Abstract
Laurus nobilis, Salvia officinalis and Salvia sclarea essential oils (EOs) and hydrolates (HYs) were investigated to define their chemical compositions and biological properties. Gas-chromatography/Mass-spectrometry (GC/MS) and Headspace-GC/MS (HS-GC/MS) techniques were used to characterize the liquid and vapor phase chemical composition of EOs and HYs. 1,8-Cineole (42.2%, 33.5%) and α-pinene (16.7%, 39.0%) were the main compounds of L. nobilis EO; 1,8-cineole (30.3%, 48.4%) and camphor (17.1%, 8.7%) were for S. officinalis EO; linalyl acetate (62.6%, 30.1%) and linalool (11.1%, 28.9%) were for S. sclarea EO for the liquid and vapor phase, respectively. Chemical profile of HYs was characterized by 1,8-cineole (65.1%, 61.4%) as a main constituent of L. nobilis and S. officinalis HYs, while linalool (89.5%) was the main constituent of S. sclarea HY. The antioxidant activity of EOs and HYs was carried out by DPPH and ABTS assays and antimicrobial properties were also investigated by microdilution and the disc diffusion method for liquid and vapor phase against five different bacterial strains such as Escherichia coli ATCC 25922, Pseudomonas fluorescens ATCC 13525 and Acinetobacter bohemicus DSM 102855 among Gram-negative and Bacillus cereus ATCC 10876 and Kocuria marina DSM 16420 among Gram-positive. L. nobilis and S. officinalis EOs demonstrated considerable antibacterial activity, while S. sclarea EO proved to be less effective. Agar diffusion method and vapor phase test showed the EOs activity with the biggest halo inhibition diameters against A. bohemicus and B. cereus. A remarkably high antioxidant activity was determined for L. nobilis showing low EC50 values and also for S. sclarea; good EO results were obtained in both of the used assays. S. officinalis EC50 values were slightly higher to which corresponds to a lower antioxidant activity. Concerning the HYs, the EC50 values for L. nobilis, S. officinalis and S. sclarea were remarkably high corresponding to an extremely low antioxidant activity, as also obtained by expressing the values in Trolox equivalent antioxidant capacity (TEAC).
1. Introduction
Medicinal and aromatic plants, characterized by a particular aroma and flavor, are among the first remedies that humans used to treat diseases and are a source of biologically active molecules [1,2,3]. Essential oils (EOs) are produced by the plant secondary metabolism and mainly obtained by the steam distillation process [4]. In the EOs isolation process, Hydrolates (HYs) are also obtained as products of the steam distillation, and a small amount of EO constituents dissolve in HYs. Precious oxygenated compounds, which provide specific organoleptic properties and flavor, as well as biological activity, make them useful for food and cosmetic industries [5,6].
Laurus nobilis, known as laurel, is a perennial shrub which is widespread in temperate and warm regions, especially in the Mediterranean area where is cultivated as an ornamental plant reaching considerable size [7]; it grows spontaneously in the environment and is a diagnostic taxon of many habitats such as Lauro nobilis–Sambucion nigrae, Lauro nobilis-Tilion platyphylli, and Lauro nobilis–Ulmion minoris [8,9]. Laurel belongs to the Lauraceae family, which comprises approximately 2500 species, and was well-known in antiquity for being a symbol of peace and victory in Greece and ancient Rome, both in the military and in sport competitions, with its intertwined branches to create crowns. The use of laurel in foods is nowadays diffused and its particularly odorous leaves and berries are added to various dish preparations. In previous studies, the chemical composition of liquid phase L. nobilis EO was investigated and 1,8-cineole, α-terpinyl acetate, α-pinene and α-terpineol resulted among the main components [10,11,12]. During time laurel gained a considerable importance in traditional medicine [13] and its leaf extracts or EO were used as gastroprotective and antidiarrheal [14,15], analgesic and anti-inflammatory [16], as antidiabetic and to decrease the risk factors of cardiovascular diseases [17,18]. The cytotoxic, antibacterial and antioxidant activities were also investigated, and different authors reported anticancer, antioxidant, insecticidal and molluscidal, antidiabetic, antimicrobial and antifungal activities by L. nobilis [19].
Among the Lamiaceae family, Salvia genus, whose name comes from the Latin “salvare” which means “to heal, to cure,” has always been considered to possess numerous medicinal properties in traditional medicine of Asia and the Middle East. About 1000 species belong to this genus and they are herbaceous, suffruticosus or shrubby perennial plants displaying a remarkable diversity in growth forms, in floral morphology and pollination biology, and in secondary metabolites production [20]. The chemical composition of Salvia species is particularly rich in sesquiterpenoids, diterpenoids, triterpenoids, steroids, polyphenols and others [21,22], and bioactive molecules were reported to exert proapototic and anticancer activities, to act in prevention and treatment of cardiovascular diseases, to be therapeutic in liver diseases, to be active in the central nervous system as a potential cognitive protective, and to prevent or disaggregate beta amyloid and fibrils as inhibitors of acetylcholinesterase [23,24,25,26,27,28,29,30,31].
To our knowledge this is the first study on the liquid and vapor phase chemical characterization of L. nobilis, S. officinalis and S. sclarea EOs and their HYs carried out by the headspace-gas-chromatography/mass-spectrometry (HS-GC/MS) technique. The antibacterial activity of EOs and HYs was also investigated by microdilution and disc diffusion methods; furthermore, DPPH and ABTS assays were performed to evaluate their antioxidant activities.
2. Results
2.1. Liquid and Vapor Phase EOs Chemical Composition
Liquid and vapor phase chemical composition of L. nobilis, S. officinalis and S. sclarea was described by Gas Chromatography-Mass Spectrometry (GC/MS) and Head-Space (HS)-GC/MS analysis. All components identified in L. nobilis EO (26 in the liquid phase and 14 in the vapor phase) are reported in Table 1. There was a prevalence of monoterpenes over sesquiterpenes which were missing in the vapor phase. In particular, 1,8-cineole was the most abundant compound (42.2%, 33.5%) followed by α-pinene (16.7%, 39.0%) and β-pinene (13.6%, 10.9%) in the liquid and vapor phase, respectively.

Table 1.
Chemical composition (%) of liquid and vapor phase L. nobilis essential oils (EO).
Twenty-three compounds were identified in the liquid phase and twelve in the vapor phase of S. officinalis EO and they are listed in Table 2. The chemical composition of EO was dominated by oxygenated monoterpenes (67.7% and 68.4%) followed monoterpenes hydrocarbons (19.1% and 31.4%) in the liquid and vapor phase, respectively. The main constituents of both phases were 1,8-cineole (30.3%, 48.4%), camphor (17.1%, 8.7%), α-thujone (9.7%, 7.0%), camphene (7.9%, 2.2%) and chrysanthenone (6.8%, 3.9%). β-Caryophyllene (0.1%) was the only sesquiterpene also detected in the vapor phase.

Table 2.
Chemical composition (%) of liquid and vapor phase S. officinalis EO.
S. sclarea EO chemical composition is reported in Table 3. Fifteen and thirteen compounds were identified in the liquid and vapor phase, respectively. Oxygenated monoterpenes were the most abundant compounds (76.6% and 62.9%), among which linalyl acetate reached the higher percentages in both phases (62.6% and 30.1%) followed by linalool (11.1%, 28.9%). β-Copaene (6.7%) and β-cubebene (5.0%) were the main sesquiterpene hydrocarbons detected in the liquid and vapor phase EO, respectively.

Table 3.
Chemical composition (%) of liquid and vapor phase S. sclarea EO.
2.2. Vapor Phase HYs Chemical Composition
By HS-GC/MS analysis, the composition of the vapor phase of all HYs was described. Seven compounds were identified in L. nobilis and in S. officinalis and only two in S. sclarea, and their percentage values are reported in Table 4. 1,8-Cineole (65.1%, 61.4%) was the main compound in both L. nobilis and S. officinalis HYs, followed by α-thujone (11.1%) and camphor (22.5%) in the first and second HY, respectively. On the other hand, linalool (89.5%) and α-terpineol (10.5%) were the only two compounds detected in S. sclarea HY. Sesquiterpenes compounds were missing in all the HYs (Figure 1). The chromatograms of HYs analyses were also reported (Figure 2, Figure 3 and Figure 4).

Table 4.
Chemical composition (%) of vapor phase L. nobilis, S. officinalis and S. sclarea hydrolates (HYs).
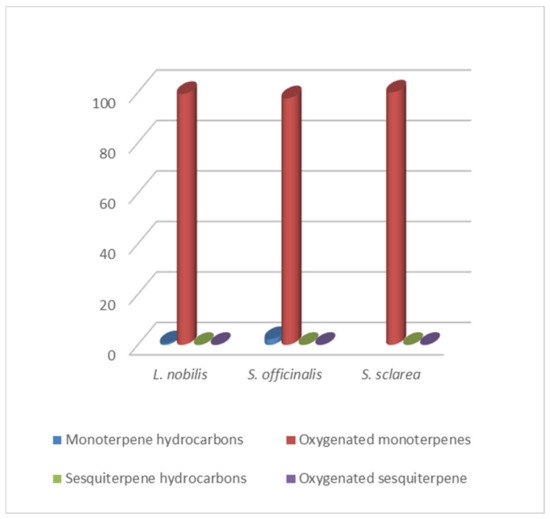
Figure 1.
Bar graph of terpene hydrocarbons percentage trend in HYs.
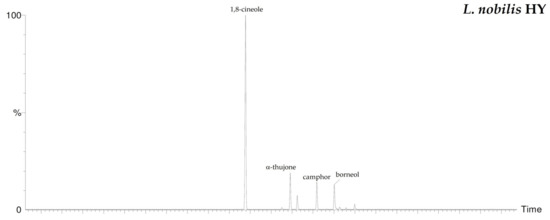
Figure 2.
HS/GC-FID chromatograms of L. nobilis HY.
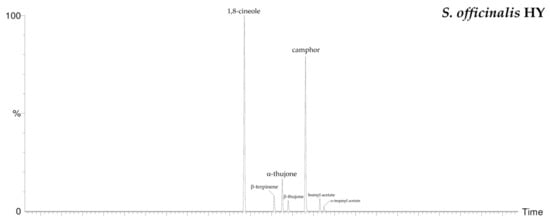
Figure 3.
HS/GC-FID chromatograms of S. officinalis HY.
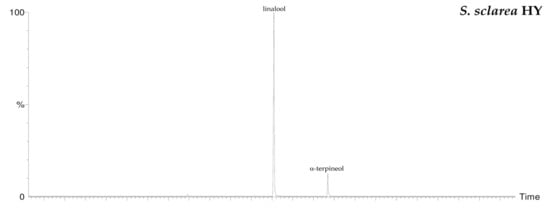
Figure 4.
HS/GC-FID chromatograms of S. sclarea HY.
2.3. Antibacterial Activity of L. nobilis, S. officinalis and S. sclarea EOs and HYs
To define the bactericidal and inhibitory activities of the investigated aromatic plants, microdilution assay, expressed as MIC (Minimum Inhibitory Concentration) and MBC (Minimal Bactericidal Concentration) and agar diffusion method and vapor phase test expressed as mm of inhibition diameter of the halo for the liquid (IZ) and vapor phase (VIZ), respectively, were carried out. Furthermore MBC/MIC ratios, which consider an agent as bacteriostatic when the MBC/MIC ratio > 4 and as bactericidal when the MBC/MIC ratio ≤ 4 [32], were reported. In Table 5, L. nobilis antibacterial activity against the tested bacterial strains is listed. EO showed MIC and MBC values of 3.13% against E. coli and P. fluorescens, MIC value of 0.78% and MBC value of 1.56% against A. bohemicus, MIC value of 1.56% and MBC value of 6.25% against K. marina, and MIC and MBC values of 1.56% against B. cereus. For all bacterial trains, MBC/MIC ratios were < 4, confirming a bactericidal activity. Concerning the inhibition halos obtained by the agar diffusion method and by the vapor phase test, the EO IZs were 18.67 ± 2.31 mm against E. coli and 7.33 ± 0.58 mm against A. bohemicus while, concerning the corresponding EO VIZ, no antibacterial activity was observed for both strains. On the contrary, EO activities against A. bohemicus, K. marina and B. cereus were observed for both liquid and vapor phase with the following inhibition halos diameter: 17.67 ± 2.31 mm, 24.67 ± 3.21 mm and 37.67 ± 2.08 mm for IZs, respectively and 45.67 ± 4.04 mm, 26.67 ± 2.52 mm and 47.33 ± 2.52 mm for VIZs, respectively. No activity was observed for L. nobilis HY in the microdilution assay, agar diffusion method and vapor phase test.

Table 5.
Antibacterial activities for EO and HY of L. nobilis.
In Table 6, the treatment with S. officinalis EO showed MIC and MBC values of 6.25% for E. coli and P. fluorescens, while MIC and MBC values were lower (0.39% and 0.78%, respectively) for A. bohemicus. The antibacterial activity against K. marina was 1.56% and 0.78% against B. cereus for both MIC and MBC values, respectively. MBC/MIC ratios defined the S. officinalis EO as bactericidal against all bacterial strains (MBC/MIC < 4). An agar diffusion method showed IZ diameters for liquid phase of 16.67 ± 1.53 mm, 8.00 ± 1.00 mm, 13.67 ± 1.53 mm, 38.33 ± 2.89 mm and 24.33 ± 3.06 mm against E. coli, P. fluorescens, A. bohemicus, K. marina and B. cereus, respectively. In the vapor phase test S. officinalis EO was active against A. bohemicus, K. marina and B. cereus with 21.67 ± 1.53 mm, 26.67 ± 1.15 mm and 23.00 ± 1.53 mm, respectively; null activity was found against E. coli and P. fluorescens. As for L. nobilis EO, S. officinalis HYs were not active against the tested bacterial strains.

Table 6.
Antibacterial activities for EO and HY of S. officinalis.
The results for S. sclarea EO and HYs antibacterial activities are reported in Table 7. MIC and MBC values for the EO were 12.50%, 1.56%, 6.25%, and 6.25% for E. coli, P. fluorescens, K. marina and B. cereus, respectively. MBC/MIC ratios defined the S. sclarea EO as bactericidal against the sensible bacterial strains. A. bohemicus was not affected by the S. sclarea EO treatment. Inhibition halos were present in the treatment with S. sclarea EO against A. bohemicus, B. cereus and K. marina with 12.67 ± 2.52 mm, 18.67 ± 0.58 mm and 10.67 ± 1.15 mm for liquid phase, while E. coli and A. bohemicus results were not affected. In the vapor phase test, S. sclarea EO did not inhibit the bacterial growth for all the tested strains, and both the liquid and the vapor phase HY were not active.

Table 7.
Antibacterial activities for EO and HY of S. sclarea.
2.4. Antioxidant Activity of L. nobilis, S. officinalis and S. sclarea for EOs and HYs
Chemical assays were used to determine the ability of the tested EOs and HYs to act as a scavenger of free radicals evaluating the decay of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+•) radicals. L. nobilis, S. officinalis and S. sclarea EOs antioxidant activity and that of the corresponding HYs are reported in Table 8, Table 9 and Table 10. In both performed tests—DPPH and ABTS assays—L. nobilis essential oil showed the highest antioxidant activity with low IC50 values (0.18 ± 0.04 µg/mL and 2.58 ± 0.08 µg/mL, respectively), while higher IC50 values (218.10 ± 29.60 µg/mL and 391.38 ± 8.72 µg/mL, respectively) were reported for its HY. Confirming this, TEAC values (Trolox equivalent antioxidant capacity) for L. nobilis EO were high (92.97 ± 6.76 mol/mg and 158.49 ± 5.15 mol/mg) for DPPH and ABTS assays, respectively, while the HY exhibited lower values (0.08 ± 0.01 mol/mg and 1.19 ± 0.21 mol/mg, respectively) (Table 8).

Table 8.
Effects of EO and HY of L. nobilis in DPPH and ABTS antioxidant assays.

Table 9.
Effects of EO and HY of S. officinalis in DPPH and ABTS antioxidant assays.

Table 10.
Effects of EO and HY of S. sclarea in DPPH and ABTS antioxidant assays.
The radical scavenging activity for S. officinalis EO was calculated and IC50 values were 14.10 ± 0.17 µg/mL and 43.64 ± 2.51 µg/mL, while the corresponding HY showed higher values (135.58 ± 33.32 µg/mL and 551.38 ± 17.33 µg/mL) for DPPH and ABTS assays, respectively. Furthermore, S. officinalis EO showed TEAC values of 1.28 ± 0.00 mol/mg and 9.26 ± 0.55 mol/mg, while its HY showed TEAC values of 0.14 ± 0.03 mol/mg and 0.76 ± 0.02 mol/mg for DPPH and ABTS assays, respectively (Table 9).
In regards to S. sclarea, EO displayed a high antioxidant activity resulting in the IC50 values of 7.79 ± 1.06 µg/mL and 2.26 ± 0.05 µg/mL in DPPH and ABTS, respectively, while the corresponding values for the HY were 200.43 ± 28.46 µg/mL and 479.27 ± 7.89 µg/mL. EO showed TEAC values of 2.34 ± 2.36 mol/mg and 186.23 ± 4.30 mol/mg, while the corresponding HY showed values of 0.09 ± 0.01 mol/mg and 0.87 ± 0.01 mol/mg in DPPH and ABTS assays, respectively (Table 10).
3. Discussion
GC/MS and HS-GC/MS techniques were carried out to describe the chemical composition of EOs (liquid and vapor phase) and HYs. In particular, using the headspace autosampler, as carried out in this case, no solvent extraction process is necessary for the analysis of HYs. The chemical characterization of L. nobilis EO showed a high eucalyptol content for both the liquid and vapor phase (30.4% and 48.4%) as well as in the HY (65.1%), and these data agreed with the reported values [33,34,35].
In our work, 1,8-cineole was the main detected component in the liquid (30.4%) and vapor (48.4%) phase of S. officinalis EO and HY (61.4%). S. officinalis EOs collected from north of Tunisia, Hatay (Turkey) and Albania showed 1,8-cineole (33.27%, 60.72% and 26.9%, respectively) as the main component [36,37,38]. On the contrary, Baydar et al. [39] reported α-thujone (20.06%) and camphor (43.38%) as the major compounds of EO and HY, respectively, in plants from Isparta, Turkey. α-Thujone was also the principal constituent in EOs from Mexico and California (18.8% and 27.4%) [38], while in two Italian sites eucalyptol (from 40.22% to 60.94%) was the chemical compound characterizing the related wild Salvia fruticosa subsp. thomasii [22].
Chemical volatile composition of S. sclarea EO was characterized by linalyl acetate as the major compound (62.6% liquid phase, 30.1% vapor phase), while HY was characterized by linalool (89.5%). Percentage values of linalyl acetate and linalool in S. sclarea EOs vary according to the geographical origin: Serbia (52.83%; 18.18%) and Turkey (11.30%; 8.50%), respectively [40,41], whereas linalyl acetate reached 34.89% and 52.7% and linalool reached 28.91% and 25.65% (inflorescences at full-flowering and at the beginning of seed ripeness stages, respectively) in S. sclarea EO from Sicily (Italy) [42].
Variations in the chemical composition of EOs depend on many factors such as the quality of the plant material, the part of the plant used for extraction, the characteristics of the climate and soil, the harvest time, as well as methods and times extraction used for the production and analysis [39,43,44,45,46,47]. Depending on the distillation time and method, the chemical profile of HYs can also vary [48].
Our biological assays, the microwell dilution method, agar diffusion method and vapor phase test, showed antibacterial activity of the investigated aromatic plants against the tested bacterial strains. L. nobilis EO showed a high antibacterial activity with MIC values ranging from 0.78% to 3.13% and MBC values from 1.56% to 3.13%. For all bacterial strains the IZ halos were detected (from 37.67 ± 2.08 mm to 7.33 ± 0.58 mm) and the vapor phase proved to be even more active against A. bohemicus (VIZ 45.67 ± 4.04 mm), K. marina (VIZ 26.67 ± 2.52 mm) and B. cereus (VIZ 47.33 ± 2.52 mm). No VIZs were detected against the E. coli and P. fluorescens.
Concerning S. officinalis EO, the obtained results confirmed the antibacterial properties of the EO, showing MIC values from 0.39% to 6.25%, MBC values from 0.78% to 6.25% and IZ values from 38.33 ± mm to 8.00 ± mm. As for L. nobilis, a high antibacterial activity for the EO vapor phase was recorded against A. bohemicus, K. marina and B. cereus with the inhibition halos of 21.67 ± 1.53 mm, 26.67 ± 1.15 mm and 23.00 ± 3.61 mm; E. coli and P. fluorescens were not affected by the treatment.
Interestingly, the larger inhibition halos were obtained in L. nobilis EO against A. bohemicus and B. cereus in vapor phase tests and in S. officinalis EO against K. marina, both in the liquid and vapor phase. The agar diffusion test showed activities for L. nobilis EO against E. coli and P. fluorescens with a smaller diameter.
These findings highlighted that L. nobilis and S. officinalis EOs were active against E. coli and P. fluorescens growth; Gram-negative bacteria, usually more resistant than Gram-positive for the presence of a complex outer membrane rich in lipopolysaccharide, does not allow the diffusion of hydrophobic molecules present in the essential oils [49]; however, some exceptions were reported [50,51].
S. sclarea EO was less effective, showing MIC and MBC values from 12.5% and 1.56%, and no activity was reported against P. fluorescens. The agar diffusion test revealed that the EO was active against A. bohemicus, K. marina and B. cereus with smaller IZ halos. On the contrary, the vapor phase test for S. sclarea revealed no activity for all bacterial strains.
On the other hand, the investigated aromatic plant HYs were not active against the bacterial strains at tested concentrations. However, it is known that HYs have a lower biological activity than the corresponding essential oils [52] and their potentially antimicrobial activity is also significantly influenced by the distillation method used [33].
As reviewed by Alejo-Armijo et al. [19], L. nobilis possess numerous biological properties and the antimicrobial activities were demonstrated against a broad spectrum of human pathogenic microorganisms, pathogens and spoilage bacteria associated with food [37,53,54]. Its antimicrobial activity is probably related to the high percentage of 1,8-cineole (42.2%) and α-pinene (39.0%) detected in the liquid and vapor phase. 1,8-Cineole was found to be active against the development of biofilms formed by the methicillin-resistant strains [55], and its antibacterial activity is enhanced when combined with carvacrol [56]. α-Pinene also demonstrated antibacterial activity [57].
In the present investigation, S. officinalis EO was also characterized by 1,8 cineole (30.4% and 48.4%) as the most abundant compound both in the liquid and in vapor phase, while S. sclarea EO, which was less active among the aromatic plants investigated, was rich in linalyl acetate (62.6%) and linalool (28.9%) in the liquid and vapor phase, respectively.
The chemical composition of different aromatic and medicinal herb EOs and their antimicrobial activities against human pathogenic bacteria were studied and a scale of antibacterial activities of their main compounds was defined; the linalyl acetate and linalool were active to a minor extent [58,59]. The liquid and the vapor phase antibacterial activity reported in this study for L. nobilis, S. officinalis and S. sclarea EOs was probably due to the synergistic action of their constituent compounds. In this regard, we assumed that the observed antibacterial action may be explained by the synergistic effect of the different components and/or by the presence of other active molecules, even in small amounts.
The development of antibiotic resistant strains caused the failure of antibiotic therapies with serious consequences on animal and human health. The use of EOs as antibacterial agents opens the prospects for new molecules and new strategies to overcome the infectious; similarly, in crop production the use of such compounds could be an alternative to chemicals against various bacteria and fungi [60,61]. In this view, deeper considerations should be given regarding the EOs mechanism of action based on long-term exposure to pathogens and the synergistic effects for the use of EOs-based antibacterial complexes [62].
In regards to the antioxidant activity, L. nobilis EO was characterized by low EC50 values, 0.18 ± 0.04 µg/mL and 2.58 ± 0.08 µg/mL (DPPH and ABTS assays, respectively), which revealed a very high antioxidant power. S. sclarea EO gave good results (7.79 ± 1.06 µg/mL and 2.26 ± 0.05 µg/mL) in both assays, respectively. Regarding S. officinalis, IC50 values were slightly higher (7.79 ± 1.06 µg/mL and 2.26 ± 0.05 µg/mL for DPPH and ABTS, respectively) which correspond to lower antioxidant activity. Concerning the HYs, the obtained EC50 values were remarkably high in the DPPH and ABTS assays which correspond to an extremely low antioxidant activity, as also obtained by expressing the values in TEAC.
Different papers reported the antioxidant activities of aromatic plant EOs due to the richness of their chemical composition [2,63,64]. L. nobilis EOs from different regions possess antioxidant properties [65,66,67], as well as Salvia species EOs [68].
Particularly, in our investigation oxygenated monoterpenes were the most abundant in L. nobilis (liquid phase) and S. sclarea (liquid and vapor phase) EOs, known for their high antioxidant activity [69,70,71].
4. Materials and Methods
4.1. Materials
EOs and HYs from inflorescences of L. nobilis, S. officinalis and S. sclarea cultivated in Tuscany (no wild site), Italy and obtained by steam distillation for 3 h (S. officinalis and S. sclarea) and 7 h (L. nobilis) extraction time, were directly provided by “èssenziale” Azienda Agricola, San Donato in Poggio (FI), Italy. Data of collection of plants: January 2020 for L. nobilis and June 2020 for S. officinalis and S. sclarea. All plLB Broth with Agar and Thiazolyl Blue Tetrazolium Bromide (MTT) were from Merck (Darmstadt, Germany). Gentamicin sulfate was purchased from Biochrom PAN-Bio-Tech GmbH (Aidenbach, Germany). Methanol, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and potassium persulfate (K2S2O8) were from Merck.
4.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
To describe the chemical composition of the EOs, a gas chromatograph with a flame ionization detector (FID) directly coupled to a mass spectrometer (MS) Perkin Elmer Clarus 500 model (Waltham, MA, USA) was used. The GC was equipped with a Varian Factor Four VF-1 capillary column. Helium was used as carrier gas at a flow rate of 1 mL/min. The injector was set to a 280 °C and the oven temperature program was as follows: from 60 °C ramped up to 220 °C at a rate of 6 °C min−1, and finally isothermal at 220 °C for 20 min. For liquid injections, 1 μL of each EO was diluted in 1 mL of methanol and 1 μL of the solution was injected. The Electron Impact-Mass Spectrometer (EI-MS), mass spectra were recorded at 70 eV (EI) and were scanned in the range 40–500 m/z. Ion source and the connection parts temperature was 220 °C. The GC-TIC mass spectra were obtained by the TurboMass data analysis software (Perkin Elmer). The identification of components was performed by matching their mass spectra with those stored in the Wiley and NIST 02 mass spectra libraries database. Furthermore, the linear retention indices (LRIs), (relative to C8–C30 aliphatic hydrocarbons, injected in the column at the same operating conditions described above) were calculated and compared with available retention data present in the literature. Relative percentages of all identified components were obtained by peak area normalization from GC-FID chromatograms without the use of an internal standard or correction factors and were expressed in percentages. All analyses were performed three times.
4.3. Head Space GC-MS Analysis
To describe the volatile fraction of the EOs and HYs vapor phase, a Perkin-Elmer Headspace Turbomatrix 40 (Waltham, MA, USA) autosampler connected to GC-MS was used for the headspace analysis. The operative conditions were performed as previously described [72,73,74]. About 1 mL of EO and 2 mL of HY were placed separately in 20 mL vials sealed with headspace PTFE-coated silicone rubber septa and caps. Quantification of identified compounds was performed by GC-FID in the same conditions described in Section 4.2.
4.4. Antibacterial Activities
To define the antibacterial activity of the liquid and vapor phase of L. nobilis, S. officinalis and S. sclarea, EOs and HYs with different methods were employed: the Minimal Inhibitory Concentration (MIC), the Minimal Bactericidal Concentration (MBC), the agar diffusion method and the Vapor Phase Test (VPT).
4.4.1. Bacterial Strains and Growth Conditions
Three Gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas fluorescens ATCC 13525 and Acinetobacter bohemicus DSM 102855) and two Gram-positive bacteria (Kocuria marina DSM 16420 and Bacillus cereus ATCC 10876) were used to test the antibacterial activity of L. nobilis, S. officinalis and S. sclarea EOs and HYs. All bacterial strains were obtained by growing cultures from the collection of the Plant Cytology and Biotechnology Laboratory (Tuscia University) grown at 26 °C (for P. fluorescens, A. bohemicus and B. cereus) and 37 °C (for K. marina and E. coli) in Lysogeny Broth (LB) agar.
4.4.2. Microwell Dilution Method
To screen antimicrobial activities of EOs and their corresponding HYs, Minimum Inhibitory Concentration (MIC) was carried out according to the microwell dilution method in triplicate. All the matrices were diluted in LB broth (from 6.25% to 0.003%). DMSO controls, growth controls without treatments, positive controls with gentamicin (100 µg/mL to 0.049 µg/mL) and sterility controls without bacteria were added to 96 microwell plates. Bacteria at 106 CFU/mL were added to each well, except to the sterility control, and were two-fold diluted. The microplates were incubated for 24 h at the corresponding growth temperature. The visualization of the bacterial growth was observed by adding 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (200 µg/mL, MTT) to each well [75,76].
Minimum Bactericidal Concentration (MBC) was carried out. Plated 10 µL of the last four dilutions from the microwell dilution method in which no bacteria growth was observed on LB agar following 24 h of incubation. The MBC value was determined by an antibacterial agent concentration for which no growth on agar was observed. The assay was carried out in triplicate. To determine the activity of the EOs and the corresponding HYs, the MBC/MIC ratio was reported. The ratio MBC/MIC > 4 reveled bacteriostatic activity, while the ratio MBC/MIC ≤ 4 revealed bactericidal activity for the tested antimicrobial agent [32].
4.4.3. Agar Diffusion Method and Vapor Phase Test (VPT)
The antibacterial activities of L. nobilis, S. officinalis and S. sclarea EOs and HYs liquid and vapor phase were investigated, determining the diameter of the inhibition halo of the bacteria growth on agar petri dishes. Concerning the agar diffusion method, sterile disks (6 mm diameter, Oxoid), impregnated with 10 µL of samples, were placed on LB broth agar surface where the bacterial strains (approximately 108 Colony Forming Unit/mL—CFU/mL) had been sown. As a positive control, 2 µL of gentamicin (10 mg/mL) was used. The petri plates were incubated for 24 h at the corresponding growth temperature, and the diameter of the inhibition halos was recorded by a vernier caliper rule [75].
The antibacterial activity of the vapor phases was evaluated by the disk volatization method. LB agar was poured into Petri plates where each bacterial suspension (108 CFU/mL) was plated once it had solidified. A lower amount of agar was poured into Petri plate cover where 6 mm sterile disks had been placed and soaked with 10 µL of the EOs and the corresponding HYs. To seal the Petri plates in order to prevent any vapor leakage, liquid agar was added in the space between the cover and the base. The Petri plates were incubated for 24 h in the upside-down position at the bacterial growth temperature. The inhibition halos were measured by a vernier caliper rule. Negative controls were carried out without the essential. Agar diffusion method and VPTs were carried out in triplicate for each bacterial strain; the means and the respective standard deviations (SD) of the measured halo were reported [72,77].
4.5. Antioxidant Activity
4.5.1. DPPH Radical Scavenging Activity Assay
To examine the radical scavenging capacity of the L. nobilis, S. sclarea, S. officinalis EOs and their HYs, a DPPH (2,2-diphenyl-1-picrylhydrazyl radical) assay was used [78]. The working solution was made by mixing 3.9 mg of DPPH reagent with 50 mL of methanol. The assay was carried out in 96-well plates in which wells with 100 μL of 12 geometric dilutions in methanol of each sample were added to 100 μL of DPPH solution. As controls, geometric dilutions of the samples without DPPH, Trolox geometric dilutions and blank DPPH were also measured. The plates were incubated for 30 min in dark conditions. The absorbance decreases were measured at 517 nm using a Tecan Sunrise™ UV-vis spectrophotometer. DPPH radical scavenging capacity was considered by the following relationship:
where % A.A—percentage of antioxidant activity, AD—absorbance of blank DPPH control, AS—absorbance of the sample.
The IC50 values of the samples (mg/L) and Trolox (μM) were calculated using calibration curves by plotting the % A.A against sample concentrations. The IC50 parameter of the samples were reported as the required concentration to scavenge 50% of DPPH radical [79]. The activity assay was repeated three times.
TEAC value, which correlates to the concentration of Trolox with the dry weigh of the tested sample, was calculated from the Trolox IC50 (µM) and the sample IC50 (mg/L):
4.5.2. ABTS Radical Scavenging Activity Assay
The radical activities of the L. nobilis, S. sclarea and S. officinalis EOs and their HYs were also calculated using the pre-formed radical monocation of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+•), as reported by Pellegrini et al. [80], with some variations. The working aqueous solution was made by mixing potassium persulfate (140 mM) with ABTS reagent (7 mM). The solution was kept for 16 h at room temperature in the dark. Before use, the ABTS+• solution was diluted in ethanol 100% to give an absorbance of 0.70 ± 0.02 at 734 nm. In total, 20 μL of each sample was added to 1980 μL of working solution; as a control, 20 μL of ethanol were used. Therefore, the obtained solutions were incubated for 5 min at room temperature and the absorbances were measured at 734 nm by a Jasco V-630 UV-Visible spectrophotometer and operating Spectra Manager™ software. The final values expressed as a percentage of inhibition against samples concentrations were likened against a standard curve of Trolox [81]. The concentrations used for Trolox range from 0.15625 μM to 2.5 μM.
4.6. Statistical Analysis
The results were expressed as means ± standard deviation (SD) and the ANOVA test (one-way analysis of variance test) using GraphPad Prism software (GraphPad Prism 5.0, GraphPad Software Inc., San Diego, CA, USA) was used to evaluate statistical discrepancies between the groups (p values < 0.05).
5. Conclusions
To the best of our knowledge, this is the first work reporting the liquid and vapor phase chemical composition of L. nobilis, S. sclarea and S. officinalis EOs from the Tuscany region (Italy) investigated by GC-MS and HS-GC/MS techniques to describe the volatile profile of the respective HYs by HS-GC/MS. The antibacterial activity was also evaluated by microdilution and the disc diffusion method, as well as the antioxidant activity by DPPH and ABTS assays. The tested EOs exhibited antibacterial and antioxidant activities, particularly L. nobilis vapor phase, which determined the major inhibition halo. HYs showed to not be antibacterial at the tested concentrations, while the antioxidant properties were extremely low compared to that of the EOs. The obtained results show the potential use of EOs as possible natural antibacterial substances and sources of bioactive molecules. To assess HY biological properties further, tests will be run on other environmental bacterial strains. From a general point of view, our findings confirm the importance of bioactive plant molecules, and as a consequence it will be important to maintain productivity and stability of cultivated plants. In this view, in situ and ex situ conservation of the progenitors of domesticated plants, crop-wild relatives, become necessary, and the gene bank to maintain biodiversity should be improved [82,83,84].
Author Contributions
Conceptualization, E.O., V.L.M., S.G.; investigation, S.G., V.L.M., M.Z.; data curation, E.O., V.L.M., S.V., S.G.; writing—original draft preparation, E.O.; V.L.M., S.G.; writing—review and editing, E.O., S.G.; funding acquisition, A.T., S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to “èssenziale” Azienda Agricola di Claudio Gori, San Donato in Poggio (FI), Italy, for providing EOs and HYs from inflorescences of L. nobilis, S. officinalis and S. sclarea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2017, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives; Academic Press: Cambridge, MA, USA, 2020; pp. 1–18. [Google Scholar]
- Giacometti, J.; Kovacevic, D.B.; Putnik, P.; Gabric, D.; Bilusic, T.; Kresic, G.; Stulic, V.; Barba, F.J.; Chemat, F.; Barbosa-Canovas, G.; et al. Extraction of bioactive compounds and essential oils from Mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245e262. [Google Scholar] [CrossRef] [PubMed]
- Rajeswara, R.B.R. Hydrosols and water-soluble essential oils of aromatic plants: Future economic products. Indian Perfum. 2012, 56, 29–33. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Analysis of the hydrosol aroma of Indian oregano. Med. Aromat. Plants 2012, 1, 7. [Google Scholar] [CrossRef]
- Batool, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Bay Leaf. Med. Plants South Asia 2019, 5, 63–74. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia, S.; Biscotti, N. Forest biodiversity of the Gargano Peninsula and a critical revision of the syntaxonomy of the mesophilous woods of southern Italy. Fitosociologia 2008, 45, 93–127. [Google Scholar]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Poldini, L.; Sburlino, G.; Vagge, I.; Venanzoni, R. New syntaxonomic contribution to the Vegetation Prodrome of Italy. Plant Biosyst. 2015, 149, 603–615. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Kilic, A.; Hafizoglu, H.; Kollmannsberger, H.; Nitz, S. Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. J. Agric. Food Chem. 2004, 52, 1601–1606. [Google Scholar] [CrossRef]
- Moghtader, M.; Farahm, A. Evaluation of the antibacterial effects of essential oil from the leaves of Laurus nobilis L. in Kerman Province. J. Microbiol. Antimicrob. 2013, 5, 13–17. [Google Scholar] [CrossRef]
- Chahal, K.K.; Kaur, M.; Bhardwaj, U.; Singla, N.; Kaur, A.; Kaur, A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. J. Pharmacogn. Phytochem. 2017, 6, 1153–1161. [Google Scholar]
- Afifi, F.U.; Khalil, E.; Tamimi, S.O.; Disi, A. Evaluation of the gastroprotective effect of Laurus nobilis seeds on ethanol induced gastric ulcer in rats. J. Ethnopharmacol. 1997, 58, 9–14. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Abdulla, F.A.; Kaddumi, E.G.; Abdalla, S.S. Antidiarrheal activity of Laurus nobilis L. leaf extract in rats. J. Med. Food 2012, 15, 51–57. [Google Scholar] [CrossRef]
- Sayyah, M.; Valizadeh, J.; Kamalinejad, M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole-and maximal electroshock-induced seizures. Phytomedicine 2002, 9, 212–216. [Google Scholar] [CrossRef]
- Khan, A.; Zaman, G.; Anderson, R.A. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J. Clin. Biochem. Nutr. 2009, 44, 52–56. [Google Scholar] [CrossRef]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. 2013, 12, 367–379. [Google Scholar]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of Laurel tree (Laurus nobilis). Nat. Prod. Comm. 2017, 12. [Google Scholar] [CrossRef]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild officinal species: A pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Patal, K.; Hu, R.; Chuang, S.; Zhang, G.; Zheng, J. Tanshinones inhibit amyloid aggregation by amyloid-β peptide, disaggregate amyloid fibrils, and protect cultured cells. ACS Chem. Neurosci. 2013, 4, 1004–1015. [Google Scholar] [CrossRef]
- Akaberi, M.; Mehri, S.; Iranshahi, M. Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia 2015, 100, 118–132. [Google Scholar] [CrossRef]
- Hung, Y.C.; Pan, T.L.; Hu, W.L. Roles of reactive oxygen species in anticancer therapy with Salvia miltiorrhiza Bunge. Oxid. Med. Cell. Longev. 2016, 2016, 5293284. [Google Scholar] [CrossRef]
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R&D 2017, 17, 53–64. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Piasecka, A.; Kujawski, R.; Bartkowiak-Wieczorek, J.; Bogacz, A.; Szulc, M.; Kaminska, E.; Kujawska, M.; Gryszczynska, A.; et al. Effect of Salvia miltiorrhiza root extract on brain acetylcholinesterase and butyrylcholinesterase activities, their mRNA levels and memory evaluation in rats. Physiol. Behav. 2017, 173, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in treating cardiovascular diseases: A review on its pharmacological and clinical applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, X.; Zhang, L.; Yang, H.; Zhang, Q. Current progress of research on neurodegenerative diseases of salvianolic acid B. Oxid. Med. Cell. Longev. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Kim, H.; Moon, S.; Lee, H.; Kim, B. Overview of Salvia miltiorrhiza as a potential therapeutic agent for various diseases: An update on efficacy and mechanisms of action. Antioxidants 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A comparative chemical and biological study of its essential oil in the mediterranean context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef] [PubMed]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.; Tamokou, J.; Nji-Nkah, B.; Tchouanguep, F.; Fodouop, S. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Di Leo Lira, P.; Retta, D.; Tkacik, E.; Ringuelet, J.; Coussio, J.D.; van Barena, C.; Bandonia, A.L. Essential oil and by-products of distillation of bay leaves (Laurus nobilis L.) from Argentina. Ind. Crops Prod. 2009, 30, 259–264. [Google Scholar] [CrossRef]
- El, S.N.; Karagozlu, N.; Karakaya, S.; Sahın, S. Antioxidant and antimicrobial activities of essential oils extracted from Laurus nobilis L. leaves by using solvent-free microwave and hydrodistillation. Food Nut. Sci. 2014, 5, 97–106. [Google Scholar] [CrossRef]
- Bouzouita, N.; Nafti, A.; Chaabouni, M.M.; Lognay, G.C.; Marlier, M.; Zghoulli, S.; Thonart, P. Chemical composition of Laurus nobilis Oil from Tunisia. J. Essent. Oil Res. 2001, 13, 116–117. [Google Scholar] [CrossRef]
- Khedher, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160–173. [Google Scholar]
- Dadalioǧlu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas l.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The chemotaxonomy of common sage (Salvia officinalis) based on the volatile constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef]
- Baydar, H.; Ozkan, G.; Erbas, S.; Altýndal, D. Yield, chemical composition, and antioxidant properties of extracts and essential oils of sage and rosemary depending on seasonal variations. Acta Hort. 2009, 826, 383–389. [Google Scholar] [CrossRef]
- Džamić, A.; Soković, M.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. Chemical composition and antifungal activity of Salvia sclarea (Lamiaceae) essential oil. Arch. Biol. Sci. 2008, 60, 233–237. [Google Scholar] [CrossRef]
- Dogan, G.; Hayta, S.; Yuce, E.; Bagci, E. Composition of the essential oil of two Salvia taxa (Salvia sclarea and Salvia verticillata subsp. verticillata) from Turkey. Nat. Sci. Discov. 2015, 1, 62–67. [Google Scholar]
- Carrubba, A.; la Torre, R.; Piccaglia, R.; Marotti, M. Characterization of an Italian biotype of clary sage (Salvia sclarea L.) grown in a semi-arid Mediterranean environment. Flavour Fragr. J. 2002, 17, 191–194. [Google Scholar] [CrossRef]
- Taarit, M.B.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant growth, essential oil and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Ind. Crops Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12. [Google Scholar] [CrossRef]
- Kamiie, Y.; Sagisaka, M.; Nagaki, M. Essential oil composition of Lavandula angustifolia “Hidcote”: Comparison of hydrodistillation and supercritical fluid extraction methods. Trans. Mater. Res. Soc. Jpn. 2014, 39, 485–489. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates—By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 1, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Goudjil, M.B.; Ladjel, S.; Bencheikh, S.E.; Zighmi, S.; Hamada, D. Study of the chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae. J. Chem. Pharm. Res. 2015, 7, 379–385. [Google Scholar]
- Catty, S. Hydrosols, The Next Aromatherapy; Healing Arts Press: Rochester, VT, USA, 2001. [Google Scholar]
- Bag, A.; Chattopadhyay, R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.Á.R.; de Sousa, J.P.; da Costa Medeiros, J.A.; de Figueiredo, R.C.B.Q.; Magnani, M.; de Siqueira Júnior, J.P.; de Souza, E.L. Synergistic inhibition of bacteria associated with minimally processed vegetables in mixed culture by carvacrol and 1,8-cineole. Food Control 2015, 47, 334–339. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Sokovic, M.; Marin, P.D.; Brkic, D.; van Griensven, L.J. Chemical composition and antibacterial activity of essential oils against human pathogenic bacteria. Food 2008, 1, 220–226. [Google Scholar]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Le, N.T.; Donadu, M.G.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Marchetti, M.; Usai, M.; et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J. Infect. Dev. Ctries. 2020, 14, 1054–1064. [Google Scholar] [CrossRef]
- Alonso-Gato, M.; Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Essential Oils as Antimicrobials in Crop Protection. Antibiotics 2021, 10, 34. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmeta, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Nogueira, J.M.F.; Nunes, M.L.; Marques, A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 26, 518–529. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef]
- Belasli, A.; Ben Miri, Y.; Aboudaou, M.; Aït Ouahioune, L.; Montañes, L.; Ariño, A.; Djenane, D. Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): Potential use as wheat preservative. Food Sci. Nutr. 2020, 8, 4717–4729. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simões, M.T.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant, and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319. [Google Scholar] [CrossRef]
- Badawy, M.E.; Marei, G.I.K.; Rabea, E.I.; Taktak, N.E. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and vapour phase of lavandin (Lavandula x intermedia) essential oil: Chemical composition and antimicrobial activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and vapor phase of four conifer-derived essential oils: Comparison of chemical compositions and antimicrobial and antioxidant properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Laghezza Masci, V.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crops Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.A. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Biomed. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M23–A3: Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters: Approved Guideline, 3rd ed.; CLSI Document; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Wang, T.-H.; Hsia, S.-M.; Wu, C.-H.; Ko, S.-Y.; Chen, M.Y.; Shih, Y.-H.; Shieh, T.-M.; Chuang, L.-C.; Wu, C.-Y. Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE 2016, 11, e0163147. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Amiri, Z.; Guo, Y.-G.; Singh, A.P.; Kenari, R.E. Fabrication of cumin loaded-chitosan particles: Characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem. 2020, 310, 125821. [Google Scholar] [CrossRef] [PubMed]
- Maliński, M.P.; Kikowska, M.A.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Thiem, B. Phytochemical screening, phenolic compounds and antioxidant activity of biomass from Lychnis floscuculi L. in vitro cultures and intact plants. Plants 2021, 10, 206. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C.A. Screening of dietary carotenoid rich fruit extracts for antioxidant activities applying ABTS radical cation decolorisation assay. Methods Enzymol. 1999, 229, 379–389. [Google Scholar]
- Vega-Ruiz, Y.C.; Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L.A. Determination of chemical constituents and antioxidant activities of leaves and stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants 2021, 10, 212. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.L.; Kell, S.P.; Scholten, M.A. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685. [Google Scholar] [CrossRef]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Genet. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- McCouch, S.; Baute, G.; Bradeen, J.; Bramel, P.; Bretting, P.; Buckler, E.; Burke, J.; Charest, D.; Cloutier, S.; Cole, G.; et al. Agriculture: Feeding the future. Nature 2013, 499, 23–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).