The Study of the Kinetics of Metalaxyl Accumulation and Dissipation in Durian (Durio zibethinus L.) Leaf Using High-Performance Liquid Chromatography (HPLC) Technique
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Metalaxyl Extraction from Durian Leaf Sample
2.2. HPLC Method Validation
2.3. Metalaxyl Concentration 24 h after Foliar Spray Application
2.4. Metalaxyl Dissipation in Durian
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Metalaxyl Treatments
3.4. Leaf Sample Extraction
3.5. HPLC Analysis
3.6. Method Validation
3.7. Calculation of Metalaxyl Concentration and Dissipation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangchote, S.; Poonpolgul, S.; Sdoodee, R.; Kanjanamaneesathian, M.; Baothong, T.; Lumyong, P. Phytophthora diseases in Thailand. In Diversity and Management of Phytophthora in Southeast Asia; Drenth, A., Guest, D.I., Eds.; BPA Print Group Pty Ltd.: Melbourne, Australia, 2004; pp. 77–82. [Google Scholar]
- Lim, T.K. Durian Diseases and Disorders; Tropical Press: Kuala Lumpur, Malaysia, 1990; ISBN 9789677300507. [Google Scholar]
- Drenth, A.; Guest, D.I. Principles of Phytophthora Disease Management. In Diversity and Management of Phytophthora in Southeast Asia; Drenth, A., Guest, D.I., Eds.; BPA Print Group Pty Ltd.: Melbourne, Australia, 2004; pp. 154–160. [Google Scholar]
- de Ruiter, H.; Kempenaar, C.; Blom, M. Foliar Absorption of Crop Protection Agents: Influence of cpa Properties, Formulation and Plant Species: A Literature Study for the Dutch Research Programme Pesticides and the Environment (DWK-359) Theme B-2; Plant Research International: Wageningen, The Netherlands, 2004; pp. 1–13. [Google Scholar]
- Baker, E.A.; Hayes, A.L.; Butler, R.C. Physicochemical properties of agrochemicals: Their effects on foliar penetration. Pestic. Sci. 1992, 34, 167–182. [Google Scholar] [CrossRef]
- de Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kerkenaar, A. On the antifungal mode of action of metalaxyl, an inhibitor of nucleic acid synthesis in Pythium splendens. Pestic. Biochem. Physiol. 1981, 16, 1–13. [Google Scholar] [CrossRef]
- Davidse, L.C.; Gerritsma, O.C.M.; Ideler, J.; Pie, K.; Velthuis, G.C.M. Antifungal modes of action of metalaxyl, cyprofuram, benalaxyl and oxadixyl in phenylamide-sensitive and phenylamide-resistant strains of Phytophthora megasperma f. sp. medicaginis and Phytophthora infestans. Crop Prot. 1988, 7, 347–355. [Google Scholar] [CrossRef]
- Kubicki, M.; Lamshöft, M.; Lagojda, A.; Spiteller, M. Metabolism and spatial distribution of metalaxyl in tomato plants grown under hydroponic conditions. Chemosphere 2019, 218, 36–41. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 42586, Metalaxyl. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Metalaxyl (accessed on 25 August 2020).
- Zelená, V.; Veverka, K. Effect of Surfactants and Liquid Fertilisers on Transcuticular Penetration of Fungicides. Plant Prot. Sci. 2007, 43, 151–156. [Google Scholar] [CrossRef]
- Cohen, Y.; Coffey, M.D. Systemic fungicides and the control of oomycetes. Annu. Rev. Phytopathol. 1986, 24, 311–338. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Zhu, H.; Li, Z.; Cui, Y.; Zhang, K.; Hu, D. Determination of Metalaxyl in Potatoes and Soil by Dispersive Solid–Phase Extraction and High–Performance Liquid Chromatography. Instrum. Sci. Technol. 2015, 43, 53–64. [Google Scholar] [CrossRef]
- Malhat, F.M. Persistence of metalaxyl residues on tomato fruit using high performance liquid chromatography and QuEChERS methodology. Arab. J. Chem. 2017, 10, S765–S768. [Google Scholar] [CrossRef]
- Velkoska-Markovska, L.; Petanovska-Ilievska, B.; Jankulovska, M.S.; Ilievski, U. Development and validation of high-performance liquid chromatography method for determination of some pesticide residues in table grape. Acta Chromatogr. 2018, 30, 250–254. [Google Scholar] [CrossRef]
- Kabir, M.H.; Abd El-Aty, A.M.; Rahman, M.M.; Chung, H.S.; Lee, H.S.; Jeong, J.H.; Wang, J.; Shin, S.; Shin, H.C.; Shim, J.H. Dissipation kinetics, pre-harvest residue limits, and dietary risk assessment of the systemic fungicide metalaxyl in Swiss chard grown under greenhouse conditions. Regul. Toxicol. Pharmacol. 2018, 92, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Cui, Y.; Zhu, H.; Li, X.; Li, Z.; Zhang, K.; Hu, D. Dissipation and residue of metalaxyl and cymoxanil in pepper and soil. Environ. Monit. Assess. 2014, 186, 5307–5313. [Google Scholar] [CrossRef]
- Ramezani, M.K.; Shahriari, D. Dissipation behaviour, processing factors and risk assessment for metalaxyl in greenhouse-grown cucumber. Pest Manag. Sci. 2015, 71, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, W.; Sun, C.; Zheng, K.; Zhang, H.; Huang, M.; Hu, D.; Zhang, K. Simultaneous determination of residues of metalaxyl, cyazofamid and a cyazofamid metabolite in tobacco leaves and soil by liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2017, 32, e4161. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Luo, X.; Qin, X.; Huang, M.; Li, J.; Zeng, S.; Zhang, K.; Hu, D. Simultaneous determination and risk assessment of metalaxyl and azoxystrobin in potato by liquid chromatography with tandem mass spectrometry. Environ. Monit. Assess. 2018, 190, 335. [Google Scholar] [CrossRef]
- Liu, C.; Wan, K.; Huang, J.; Wang, Y.; Wang, F. Behavior of mixed formulation of metalaxyl and dimethomorph in grape and soil under field conditions. Ecotoxicol. Environ. Saf. 2012, 84, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Sharma, V.K.; Rattan, G.S.; Singh, B.; Sharma, N. Dissipation of residues of mancozeb and metalaxyl in tomato (Solanum lycopersicum L.). Bull. Environ. Contam. Toxicol. 2013, 90, 248–251. [Google Scholar] [CrossRef]
- Zaki, A.I.; Zentmyer, G.A.; LeBaron, H.M. Systemic Translocation of 14C-Labeled Metalaxyl in Tomato, Avocado, and Persea indica. Phytopathology 1981, 71, 509–514. [Google Scholar] [CrossRef]
- SANCO. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residue Analysis in Food and Feed. Document SANCO/12571/2013. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 20 January 2021).
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Singh, U.S. Uptake, translocation, distribution and persistence of ¹⁴C-metalaxyl in maize (Zea mays). J. Plant. Dis. Prot. 1987, 94, 478–490. [Google Scholar]
- Singh, U.S. Studies on the systemicity of [14C]metalaxyl in cowpea (Vigna unguiculata (L.) walp.). Pestic. Sci. 1989, 25, 145–154. [Google Scholar] [CrossRef]
- Businelli, M.; Patumi, M.; Marucchini, C. Identification and determination of some metalaxyl degradation products in lettuce and sunflower. J. Agric. Food Chem. 1984, 32, 644–647. [Google Scholar] [CrossRef]
- Owen, W.J.; Donzel, B. Oxidative degradation of chlortoluron, propiconazole, and metalaxyl in suspension cultures of various crop plants. Pestic. Biochem. Physiol. 1986, 26, 75–89. [Google Scholar] [CrossRef]
- Cole, D.J.; Owen, W.J. Metabolism of metalaxyl in cell suspension cultures of Lactuca sativa L. and Vitis vinifera L. Pestic. Biochem. Physiol. 1987, 28, 354–361. [Google Scholar] [CrossRef]
- Reuveni, M.; Siegel, M.R.; Nesmith, W.C. Uptake and Distribution of [ 14C]Metalaxyl by Detached Tobacco Leaves. Pestic. Sci. 1985, 16, 251–256. [Google Scholar] [CrossRef]
- Chan, L.G.; Kwee, L.T. Comparative Invitro Sensitivity of Selected Chemicals on Phytophthora palmivora from Cocoa and Durian. Pertanika 1986, 9, 183–191. [Google Scholar]
- Matheron, M.E.; Matejka, J.C. Persistence of systemic activity for fungicides applied to citrus trunks to control Phytophthora Gummosis. Plant Dis. 1988, 72, 170–174. [Google Scholar] [CrossRef]
- Thomidis, T. Persistence of the systemic activity of metalaxyl and fosetyl-Al applied as a soil drench or foliar spray to control Phytophthora crown rot of peach. Phytopathol. Mediterr. 2002, 41, 28–32. [Google Scholar]
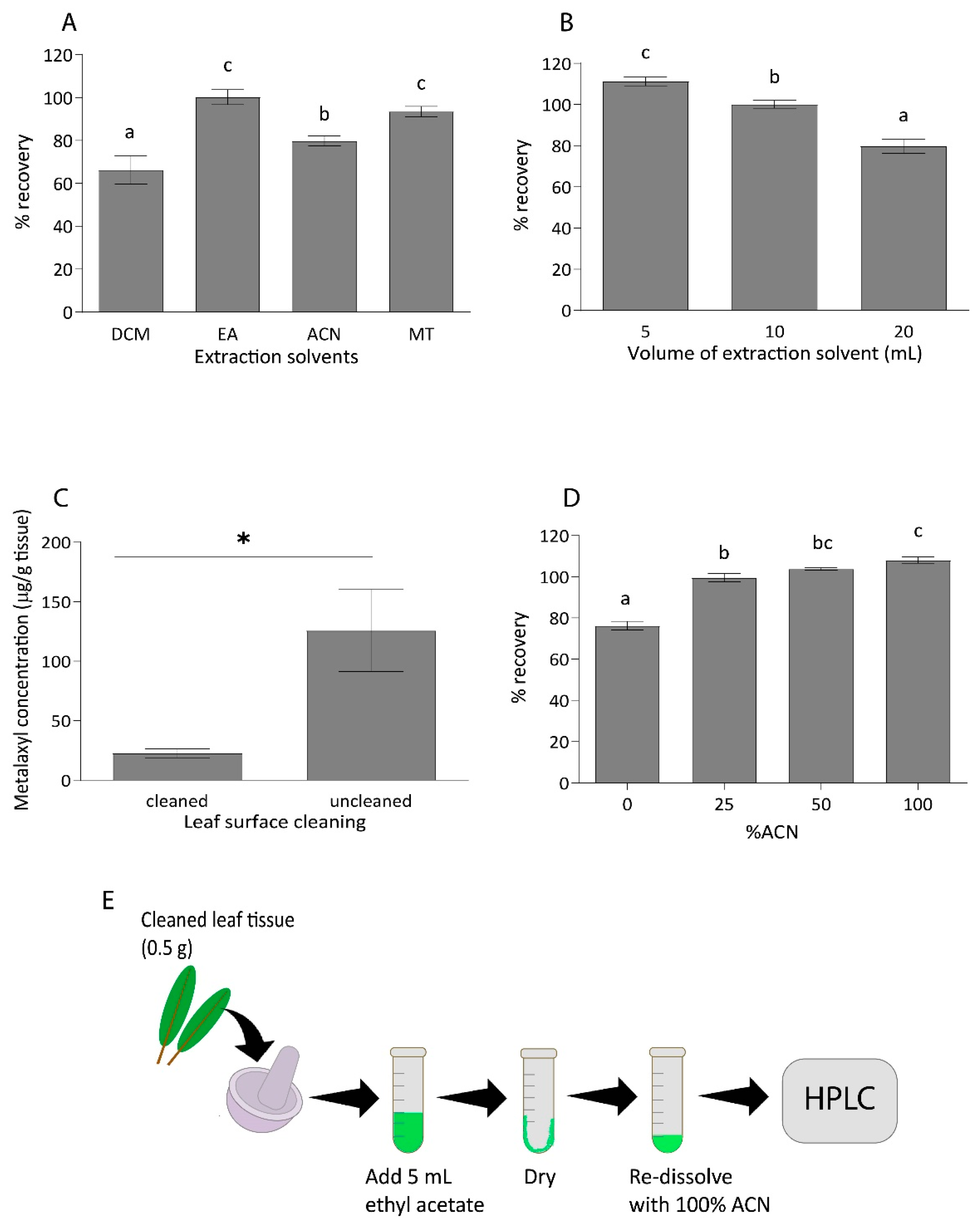
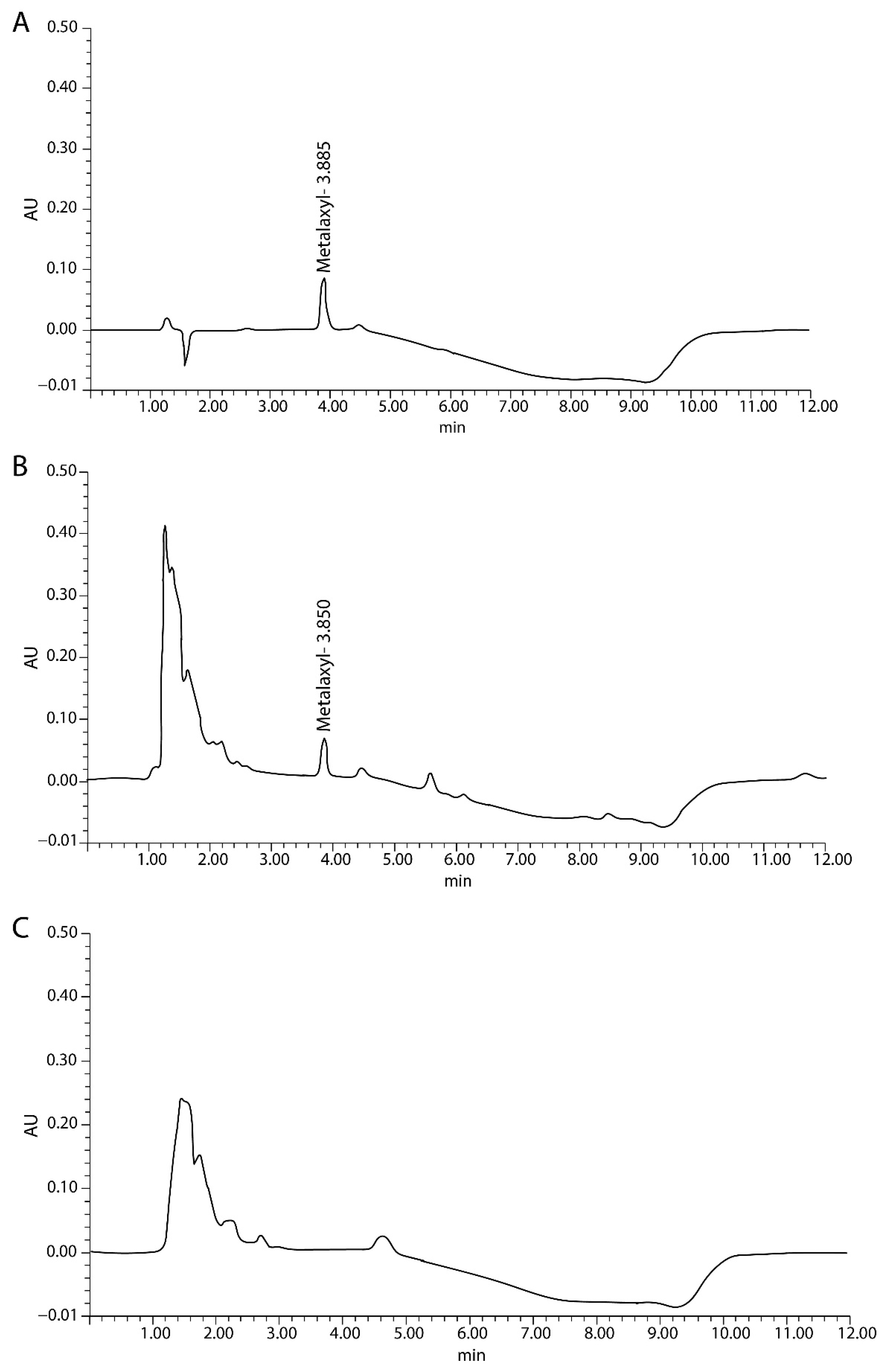
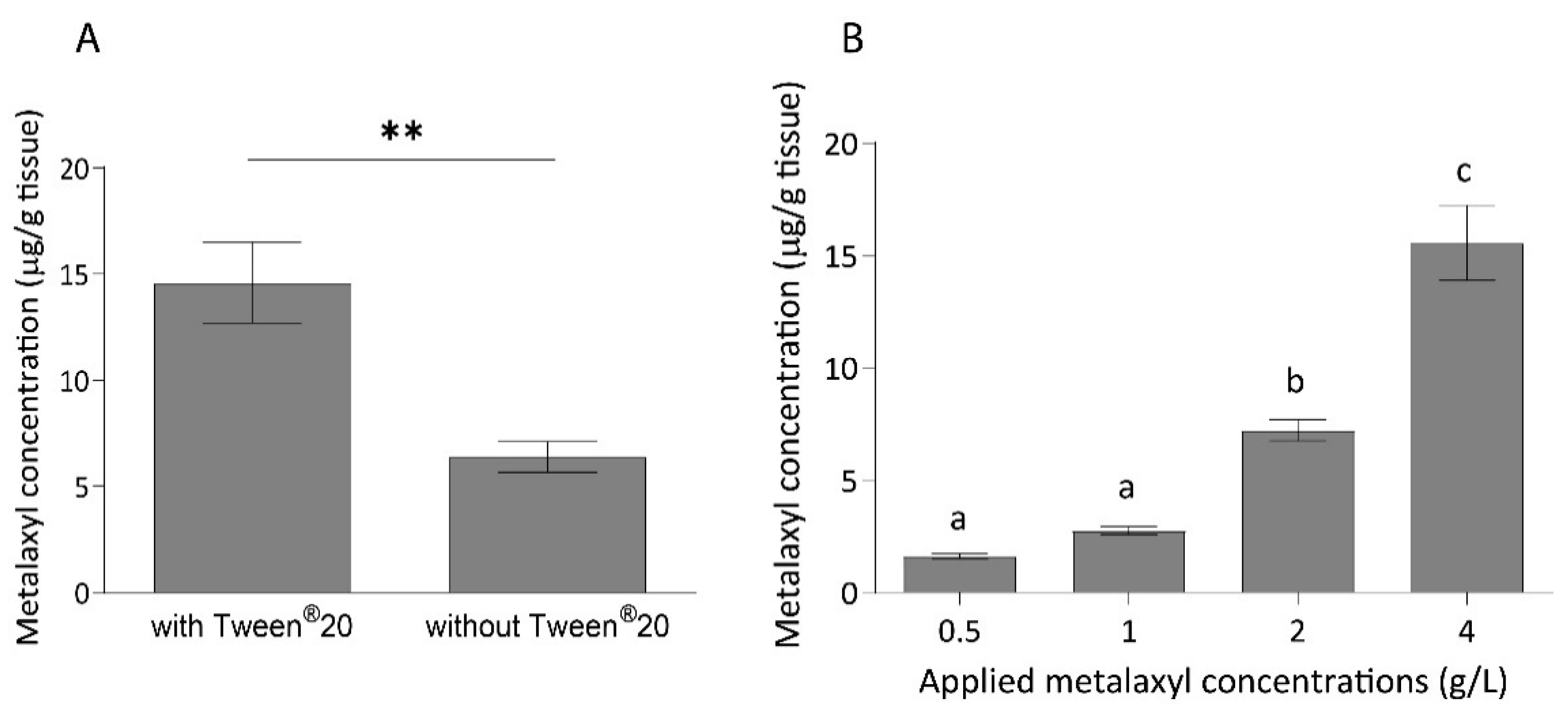
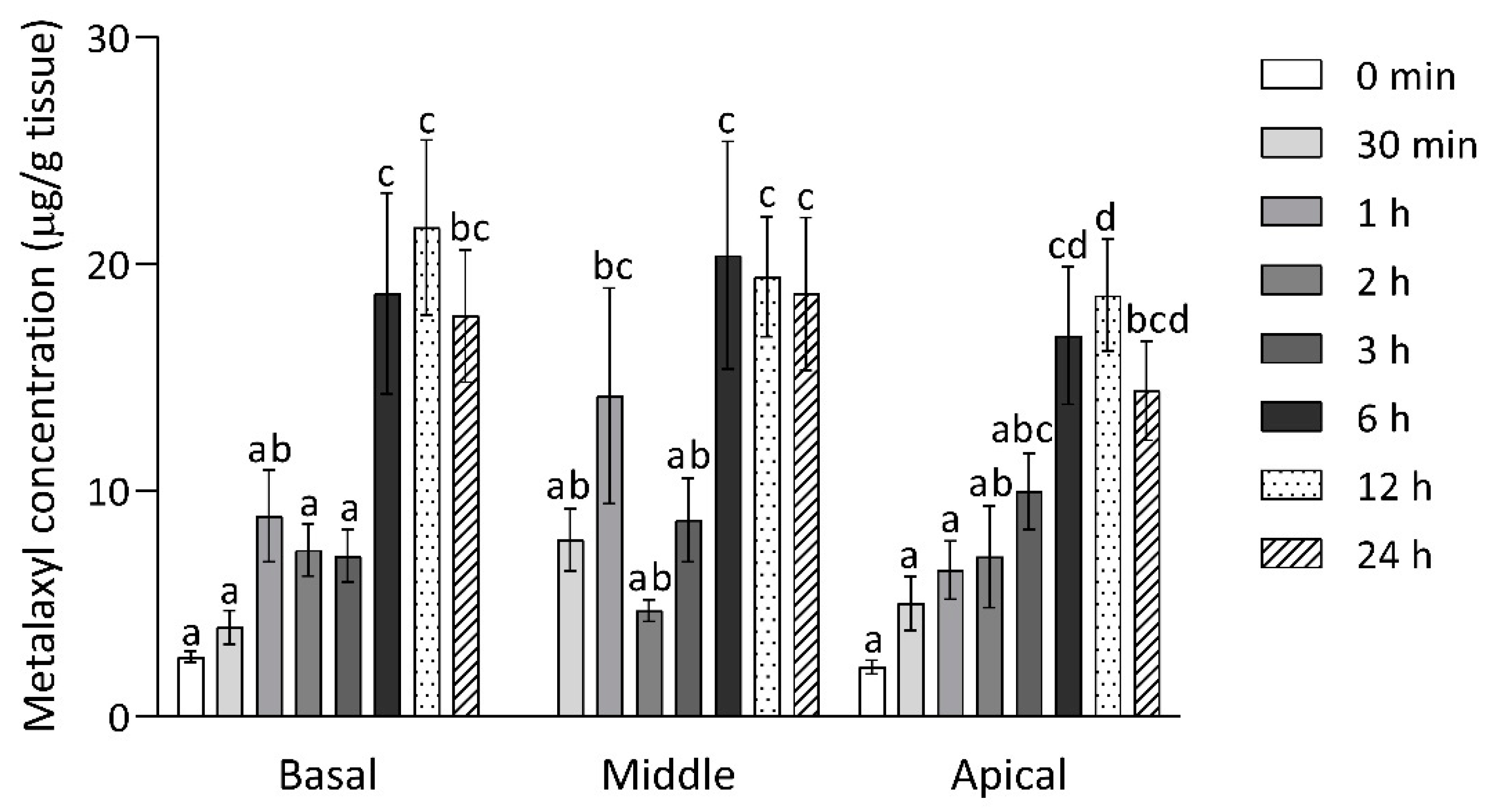

| Analyte | Matrix | Linear Equation | Linear Range (µg/mL) | R2 | LOD | Linear Range (µg/mL) |
|---|---|---|---|---|---|---|
| Metalaxyl | Acetonitrile | y = 32,787x–9550.5 | 0.5–100 | 0.999 | 0.15 | 0.51 |
| Durian leaf | y = 36,621x–653.63 | 1–100 | 0.999 | 0.27 | 0.91 |
| Spiked Level (µg/mL) | Intra-Day | Inter-Day 1 | ||
|---|---|---|---|---|
| Average (n = 6) | %RSD | Average (n = 18) | %RSD | |
| 1 | 85.36 | 2.79 | 88.07 | 9.77 |
| 84.87 | 14.56 | |||
| 93.99 | 4.10 | |||
| 10 | 98.98 | 0.41 | 103.40 | 4.06 |
| 105.31 | 2.92 | |||
| 105.91 | 2.96 | |||
| 100 | 98.24 | 0.60 | 102.13 | 3.42 |
| 102.40 | 0.88 | |||
| 105.74 | 1.76 | |||
| Application Method | Days after Application | Average Residue ± SE | %Dissipation |
|---|---|---|---|
| Foliar spray | 0 | 2.65 ± 0.21 | N/A |
| 1 | 16.46 ± 1.61 | 0 | |
| 7 | 8.54 ± 0.83 | 48.10 | |
| 15 | 5.67 ± 1.14 | 65.55 | |
| 30 | 5.53 ± 0.73 | 67.69 | |
| 60 | 3.44 ± 0.39 | 79.08 | |
| Soil drench | 0 | BDL | BDL |
| 1 | 2.25 ± 0.47 | N/A | |
| 7 | 127.40 ± 10.44 | N/A | |
| 15 | 89.31 ± 2.55 | N/A | |
| 30 | N/A | N/A | |
| 60 | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phetkhajone, S.; Pichakum, A.; Songnuan, W. The Study of the Kinetics of Metalaxyl Accumulation and Dissipation in Durian (Durio zibethinus L.) Leaf Using High-Performance Liquid Chromatography (HPLC) Technique. Plants 2021, 10, 708. https://doi.org/10.3390/plants10040708
Phetkhajone S, Pichakum A, Songnuan W. The Study of the Kinetics of Metalaxyl Accumulation and Dissipation in Durian (Durio zibethinus L.) Leaf Using High-Performance Liquid Chromatography (HPLC) Technique. Plants. 2021; 10(4):708. https://doi.org/10.3390/plants10040708
Chicago/Turabian StylePhetkhajone, Supawadee, Aussanee Pichakum, and Wisuwat Songnuan. 2021. "The Study of the Kinetics of Metalaxyl Accumulation and Dissipation in Durian (Durio zibethinus L.) Leaf Using High-Performance Liquid Chromatography (HPLC) Technique" Plants 10, no. 4: 708. https://doi.org/10.3390/plants10040708
APA StylePhetkhajone, S., Pichakum, A., & Songnuan, W. (2021). The Study of the Kinetics of Metalaxyl Accumulation and Dissipation in Durian (Durio zibethinus L.) Leaf Using High-Performance Liquid Chromatography (HPLC) Technique. Plants, 10(4), 708. https://doi.org/10.3390/plants10040708







