biochem. 2015, 81, 244–254. [Google Scholar] [CrossRef]
- Kuang, E.J.; Chi, F.Q.; Zhang, J.M.; Xu, M.G.; Colinet, G.; Xu, Q.R.; Hao, X.Y.; Zhu, B.G. Analysis of DOC component structure of black soil profile with straw deeply buried and based on fluorescence spectrum. Spectrosc. Spectral Anal. 2022, 42, 3243–3248. [Google Scholar] [CrossRef]
- Yu, W.S.; Wang, B.S.; Wang, S.C.; Meng, F.H.; Lu, C.A. Characteristics of soil labile organic carbon and carbon management index under different long-term fertilization systems in four typical soils of China. Chin. Soil. Fertil. 2018, 2, 29–34. [Google Scholar]
- Miao, C.H.; Lu, Y.Z. Spectral Characteristics and Structural Differences of DOM in Black Soil, Fluvo-Aquic Soil and Red Soil. Soils 2021, 53, 168–172. [Google Scholar] [CrossRef]
- Chen, W.R.; Liu, Q.; Yu, H.S. Fractions and Characteristics of Dissolved Organic Matter in Long-term Fertilization Paddy Soils. J. Soil. Water Conserv. 2010, 24, 111–116. [Google Scholar]
- Li, Y.M.; Wang, G.L.; Li, C.Y.; Liu, Z.Y.; Meng, X.H.; Wang, W.; Shao, G.Z.; Hu, Y.H. Effedcts of straw returning on soil WSOC fulorescence characteristics with different tillage methods. Spectrosc. Spectral Anal. 2020, 40, 1232–1237. [Google Scholar]
- Kuang, E.J.; Xu, M.G.; Colinet, G.; Chi, F.Q.; Su, Q.R.; Zhu, B.G.; Zhang, J.M. Degradation characteristics of maize straw under different buried depths in northeast black soil and their effects on soil carbon and nitrogen. Int. J. Agric. Biol. 2020, 24, 77–84. [Google Scholar]
- Andrilli, J.D.; Foreman, C.M.; Marshall, A.G.; Mcknight, D.M. Characterization of IHSS Pony Lake fulvic acid dissolved organic matter by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and fluorescence spectroscopy. Org. Geochem. 2013, 65, 19–28. [Google Scholar] [CrossRef]
- Wang, S.Y. Studies on Microbial Metabolites and Biodiversity Under Poor Nutrition Conditions. Master’s Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Li, Z.; Shang, L.P.; Deng, H.; Zhi, T.X. Extraction of characteristic parameters three-dimensional fluorescence spectra of tyrosine and tryptophan. Spectrosc. Spectral Anal. 2009, 29, 1925–1928. [Google Scholar] [CrossRef]
- Shi, K.; Xia, X.; Guan, Q.; Shen, F.Y.; Huang, Q.R.; Li, D.M.; Liu, M.Q.; Li, H.X.; Hu, F.; Jiao, J.G. Changes of dissolved organic carbon in paddy soils with different levels of carbon amended with exogenous organic matter based on fluorescence spectrum analysis. J. Soil. Water Conserv. 2016, 30, 227–233. [Google Scholar] [CrossRef]
- Li, M.T.; Wang, J.H.; Zhao, L.P. Comparative study on fluorescence characteristics of water-soluble organic compounds in soybean and maize roots. J. Soil. Water Conserv. 2013, 27, 140–144. [Google Scholar] [CrossRef]



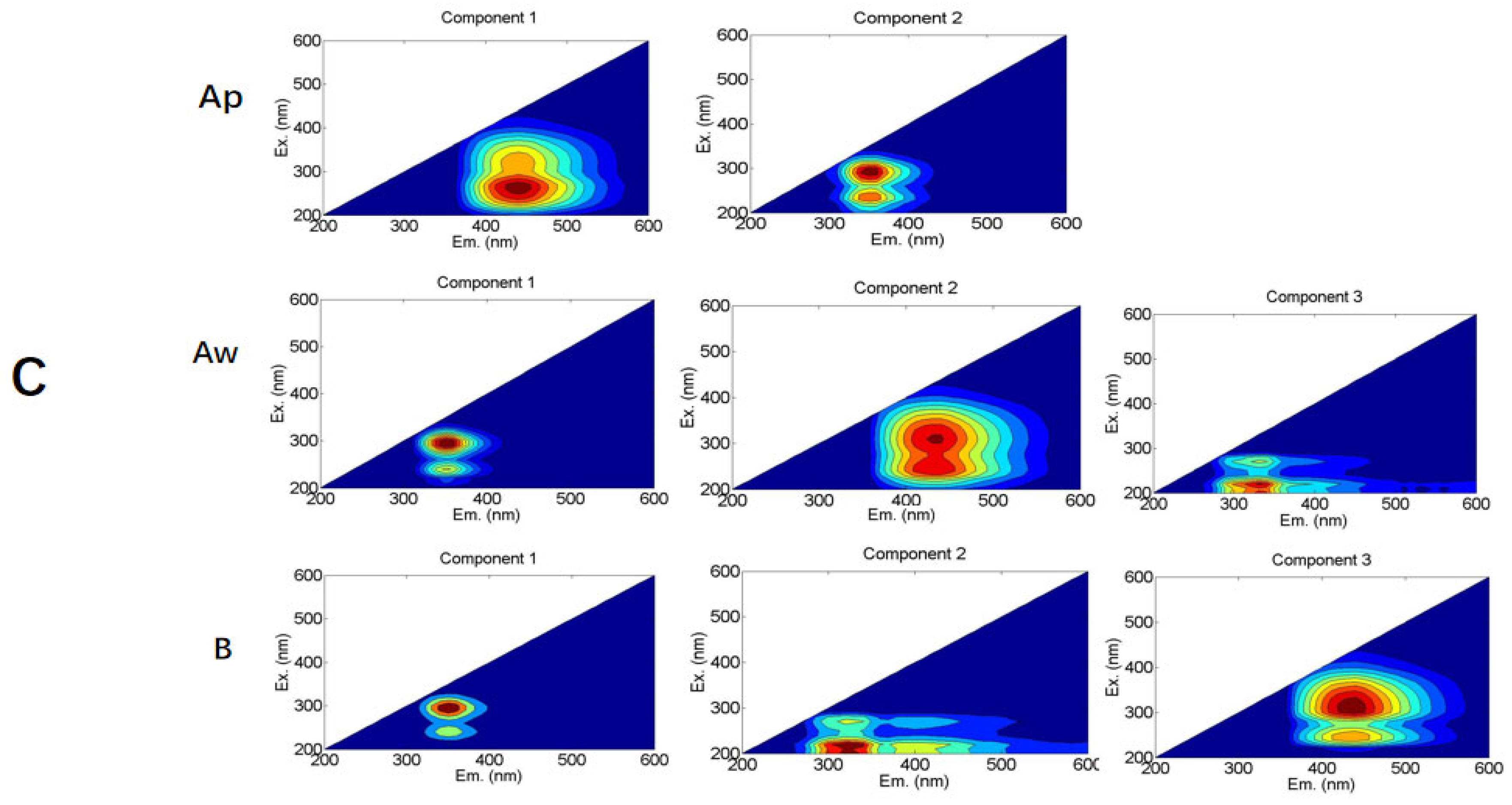

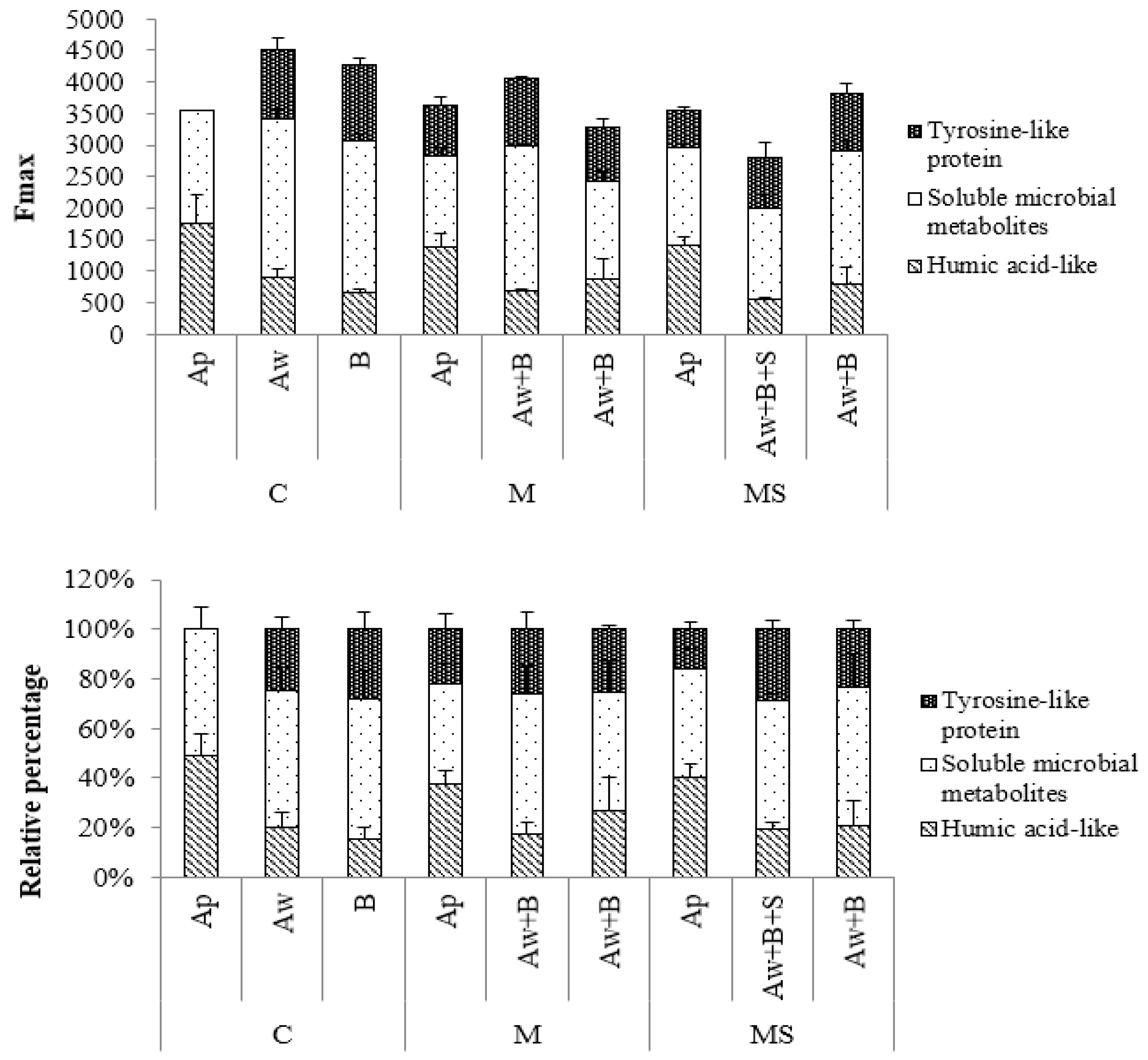
| Layers | Bulk Density (g·cm−3) | org-C (g·kg−1) | TN (g·kg−1) | C/N | CEC (cmol·kg−1) | Available Phosphate (mg·kg−1) |
|---|---|---|---|---|---|---|
| Ap (0–20 cm) | 1.18 ± 0.04 c | 13.84 ± 0.22 a | 1.17 ± 0.07 a | 14:1 | 19.3 ± 0.37 b | 21.8 ± 0.29 a |
| Aw (20–40 cm) | 1.55 ± 0.04 a | 3.77 ± 0.08 c | 0.83 ± 0.02 b | 5:1 | 14.5 ± 0.23 c | 6.0 ± 0.20 c |
| B (40–60 cm) | 1.43 ± 0.03 b | 4.58 ± 0.06 b | 0.69 ± 0.02 c | 7:1 | 31.3 ± 0.56 a | 9.2 ± 0.17 b |
| Treatment | Plant Height (cm) | Number of Pods per Plant | Number of Seeds per Plant | 100-Grain Weight (g) | Yield (kg hm2) | Increasing Yield % |
|---|---|---|---|---|---|---|
| C | 90.40 ± 0.61 c | 28.40 ± 0.26 b | 58.30 ± 0.75 c | 18.57 ± 0.19 a | 2598.8 ± 83.5 a | — |
| M | 94.54 ± 0.69 b | 30.34 ± 0.23 a | 63.93 ± 0.63 b | 18.61 ± 0.11 a | 2855.5 ± 150.2 a | 9.88 |
| MS | 97.20 ± 0.76 a | 31.20 ± 0.58 a | 65.70 ± 0.58 a | 18.62 ± 0.12 a | 2936.5 ± 208.4 a | 13.00 |
| Treatment | Soil Layer (cm) | Available N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) | pH |
|---|---|---|---|---|---|
| C | Ap | 131 ± 6.08 | 17.7 ± 0.62 | 162 ± 5.57 | 5.45 ± 0.19 |
| Aw | 78 ± 5.57 | 5.4 ± 0.35 | 121 ± 4.58 | 5.60 ± 0.08 | |
| B | 68 ± 3.61 | 9.7 ± 0.44 | 172 ± 6.24 | 5.90 ± 0.11 | |
| M | Ap | 136 ± 8.54 | 17.8 ± 0.62 | 164 ± 6.00 | 5.46 ± 0.16 |
| Aw+B | 85 ± 4.00 | 7.5 ± 0.32 | 137 ± 7.81 | 5.68 ± 0.07 | |
| Aw+B | 71 ± 3.61 | 9.8 ± 0.57 | 170 ± 6.24 | 5.94 ± 0.07 | |
| MS | Ap | 126 ± 9.17 | 16.1 ± 0.60 | 160 ± 6.56 | 5.45 ± 0.09 |
| Aw+B+S | 96 ± 7.94 | 13.6 ± 0.36 | 155 ± 5.29 | 5.69 ± 0.09 | |
| Aw+B | 79 ± 5.57 | 10.1 ± 0.33 | 172 ± 7.00 | 5.94 ± 0.13 |
| C | M | MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Ap | Aw | B | Ap | Aw+B | Aw+B | Ap | Aw+B+S | Aw+B |
| FI | 1.67 ± 0.02 c | 1.78 ± 0.05 b | 1.86 ± 0.03 a | 1.72 ± 0.03 b | 1.86 ± 0.07 a | 1.75 ± 0.03 b | 1.65 ± 0.03 c | 1.85 ± 0.02 a | 1.79 ± 0.03 b |
| BIX | 0.93 ± 0.13 c | 1.40 ± 0.34 b | 1.70 ± 0.43 a | 0.94 ± 0.16 c | 1.67 ± 0.48 a | 1.27 ± 0.06 b | 0.81 ± 0.04 c | 1.38 ± 0.09 a | 1.54 ± 0.56 a |
| HIX | 0.71 ± 0.04 a | 0.51 ± 0.06 b | 0.41 ± 0.05 bc | 0.66 ± 0.03 a | 0.41 ± 0.07 c | 0.56 ± 0.03 b | 0.75 ± 0.01 a | 0.49 ± 0.03 b | 0.46 ± 0.13 b |
| Treatments | DOC | SOC | C1 | C2 | C3 | DOC/SOC |
|---|---|---|---|---|---|---|
| DOC | 1.000 | |||||
| SOC | 0.857 ** | 1.000 | ||||
| C1 | −0.292 | −0.111 | 1.000 | |||
| C2 | −0.182 | −0.313 | −0.161 | 1.000 | ||
| C3 | 0.023 | 0.304 | 0.395 | −0.698 ** | 1.000 | |
| DOC/SOC | −0.294 | −0.694 ** | −0.207 | 0.385 | −0.609 ** | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Kuang, E.; Meng, Q.; Feng, H.; Wang, M.; Zhong, X.; Wang, Z.; Qiu, L.; Wang, Q.; Wang, Z. Characteristics of Soil Dissolved Organic Matter Structure in Albi-Boric Argosols Profiles Through Straw Incorporation: A Fluorescence Spectroscopy Study. Plants 2025, 14, 1581. https://doi.org/10.3390/plants14111581
Zhu B, Kuang E, Meng Q, Feng H, Wang M, Zhong X, Wang Z, Qiu L, Wang Q, Wang Z. Characteristics of Soil Dissolved Organic Matter Structure in Albi-Boric Argosols Profiles Through Straw Incorporation: A Fluorescence Spectroscopy Study. Plants. 2025; 14(11):1581. https://doi.org/10.3390/plants14111581
Chicago/Turabian StyleZhu, Baoguo, Enjun Kuang, Qingying Meng, Haoyuan Feng, Miao Wang, Xingjie Zhong, Zhichun Wang, Lei Qiu, Qingsheng Wang, and Zijie Wang. 2025. "Characteristics of Soil Dissolved Organic Matter Structure in Albi-Boric Argosols Profiles Through Straw Incorporation: A Fluorescence Spectroscopy Study" Plants 14, no. 11: 1581. https://doi.org/10.3390/plants14111581
APA StyleZhu, B., Kuang, E., Meng, Q., Feng, H., Wang, M., Zhong, X., Wang, Z., Qiu, L., Wang, Q., & Wang, Z. (2025). Characteristics of Soil Dissolved Organic Matter Structure in Albi-Boric Argosols Profiles Through Straw Incorporation: A Fluorescence Spectroscopy Study. Plants, 14(11), 1581. https://doi.org/10.3390/plants14111581






