Epigenetic Drugs Splitomicin, Suberohydroxamic Acid, CPTH6, BVT-948, and PBIT Moderate Fibro-Fatty Development in Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. ACM Population
2.2. CMSC Isolation and Maintenance
2.3. CMSC Differentiations
2.4. Epigenetic Drug Treatments
2.5. Fluorescence Assays
2.6. Statistical Analysis
3. Results
3.1. Epigenetic Drug Screening
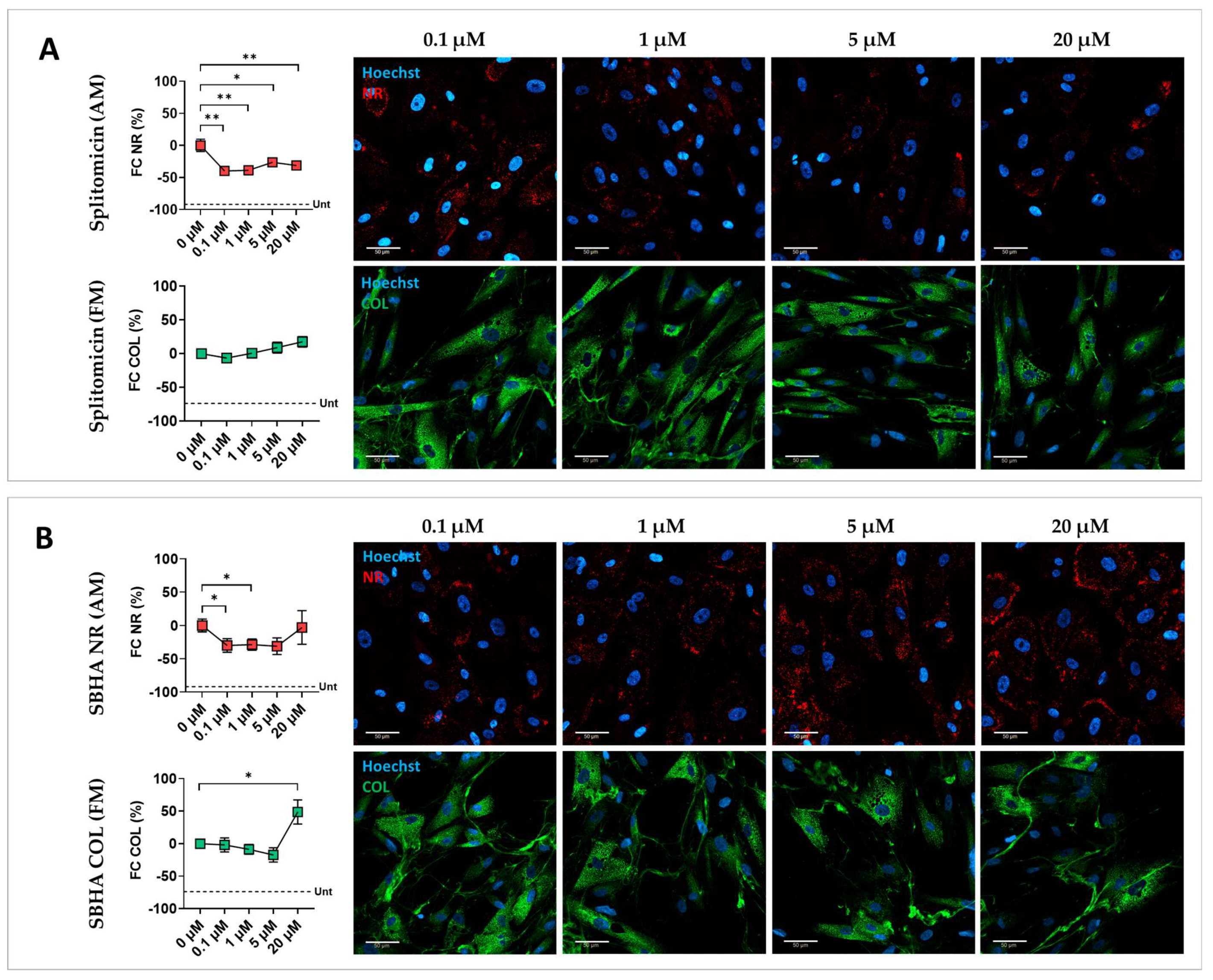
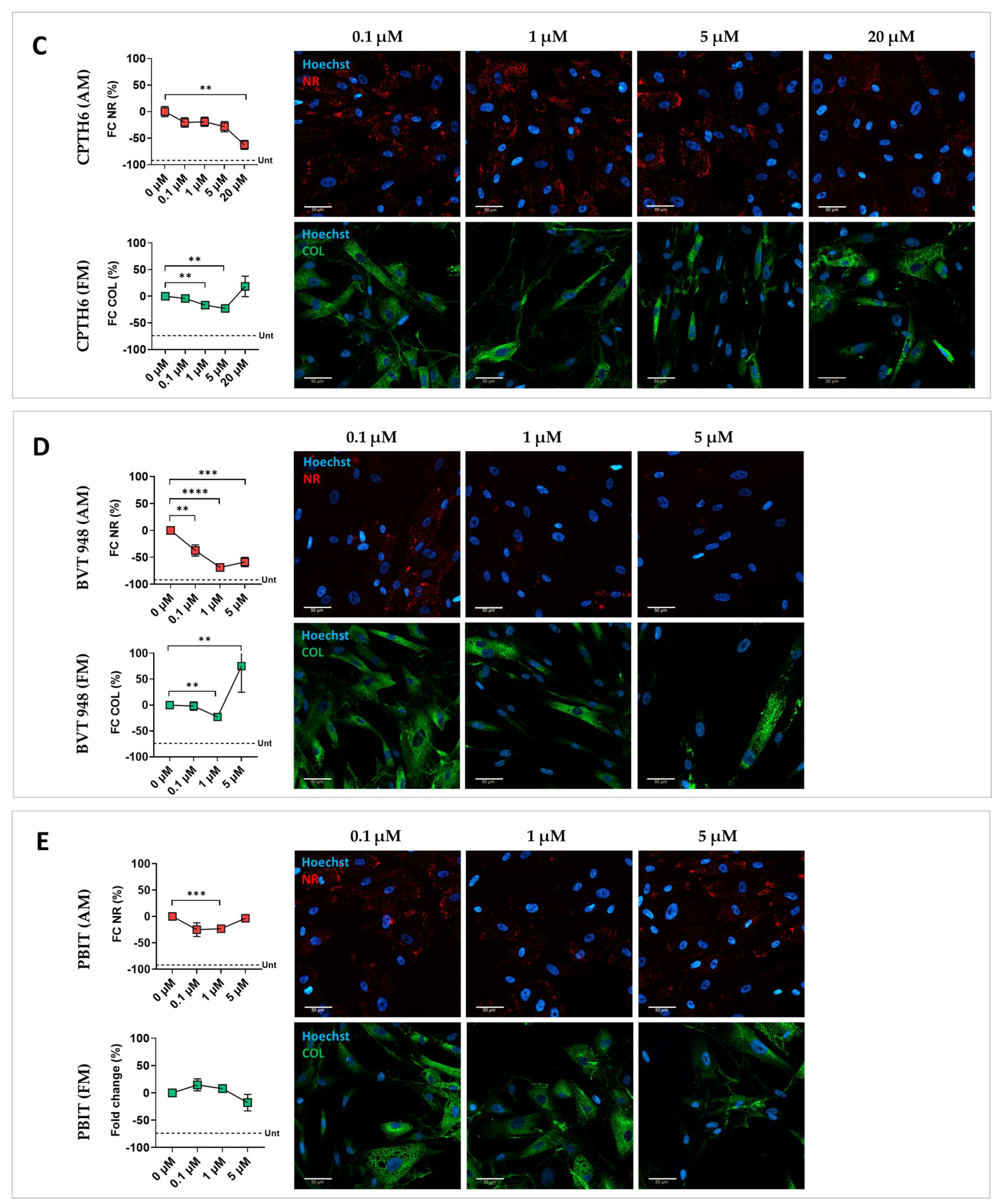
3.2. Validation of Screening Hits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACM | Arrhythmogenic cardiomyopathy |
| BSA | Bovine serum albumin |
| BVT-948 | 4-hydroxy-3,3-dimethyl-2H-benz[g]indole-2,5(3H)-dione |
| CAY10722 | N-[2-(2,4-dichlorophenyl)-5-benzoxazolyl]-benzeneacetamide |
| CMSC | Cardiac mesenchymal stromal cells |
| CPTH2 | (E)-4-(4-chlorophenyl)-2-(2-(3-methylcyclopentylidene)hydrazinyl)thiazole hydrochloride |
| CPTH6 | (E)-4-(4-chlorophenyl)-2-(2-(3-methylcyclopentylidene)hydrazinyl)thiazole hydrobromide |
| CYP | Cytochrome P450 |
| DMD | Duchenne muscular dystrophy |
| ECG | Electrocardiography |
| EPZ-5676 | (Pinometostat) (2R)-2-[(1R)-1-[5-[(4-cyclopropylpiperazin-1-yl)methyl]-1H-indazol-3-yl]-2-methylpropyl]-N,6-dimethyl-1,2-dihydro-1,3,5-triazine-4-carboxamide |
| epidrugs | Epigenetic drugs |
| FBS | Fetal bovine serum |
| GCN5 | General control non-repressed 5 protein |
| GSK126 | 4-(4-methylpiperazin-1-yl)-N-[1-(1-methylpyrazol-4-yl)cyclopentyl]-2-[(1H-pyrrolo [2,3-b]pyridin-3-yl)methyl]benzamide |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylases |
| KDM | Lysine demethylase |
| KMT | Lysine methyltransferase |
| MM | Maintenance medium |
| NI-57 | (R)-3-(2-(1H-indol-3-yl)ethyl)-N-(4-(pyrrolidin-1-ylmethyl)phenyl)-1H-pyrrolo[2,3-b]pyridine-5-carboxamide |
| PBIT | 2-4(4-methylphenyl)-1,2-benzisothiazol-3(2H)-one |
| PPAR | Peroxisome proliferator-activated receptor |
| pCAF | p300/CBP-associated factor |
| PTP | Protein tyrosine phosphatases |
| SBHA | Suberohydroxamic acid |
References
- Arrhythmogenic Cardiomyopathy: Towards Genotype Based Diagnoses and Management-Muller-Journal of Cardiovascular Electrophysiology-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jce.16519 (accessed on 24 May 2025).
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef]
- Sommariva, E.; Brambilla, S.; Carbucicchio, C.; Gambini, E.; Meraviglia, V.; Dello Russo, A.; Farina, F.M.; Casella, M.; Catto, V.; Pontone, G.; et al. Cardiac Mesenchymal Stromal Cells Are a Source of Adipocytes in Arrhythmogenic Cardiomyopathy. Eur. Heart J. 2016, 37, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.S.; Stadiotti, I.; Pilato, C.A.; Perrucci, G.L.; Saverio, V.; Catto, V.; Vettor, G.; Casella, M.; Guarino, A.; Polvani, G.; et al. Excess TGF-Β1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 2673. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.S.; Iengo, L.; Sala, L.; Massaiu, I.; Chiesa, M.; Lippi, M.; Ghilardi, S.; Florindi, C.; Lodola, F.; Zaza, A.; et al. Cardiomyocyte and Stromal Cell Cross-Talk Influences the Pathogenesis of Arrhythmogenic Cardiomyopathy: A Multi-Level Analysis Uncovers DLK1-NOTCH Pathway Role in Fibro-Adipose Remodelling. Cell Death Discov. 2024, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies: Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- James, C.A.; Jongbloed, J.D.H.; Hershberger, R.E.; Morales, A.; Judge, D.P.; Syrris, P.; Pilichou, K.; Domingo, A.M.; Murray, B.; Cadrin-Tourigny, J.; et al. International Evidence Based Reappraisal of Genes Associated with Arrhythmogenic Right Ventricular Cardiomyopathy Using the Clinical Genome Resource Framework. Circ. Genom. Precis. Med. 2021, 14, e003273. [Google Scholar] [CrossRef]
- Pinamonti, B.; Brun, F.; Mestroni, L.; Sinagra, G. Arrhythmogenic Right Ventricular Cardiomyopathy: From Genetics to Diagnostic and Therapeutic Challenges. World J. Cardiol. 2014, 6, 1234–1244. [Google Scholar] [CrossRef]
- Casella, M.; Gasperetti, A.; Sicuso, R.; Conte, E.; Catto, V.; Sommariva, E.; Bergonti, M.; Vettor, G.; Rizzo, S.; Pompilio, G.; et al. Characteristics of Patients with Arrhythmogenic Left Ventricular Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2020, 13, e009005. [Google Scholar] [CrossRef]
- Bhonsale, A.; Groeneweg, J.A.; James, C.A.; Dooijes, D.; Tichnell, C.; Jongbloed, J.D.H.; Murray, B.; te Riele, A.S.J.M.; van den Berg, M.P.; Bikker, H.; et al. Impact of Genotype on Clinical Course in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy-Associated Mutation Carriers. Eur. Heart J. 2015, 36, 847–855. [Google Scholar] [CrossRef]
- König, E.; Volpato, C.B.; Motta, B.M.; Blankenburg, H.; Picard, A.; Pramstaller, P.; Casella, M.; Rauhe, W.; Pompilio, G.; Meraviglia, V.; et al. Exploring Digenic Inheritance in Arrhythmogenic Cardiomyopathy. BMC Med. Genet. 2017, 18, 145. [Google Scholar] [CrossRef]
- Lippi, M.; Chiesa, M.; Ascione, C.; Pedrazzini, M.; Mushtaq, S.; Rovina, D.; Riggio, D.; Di Blasio, A.M.; Biondi, M.L.; Pompilio, G.; et al. Spectrum of Rare and Common Genetic Variants in Arrhythmogenic Cardiomyopathy Patients. Biomolecules 2022, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Akdis, D.; Saguner, A.M.; Shah, K.; Wei, C.; Medeiros-Domingo, A.; von Eckardstein, A.; Lüscher, T.F.; Brunckhorst, C.; Chen, H.S.V.; Duru, F. Sex Hormones Affect Outcome in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: From a Stem Cell Derived Cardiomyocyte-Based Model to Clinical Biomarkers of Disease Outcome. Eur. Heart J. 2017, 38, 1498–1508. [Google Scholar] [CrossRef]
- Beffagna, G.; Sommariva, E.; Bellin, M. Mechanotransduction and Adrenergic Stimulation in Arrhythmogenic Cardiomyopathy: An Overview of in Vitro and in Vivo Models. Front. Physiol. 2020, 11, 568535. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Stadiotti, I.; Casella, M.; Catto, V.; Dello Russo, A.; Carbucicchio, C.; Arnaboldi, L.; De Metrio, S.; Milano, G.; Scopece, A.; et al. Oxidized LDL-Dependent Pathway as New Pathogenic Trigger in Arrhythmogenic Cardiomyopathy. EMBO Mol. Med. 2021, 13, e14365. [Google Scholar] [CrossRef] [PubMed]
- Lippi, M.; Maione, A.S.; Chiesa, M.; Perrucci, G.L.; Iengo, L.; Sattin, T.; Cencioni, C.; Savoia, M.; Zeiher, A.M.; Tundo, F.; et al. Omics Analyses of Stromal Cells from ACM Patients Reveal Alterations in Chromatin Organization and Mitochondrial Homeostasis. Int. J. Mol. Sci. 2023, 24, 10017. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, Q.; Xie, P.; He, C.; Li, Q.; Yao, X.; Mao, Y.; Wu, X.; Zhang, T. Epigenetic Modifications and Emerging Therapeutic Targets in Cardiovascular Aging and Diseases. Pharmacol. Res. 2025, 211, 107546. [Google Scholar] [CrossRef]
- Rainer, J.; Meraviglia, V.; Blankenburg, H.; Piubelli, C.; Pramstaller, P.P.; Paolin, A.; Cogliati, E.; Pompilio, G.; Sommariva, E.; Domingues, F.S.; et al. The Arrhythmogenic Cardiomyopathy-Specific Coding and Non-Coding Transcriptome in Human Cardiac Stromal Cells. BMC Genom. 2018, 19, 491. [Google Scholar] [CrossRef]
- Sommariva, E.; D’Alessandra, Y.; Farina, F.M.; Casella, M.; Cattaneo, F.; Catto, V.; Chiesa, M.; Stadiotti, I.; Brambilla, S.; Dello Russo, A.; et al. MiR-320a as a Potential Novel Circulating Biomarker of Arrhythmogenic CardioMyopathy. Sci. Rep. 2017, 7, 4802. [Google Scholar] [CrossRef]
- Felisbino, M.B.; McKinsey, T.A. Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation. JACC Basic Transl. Sci. 2018, 3, 704–715. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; Wang, C.; Gao, J.; Zhai, Y. Review: Epigenetic Regulation of Adipocyte Differentiation and Adipogenesis. J. Zhejiang Univ. Sci. B 2010, 11, 784–791. [Google Scholar] [CrossRef]
- Lugenbiel, P.; Govorov, K.; Syren, P.; Rahm, A.-K.; Wieder, T.; Wunsch, M.; Weiberg, N.; Manolova, E.; Gramlich, D.; Rivinius, R.; et al. Epigenetic Regulation of Cardiac Electrophysiology in Atrial Fibrillation: HDAC2 Determines Action Potential Duration and Suppresses NRSF in Cardiomyocytes. Basic Res. Cardiol. 2021, 116, 13. [Google Scholar] [CrossRef]
- Kang, S.-H.; Seok, Y.M.; Song, M.; Lee, H.-A.; Kurz, T.; Kim, I. Histone Deacetylase Inhibition Attenuates Cardiac Hypertrophy and Fibrosis through Acetylation of Mineralocorticoid Receptor in Spontaneously Hypertensive Rats. Mol. Pharmacol. 2015, 87, 782–791. [Google Scholar] [CrossRef]
- Consalvi, S.; Mozzetta, C.; Bettica, P.; Germani, M.; Fiorentini, F.; Del Bene, F.; Rocchetti, M.; Leoni, F.; Monzani, V.; Mascagni, P.; et al. Preclinical Studies in the Mdx Mouse Model of Duchenne Muscular Dystrophy with the Histone Deacetylase Inhibitor Givinostat. Mol. Med. Camb. Mass 2013, 19, 79–87. [Google Scholar] [CrossRef]
- Mercuri, E.; Vilchez, J.J.; Boespflug-Tanguy, O.; Zaidman, C.M.; Mah, J.K.; Goemans, N.; Müller-Felber, W.; Niks, E.H.; Schara-Schmidt, U.; Bertini, E.; et al. Safety and Efficacy of Givinostat in Boys with Duchenne Muscular Dystrophy (EPIDYS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Neurol. 2024, 23, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Pilato, C.A.; Stadiotti, I.; Maione, A.S.; Saverio, V.; Catto, V.; Tundo, F.; Russo, A.D.; Tondo, C.; Pompilio, G.; Casella, M.; et al. Isolation and Characterization of Cardiac Mesenchymal Stromal Cells from Endomyocardial Bioptic Samples of Arrhythmogenic Cardiomyopathy Patients. J. Vis. Exp. JoVE 2018, 132, e57263. [Google Scholar] [CrossRef]
- Park, J.-A.; Park, S.; Park, W.-Y.; Han, M.-K.; Lee, Y. Splitomicin, a SIRT1 Inhibitor, Enhances Hematopoietic Differentiation of Mouse Embryonic Stem Cells. Int. J. Stem Cells 2019, 12, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Smesny Trtkova, K.; Luzna, P.; Weiser Drozdkova, D.; Cizkova, K.; Janovska, L.; Gursky, J.; Prukova, D.; Frydrych, I.; Hajduch, M.; Minarik, J. The Epigenetic Impact of Suberohydroxamic Acid and 5-Aza-2′-Deoxycytidine on DNMT3B Expression in Myeloma Cell Lines Differing in IL-6 Expression. Mol. Med. Rep. 2022, 26, 321. [Google Scholar] [CrossRef]
- Di Martile, M.; Desideri, M.; De Luca, T.; Gabellini, C.; Buglioni, S.; Eramo, A.; Sette, G.; Milella, M.; Rotili, D.; Mai, A.; et al. Histone Acetyltransferase Inhibitor CPTH6 Preferentially Targets Lung Cancer Stem-like Cells. Oncotarget 2016, 7, 11332–11348. [Google Scholar] [CrossRef]
- Liu, S.; Rong, G.; Li, X.; Geng, L.; Zeng, Z.; Jiang, D.; Yang, J.; Wei, Y. Diosgenin and GSK126 Produce Synergistic Effects on Epithelial–Mesenchymal Transition in Gastric Cancer Cells by Mediating EZH2 via the Rho/ROCK Signaling Pathway. OncoTargets Ther. 2020, 13, 5057–5067. [Google Scholar] [CrossRef]
- Liu, L.; Zou, J.; Guan, Y.; Zhang, Y.; Zhang, W.; Zhou, X.; Xiong, C.; Tolbert, E.; Zhao, T.C.; Bayliss, G.; et al. Blocking the Histone Lysine 79 Methyltransferase DOT1L Alleviates Renal Fibrosis through Inhibition of Renal Fibroblast Activation and Epithelial-mesenchymal Transition. FASEB J. 2019, 33, 11941–11958. [Google Scholar] [CrossRef]
- Hwang, B.-M.; Chae, H.S.; Jeong, Y.-J.; Lee, Y.-R.; Noh, E.-M.; Youn, H.Z.; Jung, S.H.; Yu, H.-N.; Chung, E.Y.; Kim, J.-S. Protein Tyrosine Phosphatase Controls Breast Cancer Invasion through the Expression of Matrix Metalloproteinase-9. BMB Rep. 2013, 46, 533–538. [Google Scholar] [CrossRef]
- Meier, J.C.; Tallant, C.; Fedorov, O.; Witwicka, H.; Hwang, S.-Y.; van Stiphout, R.G.; Lambert, J.-P.; Rogers, C.; Yapp, C.; Gerstenberger, B.S.; et al. Selective Targeting of Bromodomains of the Bromodomain-PHD Fingers Family Impairs Osteoclast Differentiation. ACS Chem. Biol. 2017, 12, 2619–2630. [Google Scholar] [CrossRef]
- Smith, T.; White, T.; Chen, Z.; Stewart, L.V. The KDM5 Inhibitor PBIT Reduces Proliferation of Castration-Resistant Prostate Cancer Cells via Cell Cycle Arrest and the Induction of Senescence. Exp. Cell Res. 2024, 437, 113991. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Wang, X.; Zhang, R.; Wang, C.; Guo, Z. Sirtuin-3 (SIRT3) Expression Is Associated with Overall Survival in Esophageal Cancer. Ann. Diagn. Pathol. 2013, 17, 483–485. [Google Scholar] [CrossRef]
- Kamiya, T.; Machiura, M.; Makino, J.; Hara, H.; Hozumi, I.; Adachi, T. Epigenetic Regulation of Extracellular-Superoxide Dismutase in Human Monocytes. Free Radic. Biol. Med. 2013, 61, 197–205. [Google Scholar] [CrossRef]
- Toro, V.; Jutras-Beaudoin, N.; Boucherat, O.; Bonnet, S.; Provencher, S.; Potus, F. Right Ventricle and Epigenetics: A Systematic Review. Cells 2023, 12, 2693. [Google Scholar] [CrossRef]
- Mazurek, S.; Kim, G.H. Genetic and Epigenetic Regulation of Arrhythmogenic Cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-N.; Wang, H.-Y.; Chen, X.-F.; Tang, X.; Chen, H.-Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient Control of Glucose Homeostasis through a Complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The Role of Sirtuins in Cardiac Disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 Functionally Interacts with the Metabolic Regulator and Transcriptional Coactivator PGC-1{alpha}. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Woster, P.M. Discovery of a New Class of Histone Deacetylase Inhibitors with a Novel Zinc Binding Group. MedChemComm 2015, 6, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Longo, R.; Peri, C.; Coppi, L.; Caruso, D.; Mai, A.; Mitro, N.; De Fabiani, E.; Crestani, M. Inhibition of Class I HDACs Imprints Adipogenesis toward Oxidative and Brown-like Phenotype. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158594. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Idelman, G.; Blanco, V.; Blomkalns, A.L.; Piegore, M.G.; Weintraub, D.S.; Kumar, S.; Rajsheker, S.; Manka, D.; Rudich, S.M.; et al. Histone Deacetylase 9 Is a Negative Regulator of Adipogenic Differentiation *. J. Biol. Chem. 2011, 286, 27836–27847. [Google Scholar] [CrossRef]
- Lv, X.; Qiu, J.; Hao, T.; Zhang, H.; Jiang, H.; Tan, Y. HDAC Inhibitor Trichostatin A Suppresses Adipogenesis in 3T3-L1 Preadipocytes. Aging 2021, 13, 17489–17498. [Google Scholar] [CrossRef]
- Vencato, S.; Romanato, C.; Forino, M.; Fossati, G.; Velle, A.; Facchinello, N.; Braghetta, P.; Licandro, S.A.; Romualdi, C.; Calore, M.; et al. Givinostat Inhibits in Vitro Differentiation of Cardiac Fibroadipogenic Precursors from a Mouse Model of Arrhythmogenic Cardiomyopathy. Biomed. Pharmacother. Biomedecine Pharmacother. 2025, 191, 118549. [Google Scholar] [CrossRef]
- Aartsma-Rus, A. Histone Deacetylase Inhibition with Givinostat: A Multi-Targeted Mode of Action with the Potential to Halt the Pathological Cascade of Duchenne Muscular Dystrophy. Front. Cell Dev. Biol. 2024, 12, 1514898. [Google Scholar] [CrossRef]
- Bettica, P.; Petrini, S.; D’Oria, V.; D’Amico, A.; Catteruccia, M.; Pane, M.; Sivo, S.; Magri, F.; Brajkovic, S.; Messina, S.; et al. Histological Effects of Givinostat in Boys with Duchenne Muscular Dystrophy. Neuromuscul. Disord. NMD 2016, 26, 643–649. [Google Scholar] [CrossRef]
- Soussi, S.; Maione, A.S.; Lefèvre, L.; Pizzinat, N.; Iacovoni, J.; Gonzalez-Fuentes, I.; Cussac, D.; Iengo, L.; Santin, Y.; Tundo, F.; et al. Analysis of Effector/Memory Regulatory T Cells from Arrhythmogenic Cardiomyopathy Patients Identified IL-32 as a Novel Player in ACM Pathogenesis. Cell Death Dis. 2025, 16, 87. [Google Scholar] [CrossRef]
- Deng, W.; Chen, H.; Su, H.; Wu, X.; Xie, Z.; Wu, Y.; Shen, H. IL6 Receptor Facilitates Adipogenesis Differentiation of Human Mesenchymal Stem Cells through Activating P38 Pathway. Int. J. Stem Cells 2020, 13, 142–150. [Google Scholar] [CrossRef]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-Activated STAT3-IL-6 Signalling in Fibro-Adipogenic Progenitors Promotes Myofibres Atrophy and Fibrosis. Nat. Cell Biol. 2018, 20, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Larragoite, E.T.; Nell, R.A.; Martins, L.J.; Barrows, L.R.; Planelles, V.; Spivak, A.M. Histone Deacetylase Inhibition Reduces Deleterious Cytokine Release Induced by Ingenol Stimulation. Biochem. Pharmacol. 2022, 195, 114844. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Ragazzoni, Y.; Pelosi, A.; Desideri, M.; Carradori, S.; Gabellini, C.; Maresca, G.; Nescatelli, R.; Secci, D.; Bolasco, A.; et al. CPTH6, a Thiazole Derivative, Induces Histone Hypoacetylation and Apoptosis in Human Leukemia Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-T.; Jin, J.; Zheng, Z.-G. Emerging Role of GCN5 in Human Diseases and Its Therapeutic Potential. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 165, 114835. [Google Scholar] [CrossRef]
- Li, S.; Shogren-Knaak, M.A. The Gcn5 Bromodomain of the SAGA Complex Facilitates Cooperative and Cross-Tail Acetylation of Nucleosomes. J. Biol. Chem. 2009, 284, 9411–9417. [Google Scholar] [CrossRef]
- Mutlu, B.; Puigserver, P. GCN5 Acetyltransferase in Cellular Energetic and Metabolic Processes. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194626. [Google Scholar] [CrossRef]
- Volani, C.; Pagliaro, A.; Rainer, J.; Paglia, G.; Porro, B.; Stadiotti, I.; Foco, L.; Cogliati, E.; Paolin, A.; Lagrasta, C.; et al. GCN5 Contributes to Intracellular Lipid Accumulation in Human Primary Cardiac Stromal Cells from Patients Affected by Arrhythmogenic Cardiomyopathy. J. Cell. Mol. Med. 2022, 26, 3687–3701. [Google Scholar] [CrossRef]
- Jin, Q.; Yu, L.-R.; Wang, L.; Zhang, Z.; Kasper, L.H.; Lee, J.-E.; Wang, C.; Brindle, P.K.; Dent, S.Y.R.; Ge, K. Distinct Roles of GCN5/PCAF-Mediated H3K9ac and CBP/P300-Mediated H3K18/27ac in Nuclear Receptor Transactivation. EMBO J. 2011, 30, 249–262. [Google Scholar] [CrossRef]
- Qiu, P.; Ritchie, R.P.; Gong, X.Q.; Hamamori, Y.; Li, L. Dynamic Changes in Chromatin Acetylation and the Expression of Histone Acetyltransferases and Histone Deacetylases Regulate the SM22alpha Transcription in Response to Smad3-Mediated TGFbeta1 Signaling. Biochem. Biophys. Res. Commun. 2006, 348, 351–358. [Google Scholar] [CrossRef]
- Wang, C.; Tian, W.; Hu, S.-Y.; Di, C.-X.; He, C.-Y.; Cao, Q.-L.; Hao, R.-H.; Dong, S.-S.; Liu, C.-C.; Rong, Y.; et al. Lineage-Selective Super Enhancers Mediate Core Regulatory Circuitry during Adipogenic and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Cell Death Dis. 2022, 13, 866. [Google Scholar] [CrossRef]
- Pott, S.; Lieb, J.D. What Are Super-Enhancers? Nat. Genet. 2015, 47, 8–12. [Google Scholar] [CrossRef]
- Siersbæk, R.; Rabiee, A.; Nielsen, R.; Sidoli, S.; Traynor, S.; Loft, A.; Poulsen, L.L.C.; Rogowska-Wrzesinska, A.; Jensen, O.N.; Mandrup, S. Transcription Factor Cooperativity in Early Adipogenic Hotspots and Super-Enhancers. Cell Rep. 2014, 7, 1443–1455. [Google Scholar] [CrossRef]
- Sayegh, J.; Cao, J.; Zou, M.R.; Morales, A.; Blair, L.P.; Norcia, M.; Hoyer, D.; Tackett, A.J.; Merkel, J.S.; Yan, Q. Identification of Small Molecule Inhibitors of Jumonji AT-Rich Interactive Domain 1B (JARID1B) Histone Demethylase by a Sensitive High Throughput Screen. J. Biol. Chem. 2013, 288, 9408–9417. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, L.; Wiese, C.B.; Zore, T.; Riestenberg, C.; Avetisyan, R.; Reue, K. Gene Regulation and Mitochondrial Activity During White and Brown Adipogenesis Are Modulated by KDM5 Histone Demethylase. J. Endocr. Soc. 2024, 8, bvae029. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Jiao, L.-M.; Qi, Y.-R.; Wang, T.-C.; Li, Y.-L.; Xu, J.-L.; Wang, Z.-W.; Yu, B.; Liu, H.-M.; Zhao, W. Discovery of Novel Pyrazole-Based KDM5B Inhibitor TK-129 and Its Protective Effects on Myocardial Remodeling and Fibrosis. J. Med. Chem. 2022, 65, 12979–13000. [Google Scholar] [CrossRef] [PubMed]
- Link, J.C.; Wiese, C.B.; Chen, X.; Avetisyan, R.; Ronquillo, E.; Ma, F.; Guo, X.; Yao, J.; Allison, M.; Chen, Y.-D.I.; et al. X Chromosome Dosage of Histone Demethylase KDM5C Determines Sex Differences in Adiposity. J. Clin. Invest. 2020, 130, 5688–5702. [Google Scholar] [CrossRef]
- Liljebris, C.; Baranczewski, P.; Björkstrand, E.; Byström, S.; Lundgren, B.; Tjernberg, A.; Warolén, M.; James, S.R. Oxidation of Protein Tyrosine Phosphatases as a Pharmaceutical Mechanism of Action: A Study Using 4-Hydroxy-3,3-Dimethyl-2H-Benzo[g]Indole-2,5(3H)-Dione. J. Pharmacol. Exp. Ther. 2004, 309, 711–719. [Google Scholar] [CrossRef]
- Blum, G.; Ibáñez, G.; Rao, X.; Shum, D.; Radu, C.; Djaballah, H.; Rice, J.C.; Luo, M. Small-Molecule Inhibitors of SETD8 with Cellular Activity. ACS Chem. Biol. 2014, 9, 2471–2478. [Google Scholar] [CrossRef]
- Shoaib, M.; Chen, Q.; Shi, X.; Nair, N.; Prasanna, C.; Yang, R.; Walter, D.; Frederiksen, K.S.; Einarsson, H.; Svensson, J.P.; et al. Histone H4 Lysine 20 Mono-Methylation Directly Facilitates Chromatin Openness and Promotes Transcription of Housekeeping Genes. Nat. Commun. 2021, 12, 4800. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.-M.; Mei, L.; Yu, Y.; Guo, Y.; Mackintosh, S.G.; Chen, J.; Allison, D.F.; Kim, A.; Storey, A.J.; et al. BAHCC1 Binds H4K20me1 to Facilitate the MCM Complex Loading and DNA Replication. Nat. Commun. 2025, 16, 5502. [Google Scholar] [CrossRef]
- Ugai, K.; Matsuda, S.; Mikami, H.; Shimada, A.; Misawa, T.; Nakamura, H.; Tatsumi, K.; Hatano, M.; Murayama, T.; Kasuya, Y. Inhibition of the SET8 Pathway Ameliorates Lung Fibrosis Even Through Fibroblast Dedifferentiation. Front. Mol. Biosci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Glondu-Lassis, M.; Dromard, M.; Chavey, C.; Puech, C.; Fajas, L.; Hendriks, W.; Freiss, G. Downregulation of Protein Tyrosine Phosphatase PTP-BL Represses Adipogenesis. Int. J. Biochem. Cell Biol. 2009, 41, 2173–2180. [Google Scholar] [CrossRef]
- Jung, H.; Kim, W.K.; Kim, D.H.; Cho, Y.S.; Kim, S.J.; Park, S.G.; Park, B.C.; Lim, H.M.; Bae, K.-H.; Lee, S.C. Involvement of PTP-RQ in Differentiation during Adipogenesis of Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2009, 383, 252–257. [Google Scholar] [CrossRef]
- Gomez, E.; Vercauteren, M.; Kurtz, B.; Ouvrard-Pascaud, A.; Mulder, P.; Henry, J.-P.; Besnier, M.; Waget, A.; Hooft Van Huijsduijnen, R.; Tremblay, M.L.; et al. Reduction of Heart Failure by Pharmacological Inhibition or Gene Deletion of Protein Tyrosine Phosphatase 1B. J. Mol. Cell. Cardiol. 2012, 52, 1257–1264. [Google Scholar] [CrossRef]
- Wade, F.; Quijada, P.; Al-Haffar, K.M.A.; Awad, S.M.; Kunhi, M.; Toko, H.; Marashly, Q.; Belhaj, K.; Zahid, I.; Al-Mohanna, F.; et al. Deletion of Low Molecular Weight Protein Tyrosine Phosphatase (Acp1) Protects against Stress-Induced Cardiomyopathy. J. Pathol. 2015, 237, 482–494. [Google Scholar] [CrossRef]
- Schulman, I.G. Liver X Receptors Link Lipid Metabolism and Inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef]
- Meyer, M.B.; Lee, S.M.; Towne, J.M.; Cichanski, S.R.; Kaufmann, M.; Jones, G.; Pike, J.W. In Vivo Contribution of Cyp24a1 Promoter Vitamin D Response Elements. Endocrinology 2024, 165, bqae134. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.H.; Choi, M.; Pham, H.G.; Yun, J.W. Cytochrome P450 2F2 (CYP2F2) Negatively Regulates Browning in 3T3-L1 White Adipocytes. Eur. J. Pharmacol. 2021, 908, 174318. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.H.; Yun, J.W. Cytochrome P450 2E1 (CYP2E1) Positively Regulates Lipid Catabolism and Induces Browning in 3T3-L1 White Adipocytes. Life Sci. 2021, 278, 119648. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ni, L.; Duan, Q.; Wang, X.; Chen, C.; Chen, S.; Chaugai, S.; Zeldin, D.C.; Tang, J.R.; Wang, D.W. CYP Epoxygenase 2J2 Prevents Cardiac Fibrosis by Suppression of Transmission of Pro-Inflammation from Cardiomyocytes to Macrophages. Prostaglandins Other Lipid Mediat. 2015, 116–117, 64–75. [Google Scholar] [CrossRef]
- Weng, J.; Cheng, Q.; Yang, J.; Jin, H.; Zhang, R.; Guan, J.; Ma, Y.; Wang, L.; Chen, C.; Wang, Z. Gal-1-Mediated Cytochrome P450 Activation Promotes Fibroblast into Myofibroblast Differentiation in Pulmonary Fibrosis. Int. Immunopharmacol. 2024, 141, 112920. [Google Scholar] [CrossRef]
- Wilson, T.S.; Scaffidi, P. Compromised Epigenetic Robustness in Cancer: Fueling Evolution, Exposing Weakness. Trends Cancer 2025, 11, 575–590. [Google Scholar] [CrossRef]
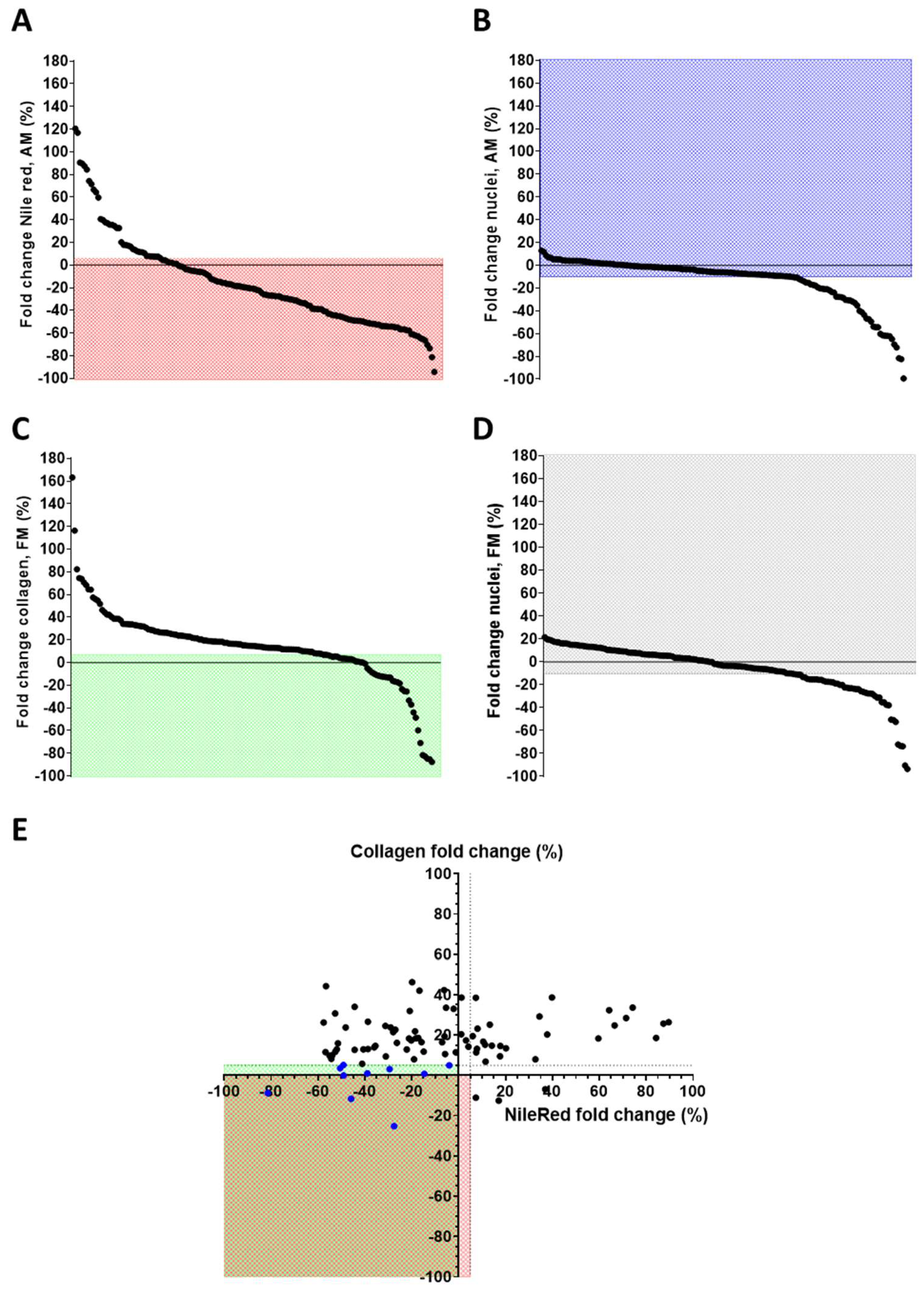
| Epigenetic Drugs | Mean of Fold Change (%) NR/Nuclei Number (AM) | Mean of Fold Change (%) Nuclei Number (AM) | Mean of Fold Change (%) COL/Nuclei Number (FM) | Mean of Fold Change (%) Nuclei Number (FM) |
|---|---|---|---|---|
| Splitomicin | −4.00 | −1.59 | 5.00 | 13.18 |
| CPTH2 (hydrochloride) | −27.52 | 0.36 | −25.18 | 14.11 |
| Suberohydroxamic acid | −14.65 | −8.04 | 0.78 | 5.52 |
| CPTH6 (hydrobromide) | −50.56 | 12.87 | 3.53 | 19.76 |
| GSK126 | −38.94 | −5.59 | 1.02 | 12.37 |
| EPZ5676 | −49.14 | 3.94 | 5.00 | 17.27 |
| BVT 948 | −81.41 | 8.69 | −8.70 | 19.32 |
| NI-57 | −49.06 | −2.47 | −0.02 | 21.58 |
| PBIT | −29.46 | −9.30 | 3.11 | 14.21 |
| CAY10722 | −45.88 | 1.55 | −11.63 | −4.14 |
| Epigenetic Drug | Dose | FC (%) Nile Red/Nuclei Number (AM) | FC (%) Nuclei Number (AM) | FC (%) COL/Nuclei Number (FM) | FC (%) Nuclei Number (FM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Splitomicin | 0.1 μM | −39.8 | 5.7 | −2.2 | 1.5 | −6.6 | 6.3 | −1.4 | 2.0 |
| 1 μM | −38.8 | 6.8 | −2.2 | 1.6 | 0.6 | 5.5 | 0.3 | 0.7 | |
| 5 μM | −26.2 | 4.8 | −2.9 | 1.6 | 8.9 | 8.0 | 1.8 | 2.6 | |
| 20 μM | −31.2 | 3.5 | −11.3 | 3.8 | 17.5 | 7.7 | −3.4 | 2.9 | |
| SBHA | 0.1 μM | −29.9 | 10.2 | 2.1 | 1.0 | −2.0 | 10.7 | 3.7 | 1.6 |
| 1 μM | −28.6 | 8.5 | 1.0 | 1.5 | −8.8 | 7.5 | 3.5 | 2.2 | |
| 5 μM | −31.1 | 12.6 | −5.1 | 0.9 | −17.4 | 11.0 | −0.1 | 3.0 | |
| 20 μM | −3.0 | 25.2 | −16.7 | 1.8 | 48.8 | 18.5 | −15.7 | 4.4 | |
| CPTH6 | 0.1 μM | −20.5 | −19.1 | 0.6 | 1.1 | −4.3 | 5.1 | 4.5 | 2.2 |
| 1 μM | 8.8 | 8.6 | 6.7 | 2.5 | −16.8 | 3.2 | 13.6 | 1.5 | |
| 5 μM | −20.5 | −19.1 | 1.9 | 1.1 | −22.9 | 3.9 | 13.8 | 2.8 | |
| 20 μM | 8.8 | 8.6 | −8.4 | 2.6 | 18.3 | 18.3 | −5.5 | 6.8 | |
| BVT 948 | 0.1 μM | −37.3 | 10.3 | −0.8 | 2.6 | −1.9 | 8.2 | 12.4 | 3.4 |
| 1 μM | −69.0 | 6.3 | 0.6 | 2.1 | −23.0 | 6.1 | 14.6 | 8.1 | |
| 5 μM | −58.8 | 8.7 | −11.5 | 3.1 | 75.3 | 50.5 | −46.7 | 12.3 | |
| PBIT | 0.1 μM | −25.0 | 12.2 | −2.4 | 2.0 | 14.6 | 10.9 | −5.6 | 1.8 |
| 1 μM | −23.3 | 3.8 | −0.9 | 1.4 | 7.8 | 6.9 | −3.3 | 2.6 | |
| 5 μM | −3.4 | 6.1 | −5.9 | 2.8 | −17.8 | 15.1 | −17.5 | 10.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, M.; Moimas, S.; Braga, L.; Santin, Y.; Galotta, A.; Giacca, M.; Pompilio, G.; Sommariva, E. Epigenetic Drugs Splitomicin, Suberohydroxamic Acid, CPTH6, BVT-948, and PBIT Moderate Fibro-Fatty Development in Arrhythmogenic Cardiomyopathy. Biomolecules 2025, 15, 1565. https://doi.org/10.3390/biom15111565
Lippi M, Moimas S, Braga L, Santin Y, Galotta A, Giacca M, Pompilio G, Sommariva E. Epigenetic Drugs Splitomicin, Suberohydroxamic Acid, CPTH6, BVT-948, and PBIT Moderate Fibro-Fatty Development in Arrhythmogenic Cardiomyopathy. Biomolecules. 2025; 15(11):1565. https://doi.org/10.3390/biom15111565
Chicago/Turabian StyleLippi, Melania, Silvia Moimas, Luca Braga, Yohan Santin, Arianna Galotta, Mauro Giacca, Giulio Pompilio, and Elena Sommariva. 2025. "Epigenetic Drugs Splitomicin, Suberohydroxamic Acid, CPTH6, BVT-948, and PBIT Moderate Fibro-Fatty Development in Arrhythmogenic Cardiomyopathy" Biomolecules 15, no. 11: 1565. https://doi.org/10.3390/biom15111565
APA StyleLippi, M., Moimas, S., Braga, L., Santin, Y., Galotta, A., Giacca, M., Pompilio, G., & Sommariva, E. (2025). Epigenetic Drugs Splitomicin, Suberohydroxamic Acid, CPTH6, BVT-948, and PBIT Moderate Fibro-Fatty Development in Arrhythmogenic Cardiomyopathy. Biomolecules, 15(11), 1565. https://doi.org/10.3390/biom15111565







