Abstract
Naturally, thiophenes represent a small family of natural metabolites featured by one to five thiophene rings. Numerous plant species belonging to the family Asteraceae commonly produce thiophenes. These metabolites possessed remarkable bioactivities, including antimicrobial, antiviral, anti-inflammatory, larvicidal, antioxidant, insecticidal, cytotoxic, and nematicidal properties. The current review provides an update over the past seven years for the reported natural thiophene derivatives, including their sources, biosynthesis, spectral data, and bioactivities since the last review published in 2015. Additionally, with the help of the SuperPred webserver, an AI (artificial intelligence) tool, the potential drug target for the compounds was predicted. In silico studies were conducted for Cathepsin D with thiophene derivatives, including ADMET (drug absorption/distribution/metabolism/excretion/and toxicity) properties prediction, molecular docking for the binding interaction, and molecular dynamics to evaluate the ligand–target interaction stability under simulated physiological conditions.
Keywords:
thiophenes; Asteraceae; biosynthesis; bioactivities; in silico studies; cathepsin D; spectral data 1. Introduction
Heterocyclic compounds display a remarkable role in the field of bioactive metabolites search. It is noteworthy that >75% of clinically utilized drugs possess heterocyclic moiety in their chemical skeleton [1]. Sulfur belongs to chalcogens that are the 16 group elements of the periodic table. Sulfur is a ubiquitous heteroatom in medicinal chemistry that can bond to various atoms, including nitrogen, oxygen, carbon, halides, and phosphorus. Several sulfur-based functionalities have become privileged pharmacophores in synthesizing new derivatives that contribute to drug discovery [2]. In living organisms, it displays a remarkable characteristic of possessing a variety of redox potentials and redox states, producing many sulfur species that take part in diverse biological processes. Thioethers and thiols can form sulfonium ions by donating electrons to other organic species, revealing their ability to stabilize a negative charge on a neighboring carbon [3]. They can undergo sequential oxidation to sulfoxides and sulfones, which have diverse biological roles. For example, S-adenosylmethionine (SAM—sulfonium compound) mediates most biochemical methylation reactions in cell metabolism [4].
S-containing species have featured a strong electron-withdrawing nature, resistance to reduction at sulfur, stability against hydrolysis, and preference for two electrons over radical processes that make this group of compounds applicable to many drug research fields [5]. Their diverse pharmacological potential makes it the first choice for incorporation by the hybrid approach, which is present in most of the required medicines accessible in the market [5]. It was reported that 41 sulfur-containing commercial drugs appeared in the Top 200 Pharmaceuticals by Retail Sales in 2019 worldwide; 20.5% contain a sulfur atom [6].
Natural products have attracted significant attention as a potential source of S-containing compounds for drug discovery. The well-known conotoxin, ecteinascidin 743 (ET-743), and penicillin are examples of natural sulfur-containing clinical drugs. Furthermore, many sulfur-containing drugs are derived from natural products, e.g., phthalascidin and ixabepilone for cancer treatments, rosuvastatin for hyperlipidemia, and dalfopristin and quinupristin for infectious diseases [7].
Thiophenes are among the heterocyclics that have been located in the focus of research interest for the last decades. They are a class of sulfur-containing molecules usually composed of one to five thiophene units connected at the α-position and often have various alkyl groups at the α’-carbon of the terminal ring [8]. Thiophene derivatives have beneficial applications in the dye, pharmaceutical, and agrochemical industries [9,10]. Interestingly, many of the approved drugs available in the markets have thiophene moiety, including antiasthma, NSAIDs (non-steroidal anti-inflammatory drugs), diuretics, anticancer, and antihistaminic drugs [11,12]. Natural occurring thiophenes represent rare constituents reported from these metabolites that have been isolated from various Asteraceae genera: Echinops, Eclipta, Pluchea, Artemisia, Tagetes, Porophyllum, Atractylodes, Atractylodes, and Xanthium. Additionally, some are reported from Ferula (family Apiaceae), as well as from actinomycetes (Streptomyces) and fungi (Penicillium) (Figure 1) [8].
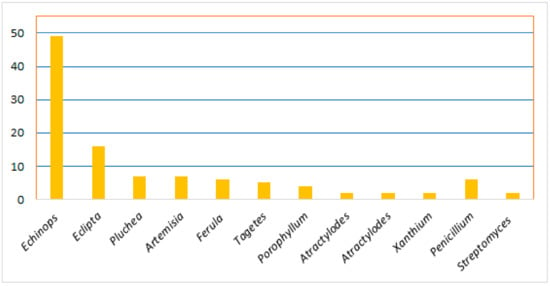
Figure 1.
Number of reported thiophenes from various sources.
They are produced as a chemical defense mechanism and are toxic to various pathogens, such as insects, nematodes, bacteria, and fungi [13,14]. Biosynthetically, they are derived from fatty acids or polyacetylenes through acetylene intermediates; therefore, they are named acetylenic thiophenes. Indeed, many of the reported derivatives possess an alkyl chain with an acetylenic unit that may contain chiral centers due to introducing a hydroxy group [8]. These metabolites have remarkable biological and pharmacological effectiveness, including antiviral, antimicrobial, antileishmanial, anti-inflammatory, larvicidal, antioxidant, insecticidal, HIV-1 (human immunodeficiency virus-1) protease inhibitory, cytotoxic, nematicidal, and phototoxic effects [8,15,16,17,18,19,20] (Figure 2).
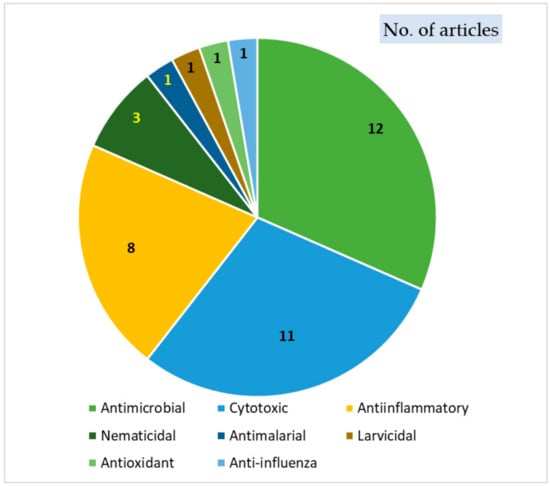
Figure 2.
Biological activities of thiophenes.
In our previous review, 96 natural thiophene derivatives were listed from various plant species belonging to the Asteraceae family till 2015, with a particular focus on their biosynthesis, bioactivities, and physical and spectral data [8]. Recently, several reviews dealing with synthetic thiophene-based derivatives, including their anti-inflammation and anticancer potentials, spectroscopic properties, and synthesis, were published [21,22,23,24]. On the other side, there is no available review on naturally occurring thiophene derivatives from plant sources.
Therefore, the current review aims to provide an update over the past seven years for the naturally reported thiophene derivatives, including their sources, biosynthesis, spectral data, and bioactivities. In total, 96 compounds have been listed that have been categorized according to the number of rings into mono-, bi-, ter, and quinque-thiophenes and miscellaneous derivatives. Additionally, their source, molecular weights and formulae, location, and fraction/extract from which they were isolated are listed in Table 1. The physical constants and spectral data of the newly reported thiophenes (Table S1) from 2015 to 2021 are included. Further, their possible biosynthetic pathways are illustrated in Scheme 1 and Scheme 2 and bioactivities are highlighted in Table 2. We hope that this review can help natural product researchers for structural characterization of these metabolites and direct the medicinal chemist to the synthesis of potentially more active new thiophene derivatives. A systematic search for the published data was performed in various databases, including Web of Science, PubMed, Scopus, and Google scholar. Moreover, published papers in different publishers such as ACS (American Chemical Society), Elsevier, Bentham, Sage, Wiley, Taylor & Francis, Thieme Medical, and Springer were surveyed. No language restrictions were applied.

Table 1.
Naturally occurring mono-, bi-, ter-, and quinquethiophenes and miscellaneous derivatives (name, source, molecular weights and formulae, and location).
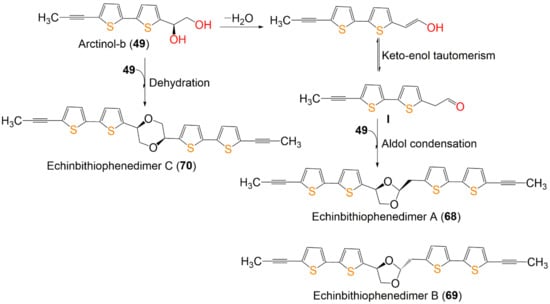
Scheme 1.
Proposed biosynthetic pathway of dimeric bithiophenes 68–70 from arctinol-b (49) [17].
Natural proteins (NP) are biologically active molecules with a myriad of structural and functional diversity. They enable the innovative design of synthetic compounds used in medicines, along with many more crucial aspects of molecular medicine, including but not limited to anti-cancer and anti-viral drugs currently in use. Many of them have proved to be incredibly useful in treating a plethora of diseases. Despite its many attributes, the speed and yields of NP-based drug discovery have significantly dropped during the golden period of 1950–1960. AI can aid in structure-dependent drug discovery by predicting the protein targets of the potential NP, thus assisting in the prediction of a compound influence on the target alongside the safety considerations. SuperPred, a prediction webserver for anatomical therapeutic chemical (ATC) code and target prediction of compounds, was used to predict the potential target for these thiophene derivatives. Based on the outcomes of the SuperPred prediction, we selected cathepsin D, one of the amplest lysosomal proteases, which is also implicated in the pathogenesis of several diseases: cancer, osteoarthritis, and possibly Alzheimer’s disease. Furthermore, in silico, including ADMET properties prediction, molecular docking for the protein ligands binding interaction, and molecular dynamics to evaluate the ligand–target interaction stability under simulated physiological conditions were also implemented.
2. Structural Characterization of Thiophenes
The structures of the reported thiophenes were elucidated by various spectral tools such as 1D (one dimensional) (1H and 13C) and 2D NMR (two-dimensional nuclear magnetic resonance spectroscopy) techniques, COSY (homonuclear correlation spectroscopy), HSQC (heteronuclear single quantum coherence), HMBC (heteronuclear multiple bond correlation), and NOESY (nuclear Overhauser effect spectroscopy) combined with other methods (UV (ultraviolet), IR (infra-red), MS (mass spectroscopy), elemental analysis). The reported spectral and physical data of the newly reported thiophenes are listed in Table S1. The relative configuration was determined by NOESY and ROESY (rotating frame Overhauser effect spectroscopy), as well as by [α]D measurement [34]. The exciton coupled circular dichroism (ECCD) analysis and electronic circular dichroism (ECD) calculations were utilized to assess the absolute configuration by comparing the theoretical and experimental CD spectra [16,17,37,49]. Additionally, the determination of the absolute configuration was carried out using Mosher’s method and analyzing chemical shift differences between (S)- and (R)-MTPA [16]. The X-ray structure crystallographic analysis of the crystalline derivatives is another tool utilized for the absolute configuration determination [49]. It was found that some compounds had no names; therefore, they are named here using the AUPAC system for nomenclature. Further, some compounds had the same molecular formulae and structures with different nomenclatures. On the other hand, some metabolites had more than one name.
3. Biosynthesis of Thiophenes
The detailed biosynthesis of thiophenes was discussed previously [8]. In this work, the recently reported biosynthetic pathways was discussed.
Wu et al. reported the biogenetic pathways of dimeric bithiophenes 68–70 (Scheme 1). These compounds had an unparalleled dimeric bithiophene skeleton containing two bithiophene units linked by uncommon cyclic diether units. It was proposed that they may be originated from arctinol-b (49). For 68 and 69, the formation of the 1,3-dioxolane ring may be obtained from an aldol condensation. Firstly, a key intermediate (I) is produced from 49 by dehydration and keto–enol tautomerism. After that, an aldol condensation among 49 and I would give 68 and 69. Additionally, an intermolecular dehydration reaction between two 49 molecules forms the 1,4-dioxane unit to give 70 [17].
Compound 46 originates from oleic acid. The latter is changed into PYE (trideca-3,5,7,9,11-pentayn-l-ene) through successive desaturation steps and shortening of the chain via crepenynic acid [52]. After that, PYE is changed into 5-BBT (5-(but-3-en-1-ynyl)-2,2′-bithiophene) via introducing a sulfur atom and ring formation that is most probably a two-step reaction [44]. Repeated elongation and desaturation of BBT yield I. Then, the double bond epoxidation produces oxirane (epoxy) intermediate II, subsequent addition of chloride by chloroperoxidase forms III, which performs additional dehydrogenation to yield 46 [53,54] (Scheme 2).
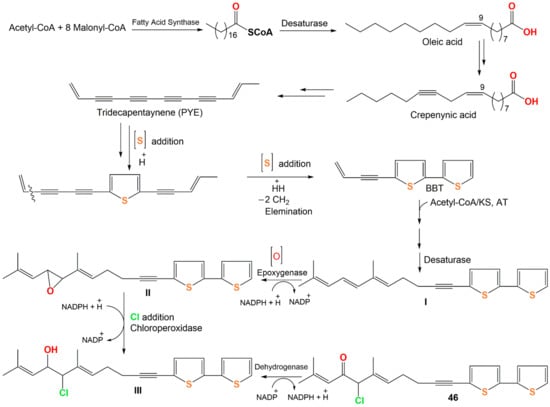
Scheme 2.
Proposed biosynthetic pathway of 46 [44,52,53,54].
4. Biological Activities of Thiophenes
The reported thiophenes were investigated for various bioactivities. In this regard, these metabolites are associated with some types of biological actions, including antimicrobial, antiviral, anti-inflammatory, larvicidal, antioxidant, insecticidal, cytotoxic, and nematicidal effects. The results of the most active metabolites are summarized.
4.1. Anti-Inflammatory Activity
Inflammation is a host body defense mechanism that enables the body to survive during injury or infection and maintains the homeostasis of tissues in noxious conditions [55].
Endogenous NO (nitric oxide) plays a critical role in maintaining the homeostasis of varied cellular functions. NO local concentrations are highly dynamic, as independent enzymatic pathways regulate the synthesis. NO has been shown to modulate inflammation, decreasing the secretion of pro-inflammatory cytokines in human alveolar macrophages challenged with bacterial lipopolysaccharides (LPS) while not altering the basal cytokine levels. Drugs used for managing inflammatory disorders relieve these ailments, but they may have life-threatening consequences [56]. Therefore, there is great enthusiasm in developing new and safe remedies for treating inflammation from natural sources. The reported studies revealed that the anti-inflammatory potential of thiophenes could be due to inhibiting the activation of the NF-κB (nuclear factor-κB) pathway that regulates the expression of pro-inflammatory cytokines and chemokines [57].
The reported studies revealed that thiophenes prohibited TNF-α (tumor necrosis factor-α), IL-6 (interleukin-6), and 5-LOX (5-lipoxygenase), as well as NO production. Thus, their inflammatory potential could be due to the inhibition of NF-κB and NO synthase [58].
Zhou et al. reported that 7 and 8 separated from Artemisia sieversiana exhibited significant anti-neuroinflammatory potential on the LPS-caused NO production in BV-2 murine microglial cells (half-maximal inhibitory concentrations (IC50s) 79.5 and 98.5 µM, respectively), compared to quercetin (IC50 16.3 µM) [26] (Figure 3 and Figure 4).
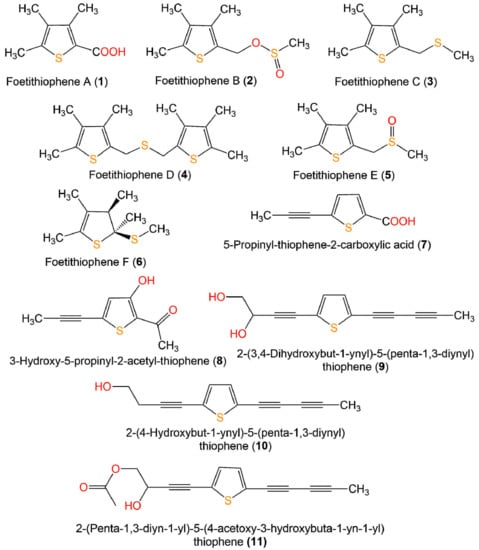
Figure 3.
Structures of monothiophenes 1–11.
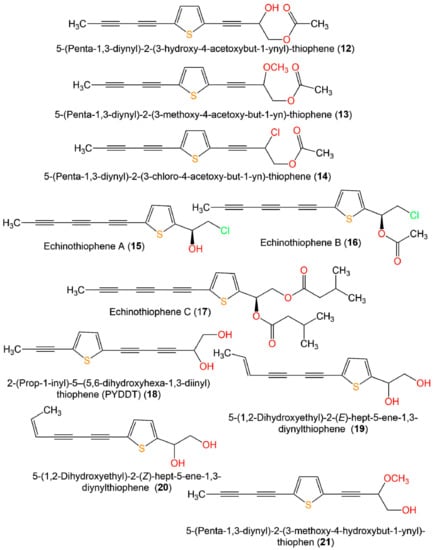
Figure 4.
Structures of monothiophenes 12–21.
In vitro anti-inflammatory assay, compounds 23–26 obtained from Pluchea indica aerial parts possessed significant inhibitory potential toward NO production caused by LPS in RAW 264.7 macrophages at a concentration of 40 µM with % inhibition ranging from 83.4% to 90.1% compared to dexamethasone (62.2%) [35] (Figure 5).
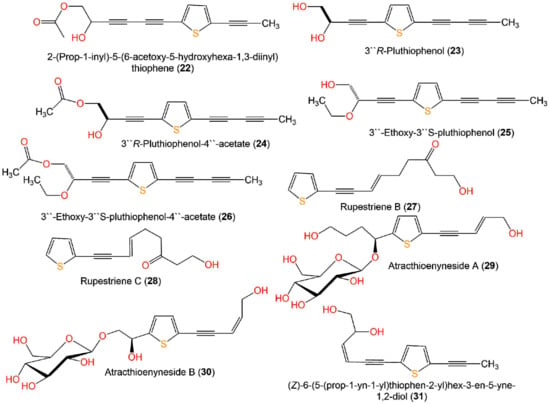
Figure 5.
Structures of monothiophenes 22–31.
On the other side, the two new thiophene polyacetylene glycosides, atracthioenynesides A (29) and B (30) isolated from Atractylodes lancea rhizomes did not show any activity in LPS-induced NO production in BV2 cells [37].
A new bithiophene, 32, along with 16 formerly separated thiophenes, 9, 10, 33–45, and 75, were purified from Echinops grijisii roots EtOAc-soluble fraction of the MeOH extract using SiO2 CC (column chromatography) eluted with n-hexane-EtOAc gradient as well as HPLC and identified by IR, UV, NMR, and HRESIMS spectroscopy [28] (Figure 6 and Figure 7).
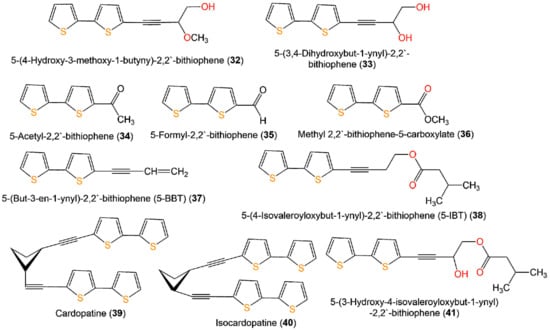
Figure 6.
Structures of compounds 32–41.
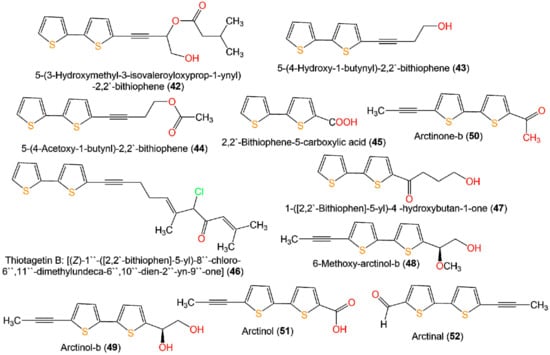
Figure 7.
Structures of bithiophenes 42–52.
These compounds were assessed for anti-inflammatory activity versus RAW 264.7 cells. Only 9, 33, and 43 (IC50s 2.5, 20.0, and 6.7 µg/mL, respectively) exhibited significant in vitro anti-inflammatory potential toward LPS-boosted NO production in RAW 264.7 cells compared to indomethacin (IC50 65.4 µg/mL) in the colorimetric assay [28]. Zhang et al. purified three new derivatives: rupestrienes A–C (86, 27, and 28), Artemisia rupestris EtOH extract by SiO2, RP-18, and Sephadex CC. Rupestrienes B and C (27 and 28) displayed significant inhibitory potential (IC50 8.5 and 5.3 μM, respectively) toward LPS-caused NO production in BV-2 microglial cells, compared to quercetin (IC50 4.3 μM), 86 was weakly active (IC50 20.3 μM) [36]. Jin et al. assessed the inhibitory potential of 19, 20, 48, 49, 51, and 55 toward NO production boosted by LPS in RAW 264.7 cells. Only 19, 20, 48, and 49 exhibited moderate inhibitory potential (IC50 12.8–48.7 µM), compared to indomethacin and aminoguanidine (IC50s 13.2 and 24.2 µM, respectively) (Table 2). On the other side, 51 and 55 did not have any activity (IC50 ˃100 µM) [34]. The structure–activity relationship revealed that the monothiophenes with two acetylene units were more potent than bithiophenes with one acetylene unit. The existence of the Δ10,11 cis double bond and 1,2-diol at C-5 enhanced the inhibitory activity [34].

Table 2.
Biological activities of naturally occurring thiophenes.
Table 2.
Biological activities of naturally occurring thiophenes.
| Compound Name | Biological Activity | Assay, Organism, or Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| Foetithiophene F (6) | Antimicrobial | Broth microdilution/B. cereus | 50 µg/mL (MIC) | Gentamicin 10 µg/mL (MIC) | [25] |
| 5-Propinyl-thiophene-2-carboxylic acid (7) | In vitro anti-inflammatory/NO | LPS-stimulated production in BV-2 microglial cells | 79.5 µM (IC50) | Quercetin 16.3 µM (IC50) | [26] |
| 3-Hydroxy-5-propinyl-2-acetyl-thiophene (8) | In vitro anti-inflammatory/NO | LPS-stimulated production in BV-2 microglial cells | 98.5 µM (IC50) | Quercetin 16.3 µM (IC50) | [26] |
| 2-(3,4-Dihydroxybut-1-ynyl)-5-(penta-1,3-diynyl)thiophene (9) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 2.5 µg/mL (IC50) | Indomethacin 65.4 µg/mL (IC50) | [28] |
| Cytotoxicity | Resazurin reduction/CEM/ADR5000 | 21.09 µM (IC50) | Doxorubicin 195.12 µM (IC50) | [30] | |
| Cytotoxicity | Resazurin reduction/CCRF-CEM | 46.96 µM (IC50) | Doxorubicin 0.20 µM (IC50) | [30] | |
| Antimicrobial | INT/E. coli | 64.0 µg/mL (MIC) | Chloramphenicol 64.0 µg/mL (MIC) | [29] | |
| Antimicrobial | INT/E. aerogenes | 64.0 µg/mL (MIC) | Chloramphenicol 16.0 µg/mL (MIC) | [29] | |
| Antimicrobial | INT/K. pneumoniae | 64.0 µg/mL (MIC) | Chloramphenicol 16.0 µg/mL (MIC) | [29] | |
| Antimicrobial | INT/P. stuartil | 64.0 µg/mL (MIC) | Chloramphenicol 128.0 µg/mL (MIC) | [29] | |
| Antimicrobial | INT/E. cloacae | 256.0 µg/mL (MIC) | Chloramphenicol 256.0 µg/mL (MIC) | [29] | |
| Antimicrobial | INT/P. aeruginosa | 256.0 µg/mL (MIC) | Chloramphenicol 16.0 µg/mL (MIC) | [29] | |
| 2-(Penta-1,3-diyn-1-yl)-5–(4-acetoxy-3-hydroxybuta-1-yn-1-yl) thiophene (11) | CYP2A6 inhibition | Enzymatic reconstitution | 6.43 µM (IC50) | Methoxsalen 0.19 µM (IC50) | [31] |
| CYP2A13 inhibition | Enzymatic reconstitution | 6.18 µM (IC50) | Methoxsalen 0.43 µM (IC50) | [31] | |
| Echinothiophene A (15) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 0.42 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 1.44 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 64.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 16.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 8.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 16.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 4.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Echinothiophene B (16) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 2.65 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 9.23 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 32.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 64.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 16.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 256.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 8.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternataalternata | 8.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Echinothiophene C (17) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 16.55 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 18.17 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. vasinfectum | 128.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. niveum | 256.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternataalternata | 32.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene (PYDDT) (18) | CYP2A6 inhibition | Enzymatic reconstitution | 3.90 µM (IC50) | Methoxsalen 0.19 µM (IC50) | [31] |
| CYP2A13 inhibition | Enzymatic reconstitution | 2.40 µM (IC50) | Methoxsalen 0.43 µM (IC50) | [31] | |
| 5-(1,2-Dihydroxyethyl)-2-(E)-hept-5-ene-1,3-diynylthiophene (19) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 28.2 µM (IC50) | -Indomethacin 13.2 µM (IC50) -Aminoguanidine 24.2 µM (IC50) | [34] |
| 5-(1,2-Dihydroxy-ethyl)-2-(Z)-hept-5-ene-1,3-diynylthiophene (20) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 12.8 µM (IC50) | -Indomethacin 13.2 µM (IC50) -Aminoguanidine 24.2 µM (IC50) | [34] |
| 2-(Prop-1-inyl)-5-(6-acetoxy-5-hydroxyhexa-1,3-diinyl) thiophene (22) | CYP2A6 inhibition | Enzymatic reconstitution | 4.44 µM (IC50) | Methoxsalen 0.19 µM (IC50) | [31] |
| CYP2A13 inhibition | Enzymatic reconstitution | 2.94 µM (IC50) | Methoxsalen 0.43 µM (IC50) | [31] | |
| 3′′R-Pluthiophenol (23) | In vitro anti-inflammatory/NO | LPS-stimulated production in RAW 264.7 macrophages cells | 84.5 (NRC % inhibition) | Dexamethasone 62.2 (NRC % inhibition) | [35] |
| 3′′R-Pluthiophenol-4′′-acetate (24) | In vitro anti-inflammatory/NO | LPS-stimulated production in RAW 264.7 macrophages cells | 83.4 (NRC % inhibition) | Dexamethasone 62.2 (NRC % inhibition) | [35] |
| 3′′-Ethoxy-3′′S-pluthiophenol (25) | In vitro anti-inflammatory/NO | LPS-stimulated production in RAW 264.7 macrophages cells | 86.9 (NRC % inhibition) | Dexamethasone 62.2 (NRC % inhibition) | [35] |
| 3′′-Ethoxy-3′′S-pluthiophenol-4′′-acetate (26) | In vitro anti-inflammatory/NO | LPS-stimulated production in RAW 264.7 macrophages cells | 90.1 (NRC % inhibition) | Dexamethasone 62.2 (NRC % inhibition) | [35] |
| Rupestriene B (27) | In vitro anti-inflammatory/NO | LPS-stimulated production in BV-2 microglial cells | 8.5 µM (IC50) | Quercetin 4.3 µM (IC50) | [36] |
| Rupestriene C (28) | In vitro anti-inflammatory/NO | LPS-stimulated production in BV-2 microglial cells | 5.3 µM (IC50) | Quercetin 4.3 µM (IC50) | [36] |
| 5-(3,4-Dihydroxybut-1-ynyl)-2,2′-bithiophene (33) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 20.0 µg/mL (IC50) | Indomethacin 65.4 µg/mL (IC50) | [28] |
| 5-(But-3-en-1-ynyl)-2,2′-bithiophene (5-BBT) (37) | Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 7.81 µg/mL (MFC) in light | Amphotericin B 0.50 µg/mL (MFC) Fluconazole ˃ 64 µg/mL (MFC) Itraconazole ˃ 16 µg/mL (MFC) | [40] |
| Larvicidal | Larval mortality/Aedes albopictus | 0.34 µg/mL (LC50) | Rotenone 3.75 µg/mL (LC50) | [41] | |
| Larvicidal | Larval mortality/Aedes albopictus | 0.72 µg/mL (LC95) | Rotenone 9.45 µg/mL (LC95) | [41] | |
| Larvicidal | Larval mortality/Anopheles sinensis | 1.36 µg/mL (LC50) | Rotenone 1.25 µg/mL (LC50) | [41] | |
| Larvicidal | Larval mortality/Anopheles sinensis | 1.93 µg/mL (LC95) | Rotenone 2.24 µg/mL (LC95) | [41] | |
| Larvicidal | Larval mortality/Culex pipiens pallens | 0.12 µg/mL (LC50) | Rotenone 1.88 µg/mL (LC50) | [41] | |
| Fungicidal | Larval mortality/Culex pipiens pallens | 0.18 µg/mL (LC95) | Rotenone 3.74 µg/mL (LC95) | [41] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 62.50 µg/mL (MFC) in low oxygen and light | - | [59] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 7.81 µg/mL (MFC) in normal oxygen and light | - | [59] | |
| 5-(4-Isovaleroyloxybut-1-ynyl)-2,2′-bithiophene (5-IBT) (38) | Larvicidal | Larval mortality/Aedes albopictus | 0.45 µg/mL (LC50) | Rotenone 3.75 µg/mL (LC50) | [41] |
| Larvicidal | 0.66 µg/mL (LC95) | Rotenone 9.45 µg/mL (LC95) | [41] | ||
| Larvicidal | Larval mortality/Anopheles sinensis | 5.36 µg/mL (LC50) | Rotenone 1.25 µg/mL (LC50) | [41] | |
| Larvicidal | 11.26 µg/mL (LC95) | Rotenone 2.24 µg/mL (LC95) | [41] | ||
| Larvicidal | Larval mortality/Culex pipiens pallens | 0.33 µg/mL (LC50) | Rotenone 1.88 µg/mL (LC50) | [41] | |
| Larvicidal | 0.54 µg/mL (LC95) | Rotenone 3.74 µg/mL (LC95) | [41] | ||
| 5-(4-Hydroxy-1-butynyl)-2,2′-bithiophene (43) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 6.7 µg/mL (IC50) | Indomethacin 65.4 µg/mL (IC50) | [28] |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 3.90 µg/mL (MFC) in light | -Amphotericin B 0.50 µg/mL (MFC) -Fluconazole ˃ 64 µg/mL (MFC) -Itraconazole ˃ 16 µg/mL (MFC) | [40] | |
| Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 8.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 64.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/C. albicans ATCC2002 | 64.0 µg/mL (MIC) | Levofloxacin 64.0 μg/mL (MIC) | [27] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 250.0 µg/mL (MFC) in low oxygen and light | - | [59] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 3.90 µg/mL (MFC) in normal oxygen and light | - | [59] | |
| Anti-inflammatory | Colorimetric/5-LOX | 41.82 µM (IC50) | Indomethacin 0.89 µM (IC50) | [43] | |
| 5-(4-Acetoxy-1-butynl)-2,2′-bithiophene (44) | Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 7.81 µg/mL (MFC) in light | -Amphotericin B 0.50 µg/mL (MFC) -Fluconazole ˃ 64 µg/mL (MFC) -Itraconazole ˃ 16 µg/mL (MFC) | [40] |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 62.50 µg/mL (MFC) in low oxygen and light | - | [59] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 7.81 µg/mL (MFC) in normal oxygen and light | - | [59] | |
| 6-Methoxy-arctinol-b (48) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 30.6 µM (IC50) | -Indomethacin 13.2 µM (IC50) -Aminoguanidine 24.2 µM (IC50) | [34] |
| Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 5.83 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] | |
| 7.05 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 128.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 256.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternataalternata | 32.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Arctinol-b (49) | In vitro anti-inflammatory/NO | LPS-stimulated production in the RAW 264.7 cell line | 48.7 µM (IC50) | -Indomethacin 13.2 µM (IC50) -Aminoguanidine 24.2 µM (IC50) | [34] |
| Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 8.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 64.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/C. albicans ATCC2002 | 64.0 µg/mL (MIC) | Levofloxacin 64.0 μg/mL (MIC) | [27] | |
| Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 13.48 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] | |
| 14.72 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. vasinfectum | 256.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. niveum | 64.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 64.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Arctinone-b (50) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 1.14 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 2.00 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. vasinfectum | 256.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | |
| Broth microdilution/Colletotrichum gloeosporioides | 64.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 128.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Arctinol (51) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 15.90 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 17.82 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. vasinfectum | 256.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. niveum | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 16.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Arctinal (52) | Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 32.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [19] |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 64.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [19] | |
| Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 2.62 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] | |
| 8.75 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. vasinfectum | 64.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. niveum | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 64.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Arctinol A (53) | Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 8.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 64.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [27] | |
| 5′-(3,4-Dihydroxybut-1-yn-1-yl)-[2,2′-bithiophene]-5-carbaldehyde (57) | Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 128.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 256.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/C. albicans ATCC2002 | 256.0 µg/mL (MIC) | Levofloxacin 64.0 μg/mL (MIC) | [27] | |
| 4-Hydroxy-1-(5′-methyl-[2,2′-bithiophen]-5-yl)butan-1-one (58) | Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 8.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] |
| Antimicrobial | Broth microdilution/E. coli ATCC 25922 | 32.0 µg/mL (MIC) | Levofloxacin 16.0 μg/mL (MIC) | [27] | |
| Antimicrobial | Broth microdilution/C. albicans ATCC2002 | 32.0 µg/mL (MIC) | Levofloxacin 64.0 μg/mL (MIC) | [27] | |
| 5′-(3,4-Dihydroxybut-1-yn-1-yl)-[2,2′-bithiophene]-5-carboxylic acid (59) | Antimicrobial | Broth microdilution/S. aureus ATCC 2592 | 256.0 µg/mL (MIC) | Levofloxacin 8.0 μg/mL (MIC) | [27] |
| Echinothiophene D (61) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 2.57 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 1.80 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 32.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 128.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 256.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 8.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 16.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Echinothiophene E (62) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 8.28 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 9.12 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 64.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 32.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 256.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 16.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Echinothiophene F (63) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 20.13 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 18.41 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/F. oxysporum f. sp. niveum | 128.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | |
| Broth microdilution/Colletotrichum gloeosporioides | 256.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 64.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| 2-Prop-1-inyl-5′-(2-hydroxy-3-chloropropyl) dithiophene (64) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 0.91 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] |
| 0.86 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| Antifungal | Broth microdilution/Fusarium solani | 64.0 µg/mL (MIC) | Carbendazim 0.5 µg/mL (MIC) | [19] | |
| Broth microdilution/F. oxysporum f. sp. vasinfectum | 32.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/F. oxysporum f. sp. niveum | 4.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Phytophthora infestans | 32.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 4.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [19] | ||
| Broth microdilution/Alternaria alternata | 4.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [19] | ||
| Ecliprostin A (65) | Antibacterial | Broth microdilution/S. aureus | 25.0 µM (MIC) | Penicillin 0.156 µM (MIC) | [18] |
| Ecliprostin B (66) | Antibacterial | Broth microdilution/S. aureus | 6.25 µM (MIC) | Penicillin 0.156 µM (MIC) | [18] |
| Ecliprostin C (67) | Antibacterial | Broth microdilution/S. aureus | 25.0 µM (MIC) | Penicillin 0.156 µM (MIC) | [18] |
| Echinbithiophenedimer A (68) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 16.53 µg/mL (LC50) in light | Ethoprophos 36.15 (LC50) in light α-Terthienyl 0.62 (LC50) in light | [17] |
| 18.17 µg/mL (LC50) in dark | Ethoprophos 31.94 (LC50) in dark α-Terthienyl 2.23 (LC50) in dark | [17] | |||
| Antifungal | Broth microdilution/Alternaria alternata; | 16.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [17] | |
| Broth microdilution/Pyricularia oryzae | 16.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Fusarium oxysporum | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 64.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [17] | ||
| Echinbithiophenedimer B (69) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 13.88 µg/mL (LC50) in light | Ethoprophos 36.15 (LC50) in light α-Terthienyl 0.62 (LC50) in light | [17] |
| 16.28 µg/mL (LC50) in dark | Ethoprophos 31.94 (LC50) in dark α-Terthienyl 2.23 (LC50) in dark | [17] | |||
| Antifungal | Broth microdilution/Alternaria alternata | 16.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [17] | |
| Broth microdilution/Pyricularia oryzae | 16.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Fusarium oxysporum | 16.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [17] | ||
| Echinbithiophenedimer C (70) | Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 8.73 µg/mL (LC50) in light | Ethoprophos 36.15 (LC50) in light α-Terthienyl 0.62 (LC50) in light | [17] |
| 9.39 µg/mL (LC50) in dark | Ethoprophos 31.94 (LC50) in dark α-Terthienyl 2.23 (LC50) in dark | [17] | |||
| Antifungal | Broth microdilution/Alternaria alternata; | 8.0 µg/mL (MIC) | Carbendazim 16.0 µg/mL (MIC) | [17] | |
| Broth microdilution/Pyricularia oryzae | 8.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Fusarium oxysporum | 32.0 µg/mL (MIC) | Carbendazim 8.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Colletotrichum gloeosporioides | 32.0 µg/mL (MIC) | Carbendazim 2.0 µg/mL (MIC) | [17] | ||
| Broth microdilution/Phytophthora infestans | 128.0 µg/mL (MIC) | Carbendazim 256.0 µg/mL (MIC) | [17] | ||
| 4-(5′-(hydroxymethyl)-[2,2′-bithiophene]-5-yl)but-3-yn-1-ol) (Thio1) (74) | Anthelmintic | Larval development test/Haemonchus contortus | 0.3243 mg/mL (EC50) | Levamisole 1.88 mg/mL (EC50) | [46] |
| Anthelmintic | Fecal egg count reduction test/Haemonchus contortus | 0.1731 mg/mL (EC50) | Levamisole 1.88 mg/mL (EC50) | [46] | |
| 2,2′:5′,2′′-Terthiophene (α-Terthienyl) (75) | Cytotoxicity | MTT/SKOV3 | 77.23 µM (IC50) | Cisplatin 11.25 µM (IC50) | [39] |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 0.24 µg/mL (MFC) in light | Amphotericin B 0.50 µg/mL (MFC) Fluconazole ˃ 64 µg/mL (MFC) Itraconazole ˃ 16 µg/mL (MFC) | [40] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 7.81 µg/mL (MFC) in low oxygen and light | - | [59] | |
| Fungicidal | Broth microdilution/C. albicans ATCC 10231 | 0.24 µg/mL (MFC) in normal oxygen and light | - | [59] | |
| Larvicidal | Larval mortality/Aedes albopictus | 1.41 µg/mL (LC50) | Rotenone 3.75 µg/mL (LC50) | [41] | |
| Larvicidal | 2.19 µg/mL (LC95) | Rotenone 9.45 µg/mL (LC95) | [41] | ||
| Larvicidal | Larval mortality/Anopheles sinensis | 1.79 µg/mL (LC50) | Rotenone 1.25 µg/mL (LC50) | [41] | |
| Larvicidal | 2.54 µg/mL (LC95) | Rotenone 2.24 µg/mL (LC95) | [41] | ||
| Larvicidal | Larval mortality/Culex pipiens pallens | 1.38 µg/mL (LC50) | Rotenone 1.88 µg/mL (LC50) | [41] | |
| Larvicidal | 2.15 µg/mL (LC95) | Rotenone 3.74 µg/mL (LC95) | [41] | ||
| Nematicidal | Nematode Mortality/J2s of Meloidogyne incognita | 0.56 µg/mL (LC50) in light | Abamectin 8.73 (LC50) in light | [19] | |
| 1.77 µg/mL (LC50) in dark | Abamectin 9.38 (LC50) in dark | [19] | |||
| 5-Formyl-2,2′:5′,2′′-terthiophene (Ecliptal) (76) | Cytotoxicity | MTT/SKOV3 | 24.57 µM (IC50) | Cisplatin 11.25 µM (IC50) | [39] |
| Cytotoxicity | MTT/Hec1A | 12.00 µM (IC50) | Cisplatin 120.42 µM (IC50) | [47] | |
| Cytotoxicity | MTT/Ishikawa | 2.20 µM (IC50) | Cisplatin 10.11 µM (IC50) | [47] | |
| Anti-inflammatory | Colorimetric/5-LOX | 26.18 µM (IC50) | Indomethacin 0.89 µM (IC50) | [43] | |
| Antibacterial | Broth microdilution/S. aureus | 25.0 µM (MIC) | Penicillin G 0.156 µM (MIC) | [38] | |
| 5-Hydroxymethyl-2,2′:5′,2′′-terthiophene (α-Terthienylmethanol) (77) | Cytotoxicity | MTT/SKOV3 | 7.73 µM (IC50) | Cisplatin 11.25 µM (IC50) | [39] |
| Cytotoxicity | MTT/Hec1A | 0.38 µM (IC50) | Cisplatin 120.42 µM (IC50) | [47] | |
| Cytotoxicity | MTT/Ishikawa | 0.35 µM (IC50) | Cisplatin 10.11 µM (IC50) | [47] | |
| Cytotoxicity | MTT/A2780 | 1.18 µM (IC50) | Cisplatin 10.80 µM (IC50) | [20] | |
| Cytotoxicity | MTT/SKOV3 | 15.51 µM (IC50) | Cisplatin 43.05 µM (IC50) | [20] | |
| Cytotoxicity | MTT/OVCAR3 | 0.20 µM (IC50) | Cisplatin 35.46 µM (IC50) | [20] | |
| Cytotoxicity | MTT/ES2 | 18.82 µM (IC50) | Cisplatin 29.58 µM (IC50) | [20] | |
| Antibacterial | Broth microdilution/S. aureus | 25.0 µM (MIC) | Penicillin G 0.156 µM (MIC) | [38] | |
| 5-Hydroxymethyl-(2,2′:5′,2′′)-terthienyl angelate (79) | Cytotoxicity | MTT/Hec1A | 129.85 µM (IC50) | Cisplatin 120.42 µM (IC50) | [47] |
| Cytotoxicity | MTT/Ishikawa | 6.87 µM (IC50) | Cisplatin 10.11 µM (IC50) | [47] | |
| 5-Hydroxymethyl-(2,2′:5′,2′′)-terthienyl tiglate (80) | Cytotoxicity | MTT/Hec1A | 2.66 µM (IC50) | Cisplatin 120.42 µM (IC50) | [47] |
| Cytotoxicity | MTT/Ishikawa | 9.68 µM (IC50) | Cisplatin 10.11 µM (IC50) | [47] | |
| 5-Methoxy-(2,2′:5′,2′′)-terthiophene (81) | Cytotoxicity | MTT/Hec1A | 1.38 µM (IC50) | Cisplatin 120.42 µM (IC50) | [47] |
| Cytotoxicity | MTT/Ishikawa | 7.12 µM (IC50) | Cisplatin 10.11 µM (IC50) | [47] | |
| 3′-Hydroxy-2,2′:5′,2′′-terthiophene-3′-O-β-D-glucopyranoside (82) | Cytotoxicity | MTT/SKOV3 | 58.20 µM (IC50) | Cisplatin 11.25 µM (IC50) | [39] |
| Thiotagetin A (83) | Cytotoxicity | MTT/KB | 2.03 μg/mL (ED50) | Adriamycin 0.26 μg/mL (ED50) | [48] |
| Cytotoxicity | MTT/MCF-7 | 3.88 μg/mL (ED50) | Adriamycin 0.07 μg/mL (ED50) | [48] | |
| Rupestriene A (86) | In vitro anti-inflammatory/NO | LPS-stimulated production in BV-2 microglial cells | 20.3 µM (IC50) | Quercetin 4.3 µM (IC50) | [36] |
| Neuraminidase inhibitory activity | Fluorescence-based assay | 351.15 µM (IC50) | Oseltamivir acid 77.91 µM (IC50) | [15] | |
| 7-[1-(Thiophene-5-yl)-1-formamido]-3-propylenyl-3-cephem-4-carboxylic acid (CAx1) (87) | Antibacterial | Broth microdilution/S. aureus MTCC 740 | 0.2 µg/mL (MIC) 2.0 µg/mL (MBC) | Penicillin 32.0 µg/mL (MIC) 64.0 µg/mL (MBC) | [40] |
| Antibacterial | Broth microdilution/B. subtilis MTCC 736 | 0.25 µg/mL (MIC) 0.5 µg/mL (MBC) | Penicillin 0.5 µg/mL (MIC) 4.0 µg/mL (MBC) | [50] | |
| Antibacterial | Broth microdilution/E. coli MTCC 739 | 4.0 µg/mL (MIC) 8.0 µg/mL (MBC) | Penicillin 4.0 µg/mL (MIC) 16.0 µg/mL (MBC) | [50] | |
| Antibacterial | Broth microdilution/K. pneumonia MTCC 661 | 4.0 µg/mL (MIC) 16.0 µg/mL (MBC) | Penicillin 16.0 µg/mL (MIC) 64.0 µg/mL (MBC) | [50] | |
| 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene (88) | Antimicrobial | Broth microdilution/E. faecalis ATCC29212 | 256.0 µg/mL (MIC) | Streptomycin 256.0 μg/mL (MIC) | [51] |
| Thiocarboxylic A (89) | Antimicrobial | Broth microdilution/E. coli ATCC35218 | 1.7 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 1.7 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 3.3 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Thiocarboxylic B (90) | Antimicrobial | Broth microdilution/E. coli ATCC35218 | 0.9 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 1.9 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 3.9 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Thiocarboxylic C1 (91) | Antimicrobial | Broth microdilution/E. coli ATCC35218 | 7.0 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 3.5 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 7.0 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Thiocarboxylic C2 (92) | Antimicrobial | Broth microdilution/E. coli ATCC35218 | 7.0 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 3.5 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 7.0 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Thiocarboxylic D1 (93) | Antimicrobial | Broth microdilution/E. coli ATCC35218 | 3.5 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 3.5 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 7.0 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Thiocarboxylic D2 (94) | Antimicrobial | Broth microdilution/E. coli (ATCC35218) | 3.5 µg/mL (MIC) | Streptomycin 2.3 µg/mL (MIC) | [16] |
| Broth microdilution/S. aureus ATCC25923 | 3.5 µg/mL (MIC) | Streptomycin 0.1 µg/mL (MIC) | [16] | ||
| Broth microdilution/C. albicans ATCC10231 | 7.0 µg/mL (MIC) | Amphotericin B 0.1 µg/mL (MIC) | [16] | ||
| Rupestriene D (95) | Neuraminidase inhibitory activity | Fluorescence-based assay | 986.54 µM (IC50) | Oseltamivir acid 77.91 µM (IC50) | [15] |
| Rupestriene E (96) | Neuraminidase inhibitory activity | Fluorescence-based assay | 365.40 µM (IC50) | Oseltamivir acid 77.91 µM (IC50) | [15] |
Compounds 43, 46, and 76 separated from aerial parts of Tagetes minuta significantly decreased NFκB p65, TNF-α, and IL-6 compared to indomethacin in the ELISA (enzyme-linked immunosorbent assay) [44]. In 2020, Ibrahim et al. reported that 43 and 76 isolated T. minuta displayed moderate anti-inflammatory potential (IC50 41.82 and 26.18 µM, respectively) in the 5-LOX colorimetric assay in comparison to indomethacin (IC50 0.89 µM) [43].
4.2. Cytotoxic Activity
Cancer is a crucial cause of death globally, accounting for ≈10 million deaths in 2020 [48,60]. There are many available medications for treating various types of cancer. However, none of them are entirely safe and effective. Many of the reported thiophenes have been assessed for cytotoxic effectiveness toward various cancer cell lines.
Four new derivatives, foetithiophenes C-F (3–6), along with foetithiophenes A (1) and B (2), were obtained from MeOH extract of Ferula foetida roots using SiO2 CC and RP-HPLC. Unfortunately, they showed no cytotoxic capacity (IC50 ˃100 µmM) versus K562 and MCF-7 cell lines in the Alamar Blue assay [25].
Additionally, 9 had more promising cytotoxic potential (IC50 21.09 µM) than doxorubicin (IC50 195.12 µM) against CEM/ADR5000 (human T-cell lymphoblast-like cell line). However, it was weakly active toward CCRF-CEM (human leukemic cell line, IC50 46.96 µM) in the resazurin reduction cytotoxic assay [30].
Compounds 11, 18, and 22 isolated from Pluchea indica aerial parts were assayed for inhibitory potential on coumarin 7-hydroxylation induced by CYP2A6 (cytochrome P450 2A6) and CYP2A13 (cytochrome P450 2A13) enzymes, using enzymatic reconstitution assay [31]. The human liver cytochrome P450 (CYP) 2A13 and 2A6 enzymes had a crucial function in nicotine metabolism and the activation of tobacco-specific nitrosamine carcinogens. Their prohibition could represent a strategy for smoking abstinence and decreasing risks of lung cancer and respiratory complaints. It was found that 18, 11, and 22 irreversibly prohibited CYP2A6- and CYP2A13-induced coumarin 7-hydroxylation (IC50 values 3.90 and 2.40 µM, respectively, for 18; IC50 6.43 and 6.18 µM, respectively for 11, and IC50 4.44 and 2.94 µM, respectively for 22). These metabolites could aid in smoking stoppage and lessened risks of lung cancer and respiratory illnesses [31].
Xu et al. reported that the treatment of SW620 (human colon cancer) cells with PYDDT (2-(pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene) (18) led to the induction of mitochondrial-mediated apoptosis that was featured by cleavage of PARP (poly ADP ribose polymerase), activating caspase-3 and 9, the release of cytochrome c from mitochondria, mitochondrial membrane potential loss, Bcl-2 (B-cell lymphoma 2) downregulation, and Bax mitochondrial translocation. A mechanism study revealed that PYDDT induced SW620 apoptosis through a JNK (c-Jun N-terminal kinase)/ROS (reactive oxygen species)-mediated mitochondrial pathway [33].
Ecliprostins A–C (65–67) new thiophene derivatives were separated from Eclipta prostrata. In contrast, ecliprostins A (65) and B (66) featured a bithiophenyl acetylenic skeleton, incorporating an isovalerate unit, whereas ecliprostin C (67) was a dimer of 65. They exerted no noticeable cytotoxicity versus Hela and MDA-MB-231 cell lines (Conc. 30 µM) [18].
Compounds 33, 75–78, and 82 were purified from the EtOH extract of Eclipta prostrata aerial parts by SiO2 CC (silica gel column chromatography) and purified using a reversed-phase CC. In the MTT assay, 77 exhibited the most potent cytotoxicity on SKOV3 cells (IC50 7.73 µM) than cisplatin (IC50 11.25 µM). The terthiopenes 75, 76, and 82 showed significant cytotoxicity (IC50 values ranging from 24.57 to 77.23 µM). However, 33 and 78 were ineffective (IC50 values ˃ 100 µM) [39].
Additionally, Preya et al. reported that 77 isolated Eclipta prostrata was a more potent cell growth inhibitor (IC50s 0.20–18.82 µM) than cisplatin (IC50 10.80 to 43.05 µM) toward a panel of human ovarian cancer cell lines; OVCAR3, SKOV3, A2780, and ES2 in the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. It caused changes in S phase-linked proteins (cyclins A and D2 and cyclin-dependent kinase 2) and induced an intracellular increase in ROS that increased the levels of p-H2AX (H2A histone family member X), resulting in DNA (deoxyribonucleic acid) damage [14,20]. A mechanism study indicated that 77 caused S-phase cell cycle arrest by inducing ROS stress and DNA damage. Therefore, 77 could be a potential therapeutic lead for treating ovarian cancer.
Sibiricumthionol (84) and (+)-xanthienopyran (85) were purified from Xanthium sibiricum fruits extract using SiO2, RP-18 (reversed phase-18), and HPLC (high-performance liquid chromatography) that were characterized by spectroscopic, X-ray, and ECCD analyses, as well as ECD calculations. These metabolites were inactive (IC50 ˃10 µM) toward HCT-116, BGC-823, HepG2, NCI-H1650, and A2780 cell lines in the MTT assay [49].
Compounds 76, 77, and 79–81 isolated from Eclipta prostrate showed prominent cytotoxic effectiveness toward Hec1A (IC50 ranging from 0.38 to 129.85 µM) and Ishikawa (IC50 ranging from 0.35 to 9.68 µM) cells compared to cisplatin (IC50 120.4 and 10.11 µM, respectively). Notably, 77 had a potent effect on Ishikawa and Hec1A cells (IC50 0.35 and 0.38 µM, respectively) [37,47]. The inhibitory effect of 77 was mediated by the induction of apoptosis, triggering caspase activation and cytochrome c release into the cytosol. Additionally, it increased the ROS intracellular level and decreased GSH (glutathione). Therefore, its apoptotic effect was attributed to the generation of reactive oxygen species via NADPH (nicotinamide adenine dinucleotide phosphate) oxidase in human endometrial cancer cells [47].
Thiotagetin A (83) purified from Tagetes minuta possessed cytotoxic capacity versus MCF-7 and KB (ED50s 3.88 and 2.03 μg/mL, respectively), compared to adriamycin (0.07 and 0.26 μg/mL, respectively) in the MTT assay [48].
4.3. Antimicrobial Activity
Infectious diseases continue to be a serious worldwide health concern. Multidrug-resistant (MDR) pathogens significantly increased morbidity and mortality rates [61]. The continuous emergence of MDR pathogens drastically reduced the efficacy of the utilized antibiotics resulting in a growth rate of therapeutic failure [62]. Accordingly, new and effective antimicrobial agents to tackle microbial infections are needed [50].
Chitsazian-Yazdi et al. assayed the antimicrobial activity of 1–6 in broth microdilution method toward B. cereus PTCC-1247, C. albicans ATCC-10231, and E. coli ATCC-8739. Whereas only 6 displayed the most potent potential (MIC 50 µg/mL) against B. cereus, compared to gentamicin (MIC 10 µg/mL) [25].
Mbaveng et al. purified 9 from the CH2Cl2 fraction of Echinops giganteus roots. It showed moderate and selective activities against E. coli ATCC-8739, E. aerogenes ATCC-13048 and -EA27, K. pneumonia ATCC11296, P. stuartii ATCC29916, E. cloacae BM47, and P. aeruginosa PA01 (MIC <100 µg/mL) in the rapid INT (p-iodonitrotetrazolium) chloride assay [29].
In 2017, Postigo et al. reported the separation and structural elucidation of 37, 43, 44, and 75 the from n-hexane extract of Porophyllum obscurum by preparative CTL (centrifugal thin layer) and TL (thin-layer) chromatography that were assayed for their fungicidal potential against C. albicans ATCC-10231 and 25 clinical strains of Candida spp. isolates as causative agents of oropharyngeal candidiasis using broth microdilution. They exhibited fungicidal effectiveness with minimum fungicidal concentrations (MFC) ranging from 0.24 to 7.81 μg/mL under UV-A irradiation, whereas 32 with (MFC 0.24 μg/mL) and 43 with (MFC 3.90 μg/mL) were the most active metabolites [40]. In 2019, Postigo et al. evaluated their photoinactivation towards C. albicans in parallel under darkness and light conditions. The results revealed that these thiophenes exhibited the highest potential under normal-light/oxygen atmosphere (MFCs ranged from 0.24 to 7.81 μg/mL). However, their effects decreased >200 times (MFCs ranged from 7.81 to 250 μg/mL) with low-oxygen conditions. On the other hand, all tested thiophenes had no antifungal potential in darkness under both oxygen conditions (MFC > 250 μg/mL). It was found that 75 was the most active photosensitizer and was the only one that generated a single oxygen at MFC. Furthermore, it did not elevate sensitivities to oxidative and osmotic stressors and did not produce leakage or apoptosis [59]. Therefore, their antifungal mechanism was proposed to be photodynamic, considering that the absence of oxygen had a passive effect on the antifungal photosensitivity capacity. Therefore, these features could encourage further assessments to confirm their potential application as photosensitizers in photodynamic antimicrobial therapy toward fungal infections [59].
Li et al. performed a broth microdilution assay for evaluating the antimicrobial potential of 7, 9, 33, 34, 43, 45, 47, 49, 52–54, and 57–60 (Figure 8) isolated from E. ritro versus E. coli, S. aureus, and C. albicans. Compounds 43, 49, 53, and 58 exhibited the same antibacterial activity toward S. aureus as levofloxacin (MIC (minimum inhibitory concentration) 8 µg/mL). Additionally, 43, 49, 52, 53, and 58 possessed activity against E. coli (MIC values of 32–64 µg/mL). On the other side, 43, 49, and 58 displayed antifungal potential toward C. albicans (MIC values of 32–64 µg/mL) that was similar or two-fold more active than levofloxacin (MIC 64 µg/mL) [27].
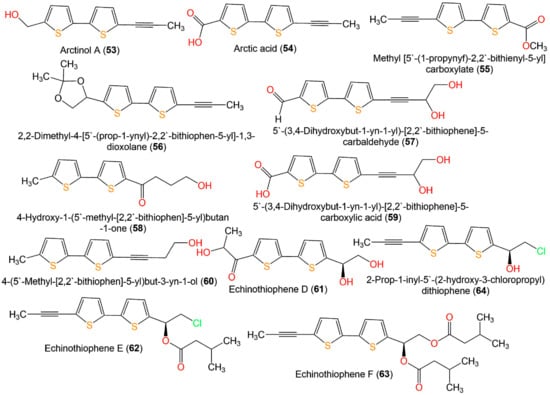
Figure 8.
Structures of bithiophenes 53–63.
Liu et al. reported that 15, 16, 48, 51, 61, 62, and 64 possessed equivalent or better antifungal capacities toward Fusarium solani, Colletotrichum gloeosporioides, F. oxysporum f. sp. vasinfectum, Phytophthora infestans, Alternaria alternata, and F. oxysporum f. sp. niveum compared to carbendazim, whereas 17, 48, 50, 52, and 63 had weak antifungal potential (MICs from 32 to >256 µg/mL). It is noteworthy that 15 (MICs 4 and 8 µg/mL, respectively) had elevated inhibitory capacity toward A. alternata and F. oxysporum f. sp. niveum compared to 16, 17, and 62 (MICs from 8 to >256 µg/mL), indicating that acylation weakened the activity. Further, the effect of 15 and 16 versus all fungi was more than that of 17, suggesting that chlorine could enhance activity [19].
Compounds 65–67 showed moderate growth inhibition against S. aureus (MICs 25.0, 6.25, and 25.0 µM, respectively) in the broth microdilution assay, compared to penicillin (MIC 0.156 µM) [18], whilst they did not have significant activity toward Vibrio vulnificus and E. coli [18].
Echinbithiophenedimers A–C (68–70) novel dimeric bithiophenes, besides 37 and 49, were separated from Echinops latifolius using SiO2, Sephadex CC, and PTLC (Figure 9). Their antifungal potential against soil-borne fungi; Pyricularia oryzae, Alternaria alternata, Colletotrichum gloeosporioides, Fusarium oxysporum, and Phytophthora infestans were assessed in light and dark by the micro-broth dilution method. Compounds 68–70 had significant antifungal capacities toward P. oryzae and A. alternata (MICs 8–16 µg/mL), whereas 70 (MIC 8 µg/mL) displayed better antifungal potential toward A. alternata than carbendazim (MIC 16 µg/mL). Additionally, they revealed more antifungal potential (MIC 28 µg/mL) against P. infestans than carbendazim (MIC 256 µg/mL). It was found that an increased thiophene rings′ number bettered the activity [17].
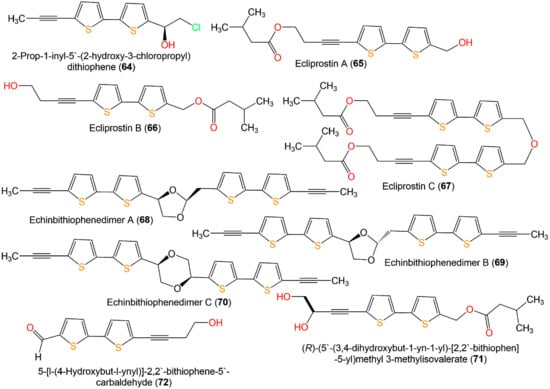
Figure 9.
Structures of bithiophenes 64–72.
Yu et al. purified two new thiophenes derivatives, 31 and 71, together with 9, 33, 48, 49, 71–73, 77, and 82 from Eclipta prostrata by SiO2, Sephadex CC, and RP-HPLC [38]. Only 77 and 82 exerted mild antibacterial potential toward S. aureus (MIC 25 µM) in the broth microdilution method, compared to penicillin G (MIC 0.156 µM) (Figure 10) [38].
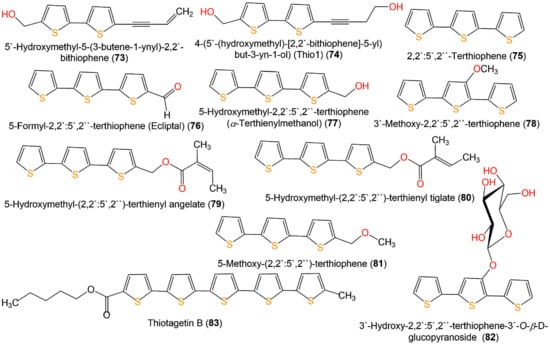
Figure 10.
Structures of bithiophenes (73 and 74), terthiophenes (75–82), and quinquethiophene 83.
Compound 87 was biosynthesized using endolithic Streptomyces sp. AL51. This compound had remarkable antibacterial potential versus both Gram-positive and -negative bacteria in the microplate broth-dilution method. It displayed higher activity than penicillin against Gram-positive S. aureus, B. subtilis, E. coli, and Klebsiella pneumonia with MIC/MBC (minimum bactericidal concentration) 0.2/2.0, 0.25/0.5, 4.0/8.0, and 4.0/16.0 µg/mL, respectively, compared to penicillin (MIC/MBC 32.0/64.0, 0.5/4.0, 4.0/16.0, and 16.0/64.0 µg/mL, respectively) [50].
Cao et al. purified 88 from the culture broth of the marine-derived actinomycete Streptomyces sp. G278 selectively prohibited Enterococcus faecalis equal to streptomycin (MIC 256 μg/mL) [51].
Six novel thiophene-furan-carboxylic acids, 89–94, were isolated from the soil-derived fungus Penicillium sp. Sb62, representing the first class of natural furan-carboxylic acids having a thiophene moiety (Figure 11). They possessed antimicrobial capacities versus E. coli, S. aureus, and C. albicans with MICs 0.9–7.0, 1.7–3.5, and 3.3–7.0 μg/mL, respectively, in the broth microdilution assay. It was observed that the absence of methoxy or a hydroxy substituent on the side chain enhanced the activity similar to 89 and 90, and the configurations of the methoxy or hydroxy groups on the side chain had a little effect as in 91, 92, 93, and 94 [16].
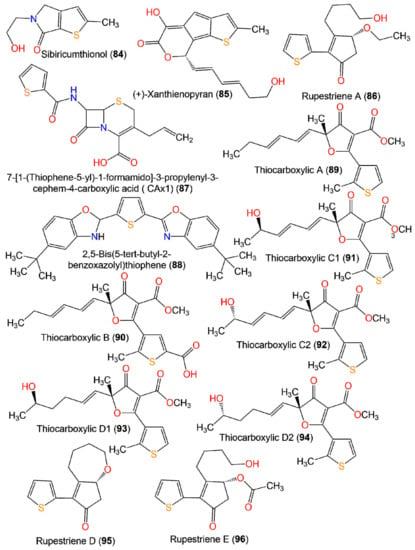
Figure 11.
Structures of miscellaneous thiophenes 84–96.
4.4. Antimalarial Activity
Malaria represents a significant parasitic disease worldwide, which is accountable for the death of at least half a million people yearly [63]. Globally, the estimated malaria cases in 2020 are 241 million in 85 malaria-endemic countries [64]. There is currently a vast augmentation of resistance to the available antimalarial drugs, which necessitates the search to pinpoint new drugs to combat malaria [65].
Bitew et al. evaluated the antimalarial activity of 9 and 14 isolated from CH2Cl2 fraction of Echinops hoehnelii roots utilizing the standard suppressive method in Plasmodium berghei-affected mice. Compounds 9 and 14 at 50 and 100 mg/kg concentrations decreased parasitemia levels by 43.2% and 50.2% and 18.8% and 32.7%, respectively, compared to chloroquine. It was suggested that the ester functional group produced a two-fold decrease in the activity as in 14 [32].
4.5. Larvicidal Activity
Currently used larvicides are synthetic pesticides with high toxic effects on humans and other non-targeted organisms. Several reports revealed that thiophenes demonstrated toxic effect toward insects, especially larval mosquitoes. It was proposed that thiophenes showed the promising possibility to be set as natural larvicides for controlling mosquitoes.
Zhao et al. reported that E. grijsii essential oil exhibited larvicidal potential versus the fourth instar larvae of Anopheles sinensis, Culex pipiens pallens, and Aedes albopictus (LC50s (lethal concentrations 50%) s 3.43, 1.47, and 2.65 µg/mL, respectively) in the larval mortality bioassay compared to rotenone. Further, the purified metabolites; 5-BBT (5-(but-3-en-1-ynyl)-2,2′-bithiophene) (37), 5-IBT (5-(4-isovaleroyloxybut-1-ynyl)-2,2′-bithiophene) (38), and α-T (α-terthienyl) (75) possessed remarkable larvicidal effectiveness (LC50 0.34, 0.45, and 1.41 µg/mL, respectively for Ae. albopictus, LC50 1.36, 5.36, and 1.79 µg/mL, respectively for An. sinensis, and LC50 0.12, 0.33, and 1.38 µg/mL, respectively for C. pipiens pallens) compared to rotenone (LC50 3.75, 1.25, and 1.88 µg/mL, respectively) [41].
4.6. Nematicidal Activity
Nematodes and plant pathogenic fungi cause diseases that can lessen the yield and quality of several crops [66]. Chemical control utilizing synthetic-produced pesticides is a commonly used way to manage these diseases. The possible imperilment of synthetic chemicals toward non-target organisms and pesticide resistance rationalized the development of eco-friendly and safe pesticides [67]. Discovering efficient and less toxic natural pesticides has given rise to a top preference in the contemporaneous pesticide industry [68].
Compounds 15, 16, 48, 50, 52, 61, 62, and 64 showed more potent nematicidal effect toward J2s (second-stage juveniles) of Meloidogyne incognita (LC50 values ranging from 0.42 to 8.28 μg/mL in light and from 0.86 to 9.23 μg/mL in dark) than abamectin (LC50 values 9.38 μg/mL in dark and 8.73 μg/mL in light). Noticeably, 61 and 64 possessed better dark potential compared to their light potential than control. Particularly, 64 was the most powerful metabolite against J2s (LC50 values 0.91 and 0.86 μg/mL, under light and dark, respectively) [13]. Compounds 48, 49, 51, and 61–64 were regarded as non-phototoxic metabolites. It was found that the thiophene unit was fundamental for the activity. However, an increase in the number of acetylenes and chlorine enhanced the effect [13,19]. Compounds 68–70 were evaluated for their nematicidal potential toward the J2s of Meloidogyne incognita under dark and light conditions in nematode mortality bioassays. They showed potent nematicidal potential (LC50 9.39–18.17 µg/mL/dark and 8.73–16.53 µg/mL/light) compared to ethoprophos (LC50 31.94 µg/mL/dark and 36.15 µg/mL/light). However, they had weaker nematicidal influences than α-terthienyl (phototoxic thiophene), suggesting that they were non-phototoxic. Furthermore, 70 exhibited more powerful activity (LC50 8.73 and 9.39 µg/mL under light and dark, respectively) than its monomeric bithiophene 49, revealing that the dimeric bithiophene framework with a 1,4-dioxane moiety in 70 enhanced the nematicidal potential [17].
Compound 74 previously reported from Tagetes patula aerial parts was synthesized by Politi et al. It had a marked in vitro anthelmintic effect toward Haemonchus contortus, exhibiting 100% efficacy in the larval development and egg hatch tests with EC50 (effective concentration 50%) 0.3243 mg/mL and 0.1731 mg/mL, respectively, compared to levamisole (EC50 1.88 mg/mL) [46].
4.7. Antioxidant and Anti-Influenza Activities
Compounds 43, 46, and 76 exhibited moderate antioxidant potential with % DPPH scavenging activity ranging from 41.87 to 45.17 at 100 µM [44].
Two new thiophene derivatives, rupestriene D (95) and rupestriene E (96), along with rupestriene A (86) isolated from the whole plants of Artemisia rupestris using SiO2 CC and RP-HPLC. They exhibited neuraminidase inhibitory potential with IC50 values ranging from 351.15 to 986.54 µM in the fluorescence-based assay compared to oseltamivir acid (IC50 77.91 µM). Compounds 86 and 96 were more potent than 95, indicating that a free OH group at the C-3 side chain might enhance the activity [15].
5. AI Target-Based Prediction vs. (Virtual Screening), and MD (Molecular Dynamics) for Thiophene Derivatives
Cathepsin D is one of the most abundant lysosomal proteases. It is implicated in protein turnover and favored apoptosis in proteostasis disruption [69,70]. The disturbance in its regulation can lead to various health disorders. Its excessive levels outside the cell membrane and lysosomes result in the growth of tumors, migration, invasion, and angiogenesis [71,72]. Many of the available inhibitors have non-specific inhibitory effects that may cause serious side effects [73]. Therefore, the currently tested thiophene derivatives as cathepsin D inhibitors could provide marked diagnostic benefits and a new therapeutic approach.
In order to detect the suitable protein targets for the thiophene derivatives, ligand-based tools were utilized for in silico target prediction [74]. In the current study, SuperPred, a prediction webserver, was used for the anatomical therapeutic chemical (ATC) code and target predication of these compounds [75]. Based on the analysis of the results for all the predicted targets, cathepsin D with PDB (protein data bank) code 4OD9 was selected, which is considered a common target for most of the thiophene derivatives with high probability and model accuracy percent (Table 3). All the listed compounds were docked, using extra precision for maximum accuracy; the docking method was validated by redocking the inhibitor N-(3,4-dimethoxybenzyl)-Nalpha-{N-[(3,4-dimethoxyphenyl)acetyl]carbamimidoyl}-D-phenylalaninamide (2RZ) that co-crystallized with 4OD9, and RMSD values were found in an acceptable range. All the redocked inhibitors revealed the same binding interaction with the active site as the original pose. Further, an in silico ADMET properties prediction of the investigated compounds was carried out. Eventually, MD simulation was conducted to assess the ligand/target interaction under simulated physiological circumstances for compound 30, which showed high docking scores.

Table 3.
The probability and model accuracy prediction for thiophene derivatives against Cathepsin D using SuperPred target prediction webserver.
5.1. In Silico ADMET Properties of Selected Ligands
The reported 96 thiophene derivatives were processed using the LigPrep of the Schrodinger suite [76]. The OPLS3 force field generated the 3D (three-dimensional) models with ionization states at 7.0 ± 0.2 pH. The QikProp module of the Schrodinger suite was utilized for predicting the ADME properties [77]. The predicted ADMET properties are summarized in Table 4. The ADMET analysis describes and determines the biological function, drug-likeness, physicochemical characters, and expected toxicity of the compounds. This is translated in terms of evaluating the usefulness of the molecules. The examined descriptors, such as drug likeness, solvent accessible surface area, dipole moment, molecular weight, hydrogen bond acceptor, and donor traits, aqueous solubility, octanol–water coefficient, number of likely metabolic reactions, brain/blood partition coefficient, human oral absorption, binding to human serum albumin, central nervous system activity, IC50 value for blockage of HERG K+ (human ether-a-go-go-related gene potassium) channels, and number of reactive functional groups were predicted for the reported thiophene derivatives. Most of the predicted values obtained for the compounds are in the recommended range, except for some highlighted parameters with yellow color.

Table 4.
In silico predicted ADME properties of the thiophenes derivatives.
5.2. Ligands and Proteins Preparations
Using LigPrep converted 2D structures to 3D, tautomerization, and ionization gave 146 minimized 3D structures that were utilized for docking with the Cathepsin D crystal structure (PDB: 4OD9). The 4OD9 prepared by the protein preparation wizard tool minimized the geometry and optimized the H-bond network. Specifying the proper force field treatment and the formal charge was accomplished by the addition of correct ionization states and missing hydrogens (Figure 12).
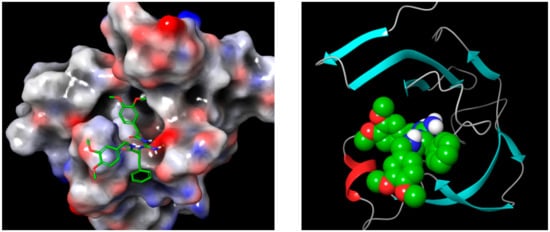
Figure 12.
N-(3,4-dimethoxybenzyl)-Nalpha-{N-[(3,4-dimethoxyphenyl)acetyl] carbamimidoyl}-D-phenylalaninamide (2RZ) complexed with Cathepsin D PDB: 4OD9 after preparation, using Protein Preparation Wizard, from Schrodinger, where hydrogen bonds are optimized, and the whole complex minimized and represented as a 3D structure.
5.3. Molecular Docking Studies
After designating the grid box in the prepared protein through Glide’s Receptor-Grid Generation tool in Maestro [78], the obtained 3D molecular structures were docked into the cathepsin D co-crystallized inhibitor binding site. Table 5 shows the results of the docked ligands that were selected owing to their most negative docking scores. These scores demonstrated the best-bonded ligand relative binding affinities and conformations. Compounds 29 and 30 displayed the highest negative docking scores of −9.439 and −9.178 kcal/mol in complex with 4OD9, respectively, while the reference inhibitors (2RZ) had a score of −6.895 kcal/mol in complex with the same protein.

Table 5.
In silico screening results of thiophene derivatives against Cathepsin D (PDB: 4OD9).
Analysis of the docking of 29 and 30 compared with the redocked reference 2RZ indicated that they interacted through hydrogen bonds (Figure 13, Figure 14, Figure 15 and Figure 16) with the binding site residues of Cathepsin D (4OD9). The binding site residues VAL 31, ASN 38, and TRP 40 of Cathepsin D had hydrogen bonding with the different hydroxyl groups of the sugar part. TRY 16 interacted with the terminal hydroxy group of 29. Whilst the binding site residues VAL 31, ASP 33, and TRP 40 of Cathepsin D possessed hydrogen bonding with the various OH groups of sugar part, and ASP 50 interacted with the terminal hydroxy group of the 30.
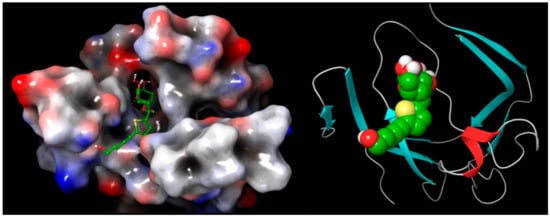
Figure 13.
Cathepsin D in complex with 29 represented as 3D molecular surface and ribbon structure.
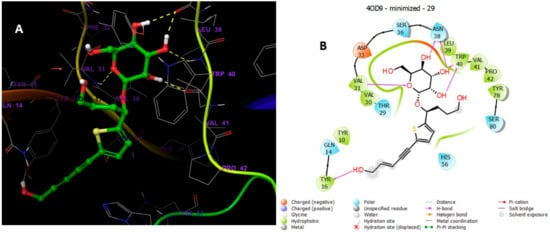
Figure 14.
(A) Putative binding mode of 29 in the binding site of cathepsin PDB: 4OD9. Compound 29 is displayed as green sticks. The amino acids residues of the binding site are represented as grey sticks, and H-bonds are represented in yellow dotted, (B) 2D depiction of the ligand–protein interactions.
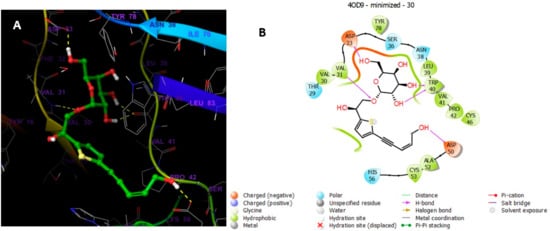
Figure 15.
(A) Putative binding mode of compound 30 in the binding site of Cathepsin PDB: 4OD9. Compound 30 is displayed as green sticks. The amino acids residues of the binding site are represented as grey sticks, and H-bonds are represented in yellow dotted, (B) 2D depiction of the ligand–protein interactions.
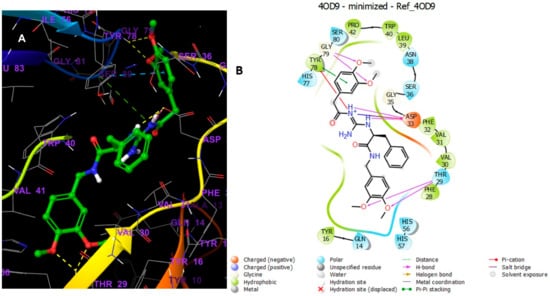
Figure 16.
(A) Putative binding mode of Reference in the binding site of Cathepsin PDB: 4OD9. The green sticks represent the Reference, whereas the grey sticks represent amino acid residues, and the yellow dotted lines represent the H-bond. (B) 2D depiction of the ligand–protein interactions.
5.4. Molecular Dynamics Simulation
The docking operation is a static view for the molecule’s binding in the active site of the specific protein. MD simulation computes the time versus atoms motions. By using Desmond software [79,80,81], the stability and frequency of compound 30 complex with Cathepsin D with PDB codes 4OD9, MD simulation was run with simulation time 100 ns. The complex structure was optimized at pH 7.0 ± 2.0. Complex stability was examined by analyzing the interaction map and the RMSD (root mean square deviation) plots of the ligand and protein. The RMSD plot in Figure 17 for the compound 30 complexed with Cathepsin D indicated the complexes tend to stabilize during simulation (100 ns) with regard to a reference frame at time 0 ns. There was a slight fluctuation during the simulation, but it lay under the permitted range of 1–3 Å; hence, it can be regarded as non-significant. Since the RMSD plots of compound 29 and protein backbone were lying over each other, the stable complex formation can be inferred. Figure 18 showed the schematic of detailed ligand atom interactions of compound 30 with Cathepsin D. The docked poses were maintained through the simulation time of 100 ns, i.e., molecular interactions with residues VAL 31, SER 36, ASN 38, TRP 40, and TYR 78.
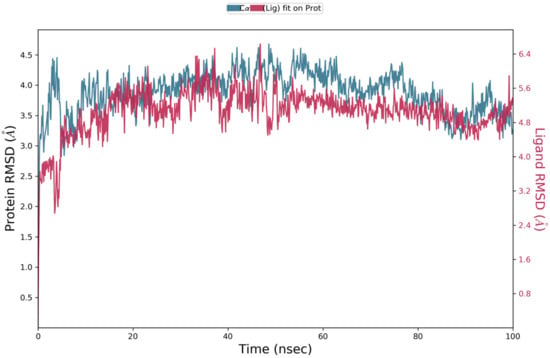
Figure 17.
RMSD analysis for compound 30 complexed with cathepsin D (PDB Code) of MD simulation trajectory. The RMSD plot was obtained for compound 30 complexed with cathepsin D (PDB ID 4OD9). The 100 ns simulation time reaffirms the stability of the complex without any significant changes in the structure.
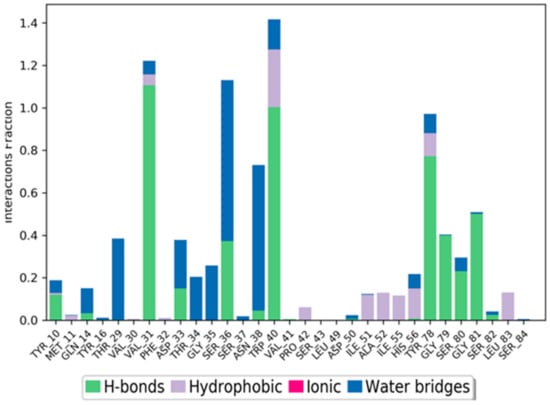
Figure 18.
Cathepsin D interactions with compound 30 throughout the simulation. The interactions between the ligand and protein are classified into hydrophobic, ionic, hydrogen bond, and water bridges. Each classification can be further sub-grouped and observed in the ‘Simulation Interactions Diagram’ panel. The stacked bar charts are normalized throughout the trajectory. The stacked bar charts are normalized over the course of the trajectory: for example, a value of 0.7 suggests that 70% of the simulation time, the specific interaction is maintained. Values over 1.0 are possible, as some protein residue may make multiple contacts of the same subtype with the ligand.
Figure 19 represents the ligand–protein interactions that are characterized into four types: ionic, hydrophobic, hydrogen bonds, and water bridges. Each interaction type includes more specified subtypes, which can be investigated via the ‘Simulation Interactions Diagram’ panel [82,83,84,85,86]. The stacked bar charts were normalized throughout the trajectory: for example, a value of 0.7 suggests that 70% of the simulation time, the specific interaction, is maintained. Values over 1.0 are possible, as some protein residue may make multiple contacts of the same subtype with the ligand. Hydrogen bonding with residues VAL 31, TRP 40, and TYR 78 was retained for more than 80% of the simulation time.
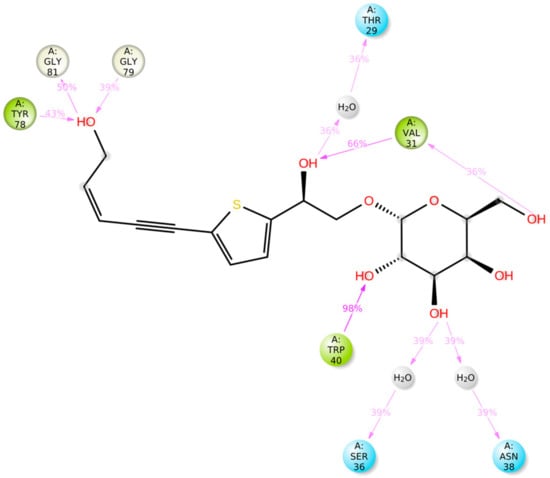
Figure 19.
The schematic diagram shows the detailed atomic interaction of compound 30 with Cathepsin D. Interactions that occur more than 30.0% of the simulation time in the selected trajectory (0.00 through 100.00 ns). It is possible to have interactions with >100% as some residues may have multiple interactions of a single type with the same ligand atom.
5.5. Materials and Methods
5.5.1. Preparation of PDB Structures
The PDB structure (PDB IDs: 4OD9) was downloaded from the Protein Data Bank [69], prepared and optimized utilizing the “Protein preparation wizard” [70] tool of Schrödinger suite [76,87]. For this reason, the bond orders for known HET groups and untemplated residues were identified, and hydrogens were added. Then, breaking bonds to metals, adding zero-order bonds among metals and nearby atoms, and correcting the formal charges to metals and neighboring atoms were carried out. From HET groups, water molecules beyond 5 Å were deleted. Disulfide bonds were generated. For ligands, metal HET states and cofactors were generated at 7.0 ± 2.0 pH using LigPrep [76]. Finally, H-bonds optimization at pH 7.0 using PROPKA [88], the removal of water molecules beyond 3 Å from HET groups, and restrained minimization using the OPLS4 force field were done.
5.5.2. ADME Properties Prediction
The drug likeness and ADME properties of the chosen compounds were estimated via the Maestro Schrodinger QikProp module in terms of metabolism, distribution, excretion, absorption, etc. [77].
5.5.3. Receptor Grids Generation and Docking
Glide [78] was utilized for both grid generation and ligands docking. For docking of the 96 thiophene derivatives, the grid was generated using the PDB: 4OD9, the region of binding was specified by selecting 2RZ. The non-polar atoms were set for the VdW radii scaling factor by 1.0 and the partial charge cut-off 0.25. The ligands docking was performed using the “ligand docking” tool of the Schrödinger suite [78,85]. The selected protocol was standard precision (SP), the ligand sampling method was flexible, and all the other settings were default.
5.5.4. MD Simulations of Compound 30 in Complex with 4OD9
MD simulations were run using the Schrödinger suite [88]. The system of compound 30 in complex with 4OD9 was retrieved from the docking results and first tuned through the “System Builder” tool. The TIP3P solvent model and then orthorhombic-shape box shape was chosen. The system was neutralized by adding Na+ ions, and the side distances box was set at 10 Å. The MD calculations were run for 100 ns per trajectory, the number of atoms, pressure, and temperature were kept constant (NPT ensemble). In contrast, the pressure was set at 1.01325 bar and temperature at 300.0 K, and the force field was set as OPLS4.
6. Conclusions
Natural products are featured by enormous scaffold diversity and structural complexity that contribute to drug discovery. Sulfur-containing natural metabolites are a large class of significant functional molecules with potent biological activities and pharmacological properties; some of them have been developed into essential drugs. In the current work, 96 naturally occurring thiophenes have been reported from 2015 till now. Most of them had one to three thiophene rings. However, dimeric bithiophenes with four thiophene rings and quinquethiophenes with five thiophene rings were extremely rare. These metabolites have been mainly evaluated for their cytotoxic, antimicrobial, anti-inflammatory, and nematicidal capacities. On the other side, there are limited reports on their antimalarial, larvicidal, antioxidant, and anti-influenza activities. Some of them showed remarkable cytotoxic effects. Therefore, they could be a potential therapeutic agent for treating various cancers. However, in vivo studies and their detailed mechanism of action need to be determined.
Furthermore, they showed marked antifungal potential against various plant pathogenic fungi and had a remarkable nematicidal effect. Hence, they could provide worthy insights for discovering effective, eco-friendly nematicides and fungicides. More studies on formulations development are needed to upgrade stability and efficacy and cut down costs. Additionally, field assessment and research on the effects of these compounds on non-target organisms are compulsory. Their antifungal mechanism was proposed to be photodynamic. However, further assessments are needed to confirm their potential for application as photosensitizers in photodynamic antimicrobial chemotherapy toward fungal infections. The structure–activity relationship study revealed that the presence of the acetylene group in the side chain increased the larvicidal and antimalarial potential. However, the attachment of the acetylenic side chain to an ester and acyl functional groups lowered the activity [19,32,34,41]. Additionally, increasing the number of acetylene groups in the side chain increased the anti-inflammatory, nematocidal, and antifungal capacities [19,32,34,41]. Further, the attachment of this chain with the chlorine group enhanced the activity [19] (Figure 20). Estimation of other potential bioactivities and derivatization of these metabolites, besides the mechanistic and in vivo studies of these metabolites, should be the target of future research. The precursor-based combinatorial biosynthesis (PCB) could be used for enhancing the production and structural modification of these metabolites. In this technique, a precursor analog has to be fed to the producing microorganism, resulting in the production of novel thiophenes with a potential pharmaceutical significance that is effective and ecologically friendly [89].
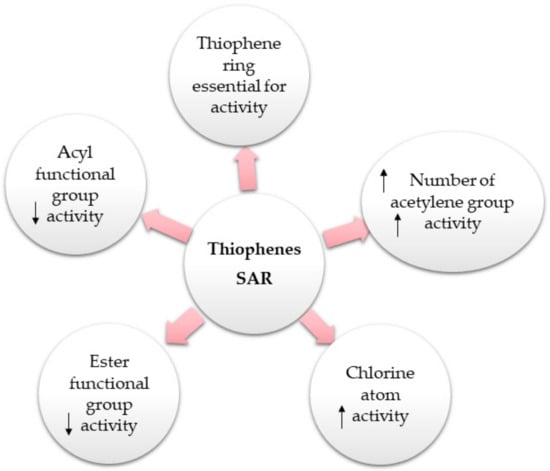
Figure 20.
Summary of the reported structure–activity relationship (SAR) of thiophenes. ↑: increase; ↓: decrease [19,32,34,41].
Based on the in silico, including ADMET properties predication, molecular docking for the protein–ligands binding interaction, and molecular dynamics, these metabolites were identified as potential inhibitors for the Cathepsin D, which will be helpful as potential leads for the treatment of several diseases that are affected by the dysregulation of this enzyme. The results of the studies described in this review are undoubtedly significant. They constitute the first stage of searching for new drug candidates and observing the strength of the tested metabolites concerning the currently used drugs. Furthermore, this review provides an overview of the research progress on naturally isolated thiophenes with special highlighting of their bioactivities that could attract the attention of many natural product researchers to further investigate and explore their mechanism, efficacy, and safety through in vivo studies. The structural diversity of thiophenes could be of considerable synthetic interest as novel chemical entities for drug discovery. Also, this work may encourage further research for isolation, characterization, and bio-evaluation of these thiophenes that may provide more candidates for the pharmaceutical industry. Additionally, it aims at providing a reference for researchers that they can use for the rapid identification of isolated thiophenes through a comparison of their physical and spectral data. Future investigations such as combinatorial chemistry and drug design will inevitably expose new avenues for the advancement of drug discovery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11040539/s1, Table S1: Physical and spectral data of newly reported naturally occurring thiophenes from 2015 to 2021.
Author Contributions
Conceptualization, S.R.M.I. and G.A.M.; resources, G.A.M., S.G.A.M., A.A.B., R.M.D. and S.R.M.I.; molecular docking and molecular dynamic, A.M.O.; data curation and funding acquisition: A.A.B., A.O.N., R.M.D. and D.M.A.; Discussion of the contents, G.A.M., S.R.M.I. and A.M.O.; writing—original draft preparation, S.R.M.I., A.O.N., G.A.M., D.M.A., S.G.A.M. and A.M.O.; writing—review and editing, S.R.M.I., S.G.A.M., G.A.M. and A.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
A2780: Human ovarian cancer cell lines; 1,3-DPBF: 1,3-Diphenylisobenzofuran: 5-LOX: 5-Lipoxygenase; AChE: Acetylcholinesterase; ACS: American Chemical Society; ADMET: absorption/distribution/metabolism/excretion/and toxicity; AI: Artificial Intelligence; ATC: Anatomical Therapeutic Chemical; Bax: Bcl-2-associated X protein; 5-BBT: 5-(But-3-en-1-ynyl)-2,2′-bithiophene; Bcl-2: B-cell lymphoma 2; BGC-823: Human gastric cancer cell line; BuOH: Butanol; CCRF-CEM: Human leukemic cell line; CEM/ADR5000: Human T-cell lymphoblast-like cell line; COSY: homonuclear correlation spectroscopy; CH2Cl2: Dichloromethane; CTLC: Preparative centrifugal thin layer chromatography; CYP2A6: Cytochrome P450 2A6; CYP2A13: Cytochrome P450 2A13; DPPH: 2,2′-Diphenyl-2-picrylhydrazyl; EC50: Effective concentration 50%; ECCD: Exciton coupled circular dichroism; ECD: Electronic circular dichroism; ED50: Effective dose 50; EIMS: Electron impact mass spectrometry; ELISA: Enzyme-linked immunosorbent assay; ERK1/2: Extracellular signal regulated kinase 1/2; ES2: Human ovarian cancer cell lines; ET-743: Ecteinascidin 743; EtOH: Ethanol; EtOAc: Ethyl acetate; GSH: Glutathione: HCT-116: Human colon cancer cell line; Hec1A: Human endometrial cancer cell line: HepG2: Human liver cancer cell line; HERG K+: Human ether-a-go-go-related gene potassium; HIV-1: human immunodeficiency virus-1; HMBC: Heteronuclear multiple bond correlation; HPLC: High pressure liquid chromatography; HSQC: Heteronuclear single quantum coherence; HRDARTMS: High resolution direct analysis real time mass spectrometry; HRESIMS: High resolution electrospray ionization mass spectrometry; 5-IBT: 5-(4-Isovaleroyloxybut-1-ynyl)-2,2′-bithiophene; IC50: Half-maximal inhibitory concentration; IR: Infra-red; INT: p-iodonitrotetrazolium: IL-6: Interleukin-6; IR: Infra-red; Ishikawa: Human endometrial cancer cell line: J2s: Second-stage juveniles; JNK: c-Jun N-terminal kinase; K562: Human erythroleukemic cell line; KB: Human oral epithelial-like cell; NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells; LC50: Lethal concentration 50%; LD50 is the amount of a material, which causes the 50% death; LC95: Lethal dose 95%, which causes the death of 95% of a group of test animals; 5-LOX: 5-Lipoxygenase; LPS: Lipopolysaccharide; MCF-7: Human breast cancer cell line; MFC: Minimal fungicide concentration; MeOH: Methanol; MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration; MDA-MB-231: Human breast cancer cell line; NCI-H1650: Human lung cancer cell line; MD: Molecular dynamics; MDR: Multidrug resistant; MTT: (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); NADPH: Nicotinamide–adenine dinucleotide phosphate: NBT: Nitro blue tetrazolium; NRC: Nitrite relative concentration; NMR: Nuclear magnetic resonance; NO: Nitric oxide; NP: Natural protein; NSAIDs: Non-steroidal anti-inflammatory drugs; OVCAR3: Human ovarian cancer cell lines; PARP: Poly ADP ribose polymerase; PCB: precursor-based combinatorial biosynthesis PDB: Protein Data Bank; PE: Petroleum ether; p-H2AX: H2A histone family member X; PHA: Phytohemagglutinin; PTLC: Preparative thin layer chromatography: PYDDT: 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene; ROS: Reactive oxygen species; RP CC: Reversed phase column chromatography; RMSD: Root mean square deviation; ROESY: Rotating frame Overhauser effect spectroscopy; SAM: S-adenosylmethionine; SKOV3: Human ovarian cancer cell lines; siRNAs: Small interfering ribonucleic acid; SiO2 CC: Silica gel column chromatography; SW620: Human colon cancer cells line; SP600125: Potent and reversible inhibitor of JNK1-3; α-T: α-Terthienyl; TNF-α: Tumor necrosis factor-α; UV: Ultraviolet; VS: Virtual screening.
References
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s toolbox. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef] [PubMed]
- Tilby, M.J.; Willis, M.C. How do we address neglected sulfur pharmacophores in drug discovery? Expert Opin. Drug Discov. 2021, 16, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.P.; DeNicola, G.M. Sulfur metabolism and its contribution to malignancy. Cell. Nutr. Util. Cancer 2019, 347, 39–103. [Google Scholar]
- Francioso, A.; Baseggio Conrado, A.; Mosca, L.; Fontana, M. Chemistry and biochemistry of sulfur natural compounds: Key intermediates of metabolism and redox biology. Oxid. Med. Cell Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef]
- Rakesh, K.P.; Wang, S.M.; Leng, J.; Ravindar, L.; Asiri, A.M.; Marwani, H.M.; Qin, H.L. Recent development of sulfonyl or sulfonamide hybrids as potential anticancer agents: A key review. Anti-Cancer Agents. Med. Chem. 2018, 18, 488–505. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and bioactivities. Phytochem. Rev. 2016, 15, 197–220. [Google Scholar] [CrossRef]
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Gramec, D.; Masic, L.P.; Sollner Dolenc, M. Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef]
- Joshi, E.M.; Heasley, B.H.; Chordia, M.D.; Macdonald, T.L. In vitro metabolism of 2-acetylbenzothiophene: Relevance to Zileuton Hepatotoxicity. Chem. Res. Toxicol. 2004, 17, 137–143. [Google Scholar] [CrossRef]
- Gil, A.; Ghersa, C.M.; Perelman, S. Root thiophenes in Tagetes minuta L. accessions from Argentina: Genetic and environmental contribution to changes in concentration and composition. Biochem. Syst. Ecol. 2002, 30, 1–13. [Google Scholar] [CrossRef]
- Champagne, D.E.; Arnason, J.T.; Philogene, B.J.R.; Campbell, G.; McLachlan, D.G. Photosensitization and feeding deterrence of Euxoa messoria (Lepidoptera: Noctuiidae) by α-terthienyl, a naturally occurring thiophene from the Asteraceae. Experientia 1984, 40, 577–578. [Google Scholar] [CrossRef]
- Cao, Y.; Zang, Y.; Huang, X.; Cheng, Z. Chemical constituents from Artemisia rupestris and their neuraminidase inhibitory activity. Nat. Prod. Res. 2021, 35, 1775–1782. [Google Scholar] [CrossRef]
- Chang, J.L.; Xu, H.Z.; Zhou, J.; Zhou, M.; Zhang, X.; Guo, Y.; Ruan, H.L. Antimicrobial furancarboxylic acids from a Penicillium sp. J. Nat. Prod. 2020, 83, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Wu, H.B.; Kuang, M.S.; Lan, H.P.; Wen, Y.X.; Liu, T.T. Novel bithiophene dimers from Echinops latifolius as potential antifungal and nematicidal agents. J. Agric. Food Chem. 2020, 68, 11939–11945. [Google Scholar] [CrossRef]
- Yu, S.J.; Yu, J.H.; He, F.; Bao, J.; Zhang, J.S.; Wang, Y.Y.; Zhang, H. New antibacterial thiophenes from Eclipta prostrata. Fitoterapia 2020, 142, 104471. [Google Scholar] [CrossRef]
- Liu, T.; Wu, H.; Jiang, H.; Zhang, L.; Zhang, Y.; Mao, L. Thiophenes from Echinops grijsii as a preliminary approach to control disease complex of root-knot nematodes and soil-borne fungi: Isolation, activities, and structure-nonphototoxic activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6160–6168. [Google Scholar] [CrossRef] [PubMed]
- Preya, U.H.; Lee, K.T.; Kim, N.J.; Lee, J.Y.; Jang, D.S.; Choi, J.H. The natural terthiophene α-terthienylmethanol induces S phase cell cycle arrest of human ovarian cancer cells via the generation of ROS stress. Chem. Biol. Interact. 2017, 272, 72–79. [Google Scholar] [CrossRef]
- Da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-based compounds with potential anti-inflammatory activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-based covalent organic frameworks: Synthesis, photophysics and light-driven applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef] [PubMed]
- Bhilare, N.V.; Auti, P.B.; Marulkar, V.S.; Pise, V.J. Diverse thiophenes as scaffolds in anti-cancer drug development: A concise review. Mini-Rev. Med. Chem. 2021, 2, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Abedinifar, F.; Babazadeh Rezaei, E.; Biglar, M.; Larijani, B.; Hamedifar, H.; Ansari, S.; Mahdavi, M. Recent strategies in the synthesis of thiophene derivatives: Highlights from the 2012–2020 literature. Mol. Divers. 2021, 25, 2571–2604. [Google Scholar] [CrossRef] [PubMed]
- Chitsazian-Yazdi, M.; Agnolet, S.; Lorenz, S.; Schneider, B.; Es’haghi, Z.; Kasaian, J.; Khameneh, B.; Iranshahi, M. Foetithiophenes C-F, thiophene derivatives from the roots of Ferula foetida. Pharm. Biol. 2015, 53, 710–714. [Google Scholar] [CrossRef]
- Zhou, X.D.; Zhang, C.; He, S.; Zheng, B.; Zeng, K.W.; Zhao, M.B.; Jiang, Y.; Tu, P.F. New terpenoids and thiophene derivatives from the aerial parts of Artemisia sieversiana. Bioorg. Med. Chem. Lett. 2017, 27, 5441–5445. [Google Scholar] [CrossRef]
- Li, L.B.; Xiao, G.D.; Xiang, W.; Yang, X.; Cao, K.X.; Huang, R.S. Novel substituted thiophenes and sulf-polyacetylene ester from Echinops ritro L. Molecules 2019, 24, 805. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.P.; Chen, C.C.; Huang, H.C.; Wang, S.Y.; Chen, J.J.; Yang, C.S.; Ou, C.Y.; Wu, J.B.; Huang, G.J.; Kuo, Y.H. A new bithiophene from the root of Echinops grijsii. Nat. Prod. Commun. 2015, 10, 2147–2149. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Sandjo, L.P.; Tankeo, S.B.; Ndifor, A.R.; Pantaleon, A.; Nagdjui, B.T.; Kuete, V. Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes. Springerplus 2015, 4, 823. [Google Scholar] [CrossRef] [Green Version]
- Sandjo, L.P.; Kuete, V.; Siwe, X.N.; Poumale, H.M.P.; Efferth, T. Cytotoxicity of an unprecedented brominated oleanolide and a new furoceramide from the Cameroonian spice, Echinops giganteus. Nat. Prod. Res. 2016, 30, 2529–2537. [Google Scholar] [CrossRef]
- Boonruang, S.; Prakobsri, K.; Pouyfung, P.; Srisook, E.; Prasopthum, A.; Rongnoparut, P.; Sarapusit, S. Inhibition of human cytochromes P450 2A6 and 2A13 by flavonoids, acetylenic thiophenes and sesquiterpene lactones from Pluchea indica and Vernonia cinerea. J. Enzyme Inhib. Med. Chem. 2017, 32, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Bitew, H.; Mammo, W.; Hymete, A.; Yeshak, M.Y. Antimalarial activity of acetylenic thiophenes from Echinops hoehnelii Schweinf. Molecules 2017, 22, 1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.G.; Lv, W.; Dai, C.Y.; Zhu, F.F.; Xu, G.H.; Ma, Z.J.; Chen, Z. 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene induces apoptosis through reactive oxygen species-mediated JNK activation in human colon cancer SW620 cells. Anat. Rec. 2015, 298, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lee, J.W.; Jang, H.; Choi, J.E.; Kim, H.S.; Lee, D.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Dimeric sesquiterpene and thiophenes from the roots of Echinops latifolius. Bioorg. Med. Chem. Lett. 2016, 26, 5995–5998. [Google Scholar] [CrossRef]
- Ruan, J.; Li, Z.; Yan, J.; Huang, P.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Bioactive constituents from the aerial parts of Pluchea indica Less. Molecules 2018, 23, 2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Liu, B.Y.; Zeng, K.W.; Guo, X.Y.; Jiang, Y.; Tu, P.F. New sesquiterpene and thiophene derivatives from Artemisia rupestris. J. Asian Nat. Prod. Res. 2015, 17, 1129–1136. [Google Scholar] [CrossRef]
- Feng, Z.M.; Xu, K.; Wang, W.; Du, N.; Zhang, J.H.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Two new thiophene polyacetylene glycosides from Atractylodes lancea. J. Asian Nat. Prod. Res. 2018, 20, 531–537. [Google Scholar] [CrossRef]
- Yu, S.J.; Zhang, J.S.; He, H.; Yu, J.H.; Bao, J.; Zhang, H. Thiophene enantiomers from the aerial parts of Eclipta prostrata. J. Asian Nat. Prod. Res. 2021, 23, 745–753. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.M.; Ryu, B.; Lee, J.S.; Choi, J.H.; Jang, D.S. Constituents of the aerial parts of Eclipta prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch. Pharm. Res. 2015, 38, 1963–1969. [Google Scholar] [CrossRef]
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagn. Photodyn. Ther. 2017, 20, 263–272. [Google Scholar] [CrossRef]
- Zhao, M.P.; Liu, Q.Z.; Liu, Q.; Liu, Z.L. Identification of larvicidal constituents of the essential oil of Echinops grijsii roots against the three species of mosquitoes. Molecules 2017, 22, 205. [Google Scholar] [CrossRef] [Green Version]
- Kiyekbayeva, L.; Mohamed, N.M.; Yerkebulan, O.; Mohamed, E.I.; Ubaidilla, D.; Nursulu, A.; Assem, M.; Srivedavyasasri, R.; Ross, S.A. Phytochemical constituents and antioxidant activity of Echinops albicaulis. Nat. Prod. Res. 2018, 32, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Tagetnoic acid, a new lipoxygenase inhibitor peroxy fatty acid from Tagetes minuta growing in Saudi Arabia. Nat. Prod. Res. 2020, 34, 474–481. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Esmat, A.; Mohamed, G.A. Thiotagetin B and tagetannins A and B, new acetylenic thiophene and digalloyl glucose derivatives from Tagetes minuta and evaluation of their in vitro antioxidative and anti-inflammatory activity. Fitoterapia 2018, 125, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Zhang, Z.; Sauriol, F.; Shi, Q.; Yang, J. New thiophene acetylene from Echinops spinosissimus subsp. Spinosus. Chem. Nat. Compd. 2017, 53, 933–934. [Google Scholar] [CrossRef]
- Politi, F.A.S.; Bueno, R.V.; Zeoly, L.A.; Fantatto, R.R.; Eloy, J.O.; Chorilli, M.; Coelho, F.; Guido, R.V.C.; Chagas, A.C.S.; Furlan, M. Anthelmintic activity of a nanoformulation based on thiophenes identified in Tagetes patula L. (Asteraceae) against the small ruminant nematode Haemonchus contortus. Acta Trop. 2021, 219, 105920. [Google Scholar] [CrossRef]
- Lee, J.S.; Ahn, J.H.; Cho, Y.J.; Kim, H.Y.; Yang, Y.I.; Lee, K.T.; Jang, D.S.; Choi, J.H. α-Terthienylmethanol, isolated from Eclipta prostrata, induces apoptosis by generating reactive oxygen species via NADPH oxidase in human endometrial cancer cells. J Ethnopharmacol. 2015, 169, 426–434. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Thiotagetin A, new cytotoxic thiophene from Tagetes minuta. Nat. Prod. Res. 2017, 31, 543–547. [Google Scholar] [CrossRef]
- Shi, Y.S.; Li, L.; Liu, Y.B.; Ma, S.G.; Li, Y.; Qu, J.; Liu, Q.; Shen, Z.F.; Chen, X.G.; Yu, S.S. A new thiophene and two new monoterpenoids from Xanthium sibiricum. J. Asian Nat. Prod. Res. 2015, 17, 1039–1047. [Google Scholar] [CrossRef]
- Bhattacharjee, K.; Palepu, N.R.; Rao, K.M.; Joshi, S.R. Precursor-directed combinatorial biosynthesis of cephalosporin analogue by endolithic actinobacterium Streptomyces sp. AL51 by utilizing thiophene derivative. 3 Biotech 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.T.; Tran, V.H.; Vu, V.N.; Mai, H.D.T.; Le, T.H.M.; Vu, T.Q.; Nguyen, H.H.; Chau, V.M.; Pham, V.C. Antimicrobial metabolites from a marine-derived actinomycete Streptomyces sp. G278. Nat. Prod. Res. 2019, 33, 3223–3230. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.-J.; Chen, J.; Zhao, Q.-Q.; Li, Y.; Gao, K. Thiophene acetylenes and furanosesquiterpenes from Xanthopappus subacaulis and their antibacterial activities. Phytochemistry 2014, 106, 134–140. [Google Scholar] [CrossRef]
- Velíšek, J.; Cejpek, K. Biosynthesis of food constituents: Lipids. 1. Fatty acids and derived compounds—A review. Czech J. Food Sci. 2006, 24, 193–216. [Google Scholar] [CrossRef] [Green Version]
- Field, J.A. Natural Production of Organohalide Compounds in the Environment. In Organohalide-Respiring Bacteria, 1st ed.; Adrian, L., Löffler, F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–29. [Google Scholar]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.G.; Muntean, D.M.; Mirel, S.; Vostinaru, O.; Kiss, B.; Popa, D.S. Antitussive, antioxidant, and anti-inflammatory effects of a walnut (Juglans regia L.) septum extract rich in bioactive compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef]
- Ranaweera, S.S.; Dissanayake, C.Y.; Natraj, P.; Lee, Y.J.; Han, C.H. Anti-inflammatory effect of sulforaphane on LPS-stimulated RAW 264.7 cells and ob/ob mice. J. Vet. Sci. 2020, 21, e91. [Google Scholar] [CrossRef] [PubMed]
- Postigo, A.; Schiavi, P.C.; Funes, M.; Sortino, M. Mechanistic studies of Candida albicans photodynamic inactivation with Porophyllum obscurum hexanic extract and its isolated thiophenic compounds. Photodiagn. Photodyn. Ther. 2019, 26, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 25 December 2021).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Gay, F.; Zougbédé, S.; N’dilimabaka, N.; Rebollo, A.; Mazier, D.; Moreno, A. Cerebral malaria: What is known and what is on research. Rev. Neurol. 2012, 68, 239–256. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 25 December 2021).
- Ibrahim, S.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti-malarial agents from endophytic fungi: A Review. Mini-Rev. Med. Chem. 2018, 18, 1110–1132. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Gao, C.; Yu, Z. Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Gan, X.; Song, B.; Hu, D.; Song, B. Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole-cinnamic acid hybrids. J. Agric. Food Chem. 2018, 66, 9616–9623. [Google Scholar] [CrossRef] [PubMed]
- Tocco, G.; Eloh, K.; Onnis, V.; Sasanelli, N.; Caboni, P. Haloacetophenones as newly potent nematicides against Meloidogyne incognita. Ind. Crops Prod. 2017, 110, 94–102. [Google Scholar] [CrossRef]
- Castino, R.; Davies, J.; Beaucourt, S.; Isidoro, C.; Murphy, D. Autophagy is a prosurvival mechanism in cells expressing an autosomal dominant familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J. 2005, 19, 1021–1023. [Google Scholar] [CrossRef] [Green Version]
- Houštecká, R.; Hadzima, M.; Fanfrlík, J.; Brynda, J.; Pallová, L.; Hánová, I.; Mertlíková-Kaiserová, H.; Lepšík, M.; Horn, M.; Smrčina, M.; et al. Biomimetic macrocyclic inhibitors of human cathepsin D: Structure-activity relationship and binding mode analysis. J. Med. Chem. 2020, 63, 1576–1596. [Google Scholar] [CrossRef]
- Pranjol, M.Z.I.; Gutowski, N.; Hannemann, M.; Whatmore, J. The potential role of the proteases cathepsin D and cathepsin L in the progression and metastasis of epithelial ovarian cancer. Biomolecules 2015, 5, 3260–3279. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Roth, J.M.; Brooks, P.; Luty, J.; Karpatkin, S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008, 68, 4666–4673. [Google Scholar] [CrossRef] [Green Version]
- Lowry, J.R.; Klegeris, A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull. 2018, 139, 144–156. [Google Scholar] [CrossRef]
- Yang, S.; Ye, Q.; Ding, J.; Yin, M.; Lu, A.; Chen, X.; Hou, T.; Cao, D. Current advances in ligand-based target prediction. WIREs Comput. Mol. Sci. 2021, 11, e1504. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, 26. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. Schrödinger Release 2021-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: QikProp; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: Glide; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: Desmond Molecular Dynamics System; D. E. Shaw Research: New York, NY, USA, 2021. [Google Scholar]
- Maestro-Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2021.
- RCSB PDB: Homepage. Available online: https://www.rcsb.org/ (accessed on 5 January 2022).
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Schrödinger Release 2021-4: Protein Preparation Wizard; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: Epik; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: Impact; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4: Prime; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Zhao, H.; Ang, E.L. Recent advances in combinatorial biosynthesis for drug discovery. Drug Des. Dev. Ther. 2015, 9, 823–833. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).