Abstract
This study delves into the ethnoveterinary medicine (EVM) practiced by pastoralists along the transhumance routes in southwestern Angola. Within the framework of three cooperation projects, we conducted 434 interviews, collecting information on 89 taxa used for treating 16 livestock diseases. The most cited species was Ptaeroxylon obliquum (132 citations), followed by Salvadora persica (59) and Elaeodendron transvaalense (49). Contagious bovine pleuropneumonia (CBPP) was the disease most cited (223 citations; 44 species), followed by wounds (95; 20) and Newcastle (86; 14). We found that 30 species and 48 uses have not been previously reported in the ethnoveterinary literature. Jaccard index (mean value = 0.13) showed a greatly diversified knowledge among the ethnic groups: Kuvale and Nyaneka were the most knowledgeable and should be included in the various strategies for disseminating EVM in the area. Most informants recognized that abundance of some species decreased in the last years as a result of human activities and climatic changes. Finally, we discuss challenges in preserving the EVM in the area. Our findings suggest that preservation of the EVM in southwestern Angola is widely impacted by the access to biomedicine. Future studies should investigate the opportunity to integrate traditional medicine into mainstream development projects, which is crucial for decolonizing the veterinary sector in Angola.
1. Introduction
Pastoralism in southwestern Angola is a viable part of the local cultures where livestock herding not only plays a crucial role in ensuring livelihood and food security but is also the core of social life and culture as well as the base of religious and cultural identity of the indigenous communities [1]. The southwestern provinces have the largest number of livestock, accounting for 97% of total national bovine production; this region is characterized by erratic precipitations and high temperatures [2]. As a result of these climatic constraints, pastoralists relocate alongside their livestock on a daily and seasonal basis according to the distribution of fodder and water points [3] (Figure 1).
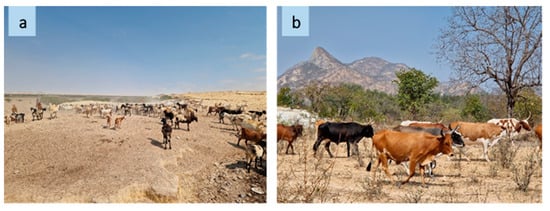
Figure 1.
The landscape of a grazing area along transhumance routes ((a) Namibe, source: R. Bozzi, 2023; (b) Huila, source: R. Bozzi, 2023).
The rise of nomadism and transhumance in this region dates back to very ancient times [4]; for millennia, mobility allowed opportunistic use of key resources and helped in reducing the impact of drought on the livelihood of communities living in the area. Angola is now experiencing its most severe drought period in 40 years [5], and approximately 3.8 million individuals are facing inadequate availability of food in the southwestern provinces [6]. Drought and lack of resources forced thousands of people to cross the Namibia borders or to migrate to urban areas, causing the process of “human desertification” of the rural communities. Despite the past month’s increased precipitation, long-term rainfall shortage and deterioration of the vegetation biomass (20–40% below the average) persist in Huíla, Cunene, and, in particular, Namibe [7]. Climatic events have detrimental impacts on the availability and quality of pasture, on the accessibility of water points, and, consequently, on the production and survival of livestock. Cattle, in particular, have been shown to be more affected due to their feeding habits and susceptibility to heat stress [8]. In addition, drought exacerbates the effects of parasites and infectious diseases caused by reduced immunity associated with poor nutrition and the increased risk of transmission in overcrowding conditions near water and food sources. The introduction of public measures to encourage animal vaccinations and the use of pharmacological treatments have contributed significantly to the prevention and control of the most serious animal diseases in Angola [9]. However, several political and socioeconomic barriers continue to limit access to veterinary medicines in Angola and sub-Saharan Africa in general [10]. Angola, for example, is completely dependent on imports of veterinary medicines from other countries and lacks an effective regulatory system addressing veterinary drugs [10]. Another problem is related to the difficulty of organizing a network of infrastructures and services able to rapidly meet the needs of all farmers; poor infrastructure and the limited availability of cold chain equipment make it difficult to reach cattle heads in remote areas before the vaccine is degraded to the point that it cannot ensure immunity. All these limitations make it urgent to search for alternative and complementary approaches to heal livestock diseases. In the last years, there has been an increased focus on ethnoveterinary medicine (EVM) due to the scientific validation of the efficacy of some traditional herbal remedies as well the awareness that this precious knowledge is rapidly disappearing [11,12]. EVM has been mostly investigated in Africa, Asia, and North America [13]. Regarding the southern areas of Africa, most of the research is from South Africa (see, for example, the review by [11] and the papers by [14,15,16,17]). More sporadically, studies have been carried out in Zimbabwe [18,19,20,21,22,23], Botswana [24,25,26], Mozambique [27], Namibia [12,28], and Zambia [29]. To the best of our knowledge, only one paper has been published on the ethnoveterinary uses in Angola [30]. The research presented in this paper is the result of a partnership between international cooperation and the scientific community, within the framework of three different projects: PIRAN, RETESA, and FRESAN.
We studied ethnoveterinary medicine practiced by pastoralists along the transhumance routes (Figure 2) crossing the southwestern provinces of Angola in order to outline the importance of the local ecological knowledge (LEK) for improving animal health and to discuss the opportunities and challenges of incorporating traditional knowledge-based practices into projects of development assistance to veterinary sector. We think that a rights-based strategy must integrate and underscore the LEK as a pivotal element for both decision-making and understanding of the actual needs of the local communities. The specific objects of this paper are (1) to record veterinary plants and their uses; (2) to identify differences and similarities of ethnoveterinary uses among ethnic groups living in the area; (3) to get information on the conservation status of the plants mentioned by the informants; and (4) to discuss challenges and constraints of preserving the EVM in the studied area.
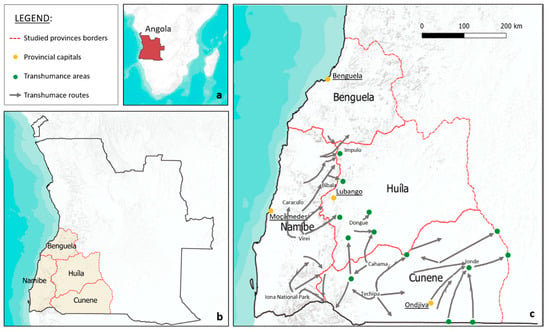
Figure 2.
Map of Angola (a); provinces where the study occurred (b); key transhumance routes (grey arrows) and significant grazing locations (green dots) (c).
2. Results and Discussion
2.1. Informants
We interviewed a total of 434 pastoralists: 279 in the first phase of the fieldwork (2014–2017) and 155 in the second phase (2023) (Table 1; Figure 3). Three thousand and eleven people (72%) reported the veterinary use of one species. Out of the knowledgeable informants, 24 (7.7%) were from Benguela, 62 (20%) from Cunene, 66 (21.3%) from Huila, and 159 (51%) from Namibe (Table 1).

Table 1.
Informants reporting ethnoveterinary uses in the studied area.

Figure 3.
(a) The first author interviewing Mumuhuila people about local plants and their veterinary uses (Bibala, source: M. Tonini, 2015). (b) A research assistant from the Veterinary Services Institute (ISV) interviewing a shepherd from Bibala (source: P. Bruschi, 2015). The participants of the study consented to the publication of the photos.
Most of them (54.7%) live in the dry steppes of the Namib savanna woodlands and Angolan Escarpment savanna ecoregions, 39% live in the dry woodlands of Angola Mopane ecoregion, and only 6% in the area of Angolan Miombo woodland ecoregion (see [2]) for the classification of Angolan ecoregions and vegetation types). Most of the informants were Nyaneka (21.5%), Kuvale (14.7%), and Muhumbi (14.5%) (Table 1); Kwanyama and Ovimbundu were other well-represented groups (10.2% and 9.6%, respectively (Table 1). Twenty-six informants did not report any information regarding their ethnicity. The observed distribution of informants by gender (91% males and 9% females) confirms the common pattern of greater male involvement in pastoral activities [12,17]. Livestock-related tasks, including herding and trading, are exclusively conducted by males, while females play crucial roles in milking cows, managing household chores, harvesting crops, and caring for children. Sixty participants did not know or remember their age; this is a common occurrence in rural areas of Angola [31]. The age of the other informants ranged between 17 and 88 (mean age = 46.9 ± 15.24) with the age group of 40–49 being the dominant one (22%), followed by the group of 50–59 (21%), and the group of 30–39 (19%). Thirty-five % of the interviewed people claimed to have received no education at all; the overall level of education is still poor and limited to the first grades of primary school.
2.2. Diversity of Veterinary Plant Species in South Angola
We identified 89 taxa belonging to 85 genera and 41 families for healing 16 different animal diseases (Table 2). Other eight identified species (Boscia integrifolia J.St.-Hil., Brachystegia tamarindoides Welw. ex Benth., Croton mubango Müll.Arg., Entandrophragma angolense C.DC., Pittosporum viridiflorum Sims, Senegalia schweinfurthii (Brenan and Exell) Seigler and Ebinger, Solanum anomalum Thonn., Zanthoxylum leprieurii Guill. and Perr.) have not been included in Table 2 because informants did not report the specific medicinal use. The number of species in our study is higher than reported in the same area [30] or in other Southern Africa regions [12,15,16,17,27]. This can be due to the greater number of informants interviewed or to the broader variety of ethnic groups involved in our study, which could have enabled us to cover a broader range of knowledge systems. Moreover, transhumance routes in southwestern Angola cross diverse ecosystems (dry steppes, Miombo and Mopane), and shepherds can have the chance to know and experience a high variety of plants. Plant species were compared with the available data from other ethnoveterinary studies carried out in other Southern Africa areas. The results showed low values of similarity (mean JI = 6.2 ± 1.7), confirming the richness and peculiarity of ethnoveterinary wisdom owned by the pastoralists of southwestern Angola. The highest value was found with studies conducted in Namibia [12,28] and Zimbabwe [18,19,20,21,22,23] (JI = 8.2), while the low values were obtained for Botswana [24,25,26] (JI = 3.5) and Zambia [29] (JI = 4.9). Comparison with South Africa [11,14,15,16,17] and Mozambique [27] showed a JI = 6.5 and a JI = 6.1, respectively. Most species (51%) had only one or two citations (Table 2) and only 20% of the species were cited more than ten times.

Table 2.
List of veterinary taxa used in the studied area.
The most cited species was Ptaeroxylon obliquum (Thunb.) Radlk (Figure 4c) (132 citations; 17% of the total citations), followed by Salvadora persica L. (59; 7.7%), Elaeodendron transvaalense (Burtt Davy) R.H.Archer (49; 6.5%), Capsicum frutescens L. (47; 6.1%), Aloe littoralis Baker (Figure 5a) (46; 6%), and Zygophyllum simplex L. (Figure 5b) (43; 5.6%) (Table 2).

Figure 4.
Some useful medicinal plants indicated by the informants: (a) Pterocarpus angolensis (source: P. Bruschi, Bibala 2015), (b) Pachypodium lealii (source P. Bruschi, Bibala 2015), (c) Ptaeroxylon obliquum (source P. Bruschi, Bibala 2015), and (d) Ximenia americana (source: P. Bruschi, Bibala 2015).

Figure 5.
Useful medicinal plants indicated by the informants (a) Aloe littoralis (source: P. Bruschi, Namibe 2015); (b) Zygophyllum simplex (source: K. Kamba, Bibala 2014).
Most of the species belong to Fabaceae (22 species; 25%), followed by Asteraceae (6; 7%), Euphorbiaceae, Malvaceae, and Solanaceae (4 each; 4.5%). Similar findings were reported in the study conducted by [32] on the plants used for the human disease treatment in the same area. According to [33] Fabaceae and Asteraceae are the largest botanical families in Angola with 934 and 463 species, respectively; moreover, Fabaceae and Asteraceae have been reported to have several ethnopharmacological uses both in Angola and worldwide (see among others, [11,32,34]). Most of the Fabaceae mentioned in this study are important, or even dominant, species of the Miombo and Mopane ecosystems [34,35] where the interviewed shepherds live or that they cross during their seasonal migration. The floristic composition and physiognomic structure of these dry woodland ecosystems could also explain the results of the life forms analysis: perennial woody plants are the most numerous (70%; 54% trees and 16% shrubs), followed by herbs (21%, of which 17% are perennial), succulents (5.7%), and lianas (2.3%). Moreover, many studies [32,36] have shown that in strongly seasonal environments, woody plants are more frequently used than herbs. Trees and shrubs can supply a variety of useful products all year round, especially in the arid months, due to the biological and ecological characteristics making them more resistant to drought and other abiotic disturbances. As a confirmation of this, informants reported that 87% of the mentioned remedies (85% of all the citations) were available all year round, while 10% and 2% were available during rainfall and dry seasons, respectively. Among the 89 identified species, 74 are native and 15 are exotic. Most annual herbs were cultivated species that can be grown in gardens throughout the year, like Solanum lycopersicon L., Nicotiana tabacum L., and Zea mays L. Leaves were the most frequently quoted used parts (255 citations; 36% of the total citations). followed by roots (206; 29%), bark (158; 22%), and fruits (57; 8%). These results are consistent with those reported in many other ethnoveterinary studies [16,17]. The most frequent preparation method was maceration (379 citations; 50% of the total citations), followed by burn (71; 9.5%), paste (69; 9.2%) powder (63; 8%), infusion (48, 6%), and raw (40; 5%). The oral route was the main way of administration (467 citations; 62% of the total citations), followed by direct application (213; 28%), inhalation (56; 7%), and bath (19; 2.5%).
2.3. Diseases Treated by Plant Preparations
Plant remedies were mostly used for treating cattle (91% of the total number of citations; 84 species) and, in a secondary way, chicken diseases (7.9%; 14). Only a few citations were recorded for the veterinary uses concerning goats and pigs. The magnitude of this discrepancy confirms the importance that cattle herding plays in the economy and the sociocultural context of these people [37]. A man’s wealth depends on the number of heads he owns; cattle are an integral part of the life of each member of the community, and they are the main reason for physical and social mobility [38]. The economic use of cattle is mostly milk production, which is the basis of the agropastoralists’ diet and provides cash income during the year. As an example, the fermented buttermilk produced by Herero and Ovambo of northern Namibia and southern Angola plays an important cultural and economic role [39]. Poultry production in rural areas is a traditional household activity carried out mainly by women and children [40]. Caprines and ovines are not particularly important either culturally or economically, although they represent the currency of exchange and are consumed more frequently than bovine meat. Most ethnoveterinary uses (93%) had only one or two citations (Table 3) and only 2% of them were cited more than ten times. The higher number of citations (223 citations; 29% of the total number of citations; 44 species; see Figure 6b) regarded the treatment of CBPP (contagious bovine pleuropneumonia, locally known as Cawenha) (Table 3). This finding is in accordance with [30] and reflects the epidemiological situation of the area. An investigation carried out by [41] showed that 64% of farmers interviewed in Namibe identified CBPP as a primary factor of cattle death and estimated the prevalence of the disease to be more than 94%. More recently, ref. [42] found that 83% of total rejections in Benguela slaughterhouse were caused by the manifestation of CBPP symptoms. The most cited species for treating CBPP were Salvadora persica (54 citations; FL = 98%), Ptaeroxylon obliquum (50; 48%), Spirostachys africana Sond. (17; 81%), Oldfieldia dactylophylla (Welw. ex Oliv.) J. Léonard (12; 100%), Peltophorum africanum Sond. (11; 61%), and Cissus quadrangularis L. (10; 91%). The only literature references supporting this veterinary use were for P. obliquum [30] and C. quadrangularis [43]; however, extracts of S. persica [12,44], P. obliquum [45,46], S. africana [47], and P. africanum [48] have been shown to have noticeable antimicrobial activity. O. dactylophylla is reported as a remedy for pneumonia in humans [49]. Wounds were the second most cited disease category (95 citations; 12.5% of the total number of citations; 20 species) (Table 3) and included sores, abscesses, warts, and inflamed skin lesions. According to the informants, wounds are mainly caused by tick bites and thorny bushes or other plant materials.

Table 3.
List of treated diseases.
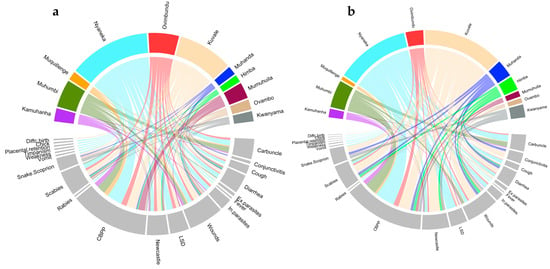
Figure 6.
Circular plots showing the relationship between the reported livestock disease and the different ethnic groups. Color bands summarize relationships between each disease and ethnic group (ticks represent the number of species (a) and the number of citations (b) for each disease and ethnic group, see Table 3).
The main plant used for treating wounds was Elaeodendron transvaalense (49 citations; FL = 100%), followed by Peltophorum africanum (7; 39%) and Erlangea misera S. Moore (6; 100%). Ethnoveterinary uses of E. transvaalense reported in the literature concern the treatment of diarrhea [50,51] and worms [51]. However, this species is traditionally used in human medicine to treat skin rashes and wounds [52] and as an emetic [53]. Several studies have demonstrated that E. transvaalense extracts have antibacterial and anti-inflammatory activities, thereby supporting their traditional uses [54]. P. africanum is used to treat wounds in humans [48]. No literature information is available on E. misera as a veterinary remedy. The only medicinal use reported in the literature for this species is related to vomiting and fever relief in Kwanyama children [55]. Newcastle was the only disease mentioned for poultry (86 citations; 11% of the total number of citations; 14 species) (Table 3). Newcastle is the major cause of chicken mortality in rural villages and represents an important constraint to traditional poultry production in Angola [40]. Capsicum frutescens (46 citations; FL = 98%) was the main drug used to treat Newcastle, followed by Aloe littoralis (12; 32%) and Ptaeroxylon obliquum (9; 9%). Remedies against Newcastle, based on fruits of C. frutescens and leaf sap of Aloe spp., have been reported in studies conducted in other African countries [23,56,57,58]. While no prophylactic or therapeutic value of C. frutescens extract against Newcastle virus has been found [59], a reduced mortality rate has been observed in chickens treated with Aloe secundiflora Engl. leaf extract [60]. Other frequently mentioned treatments concerned scabies (83 citations; 10.8% of the total number of citations; 16 species), viral and bacterial diarrhea (60; 7.8%; 14), symptomatic carbuncle (46; 6%; 17), snake or scorpion bites (41; 5.4%; 12), and LSD (lumpy skin disease) (40; 5.2%; 12). The most frequently cited plant against scabies was Zygophyllum simplex (43 citations; FL = 100%), followed by Securidaca longipedunculata Fresen. (9; 69%), Otomeria elatior (A.Rich.) Verdc. (7; 54%), and Ximenia americana L. in Figure 4d (6; 75%). Z. simplex is reported by Cholistan pastoralists to treat livestock skin problems [61], but it is also documented as a traditional remedy for the treatment of dry scaly patches and cleaning the skin in humans [62]. S. longipedunculata has been proven to have high pesticidal effects against several insect species [63]. X. americana is used to treat internal parasites in cows and has been proven to have acaricide activity against the cattle parasite Rhipicephalus microplus [64]. In southern Angola, an oil obtained from the kernels of this species is widely employed to cure skin problems in humans. P. obliquum was the most reported species to treat diarrhea (28 citations; FL = 27%), followed by P. angolensis (6; 100%). P. obliquum is used against intestinal parasites in goats and, also, to treat diarrhea in humans [32]. P. angolensis is reported to have anthelmintic, antibacterial, and anti-inflammatory activities [65,66]. P. obliquum was also the most used species to treat symptomatic carbuncle affection (or anthrax) (16 citations; FL = 15%). This is in accordance with what was reported by [67] and [45]. The main species against snake or scorpion bites were Senna occidentalis (L.) Link (15 citations; FL = 75%) and Sclerocarya birrea Hochst. (11; 55%). S. occidentalis is widely employed in traditional medicine for the treatment of local tissue damages in humans induced by snake and insect bites [68]. Ref. [32] reported the same use in the Namibe area. S. birrea is known as a remedy in human medicine for the treatment of snake bites in Senegal [69], Tanzania [70], and Ghana [71]. P. obliquum (12 citations; FL = 11%) was the most frequently cited species for treating LSD, followed by Spirostachys africana (7; 33%) and Peltophorum africanum (5; 28%). Other interesting and new ethnoveterinary uses were recorded for the treatment of conjunctivitis with the endemic species Thesium cinereum A. W. Hill (16 citations; FL = 100%) and Cenchrus americanus (L.) Morrone R. Br. (6; 100%). The only traditional medicinal use of T. cinereum reported in the literature is for the treatment of human bronchitis in southern Angola [72]. Other species belonging to the genus Thesium are used to enhance the sight [73]. Ref. [16] report Thesium sp. in the treatment of diarrhea and internal parasites in calves and cows. C, americanus is used to heal brucellosis in cattle. However, a similar species, Pennisetum pedicellatum Trin., is used to treat human eye infections in northern Nigeria [74]. The most versatile species was P. obliquum (11 uses), followed by Aloe littoralis (9), S. longipedunculata (6), P. africanum, S. occidentalis, and S. africana (5 each). By comparing our research results with previous studies, we found that 30 species (58% of the total number of species) and 48 uses (67% of uses recorded for the already known species) have not been previously reported in the ethnoveterinary literature (Table A2 in Appendix B). These findings show that our study provides an important contribution to the ethnoveterinary medicine of southern regions of Africa and, also, highlights the importance of recording new ethnobotanical information for already known medicinal plants.
2.4. Plants and Uses among Different Ethnic Groups
There were 63% of plants and 58% of uses recorded from at least two ethnic groups. Ptaeroxylon obliquum was reported by 10 ethnic groups; Aloe littoralis, Capsicum frutescens, Elaeodendron transvaalense, Peltophorum africanum, Salvadora persica, and Zygophyllum simplex were reported by 6 ethnic groups; and Cissus quadrangularis and Spirostachys Africana, by 5 ethnic groups. P. obliquum against CBPP was the most common throughout different ethnic groups (8 ethnic groups); C. frutescens to treat Newcastle, E. transvaalense to heal wounds, P. obliquum to treat diarrhea, S. persica to treat CBPP, and Z. simplex to treat scabies were reported by 6 ethnic groups. C. quadrangularis against CBPP, P. obliquum against symptomatic carbuncle, and S. africana against CBPP were reported by 5 ethnic groups. The high diversity of ethnoveterinary knowledge existing in the area was confirmed by the low values of the Jaccard similarity index (JI mean value = 0.13 ± 0.12). The highest value was 0.50 when comparing Mumwila (Nyaneka-Humbi ethnolinguistic group) and Himba (Herero ethnolinguistic group). Muhanda (Nyaneka-Humbi ethnolinguistic group) was the most different with JI values ranging from 0.0 to 0.09. Ovambo and Kwanyama, belonging to the Ambo ethnolinguistic group, showed a certain similarity (JI = 0.44) and were strongly different from the other ethnic groups. Kuvale and Nyaneka were particularly skilled in treating CBPP, Newcastle, scabies, LSD, and cough (Figure 6). Kuvale also had the higher number of unique uses (6), followed by Muhanda (4) and Nyaneka (3).
These results suggest that Kuvale and Nyaneka should be included in the various strategies for promoting and disseminating knowledge about the use of ethnoveterinary plants. Kuvale has developed a lot of knowledge about the rearing and management of livestock. Although their society is greatly conservative, it is possible to find some similarities with other groups living in the same area like the Himba, the Nyaneka, the Mwila, and the Muhimbi [75]. Similarities with these groups can also be found in the use of veterinary plants, suggesting an exchange of knowledge and experiences on the treatment of animal diseases along the transhumance routes. On the other hand, the same linguistic ancestry characterizing all the Bantu languages can provide important points of convergence between ethnic groups belonging to the Nyaneka-Humbi ethnolinguistic group; this also true for Kuvale and Himba that are believed to speak the same Herero dialect, which, along with Nyaneka-Humbi languages, has been included in a subcategory of Southwest Bantu languages [76].
2.5. Conservation Status of the Mentioned Species
Most interviewees (78%) recognized that the abundance of some species (Figure 7) (e.g., Elaeodendron transvaalense, Ptaeroxylon obliquum, Salvadora persica, Zygophyllum simplex) decreased in the last years and that these changes are mainly related to human activities and climatic changes. Distribution of abundance categories based on informants’ perception showed that 202 (42%) citations accounted for high abundance, 150 (31%) for medium abundance, and 131 (27%) for low abundance (see Table A1 in Appendix A; Figure 7). Only 16 (18%) mentioned species were reported in an inventory study conducted by [77] on Mopane vegetation in the same area (Table A1 in Appendix A). Species having higher density in the inventory plots were Colophospermum mopane (J.Kirk ex Benth.) (PAI: 2.7), Croton gratissimus var. gratissimus (2.4), Ximenia americana (2.3), Salvadora persica (2.0), and Spirostachys africana (2.8). Rarely encountered species were Peltophorum africanum (PAI: 1.9), Sclerocarya birrea (1.7), and Elaeodendron transvaalense (1.7). Ptaeroxylon obliquum, which was the species most cited by our informants, had an abundance of about 13 individuals/ha (PAI: 2.2). Similar density values have been found by [78].
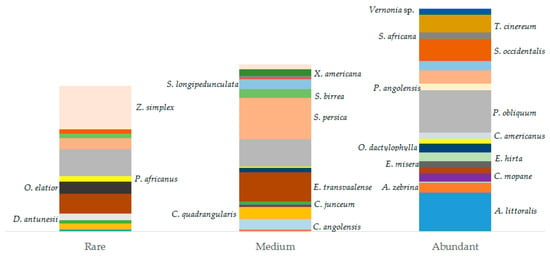
Figure 7.
Distribution of species (≥5 citations) abundance according to the informants’ perception. Species were grouped into three categories (x-axis): rare, medium, and abundant. Each colored box represents a species and box sizes represent the number of interviewees reporting a certain abundance category for each species. Species can occur in more than one category (e.g., P. obliquum is reported in the three abundance categories) because the graph is based on the number of interviewees indicating a certain abundance category for each species. We included the scientific name of the species in the box of species with a higher number of reports in each category.
Thirty-eight wild species mentioned in our study are included in the Red List [79] (see Table A1 in Appendix A); all of them are reported as the least concern (LC) and stable with the exception of Philenoptera pallescens (Welw. ex Baker) Schrire, which is categorized as vulnerable (VU) and decreasing. Ref. [80] reported fifteen species as VU, one as EN (Diospyros mespiliformis Hochst. ex A.DC.), and five as LC. Among the most cited species in our study, six are classified as VU by these authors (see Table A1 in Appendix A). The analysis of endemism showed that four species (Dicoma antunesii O. Hoffm., Helichrysum benguellense Hiern, Philenoptera pallescens, Thesium cinereum) are endemic to Angola.
The relatively restricted distribution of these species makes them potentially more susceptible to environmental threats. Knowledge of perceptions can be used to establish baselines in the first phases of a conservation project and to have a rapid overview of the conservation status of species when other data are not available [81]. However, local perceptions can be greatly subjective and do not always represent the actual status of the environment [82]; this can explain the lack of a significant correlation (R = 0.17; p > 0.05) between PAI and density values in the inventory studies. Despite experiencing the same ecosystems, different cultural backgrounds may have different views on the conservation status of ecosystems. In the case of Securidaca longipeduncolata, Nhanyeka reported the species as rare, while Kuvale indicated it as highly abundant. Ptaeroxylon obliquum was abundant according to Kuvale, Muanha, and Muhanda perceptions, and as rare according to Nyaneka. Oldfieldia dactylophilla was reported as abundant by Kuvale and as medium by Muhumbi. When plants were mentioned only by one group, we often observed absolute concordance in terms of abundance categories. For example, Cenchrus americanus and Pterocarpus angolensis (Figure 4a) were mentioned only by Muhanda and all the six informants reported these species as abundant in the area. The same concordance pattern was observed for Thesium cinereum and Cassia angolensis Hiern. that were reported by Kuvale (T. cinereum) and Nyaneka (C. angolensis) as abundant and medium, respectively.
2.6. Folk Names of Reported Plants
The scientific identification of plants is a process demanding a considerable amount of time and it requires well-trained and highly skilled professionals. In this regard, a repertoire of folk names with the corresponding scientific names may be a very useful tool for a rapid assessment of resource uses in remote areas, especially during Rapid Rural Appraisal (RRA) surveys [83]. However, while recognizing the significant role of folk nomenclature in providing valuable insights on LEK, many authors have pointed out the risk of misidentification and misinterpretation by an ethnobotanical approach based only on the collection of folk names [84,85]. In our study, 149 folk names were recorded for the 89 identified taxa. Fifty-four percent of these folk names matched those included by [86] in their list and could be ascribed to the same species; however, when comparison was made with data reported in [30,32], we found that only 9 folk names could be consistently equated with the 18 species common to these datasets. These results suggest that folk names could provide a valuable contribution to the rapid identification of the ethnospecies. At the same time, a critical approach when using this type of data for ethnobotanical inventory purposes is crucial. We observed that some folk names had correspondence with two or more species in [86]. For example, the Nyaneka name “onambumbu”, identified as Croton gratissimus var. gratissimus in our study, is ascribed by [86] both to Croton gratissimus var. gratissimus and Croton integrifolius Pax.; the Mumuhuila name “katete”, identified as C. integrifolia in our study, was reported both as C. integrifolia and Eugenia malangensis (O. Hoffm.) Nied. in [86]. Thirty percent of folk names were not reported at all by [86] and thirteen percent were reported with a slightly different transcription (e.g., “chifuaca” vs. “tchifuica” = Fadogia cienkowski var. lanceolata Robyns; “muipanhoca” vs. “murianhoca” = Senna occidentalis; “nganga” vs. “unganga” = Burkea africana Hook.; “opandambala” vs. “omupndambale” = Philenoptera pallescens; “opukange” vs. “omupange” = Dichrostachys cinerea (L.) Wight and Arn.). Last, 3% of the folk names were ascribed to different species in [86]. For example, the Umbundu name “ongombe” that we identified as Commiphora mollis (Oliv.) Engl. was ascribed by [86] to three different species: Argyrolobium aequinoctiale Welw. ex Baker, Cordia sinensis Lam., and Rothmannia engleriana var. ternifolia (Ficalho and Hiern) Somers. These incongruences could be the result of linguistic issues [72,86] or, in some cases, of errors in transcribing the information by the different interviewers involved in this study (e.g., the names “Lumbungululu”, “Lumungululu”, “Mbungululu”, “Mbungulolo”, “Mumbungululu” reported during the interviewers are all spelling variations of the same folk Nyanheca name for Ptaeroxylon obliquum) (Figure 4c). It is undeniable that the risk of confusion is particularly high in a country like Angola where a multitude of different languages is spoken [87] and local variants can be found within the same linguistic group; in this regard, familiarity of the interviewer with the local languages is a factor strongly affecting the process of data collection. Moreover, morphological similarities and ecological convergence can amplify the risks associated with the abundance and variety of folk names. The use of folk names remains crucial for communicating with local people and public institutions about the importance of preserving LEK and can provide guidance in identifying a taxon when no other information is available. However, our results indicate that each record should be supported by a voucher specimen and validated through identification by an expert on the local flora.
2.7. Challenges and Constraints along Transhumance Routes
The practice of transhumance has changed over the years due to environmental, economic, and social factors, as reported by the informants. The main problems affecting the availability of resources in the region are habitat destruction due to timber harvesting/charcoal production and overgrazing by livestock [88]. Since 2008, the south of Angola has been repeatedly hit by droughts and floods, affecting the quality of pastures and the health of livestock. This situation is expected to worsen in the coming years. Average annual temperatures are projected to warm by 2.0 and 3.0 °C between 2040 and 2060 [89]. The impact of these changes on people’s livelihoods is already being felt, particularly in terms of water availability and vegetation cover. Participants in our study largely agreed that the decline in rainfall and the unpredictable and shortened rainy season are leading to water scarcity and a reduction in grazing land in the region. This view is exemplified by the following quotations: “It’s been 10 years without rain, water and pasture are now rare” and “Now it does not rain anymore and we are forced to walk livestock over long distances”. In addition, land grabbing and the occupation of common land, which includes trails connecting seasonal grazing areas, are fuelling landscape fragmentation and threatening the survival of transhumance routes [90]. A shepherd from Tombwa told us: “Many losses on the road, as often the animal doesn’t make it and die” This refers to the exhaustion of the animals due to fatigue and high temperatures and the struggle for grazing and water along the transhumance routes. Some informants also reported that attitudes toward mutual aid and solidarity, which have historically played a key role in the social cohesion and resilience of pastoralist communities [91], have recently declined due to climate change and land rights issues. Disputes and conflicts over grazing land and water points often occur along the border areas. Surveys revealed that conflict-prone areas also include regions close to provincial and municipal borders where overlapping resource claims lead to disputes. Frequent areas of conflict are the main destinations of transhumance, i.e., Pediba, Quilemba Velha, Lola, and Mutipa, where herd movements overlap with sedentary communities and their agricultural activities. Conflicts are more common among young people, who are less willing to engage in dialogue and respect rules and traditions and are often exacerbated by ethnic differences. Conflict resolution mechanisms include community meetings convened by the traditional authorities. Resolutions usually involve compensatory measures, such as the payment of livestock or financial compensation, to redress grievances and restore harmony between communities and/or pastoralists. According to interviewees, adaptation strategies that emphasize dialogue, cooperation, and negotiation between pastoralists and local communities, always with the involvement of traditional authorities and leaders, have been used in response to these changes. Proposals to adapt to the changing climatic conditions include creating water reserves, planting more fodder trees for livestock, and expanding grazing areas. Preserving cultural aspects remains a priority, but adaptations could ensure the sustainability of practices in the face of changing circumstances. The resilience of the community and its ability to overcome challenges through dialogue and collaboration are the key themes of the analysis.
2.8. Western Veterinary Medicine and Indigenous Practices
Our findings suggest that the management of livestock diseases in southwestern Angola is widely influenced by the access of shepherds to biomedicine. The recent promotion of free mass vaccination campaigns in Angola and the efforts made by the Angolan authorities to make the veterinary service more efficient throughout the whole territory have significantly improved the prevention and containment of CBPP, hematic and symptomatic carbuncle, and LSD [9]. In addition to causing livestock death, lack of regular vaccination and parasite control can contribute to organs and carcass rejections in slaughterhouses [42]; this severely impacts livestock productivity and, therefore, farmers’ economic welfare. Then, it is unsurprising that most of the respondents claimed to currently rely on Western veterinary medicine rather than on traditional plant remedies. A Nanyeka informant stated that “if a cow does get sick, we call the doctor”. A Kuvale woman said: “We buy medicines in the animal store”. Another informant: “We use conventional medicine because it works well”. For these and other shepherds stating similar assertions, the use of traditional EVM was apparently of no use. They believe that the government veterinary service has better expertise for the prevention, detection, and management of animal illnesses and that Western medicine is more effective than traditional practices. This perception is transversal to all ethnic groups and can be presumed to be basically independent of any cultural membership. Some studies have shown that farmers are pragmatic and evaluate/absorb information in a practical and utilitarian way regardless of whether it comes from Western science or any other source [92,93]. When a particular piece of knowledge proves to be useful, economically sound, and socioculturally acceptable, it will be adopted [94]. A predominance of livestock treatments through conventional medicine was also observed by [30,95] in the same provinces where we conducted this study. Conversely, refs. [16,96] showed that in South Africa, most indigenous people preferred to use traditional approaches where Western medicine was available. As suggested by [97], the hegemony of the Western approach can have a strong impact on the conservation of indigenous health traditions, but at the same time, it can cause the opposite effect of cultural resistance and revalorization of traditional medicine. Some herders interviewed in our study seemed to be reluctant toward the adoption of Western medicine, expressing trust in the traditional approach. This perception is exemplified by the following quotation: “Plant remedies work well for me; there were times we had only plants to heal animals. Now there are vaccines but I continue to use plants because they are effective”. This resistance to Western science could be due to deep-rooted beliefs transmitted within the clan or the community from generation to generation: “Our forefathers used plants, our fathers used plants, we continue to use plants”; “elders of the community passed on their knowledge to the younger ones, and that is how it works to this day”(Figure 8a).
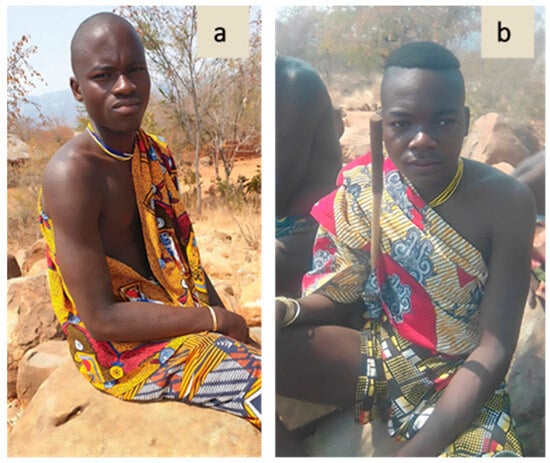
Figure 8.
Young shepherds from Kamacuio (a) and Bentiaba (b) interviewed in Namibe by ISCED technicians in 2023. The participants of the study consented to the publication of the photos.
Responses from other informants suggest that government veterinary procedures are sometimes viewed with suspicion by some shepherds; this lack of trust in institutions could have led to the resistance of people to the acceptance of veterinary services and to the adoption of chemicals to treat livestock diseases. One shepherd mentioned that plant medicines are healthier whereas pharmaceuticals have a negative impact, suggesting that another possible constraint to the use of Western medicine is the lack of notions about how to manage and apply veterinary medicines to livestock. An uncorrected utilization can produce counterproductive effects on the animal health causing distrust in conventional products. These adverse impacts can be exacerbated by illegal veterinary medicines, including falsified, unregistered/unauthorized products with low active molecule concentration or low bioavailability [98].
2.9. Importance of Preserving EVM
It is conceivable that the introduction of Western practices might have impacted the traditional understanding of livestock health care and on the conservation of ethnoveterinary knowledge in the studied area. In a similar way, ref. [99] suggests that the development of veterinary services promoted by state subsidies would have caused the abandonment of EVM in Russian Karelia. Ref. [100] supposes that modernization of veterinary practices in Kenya relegated EVM to a subordinate position where traditional approaches are considered primitive and anachronistic. Two Kuvale shepherds encountered in 2023 said to us: “What do we need plants for when vaccines are available? Our elders know the uses of healing plants but we do not need to know them”. Other informants reported that many more plants were used in the past to treat livestock diseases. By comparing data from the two recording periods, we observed that the informants quoting almost one veterinary species were 82% in 2014–2017 and 51% in 2023. A great reduction in the number of reported species and uses was also observed: species were 79 in 2014–2017 and 24 in 2023; uses were 148 in 2014–2017 and 52 in 2023. In particular, this reduction is associated with the disappearance of plants against internal parasites (8 species in 2014–2017) and LSD (12), and a lower number of species for treating carbuncle (14 in 2014–2017 vs. 4 in 2023), Newcastle (13 vs. 3) and wounds (20 vs. 1). Even taking into account the limitations due to design effects (i.e., different number of informants interviewed and data collected by different interviewers), these findings raise concerns on the conservation of EVM in the studied area. The risk of losing traditional practices does not regard only the healing practices; 42% of the interviews also revealed that cultural items traditionally associated with transhumance, such as ritualistic sacrifices and community celebrations before departure, have vanished in some areas. In this regard, an informant said that “in the past, a goat was killed before leaving with the cattle, now they no longer do that, they just take the cattle and go” (Figure 8b). Preserving and valorizing this piece of indigenous knowledge is crucial for ensuring the resilience of the pastoral communities coping with harsh environmental conditions like those found in southern Angola. EVM might provide valuable solutions when addressing minor health issues where the cost of veterinary services is unaffordable and where, especially in remote communities, access is limited [101]. Angola is dependent on imports of veterinary medicines from other countries [10] Medicine shortage makes it hard to meet the medical needs of local communities, so some livestock diseases cannot be treated according to the canons of Western medicine. For example, the country has experienced five years of consecutive economic recession since 2016, which led to a discontinuity in the vaccination rate and to the spread of some epidemic diseases causing high production losses [42]. Ref. [102] reports that Newcastle vaccinations, even if available since 2012, have not reached remote villages of southwestern regions of the country. Another important issue to be considered is the high and often unaffordable cost of medicines not covered by the National Veterinary Service. The cost of antibiotics used for healing wounds ranges from AOA 1550 to AOA 18,600. An acaricide may cost AOA 19,700. As a comparison, the selling price of milk ranges between AOA 200 and AOA 400 per liter; the market price of cattle ranges between AOA 150,000 and AOA 300,000, depending on the body weight and quality. This practice not only increases the costs associated with the vaccination but also exposes herders to the risk of theft and robbery while traveling. Similar constraints to the use of orthodox livestock medicines have been underlined by [103] in South Africa. The need to travel to a veterinary pharmacy, which is often far away, means additional transport costs, which worsens the herder’s economy [104]. Another motivation for preserving EVM knowledge is related to the need for alternatives to conventional medicines. The inappropriate and repeated use of antibiotics and acaricides is known to increase the number of drug-resistant pathogens, the accumulation of chemical residues in the food chain, and negative environmental effects. The use of plant remedies can contribute to the reduction of this problem; plants have a complex and refined mix of different active biomolecules able to cover a wide spectrum of therapeutic indications. In this regard, ref. [105] affirms the need to find substitutive treatments for the control of endo- and ectoparasites in Angolan livestock by using bioactive plants. Moreover, due to their easier and more rapid metabolization rate, phytochemicals reduce the risk of contaminants in meat and dairy products [106].
2.10. EVM in the Framework of Development Projects
Some papers [107,108] stress the importance of making the interests and requests of the academy match those of people living in the studied communities. This concept encompasses different knowledge domains and implies a continuous bidirectional exchange of information between professionals/researchers and local actors, with mutual learning opportunities for mutual benefits. Ref. [109] suggests that recording information on indigenous veterinary practices is the most positive way to face problems of tropical animal health within the framework of assistance programs. Ref. [100] stresses the importance of a bottom-up approach where the indigenous perception of livestock health problems plays a crucial role in planning and developing projects, making interventions more adapted to the sociocultural context in which people live and work. How to integrate indigenous knowledge into the practice of the developmental process is the real challenge. According to [11], both modern medicine and EVM have their advantages and limitations. As already pointed out, the application of Western medicine has several constraints in African countries; on the other hand, plants cannot eradicate epidemics like CBPP or carbuncle and do not serve as a universal solution for all animal diseases. This awareness was also shared by shepherds interviewed in this research, who mostly claimed to rely on conventional Western medicine. Preserving traditional culture remains a priority, but calibrations are needed to allow indigenous communities to cope with the ever-changing environmental and socioeconomic conditions. For this reason, focusing on EVM as a radical alternative to the Western veterinary health care system is not a viable option for local communities. As observed by [94], a representation of indigenous knowledge as something untainted to be preserved against the progress of Western science is misleading because it creates an image of the indigenous culture as conservative and resistant to innovation. Moreover, the view that indigenous knowledge produces a more reliable and sustainable solution to the problems of local communities than Western practices is illogical and dangerous. The crucial action to take to preserve EVM in African countries is to negotiate a way of integrating traditional medicine into mainstream development projects and health policies. This strategy could allow curbing the erosion of ethnoveterinary knowledge and fostering decolonization of the veterinary and paraveterinary sectors. Difficulties in bridging the gap between indigenous and Western scientific paradigms have been widely discussed in the literature [110,111]. However, some examples of collaborative initiatives concerning the human health care system demonstrate that the dichotomy between Western veterinary medicine and African healing methods can be overcome, allowing for the development of hybrid knowledge that evolves and is continually redefined [111,112]. Ref. [110] suggests working at the interface, identifying convergence points between the two paradigms, and working jointly toward the same health goal. Exploring EVM practices, as we have accomplished in this study, can be considered a first step in this approach because it helps to identify the problems regarding the management of livestock health on the ground and provides scientific information on therapeutic solutions based on indigenous perception and values reflecting local observations and knowledge system. Western science can supply tools and expertise functional to the validation and standardization of traditional procedures. Working within the two health systems avoiding a Eurocentric approach or, on the contrary, a romanticized view of traditional practices allows for a mutual acknowledging of the epistemologies upon which these systems are founded [111]. It is crucial to underline that the role of research, in this perspective, is to allow for a better contextualization and integration of reality, not supplanting it, and as highlighted by [113], the reality is more complex than drawn by a laboratory essay. The efficacy of a plant extract must be validated taking into account not only the synergistic action of different molecules and its effect on the organism in situ but also the specific economic, sociocultural, and spiritual context where the knowledge has been produced and where the reworked knowledge will be brought back. Ethnobiology, as a multidisciplinary science operating at the interface between biology and culture, plays a pivotal role in mediation by promoting mutual understanding between diverse knowledge systems and developing strategies to legitimate each other’s way of viewing animal health care. Ultimately, this can lead to enhanced health outcomes for livestock within the framework of assistance projects implemented in culturally different indigenous communities.
3. Conclusions
In our paper, the use of 89 taxa has been reported and a valuable number of new ethnoveterinary species and uses have been detected, which must be added to the current repertoire of veterinary plant uses recorded in the Southern African regions. This means that EVM is an important part of the local cultural heritage and can significantly contribute to the resilience of the local livestock-oriented livelihood systems. On the other hand, our findings suggest that the preservation of EVM in southwestern Angola risks being negatively impacted by access to biomedicine. Information collected and discussed in this paper can be a starting point for building a syncretic framework, in which diverse veterinary knowledge systems are allowed to coexist in different ways. The rather recent Angolan law (Presidential Decree No. 253/20) that recognizes the importance of traditional medicine as a tool to be incorporated into the national health system in Angola can be a further encouraging step. Further research is needed for a deeper understanding of the pharmacological activity, therapeutic use, and possible toxic effects of the less-known mentioned species. We want to conclude this paper by highlighting that medical pluralism has been a characteristic feature of Atlantic African societies (and Angola was not an exception) even before the European settlement [114]. The arrival of colonists did nothing more than add new medicinal knowledge to this plurality. As observed by [114], cross-cultural interaction and mutual acceptance of medical practices were still the norm in early modern times; Africans and Europeans were open to trying out new healing practices and new drugs, both perceiving these interactions as chances to learn new skills. Now, we should restart from this “innocence age” where a melting pot of different cultures permeated the health systems of African colonies before Europeans, seduced by the ideas of their cultural supremacy, began to deny traditional knowledge.
4. Material and Methods
4.1. Study Area
The study area covers 15 municipalities in 4 provinces in the southwest of Angola: namely, Namibe, Huila, Cunene, and Benguela. (Figure 2 and Table 1).
The region, commonly referred to as Sudoeste Angolano [115], is bounded by the Atlantic Ocean in the west and the Marginal Mountain Range of Huila and Cunene in the east; it borders Namibia in the south and is delimited by the Coporolo intermittent river in Benguela Province in the north [116]. According to an estimate by the Angolan Institute of Statistics (INE), the Province of Namibe is home to approximately 672,086 inhabitants [117], Cunene hosts approximately 990,087 inhabitants, Huila 2,497,422, and Benguela 2,231,385 [118]. The study area shows a large variety of climatic conditions: from the sub-humid zones affected by the Serra da Chela Escarpment and the Humpata Plateau’s influence [119] to the hyper-arid desertic climate of the south part of Namibe Province. The area experiences the impact of the interplay between the cold Benguela current, the Namib Desert, and the elevated terrain [2]. The mean annual temperature is 20 °C and the mean annual precipitation is 37 mm [2]. The rainy hot season occurs from January to March, while the long dry season lasts about nine months [2].
4.2. Ethnic Groups Living in the Area
People living in the area belong to 4 main ethnolinguistic groups and approximately 12 distinct ethnic groups (povos according to [87]). The Nyaneka-Humbi ethnolinguistic group includes the following ethnic groups: Muhanda, Muhumbi, Mumuhuila, Mungambwe, and Muquilengue. They are mainly settled in the eastern foothills of the Chela mountain range [120] and have a semi-nomadic lifestyle, mostly practicing subsistence agriculture while also keeping livestock such as poultry, goats, and cattle. They are widely known for their beekeeping practices and honey production. The Herero group comprises Kuvale (also known as Mucubal), Himba, Muacahona, Mundimba, Tjimba (Cimba), Mbanderu, and Kwandu. Herero are widespread in Southern Africa and live mainly in Namibia, while smaller communities can also be found in Botswana and southern Angola. Unlike most Bantu groups, the Herero are traditionally nomadic pastoralists whose main occupation is herding cattle and whose social status is determined by the number of animals they own. The Herero have mastered the art of food preservation, using techniques such as souring milk and drying meat to extend the shelf life of these perishable foods. Ovimbundu/Umbundu live in the Benguela highlands and the wooded savannas along the coastal areas west of these highlands. They are considered the largest ethnic group in Angola (40% of the whole population). Ovimbundu are mostly farmers, with a notable contribution from women. Livestock rearing involves cattle, goats, pigs, and poultry, with cattle being an important form of investment. Traditional activities such as fishing, hunting, and trapping are practiced during the dry season, reflecting the diverse livelihood strategies within this community. The Ambó ethnolinguistic group, also known as the Ovambo, comprises various subgroups, including the Kwanyama ethnic groups, who live in the flat sandy grass plains of Cunene Province [121]. They lead a sedentary life and live mainly on a combination of agriculture and livestock rearing, cultivating staple crops such as millet, sorghum, and beans. In drier seasons, livestock farming with herds of cattle, goats, and sheep plays an important role as a source of milk, contributing to the livelihood of people. The Ambó are also skilled artisans who make and sell baskets, pottery, jewelry, wooden combs, wooden iron spears, arrows, and other items.
4.3. Transhumance Practices
All ethnic groups interviewed (Table 1) are involved in transhumance; in particular, Kuvale, Nyaneka, and Umbundu are known to have a deeper knowledge and experience in traditional transhumance practices. The movement of cattle spans several municipalities within key areas (Figure 2), including breaks for cattle about every 15 km. The transhumance locations are generally the same from one time to another, although the routes can undergo modifications depending on the annual availability of pasture and water points. The cattle are often brought to specific grazing grounds, such as vast plains, foothills, and riverbanks, providing a diverse range of vegetation for the animals and watering resources. The timing related to departures and returns of the herds varies depending on the needs of the shepherds, the climatic events (i.e., timing and length of rainfall events), and the areas of departure.
4.4. Data Collection
The ethnobotanical investigation was conducted in collaboration with the Veterinary Services Institute (ISV) and the Herbarium of ISCED (Instituto Superior de Ciências de Educação). The study combines two surveys carried out in different periods but in the same geographical areas during the migration along the main routes. In the first survey, data collection was conducted by the technicians of lSV from 2014 to 2017.
The second phase of data collection was carried out by ISCED personnel in 2023. Informants were selected using snowball sampling. After explaining the framework and the aims of the study, informed consensus was obtained from all the participants. A semi-structured questionnaire was used to record the following information on the ethnoveterinary uses: (1) informant name, (2) age, (3) ethnicity, (4) date of the interview, (5) geographic location, (6) education level, (7) plant folk name, (8) part of the plant used, (9) disease treated, (10) preparation method, and (11) administration mode. The informants were asked to give their perceptions of the availability of each species as abundant, moderately abundant, and rare. The participants were also encouraged to freely talk about other topics related to pastoralism, including practical aspects of transhumance, conflict resolution, changes over time, and cultural preservation.
Each interview was conducted in the local language and later translated into Portuguese. All interviews adhered to ethical standards as outlined in [122]. Specimens of the mentioned plants were gathered with the help of the participant with the exception of common or cultivated plants. Although quoted during the interviews, some plants were not shown to the researchers and for this reason, they were excluded from the dataset. Vouchers were deposited at the ISCED Herbarium (Lubango, Huila, Angola) except for some samples badly damaged during preparation and/or transport to the Herbarium, which had to be discarded. Identification was carried out by using taxonomic keys and field guides (see information reported in [32]). Taxonomic nomenclature was in accordance with World Flora Online (https://www.worldfloraonline.org/, accessed on 13 September 2023).
4.5. Data Analysis
Data collected in the field were entered into a database (Microsoft Excel, 2019), which was organized as a spreadsheet. Each row, considered as a single record, represents a citation, which is a single use reported for a single plant by a single informant [123]. Diseases were grouped into 16 categories according to the symptoms reported during the interviews (Table 3). The experience of veterinary technicians was crucial in translating the symptoms and the folk names of diseases mentioned by the informants into disease categories. The fidelity level was calculated for assessing the specificity of a treatment [124]. Jaccard’s index was used to compare (1) species similarity between our study and studies conducted in other regions of Southern Africa and (2) the usages of plants in the different ethnic groups. This index was corrected for maximum possible values to limit potential artifacts due to the different number of informants interviewed within each group [125]. An abundance index according to the informants’ perception (PAI) was calculated for species having ≥5 citations as follows: ((nl × 1) + (nm × 2) + (na × 3)/(nl + nm + na) where nl, nm, and na represent the number of times each abundance category (low, moderate, and abundant) was assigned to a given species according to the informants’ perception. The relationships between ethnic groups and used categories were examined by a circular plot, using the circlize package (vers. 0.4–2 [126]).
Author Contributions
Conceptualization, D.S., M.T., T.B. and P.B.; methodology, D.S., M.V.M. and P.B.; fieldwork and data collection, D.S., J.J.T., M.F.F.R., M.T. and T.B.; data curation, D.S., M.V.M. and P.B.; formal analysis, D.S., M.V.M., L.M. and P.B.; writing—original draft preparation, M.V.M. and P.B.; writing—review and editing, G.F., M.V.M., P.B. and S.L.; projects administration, D.S., M.T., M.V.M. and T.B., supervision D.S., M.T., M.V.M., T.B. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was developed as part of three different projects, financed by different institutions: the PIRAN (Integrated Resilience Project Angola Namibia) project (OSRO/RAF/504/USA), funded by USAID and implemented by FAO; the RETESA (Land rehabilitation and management of pasture areas in the agropastoral production systems of smallholders in southwest Angola) project (GCP/ANG/048/GFF), which had technical and methodological support from the implementer FAO and was financed by the Global Environment Fund (GEF); and the current PIRA Namibe (Integrated Environmental Resilience Project) Project (FED/2017/389-710) funded by the EU and implemented by Instituto Camões. The funders were not involved in study design, data collection, analysis, interpretation, and preparation of the manuscript, or decision to submit for publication.
Informed Consent Statement
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study, according to International Society of Ethnobiology, 2006. All ethical principles of data collection were in line with the International Society of Ethnobiology (2008) concerning traditional resource rights.
Data Availability Statement
All data supporting the results of this research are included within the article.
Acknowledgments
We extend our sincere gratitude to all the pastoralists whose invaluable knowledge and cooperation made this research possible. We are also thankful to the individuals involved in the previous research endeavors within the framework of the PIRAN and RETESA projects, whose contributions laid the foundation for this study. Special thanks are extended to ISV (Instituto dos Serviços de Veterinária—Veterinary Services Institute) for their support and collaboration throughout the research process. We are also thankful to Instituto Camões, implementer of the PIRA Namibe project (within FRESAN—Fortalecimento da Resiliência e da Segurança Alimentar e Nutricional—Program), for their supervision and support. Last, we acknowledge the pivotal role played by ADESPOV (Associação para o Desenvolvimento e Enquadramento Social de Populações Vulneráveis—Association for the Development and Social Framing of Vulnerable Populations), the local NGO coordinating the project, for their dedication to community development and their facilitation of this research initiative.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Perceived Abundance Index (PAI) of the most cited species, IUCN categories and vegetation inventories. nr = not reported.
Table A1.
Perceived Abundance Index (PAI) of the most cited species, IUCN categories and vegetation inventories. nr = not reported.
| Species | PAI | Conservation Categories | Inventory (Urso 2014) [77]: Only Adult Stems | Inventory (Kissanga et al. 2024) [78]: Adult and Young Stems | |
|---|---|---|---|---|---|
| Da Costa et al. (2019) [80] | IUCN (2023) [79] | Average density (stems/ha) | |||
| Aloe littoralis | 2.9 | LC | 12.2 | nr | |
| Aloe zebrina | 2.8 | LC | nr | nr | |
| Cassia angolensis | 2.1 | nr | nr | ||
| Cassytha filiformis | 2.0 | nr | nr | ||
| Cissampelos mucronata | 1.0 | nr | nr | ||
| Cissus quadrangularis | 1.7 | LC | nr | nr | |
| Colophospermum mopane | 2.7 | VU | LC | 295.7 | 514.7 adult; 958.8 young |
| Croton gratissimus var. gratissimus | 2.4 | LC | 131 | nr | |
| Cyphostemma junceum | 1.9 | nr | nr | ||
| Dalbergia nitidula | 2.0 | LC | nr | nr | |
| Dicoma antunesii | 1.0 | nr | nr | ||
| Elaeodendron transvaalense | 1.7 | 1.30 | nr | ||
| Erlangea misera | 3.0 | nr | nr | ||
| Euphorbia hirta | 3.0 | nr | nr | ||
| Ipomoea prismatosyphon | 3.0 | LC | nr | nr | |
| Landolphia parviflora | 1.0 | LC | nr | nr | |
| Oldfieldia dactylophylla | 2.7 | nr | nr | ||
| Otomeria elatior | 1.0 | nr | nr | ||
| Peltophorum africanum | 1.9 | VU | LC | 0.64 | 12.0 young |
| Pennisetum glaucum | 3.0 | nr | nr | ||
| Ptaeroxylon obliquum | 2.2 | VU | LC | 13.3 | 5.0 adult; 18.0 young |
| Pterocarpus angolensis | 3.0 | VU | LC | nr | 10.0 young |
| Salvadora persica | 2.0 | LC | 23.7 | nr | |
| Sclerocarya birrea | 1.7 | 1.9 | 4.0 adult; 6.0 young | ||
| Securidaca longepedunculata | 2.5 | VU | nr | nr | |
| Senna occidentalis | 2.6 | 5.1 | nr | ||
| Spirostachys africana | 2.8 | VU | LC | 23.7 | 248.2 adult; 358.8 young |
| Thesium cinereum | 3.0 | nr | nr | ||
| Ximenia americana | 2.3 | 42.2 | 1.0 adult | ||
| Zygophyllum simplex | 1.1 | nr | nr | ||
Appendix B

Table A2.
New species and uses compared to literature; (citations by informants).
Table A2.
New species and uses compared to literature; (citations by informants).
| New Species | New Uses (Citations) | Already Known Uses (Citations) | |
|---|---|---|---|
| Adansonia digitata | NO | CBPP (1) | NO |
| Afzelia quanzensis | NO | CBPP (2), Diarrhea (1) | NO |
| Allium sativum | NO | Newcastle (1) | NO |
| Aloe littoralis | NO | Cough (1), CBPP (5), Diarrhea (8), Newcastle (12), Ext. and int. Parasites (2, 6), Scabies (2), Symptomatic carbuncle (1), Wounds (5) | NO |
| Aloe zebrina | NO | CBPP (1), Newcastle (6), Placental retantion (1) | Conjunctivitis (1) (van der Merwe et al., 2001) [50]; Wounds (2) (Luseba and van der Merwe, 2006 [66]; Chinsembu et al., 2014 [28]; Madisha and McGaw 2023 [17]) |
| Balanites angolensis | YES | ||
| Brachystegia spiciformis | NO | CBPP (1) | |
| Brackenridgea areanaria | YES | ||
| Burkea africana | NO | Wounds (1) | |
| Cajanus cajan | NO | CBPP (1) | |
| Cannabis sativa | NO | CBPP (2) | Cough (1) (Uprety et al., 2022) [127] |
| Capsicum frutescens | NO | Snakebite (1) | Newcastle (46) (Dharani et al. 2015 [128]; Guèye 2002 [56]; Lagu and Kayanja 2010 [57]; Gobvu et al. 2023 [23]) |
| Cassia angolensis | YES | ||
| Cassytha filiformis | NO | CBPP (1) | |
| Cenchrus americanus | NO | Conjunctivitis (6) | |
| Cissampelos mucronata | NO | Wounds (1) | |
| Cissus quadrangularis | NO | Carbuncle (2) | CBPP (10) (Gradè et al., 2009) [43], Int. parasites (2) (Gradè et al., 2009) [43], Newcastle (2) (Ribeiro et al., 2010 [129]) |
| Colophospermum mopane | NO | CBPP (1), Int. parasites (1), Scabies (2), Wounds (2) | Ext. Parasites (1) (Nyahangare et al., 2015) [20] |
| Combretum imberbe | NO | Diarrhea (2) | |
| Commiphora mollis | YES | ||
| Croton gratissimus | NO | Carbuncle (4), Snake bite (1) | CBPP (3) (van der Merwe et al., 2001) [50] |
| Croton integrifolius | YES | ||
| Cussonia angolensis | YES | ||
| Cyperus articulatus | NO | LSD (2) | |
| Cyphostemma junceum | NO | CBPP (3), Diarrhea (1), Scabies (1), Snakebite (1) | Symptomatic carbuncle (2) (Abrha et al., 2020) [130] |
| Dalbergia nitidula | NO | CBPP (2), Diarrhea (1), Symptomatic carbuncle (3) | |
| Dialium englerianum | YES | ||
| Dichrostachys cinerea | NO | Conjunctivitis (1) | |
| Dicoma antunesii | YES | ||
| Diospyros kirkii | YES | ||
| Diospyros mespiliformis | NO | CBPP (1) | |
| Elaeodendron transvaalense | NO | Wounds (49) | |
| Entandrophragma spicatum | YES | ||
| Erlangea misera | YES | ||
| Erythrina abyssinica | NO | CBPP (1) | |
| Erythrophleum africanum | NO | Scabies (1) | |
| Euclea crispa | YES | ||
| Euphorbia hirta | NO | CBPP (2), Vomit (2), Weakness (2) | Fever (2) (Galav et al., 2013) [131] |
| Fadogia cienkowskii var. lanceolata | YES | ||
| Faidherbia albida | NO | Wounds (1) | |
| Gomphocarpus tomentosum | YES | ||
| Helichrysum benguellense | YES | ||
| Hyphaene petersiana | NO | Cough (1) | |
| Ipomoea prismatosyphon var. prismatosyphon | YES | ||
| Jacaranda mimosifolia | NO | Newcastle (1) | |
| Julbernardia paniculata | YES | ||
| Landolphia parvifolia | YES | ||
| Musa acuminata | NO | Int. Parasites (2) (Maroy, 2012) [19] | |
| Nicotiana tabacum | NO | Snakebites (1) (Yigezu et al., 2014) [132] | |
| Oldfieldia dactylophylla | YES | ||
| Opuntia ficus-indica | NO | CBPP (1), Scabies (1), Wounds (1) | |
| Otomeria elatior | YES | ||
| Pachycarpus lineolatus | YES | ||
| Pachypodium lealii | YES | ||
| Parinari curatellifolia | NO | Wounds (1) | |
| Pavetta schumanniana | YES | ||
| Peltophorum africanum | NO | CBPP (11), Conjunctivitis (1), LSD (5), Scabies (1), Wounds (7) | |
| Pericopsis angolensis | YES | ||
| Philenoptera pallescens | YES | ||
| Physalis peruviana | NO | Carbuncle (1) | |
| Psidium guajava | NO | Int. Parasites (1) | |
| Ptaeroxylon obliquum | NO | CHICK (1), Cough (2), LSD (12), Newcastle (9), Timpanism (1), Wounds (2) | CBPP (50) (Bruschi et al., 2017) [30], Diarrhea (28) (Maphosa and Masika, 2010) [133], Internal parasites (3) (Selogatwe et al., 2021) [134], Scabies (4) (Nyahangare te al., 2015) [20], Symptomatic carbuncle (16) (Hutchings et al., 1996) [67] |
| Pterocarpus angolensis | NO | Diarrhea (6) | |
| Salvadora persica | NO | CBPP (54) | Ext. parasites (5) (Dharani et al., 2015) [128] |
| Sclerocarya birrea | NO | Snakebite (11), Symtomatic carbuncle (1) | |
| Searsia tenuinervis | YES | ||
| Securidaca longipedunculata | NO | CBPP (1), Inflammations (2), Plancental retantion (1), Wounds (2), Scabies (9) | Int. parasites (2) (Djoueche et al., 2011 [135]; Hassan and Yuguda, 2021 [136]; Fidèle et al., 2023 [137]) |
| Senna occidentalis | NO | CBPP (4), LSD (3), Newcastle (1), Snakebite (15) | Int. parasites (3) (Dansou et al., 2021 [138]) |
| Senna singueana | NO | LSD (1) | Cough (1) (Syakalima et al., 2018) [29] |
| Solanum lycopersicum | NO | Ext. parasites (1) | |
| Spirostachys africana | NO | CBPP (17), Cough (3), LSD (7), Symtomatic carbuncle (2) | |
| Stachytarpheta mutabilis | YES | ||
| Steganotaenia araliacea | NO | Symtomatic carbuncle (2) | |
| Sterculia setigera | NO | Diarrhea (1), LSD (2) | |
| Strychnos henningsi | NO | Symtomatic carbuncle (1) | |
| Tephrosia vogelii | NO | CBPP (1), Cough (1), Newcastle (1) | |
| Thesium cinereum | YES | ||
| Vachellia erioloba | NO | CBPP (2) | Wounds (2) (Eiki et al., 2022) [12] |
| Vernonia steetziana | YES | ||
| Vernoniastrum latifolium | YES | ||
| Ximenia americana | NO | Scabies (6) | CBPP (1) (Bruschi et al., 2017) [30], Snakebite (Ismaila and Adamu, 2012) [139] |
| Zea mays | NO | CBPP (2) | |
| Zygophyllum simplex | NO | Scabies (43) |
References
- De Carvalho, E.C. “Traditional” and “Modern” Patterns of Cattle Raising in Southwestern Angola: A Critical Evaluation of Change from Pastoralism to Ranching. J. Dev. Areas 1974, 8, 199–226. [Google Scholar]
- Huntley, B.J. Ecology of Angola: Terrestrial Biomes and Ecoregions; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Jenet, A.; Buono, N.; Di Lello, S.; Gomarasca, M.; Heine, C.; Mason, S.; Nori, M.; Saavedra, R.; Van Troos, K. The Path to Greener Pastures: Pastoralism, the Backbone of the World’s Drylands. SSRN Electron. J. 2016, 1–105. [Google Scholar] [CrossRef]
- Kinahan, J. The Rise and Fall of Nomadic Pastoralism in the Central Namib Desert. In The Archaeology of Africa: The Archaeology of Africa: Foods, Metals and Towns; Shaw, T., Sinclair, P., Andah, B., Okpoko, A., Eds.; Routledge: London, UK, 1993; pp. 372–385. [Google Scholar]
- The International Federation of Red Cross and Red Crescent Societies (IFRC). Angola: Drought—Operation Update Report; IFRC: Paris, France, 2022. [Google Scholar]
- UNICEF. Humanitarian Action for Children: Angola Highlights; UNICEF: New York, NY, USA, 2023. [Google Scholar]
- FEWS NET. A Drier-Than-Normal 2023/2024 Rainy Season Likely amid Forecast El Niño. 2023. Available online: https://fews.net/southern-africa/angola/remote-monitoring-report/october-2023 (accessed on 7 November 2023).
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest Sci 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Parodi, P.; Barbosa, M.; Samo, D.; Ferri, N.; Mancuso, A.; Possenti, L.; Santos, M.; Scacchia, M.; Venturi, L. Veterinary Public Health: A long lasting collaboration between Angola and Italy. Quad. Soc. Ital. Med. Trop. Sal. Glob. 2016, 1, 59–66. [Google Scholar]
- Jaime, G.; Hobeika, A.; Figuié, M. Access to Veterinary Drugs in Sub-Saharan Africa: Roadblocks and Current Solutions. Front. Vet. Sci. 2022, 8, 558973. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Famuyide, I.M.; Khunoana, E.T.; Aremu, A.O. Ethnoveterinary Botanical Medicine in South Africa: A Review of Research from the Last Decade (2009 to 2019). J. Ethnopharmacol. 2020, 257, 112864. [Google Scholar] [CrossRef] [PubMed]
- Eiki, N.; Maake, M.; Lebelo, S.; Sakong, B.; Sebola, N.; Mabelebele, M. Survey of Ethnoveterinary Medicines Used to Treat Livestock Diseases in Omusati and Kunene Regions of Namibia. Front. Vet. Sci. 2022, 9, 762771. [Google Scholar] [CrossRef]
- McGaw, L.J.; Abdalla, M.A. Ethnoveterinary Medicine; McGaw, L.J., Abdalla, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-32269-4. [Google Scholar]
- Chakale, M.V.; Mwanza, M.; Aremu, A.O. Ethnoveterinary Knowledge and Biological Evaluation of Plants Used for Mitigating Cattle Diseases: A Critical Insight Into the Trends and Patterns in South Africa. Front. Vet. Sci. 2021, 8, 710884. [Google Scholar] [CrossRef]
- Chakale, M.V.; Asong, J.A.; Struwig, M.; Mwanza, M.; Aremu, A.O. Ethnoveterinary Practices and Ethnobotanical Knowledge on Plants Used against Cattle Diseases among Two Communities in South Africa. Plants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Ndou, R.V.; Materechera, S.A.; Mwanza, M.; Otang-Mbeng, W.; Ijane, M.F. Indigenous Knowledge and Use of Medicinal Plants for Ethnoveterinary within the North West Province, South Africa. Front. Vet. Sci. 2023, 10, 1273562. [Google Scholar] [CrossRef]
- Madisha, J.K.; McGaw, L.J. Ethnoveterinary Survey of Medicinal Plants Used for the Management of Respiratory and Dermatological Infections in Livestock by Bapedi People of Sekhukhune, Limpopo Province, South Africa. S. Afr. J. Bot. 2023, 155, 241–248. [Google Scholar] [CrossRef]
- Matekaire, T.; Bwakura, T.M. Ethnoveterinary Medicine: A Potential Alternative to Orthodox Animal Health Delivery in Zimbabwe. Intern. J. Appl. Res. Vet. Med. 2004, 2, 269–273. [Google Scholar]
- Maroyi, A. Enhancing Food Security through Cultivation of Traditional Food Crops in Nhema Communal Area, Midlands Province, Zimbabwe. Afr. J. Agric. Res. 2012, 7, 5412–5420. [Google Scholar] [CrossRef]
- Nyahangare, E.T.; Mvumi, B.M.; Mutibvu, T. Ethnoveterinary Plants and Practices Used for Ecto-Parasite Control in Semi-Arid Smallholder Farming Areas of Zimbabwe. J. Ethnobiol. Ethnomed. 2015, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Marandure, T. Concepts and Key Issues of Ethnoveterinary Medicine in Africa: A Review of Its Application in Zimbabwe. Afr. J. Agric. Res. 2016, 11, 1836–1841. [Google Scholar] [CrossRef]
- Jambwa, P.; Nyahangare, E.T. Ethnoveterinary Medicine: A Zimbabwean Perspective. In Ethnoveterinary Medicine; Springer International Publishing: Cham, Switzerland, 2020; pp. 269–283. [Google Scholar]
- Gobvu, V.; Pote, W.; Poshiwa, X.; Benhura, M.A. A Review of Ethnoveterinary Medicines Used for Poultry Health Management in Zimbabwe. Worlds Poult. Sci. J. 2023, 79, 851–865. [Google Scholar] [CrossRef]
- Gabanakgosi, K.; Moreki, J.C.; Nsoso, S.J.; Tsopito, C. Ethnoveterinary Medicine Usage in Family Chickens in the Selected Four Villages of Botswana. J. Vet. Adv. 2012, 2, 586–594. [Google Scholar]
- Gabalebatse, M.; Ngwenya, B.N.; Teketay, D.; Kolawole, O.D. Ethno-Veterinary Practices amongst Livestock Farmers in Ngamiland District, Botswana. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 490–502. [Google Scholar] [CrossRef]
- Madibela, O.R. A Perspective on Ethnomedicine Research for Livestock Production: Focus on Botswana. PULA Botsw. J. Afr. Stud. 2017, 31, 1–7. [Google Scholar]
- Barbosa, F.M.A.; Cala, A.C.; Sevastyanov, V.; Boane, E.; Hlashwayo, D.F. Ethnoveterinary Study of Plant-Based Remedies for Treating Diseases in Small Ruminants in Maputo Province, Mozambique. Evid.-Based Complement. Altern. Med. 2023, 2023, 1842870. [Google Scholar] [CrossRef]
- Chinsembu, K.C.; Negumbo, J.; Likando, M.; Mbangu, A. An Ethnobotanical Study of Medicinal Plants Used to Treat Livestock Diseases in Onayena and Katima Mulilo, Namibia. S. Afr. J. Bot. 2014, 94, 101–107. [Google Scholar] [CrossRef]
- Syakalima, M.; Simuunza, M.; Zulu, V.C. Ethnoveterinary Treatments for Common Cattle Diseases in Four Districts of the Southern Province, Zambia. Vet. World 2018, 11, 141–145. [Google Scholar] [CrossRef]
- Bruschi, P.; Urso, V.; Solazzo, D.; Tonini, M.; Signorini, M.A. Traditional Knowledge on Ethno-Veterinary and Fodder Plants in South Angola: An Ethnobotanic Field Survey in Mopane Woodlands in Bibala, Namibe Province. J. Agric. Environ. Int. Dev. 2017, 111, 93–109. [Google Scholar] [CrossRef]
- Jendras, G.; Monizi, M.; Neinhuis, C.; Lautenschläger, T. Plants, Food and Treatments Used by BaKongo Tribes in Uíge (Northern Angola) to Affect the Quality and Quantity of Human Breast Milk. Int. Breastfeed. J. 2020, 15, 88. [Google Scholar] [CrossRef]
- Urso, V.; Signorini, M.A.; Tonini, M.; Bruschi, P. Wild Medicinal and Food Plants Used by Communities Living in Mopane Woodlands of Southern Angola: Results of an Ethnobotanical Field Investigation. J. Ethnopharmacol. 2016, 177, 126–139. [Google Scholar] [CrossRef]
- Goyder, D.; Gonçalves, F.M. The Flora of Angola: Collectors, Richness and Endemism. In Biodiversity of Angola: Science and Conservation: A Modern Synthesis; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 79–96. ISBN 9783030030834. [Google Scholar]
- Catarino, S.; Duarte, M.C.; Costa, E.; Carrero, P.G.; Romeiras, M.M. Conservation and Sustainable Use of the Medicinal Leguminosae Plants from Angola. PeerJ 2019, 7, e6736. [Google Scholar] [CrossRef]
- Maquia, I.; Catarino, S.; Pena, A.R.; Brito, D.R.A.; Ribeiro, N.S.; Romeiras, M.M.; Ribeiro-Barros, A.I. Diversification of African Tree Legumes in Miombo–Mopane Woodlands. Plants 2019, 8, 182. [Google Scholar] [CrossRef]
- Bruschi, P.; Morganti, M.; Mancini, M.; Signorini, M.A. Traditional Healers and Laypeople: A Qualitative and Quantitative Approach to Local Knowledge on Medicinal Plants in Muda (Mozambique). J. Ethnopharmacol. 2011, 138, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Bagnol, B.; Verhoesel, K. O Gado: Capital Simbólico. Relações de Género Nas Comunidades de Pastores; GFA Consulting Group: Hamburg, Germany, 2009. [Google Scholar]
- de Carvalho, R.D. Vou Lá Visitar Pastores: Exploração Epistolar de um Percurso Angolano em Território Kuvale (1992–1997); Cotovia: Lisbon, Portugal, 1999. [Google Scholar]
- Bille, P.G.; Buys, E.; Taylor, J.R.N. The Technology and Properties of Omashikwa, a Traditional Fermented Buttermilk Produced by Small-holder Milk Producers in Namibia. Int. J. Food Sci. Technol. 2007, 42, 620–624. [Google Scholar] [CrossRef]
- Kama, K.L.; Massampo, M.C.H.; N’salambi, D. Country Report: Angola. 2000. Available online: https://ageconsearch.umn.edu/record/135373/files/PR103.pdf (accessed on 13 November 2023).
- Daniel, S.; Antonia Abeledo-Abreu, M.; Lobo-Rivero, E. Comunicación Corta Seroprevalencia de Pleuroneumonía Contagiosa Bovina En Tres Municipios de La Provincia Namibe, Angola Seroprevalence of Contagious Bovine Pleuropneumonia (CBPP) in Three Municipalities of Namibe Province, Angola. Rev. Salud Anim. 2017, 39, 62–67. [Google Scholar]
- Cadete, C.J.C.; da Costa, A.; António, A.P. Principais Causas de Rejeições Nos Bovinos, Caprinos e Suínos Registradas Nos Matadouros Do Município de Benguela (Angola), No Período de Janeiro a Abril de 2021. Rev. Educ. Contin. Med. Veterinária E Zootec. CRMV-SP 2022, 20, 1–8. [Google Scholar] [CrossRef]
- Gradé, J.T.; Tabuti, J.R.S.; Van Damme, P. Ethnoveterinary Knowledge in Pastoral Karamoja, Uganda. J. Ethnopharmacol. 2009, 122, 273–293. [Google Scholar] [CrossRef]
- Mudzengi, C.P.; Murwira, A.; Tivapasic, M.; Murungweni, C.; Burumue, J.V.; Halimani, T. Antibacterial Activity of Aqueous and Methanol Extracts of Selected Species Used in Livestock Health Management. Pharm. Biol. 2017, 55, 1054–1060. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. The Ultrastructural Damage Caused by Eugenia Zeyheri and Syzygium Legatii Acetone Leaf Extracts on Pathogenic Escherichia Coli. BMC Vet. Res. 2020, 16, 326. [Google Scholar] [CrossRef]
- Ramadwa, T.E.; McGaw, L.J.; Adamu, M.; Madikizela, B.; Eloff, J.N. Anthelmintic, Antimycobacterial, Antifungal, Larvicidal and Cytotoxic Activities of Acetone Leaf Extracts, Fractions and Isolated Compounds from Ptaeroxylon Obliquum (Rutaceae). J. Ethnopharmacol. 2021, 280, 114365. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Chanchal, D.K. Formulation and In-Vitro Drug Release Study of Controlled Release Tablet of Nimodipine. Int. J. Pharm. Sci. Res. 2020, 11, 5027. [Google Scholar] [CrossRef]
- Bizimenyera, S.E.; Swan, G.E.; Chikoto, H.; Eloff, J.N. Rationale for Using Peltophorum Africanum (Fabaceae) Extracts in Veterinary Medicine. J. S. Afr. Vet. Assoc. 2005, 76, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Manda, L.; Tunon, H.; Godwin, D.; Mkamanga, Y.; Magombo, Z.L. Status and Uses of Oldfieldia Dactylophylla (Euphorbiaceae) in Malawi. Master’s Thesis, International Master Programme at the Swedish Biodiversity Centre, Uppsala, Sweden, 2007. [Google Scholar]
- Van der Merwe, D.; Swan, G.E.; Botha, C.J. Use of Ethnoveterinary Medicinal Plants in Cattle by Setswana-Speaking People in the Madikwe Area of the North West Province of South Africa. J. S. Afr. Vet. Assoc. 2001, 72, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kunene, N.; Wilson, R.; Myeni, N. The Use of Trees, Shrubs and Herbs in Livestock Production by Communal Farmers in Northern KwaZulu-Natal, South Africa. Afr. J. Range Forage Sci. 2003, 20, 271–274. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. A Review of the Traditional Use of Southern African Medicinal Plants for the Treatment of Inflammation and Inflammatory Pain. J. Ethnopharmacol. 2022, 283, 114436. [Google Scholar] [CrossRef] [PubMed]
- Amusan, O.O.G.; Sukati, N.A.; Dlamini, P.S.; Sibandze, F.G. Some Swazi Phytomedicines and Their Constituents. Afr. J. Biotechnol. 2007, 6, 267–272. [Google Scholar]
- Semenya, S.S.; Maroyi, A. Source, Harvesting, Conservation Status, Threats and Management of Indigenous Plant Used for Respiratory Infections and Related Symptoms in the Limpopo Province, South Africa. Biodiversitas 2019, 20, 790–811. [Google Scholar] [CrossRef]
- Loeb, E.M.; Koch, C.; Loeb, E.-M.K. Kuanyama Ambo Magic. 6. Medicinal, Cosmetical, and Charm Flora and Fauna. J. Am. Folk. 1956, 69, 147–174. [Google Scholar] [CrossRef]
- Guèye, E.F. Newcastle Disease in Family Poultry: Prospects for Its Control through Ethnoveterinary Medicine. Livest. Res. Rural Dev. 2002, 14, 80–91. [Google Scholar]
- Lagu, C.; Kayanja, F.I.B. Medicinal plant extracts widely used in the control of Newcastle disease (NCD) and helminthosis among village chickens of South Western Uganda. Livest. Res. Rural Dev. 2010, 22, 1–14. [Google Scholar]
- Aremu, A.O.; Lawal, I.O. An Analysis of the Ethnoveterinary Medicinal Uses of the Genus Aloe L. for Animal Diseases in Africa. S. Afr. J. Bot. 2022, 147, 976–992. [Google Scholar] [CrossRef]
- Mtambo, M.M.A.; Mushi, E.J.; Kinabo, L.D.B.; Maeda-Machang’u, A.; Mwamengele, G.L.M.; Yongolo, M.G.S.; Temu, R.P.C. Evaluation of the Efficacy of the Crude Extracts of Capsicum Frutescens, Citrus Limon and Opuntia 6ulgaris against Newcastle Disease in Domestic Fowl in Tanzania. J. Ethnopharmacol. 1999, 68, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Waihenya, R.K.; Mtambo, M.M.A.; Nkwengulila, G.; Minga, U.M. Efficacy of Crude Extract of Aloe Secundiflora against Salmonella Gallinarum in Experimentally Infected Free-Range Chickens in Tanzania. J. Ethnopharmacol. 2002, 79, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Faheem, M.; Uddin Rajer, F.; Yousaf, S.; Yameen, M. Volatile and Non-Volatile Antifungal Compounds Produced by Trichoderma Harzianum SQR-T037 Suppressed the Growth of Fusarium Oxysporum f. Sp. Niveum. Sci. Lett. 2013, 1, 21–24. [Google Scholar]
- Ahmed, A.; Hameed, A.; Saeed, S. Biochemical Profile and Bioactive Potential of Thirteen Wild Folk Medicinal Plants from Balochistan, Pakistan. PLoS ONE 2020, 15, e0231612. [Google Scholar] [CrossRef]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Staden, J. Van Securidaca Longipedunculata Fresen (Polygalaceae): A Review of Its Ethnomedicinal Uses, Phytochemistry, Pharmacological Properties and Toxicology. J. Ethnopharmacol. 2015, 165, 215–226. [Google Scholar] [CrossRef]
- de Menezes, I.R.A.; da Costa, R.H.S.; Augusti Boligon, A.; Rolón, M.; Coronel, C.; Vega, C.; Melo Coutinho, H.D.; da Costa, M.S.; Tintino, S.R.; Silva Pereira, R.L.; et al. Ximenia Americana L. Enhances the Antibiotic Activity and Inhibit the Development of Kinetoplastid Parasites. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 40–46. [Google Scholar] [CrossRef]
- McGaw, L.J.; Van der Merwe, D.; Eloff, J.N. In Vitro Anthelmintic, Antibacterial and Cytotoxic Effects of Extracts from Plants Used in South African Ethnoveterinary Medicine. Vet. J. 2007, 173, 366–372. [Google Scholar] [CrossRef]
- Luseba, D.; Elgorashi, E.E.; Ntloedibe, D.T.; Van Staden, J. Antibacterial, Anti-Inflammatory and Mutagenic Effects of Some Medicinal Plants Used in South Africa for the Treatment of Wounds and Retained Placenta in Livestock. S. Afr. J. Bot. 2007, 73, 378–383. [Google Scholar] [CrossRef]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A.B. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermartzburg, South Africa, 1996. [Google Scholar]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Medicinal Plants for the Treatment of Local Tissue Damage Induced by Snake Venoms: An Overview from Traditional Use to Pharmacological Evidence. Evid.-Based Complement. Altern. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef] [PubMed]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Royal Botanic Gardens: Edinburgh, UK, 1985. [Google Scholar]
- Hamza, O.J.M.; van den Bout-van den Beukel, C.J.P.; Matee, M.I.N.; Moshi, M.J.; Mikx, F.H.M.; Selemani, H.O.; Mbwambo, Z.H.; Van der Ven, A.J.A.M.; Verweij, P.E. Antifungal Activity of Some Tanzanian Plants Used Traditionally for the Treatment of Fungal Infections. J. Ethnopharmacol. 2006, 108, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mshana, N.; Abbiw, K.; Addae-Mensah, I.; Adjanohoun, E.; Ahyi, M.; Ekpere, J.; Enow-Orock, E.; Gbile, Z.; Noamesi, G.; Odei, M.; et al. Traditional Medicine and Pharmacopoeia. Contribution to the Revision of Ethnobotanical and Floristic Studies in Ghana; OAU/STRC: Adissabeba, Ethiopia, 2000; pp. 676–677. [Google Scholar] [CrossRef]
- Bossard, E. La Médecine Traditionnelle Au Centre et à l’ouest de l’Angola; Ministério da Ciência e da Tecnologia, Instituto de Investigação Científica Tropical: Lisbon, Portugal, 1996. [Google Scholar]
- Lombard, N.; van Wyk, B.E.; Marianne le Roux, M. A Review of the Ethnobotany, Contemporary Uses, Chemistry and Pharmacology of the Genus Thesium (Santalaceae). J. Ethnopharmacol. 2020, 256, 112745. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Ojo, A.B.; Barnabas, M.; Iyobhebhe, M.; Elebiyo, T.C.; Evbuomwan, I.O.; Michael, T.; Ajiboye, B.O.; Oyinloye, B.E.; Oloyede, O.I. Phytochemical Properties and Pharmacological Activities of the Genus Pennisetum: A Review. Sci. Afr. 2022, 16, e01132. [Google Scholar] [CrossRef]
- Tchikola, D.G.J. Chinua Achebe and Ruy Duarte de Carvalho: A Comparative Study of Things Fall Apart and Vou Lá Visitar Pastores. 2017. Available online: http://hdl.handle.net/10362/20424 (accessed on 13 November 2023).
- Vansina, J. How Societies Are Born: Governance in West Central Africa Before 1600, 1st ed.; University of Virginia Press: Charlottesville, VA, USA, 2004. [Google Scholar]
- Urso, V. Conoscenze Etnobotaniche e Valorizzazione Delle Risorse Forestali in Angola; Dottorato di ricerca in biosistematica ed cecologia vegetale; Università degli studi di Firenze: Florence, Italy, 2014. [Google Scholar]
- Kissanga, R.; Catarino, L.; Máguas, C.; Cabral, A.I.R.; Chozas, S. Assessing the Impact of Charcoal Production on Southern Angolan Miombo and Mopane Woodlands. Forests 2023, 15, 78. [Google Scholar] [CrossRef]
- IUCN The Iucn Red List Of Threatened Species—Version 2023. Available online: www.iucnredlist.org (accessed on 7 December 2023).
- Da Costa, E.; Adão, T.; Catarino, S.; Pedro, M.P.C.; Romeiras, M. Plantas Ameaçadas Em Angola—Estado Actual. Rev. Int. Em Língua Port. 2019, 31–43. [Google Scholar] [CrossRef]
- Braga-Pereira, F.; Morcatty, T.Q.; El Bizri, H.R.; Tavares, A.S.; Mere-Roncal, C.; González-Crespo, C.; Bertsch, C.; Rodriguez, C.R.; Bardales-Alvites, C.; von Mühlen, E.M.; et al. Congruence of Local Ecological Knowledge (LEK)-based Methods and Line-transect Surveys in Estimating Wildlife Abundance in Tropical Forests. Methods Ecol. Evol. 2022, 13, 743–756. [Google Scholar] [CrossRef]
- Bennett, J.A.; Riibak, K.; Tamme, R.; Lewis, R.J.; Pärtel, M. The Reciprocal Relationship between Competition and Intraspecific Trait Variation. J. Ecol. 2016, 104, 1410–1420. [Google Scholar] [CrossRef]
- Khasbagan; Soyolt. Indigenous Knowledge for Plant Species Diversity: A Case Study of Wild Plants’ Folk Names Used by the Mongolians in Ejina Desert Area, Inner Mongolia, P.R. China. J. Ethnobiol. Ethnomed. 2008, 4, 2. [Google Scholar] [CrossRef]
- Heinrich, M.; Edwards, S.; Moerman, D.E.; Leonti, M. Ethnopharmacological Field Studies: A Critical Assessment of Their Conceptual Basis and Methods. J. Ethnopharmacol. 2009, 124, 1–17. [Google Scholar] [CrossRef]
- Rivera, D.; Allkin, R.; Obón, C.; Alcaraz, F.; Verpoorte, R.; Heinrich, M. What Is in a Name? The Need for Accurate Scientific Nomenclature for Plants. J. Ethnopharmacol. 2013, 152, 393–402. [Google Scholar] [CrossRef]
- Figueiredo, E.; Smith, G. Common Names of Angolan Plants; Protea Book House: Pretoria, South Africa, 2018; ISBN 1485306809, 9781485306801. [Google Scholar]
- Coelho, V. A Classificação Etnográfica Dos Povos de Angola (1.a Parte). Mulemba 2015, 5, 203–220. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Figueira, R.; Duarte, M.C.; Beja, P.; Darbyshire, I. Documenting Biogeographical Patterns of African Timber Species Using Herbarium Records: A Conservation Perspective Based on Native Trees from Angola. PLoS ONE 2014, 9, e103403. [Google Scholar] [CrossRef]
- The World Bank Group. Angola Country Climate and Development Report; The World Bank Group: Washington, DC, USA, 2022. [Google Scholar]
- Sulieman, H.M. Land Grabbing along Livestock Migration Routes in Gadarif State, Sudan: Impacts on Pastoralism and the Environment; The Land Deal Politics Initiative: Hague, The Netherlands, 2013. [Google Scholar]
- Satoto, G.F.; Araújo, M.E.M. Estudo do óLeo de Ximenia Americana L. de Produção Artesanal Angolana: Avaliação das Propriedades Químicas E Biológicas. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Bellon, M.R. Farmers’ Knowledge and Sustainable Agroecosystem Management: An Operational Definition and an Example from Chiapas, Mexico. Hum. Organ. 1995, 54, 263–272. [Google Scholar] [CrossRef]
- Briggs, J. Environmental Resources: Their Use and Management by the Bedouin of the Nubian Desert of Southern Egypt. In People and Environment in Africa; Binns, T., Ed.; John Wiley & Sons: Glasgow, UK, 1995; pp. 61–67. ISBN 9780471951001. [Google Scholar]
- Briggs, J. The Use of Indigenous Knowledge in Development: Problems and Challenges. Prog. Dev. Stud. 2005, 5, 99–114. [Google Scholar] [CrossRef]
- Collins, A. Inquérito de Base—2011, Angola; FAO: Luanda, Angola, 2011. [Google Scholar]
- Masika, P.J.; Sonandi, A.; Van Averbeke, W. Perceived Causes, Diagnosis and Treatment of Babesiosis and Anaplasmosis in Cattle by Livestock Farmers in Communal Areas of the Central Eastern Cape Province, South Africa. J. S. Afr. Vet. Assoc. 1997, 68, 40–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathez-Stiefel, S.-L.; Vandebroek, I.; Rist, S. Can Andean Medicine Coexist with Biomedical Healthcare? A Comparison of Two Rural Communities in Peru and Bolivia. J. Ethnobiol. Ethnomed. 2012, 8, 26. [Google Scholar] [CrossRef]
- Vidhamaly, V.; Bellingham, K.; Newton, P.N.; Caillet, C. The Quality of Veterinary Medicines and Their Implications for One Health. BMJ Glob. Health 2022, 7, e008564. [Google Scholar] [CrossRef]
- Mattalia, G.; Belichenko, O.; Kalle, R.; Kolosova, V.; Kuznetsova, N.; Prakofjewa, J.; Stryamets, N.; Pieroni, A.; Volpato, G.; Sõukand, R. Multifarious Trajectories in Plant-Based Ethnoveterinary Knowledge in Northern and Southern Eastern Europe. Front. Vet. Sci. 2021, 8, 710019. [Google Scholar] [CrossRef]
- Nyamanga, P.A.; Suda, C.; Aagaard-Hansen, J. The Socio-Cultural Context and Practical Implications of Ethnoveterinary Medical Pluralism in Western Kenya. Agric Hum. Values 2008, 25, 513–527. [Google Scholar] [CrossRef]
- Martin, M.; McCorkle, M.C.; Mathias, E. Ethnoveterinary Medicine. An Annotated Bibliography of Community Animal Healthcare; ITDG Publishing: London, UK, 2001. [Google Scholar]
- Henriques, A.M.; Neto, A.; Fagulha, T.; Almeida, V.; Fevereiro, M. Molecular Characterization and Phylogenetic Analysis of Newcastle Disease Viruses Isolated in Southern Angola, 2016–2018. Infect. Genet. Evol. 2023, 113, 105481. [Google Scholar] [CrossRef] [PubMed]
- Ramovha, L.I.; Van Wyk, A.E. Ethnoveterinary Practices of the VhavenḓA, South Africa, in the Treatment of Redwater (Mali) In Cattle. Indilinga Afr. J. Indig. Knowl. Syst. 2016, 15, 314–327. [Google Scholar]
- Gomes, A.F.; Satiaca, C.S. Okulima Kuvala. Campesinato e Meios de Vida No Município de Cacula, Província da Huíla Angola; Publicações Isptundavala: Lubango, Angola, 2019; pp. 1–125. [Google Scholar]
- Paixão, A.; Silvino, R.; Sánchez, L.M.; Loução Bongo, A.; Simões, C.; Lucombo Maria, D.; Cunga, A.; Inácio, E. Actividad Antihelmíntica in Vivo de Tephrosia Vogelii Sobre Estrongílidos Gastrointestinales En Caprinos Infectados Naturalmente. Rev. Med. Vet. 2021, 1, 51–60. [Google Scholar] [CrossRef]
- Eloff, J.N.; McGaw, L.J. Application of Plant Extracts and Products in Veterinary Infections. In New Strategies Combating Bacterial Infection; Wiley: Hoboken, NJ, USA, 2008; pp. 205–228. [Google Scholar]
- Pérez-Ojeda Del Arco, A.; Pérez-Ojeda Arco, D.M.; Tapia-Cortese, S.; Blancas, N.I. What Have We Forgotten? Returning Data from Ethnobiological Research to Local Communities. Bioremediat. Biodivers. Bioavailab. 2011, 5, 22–27. [Google Scholar]
- Patzlaff, R.G.; Peixoto, A.L. A Pesquisa Em Etnobotânica e o Retorno Do Conhecimento Sistematizado à Comunidade: Um Assunto Complexo. Hist. Cienc. Saude Manguinhos 2009, 16, 237–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leeflang, P. Some Observations on Ethnoveterinary Medicine in Northern Nigeria. Vet. Q. 1993, 15, 72–74. [Google Scholar] [CrossRef]
- Durie, M. Understanding Health and Illness: Research at the Interface between Science and Indigenous Knowledge. Int. J. Epidemiol. 2004, 33, 1138–1143. [Google Scholar] [CrossRef]
- Waldron, I. The Marginalization of African Indigenous Healing Traditions within Western Medicine: Reconciling Ideological Tensions and Contradictions along the Epistemological Terrain. Women’s Health Urban Life 2010, 9, 50–71. [Google Scholar]
- Ampomah, I.G.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Emeto, T.I. Effectiveness of Integrated Health Systems in Africa: A Systematic Review. Medicina 2020, 56, 271. [Google Scholar] [CrossRef] [PubMed]
- Zorloni, A. Toward a Better Understanding of African Ethnoveterinary Medicine and Husbandry. In Ethnoveterinary Medicine; Springer International Publishing: Cham, Switzerland, 2020; pp. 151–172. [Google Scholar]
- Kananoja, K. Healing Knowledge in Atlantic Africa; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781108868020. [Google Scholar]
- Missão de Inquéritos Agrícolas de Angola (MIAA). Recenseamento Agrícola de Angola. 28—Zona Subdesértica do Litoral (Zona Agrícola No 22/29); MIAA: Luanda, Angola, 1970. [Google Scholar]
- César, J.; Manuel Armando Valeriano, M.; Augusto Manue, N.G.C.; Fernando Manuel, G.M. Avaliação Das Terras de Pastoreio Extensivo Na Província Do Namibe-Angola; Instituto Superior de Agronomia (ISA): Lisbon, Portugal, 2014. [Google Scholar]
- Instituto Nacional de Estatística (INE). Projecção da População Província do Namibe 2014–2050; INE: Luanda, Angola, 2016. [Google Scholar]
- Governo de Angola. Plano de Desenvolvimento Nacional 2018–2022; Governo de Angola: Luanda, Angola, 2018. [Google Scholar]
- Diniz, A.C.; Aguiar, F.Q. de B. Regiões Naturais de Angola. Fomento 1967, 5, 45–50. [Google Scholar]
- Sousa, F. Etnografia de Angola Entre a Pesquisa e o Desenvolvimento de Políticas Culturais; Mayamba Editora: Luanda, Angola, 2012. [Google Scholar]
- Martins, V. The Plateau of Trials: Modern Ethnicity in Angola. Ph.D. Thesis, ISCTE IUL Instituto Universitario de Lisboa, Lisbon, Portugal, 2015. [Google Scholar]
- ISE—International Society of Ethnobiology International Society of Ethnobiology Code of Ethics (with 2008 Additions). 2006. Available online: https://www.ethnobiology.net/what-we-do/core-programs/ise-ethics-program/code-of-ethics/#:~:text=It%20affirms%20the%20commitment%20of,linguistic%20and%20biological%20diversity%3B%20and (accessed on 16 January 2015).
- Signorini, A.; Lombardini, C.; Bruschi, P.; Vivona, L. Conoscenze Etnobotaniche e Saperi Tradizionali Territorio Di San Miniato (Pisa). Atti. Soc. Tosc. Sci. Nat. Mem. Serie 2007, 114, 65–83. [Google Scholar]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A Preliminary Classification of the Healing Potential of Medicinal Plants, Based on a Rational Analysis of an Ethnopharmacological Field Survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef]
- Milani, F.; Bottoni, M.; Bardelli, L.; Colombo, L.; Colombo, P.S.; Bruschi, P.; Giuliani, C.; Fico, G. Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy). Plants 2023, 12, 2748. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. “Circlize” Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Uprety, Y.; Karki, S.; Poudel, R.C.; Kunwar, R.M. Ethnoveterinary use of plants and its implication for sustainable livestock management in Nepal. Front. Vet. Sci. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Dharani, N.; Yenesew, A.; Aynekulu, E.; Tuei, B.; Jamnadass, R.; Dawson, I.K. Traditional Ethnoveterinary Medicine in East Africa. A Manual on the Use of Medicinal Plants; The World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2015; pp. 1–199. [Google Scholar]
- Ribeiro, A.; Romeiras, M.M.; Tavares JFaria, M.T. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J. Ethnobiol. Ethnomed. 2010, 6, 33. [Google Scholar] [CrossRef]
- Abrha, H.K.; Gerima, Y.G.; Gebreegziabher, S.T. Indigenous Knowledge of Local Communities in Utilization of Ethnoveterinary Medicinal Plants and Their Conservation Status in Dess’a Priority Forest, Northeastern Escarpment of Ethiopia. Res. Square 2020, 1–51. [Google Scholar] [CrossRef]
- Galav, P.; Jain, A.; Katewa, S.S. Traditional veterinary medicines used by livestock owners of Rajasthan India. Indian J. Tradit. Knowl. 2013, 12, 47–55. [Google Scholar]
- Yigezu, Y.; Haile, D.B.; Ayen, W.Y. Ethnoveterinary medicines in four districts of Jimma zone, Ethiopia: Cross sectional survey for plant species and mode of use. BMC Vet. Res. 2014, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, V.; Masika, P.J. Ethnoveterinary uses of medicinal plants: A survey of plants used in the ethnoveterinary control of gastro-intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharma. Biol. 2010, 48, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Selogatwe, K.M.; Asong, J.A.; Struwig, M.; Ndou, R.V.; Aremu, A.O. A Review of Ethnoveterinary Knowledge, Biological Activities and Secondary Metabolites of Medicinal Woody Plants Used for Managing Animal Health in South Africa. Vet. Sci. 2021, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Djoueche, C.M.; Azebaze, A.B.; Dongmo, A.B. Investigation of plants used for the ethnoveterinary control of gastro-intestinal parasites in Benoue Region, Cameroon. Tropicultura 2011, 29, 205–211. [Google Scholar]
- Hassan, M.; Yuguda, U.A. Study of Ethnoveterinary Medicinal Plants Used by Pastoralists in Northern Gombe State, Nigeria. Asian J. Plant Biol. 2021, 3, 1–5. [Google Scholar] [CrossRef]
- Fidèle, T.D.; Roland, M.N.; Almamy, K.; Adama, K.; Hamidou, T.H.; Gaston, B.A.M. Ethnoveterinary study on livestock practices and the use of medicinal plants against gastrointestinal parasites of small ruminants in the BWA community in Burkina Faso. Int. J. Vet. Sci. Anim. Husb. 2023, 8, 100–107. [Google Scholar] [CrossRef]
- Dansou, C.; Olounladé, P.A.; Konmy, B.; Songbé, O.; Arigbo, K.; Aboh, A.; Lagnika, L.; Hounzangbé-Adoté, S. Ethno-Veterinary Survey and Quantitative Study of Medicinal Plants with Anthelmintic Potential Used by Sheep and Goat Breeders in the Cotton Zone of Central Benin (West Africa). J—Multidiscip. Sci. J. 2021, 4, 544–563. [Google Scholar] [CrossRef]
- Ismaila, M.; Adamu, S. The impact of traditional methods of managing snake bite in humans and livestock among the Hausa-Fulani communities of Sokoto State (North-western Nigeria). J. Med. Plants Res. 2012, 6, 4489–4493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).