Abstract
Seed priming has become a practical pre-sowing strategy to deal with abiotic stresses. This study aims to explore the effects of polyethylene glycol (PEG) priming on seed germination and seedling growth of Scutellaria baicalensis Georgi under salt stress. Regardless of seed priming, salt stress significantly inhibited the seed germination and seedling growth of S. baicalensis. PEG priming significantly alleviates the inhibitory effects of salt stress on seed germination and seedling growth when compared to non-priming and water priming. Among all treatments, PEG priming exhibited the highest germination rate, germination potential, seed vigor index, fresh weight, dry weight, and plant length; the highest contents of proline, soluble sugar, and soluble protein; the highest K+/Na+ ratio and relative water content; the highest antioxidant activities and contents; but the lowest H2O2, malondialdehyde (MDA) content, and relative electrical conductivity in response to salt stress. In addition, PEG priming had the highest transcript levels of antioxidant-related genes among all treatments under NaCl stress. Taken together, the results demonstrated that seed priming with PEG could be recommended as an effective practice to enhance the germination and early seedling growth of S. baicalensis under saline conditions.
1. Introduction
Soil salinization is an important environmental factor for crop planting, as it not only decreases planting area but also reduces the yield and quality of crops [1]. Globally, around one billion hectares of land are in high salinity, and the salinization of soil is becoming more and more serious during these decades, which is due to climate change, improper irrigation measures, and so on [1,2]. In China, about 1.3 × 107 hectares of saline soils are potential agricultural land, accounting for about 10% of the total cultivated land area [3]. The fair use of these saline soils can increase cultivated land area effectively, meeting the diverse demands of the growing population.
The mechanisms of salt-induced damage in plants are well documented, including osmotic stress, oxidation stress, and ion toxicity. These deleterious effects ultimately lead to reduced water and nutrient absorption by plants [4,5]. To deal with the unfavorable stresses, plants have evolved a variety of adaptive mechanisms, such as up-regulating osmolyte contents, antioxidant systems, and ion homeostasis or regionalization. Notably, the accumulation of osmolytes, such as proline content, helps to improve water uptake, and the activation of antioxidant enzymes is beneficial to reduce ROS-induced oxidative damage [6,7].
Seed germination has been considered the most vulnerable stage in a plant’s life cycle, because salt stress can severely hinder seed sprouting and subsequent seedling establishment [8]. So, rapid seed germination and seedling establishment are vital for plants to survive in a saline environment [9]. To tackle this challenge, two methods have proven effective in agricultural practices: highly salt-tolerant varieties and the direct application of exogenous chemicals [10]. Seed priming is a pre-sowing treatment measure that involves initially controlling the water absorption of seeds and finally re-drying them [11]. And mounting evidence has shown that primed seeds are stronger than unprimed seeds in abiotic stresses [12,13,14]. Some chemical agents, such as phytohormones, reactive chemical species (H2O2 and NO), osmolytes, and nanoparticles, act in seed priming [15,16]. And the agent type, concentration, and treatment time all influence the effects of treatments [11]. Therefore, it is necessary to choose an optimal agent and the concentration for a certain crop in seed priming. Benadjaoud et al. [14] reported that 4% potassium chloride used in butterfly-lavender seed priming dramatically mitigated the negative impacts of salt or drought stress on germination.
Scutellaria baicalensis Georgi is a popular medicinal plant that is widely cultivated in China. Its dry root has been used as a kind of primary medicinal component to treat many diseases, such as hepatitis, dysentery, and epilepsy, for more than 2000 years [17,18]. Recent studies demonstrate that the active ingredients from the root of S. baicalensis exhibit preventive and therapeutic properties against a series of inflammatory responses triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19 [19]. Currently, due to rapidly increasing demand, cultivated S. baicalensis has surpassed its wild counterpart as the primary market supply. However, soil salinization has seriously endangered the planting area and yield of S. baicalensis. A high level of seed germination and seedling establishment is essential for S. baicalensis to survive in a saline habitat. This study aims to explore the effects of polyethylene glycol (PEG) priming on seed germination and seedling growth of S. baicalensis under salt stress. It provides an empirical foundation for the practical production and selection of salt-tolerant varieties of S. baicalensis.
2. Results
2.1. PEG Priming Significantly Alleviates the Germination Inhibition of S. baicalensis Seeds under Salt Stress
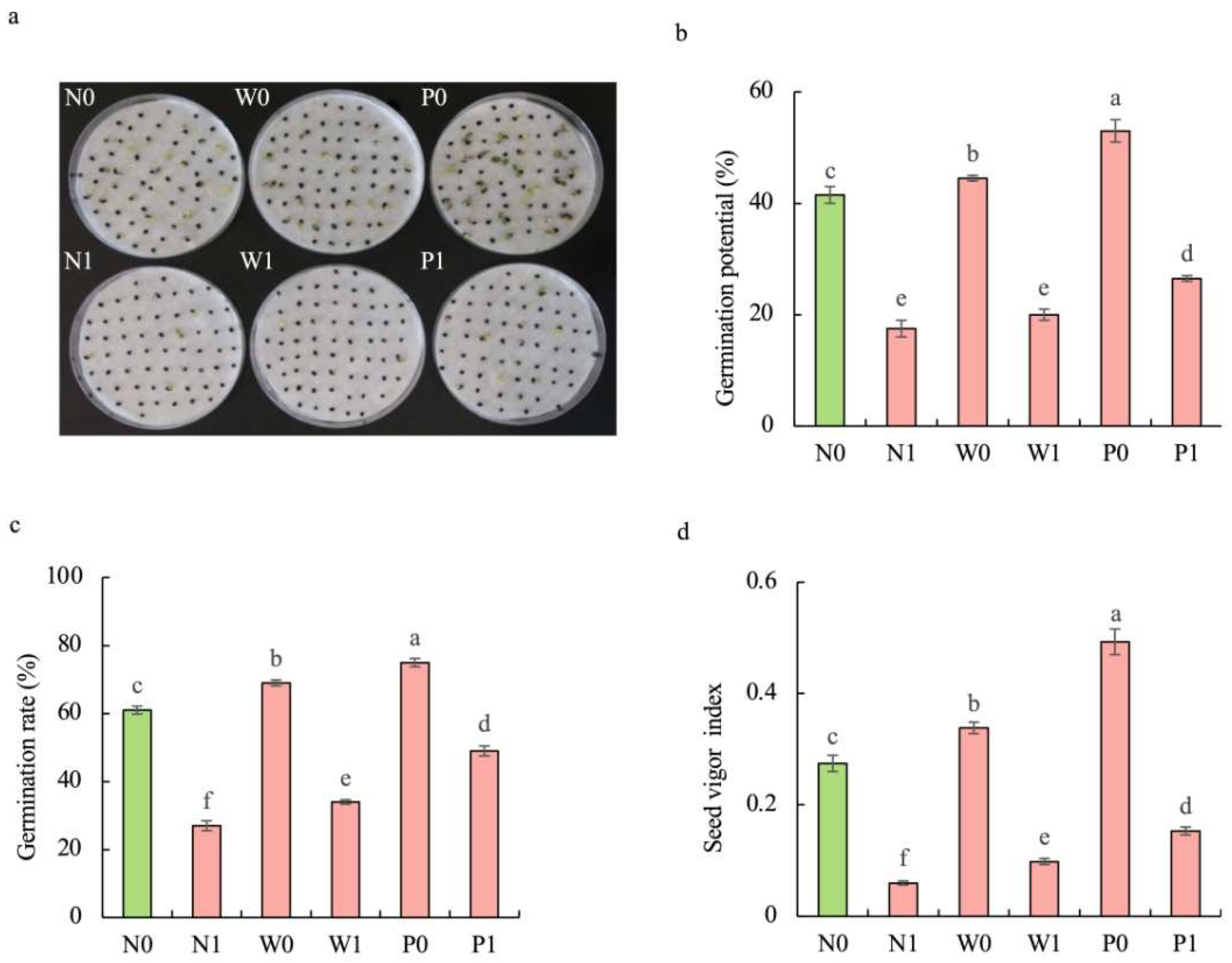
Regardless of seed priming, salt stress significantly inhibited the germination of S. baicalensis seeds. Compared to the non-priming control, priming treatments alleviated the negative effect of salt stress on seed germination (Figure 1). The germination potential (GP), germination rate (GR), and seed vigor index (SVI) under W1 treatment were increased by 14.3%, 25.9%, and 64.2%, respectively, compared to that under N1 treatment. The GP, GR, and SVI under P1 treatment were enhanced by 51.4%, 81.5%, and 155.7%, respectively, over N1 treatment.
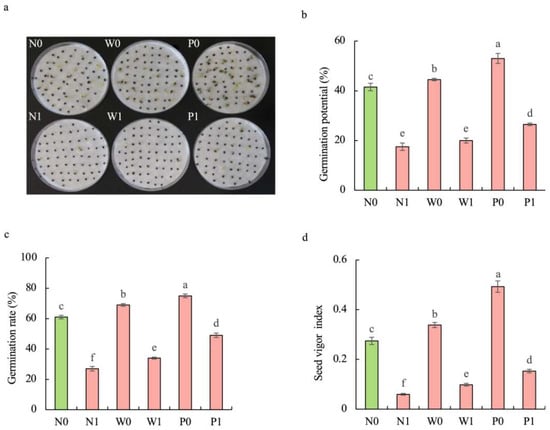
Figure 1.
Effects of PEG priming on the germination of S. baicalensis seeds in response to salt stress. (a) Phenotype; (b) germination potential; (c) germination rate; (d) seed vigor index. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
2.2. PEG Priming Significantly Enhanced the Growth of S. baicalensis Seedlings under Salt Stress
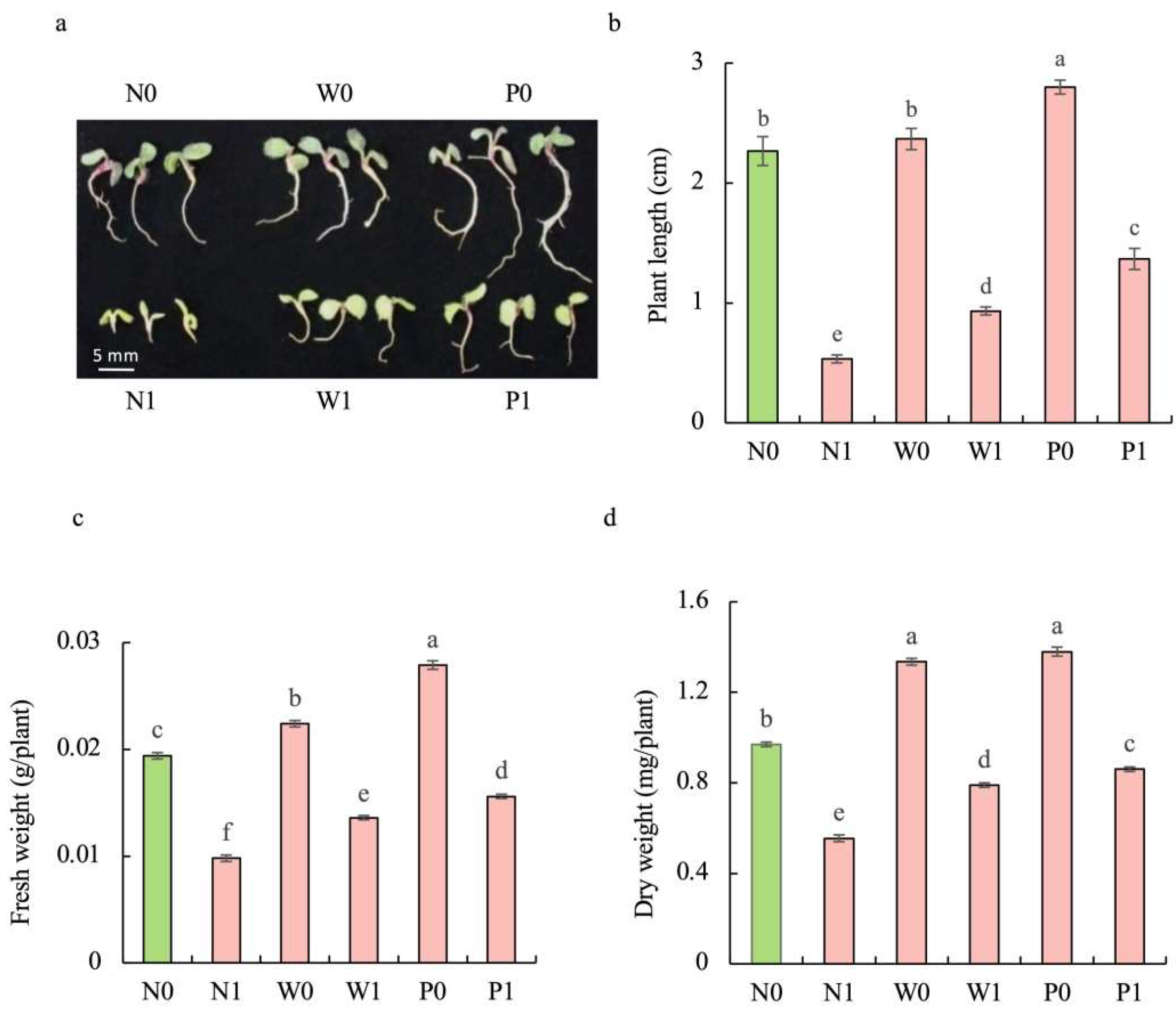
Regardless of seed priming, salt stress significantly impaired the growth of S. baicalensis seedlings. Compared to the non-priming control, priming treatments significantly mitigated the inhibitory effect of salt stress on the growth of seedlings (Figure 2a). The plant length (PL) of S. baicalensis seedlings under salt stress was significantly decreased. Compared to the N0 treatment, the PL under N1, W1, and P1 treatments was decreased by 76.5%, 58.8%, and 39.7%, respectively. Upon exposure to salt stress, the seedlings under priming treatments showed higher PL than those in the non-priming control. The PLs under W1 and P1 treatments were 1.75- and 2.56 times that under N1 treatment, respectively (Figure 2b).
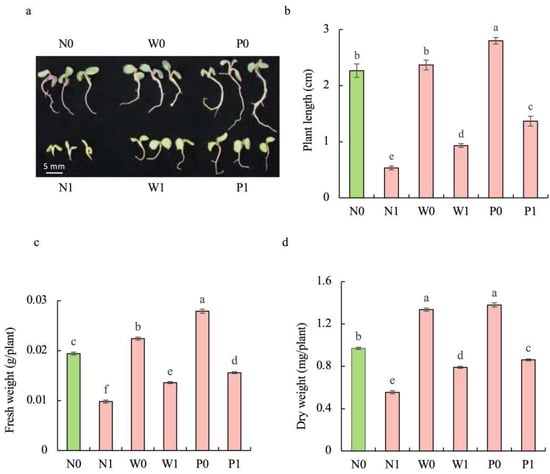
Figure 2.
Effects of PEG priming on the growth of S. baicalensis seedling in response to salt stress. (a) Phenotype; (b) plant length; (c) fresh weight; (d) dry weight. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
The fresh weight (FW) and dry weight (DW) of seedlings under salt stress were significantly decreased. Compared to the N0 treatment, N1, W1, and P1 treatments resulted in significant decreases in the FW (49.5%, 29.9%, and 19.6%) and DW (42.8%, 18.6%, and 11.3%), respectively. Compared to the non-priming control, priming treatments significantly enhanced the FW and DW under salt stress. W1 and P1 treatments caused significant enhancements in the FW (38.8% and 59.2%, respectively) and DW (42.3% and 54.9%) compared to the N1 treatment (Figure 2c,d).
2.3. Effect of PEG Priming on Osmolyte Content and Relative Water Content of S. baicalensis Shoots under Salt Stress
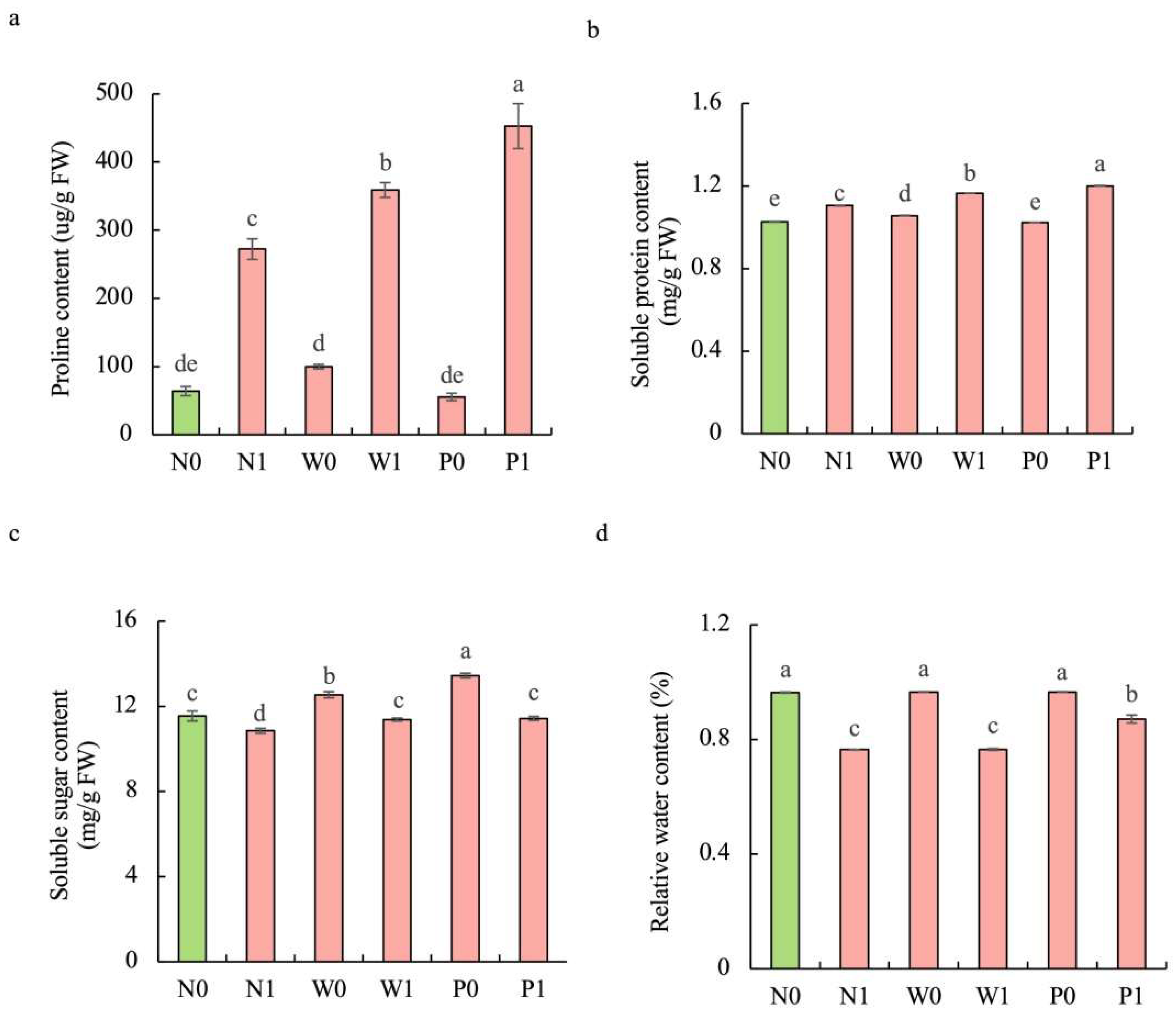
Salt stress significantly enhanced the contents of proline and soluble protein while diminishing the soluble sugar concentration and relative water content (RWC) in S. baicalensis shoots compared to non-salt treatment (Figure 3). Upon exposure to salt stress, seedlings in the PEG priming treatment exhibited higher contents of proline, soluble protein, soluble sugar, and RWC than in the non-priming control. The contents of proline, soluble protein, soluble sugar, and RWC under P1 treatment were increased by 66.3%, 8.6%, 5.4%, and 13.9%, respectively, over the N1 treatment.
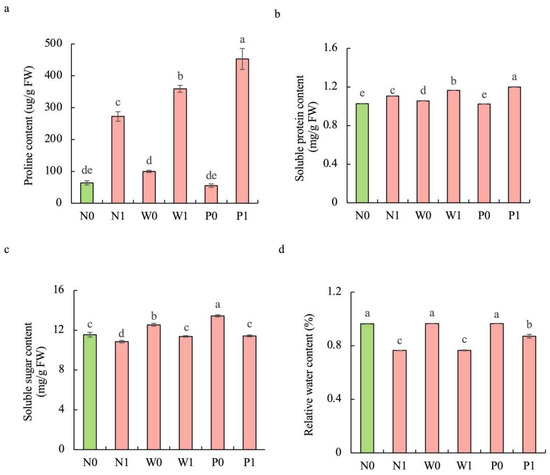
Figure 3.
Effects of PEG priming on the osmolyte content and relative water content (RWC) of S. baicalensis shoots in response to salt stress. (a) Proline content; (b) soluble protein content; (c) soluble sugar content; (d) RWC. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
2.4. Effect of PEG Priming on Lipid Peroxidation and Membrane Permeability of S. baicalensis Shoots under Salt Stress
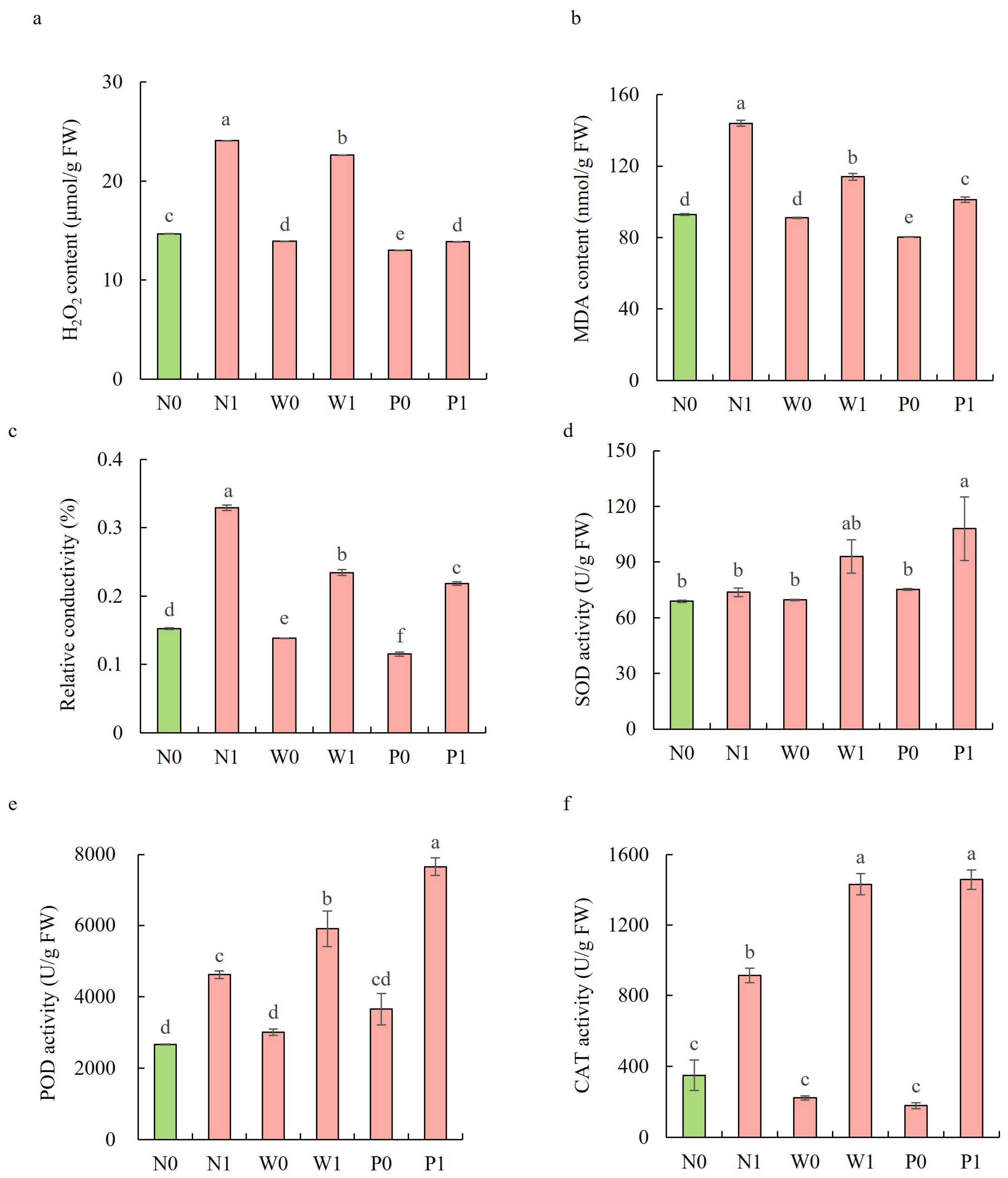
In the non-priming treatments, salt stress significantly increased the H2O2 content, MDA content, and REC in the shoots of S. baicalensis (Figure 4a–c). The H2O2 content, MDA content, and REC under N1 treatment were significantly enhanced by 64.1%, 55.0%, and 115.9%, respectively, over the N0 treatment. Both water and PEG priming significantly reduced the H2O2 content, MDA content, and REC, with PEG priming exhibiting a larger decline than water priming. Compared to the N1 treatment, the W1 and P1 treatments led to significant decreases in the H2O2 content (6.0% and 42.4%, respectively), MDA (20.8% and 29.7%), and REC (28.8% and 33.7%).
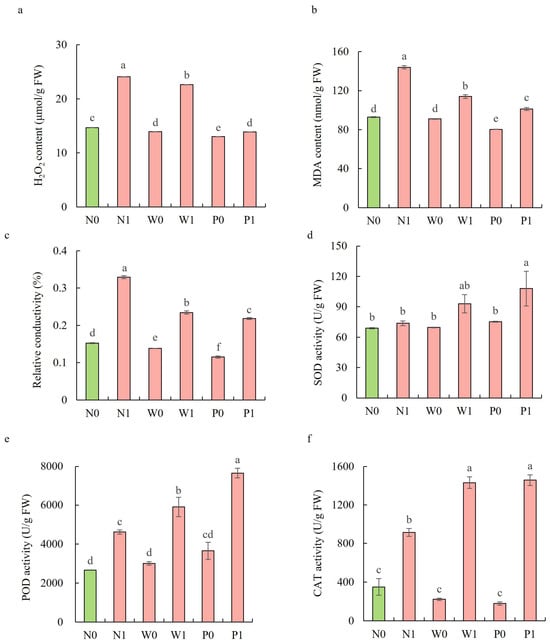
Figure 4.
Effects of PEG priming on lipid peroxidation, membrane permeability, and enzymatic antioxidant activity of S. baicalensis shoots in response to salt stress. (a) H2O2 content; (b) MDA content; (c) relative electrical conductivity (REC); (d) SOD activity; (e) POD activity; (f) CAT activity. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
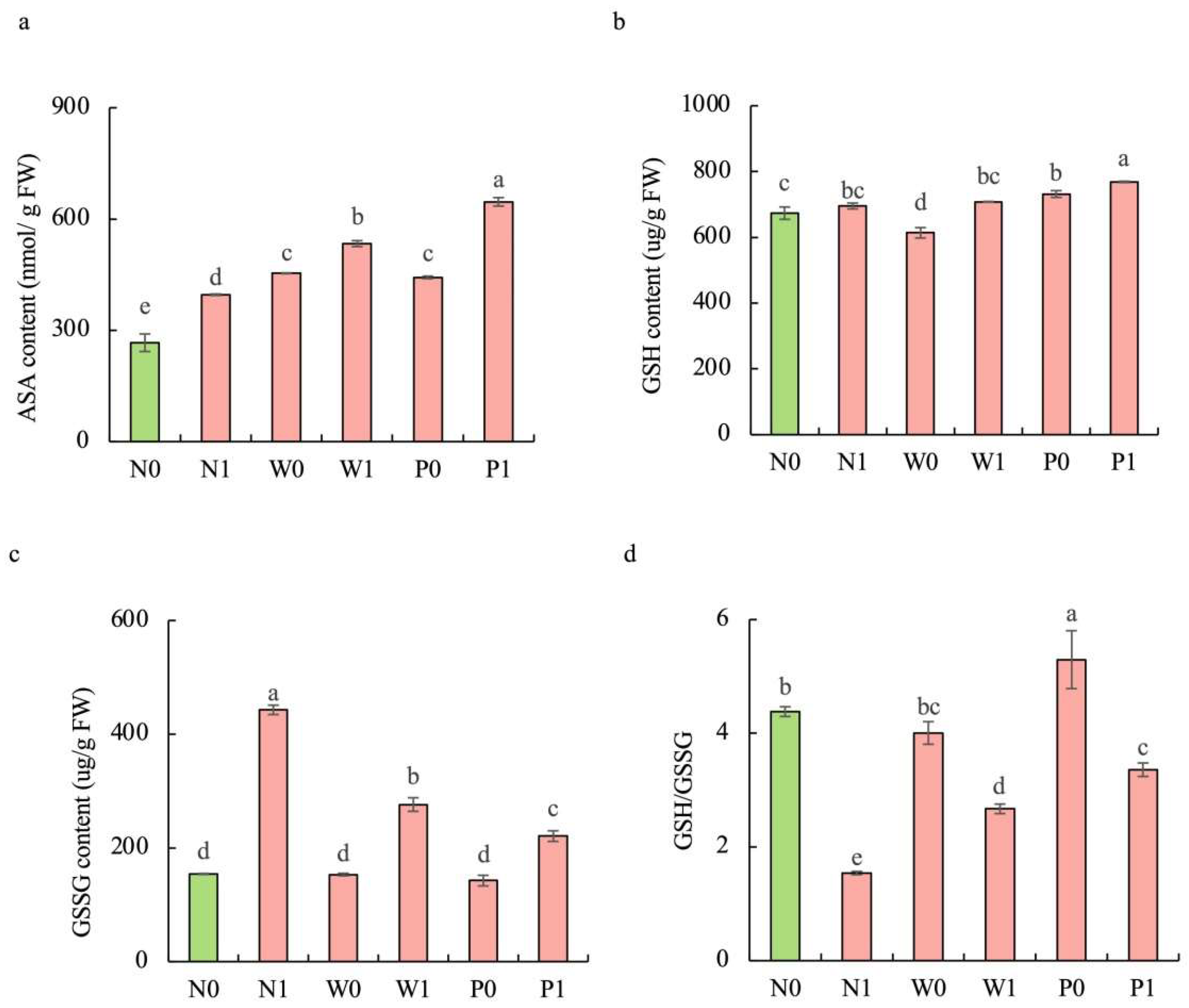
2.5. Effect of PEG Priming on Antioxidant Systems of S. baicalensis Shoots under Salt Stress
Upon exposure to salt stress, PEG priming significantly increased the activities of enzymatic antioxidants and the content of non-enzymatic antioxidants in the shoots of S. baicalensis, compared to the non-priming control (Figure 4d–f and Figure 5). The activities of SOD, POD, and CAT under P1 treatment were enhanced by 46.4%, 65.9%, and 59.6%, respectively, over the N1 treatment. The ascorbic acid (AsA) content, glutathione (GSH) content, and GSH/GSSG ratio under P1 treatment were respectively enhanced by 63.2%, 10.5%, and 117.9%, but the GSSG content decreased by 50.1% compared to the N1 treatment.
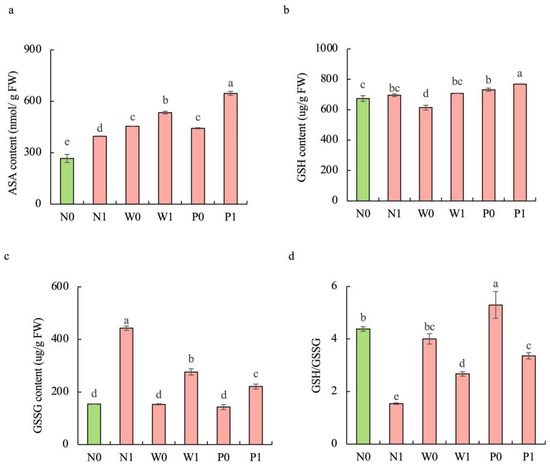
Figure 5.
Effects of PEG priming on the non-enzymatic antioxidant content of S. baicalensis shoots in response to salt stress. (a) AsA content; (b) GSH content; (c) GSSG content; (d) GSH/GSSG ratio. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
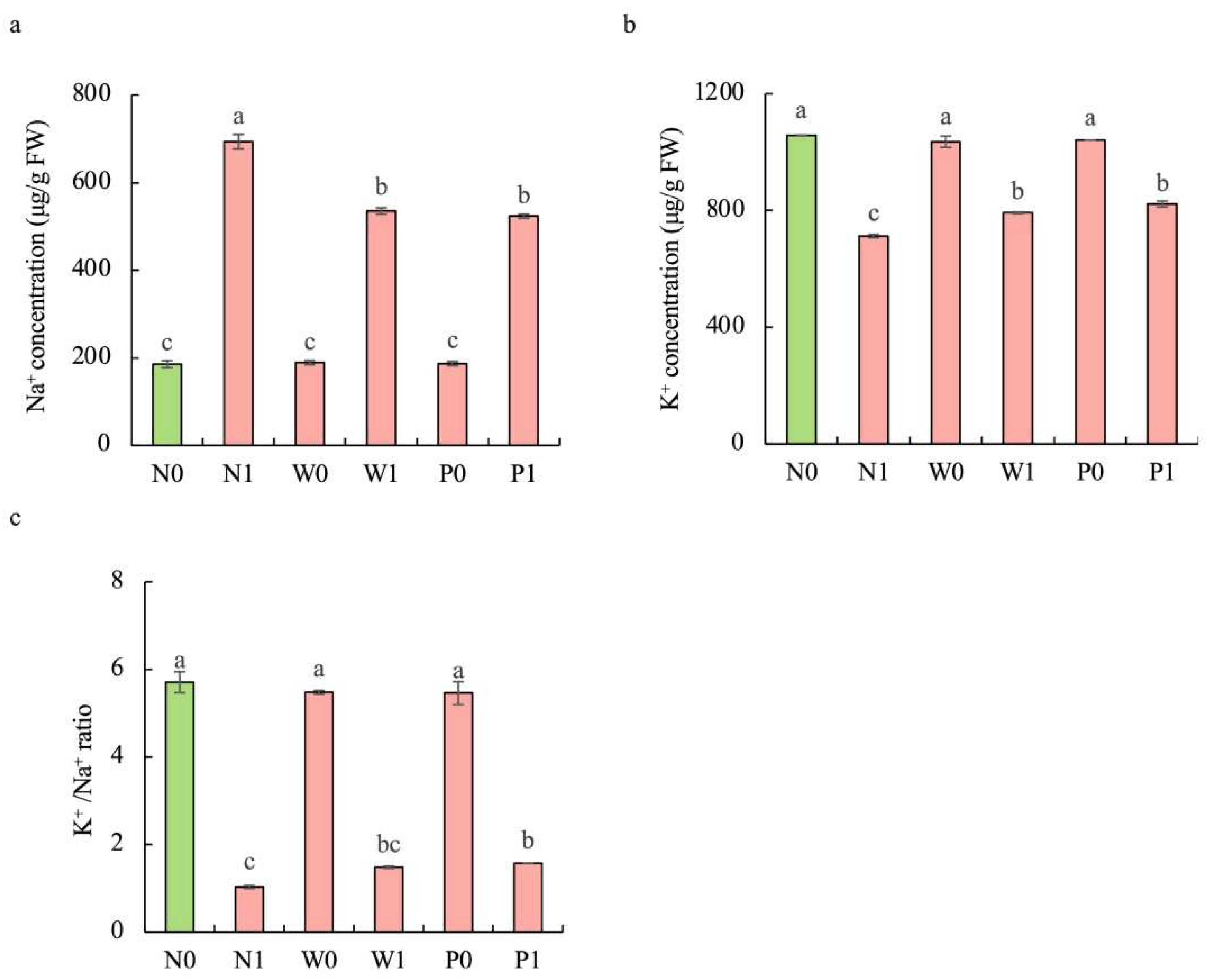
2.6. Effect of PEG Priming on Contents of Sodium and Potassium Ions in S. baicalensis Shoots under Salt Stress
Compared to the non-salt treatment, salt stress significantly enhanced the Na+ content but decreased the K+ content in the shoots of S. baicalensis (Figure 6). The contents of Na+ under N1, W1, and P1 treatments were respectively increased by 273.9%, 188.5%, and 182.2%, while the contents of K+ were respectively decreased by 32.6%, 25.1%, and 22.2%, over the N0 treatment. Compared to the non-priming treatment, priming treatment significantly decreased the Na+ content but enhanced the K+ content under salt stress. The Na+ contents under W1 and P1 treatments were respectively decreased by 22.9% and 24.5%, while the K+ contents were respectively increased by 11.2% and 15.4%, over the N1 treatment. Furthermore, PEG priming and water priming showed a higher K+/Na+ ratio than in the non-priming treatment under salt stress. The K+/Na+ ratios in W1 and P1 were enhanced by 44.1% and 52.9%, respectively, over N1 treatment.
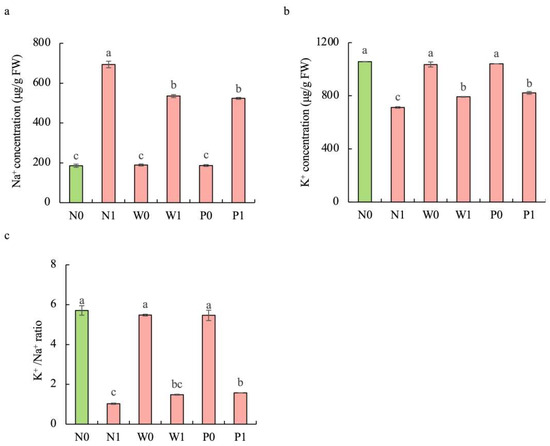
Figure 6.
Effects of PEG priming on the Na+ content, K+ content, and K+/Na+ ratio of S. baicalensis shoots in response to salt stress. (a) Na+ content; (b) K+ content; (c) K+/Na+ ratio. Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
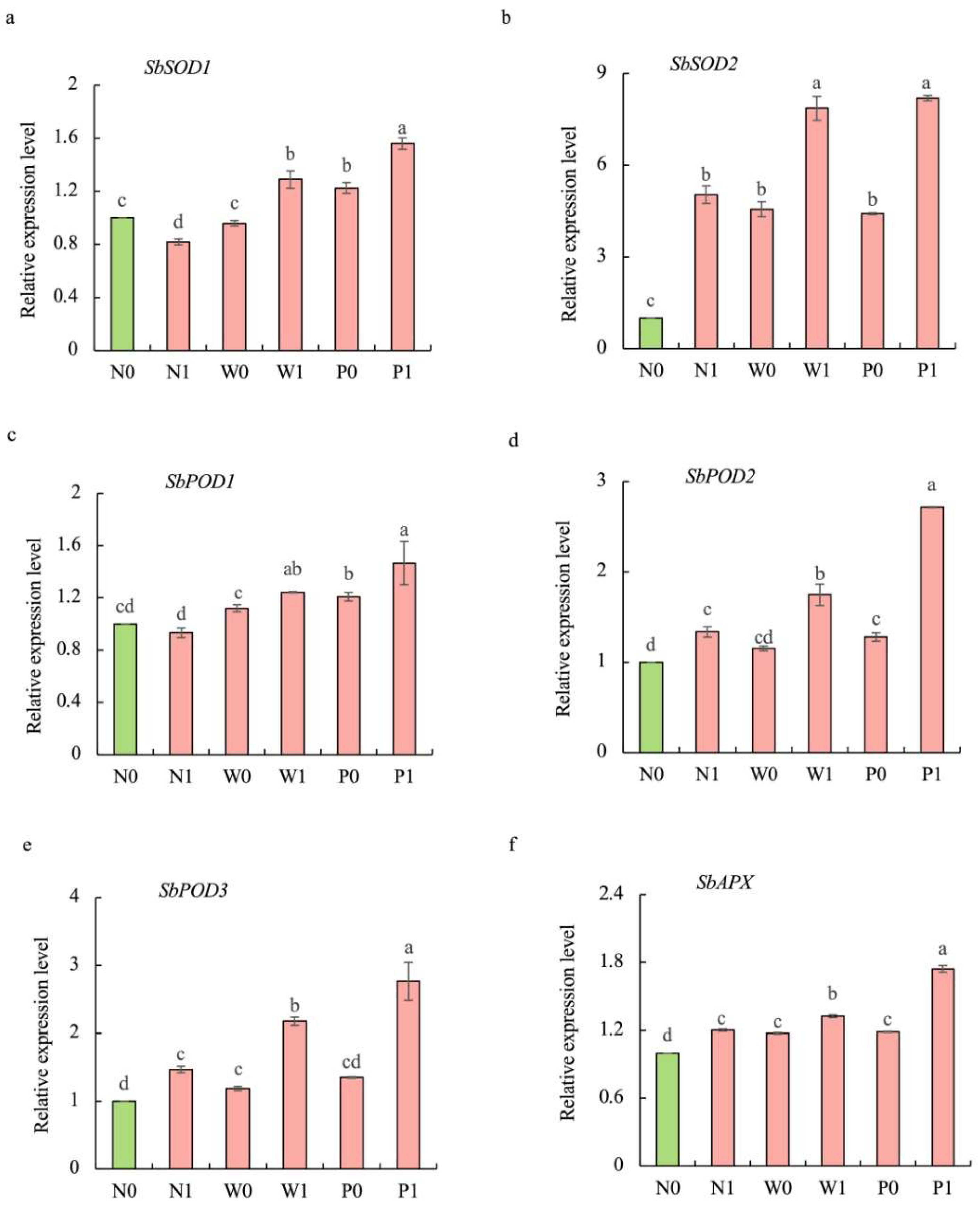
2.7. Effect of PEG Priming on Transcript Levels of Antioxidant-Related Genes in S. baicalensis Shoots under Salt Stress
Compared to the non-priming control, priming treatments significantly increased the transcript levels of antioxidant-related genes, and PEG priming demonstrated greater effectiveness than water priming under salt stress (Figure 7). The transcript levels of SbPOD1, SbPOD2, SbPOD3, SbSOD1, SbSOD2, and SbAPX under P1 treatment were significantly enhanced by 57.1%, 103.2%, 87.9%, 90.5%, 62.6%, and 44.9%, respectively, over N1 treatment.
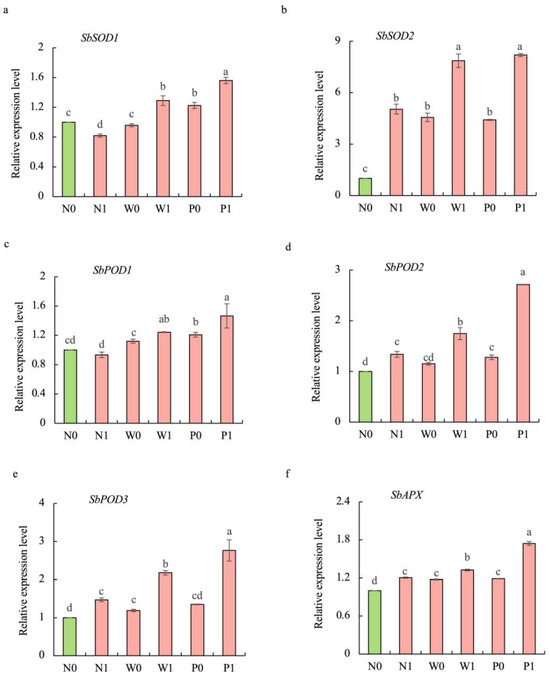
Figure 7.
Effects of PEG priming on the transcription levels of antioxidant-related genes of S. baicalensis shoots in response to NaCl stress. (a) SbSOD1; (b) SbSOD2; (c) SbPOD1; (d) SbPOD2; (e) SbPOD3; (f) SbAPX. Data represent mean ± SD (n = 3). Bars annotated by different letters indicate statistically significant differences between treatments, as determined by the LSD test at a significance level of p < 0.05. N0: non-priming without salt treatment. N1: non-priming and cultivated with 75 mM of NaCl solution. W0: water priming without salt treatment. W1: water priming and cultivated with 75 mM of NaCl solution. P0: PEG priming without salt treatment. P1: PEG priming and cultivated with 75 mM of NaCl solution.
3. Discussion
Abiotic stresses, such as salt and drought, are the key ecological factors that limit the growth and quality of traditional Chinese medicine [20]. Seed priming is considered a promising technique for increasing seed germination and seedling growth under challenging environmental conditions. This technique has been proven effective in a variety of plant species, including food crops, certain vegetables, and flowers [9,15,21]. Polyethylene glycol (PEG) is an osmotic agent commonly used for simulating the conditions of drought stress in plants [22]. PEG priming efficiently improved the germination rate and seedling growth of barley in response to drought stress [23]. In the presented study, salt stress significantly inhibited the seed germination and seedling growth of S. baicalensis, regardless of whether the seeds were primed or not. This is in agreement with the other studies that demonstrated negative effects of salt stress on plant growth [14,24]. PEG priming showed significantly enhanced seed germination rate and seedling growth of S. baicalensis under salt stress, certificating the positive role of seed priming in plant response to environmental stress.
Although it is a highly energy-consuming process, the rapid biosynthesis of certain compatible solutes, such as soluble sugar and proline, remains an efficient strategy for plants to cope with osmotic stress [25]. These compatible solutes play important roles in the enhancement of salt and drought tolerance in many plant species [26,27]. Parallel to the observations, our studies revealed that the contents of proline and soluble protein in S. baicalensis seedlings were significantly increased in response to salt stress and seed priming, with either water or PEG further enhancing their contents. Soluble sugar, an important osmolyte, does not always show an increasing trend in response to salt stress. The possible reason is that the decomposition of these carbohydrates is conducive to providing sufficient carbon backbone and energy supply for the synthesis of other osmotic protectants containing nitrogen compounds to cope with the deepening of salt stress [28]. Wang et al. [29] reported that salt stress resulted in the decrease in soluble sugar content of G. uralensis seedlings. RWC is an important indicator to assess environmental stress tolerance. Vazayef et al. [30] reported that a high level of salinity resulted in a significantly reduced RWC in leaves of Brassica napus. In this study, salt stress significantly decreased the soluble sugar content and RWC in S. baicalensis shoots but seed priming with PEG promoted the accumulation of soluble sugar and RWC, which agreed with previous studies reported by Wang et al. [29] and Vazayef et al. [30].
Ion homeostasis plays a vital role in plant salt tolerance. An excess accumulation of sodium ions (Na+) under salt stress would disturb the balance between Na+ and other ions, such as potassium ions (K+) and calcium ions, (Ca2+), and eventually lead to ion toxicity [30]. In this study, we also found a significantly enhanced Na+ content and a significantly reduced K+ content in S. baicalensis shoots upon exposure to salt stress. Potassium, an essential macronutrient, plays an important role in regulating the activity of various enzymes during plant growth and development. A high ratio of K+/Na+ is required for plants to minimize Na+ toxicity and maintain ion homeostasis in saline environments [31]. In the presented study, the ratio of K+/Na+ in seedlings with seed priming was significantly higher than that in seedlings without any priming treatment under salt stress. It was helpful for S. baicalensis seedlings to maintain the ion homeostasis and minimize Na+ toxicity caused by salt stress.
Besides osmotic stress and ion toxicity, salt stress generates excess reactive oxygen species (ROS), such as H2O2 in plants, and easily leads to oxidative stress [32]. The changes in MDA levels and REC can be served as important markers for evaluating the degree of oxidative damage of cell membranes [33]. So, in the experiments presented here, the contents of H2O2, MDA, and REC were significantly enhanced in the seedlings treated by salt stress, suggesting that salt stress markedly increased lipid peroxidation. However, the degree of lipid peroxidation was greatly alleviated in the seedlings with seed priming, and PEG priming was more efficient than water priming. In plants, two sets of antioxidant systems are developed to minimize the adverse impacts from ROS: one is enzymatic antioxidants, such as SOD and CAT, and the other is non-enzymatic antioxidants, such as GSH and AsA [7,34]. Here, we found that salt stress mildly increased SOD activity and GSH content but significantly enhanced POD, CAT activities, and AsA content. The positive effects of PEG priming on the antioxidant systems of S. baicalensis shoots were stronger than those in non-priming and water-priming under salt stress. This is in line with the previous results that seed priming conferred drought tolerance to Chinese cabbage seedlings by up-regulating the activities of the antioxidant enzymes [12].
In the S. baicalensis genome, we found six genes encoding antioxidant enzymes (SbSOD1, SbSOD2, SbPOD1, SbPOD2, SbPOD3, and SbAPX) by exploring the National Centre for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov). In the presented experiment, we found that salt stress mildly down-regulated the expression of SbPOD1 and significantly decreased the expression of SbSOD1 but significantly increased the expression of the others (SbSOD2, SbPOD1, SbPOD2, SbPOD3, and SbAPX). This suggested that the expression pattern of these genes varied in response to salt stress. In addition, the transcript levels of SbSOD1, SbSOD2, SbPOD1, SbPOD2, SbPOD3, and SbAPX were found to be significantly enhanced in seedlings treated with PEG priming, indicating that PEG priming greatly up-regulated these genes’ expression levels. This can explain the highest activities of SOD, POD, and CAT in S. baicalensis shoots treated with PEG priming.
4. Materials and Methods
4.1. Plant Materials and Priming
Seeds of S. baicalensis Georgi were collected from its native producing area in Longxi County, Gansu Province, China. A total of 360 healthy and uniform seeds underwent surface sterilization with a 2% sodium hypochlorite solution for 10 min. Following this, they were rinsed four or five times with distilled water. The seeds were divided equally into three groups, two of which were soaked with distilled water or 20% PEG-6000 at room temperature for 12 h, respectively. After that, these seeds were washed thoroughly with distilled water and air-dried at room temperature for two days back to their initial moisture content. Another group without soaking served as the unprimed control. Both primed and unprimed seeds were set to germinate in Petri dishes, each lined with two layers of filter paper (Whatman #1, 60 seeds per dish). The filter paper was dampened with 2 mL of distilled water or 75 mM of NaCl solution.
There were six seed treatments in total: (1) non-priming without salt treatment (N0); (2) non-priming and cultivated with 75 mM of NaCl solution (N1); (3) water priming without salt treatment (W0); (4) water priming and cultivated with 75 mM of NaCl solution (W1); (5) PEG priming without salt treatment (P0); (6) PEG priming and cultivated with 75 mM of NaCl solution (P1). These seeds were placed in a germinator at 25 °C under 16 h/8 h of light/dark condition. The seeds per dish were sprayed with 1.0 mL of water or 75 mM of NaCl solution every 2 days. At least three independent biological replicates were used for each treatment.
4.2. Germination Test
The germination rate (GR), germination potential (GP), and seedling vigor index (SVI) were measured according to the methods described by Yan [12]. Seeds with 1 mm long radicle protruded through the seed coat were considered germinated. The GP was investigated on the 5th day and GR was on the 12th day.
4.3. Measurements of Growth Parameters and Sampling
After the germination test, three seedlings from each treatment were randomly collected and photographed. For each treatment, ten seedlings were selected to measure the plant length (PL), fresh weight (FW), and dry weight (DW). Relative water content (RWC) in shoots was determined as described by Li et al. [10]. PL was the distance from taproot tip to shoot unbent. The other seedlings were separated into shoots and roots, and the shoots were for later determining the physiological index and performing the molecular detection.
4.4. Assay of Physiological Indicators and Ion Contents
The MDA content, soluble protein content, and soluble sugar content were quantified following the methodologies delineated by Yusuf et al. [35], Aminian et al. [36], and Hackmann et al. [37], respectively. REC was determined by the conductance method. Proline content was measured according to the method described by Benitez et al. [38]. The contents of AsA, GSH, and GSSG (GSH, oxidized) were determined using the corresponding assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The H2O2 content was assayed following the methods in Martinez-Gutierrez et al. [39]. The activities of SOD, POD, and CAT were measured following the methods described by Usluoglu et al. [40]. The contents of Na+ and K+ were determined by flame photometry (ICP-OES, Optima 8000, PerkinElmer Instruments Co., Ltd., Waltham, MA, USA) as described by Williams and Twine [41].
4.5. qRT-PCR Analysis
Total RNA was isolated with an RNA extraction kit (Tiangen, Beijing, China) and then reverse transcribed into cDNA using a PrimeScript RT Reagent Kit (Tiangen, Beijing, China). Using SbActin as an internal reference, qRT-PCR was conducted to assess the expression levels of genes associated with antioxidant enzymes via specific primers (Table S1). The antioxidant-enzyme-related genes included SbSOD1 (HQ395746), SbSOD2 (HQ395747), SbPOD1 (AB024437), SbPOD2 (AB024438), SbPOD3 (AB024439), and SbAPX (HQ395752). The relative expression level was calculated using the 2–ΔΔt method [42]. All data are presented as the mean ± SD after normalization.
4.6. Statistical Analysis
Analysis-of-variance (ANOVA) procedures were performed with the SPSS 22.0 software (IBM, Armonk, NY, USA). Using the SAS program, the means were distinguished based on the least-significant-difference (LSD) test at a significance level of 0.05.
5. Conclusions
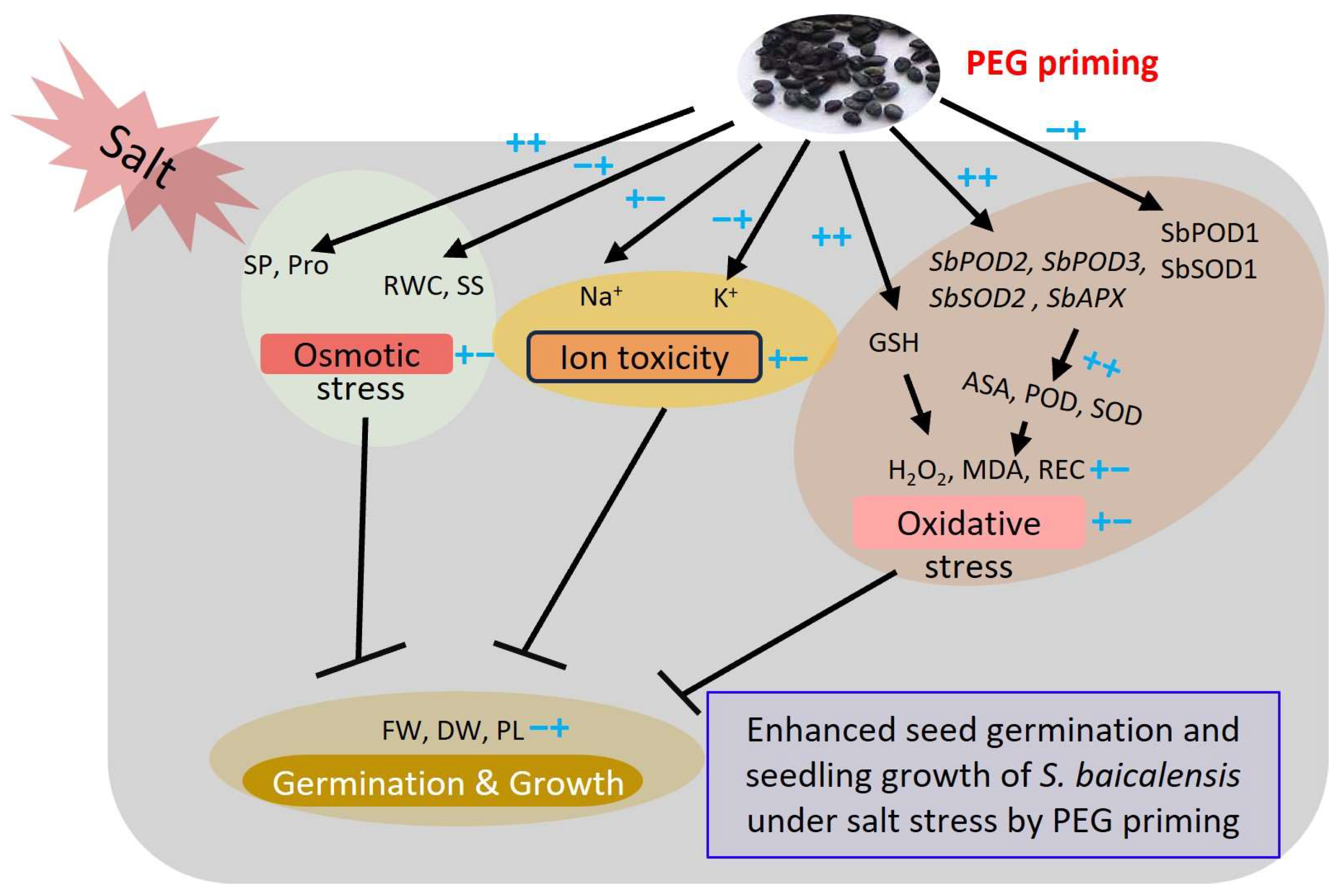
In general, PEG priming effectively alleviates the adverse effect of salt stress on the seed germination and seedling growth of S. baicalensis (Figure 8). This was due to PEG priming enhancing the accumulation of osmolytes, such as proline content, and improving the K+/Na+ ratio to tune down osmotic stress and ion toxicity; PEG priming also increased the AsA content and enzymatic activities through up-regulating the expression levels of antioxidant-related genes. In addition, PEG priming reduced the contents of H2O2 and MDA to minimize the oxidative stresses of S. baicalensis seedlings.
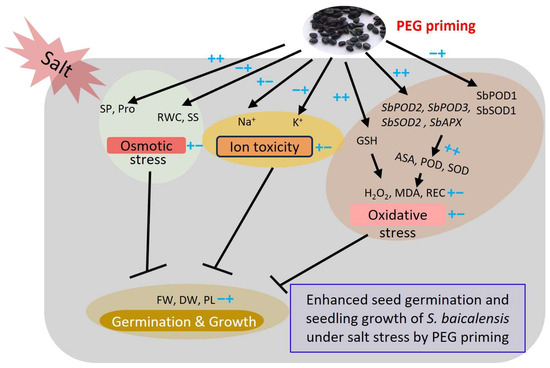
Figure 8.
Working model of enhanced seed germination and seedling growth of S. baicalensis under salt stress induced by PEG priming. ++ or −− refers to the strong increase or decrease treated by both salt stress and PEG priming, respectively. +− indicates the initial increase by salt stress and subsequent decrease caused by PEG priming. −+ indicates the initial decrease by salt stress and subsequent increase by PEG priming. SOD, POD, CAT, PEG, Pro, RWC, GSH, AsA, REC, MDA, FW, DW, SP, SS, and PL are the abbreviations of superoxide dismutase, peroxidase, catalase, polyethylene glycol, proline, relative water content, glutathione, ascorbic acid, relative electrical conductivity, malondialdehyde, fresh weight, dry weight, soluble protein, soluble sugar, and plant length, respectively.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13050565/s1, Table S1: Primers used for qRT-PCR analysis.
Author Contributions
Conceptualization, J.L.; Data curation, R.W. and C.L.; Formal analysis, J.L.; Funding acquisition, L.L.; Investigation, R.W., C.L., L.Z. and J.X.; Methodology, R.W., C.L. and J.X.; Project administration, J.L. and L.L.; Software, R.W. and C.L.; Supervision, J.L. and L.L.; Validation, L.L.; Visualization, L.L.; Writing—original draft, J.L.; Writing—review and editing, J.L., R.W., C.L., L.Z., L.L. and J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Beijing Joint Research Program for Germplasm Innovation and New Variety Breeding, grant number G20220628003-01.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Sousa, L.D. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Wang, J.L.; Huang, X.J.; Zhong, T.Y.; Chen, Z.G. Review on sustainable utilization of salt-affected land. Acta Geogr. Sin. 2011, 66, 673–684. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; Alsubeie, M.S.; Hafez, Y.; Emeran, A.; Moghanm, F.; Okasha, S.; Omara, R.; Basahi, M.A.; Darwish, D.B.E.; Ibrahim, M.F.M.; et al. Physiological and biochemical changes in vegetable and field crops under drought, salinity and weeds stresses: Control strategies and management. Agriculture 2022, 12, 2084. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.A.; Liu, L.G.; Guo, X.M.; Pei, Y.H.; Wang, C.X.; Song, X.Y. Exogenous sorbitol application confers drought tolerance to maize seedlings through up-regulating antioxidant system and endogenous sorbitol biosynthesis. Plants 2023, 12, 2456. [Google Scholar]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience 1986, 21, 1105–1112. [Google Scholar] [CrossRef]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Thuenprom, N.; Tisarum, R.; Cha-um, S.; Datta, A. Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Benadjaoud, A.; Dadach, M.; El-Keblawy, A.; Mehdadi, Z. Impacts of osmopriming on mitigation of the negative effects of salinity and water stress in seed germination of the aromatic plant Lavandula stoechas L. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100407. [Google Scholar] [CrossRef]
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol. 2021, 61, 1995–2003. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, J.; Jiang, C.; Li, G.; Lu, H.; Meng, F.; Shi, L. Deep sequencing of the Scutellaria baicalensis Georgi transcriptome reveals flavonoid biosynthetic profiling and organ-specific gene expression. PLoS ONE 2015, 10, e0136397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Wang, D.; Hu, S.; Zhang, Q.; Wang, Z.; Cui, L. Genome-wide identification and characterization of the WRKY gene family in Scutellaria baicalensis Georgi under diverse abiotic stress. Int. J. Mol. Sci. 2022, 23, 4225. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, S.; Cao, Y.; Chen, R.; Wang, Z.; Cao, X. Selection and evaluation of reference genes for qRT-PCR of Scutellaria baicalensis Georgi under different experimental conditions. Mol. Biol. Rep. 2021, 48, 1115–1126. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, X.; Chen, H.; Yang, R.; Chen, J.; Zou, L.; Wang, C.; Chen, J.; Tan, M.; Mei, Y.; et al. Variations in morphology, physiology, and multiple bioactive constituents of Lonicerae Japonicae Flos under salt stress. Sci. Rep. 2021, 11, 3939. [Google Scholar] [CrossRef]
- Cayuela, E.; Perezalfocea, F.; Caro, M.; Bolarin, M.C. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol. Plant. 1996, 96, 231–236. [Google Scholar] [CrossRef]
- Rubinstein, B. Regulation of H+ excretion: Effects of osmotic shock. Plant Physiol. 1982, 69, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Amini, R. Drought stress tolerance of barley (Hordeum vulgare L.) affected by priming with PEG. Int. J. Farming Allied Sci. 2013, 2, 803–808. [Google Scholar]
- Al-Shammari, W.B.; Altamimi, H.R.; Abdelaal, K. Improvement in physiobiochemical and yield characteristics of Pea plants with nano-silica and melatonin under salinity stress conditions. Horticulturae 2023, 9, 711. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hao, D.L.; Jin, M.; Li, Y.; Liu, Z.T.; Huang, Y.N.; Chen, T.X.; Su, Y.H. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, X.; Lang, D.; Ma, X.; Zhang, X. Physio-biochemical and transcriptomic analysis reveals that the mechanism of Bacillus cereus G2 alleviated oxidative stress of salt-stressed Glycyrrhiza uralensis Fisch. seedlings. Ecotoxicol. Environ. Saf. 2022, 247, 114264. [Google Scholar] [CrossRef]
- Vazayefi, M.; Shekari, F.; Zangani, E.; Dolatabadian, A.; Janda, T.; Mastinu, A. Seed treatment with chlormequat chloride improves the physiological and biochemical characteristics of Brassica napus L. under salt stress. Plant Stress 2023, 9, 100175. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, M.C.; Zhang, J.C.; Duan, L.S.; Li, Z.H. SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio. J. Plant Physiol. 2012, 169, 255–261. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, C.; Liang, Y.; Wang, C.; Yang, C.; Liu, G. A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress in tobacco plants. J. Plant Physiol. 2010, 167, 222–330. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Yusuf, C.S.; Chand, R.; Mishra, V.K.; Joshi, A.K. The association between leaf malondialdehyde and lignin content and re-sistance to spot blotch in wheat. J. Phytopathol. 2016, 164, 896–903. [Google Scholar] [CrossRef]
- Aminian, M.; Nabatchian, F.; Vaisi-Raygani, A.; Torabi, M. Mechanism of Coomassie Brilliant Blue G-250 binding to cetyltrimethylammonium bromide: An interference with the Bradford assay. Anal. Biochem. 2013, 434, 287–291. [Google Scholar] [CrossRef]
- Hackmann, T.J.; Keyser, B.L.; Firkins, J.L. Evaluation of methods to detect changes in reserve carbohydrate for mixed rumen microbes. J. Microbiol. Methods 2013, 93, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.C.; Vighi, I.L.; Auler, P.A.; Amaral, M.N.; Moraes, G.P.; Rodrigues, G.S.; Maia, L.C.; Júnior, A.M.; Braga, E.J. Correlation of proline content and gene expression involved in the metabolism of this amino acid under abiotic stress. Acta Physiol. Plant. 2016, 38, 267. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, R.; Mora-Herrera, M.E.; Lopez-Delgado, H.A. Exogenous H2O2 in phytoplasma-infected potato plants promotes antioxidant activity and tuber production under drought conditions. Am. J. Potato Res. 2012, 89, 53–62. [Google Scholar] [CrossRef]
- Usluoglu, A.; Arabaci, G. The effect of acid dyes on antioxidant enzymes from cress (Lepidum sativum). Curr. Opin. Biotechnol. 2011, 22, 140. [Google Scholar] [CrossRef]
- Williams, V.; Twine, S. Flame photometric method for sodium, potassium and calcium. In Modern Methods of Plant Analysis; Peach, K., Tracey, M.V., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1960; pp. 3–5. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).