Microglia-Mediated Phagocytosis in Alzheimer’s Disease: Mechanisms, Heterogeneity, and Therapeutic Insights
Abstract
1. Introduction
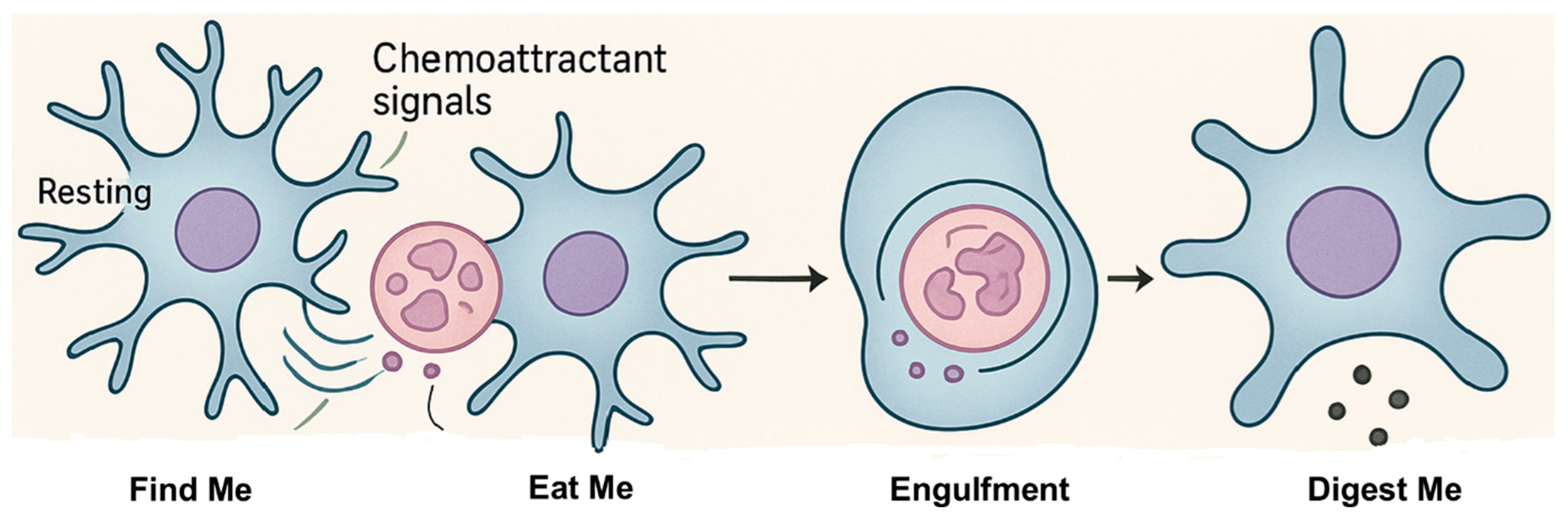
2. Microglial Function and Phagocytosis
2.1. ”Find-Me” Phase
2.2. “Eat-Me” Phase
2.2.1. “Eat-Me” Signals
2.2.2. ”Do-Not-Eat-Me” Signals
2.3. ”Digest-Me” Phase
3. Roles and Responses of Microglia to Alzheimer’s Disease Pathogenesis
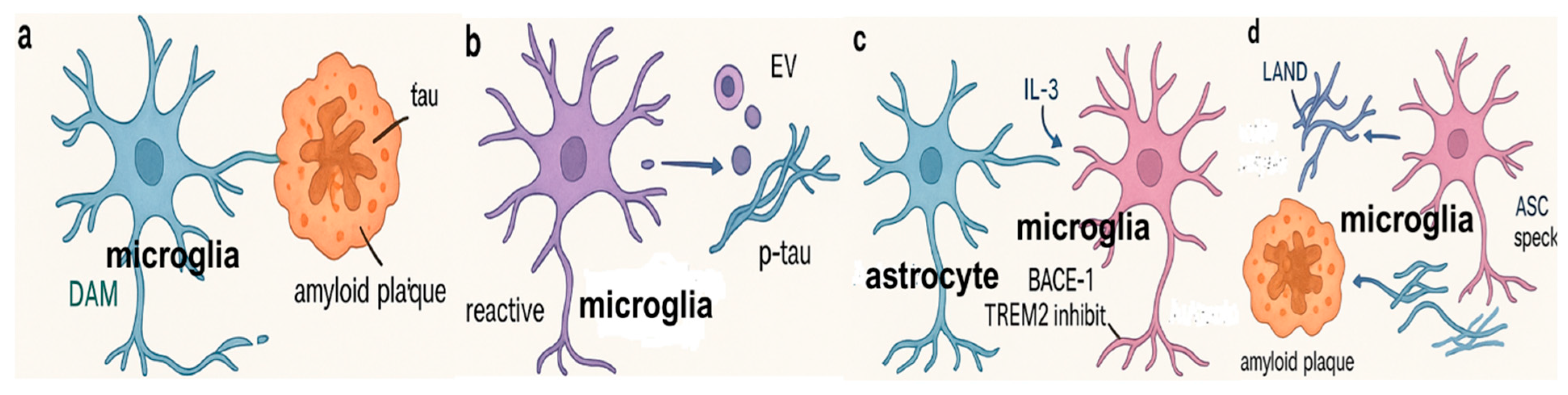
3.1. Microglial Effects on Aβ Pathology
3.2. Effect of Microglia on Tau Pathology
3.3. Molecular Sensors for Microglial Phagocytosis in Alzheimer’s Disease
4. Impaired Phagocytosis in Alzheimer’s Disease
4.1. Mechanisms Underlying Impaired Phagocytosis in Alzheimer’s Disease
4.1.1. Genetic Factors and Receptor Dysfunction
4.1.2. Chronic Inflammation, Oxidative Stress and Microglia Senescence
4.1.3. Dysfunction of Phagocytic Receptors and Signaling Pathways
4.2. Consequences of Impaired Phagocytosis in Alzheimer’s Disease
4.2.1. Accumulation of Amyloid-Beta Plaques and Neurofibrillary Tangles
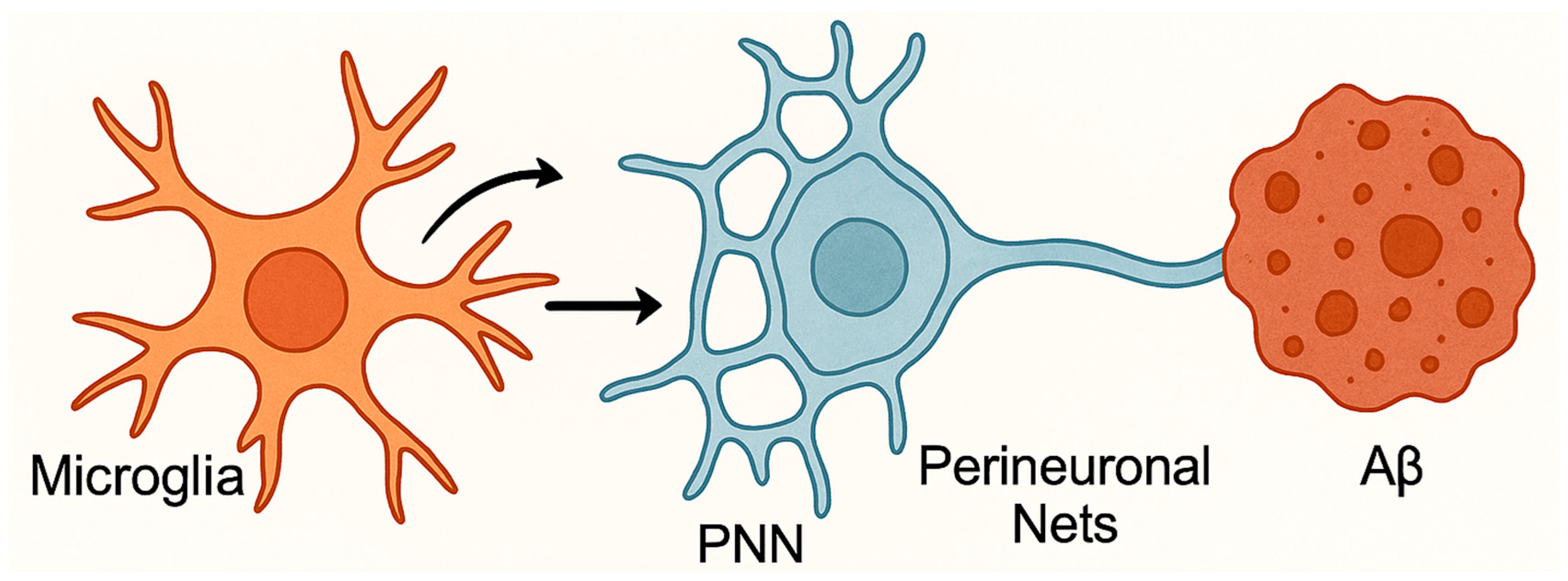
4.2.2. Microglial Dysfunction Impairs Neuronal Plasticity
4.3. Conflicting Evidence on Microglial Phagocytosis in Alzheimer’s Disease
5. Targeting Microglial Phagocytosis for Treating Alzheimer’s Disease
5.1. Targeting “Eat Me” Signals
5.2. Targeting TREM2
5.3. Restoring Lysosomal Function
5.4. Toll-like Receptor Agonists
5.5. Targeting Oxidized Lipoproteins and Complement Pathways
6. Drug Therapies That Modulate Microglial Phagocytosis for Treating Alzheimer’s Disease
6.1. TREM2-Targeting Antibodies
6.2. PPARγ Agonists
6.3. CD33 Inhibitors
6.4. NLRP3 Inflammasome Inhibitors
6.5. Interleukin-33
6.6. CSF1R Inhibitors
6.7. Complement System Inhibitors
7. Barriers to Clinical Translation
7.1. Timing and Disease Stage
7.2. Species Gaps and Model Limitations
7.3. Heterogeneity Across AD Subtypes
7.4. Off-Target Toxicity and Overactivation
7.5. Emerging Metabolic and Peripheral Influences
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beiter, R.M.; Sheehan, P.W.; Schafer, D.P. Microglia phagocytic mechanisms: Development informing disease. Curr. Opin. Neurobiol. 2024, 86, 102877. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Iglesias, M.; Maldonado-Teixido, J.; Melero, A.; Piriz, J.; Galea, E.; Ransohoff, R.M.; Sierra, A. Microglia as hunters or gatherers of brain synapses. Nat. Neurosci. 2024, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zengeler, K.E.; Lukens, J.R. Microglia pack a toolbox for life. Trends Immunol. 2024, 45, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Crapser, J.D.; Spangenberg, E.E.; Barahona, R.A.; Arreola, M.A.; Hohsfield, L.A.; Green, K.N. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine 2020, 58, 102919. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Mi-croglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Parakalan, R.; Jiang, B.; Nimmi, B.; Janani, M.; Jayapal, M.; Lu, J.; Tay, S.S.; Ling, E.-A.; Dheen, S.T. Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neurosci. 2012, 13, 64. [Google Scholar] [CrossRef]
- Provenzano, F.; Pérez, M.J.; Deleidi, M. Redefining microglial identity in health and disease at single-cell resolution. Trends Mol. Med. 2021, 27, 47–59. [Google Scholar] [CrossRef]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A unique microglia type associated with restricting development of alzheimer’s disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Mathys, H.; Adaikkan, C.; Gao, F.; Young, J.Z.; Manet, E.; Hemberg, M.; De Jager, P.L.; Ransohoff, R.M.; Regev, A.; Tsai, L.-H. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017, 21, 366–380. [Google Scholar] [CrossRef]
- Gerrits, E.; Brouwer, N.; Kooistra, S.M.; Woodbury, M.E.; Vermeiren, Y.; Lambourne, M.; Mulder, J.; Kummer, M.; Möller, T.; Biber, K.; et al. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 2021, 141, 681–696. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; Hahn-Pedersen, J.H.; Eichinger, C.S.; Freeman, C.; Clark, A.; Tarazona, L.R.S.; Cummings, J. Burden of illness in people with alzheimer’s disease: A systematic review of epidemiology, comorbidities and mortality. J. Prev. Alzheimer’s Dis. 2024, 11, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- van der Flier, W.M.; de Vugt, M.E.; Smets, E.M.A.; Blom, M.; Teunissen, C.E. Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat. Aging 2023, 3, 494–505. [Google Scholar] [CrossRef]
- Kaur, S.K.M.; Sharma, A.; Giridharan, V.V.; Dandekar, M.P. Brain resident microglia in Alzheimer’s disease: Foe or friends. Inflammopharmacology 2024, 32, 2781–2800. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Rawlinson, C.; Jenkins, S.; Thei, L.; Dallas, M.L.; Chen, R. Post-ischaemic immunological response in the brain: Targeting microglia in ischaemic stroke therapy. Brain Sci. 2020, 10, 159. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An immunobiologic process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Chocarro, L.; Echaide, M.; Ausin, K.; Escors, D.; Kochan, G. Fractalkine in health and disease. Int. J. Mol. Sci. 2024, 25, 8007. [Google Scholar] [CrossRef]
- Winter, A.N.; Subbarayan, M.S.; Grimmig, B.; Weesner, J.A.; Moss, L.; Peters, M.; Weeber, E.; Nash, K.; Bickford, P.C. Two forms of CX3CL1 display differential activity and rescue cognitive deficits in CX3CL1 knockout mice. J. Neuroinflamm. 2020, 17, 157. [Google Scholar] [CrossRef]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF production and release from microglia via extracellular vesicles: Impact on brain functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef]
- Komori, T.; Okamura, K.; Ikehara, M.; Yamamuro, K.; Endo, N.; Okumura, K.; Yamauchi, T.; Ikawa, D.; Ouji-Sageshima, N.; Toritsuka, M.; et al. Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior. Mol. Psychiatry 2024, 29, 1338–1349. [Google Scholar] [CrossRef]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef]
- Moon, B.; Yang, S.; Moon, H.; Lee, J.; Park, D. After cell death: The molecular machinery of efferocytosis. Exp. Mol. Med. 2023, 55, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, F.; Snaidero, N.; Kleinberger, G.; Madore, C.; Daria, A.; Werner, G.; Krasemann, S.; Capell, A.; Trümbach, D.; Wurst, W.; et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. Embo Rep. 2017, 18, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.B.; Kremen, V.; Haruwaka, K.; Zhao, S.; Wang, L.; Ebner, B.A.; Zheng, J.; Xie, M.; Dheer, A.; Perry, J.F.; et al. Microglial TREM2 promotes phagocytic clearance of damaged neurons after status epilepticus. Brain Behav. Immun. 2025, 123, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Serrano, J.R.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Honda, S.; Sasaki, Y.; Ohsawa, K.; Imai, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 2001, 21, 1975–1982. [Google Scholar] [CrossRef]
- Koizumi, S.; Shigemoto-Mogami, Y.; Nasu-Tada, K.; Shinozaki, Y.; Ohsawa, K.; Tsuda, M.; Joshi, B.V.; Jacobson, K.A.; Kohsaka, S.; Inoue, K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 2007, 446, 1091–1095. [Google Scholar] [CrossRef]
- Wendt, S.; Maricos, M.; Vana, N.; Meyer, N.; Guneykaya, D.; Semtner, M.; Kettenmann, H. Changes in phagocytosis and potassium channel activity in microglia of 5xFAD mice indicate alterations in purinergic signaling in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2017, 58, 41–53. [Google Scholar] [CrossRef]
- Miksa, M.; Amin, D.; Wu, R.; Dong, W.; Ravikumar, T.S.; Wang, P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol. Med. 2007, 13, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Innamorato, N.G.; Jaworski, T.; Rábano, A.; Kügler, S.; Van Leuven, F.; Cuadrado, A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain 2014, 137, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Neher, J.J.; Zhao, J.-W.; Théry, C.; Tolkovsky, A.M.; Brown, G.C. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J. Neurosci. 2012, 32, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, L.; Través, P.G.; Tufail, Y.; Leal-Bailey, H.; Lew, E.D.; Burrola, P.G.; Callaway, P.; Zagórska, A.; Rothlin, C.V.; Nimmerjahn, A.; et al. TAM receptors regulate multiple features of microglial physiology. Nature 2016, 532, 240–244. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, C.R.; Park, K.; Kim, S.; Roh, E.; Kim, M.J.; Lee, J.; Park, S.; Lee, J.H.; Kim, S.; et al. Microglia gravitate toward amyloid plaques surrounded by externalized phosphatidylserine. Adv. Sci. 2024, 11, e2420676. [Google Scholar] [CrossRef]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat. Commun. 2014, 5, 4046. [Google Scholar] [CrossRef]
- Lemke, G. Phosphatidylserine is the signal for TAM receptors and their ligands. Trends Biochem. Sci. 2017, 42, 738–748. [Google Scholar] [CrossRef]
- Galvan, M.D.; Greenlee-Wacker, M.C.; Bohlson, S.S. C1q and phagocytosis: The perfect complement to a good meal. J. Leukoc. Biol. 2012, 92, 489–497. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Park, Y.-J.; Tsuruta, Y.; Lorne, E.F.; Zhao, X.; Abraham, E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 2008, 181, 4240–4246. [Google Scholar] [CrossRef]
- Liang, T.; Yang, S.-X.; Qian, C.; Du, L.-D.; Qian, Z.-M.; Yung, W.-H.; Ke, Y. HMGB1 mediates inflammation-induced DMT1 increase and dopaminergic neurodegeneration in the early stage of parkinsonism. Mol. Neurobiol. 2024, 61, 2006–2020. [Google Scholar] [CrossRef]
- Ohnishi, H.; Kaneko, Y.; Okazawa, H.; Miyashita, M.; Sato, R.; Hayashi, A.; Tada, K.; Nagata, S.; Takahashi, M.; Matozaki, T. Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J. Neurosci. 2005, 25, 2702–2711. [Google Scholar] [CrossRef]
- Yamao, T.; Noguchi, T.; Takeuchi, O.; Nishiyama, U.; Morita, H.; Hagiwara, T.; Akahori, H.; Kato, T.; Inagaki, K.; Okazawa, H.; et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 2002, 277, 39833–39839. [Google Scholar] [CrossRef]
- Han, M.H.; Lundgren, D.H.; Jaiswal, S.; Chao, M.; Graham, K.L.; Garris, C.S.; Axtell, R.C.; Ho, P.P.; Lock, C.B.; Woodard, J.I.; et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J. Exp. Med. 2012, 209, 1325–1334. [Google Scholar] [CrossRef]
- Claude, J.; Linnartz-Gerlach, B.; Kudin, A.P.; Kunz, W.S.; Neumann, H. Microglial CD33-related siglec-E inhibits neurotoxicity by preventing the phagocytosis-associated oxidative burst. J. Neurosci. 2013, 33, 18270–18276. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.I.; Elguero, E.J.; Tran, J.A.; Sinatra, V.; Feany, M.B.; McCall, K. Defective phagocytic corpse processing results in neurodegeneration and can be rescued by TORC1 activation. J. Neurosci. 2016, 36, 3170–3183. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Gutierrez, D.; Hughes, A.L.; Madeo, F.; Ruckenstuhl, C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 2016, 32, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Son, S.M.; Mook-Jung, I. Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer’s disease mouse model. Glia 2016, 64, 2274–2290. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- St-Pierre, M.-K.; Carrier, M.; Ibáñez, F.G.; Šimončičová, E.; Wallman, M.-J.; Vallières, L.; Parent, M.; Tremblay, M. Ultrastructural characterization of dark microglia during aging in a mouse model of Alzheimer’s disease pathology and in human post-mortem brain samples. J. Neuroinflamm. 2022, 19, 235. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial ac-tivation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018, 141, 2740–2754. [Google Scholar]
- Smith, A.M.; Davey, K.; Tsartsalis, S.; Khozoie, C.; Fancy, N.; Tang, S.S.; Liaptsi, E.; Weinert, M.; McGarry, A.; Muirhead, R.C.J.; et al. Diverse human astrocyte and microglial transcriptional responses to Alzheimer’s pathology. Acta Neuropathol. 2022, 143, 75–91. [Google Scholar] [CrossRef]
- El Khoury, J.B.; Moore, K.J.; Means, T.K.; Leung, J.; Terada, K.; Toft, M.; Freeman, M.W.; Luster, A.D. CD36 mediates the innate host response to β-amyloid. J. Exp. Med. 2003, 197, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; D’ABramo, C.; Zingg, J.-M.; Giliberto, L.; Markesbery, W.; Azzi, A.; Marinari, U.M.; Pronzato, M.A.; Tabaton, M. CD36 overexpression in human brain correlates with β-amyloid deposition but not with Alzheimer’s disease. Free Radic. Biol. Med. 2004, 36, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; O’bRien, C.E.; Bieri, G.; Czirr, E.; Mosher, K.I.; Abbey, R.J.; Mastroeni, D.F.; Rogers, J.; Spencer, B.; Masliah, E.; et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in alzheimer’s disease. Neuron 2013, 79, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Teubner, B.J.; Tummers, B.; Boada-Romero, E.; Harris, L.; Yang, M.; Guy, C.S.; Zakharenko, S.S.; Green, D.R. LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine alzheimer’s disease. Cell 2019, 178, 536–551.e14. [Google Scholar] [CrossRef]
- Wang, J.; Qin, X.; Sun, H.; He, M.; Lv, Q.; Gao, C.; He, X.; Liao, H. Nogo receptor impairs the clearance of fibril amyloid-β by microglia and accelerates Alzheimer’s-like disease progression. Aging Cell 2021, 20, e13515. [Google Scholar] [CrossRef]
- Singh, N.; Benoit, M.R.; Zhou, J.; Das, B.; Davila-Velderrain, J.; Kellis, M.; Tsai, L.-H.; Hu, X.; Yan, R. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci. Adv. 2022, 8, eabo1286. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 2021, 595, 701–706. [Google Scholar] [CrossRef]
- Yang, D.S.; Stavrides, P.; Mohan, P.S.; Kaushik, S.; Kumar, A.; Ohno, M.; Schmidt, S.D.; Wesson, D.; Bandyopadhyay, U.; Jiang, Y.; et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 2011, 134, 258–277. [Google Scholar] [CrossRef]
- Yang, D.-S.; Stavrides, P.; Saito, M.; Kumar, A.; Rodriguez-Navarro, J.A.; Pawlik, M.; Huo, C.; Walkley, S.U.; Saito, M.; Cuervo, A.M.; et al. Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: Prevention by correcting lysosomal proteolytic deficits. Brain 2014, 137, 3300–3318. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Akatsu, H.; Fraser, G.; Crowther, R.A.; Frank, S.; Hench, J.; Probst, A.; Winkler, D.T.; Reichwald, J.; Staufenbiel, M.; et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA 2013, 110, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Hernández, F.; Ávila, J. Tau Spreading Mechanisms; Implications for Dysfunctional Tauopathies. Int. J. Mol. Sci. 2018, 19, 645. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Perea, J.R.; Jurado-Arjona, J.; Rábano, A.; Hernández, F.; Avila, J. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol. Neurodegener. 2017, 12, 59. [Google Scholar] [CrossRef]
- Mondragonrodriguez, S.; Perry, G.; Lunamunoz, J.; Acevedo-Aquino, M.C.; Williams, S.E. Phosphorylation of tau protein at sites Ser396-404is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Wang, C.; Fan, L.; Khawaja, R.R.; Liu, B.; Zhan, L.; Kodama, L.; Chin, M.; Li, Y.; Le, D.; Zhou, Y.; et al. Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat. Commun. 2022, 13, 1969. [Google Scholar] [CrossRef]
- Pomilio, C.; Gorojod, R.M.; Riudavets, M.; Vinuesa, A.; Presa, J.; Gregosa, A.; Bentivegna, M.; Alaimo, A.; Alcon, S.P.; Sevlever, G.; et al. Microglial autophagy is impaired by prolonged exposure to β-amyloid peptides: Evidence from experimental models and Alzheimer’s disease patients. GeroScience 2020, 42, 613–632. [Google Scholar] [CrossRef]
- Xu, Y.; Propson, N.E.; Du, S.; Xiong, W.; Zheng, H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc. Natl. Acad. Sci. USA 2021, 118, e2023418118. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Iwahara, N.; Abdullah, M.; Onos, K.D.; Keezer, K.J.; Hu, J.; Ikezu, S.; Howell, G.R.; Gygi, S.P.; et al. Enrichment of Neurodegenerative Microglia Signature in Brain-Derived Extracellular Vesicles Isolated from Alzheimer’s Disease Mouse Models. J. Proteome Res. 2021, 20, 1733–1743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruan, Z.; Delpech, J.-C.; Kalavai, S.V.; Van Enoo, A.A.; Hu, J.; Ikezu, S.; Ikezu, T. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol. Neurodegener. 2020, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective β-amyloid clearance pathways in aging alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Frenkel, D.; Wilkinson, K.; Zhao, L.; Hickman, S.E.; Means, T.K.; Puckett, L.; Farfara, D.; Kingery, N.D.; Weiner, H.L.; El Khoury, J. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 2013, 4, 2030. [Google Scholar] [CrossRef]
- Fu, H.; Liu, B.; Frost, J.L.; Hong, S.; Jin, M.; Ostaszewski, B.; Shankar, G.M.; Costantino, I.M.; Carroll, M.C.; Mayadas, T.N.; et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 2012, 60, 993–1003. [Google Scholar] [CrossRef]
- García-Alberca, J.M.; de Rojas, I.; Sanchez-Mejias, E.; Garrido-Martín, D.; Gonzalez-Palma, L.; Jimenez, S.; Pino-Angeles, A.; Cruz-Gamero, J.M.; Mendoza, S.; Alarcón-Martín, E.; et al. An Insertion Within SIRPβ1 Shows a Dual Effect Over Alzheimer’s Disease Cognitive Decline Altering the Microglial Response. J. Alzheimer’s Dis. 2024, 98, 601–618. [Google Scholar] [CrossRef]
- D’errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezö, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, Y.; Zhuo, R.; Wang, T.; Wang, K.; Huang, R.; Wang, D.; Gao, Y.; Zhu, Y.; Sheng, X.; et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Neniskyte, U.; Neher, J.J.; Brown, G.C. Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 2011, 286, 39904–39913. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Larionov, S.; Wang, Y.; Dannenberg, H.; Matozaki, T.; Monsonego, A.; Thal, D.R.; Neumann, H. Signal regulatory pro-tein-beta1: A microglial modulator of phagocytosis in Alzheimer’s disease. Am. J. Pathol. 2009, 175, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lue, L.-F.; Yan, S.; Xu, H.; Luddy, J.S.; Chen, D.; Walker, D.G.; Stern, D.M.; Yan, S.; Schmidt, A.M.; et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010, 24, 1043–1055. [Google Scholar] [CrossRef]
- Jones, R.S.; Minogue, A.M.; Connor, T.J.; Lynch, M.A. Amyloid-β-Induced Astrocytic Phagocytosis is Mediated by CD36, CD47 and RAGE. J. Neuroimmune Pharmacol. 2013, 8, 301–311. [Google Scholar] [CrossRef]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2017, 603, 885–892. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Wang, K.; Hu, G.; Wang, X.; Miao, Z.; Azevedo, J.A.; Suh, E.; Van Deerlin, V.M.; Choi, D.; Roeder, K.; et al. APOE and TREM2 regulate amyloid-responsive microglia in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 477–493. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Lish, A.M.; Grogan, E.F.; Benoit, C.R.; Pearse, R.V.; Heuer, S.E.; Luquez, T.; Orme, G.A.; Galle, P.C.; Milinkeviciute, G.; Green, K.N.; et al. CLU alleviates Alzheimer’s disease-relevant processes by modulating astrocyte reactivity and microglia-dependent synaptic density. Neuron 2025, 113, 1925–1946.e11. [Google Scholar] [CrossRef]
- McQuade, A.; Kang, Y.J.; Hasselmann, J.; Jairaman, A.; Sotelo, A.; Coburn, M.; Shabestari, S.K.; Chadarevian, J.P.; Tu, C.H.; Fote, G.; et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 2020, 11, 5370. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Leyns, C.E.G.; Gratuze, M.; Narasimhan, S.; Jain, N.; Koscal, L.J.; Jiang, H.; Manis, M.; Colonna, M.; Lee, V.M.Y.; Ulrich, J.D.; et al. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci. 2019, 22, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tan, L.; Chen, Q.; Tan, M.-S.; Zhou, J.-S.; Zhu, X.-C.; Lu, H.; Wang, H.-F.; Zhang, Y.-D.; Yu, J.-T. A rare coding variant in TREM2 increases risk for Alzheimer’s disease in Han Chinese. Neurobiol. Aging 2016, 42, 217.e1. [Google Scholar] [CrossRef] [PubMed]
- Olive, C.; Ibanez, L.; Farias, F.H.G.; Wang, F.; Budde, J.P.; Norton, J.B.; Gentsch, J.; Morris, J.C.; Li, Z.; Dube, U.; et al. Examination of the effect of rare variants in TREM2, ABI3, and PLCG2 in LOAD through multiple phenotypes. J. Alzheimer’s Dis. 2020, 77, 1469–1482. [Google Scholar] [CrossRef]
- Ewers, M.; Biechele, G.; Suárez-Calvet, M.; Sacher, C.; Blume, T.; Morenas-Rodriguez, E.; Deming, Y.; Piccio, L.; Cruchaga, C.; Kleinberger, G.; et al. Higher CSF sTREM2 and microglia activation are associated with slower rates of beta-amyloid accumulation. EMBO Mol. Med. 2020, 12, e12308. [Google Scholar] [CrossRef]
- Vilalta, A.; Zhou, Y.; Sevalle, J.; Griffin, J.K.; Satoh, K.; Allendorf, D.H.; De, S.; Puigdellívol, M.; Bruzas, A.; Burguillos, M.A.; et al. Wild-type sTREM2 blocks Aβ aggregation and neurotoxicity, but the Alzheimer’s R47H mutant increases Aβ aggregation. J. Biol. Chem. 2021, 296, 100631. [Google Scholar] [CrossRef]
- Thornton, P.; Sevalle, J.; Deery, M.J.; Fraser, G.; Zhou, Y.; Ståhl, S.; Franssen, E.H.; Dodd, R.B.; Qamar, S.; Perez-Nievas, B.G.; et al. TREM 2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer’s disease-associated H157Y variant. EMBO Mol. Med. 2017, 9, 1366–1378. [Google Scholar] [CrossRef]
- Moutinho, M.; Coronel, I.; Tsai, A.P.; Di Prisco, G.V.; Pennington, T.; Atwood, B.K.; Puntambekar, S.S.; Smith, D.C.; Martinez, P.; Han, S.; et al. TREM2 splice isoforms generate soluble TREM2 species that disrupt long-term potentiation. Genome Med. 2023, 15, 11. [Google Scholar] [CrossRef]
- Tsai, H.H.; Li, H.; Fu, A.K.Y.; Ip, N.Y. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 2022, 29, 1197–1212. [Google Scholar] [CrossRef]
- Huang, H.; Xiang, R.; Yan, R. Linking APOE4/4 genotype to microglial lipid droplets and neurotoxicity in Alzheimer’s disease. Transl. Neurodegener. 2024, 13, 38. [Google Scholar] [CrossRef]
- Zhang, B.; Gaitán-Zeballos, M. Modulation of Microglial Phagocytosis: Molecular Mechanisms and Implications for Alzheimer’s Disease. J. Neuroinflamm. 2020, 17, 18. [Google Scholar] [CrossRef]
- Madsen, P.M.; Pinto, M. Targeting the NLRP3 inflammasome in Alzheimer’s disease: A possible therapeutic approach. J. Neurosci. Res. 2019, 97, 1187–1200. [Google Scholar]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.; Mustafa, S.; Collins-Praino, L.E. The Hallmarks of Ageing in Microglia. Cell. Mol. Neurobiol. 2025, 45, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The role of advanced glycation end products in aging and metabolic diseases: Bridging association and causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Arancio, O.; Zhang, H.P.; Chen, X.; Lin, C.; Trinchese, F.; Puzzo, D.; Liu, S.; Hegde, A.; Yan, S.F.; Stern, A.; et al. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004, 23, 4096–4105. [Google Scholar] [CrossRef]
- Origlia, N.; Righi, M.; Capsoni, S.; Cattaneo, A.; Fang, F.; Stern, D.M.; Chen, J.X.; Schmidt, A.M.; Arancio, O.; Du Yan, S.; et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-β-mediated cortical synaptic dysfunction. J. Neurosci. 2008, 28, 3521–3530. [Google Scholar] [CrossRef]
- Floden, A.M.; Combs, C.K. Microglia demonstrate age-dependent interaction with amyloid-β fibrils. J. Alzheimer’s Dis. 2011, 25, 279–293. [Google Scholar] [CrossRef]
- Karki, S.; Nichols, M.R. CD47 does not mediate amyloid-β(1–42) protofibril-stimulated microglial cytokine release. Biochem. Biophys. Res. Commun. 2014, 454, 239–244. [Google Scholar] [CrossRef]
- Ulland, T.K.; Colonna, M. TREM2—A key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef]
- Pan, R.-Y.; Liu, Y.; Liu, Z.-Y.; Qu, W.; Zhou, D.-Y.; Wang, J.; Xu, Y.; Hu, Y.-J.; Qiao, H.; Xu, Q.-Q.; et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022, 34, 634–648.e63. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Hu, S.J.; Alonge, K.M. The “loss” of perineuronal nets in Alzheimer's disease: Missing or hiding in plain sight? Front. Integr. Neurosci. 2022, 16, 896400. [Google Scholar] [CrossRef]
- de Vries, L.E.; Bahnerth, A.; Swaab, D.F.; Verhaagen, J.; Carulli, D. Resilience to Alzheimer’s disease associates with alterations in perineuronal nets. Alzheimer’s Dement. 2025, 21, e14504. [Google Scholar] [CrossRef] [PubMed]
- Egorova, D.; Kerever, A.; Inada, M.; Itoh, Y.; Arikawa-Hirasawa, E.; Miyata, S. Microglial depletion increases aggrecan and hyaluronan levels in the diffuse and aggregated extracellular matrix of the mouse brain. Sci. Rep. 2025, 15, 9376. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mumford, P.; Noy, S.; Cleverley, K.; Mrzyglod, A.; Luo, D.; van Dalen, F.; Verdoes, M.; Fisher, E.M.C.; Wiseman, F.K. Cathepsin B abundance, activity and microglial localisation in Alzheimer’s disease-Down syndrome and early onset Alzheimer’s disease; the role of elevated cystatin B. Acta Neuropathol. Commun. 2023, 11, 132. [Google Scholar] [CrossRef]
- Baligács, N.; Albertini, G.; Borrie, S.C.; Serneels, L.; Pridans, C.; Balusu, S.; De Strooper, B. Homeostatic microglia initially seed and activated microglia later reshape amyloid plaques in Alzheimer’s Disease. Nat. Commun. 2024, 15, 10634. [Google Scholar] [CrossRef]
- Feringa, F.M.; Koppes-den Hertog, S.J.; Wang, Y.L.; Derks, R.J.; Kruijff, I.; Erlebach, L.; Heijneman, J.; Miramontes, R.; Blomberg, N.; Olivier-Jimenez, D.; et al. The Neurolipid Atlas: A lipidomics resource for neurodegenerative diseases. Nat. Metab. 2025, 1–23. [Google Scholar] [CrossRef]
- Lepiarz-Raba, I.; Musial, A.; Grudzien, A.; Pera, J. Metabolic regulation of microglial phagocytosis: Implications for Alzheimer’s disease therapeutics. Transl. Neurodegener. 2023, 12, 39. [Google Scholar] [CrossRef]
- Noh, M.Y.; Kim, S.; Choi, S.; Kim, H.J.; Kim, S.H. Biomarkers and therapeutic strategies targeting microglia in neurodegenerative diseases: Current status and future directions. Mol. Neurodegener. 2025, 20, 41. [Google Scholar] [CrossRef]
- Sun, Z.; Luo, J.; Li, Q.; Li, Y. Targeting microglia in Alzheimer’s disease: Pathogenesis and therapeutic strategies. Front. Aging. Neurosci. 2024, 16, 1383854. [Google Scholar] [PubMed]
- Liu, G.; Fu, Y. The Roles of Phosphatidylserine and Phosphatidylserine Receptors in Apoptotic Cell Clearance: Mechanisms and Implications. Front. Immunol. 2019, 10, 1039. [Google Scholar] [CrossRef]
- Horiuchi, K.; Jinushi, M. Calreticulin: A Possible Novel Target in Neurodegenerative Disorders and Cancer Therapy. Cell Stress Chaperones 2018, 23, 361–372. [Google Scholar]
- Madhu, L.N.; Attaluri, S. Neuro-Inflammation and Microglial Activation in Alzheimer’s Disease: Implications for Therapeutic Intervention. J. Clin. Med. 2020, 9, 749. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Manolopoulos, A.; Ferrucci, L.; Kapogiannis, D. Critical assessment of anti-amyloid-β monoclonal antibodies effects in Alzheimer’s disease: A systematic review and meta-analysis highlighting target engagement and clinical meaningfulness. Sci. Rep. 2024, 14, 25741. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Fujita, K.; Motoki, K.; Tagawa, K.; Chen, X.; Hama, H.; Nakajima, K.; Homma, H.; Tamura, T.; Watanabe, H.; Katsuno, M.; et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 2016, 6, 31895. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): From risk factors to therapeutic targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Ding, X.; Wang, J.; Huang, M.; Chen, Z.; Liu, J.; Zhang, Q.; Zhang, C.; Xiang, Y.; Zen, K.; Li, L. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat. Commun. 2021, 12, 2030. [Google Scholar] [CrossRef]
- Ju, J.; Liu, X.; Zhao, C.; Jin, Q.; Zhang, X.; Chen, Z. The “don’t eat me” signal CD47 is associated with synaptic dysfunction; reducing CD47 signaling enhances microglia-mediated synapse phagocytosis. Proc. Natl. Acad. Sci. USA 2025, 122, e2411080122. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, Y.; Tang, Q.; Yuan, Z.; Jiang, K.; Zhang, Z.; Chen, S.; Wu, Q.; Zhou, X.; Zhang, X. SIRPα modulates microglial efferocytosis and neuroinflammation following experimental subarachnoid hemorrhage via the SHP1/STAT6 axis. J. Neuroinflamm. 2025, 22, 314. [Google Scholar] [CrossRef] [PubMed]
- Schoch, K.M.; Ezerskiy, L.A.; Morhaus, M.M.; Bannon, R.N.; Sauerbeck, A.D.; Shabsovich, M.; Jafar-Nejad, P.; Rigo, F.; Miller, T.M. Acute Trem2 reduction triggers increased microglial phagocytosis, slowing amyloid deposition in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2100356118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, P.; Xu, Y.; Jiang, L.; Fan, X.; Li, L.; Li, X.; Arase, H.; Zhao, Y.; Cao, W.; Zheng, H.; et al. A tetravalent TREM2 agonistic antibody reduced amyloid pathology in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabq0095. [Google Scholar] [CrossRef]
- Lee, S.-H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lee, S.H. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef]
- Atagi, Y.; Liu, C.-C.; Painter, M.M.; Chen, X.-F.; Verbeeck, C.; Zheng, H.; Li, X.; Rademakers, R.; Kang, S.S.; Xu, H.; et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J. Biol. Chem. 2015, 290, 26043–26050. [Google Scholar] [CrossRef]
- Quick, J.D.; Silva, C.; Wong, J.H.; Lim, K.L.; Reynolds, R.; Barron, A.M.; Zeng, J.; Lo, C.H. Lysosomal acidification dysfunction in microglia: An emerging pathogenic mechanism of neuroinflammation and neurodegeneration. J. Neuroinflamm. 2023, 20, 379. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Zhang, J.; Shao, Y.; Wu, B.; Liu, J. TFEB acetylation promotes lysosome biogenesis and ameliorates Alzheimer’s disease–relevant phenotypes in mice. Signal Transduct Target Ther. 2022, 7, 379. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, X.; Liu, H.; Zhang, Q.; Wang, J.; Zhang, H. TFEB agonist clomiphene citrate activates the autophagy-lysosomal pathway and improves pathology in an AD mouse model. J. Biol. Chem. 2024, 300, 105440. [Google Scholar] [CrossRef]
- Long, H.; Simmons, A.; Mayorga, A.; Burgess, B.; Nguyen, T.; Budda, B.; Rychkova, A.; Rhinn, H.; Tassi, I.; Ward, M.; et al. Preclinical and first-in-human evaluation of AL002, a novel TREM2 agonistic antibody for Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 235. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Hu, X.; Karako, K.; Song, P.; Tang, W.; Xia, Y. The potential and challenges of TREM2-targeted therapy in Alzheimer’s disease: Insights from the INVOKE-2 study. Front. Aging Neurosci. 2025, 17, 1576020. [Google Scholar] [CrossRef]
- Song, W.M.; Joshita, S.; Zhou, Y.; Ulland, T.K.; Gilfillan, S.; Colonna, M. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 2018, 215, 745–760. [Google Scholar] [CrossRef]
- Heneka, M.T.; Reyes-Irisarri, E.; Hüll, M.; Kummer, M.P. Impact and therapeutic potential of PPARγ modulation in microglial cells during AD. Curr. Alzheimer Res. 2005, 2, 465–473. [Google Scholar]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Swain, P. Rosiglitazone monotherapy in mild-to-moderate alzheimer’s disease: Results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, E.M.; Chibnik, L.B.; Keenan, B.T.; Ottoboni, L.; Raj, T.; Tang, A.; De Jager, P.L. CD33 Alzheimer’s disease locus: Altered monocyte function and amyloid biology. Nat. Neurosci. 2013, 16, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Griciuc, A.; Serrano-Pozo, A.; Parrado, A.R.; Lesinski, A.N.; Asselin, C.N.; Mullin, K.; Tanzi, R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 2013, 78, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Prakash, R.; Kumari, N.; Ali Khan, M.; Quaiyoom Khan, A.; Uddin, S.; Verma, S.; Robertson, A.A.B.; Boltze, J.; Raza, S.S. MCC950 reduces autophagy and improves cognitive function by inhibiting NLRP3-dependent neuroinflammation in a rat model of Alz-heimer’s disease. Brain Behav. Immun. 2024, 116, 70–84. [Google Scholar] [CrossRef]
- Han, R.T.; Vainchtein, I.D.; Schlachetzki, J.C.; Cho, F.S.; Dorman, L.C.; Ahn, E.; Kim, D.K.; Barron, J.J.; Nakao-Inoue, H.; Molofsky, A.B.; et al. Microglial pattern recognition via IL-33 promotes synaptic refinement in developing corticothalamic circuits in mice. J. Exp. Med. 2023, 220, e20220605. [Google Scholar] [CrossRef]
- Saresella, M.; La Rocca, D.; Marventano, I.; Piancone, F.; Zoppis, M.; Calabrese, E.; Fenoglio, C.; Galimberti, D.; Scarpini, E.; Clerici, M. IL-33 and sST2 in patients with Alzheimer’s disease and mild cognitive impairment. J. Neuroinflamm. 2020, 17, 59. [Google Scholar] [CrossRef]
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.P.; Blurton-Jones, M.; Green, K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2019, 142, 726–743. [Google Scholar] [CrossRef]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.-A.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of alzheimer’s disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef]
- Lansita, J.A.; Mease, K.M.; Qiu, H.; Yednock, T.; Sankaranarayanan, S.; Kramer, S. Nonclinical Development of ANX005: A Humanized Anti-C1q Antibody for Treatment of Autoimmune and Neurodegenerative Diseases. Int. J. Toxicol. 2017, 36, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, Q.D.; Islam, Z.; Papri, N.; Hayat, S.; Jahan, I.; Azad, K.A.K.; Artis, D.R.; Hoehn, B.; Humphriss, E.; Lin, P.; et al. Results From a Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ANX005, a C1q Inhibitor, in Patients With Guillain–Barré Syndrome. J. Peripher. Nerv. Syst. 2025, 30, e70009, Erratum in J. Peripher. Nerv. Syst. 2025, 30, e70024. https://doi.org/1010.1111/jns.70024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, M.Z.; Peng, T.; Duarte, M.L.; Wang, M.; Cai, D. Updates on mouse models of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 23. [Google Scholar] [CrossRef]
- Chen, Y.; Colonna, M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J. Exp. Med. 2021, 218, e20202717. [Google Scholar] [CrossRef]
- Sun, N.; Victor, M.B.; Park, Y.P.; Xiong, X.; Scannail, A.N.; Leary, N.; Prosper, S.; Viswanathan, S.; Luna, X.; Boix, C.A.; et al. Human microglial state dynamics in Alzheimer’s disease progression. Cell 2023, 186, 4386–4403.e29. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Berlanga, M.L.; Morsey, B.; Fox, H.S. Species-specific metabolic reprogramming in human and mouse iPSC-derived microglia supported by multi-omics analyses. Front. Cell. Neurosci. 2023, 17, 1254735. [Google Scholar]
- Dolan, M.J.; Li, M.; Xiong, X.; Victor, M.B.; Park, Y.P.; Bennett, D.A.; Kellis, M.; Tsai, L.H. Exposure of iPSC-derived human microglia to brain substrates enables the generation and manipulation of diverse transcriptional states in vitro. Nat. Immunol. 2023, 24, 1794–1806. [Google Scholar] [CrossRef]
- Clark, C.; Jurado-Garcia, J.; Huentelman, M. The promise of multi-omics approaches to discover new biomarker signatures and therapeutic targets in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1065904. [Google Scholar] [CrossRef]
- Tijms, B.M.; Vromen, E.M.; Mjaavatten, O.; Holstege, H.; Reus, L.M.; van der Lee, S.; Wesenhagen, K.E.J.; Lorenzini, L.; Vermunt, L.; Venkatraghavan, V.; et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat. Aging 2024, 4, 33–47. [Google Scholar] [CrossRef]
- Fitz, N.F.; Nam, K.N.; Wolfe, C.M.; Letronne, F.; Playso, B.E.; Iordanova, B.E.; Kozai, T.D.Y.; Biedrzycki, R.J.; Kagan, V.E.; Tyurina, Y.Y.; et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer’s disease. Nat. Commun. 2021, 12, 3416. [Google Scholar] [CrossRef] [PubMed]
- Yousefizadeh, A.; Piccioni, G.; Saidi, A.; Triaca, V.; Mango, D.; Nisticò, R. Pharmacological targeting of microglia dynamics in Alzheimer’s disease: Preclinical and clinical evidence. Pharmacol. Res. 2022, 184, 106404. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dys-functional and pro-inflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Arbaizar-Rovirosa, M.; Mora-Grau, B.; Fernández-Martínez, A.B.; Hernández, S.; Martín-Suárez, S.; Iglesias, G.; Ávila, C.; Saavedra, C.; Iglesias, T.; Casas, C.; et al. Aged lipid-laden microglia display impaired responses to metabolic challenge. EMBO Mol. Med. 2023, 15, e17175. [Google Scholar] [CrossRef]
- Loh, Y.P.; Wong, P.T.; Lai, M.K.P.; Ling, E.A. Microbiota–gut–brain axis and its therapeutic applications in Alzheimer’s disease. Signal Transduct. Target. Ther. 2024, 9, 122. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Chen, L.; Liu, C.; Zhang, Y.; Zhang, J. Advances in the study of the effects of gut microflora on neuroinflammation and microglia in Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 1295916. [Google Scholar] [CrossRef]

| Molecule | Substrate | Effect on Phagocytosis | Reference(s) |
|---|---|---|---|
| CXCL1/CX3CR1 | Tau | Upregulation | [77] |
| TREM2 | Aβ | Upregulation | [36,91] |
| Apoptotic cells | Upregulation | ||
| MFG-E8 | Apoptotic cells | Upregulation | [41,42,92] |
| C3 | Aβ | Upregulation | [88] |
| SIRPβ1 | Aβ | Upregulation | [93] |
| Scara1 | Aβ | Upregulation | [87] |
| RAGE | Aβ | Upregulation | [94,95] |
| Aβ | Downregulation | ||
| CD47 | Aβ | Upregulation | [52] |
| Apoptotic cells | Downregulation | ||
| NLRP3 | Aβ | Downregulation | [84] |
| Caspase-1 | Aβ | Downregulation | [84] |
| ASC | Aβ | Downregulation | [85] |
| Cystatin B | Aβ | Downregulation | [96] |
| P2Y6 | Aβ | Upregulation | [40] |
| Phosphatidylserine (PS) | Enhancing PS-receptor interaction may restore phagocytic function [132]. |
| Calreticulin | Exposed on stressed neurons due to Aβ and tau toxicity; insufficient clearance contributes to neuroinflammation [133]. Enhancing microglial sensitivity to calreticulin may reduce debris accumulation. |
| Oxidized LDL (oxLDL) | Oxidative stress increases oxLDL formation, which binds microglial receptors like CD36. Boosting CD36 function may improve clearance and reduce inflammation [134]. |
| Complement Proteins | Dysregulated complement activation contributes to neuroinflammation. Therapeutic inhibition of excessive complement activity while preserving clearance functions shows promise [134]. |
| Amyloid-beta (Aβ) | Immunotherapies designed to promote Aβ clearance are now in clinical use. This involves monoclonal antibodies, e.g., Aducanumab (Aduhelm), Lecanemab (Leqembi), Donanemab, facilitating their clearance through microglial phagocytosis or peripheral sink mechanisms [135]. |
| Neuron-Specific Enolase (NSE) | Elevated NSE reflects neuronal damage and overwhelmed microglial clearance; enhancing response to NSE could mitigate inflammation [136]. |
| High Mobility Group Box 1 (HMGB1) | Targeting HMGB1 or its receptors (e.g., RAGE, TLR4) may modulate inflammation while supporting clearance [137,138]. |
| Signal Regulatory Protein Alpha (SIRPα) | Blocking CD47-SIRPα interaction enhances microglial clearance and reduces inflammation, representing a novel therapeutic target [139,140,141]. |
| Strategy | Mechanism | Outcome in Preclinical/Clinical Models |
|---|---|---|
| TREM2 Agonists (e.g., AL002) | Enhances phagocytosis, promotes DAM phenotype | Reduced Aβ, improved cognition |
| PPARγ Agonists (e.g., Pioglitazone) | Anti-inflammatory and pro-phagocytic gene regulation | Decreased plaque burden, improved memory |
| CD33 Inhibitors | Blocks negative regulation of phagocytosis | Enhanced Aβ clearance, cognitive improvement |
| NLRP3 Inhibitors (e.g., MCC950) | Reduces inflammasome activation | Reduced inflammation, enhanced Aβ/tau clearance |
| IL-33 | Enhances phagocytosis via ST2 receptor | Reduced Aβ, improved synaptic health |
| CSF1R Inhibitors (e.g., PLX5622) | Depletes or resets microglial populations | Reduced neuroinflammation, potential repopulation benefit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, H.; Rawlinson, C.; Lan, Y.-L.; Jenkins, S.; Chen, R. Microglia-Mediated Phagocytosis in Alzheimer’s Disease: Mechanisms, Heterogeneity, and Therapeutic Insights. Biomolecules 2025, 15, 1629. https://doi.org/10.3390/biom15111629
Hassan H, Rawlinson C, Lan Y-L, Jenkins S, Chen R. Microglia-Mediated Phagocytosis in Alzheimer’s Disease: Mechanisms, Heterogeneity, and Therapeutic Insights. Biomolecules. 2025; 15(11):1629. https://doi.org/10.3390/biom15111629
Chicago/Turabian StyleHassan, Halimatu, Charlotte Rawlinson, Yu-Long Lan, Stuart Jenkins, and Ruoli Chen. 2025. "Microglia-Mediated Phagocytosis in Alzheimer’s Disease: Mechanisms, Heterogeneity, and Therapeutic Insights" Biomolecules 15, no. 11: 1629. https://doi.org/10.3390/biom15111629
APA StyleHassan, H., Rawlinson, C., Lan, Y.-L., Jenkins, S., & Chen, R. (2025). Microglia-Mediated Phagocytosis in Alzheimer’s Disease: Mechanisms, Heterogeneity, and Therapeutic Insights. Biomolecules, 15(11), 1629. https://doi.org/10.3390/biom15111629







