Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants
Abstract
:1. Introduction
2. Results
2.1. Plant Growth
2.2. Photochemical Efficiency
2.3. Morphological and Production Parameters
2.4. Root Characterization
2.5. Soil Biological Properties
2.6. Cd Concentration in the Roots and Soil
2.7. Correlations
3. Discussion
3.1. Plant Growth and Yield
3.2. Soil Parameters
3.3. Influence of Variables
4. Materials and Methods
4.1. Experimental Setup
4.2. Soil Description and Preparation
4.3. Microplastics Particles and Heavy Metals
4.4. Plant Analysis
4.5. Microbiological Soil Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy Considerations for Limiting Unintended Residual Plastic in Agricultural Soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE Microplastic Films Alter Microbial Community Composition and Enzymatic Activities in Soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Schirmel, J.; Albert, J.; Kurtz, M.P.; Muñoz, K. Plasticulture Changes Soil Invertebrate Assemblages of Strawberry Fields and Decreases Diversity and Soil Microbial Activity. Appl. Soil Ecol. 2018, 124, 379–393. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and Micro- Plastics in Soil-Plant System: Effects of Plastic Mulch Film Residues on Wheat (Triticum aestivum) Growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of Plastic Film Residues on Occurrence of Phthalates and Microbial Activity in Soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef]
- Daugaard, H. The Effect of Mulching Materials on Yield and Berry Quality in Organic Strawberry Production. Biol. Agric. Hortic. 2008, 26, 139–147. [Google Scholar] [CrossRef]
- Ekebafe, L.O.; Ogbeifun, D.E.; Okieimen, F.E. Polymer Applications in Agriculture. Biokemistri 2011, 23, 81–89. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 1 February 2022).
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Environment Programme, UNEP. Year Book 2014: Emerging Issues in Our Global Environment. Available online: http://www.unep.org/resources/report/unep-year-book-2014-emerging-issues-our-global-environment-0 (accessed on 9 January 2022).
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, Q.; Khan, A.; Nawaz, A.; Baig, K.S.; Chen, J.-T. Enhancing Cadmium Tolerance and Pea Plant Health through Enterobacter Sp. MN17 Inoculation Together with Biochar and Gravel Sand. Plants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological Restoration of Mine Degraded Soils, with Emphasis on Metal Contaminated Soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, T.; Baveye, P.C.; Zhu, J.; Ning, Z.; Li, H. Potential Health Risk in Areas with High Naturally-Occurring Cadmium Background in Southwestern China. Ecotoxicol. Environ. Saf. 2015, 112, 122–131. [Google Scholar] [CrossRef]
- Ammar, W.B.; Nouairi, I.; Zarrouk, M.; Ghorbel, M.H.; Jemal, F. Antioxidative response to cadmium in roots and leaves of tomato plants. Biol. Plant. 2008, 52, 727–731. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium Toxicity in Maize (Zea Mays L.): Consequences on Antioxidative Systems, Reactive Oxygen Species and Cadmium Accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Santos, C.; Soares, A.M.V.M.; Mann, R.M. Assessment of Biomarkers of Cadmium Stress in Lettuce. Ecotoxicol. Environ. Saf. 2009, 72, 811–818. [Google Scholar] [CrossRef]
- Joint Expert Committee on Food Additives. Safety Evaluation of Certain Contaminants in Food: Prepared by the Sixty-Fourth Meeting of the Joint FAO; WHO Food Additives Series; World Health Organization: Geneva, Switzerland, 2006; ISBN 9789251054260. [Google Scholar]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of Microplastics and Cadmium on Plant Growth and Arbuscular Mycorrhizal Fungal Communities in an Agricultural Soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and Remediation of Phthalic Acid Esters in Agricultural Soils in China: A Review. Agron. Sustain. Dev. 2015, 35, 519–534. [Google Scholar] [CrossRef]
- Gianfreda, L.; Antonietta Rao, M.; Piotrowska, A.; Palumbo, G.; Colombo, C. Soil Enzyme Activities as Affected by Anthropogenic Alterations: Intensive Agricultural Practices and Organic Pollution. Sci. Total Environ. 2005, 341, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia Faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of Common Bean (Phaseolus Vulgaris L.) Growth to Soil Contaminated with Microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt Frank, N.A.; Marcel, A.K.J. Polyethylene Microplastics Adhere to Lemna minor (L.), yet Have No Effects on Plant Growth or Feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Fu, H.; Ji, R.; Qu, X. Sunlight Mediated Cadmium Release from Colored Microplastics Containing Cadmium Pigment in Aqueous Phase. Environ. Pollut. 2020, 263, 114484. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xu, G.; Wang, Y.; Yu, Y. Impacts of Polyethylene Microplastics on Bioavailability and Toxicity of Metals in Soil. Sci. Total Environ. 2021, 760, 144037. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A.; Turner, A.; Holmes, L.A. Adsorption of Trace Metals by Microplastic Pellets in Fresh Water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal Solid Waste (MSW) Landfill: A Source of Microplastics? -Evidence of Microplastics in Landfill Leachate. Water Research 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Zhang, G.; Wu, F. Identification of Barley Varieties Tolerant to Cadmium Toxicity. Biol. Trace Elem. Res. 2008, 121, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-B.; He, J.; Polle, A.; Rennenberg, H. Heavy Metal Accumulation and Signal Transduction in Herbaceous and Woody Plants: Paving the Way for Enhancing Phytoremediation Efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A Proposal of “Core Enzyme” Bioindicator in Long-Term Pb-Zn Ore Pollution Areas Based on Topsoil Property Analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhang, W.; Miao, L.; Ji, T.; Wang, Y.; Zhang, H.; Ding, Y.; Zhu, W. The Effects of Vermicompost and Shell Powder Addition on Cd Bioavailability, Enzyme Activity and Bacterial Community in Cd-Contaminated Soil: A Field Study. Ecotoxicol. Environ. Saf. 2021, 215, 112163. [Google Scholar] [CrossRef]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C.; et al. Comparative Transcriptome Combined with Morpho-Physiological Analyses Revealed Key Factors for Differential Cadmium Accumulation in Two Contrasting Sweet Sorghum Genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef]
- Xian, J.; Wang, Y.; Niu, K.; Ma, H.; Ma, X. Transcriptional Regulation and Expression Network Responding to Cadmium Stress in a Cd-Tolerant Perennial Grass Poa Pratensis. Chemosphere 2020, 250, 1–12. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Vig, K.; Megharaj, M.; Sethunathan, N.; Naidu, R. Bioavailability and Toxicity of Cadmium to Microorganisms and Their Activities in Soil: A Review. Adv. Environ. Res. 2003, 8, 121–135. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment; IntechOpen: Rijeka, Croatia, 2012; ISBN 9789533070193. [Google Scholar]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids as a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Wang, Z.; Lu, G.; He, W.; Wei, G.; Huang, F.; Xu, X.; Shen, W. Kinetics of Soil Dehydrogenase in Response to Exogenous Cd Toxicity. J. Hazard. Mater. 2017, 329, 299–309. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Klose, S.; Ajwa, H.A. Enzyme Activities in Agricultural Soils Fumigated with Methyl Bromide Alternatives. Soil Biol. Biochem. 2004, 36, 1625–1635. [Google Scholar] [CrossRef] [Green Version]
- Xian, Y.; Wang, M.; Chen, W. Quantitative Assessment on Soil Enzyme Activities of Heavy Metal Contaminated Soils with Various Soil Properties. Chemosphere 2015, 139, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Tang, D.; Feng, H.; Gao, Y.; Zhou, P.; Xu, L.; Wang, L. Determining Soil Enzyme Activities for the Assessment of Fungi and Citric Acid-Assisted Phytoextraction under Cadmium and Lead Contamination. Environ. Sci. Pollut. Res. Int. 2015, 22, 19860–19869. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; He, Y.; Wang, Z.; Li, C.; Kong, L.; Tian, H.; Shen, W.; Megharaj, M.; He, W. Soil Mineral Alters the Effect of Cd on the Alkaline Phosphatase Activity. Ecotoxicol. Environ. Saf. 2018, 161, 78–84. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid Bacterial Colonization of Low-Density Polyethylene Microplastics in Coastal Sediment Microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Renella, G.; Mench, M.; Landi, L.; Nannipieri, P. Microbial Activity and Hydrolase Synthesis in Long-Term Cd-Contaminated Soils. Soil Biol. Biochem. 2005, 37, 133–139. [Google Scholar] [CrossRef]
- Shentu, J.; He, Z.; Yang, X.; Li, T. Microbial Activity and Community Diversity in a Variable Charge Soil as Affected by Cadmium Exposure Levels and Time. J. Zhejiang Univ. Sci. B 2008, 9, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Castle, S.C.; Sullivan, B.W.; Knelman, J.; Hood, E.; Nemergut, D.R.; Schmidt, S.K.; Cleveland, C.C. Nutrient Limitation of Soil Microbial Activity during the Earliest Stages of Ecosystem Development. Oecologia 2017, 185, 513–524. [Google Scholar] [CrossRef]
- Caravaca, F.; Lozano, Z.; Rodríguez-Caballero, G.; Roldán, A. Spatial Shifts in Soil Microbial Activity and Degradation of Pasture Cover Caused by Prolonged Exposure to Cement Dust. Land Degrad. Dev. 2017, 28, 1329–1335. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Bolan, N.; Sarkar, B.; Ok, Y.S.; Zhang, W.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; et al. Microbial Functional Diversity and Carbon Use Feedback in Soils as Affected by Heavy Metals. Environ. Int. 2019, 125, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Skórzyńska-Polit, E.; Baszyński, T. Differences in Sensitivity of the Photosynthetic Apparatus in Cd-Stressed Runner Bean Plants in Relation to Their Age. Plant Sci. 1997, 128, 11–21. [Google Scholar] [CrossRef]
- Dede, G.; Ozdemir, S. Effects of Elemental Sulphur on Heavy Metal Uptake by Plants Growing on Municipal Sewage Sludge. J. Environ. Manag. 2016, 166, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Chaperon, S.; Sauvé, S. Toxicity Interactions of Cadmium, Copper, and Lead on Soil Urease and Dehydrogenase Activity in Relation to Chemical Speciation. Ecotoxicol. Environ. Saf. 2008, 70, 1–9. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014, e752708. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of Nitrogen Utilization by Soil Microorganisms—A Review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Keys to Soil Taxonomy; USDA: Washington, DC, USA, 2010; ISBN 9780160854279. [Google Scholar]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N.; Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry (Vaccinium Virgatum Aiton) ‘Ochlockonee’ Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential Use of Dehydrogenase Activity as an Index of Microbial Activity in Degraded Soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Proteases, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Naseby, D.C.; Lynch, J.M. Rhizosphere Soil Enzymes as Indicators of Perturbations Caused by Enzyme Substrate Addition and Inoculation of a Genetically Modified Strain of Pseudomonas Fluorescens on Wheat Seed. Soil Biol. Biochem. 1997, 29, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) 5—Estimation of Microbial Activities. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 193–270. ISBN 9780125138406. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzales, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2013; Grupo InfoStat, FCA; Universidad Nacional de Córdova: Córdova, Argentina, 2013; Available online: http://www.infostat.com.ar (accessed on 21 June 2021).
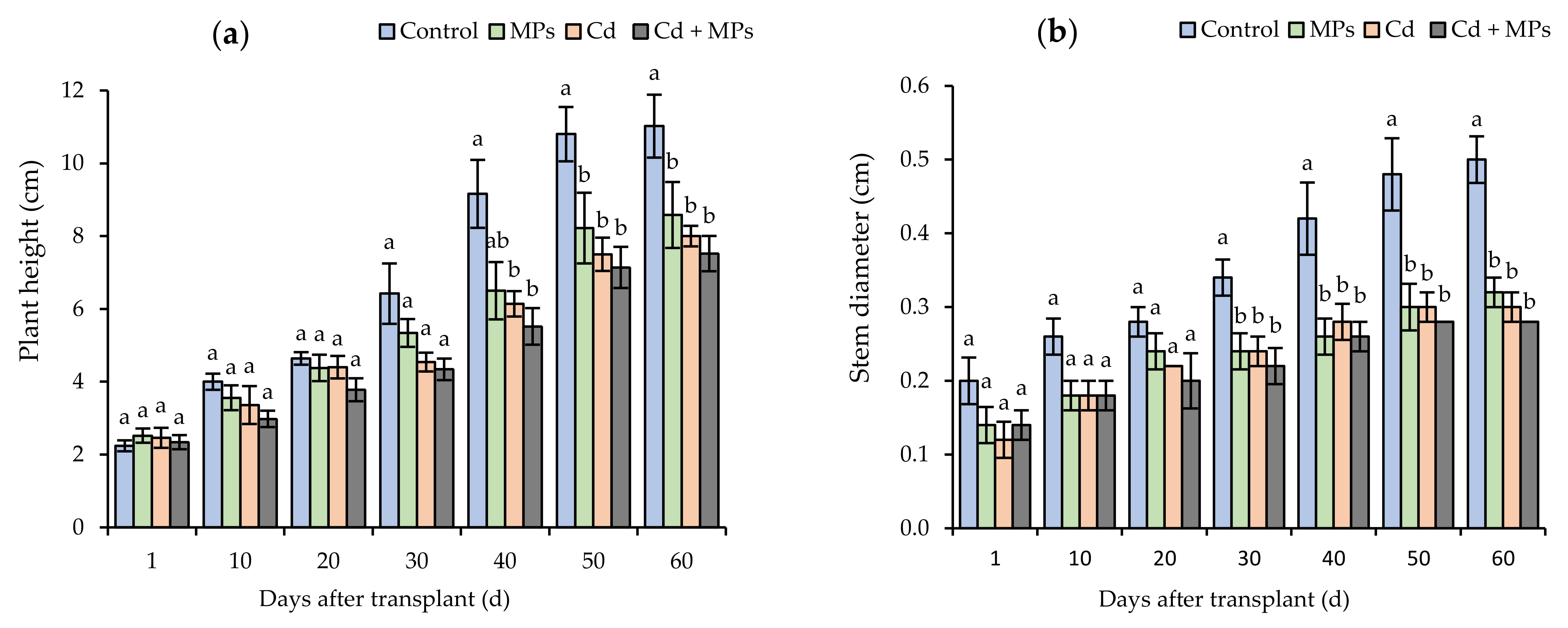
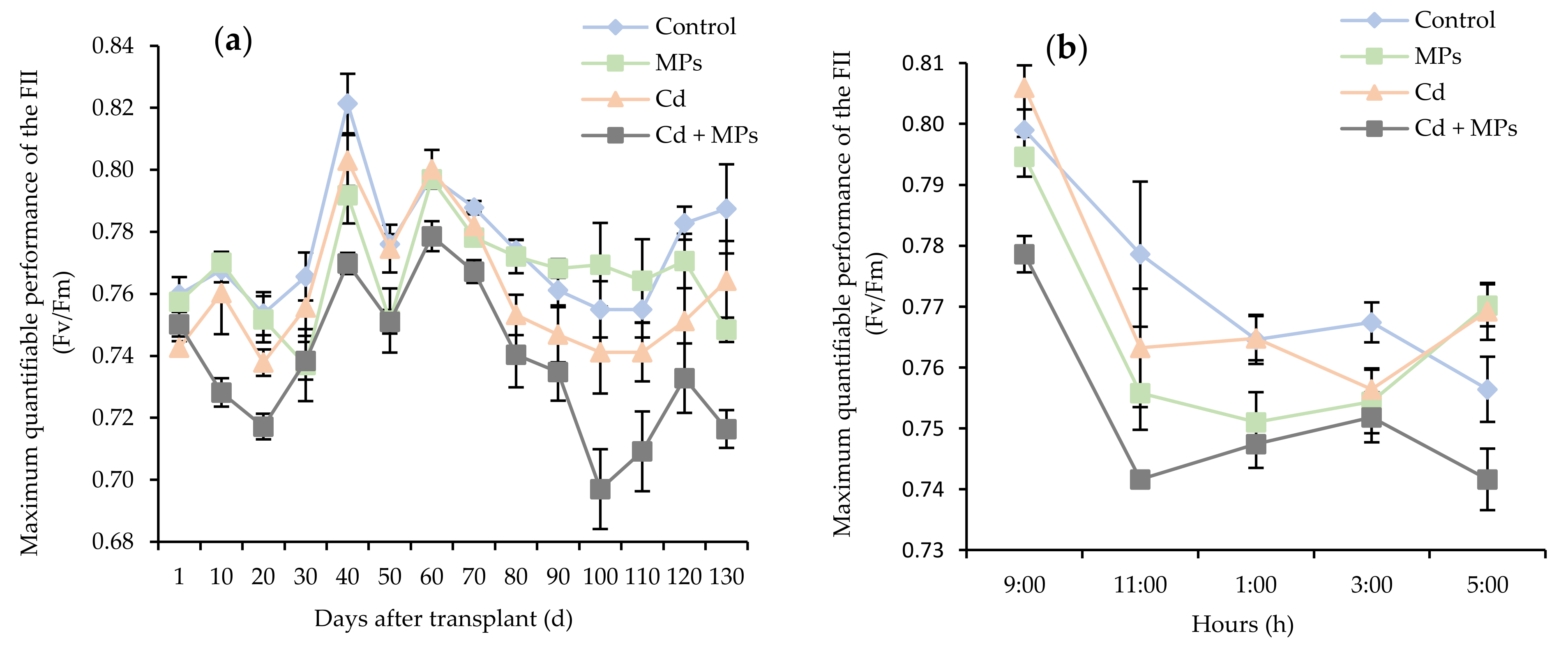

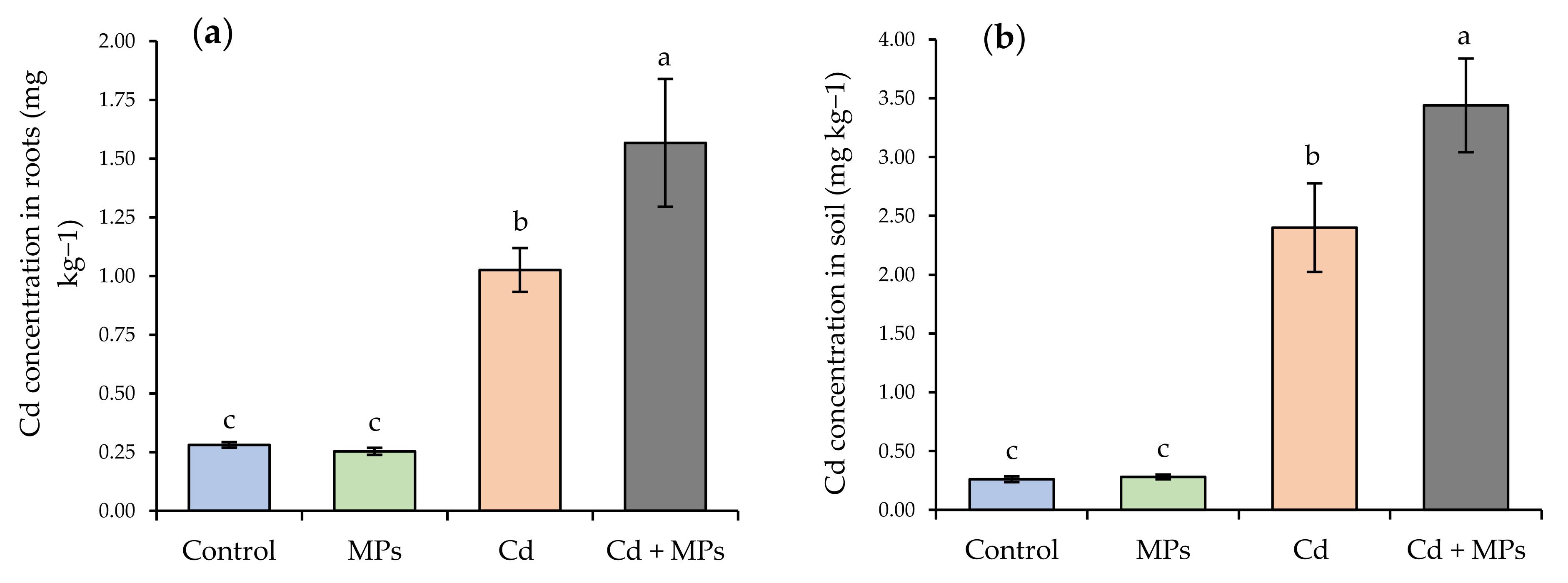

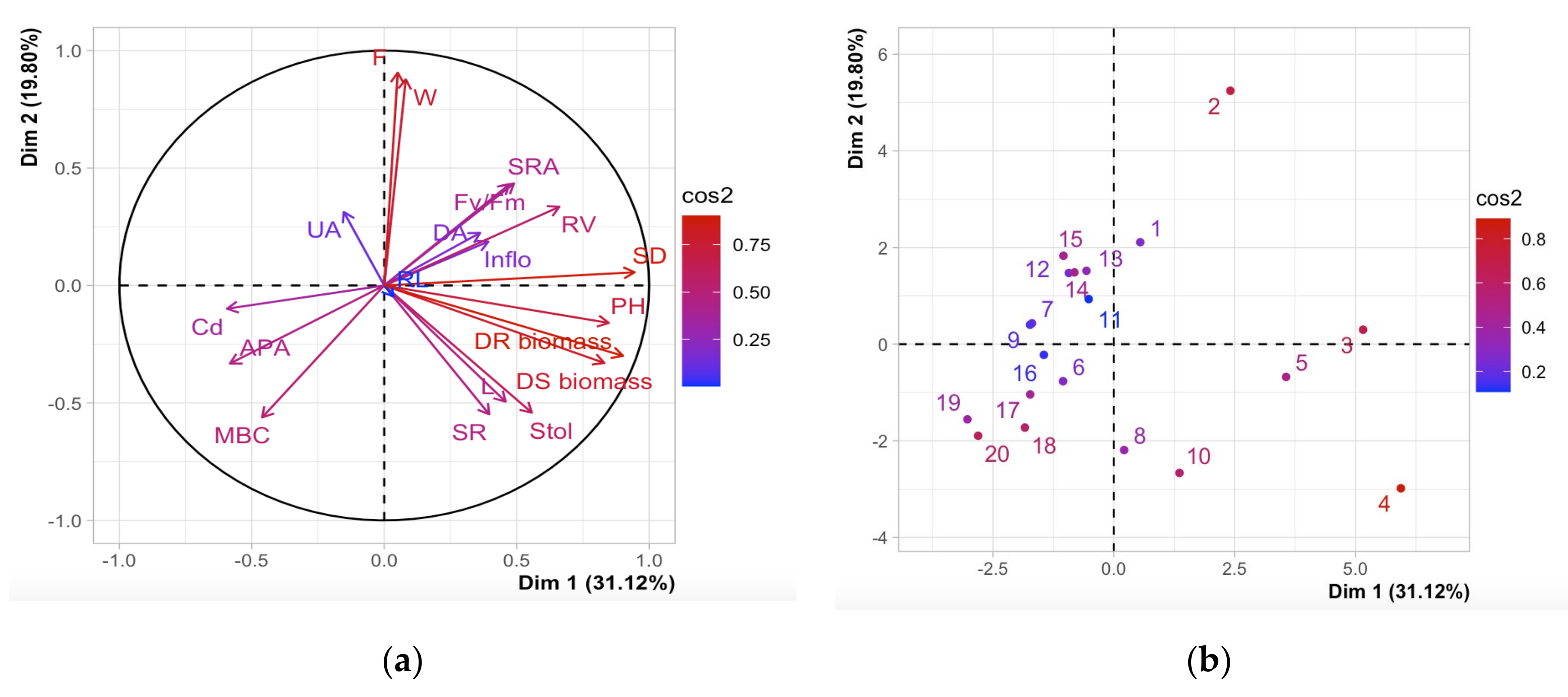
| Treatments | Number of Inflorescences (Flowers Plant−1) | Total Number of Stolons (Stolons Plant−1) | Stolon Length (cm Plant−1) | Total Number of Fruits (Fruits Plant−1) | Fruit Weight (g Plant−1) | Total Plant Biomass (g Plant−1) |
|---|---|---|---|---|---|---|
| Control | 5.4 ± 0.67 a | 1.6 ± 0.67 a | 40.1 ± 10.10 a | 4.4 ± 1.50 a | 20.9 ± 8.80 a | 7.3 ± 1.00 a |
| MPs | 3.2 ± 0.80 a | 1.4 ± 0.51 a | 26.6 ± 8.60 a | 2.0 ± 0.50 b | 8.8 ± 2.80 a | 4.4 ± 0.30 b |
| Cd | 3.8 ± 0.80 a | 1.0 ± 0.31 a | 23.7 ± 6.70 a | 3.8 ± 0.50 a | 20.4 ± 2.10 a | 4.2 ± 0.30 b |
| Cd + MPs | 3.2 ± 0.37 a | 1.2 ± 0.20 a | 29.9 ± 3.40 a | 2.0 ± 0.30 b | 9.8 ± 1.20 a | 4.8 ± 0.30 b |
| Anova p values | 0.1165 | 0.9160 | 0.4767 | 0.0426 | 0.1783 | 0.0073 |
| Treatments | Acid Phosphatase Activity (μmol PNP g−1h−1) | Urease Activity (μmol NH4+g −1h−1) | Dehydrogenase Activity (μg INTF g−1) | Microbial Biomass C (µg Kg−1) | Soil Respiration (μg CO2 g−1 Day) |
|---|---|---|---|---|---|
| Control | 21.3 ± 1.8 b | 2.2 ± 0.10 a | 39.5 ± 2.70 a | 1.5 ± 0.2 b | 81.5 ± 11.2 a |
| MPs | 29.3 ± 2.7 ab | 2.1 ± 0.09 a | 37.1 ± 5.20 a | 2.5 ± 0.2 a | 85.8 ± 8.3 a |
| Cd | 25.6 ± 2.4 b | 2.3 ± 0.10 a | 23.2 ± 2.08 b | 1.2 ± 0.2 b | 69.9 ± 3.4 a |
| Cd + MPs | 39.9 ± 3.8 a | 2.1 ± 0.10 a | 22.9 ± 4.80 b | 3.1 ± 0.4 a | 72.3 ± 3.6 a |
| Anova p values | 0.001 | 0.5948 | 0.0125 | 0.0023 | 0.4138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Poblete, A.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. https://doi.org/10.3390/plants11040536
Pinto-Poblete A, Retamal-Salgado J, López MD, Zapata N, Sierra-Almeida A, Schoebitz M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants. 2022; 11(4):536. https://doi.org/10.3390/plants11040536
Chicago/Turabian StylePinto-Poblete, Andrés, Jorge Retamal-Salgado, María Dolores López, Nelson Zapata, Angela Sierra-Almeida, and Mauricio Schoebitz. 2022. "Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants" Plants 11, no. 4: 536. https://doi.org/10.3390/plants11040536
APA StylePinto-Poblete, A., Retamal-Salgado, J., López, M. D., Zapata, N., Sierra-Almeida, A., & Schoebitz, M. (2022). Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants, 11(4), 536. https://doi.org/10.3390/plants11040536










