Ethanol Exposure Increases Oxygen Consumption by Developing Cerebral Arteries in a Trimester-, Concentration- and Sex-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Subjects and Experimental Groups
2.2. Measurement of Progeny Brain and Body Weight
2.3. Measurement of Blood Ethanol Levels
2.4. Oxygen Consumption and Analysis of Mitochondrial Respiration Parameters in Developing Cerebral Arteries
2.5. Quantification of Total DNA Content in Progeny Cerebral Arteries
2.6. Measurement of Corticosterone Levels
2.7. RNA Isolation and Quantitative PCR (qPCR) Analysis of Sry (Sex-Determining Region Y) Gene Expression
2.8. Chemicals Reagents
2.9. Statistical Analysis
3. Results
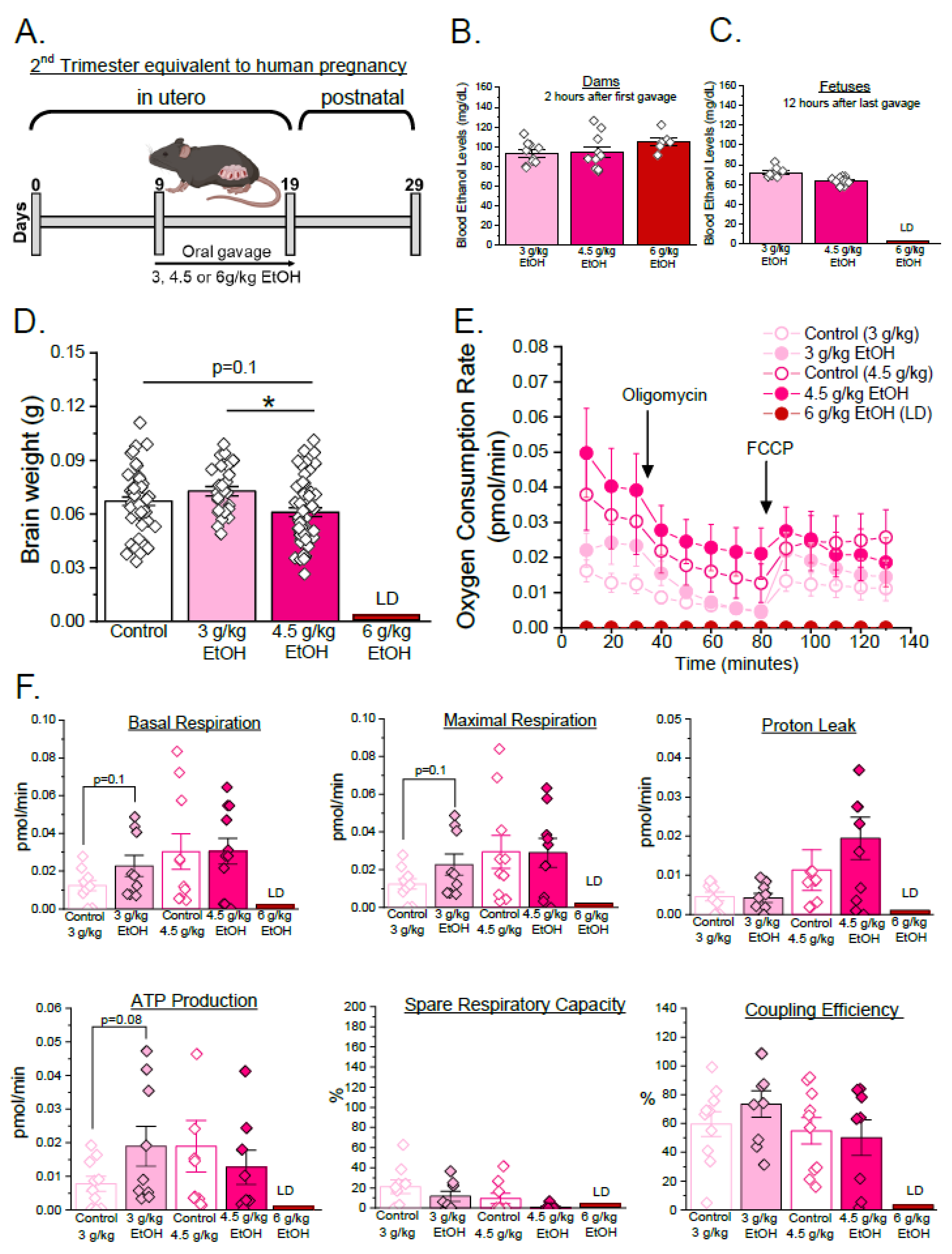
3.1. Developmental Stage- and Concentration-Specific Effect of Ethanol on Mitochondrial Respiratory Parameters of Developing Cerebral Arteries
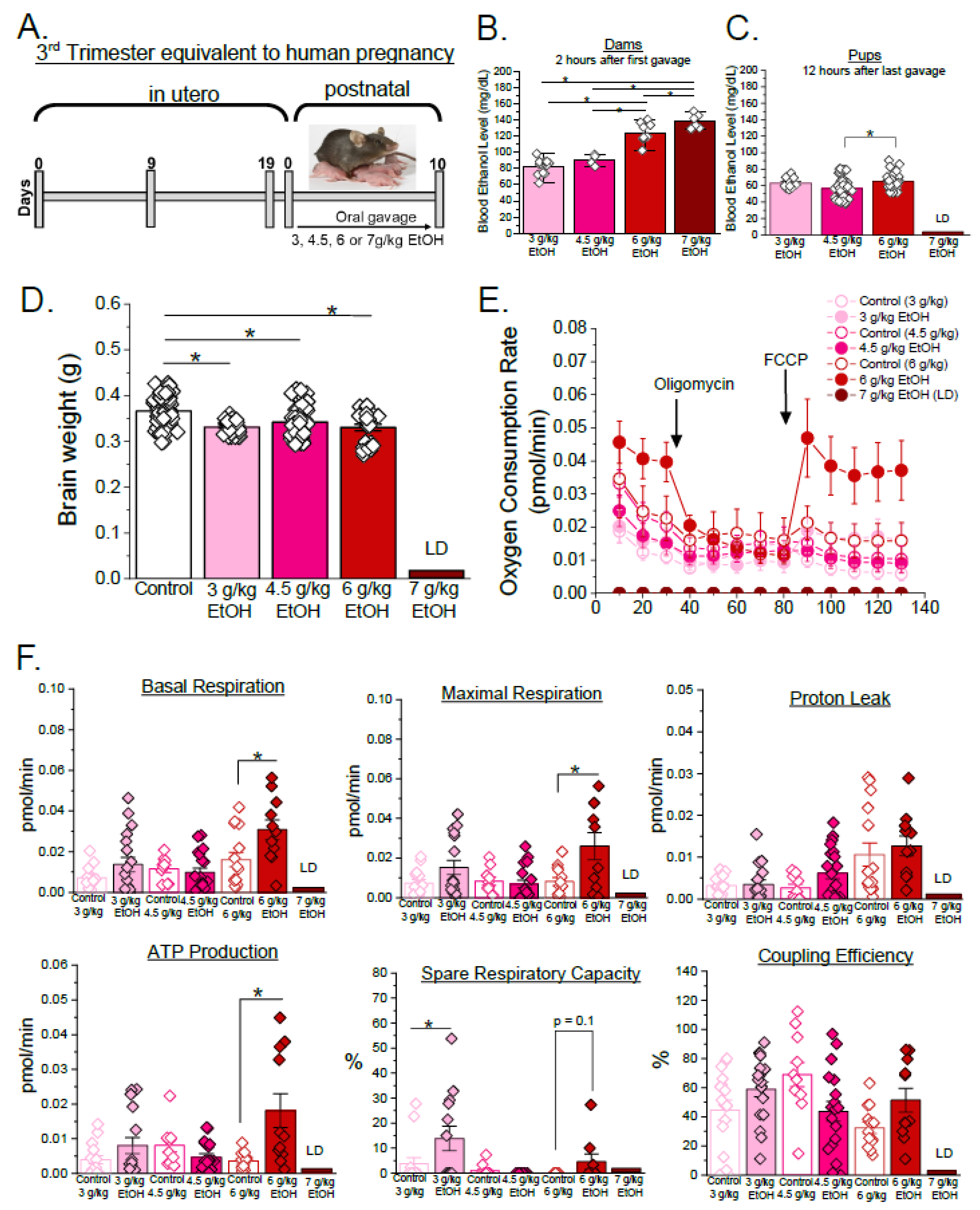
3.2. Sex-Specific Effects of Ethanol on Mitochondrial Respiration Parameters in Developing Cerebral Arteries During Third Trimester Equivalent of Human Pregnancy
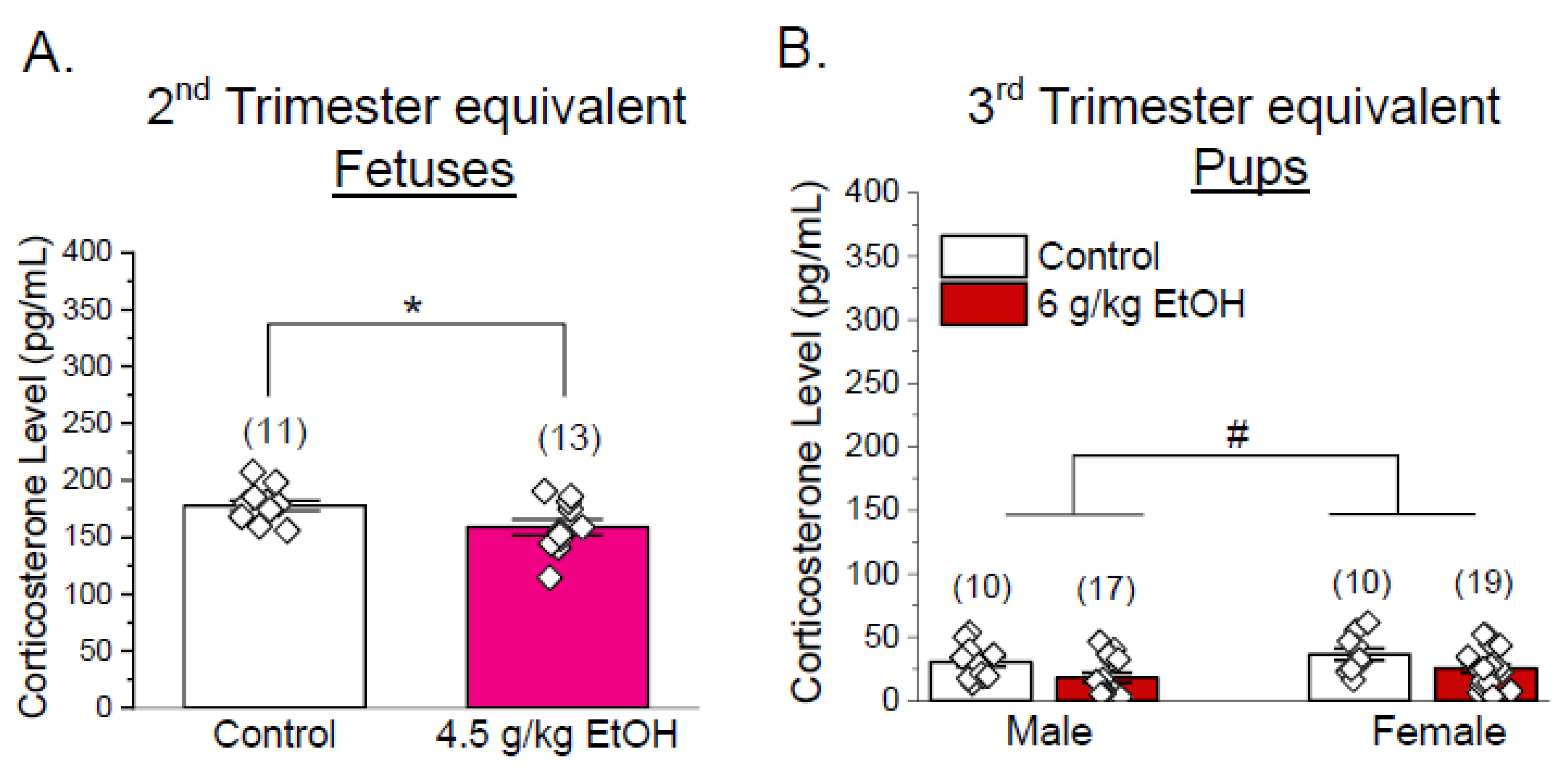
3.3. Corticosterone Levels Are Not Increased in Progeny of Ethanol-Exposed Dams
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EtOH | Ethanol |
| FASD | Fetal alcohol spectrum disorders |
| PEE | Prenatal ethanol exposure |
| GD | Gestational days |
| PD | Postnatal days |
| OCR | Oxygen consumption rate |
| FCCP | Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone |
| qPCR | Quantitative polymerase chain reaction |
| HSD | Honestly significant difference |
| LD | Lethal dose |
References
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef]
- Olivier, L.; Curfs, L.M.; Viljoen, D.L. Fetal alcohol spectrum disorders: Prevalence rates in South Africa. S. Afr. Med. J. 2016, 106 (Suppl. S1), S103–S106. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder. Biochem. Cell Biol. 2018, 96, 237–240. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Shield, K.; Burd, L.; Rehm, J. Prevalence of fetal alcohol spectrum disorder among special subpopulations: A systematic review and meta-analysis. Addiction 2019, 114, 1150–1172. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 2023, 9, 11. [Google Scholar] [CrossRef]
- Gosdin, L.K.; Deputy, N.P.; Kim, S.Y.; Dang, E.P.; Denny, C.H. Alcohol Consumption and Binge Drinking During Pregnancy Among Adults Aged 18–49 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Jarmasz, J.S.; Basalah, D.A.; Chudley, A.E.; Del Bigio, M.R. Human Brain Abnormalities Associated With Prenatal Alcohol Exposure and Fetal Alcohol Spectrum Disorder. J. Neuropathol. Exp. Neurol. 2017, 76, 813–833. [Google Scholar] [CrossRef]
- Lotfullina, N.; Khazipov, R. Ethanol and the Developing Brain: Inhibition of Neuronal Activity and Neuroapoptosis. Neuroscientist 2018, 24, 130–141. [Google Scholar] [CrossRef]
- Parnell, S.E.; Ramadoss, J.; Delp, M.D.; Ramsey, M.W.; Chen, W.A.; West, J.R.; Cudd, T.A. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: Ovine model. Exp. Physiol. 2007, 92, 933–943. [Google Scholar] [CrossRef]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the Mammalian Aortic Arch Arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D. Circulation and energy metabolism of the brain. In Basic Neurochemistry: Molecular, Cellular, and Medical Aspects; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Menshawi, K.; Mohr, J.P.; Gutierrez, J. A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J. Stroke 2015, 17, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. Developmental origins of cerebrovascular disease I: Prenatal cerebrovascular development--classic findings in the context of advances in genetic and fetal surveillance evaluations. J. Child. Neurol. 2012, 27, 121–131. [Google Scholar] [CrossRef]
- Solonskii, A.V.; SLogvinov, V.; Kutepova, N.A. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci. Behav. Physiol. 2008, 38, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Tingling, J.D.; Miranda, R.C. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: Evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol. Clin. Exp. Res. 2012, 36, 748–758. [Google Scholar] [CrossRef]
- Bisen, S.; Kakhniashvili, D.; Johnson, D.L.; Bukiya, A.N. Proteomic Analysis of Baboon Cerebral Artery Reveals Potential Pathways of Damage by Prenatal Alcohol Exposure. Mol. Cell Proteom. 2019, 18, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Bukiya, A.N.; Dopico, A.M. Fetal Cerebral Circulation as Target of Maternal Alcohol Consumption. Alcohol. Clin. Exp. Res. 2018, 42, 1006–1018. [Google Scholar] [CrossRef]
- Kochunov, P.; Castro, C.; Davis, D.M.; Dudley, D.; Wey, H.-Y.; Purdy, D.; Fox, P.T.; Simerly, C.; Schatten, G. Fetal brain during a binge drinking episode: A dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport 2010, 21, 716–721. [Google Scholar] [CrossRef]
- Saha, P.S.; Mayhan, W.G. Prenatal exposure to alcohol: Mechanisms of cerebral vascular damage and lifelong consequences. Adv. Drug Alcohol Res. 2022, 2, 10818. [Google Scholar] [CrossRef]
- Seleverstov, O.; Tobiasz, A.; Jackson, J.S.; Sullivan, R.; Ma, D.; Sullivan, J.P.; Davison, S.; Akkhawattanangkul, Y.; Tate, D.L.; Costello, T.; et al. Maternal alcohol exposure during mid-pregnancy dilates fetal cerebral arteries via endocannabinoid receptors. Alcohol 2017, 61, 51–61. [Google Scholar] [CrossRef]
- Tobiasz, A.M.; Duncan, J.R.; Bursac, Z.; Sullivan, R.D.; Tate, D.L.; Dopico, A.M.; Bukiya, A.N.; Mari, G. The Effect of Prenatal Alcohol Exposure on Fetal Growth and Cardiovascular Parameters in a Baboon Model of Pregnancy. Reprod. Sci. 2018, 25, 1116–1123. [Google Scholar] [CrossRef]
- Jégou, S.; El Ghazi, F.; de Lendeu, P.K.; Marret, S.; Laudenbach, V.; Uguen, A.; Marcorelles, P.; Roy, V.; Laquerrière, A.; Gonzalez, B.J. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann. Neurol. 2012, 72, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, H.V.; Kovetsky, N.S.; Bobryshev, Y.V.; Ashwell, K.W.S. Disorders of brain development in the progeny of mothers who used alcohol during pregnancy. Early Hum. Dev. 1997, 48, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Busija, D.W.; Rutkai, I.; Dutta, S.; Katakam, P.V. Role of Mitochondria in Cerebral Vascular Function: Energy Production, Cellular Protection, and Regulation of Vascular Tone. Compr. Physiol. 2016, 6, 1529–1548. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best. Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Shao, Y.; Mai, L.; Qiao, R.; Liang, Y.; Jiao, Y.; Homburg, J.; Jiang, Z.; Song, L. Endothelial mitochondria in the blood-brain barrier. Fluids Barriers CNS 2025, 22, 88. [Google Scholar] [CrossRef]
- Momin, S.Z.; Le, J.T.; Miranda, R.C. Vascular Contributions to the Neurobiological Effects of Prenatal Alcohol Exposure. Adv. Drug Alcohol Res. 2023, 3, 10924. [Google Scholar] [CrossRef] [PubMed]
- Girault, V.; Gilard, V.; Marguet, F.; Lesueur, C.; Hauchecorne, M.; Ramdani, Y.; Laquerrière, A.; Marret, S.; Jégou, S.; Gonzalez, B.J.; et al. Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis. 2017, 8, e2610. [Google Scholar] [CrossRef]
- Marcu, R.; Zheng, Y.; Hawkins, B.J. Mitochondria and Angiogenesis. Adv. Exp. Med. Biol. 2017, 982, 371–406. [Google Scholar] [PubMed]
- Labonne, B.E.; Gutiérrez, M.; Gómez-Quiroz, L.E.; Fainstein, M.K.; Bucio, L.; Souza, V.; Flores, O.; Ortíz, V.; Hernández, E.; Kershenobich, D.; et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 2009, 25, 599–609. [Google Scholar] [CrossRef]
- Karadayian, A.G.; Carrere, L.; Czerniczyniec, A.; Lores-Arnaiz, S. Molecular mechanism underlying alcohol’s residual effects: The role of acetaldehyde in mitochondrial dysfunction at synapses in mouse brain cortex. Alcohol 2025, 129, 79–91. [Google Scholar] [CrossRef]
- Tsermpini, E.E.; Ilješ, A.P.; Dolžan, V. Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Boschen, K.E.; Ruggiero, M.J.; Klintsova, A.Y. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 2016, 324, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Darbinian, N.; Darbinyan, A.; Merabova, N.; Kassem, M.; Tatevosian, G.; Amini, S.; Goetzl, L.; Selzer, M.E. In utero ethanol exposure induces mitochondrial DNA damage and inhibits mtDNA repair in developing brain. Front. Neurosci. 2023, 17, 1214958. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.B.; Mitchell, J.J.; Paiva, M. Ethanol-induced alterations in neurotrophin expression in developing cerebellum: Relationship to periods of temporal susceptibility. Alcohol. Clin. Exp. Res. 1999, 23, 1637–1642. [Google Scholar] [CrossRef]
- Hwang, H.; Liu, R.; Eldridge, R.; Hu, X.; Forghani, P.; Jones, D.P.; Xu, C. Chronic ethanol exposure induces mitochondrial dysfunction and alters gene expression and metabolism in human cardiac spheroids. Alcohol Clin. Exp. Res. 2023, 47, 643–658. [Google Scholar] [CrossRef]
- O’LEary-Moore, S.K.; Parnell, S.E.; Godin, E.A.; Dehart, D.B.; Ament, J.J.; Khan, A.A.; Johnson, G.A.; Styner, M.A.; Sulik, K.K. Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 953–964. [Google Scholar] [CrossRef]
- Arzua, T.; Yan, Y.; Liu, X.; Dash, R.K.; Liu, Q.-S.; Bai, X. Synaptic and mitochondrial mechanisms behind alcohol-induced imbalance of excitatory/inhibitory synaptic activity and associated cognitive and behavioral abnormalities. Transl. Psychiatry 2024, 14, 51. [Google Scholar] [CrossRef]
- Bukiya, A.N. Fetal Cerebral Artery Mitochondrion as Target of Prenatal Alcohol Exposure. Int. J. Environ. Res. Public Health 2019, 16, 1586. [Google Scholar] [CrossRef]
- Laboratory, T.J. Timed-Pregnant Mice. Available online: https://www.jax.org/jax-mice-and-services/surgical-and-preconditioning/timed-pregnant-mice (accessed on 17 August 2025).
- Heyne, G.W.; Plisch, E.H.; Melberg, C.G.; Sandgren, E.P.; A Peter, J.; Lipinski, R.J. A Simple and Reliable Method for Early Pregnancy Detection in Inbred Mice. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 368–371. [Google Scholar] [PubMed]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; A Taft, R. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Bake, S.; Gardner, R.; Tingling, J.D.; Miranda, R.C.; Sohrabji, F. Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol. Clin. Exp. Res. 2017, 41, 117–127. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.H.; Fitzgerald, A.C.; Ponnapula, S.; Dopico, A.M.; Bukiya, A.N. Differential distribution of cholesterol pools across arteries under high-cholesterol diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159235. [Google Scholar]
- Cottam, N.C.; Ofori, K.; Stoll, K.T.; Bryant, M.; Rogge, J.R.; Hekmatyar, K.; Sun, J.; Charvet, C.J. From Circuits to Lifespan: Translating Mouse and Human Timelines with Neuroimaging-Based Tractography. J. Neurosci. 2025, 45, e1429242025. [Google Scholar] [CrossRef] [PubMed]
- Pressler, R.; Auvin, S. Comparison of Brain Maturation among Species: An Example in Translational Research Suggesting the Possible Use of Bumetanide in Newborn. Front. Neurol. 2013, 4, 36. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Env. Environ. Health Perspect. 2000, 108 (Suppl. S3), 511–533. [Google Scholar]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Agarwal, N.; Carare, R.O. Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes. Front. Neurol. 2020, 11, 611485. [Google Scholar] [CrossRef]
- Katakam, P.V.; Wappler, E.A.; Katz, P.S.; Rutkai, I.; Institoris, A.; Domoki, F.; Gáspár, T.; Grovenburg, S.M.; Snipes, J.A.; Busija, D.W. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 752–759. [Google Scholar] [CrossRef]
- Qian, Y.; Du, Y.-H.; Tang, Y.-B.; Lv, X.-F.; Liu, J.; Zhou, J.-G.; Guan, Y.-Y. ClC-3 chloride channel prevents apoptosis induced by hydrogen peroxide in basilar artery smooth muscle cells through mitochondria dependent pathway. Apoptosis 2011, 16, 468–477. [Google Scholar] [CrossRef]
- Xi, Q.; Cheranov, S.Y.; Jaggar, J.H. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ. Res. 2005, 97, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.A.; Maier, S.E.; Parnell, S.E.; West, J.R. Alcohol and the developing brain: Neuroanatomical studies. Alcohol. Res. Health 2003, 27, 174–180. [Google Scholar] [PubMed]
- Morales, R.; Thapa, S.; Bukiya, A.N. Prenatal alcohol exposure and mitochondrial function in the brain. In Molecular Mechanisms and Lifelong Consequences of Prenatal Exposure to Psychoactive Substances; Bukiya, A.N., Ali, D.W., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2026. [Google Scholar]
- Heatley, M.K.; Crane, J. The blood alcohol concentration at post-mortem in 175 fatal cases of alcohol intoxication. Med. Sci. Law. 1990, 30, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Koob, G.F. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol. Res. Health 2008, 31, 185–195. [Google Scholar]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Best, K.; Belknap, J.K.; Finn, D.A.; Crabbe, J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005, 84, 53–63. [Google Scholar] [CrossRef]
- NIAAA. Understanding Binge Drinking. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/binge-drinking (accessed on 30 October 2025).
- Perez, R.F.; Conner, K.E.; Erickson, M.A.; Nabatanzi, M.; Huffman, K.J. Alcohol and lactation: Developmental deficits in a mouse model. Front. Neurosci. 2023, 17, 1147274. [Google Scholar] [CrossRef]
- Song, S.B.; Hwang, E.S. A Rise in ATP, ROS, and Mitochondrial Content upon Glucose Withdrawal Correlates with a Dysregulated Mitochondria Turnover Mediated by the Activation of the Protein Deacetylase SIRT1. Cells 2018, 8, 11. [Google Scholar] [CrossRef]
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Goikoetxea-Usandizaga, N.; Bravo, M.; Egia-Mendikute, L.; Abecia, L.; Serrano-Maciá, M.; Urdinguio, R.G.; Clos-García, M.; Rodríguez-Agudo, R.; Araujo-Legido, R.; López-Bermudo, L.; et al. The outcome of boosting mitochondrial activity in alcohol-associated liver disease is organ-dependent. Hepatology 2023, 78, 878–895. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tanvir, M.F.; Jiang, H. Investigating the Impact of Chronical Prenatal Alcohol Exposure on Fetal Vascular Development Across Pregnancy Stages Using Photoacoustic Tomography. J. Biophotonics 2025, 18, e202400410. [Google Scholar] [CrossRef]
- Chang, L.; Oishi, K.; Skranes, J.; Buchthal, S.; Cunningham, E.; Yamakawa, R.; Hayama, S.; Jiang, C.S.; Alicata, D.; Hernandez, A.; et al. Sex-Specific Alterations of White Matter Developmental Trajectories in Infants With Prenatal Exposure to Methamphetamine and Tobacco. JAMA Psychiatry 2016, 73, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.V.; Green, R.; DaCosta, A.; Flora, D.; Lanphear, B.; Till, C. Sex difference of pre- and post-natal exposure to six developmental neurotoxicants on intellectual abilities: A systematic review and meta-analysis of human studies. Environ. Health 2023, 22, 80. [Google Scholar] [CrossRef]
- Legault, L.M.; Doiron, K.; Breton-Larrivée, M.; Langford-Avelar, A.; Lemieux, A.; Caron, M.; Jerome-Majewska, L.A.; Sinnett, D.; McGraw, S. Pre-implantation alcohol exposure induces lasting sex-specific DNA methylation programming errors in the developing forebrain. Clin. Epigenetics 2021, 13, 164. [Google Scholar] [CrossRef]
- Rabiant, K.; Antol, J.; Naassila, M.; Pierrefiche, O. Sex difference in the vulnerability to hippocampus plasticity impairment after binge-like ethanol exposure in adolescent rat: Is estrogen the key? Addict. Biol. 2021, 26, e13002. [Google Scholar] [CrossRef]
- Devillers, M.M.; Mhaouty-Kodja, S.; Guigon, C.J. Deciphering the Roles & Regulation of Estradiol Signaling during Female Mini-Puberty: Insights from Mouse Models. Int. J. Mol. Sci. 2022, 23, 13695. [Google Scholar] [CrossRef]
- Gaignard, P.; Fréchou, M.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Sex differences in brain mitochondrial metabolism: Influence of endogenous steroids and stroke. J. Neuroendocr. Neuroendocrinol. 2018, 30. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Eachus, H.; Ryu, S. Glucocorticoid effects on the brain: From adaptive developmental plasticity to allostatic overload. J. Exp. Biol. 2024, 227 (Suppl. S1), jeb246128. [Google Scholar] [CrossRef]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Siggins, R.W.; McTernan, P.M.; Simon, L.; Souza-Smith, F.M.; Molina, P.E. Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury. Int. J. Mol. Sci. 2023, 24, 8650. [Google Scholar] [CrossRef]
- Derme, M.; Briante, M.; Ceccanti, M.; Giannini, G.; Vitali, M.; Messina, M.P.; Piccioni, M.G.; Mattia, A.; Nicotera, S.; Crognale, A. Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics: The Role of the Oxidative Stress-A Review of the Literature. Children 2024, 11, 269. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef]
- Liu, W.; Qi, Z.; Li, W.; Liang, J.; Zhao, L.; Shi, Y. M1 Microglia Induced Neuronal Injury on Ischemic Stroke via Mitochondrial Crosstalk between Microglia and Neurons. Oxid. Med. Cell Longev. 2022, 2022, 4335272. [Google Scholar] [CrossRef]
- Benítez-Marín, M.J.; Marín-Clavijo, J.; Blanco-Elena, J.A.; Jiménez-López, J.; González-Mesa, E. Brain Sparing Effect on Neurodevelopment in Children with Intrauterine Growth Restriction: A Systematic Review. Children 2021, 8, 745. [Google Scholar] [CrossRef]
- Beukers, F.; Aarnoudse-Moens, C.S.; van Weissenbruch, M.M.; Ganzevoort, W.; van Goudoever, J.B.; van Wassenaer-Leemhuis, A.G. Fetal Growth Restriction with Brain Sparing: Neurocognitive and Behavioral Outcomes at 12 Years of Age. J. Pediatr. 2017, 188, 103–109.e2. [Google Scholar] [CrossRef]
- Figueras, F.; Cruz-Martinez, R.; Sanz-Cortes, M.; Arranz, A.; Illa, M.; Botet, F.; Costas-Moragas, C.; Gratacos, E. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet. Gynecol. 2011, 38, 288–294. [Google Scholar] [CrossRef]
- Gao, L.; Grebogi, C.; Lai, Y.-C.; Stephen, J.; Zhang, T.; Li, Y.; Ren, H.; Li, D.; Wang, J.; Schelter, B.; et al. Quantitative assessment of cerebral connectivity deficiency and cognitive impairment in children with prenatal alcohol exposure. Chaos 2019, 29, 041101. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Akkaya-Hocagil, T.; Ryan, L.M.; Dodge, N.C.; Richardson, G.A.; Olson, H.C.; Coles, C.D.; Day, N.L.; Cook, R.J.; Jacobson, S.W. Effects of prenatal alcohol exposure on cognitive and behavioral development: Findings from a hierarchical meta-analysis of data from six prospective longitudinal U.S. cohorts. Alcohol. Clin. Exp. Res. 2021, 45, 2040–2058. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Thomas, K.G.F.; Molteno, C.D.; Kliegel, M.; Meintjes, E.M.; Jacobson, J.L.; Jacobson, S.W. Prospective Memory Impairment in Children with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2016, 40, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Monteith, C.; Flood, K.; Pinnamaneni, R.; Levine, T.A.; Alderdice, F.A.; Unterscheider, J.; McAuliffe, F.M.; Dicker, P.; Tully, E.C.; Malone, F.D.; et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 2019, 221, 273.e1–273.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Martin, C.D.; Lei, A.L.; Hausknecht, K.A.; Ishiwari, K.; Richards, J.B.; Haj-Dahmane, S.; Shen, R.-Y. Prenatal Ethanol Exposure Leads to Attention Deficits in Both Male and Female Rats. Front. Neurosci. 2020, 14, 12. [Google Scholar] [CrossRef]

| Parameters | Equation |
|---|---|
| Non-mitochondrial Respiration | Minimum OCR measured throughout |
| Basal Respiration | (Last OCR measured before oligomycin injection)—(Non-Mitochondrial Respiration) |
| Maximal OCR (Maximal Respiration) | (Maximum rate measured after FCCP injection)—(Non-Mitochondrial Respiration) |
| Proton (H+) Leak | (Minimum rate measured after oligomycin injection)—(Non-Mitochondrial Respiration) |
| OCR associated with ATP Production | (Last rate measured before oligomycin injection)—(Minimum rate measured after oligomycin injection) |
| Spare Respiratory Capacity (%) | (Maximal Respiration-Basal Respiration)/(Basal Respiration) × 100 |
| Coupling Efficiency (%) | (OCR associated with ATP Production)/(Basal Respiration) × 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, S.; Morales, R.M.; Smallwood, H.S.; Bukiya, A.N. Ethanol Exposure Increases Oxygen Consumption by Developing Cerebral Arteries in a Trimester-, Concentration- and Sex-Dependent Manner. Biomolecules 2025, 15, 1566. https://doi.org/10.3390/biom15111566
Thapa S, Morales RM, Smallwood HS, Bukiya AN. Ethanol Exposure Increases Oxygen Consumption by Developing Cerebral Arteries in a Trimester-, Concentration- and Sex-Dependent Manner. Biomolecules. 2025; 15(11):1566. https://doi.org/10.3390/biom15111566
Chicago/Turabian StyleThapa, Shiwani, Rika M. Morales, Heather S. Smallwood, and Anna N. Bukiya. 2025. "Ethanol Exposure Increases Oxygen Consumption by Developing Cerebral Arteries in a Trimester-, Concentration- and Sex-Dependent Manner" Biomolecules 15, no. 11: 1566. https://doi.org/10.3390/biom15111566
APA StyleThapa, S., Morales, R. M., Smallwood, H. S., & Bukiya, A. N. (2025). Ethanol Exposure Increases Oxygen Consumption by Developing Cerebral Arteries in a Trimester-, Concentration- and Sex-Dependent Manner. Biomolecules, 15(11), 1566. https://doi.org/10.3390/biom15111566








