FTO Suppresses Dental Pulp Stem Cell Senescence by Destabilizing NOLC1 mRNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmids
2.3. RNA Interference
2.4. Quantitative RT-PCR and Semi-Quantitative RT-PCR
2.5. Western Blot Analysis
2.6. Reactive Oxygen Species Detection
2.7. β-Galactosidase Staining
2.8. Cell Cycle
2.9. RNA Sequencing
2.10. MeRIP Assay
2.11. RNA Stability Assay
2.12. Statistical Analysis
3. Results
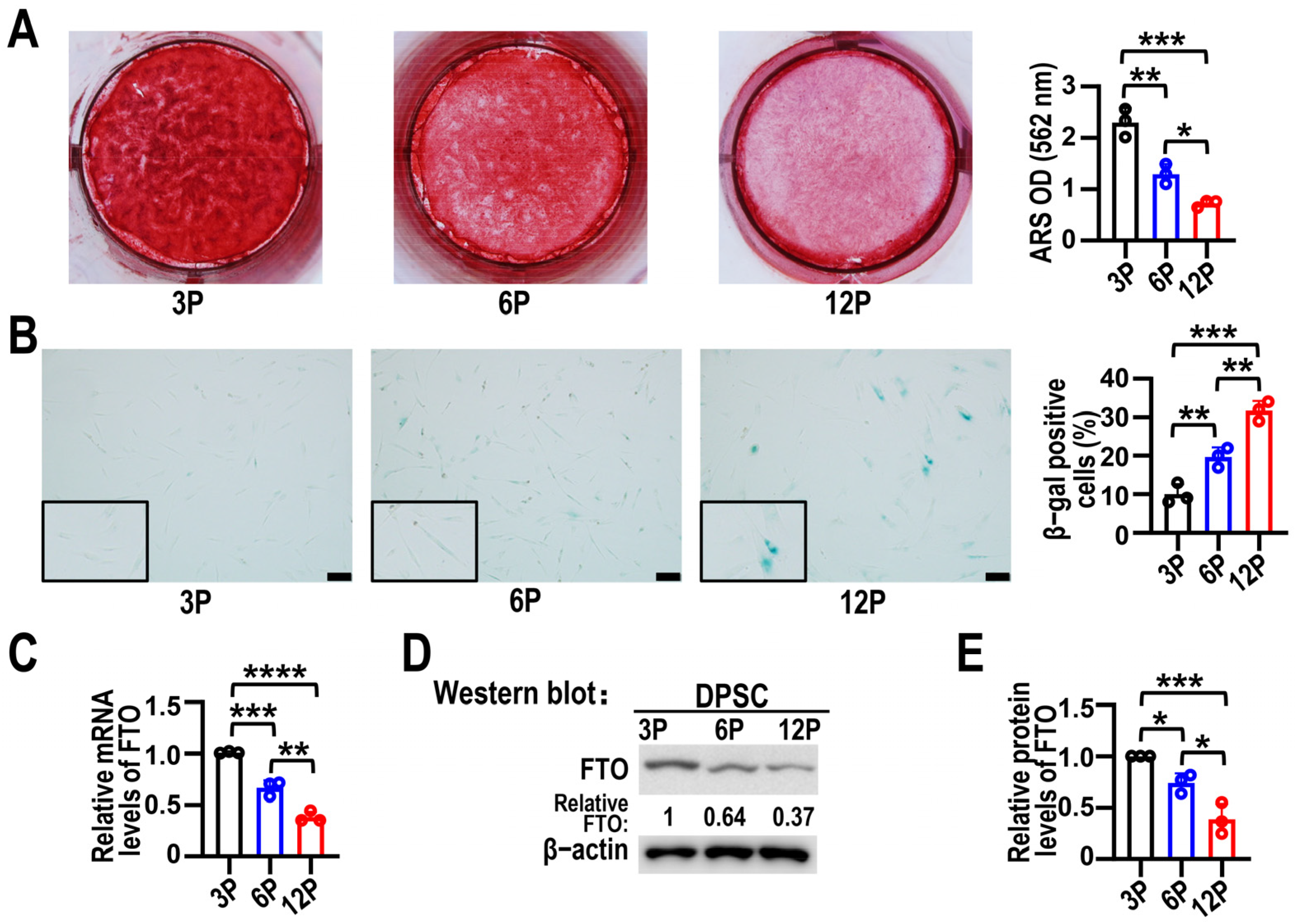
3.1. FTO Expression Levels Were Reduced in Senescent DPSCs
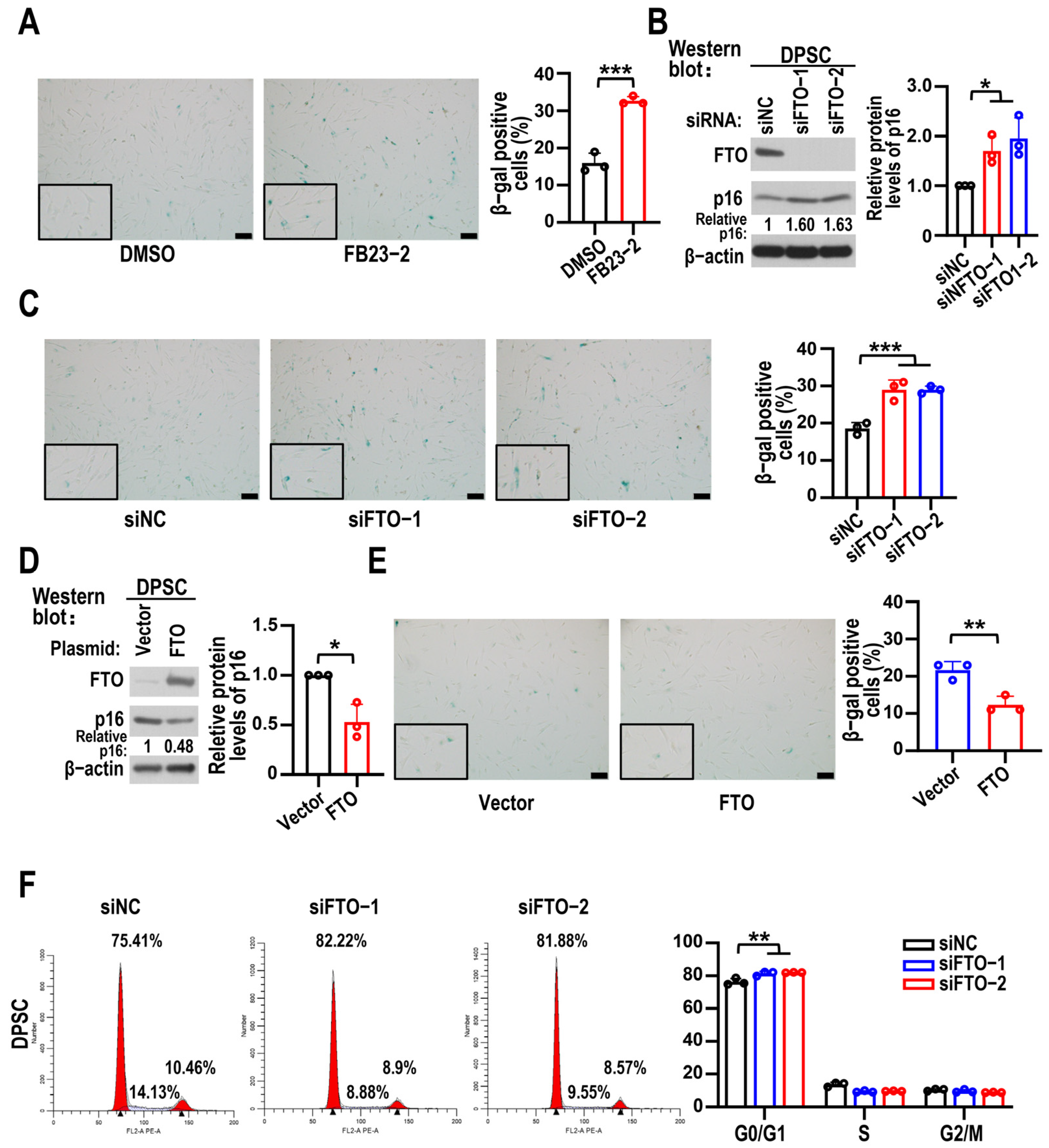
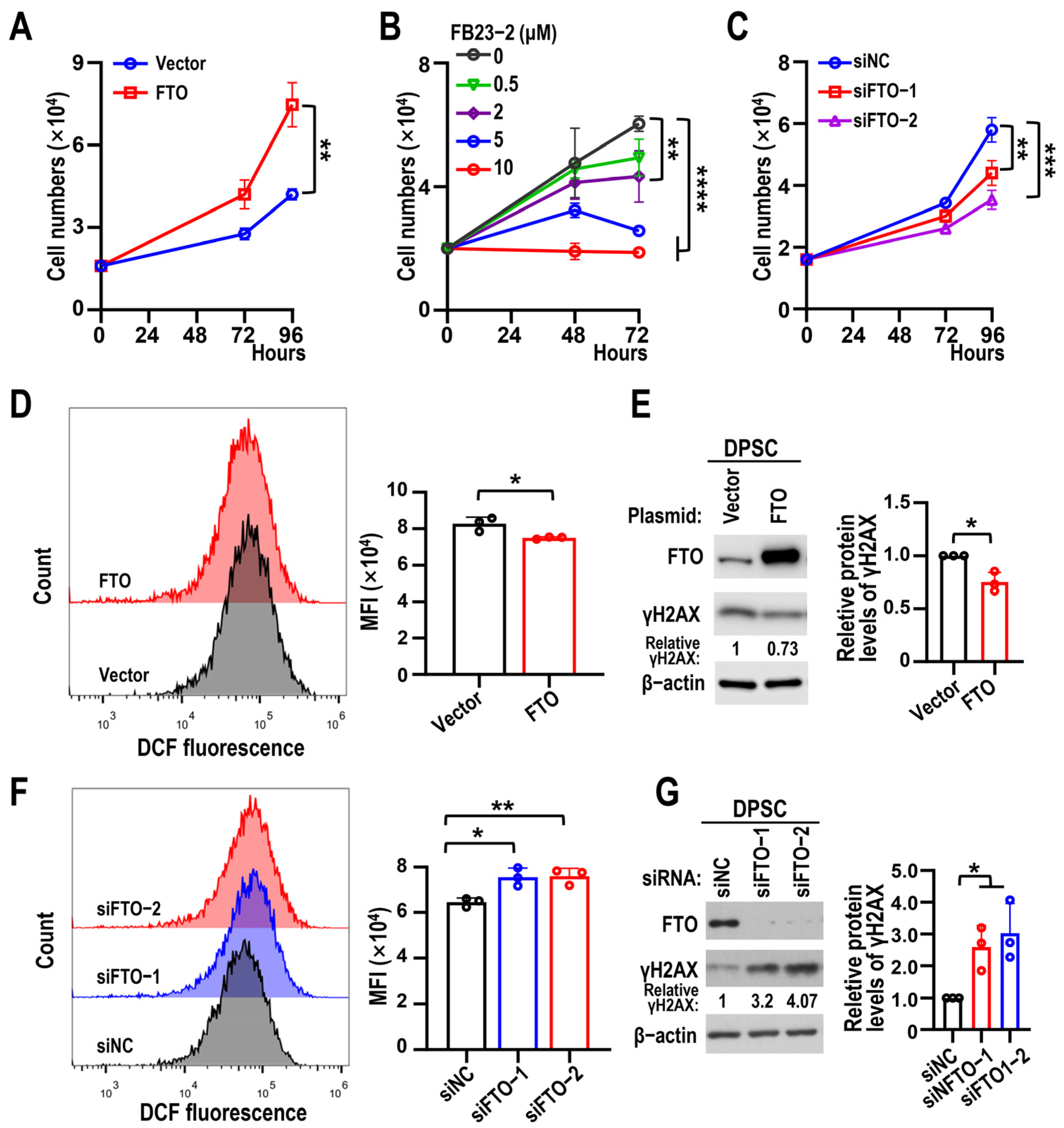
3.2. Depletion of FTO Accelerated DPSC Senescence
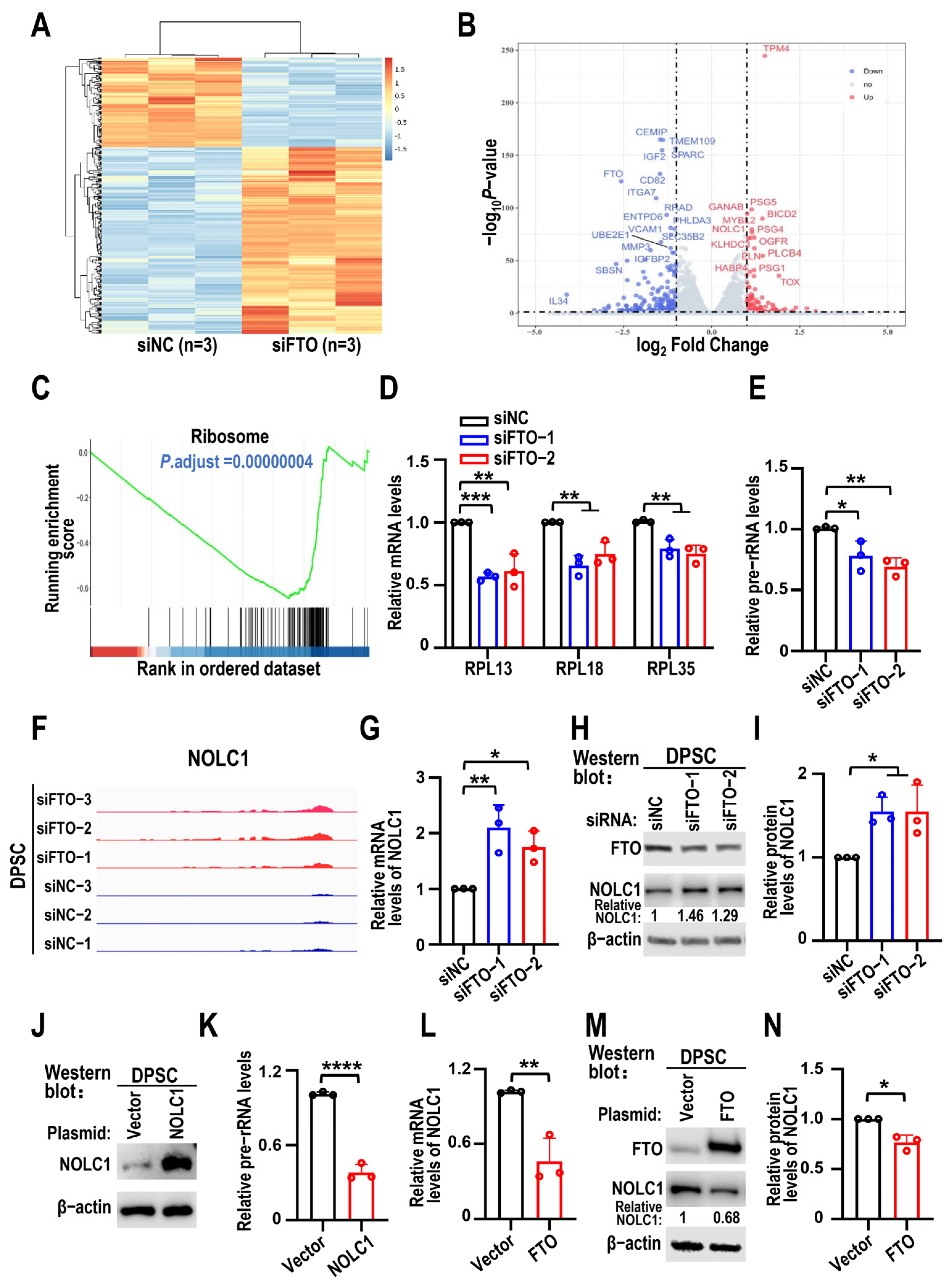
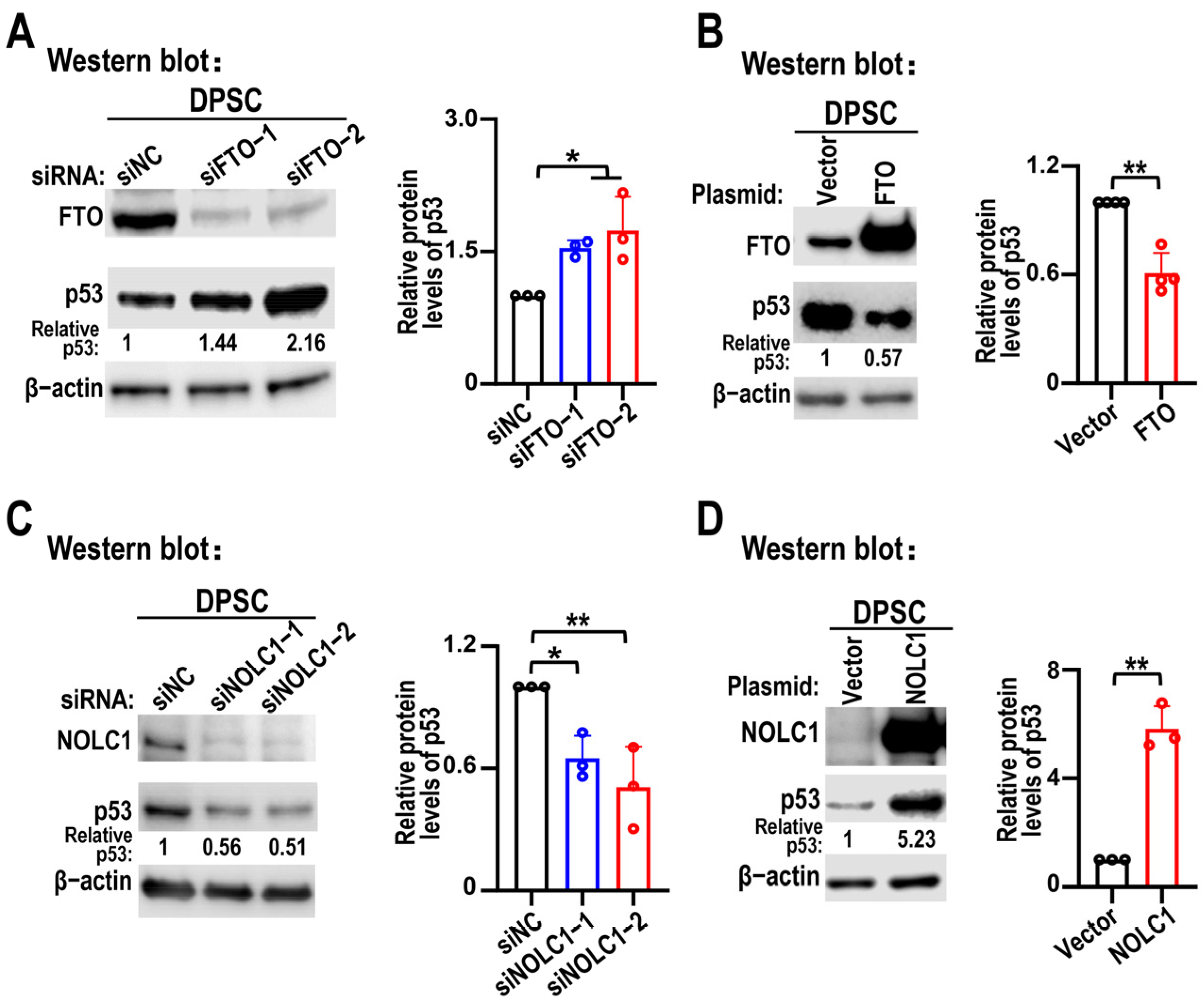
3.3. FTO KD Suppresses Pre-rRNA Synthesis Through NOLC1
3.4. NOLC1 Promotes DPSC Senescence
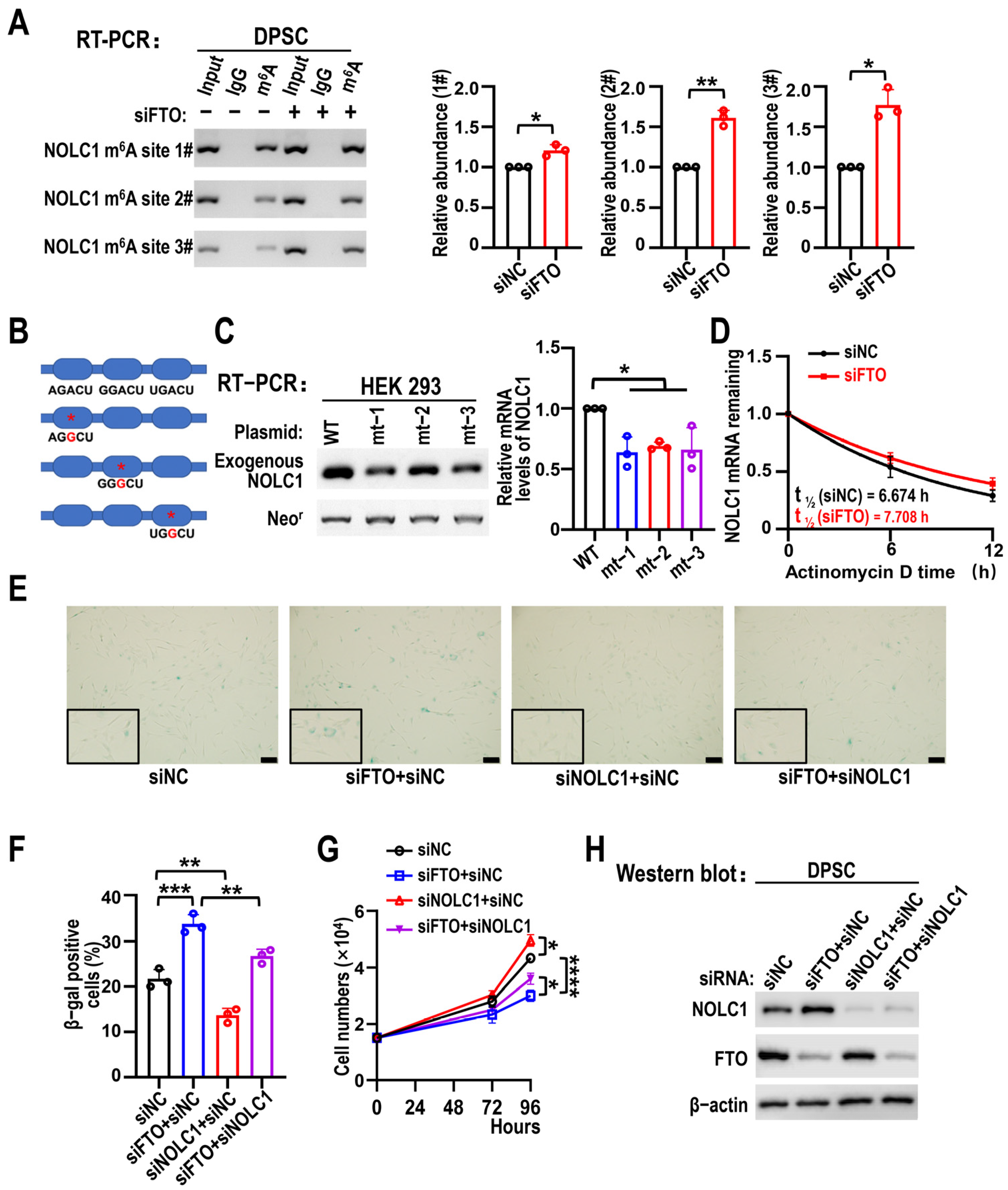
3.5. NOLC1 Is Regulated by an FTO-m6A-Dependent Mechanism, and NOLC1 KD Partially Rescues FTO Silencing-Induced DPSC Senescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Torres, Y.; Benguría, A.; Dopazo, A.; Roche, E.; Carrera-Quintanar, L.; Pérez, R.A.; Enríquez, J.A.; Torres, R.; Ramírez, J.C.; et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013, 4, e691. [Google Scholar] [CrossRef]
- Liang, H.; Li, W.; Yang, H.; Cao, Y.; Ge, L.; Shi, R.; Fan, Z.; Dong, R.; Zhang, C. FAM96B inhibits the senescence of dental pulp stem cells. Cell Biol. Int. 2020, 44, 1193–1203. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Yang, Z.; Chu, X.; Wu, J.; Wang, S.; He, X.; Gao, C.; Chen, X.; Yang, K.; et al. Downregulation of ROR2 promotes dental pulp stem cell senescence by inhibiting STK4-FOXO1/SMS1 axis in sphingomyelin biosynthesis. Aging Cell 2021, 20, e13430. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, Y.; Wei, P.; Yan, X.; Qiu, Q.; Qiu, L. Melatonin attenuates dental pulp stem cells senescence due to vitro expansion via inhibiting MMP3. Oral Dis. 2024, 30, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.L.; Huang, H.M.; Han, C.S.; Cui, S.J.; Zhou, Y.K.; Zhou, Y.H. Serine Metabolism Controls Dental Pulp Stem Cell Aging by Regulating the DNA Methylation of p16. J. Dent. Res. 2021, 100, 90–97. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.; Hao, Y.; Sun, B.; Sun, H.; Li, A.; Ping, X.; Lai, W.; et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.; Ping, X.; Chen, Y.; Wang, W.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, R.; Sheng, Y.; Dong, L.; Dong, Z.; Xu, H.; Ni, T.; Zhang, Z.S.; Zhang, T.; Li, C.; et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell 2019, 35, 677–691.E10. [Google Scholar] [CrossRef]
- Yang, S.; Wei, J.; Cui, Y.; Park, G.; Shah, P.; Deng, Y.; Aplin, A.E.; Lu, Z.; Hwang, S.; He, C.; et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 2019, 10, 2782. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Li, Z.; Dai, Z.; He, Y.; Chu, K.; Gu, J.; Ji, Y.; Sun, N.; Yang, F.; et al. The m6A mRNA demethylase FTO in granulosa cells retards FOS-dependent ovarian aging. Cell Death Dis. 2021, 12, 744. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Shi, Y.; Wang, S.; Ren, J.; Yu, Z.; Huang, D.; Yan, K.; He, Y.; Liu, X.; et al. FTO stabilizes MIS12 and counteracts senescence. Protein Cell 2022, 13, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, B.; Huang, J.; Jia, R.; Guo, J. The N6-methyladenosine demethylase FTO is required for odontoblast differentiation in vitro and dentine formation in mice by promoting RUNX2 exon 5 inclusion through RBM4. Int. Endod. J. 2023, 56, 1534–1549. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.T.; Blobel, G. A nuclear localization signal binding protein in the nucleolus. J. Cell Biol. 1990, 111, 2235–2245. [Google Scholar] [CrossRef]
- Meier, U.T.; Blobel, G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell 1992, 70, 127–138. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, Y.; Ma, L.; Cheng, Q.; Li, G.; Tong, T. Enhanced NOLC1 promotes cell senescence and represses hepatocellular carcinoma cell proliferation by disturbing the organization of nucleolus. Aging Cell 2017, 16, 726–737. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Salmon-Divon, M.; Amariglio, N.; Rechavi, G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013, 8, 176–189. [Google Scholar] [CrossRef]
- Ma, L.; Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Maintained Properties of Aged Dental Pulp Stem Cells for Superior Periodontal Tissue Regeneration. Aging Dis. 2019, 10, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ji, S. Cellular senescence: Molecular mechanisms and pathogenicity. J. Cell. Physiol. 2018, 233, 9121–9135. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Tang, N.; Yang, J.; Liu, S.; Cheng, C.; Wang, Y.; Chen, C.; Guo, Y.; Wang, H.; Zhao, W.; et al. Deficiency of ribosomal proteins reshapes the transcriptional and translational landscape in human cells. Nucleic Acids Res. 2022, 50, 6601–6617. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, L.; Lin, R.; Guo, X.; Yin, J.; Xie, H.; Lu, Y.; Zhang, Z.; Zhang, H.; Yao, Z.; et al. RPL35 downregulated by mechanical overloading promotes chondrocyte senescence and osteoarthritis development via Hedgehog-Gli1 signaling. J. Orthop. Transl. 2024, 45, 226–235. [Google Scholar] [CrossRef]
- Wei, J.; Yu, X.; Yang, L.; Liu, X.; Gao, B.; Huang, B.; Dou, X.; Liu, J.; Zou, Z.; Cui, X.; et al. FTO mediates LINE1 m6A demethylation and chromatin regulation in mESCs and mouse development. Science 2022, 376, 968–973. [Google Scholar] [CrossRef]
- Sepúlveda, J.C.; Tomé, M.; Fernández, M.E.; Delgado, M.; Campisi, J.; Bernad, A.; González, M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells 2014, 32, 1865–1877. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, O.; Yan, F.; Lin, X.; Diao, S.; Wang, L.; Jin, L.; Wang, S.; Lu, Y.; Fan, Z. Analysis of Senescence-Related Differentiation Potentials and Gene Expression Profiles in Human Dental Pulp Stem Cells. Cells Tissues Organs 2017, 203, 1–11. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Zhong, Y.; Cai, Y.; Meng, X.; Chen, M.; Mao, R.; Xiao, X.; Yi, C.; Guo, Y.; et al. Depletion of Fat Mass and Obesity-Associated Protein (FTO) Drives Heterochromatin Loss via Lysine Acetyltransferase 8 (KAT8)-Mediated Remodeling and Spacing Factor 1 (RSF1) Acetylation in Skin Aging. MedComm 2025, 6, e70205. [Google Scholar] [CrossRef] [PubMed]
- Attaway, A.H.; Bellar, A.; Welch, N.; Sekar, J.; Kumar, A.; Mishra, S.; Hatipoğlu, U.; McDonald, M.-L.; Regan, E.A.; Smith, J.D.; et al. Gene polymorphisms associated with heterogeneity and senescence characteristics of sarcopenia in chronic obstructive pulmonary disease. J. Cachexia Sarcopenia Muscle 2023, 14, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Lafita-Navarro, M.C.; Conacci-Sorrell, M. Nucleolar stress: From development to cancer. Semin. Cell Dev. Biol. 2023, 136, 64–74. [Google Scholar] [CrossRef]
- Turi, Z.; Lacey, M.; Mistrik, M.; Moudry, P. Impaired ribosome biogenesis: Mechanisms and relevance to cancer and aging. Aging 2019, 11, 2512–2540. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, X.; Zhang, J.; Liang, L.; Gao, C.; Zhang, P.; Liu, Y.; Sun, J.; Ruan, B.; Duan, J.; et al. Repression of rRNA gene transcription by endothelial SPEN deficiency normalizes tumor vasculature via nucleolar stress. J. Clin. Investig. 2023, 133, e159860. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Huang, S.; Wang, J.; Wang, X.; Liang, Y.; Han, H. FTO inhibition represses B-cell acute lymphoblastic leukemia progression by inducing nucleolar stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2025, 238, 507–521. [Google Scholar] [CrossRef]
- Yao, Z.; Duan, S.; Hou, D.; Wang, W.; Wang, G.; Liu, Y.; Wen, L.; Wu, M. B23 acts as a nucleolar stress sensor and promotes cell survival through its dynamic interaction with hnRNPU and hnRNPA1. Oncogene 2010, 29, 1821–1834. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, H.; Zhao, J.; Tan, T.; Pan, H.; Zhu, Y.; Chen, L.; Zhang, C.; Zhang, L.; Lei, A.; et al. LIN28 coordinately promotes nucleolar/ribosomal functions and represses the 2C-like transcriptional program in pluripotent stem cells. Protein Cell 2022, 13, 490–512. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, J.; Yu, W.; Xie, Z.; Zheng, G.; Liu, W.; Wang, S.; Cao, Q.; Lin, J.; Su, Z.; et al. ALKBH5 facilitates CYP1B1 mRNA degradation via m6A demethylation to alleviate MSC senescence and osteoarthritis progression. Exp. Mol. Med. 2023, 55, 1743–1756. [Google Scholar] [CrossRef]
- Hwang, Y.; Lu, T.; Huang, D.; Kuo, Y.; Kao, C.; Yeh, N.; Wu, H.; Lin, C. NOLC1, an enhancer of nasopharyngeal carcinoma progression, is essential for TP53 to regulate MDM2 expression. Am. J. Pathol. 2009, 175, 342–354. [Google Scholar] [CrossRef]
- Zhai, F.; Li, Y.; Zheng, J.; Yan, C.; Wang, S.; Yang, W.; Jin, J.; Luo, X.; Zhan, Z.; Shi, J.; et al. SPOP/NOLC1/B4GALT1 signaling axis enhances paclitaxel resistance in endometrial cancer by inducing O-dysglycosylation. Oncogene 2025, 44, 1694–1708. [Google Scholar] [CrossRef]
- Yuan, F.; Li, G.; Tong, T. Nucleolar and coiled-body phosphoprotein 1 (NOLC1) regulates the nucleolar retention of TRF2. Cell Death Discov. 2017, 3, 17043. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Xu, M.; Huang, J.; Jia, R. FTO Suppresses Dental Pulp Stem Cell Senescence by Destabilizing NOLC1 mRNA. Biomolecules 2025, 15, 1627. https://doi.org/10.3390/biom15111627
Li B, Xu M, Huang J, Jia R. FTO Suppresses Dental Pulp Stem Cell Senescence by Destabilizing NOLC1 mRNA. Biomolecules. 2025; 15(11):1627. https://doi.org/10.3390/biom15111627
Chicago/Turabian StyleLi, Bingrong, Mi Xu, Junjun Huang, and Rong Jia. 2025. "FTO Suppresses Dental Pulp Stem Cell Senescence by Destabilizing NOLC1 mRNA" Biomolecules 15, no. 11: 1627. https://doi.org/10.3390/biom15111627
APA StyleLi, B., Xu, M., Huang, J., & Jia, R. (2025). FTO Suppresses Dental Pulp Stem Cell Senescence by Destabilizing NOLC1 mRNA. Biomolecules, 15(11), 1627. https://doi.org/10.3390/biom15111627





