Abstract
Infertility represents a significant global health challenge affecting both men and women. Despite regular unprotected sexual intercourse, approximately 15% of couples of reproductive age struggle to conceive within 12 months, with 10% of infertility cases attributed to unknown causes worldwide. As a result, numerous studies have turned their attention to exploring the use of natural products for the prevention and treatment of infertility. Among these natural remedies is date palm pollen (DPP), a male reproductive powder derived from the blossoms of the Phoenix dactylifera L. palm tree, which has a long history of use as a dietary supplement, particularly as an aphrodisiac and fertility enhancer for both men and women. This review critically examines the diverse components of DPP, including metabolites, proteins, amino acids, fatty acids, to elucidate its potential impact on human reproduction. The analysis thoroughly assesses the composition of DPP in relation to its effects on enhancing reproductive processes and delves into its traditional uses and therapeutic benefits in male fertility, such as the enhancement of sexual desire, semen quality, and hormonal equilibrium. Similarly, it explores the influence of DPP on female fertility, emphasizing its potential to improve factors such as lubrication, desire, ovulation, and hormonal balance. Overall, this review underscores the potential of DPP as a natural remedy for addressing reproductive disorders.
1. Introduction
Infertility stands as a significant global health concern affecting both men and women. Approximately 15% of couples of reproductive age, despite regular unprotected sexual contact, face difficulties in conceiving within 12 months, with 10% of cases attributed to unknown factors [1]. Consequently, there has been a growing interest in exploring natural products for the prevention and treatment of infertility [2]. Furthermore, the prevalence of infertility in both developing and developed countries has prompted research into alternative approaches to address fertility issues. Within this framework, natural products such as date palm pollen (DPP) have garnered attention as potential interventions [3]. Extensive investigations into the effects of DPP on male and female reproductive parameters have been conducted to mitigate fertility-related problems. As a result, its notable ameliorative effects have been underscored in numerous clinical and subclinical studies [4,5,6]. Nevertheless, a comprehensive understanding of its impacts on male and female reproductive parameters is imperative for the effective utilization of the therapeutic potential of DPP.
The date palm (Phoenix dactylifera L.), a monocotyledonous plant belonging to the Arecaceae family, is primarily found in arid regions of the Middle East and North Africa [7]. Date palm pollen (DPP), extracted from the date palm, accounts for approximately 1000 tons of production annually, derived from millions of palm trees cultivated in Arabian regions. DPP represents the male reproductive cells of palm flowers and serves as a natural reservoir of various bioactive compounds, including estradiol, estriol, cholesterol, estrone, saponins, carbohydrates, fatty acids, tannins, and flavonoids [8,9,10,11]. These diverse compounds contribute to its considerable nutritional value and diverse therapeutic effects [9,12]. Moreover, DPP exhibits significant antioxidant and antimicrobial activities owing to the presence of volatile unsaturated fatty acids, phenolic compounds, carotenoids, and tocopherols [9,13].
This review aims to comprehensively analyze the correlation between the composition of DPP and its impacts on reproduction by conducting a thorough examination of the various secondary metabolites, proteins, amino acids, carbohydrates, fatty acids, minerals, and vitamins found in DPP. Additionally, it investigates the traditional uses of DPP and its therapeutic effects on male fertility, including its potential to enhance sexual desire, semen quality, and hormonal levels. Subsequently, it delves into its influence on female fertility, highlighting its potential to improve lubrication, desire, ovulation, as well as various biochemical and hormonal factors. This study extends to unraveling the possible mechanisms implicated in these effects on both male and female reproductive parameters. While existing reviews have explored the effects of date palm pollen on reproductive parameters, this review stands out by delving into potential mechanisms and the involvement of specific components of date palm pollen in shaping these reproductive outcomes.
2. Status of Date Palm in the World
The date palm (Phoenix dactylifera L.), a popular perennial fruit tree belonging to the Arecaceae family, holds considerable agricultural and economic importance globally [14]. Renowned for its ability to thrive in harsh desert conditions, the date palm has been cultivated in regions such as North Africa, the Arabian Peninsula, and the Middle East, where it plays a vital role as a representative species within desert ecosystems [15,16]. Over the past three centuries, its cultivation has expanded to various parts of the world, including Australia, India, Mexico, Pakistan, Southern Africa, South America, and the United States of America [7]. Presently, the global date palm population has exceeded 120 million trees, with the Middle East region alone contributing to 70% of this total [17]. Within this diverse palm population, 5000 different date varieties are cultivated worldwide. Notably, Morocco holds a significant share of this diversity, with the INRA cataloging a total of 453 Moroccan date palm varieties. Among these, the Mejhoul, Boufeggous, Bouskri, and Jihel varieties stand out prominently [18]. Additionally, internationally recognized date varieties include Ajwa, Zahidi, Aseel, Majdool, Mabrook, Dhakki, Halawi, Lasht, Deggla, and Bamy [19,20].
Apart from its fruits, the date palm offers various secondary by-products such as leaves, coir, petioles, spadix stems, and trunks, serving multiple traditional purposes [21]. Originally cultivated for its date fruits, the date palm is renowned for its nutritional and medicinal properties owing to its richness in biomolecules [3]. It has been utilized to create value-added products like date flour, fiber concentrate, juices, jam, date fruit bars, sugar, and various dairy and bakery products, rendering the date palm an economically valuable commodity [22]. In the sphere of date palm pollen, only a few male date palm trees are needed to ensure the pollination process, with one male producing enough pollen to fertilize 50 female trees [23]. The remaining pollen is utilized in the Moroccan pharmacopeia to treat or prevent different diseases, mainly those related to reproduction in both males and females, further enhancing its economic value (Figure 1). It has attracted attention for its potential therapeutic effects, particularly in the realm of fertility. Studies suggest that DPP may positively impact reproductive health by enhancing sperm quality and count in males, as well as regulating menstrual cycles and improving ovulatory function in females [4,24,25]. Moreover, the historical application of date palm pollen as a revitalizing medicinal agent by early Egyptians and Chinese adds a rich layer to its narrative [10]. Previous studies delving into pollen’s impact on the reproductive system have further demonstrated its enhancing effects, encompassing anticoccidial, antiapoptotic, and antimicrobial properties [26,27].

Figure 1.
Date palm pollen powder.
3. Exploring Mechanisms: How Date Palm Pollen Composition Influences Reproduction?
The nutritional value of DPP has been recognized for an extensive period. Early Egyptians and ancient Chinese civilizations utilized DPP as a rejuvenating medicinal agent, often referring to it as the “fountain of youth”. Furthermore, owing to its nutritional content, DPP has traditionally served as an aphrodisiac and fertility enhancer [4]. Consequently, recent research has focused on the biochemical and nutritional characterization of this natural product. These studies collectively underscore the abundance of secondary metabolites in DPP, primarily antioxidants. The quantity of these metabolites varies depending on factors such as the DPP variety, growth soil, and climatic conditions [28,29,30]. Additionally, DPP represents a significant source of proteins, essential amino acids, carbohydrates, fatty acids, minerals, and vitamins [31].
3.1. Secondary Metabolites
DPP contains a diverse array of secondary metabolites (Table 1), including phenolic compounds [11], triterpenoids, sterols, and carotenoids. These organic compounds serve functions that extend beyond plant growth and reproduction, playing roles such as pest defense, pollinator attraction, and adaptation to environmental stress [32,33]. Consequently, these secondary metabolites possess a spectrum of bioactive properties that render them valuable for various human applications [34]. Certain secondary metabolites of DPP have been the subject of research for their potential effects on various aspects of reproduction, including fertility, hormonal balance, and reproductive health (Table 1).

Table 1.
Approximate composition of DPP.
3.1.1. Phenolic Compounds
Considerable quantities of phenolic compounds, including caffeic acid, gallic acid, coumaric acid, catechin, chlorogenic acid, quercetin, and rutin, along with flavonoids such as isorhamnetin, apigenin, lutein, and naringin, have been identified in Egyptian, Tunisian, Moroccan, and Iraqi DPP [38,40,41] (Table 2). Additionally, other studies have highlighted pyrogallol and catechin as the major phenolic compounds present in DPP [9,42]. Throughout these investigations, substantial variability in terms of the concentration and types of phenolic compounds has been observed. This variability can be ascribed to various biological factors, including differences in genetics and cultivation practices, as well as environmental factors such as soil conditions, maturation stages, salinity levels, temperature, water availability, and light intensity [43]. Several experimental studies have examined the effects of phenolic compounds from DPP (administered at doses ranging from 100 to 360 mg/kg body weight (BW)/day) on sexual and reproductive functions in laboratory animals) [44,45,46,47]. All of these studies have reported that aqueous and ethanolic extracts of DPP enhance testicular health, hormone levels, and sexual behavior. Notably, the research has documented an improvement in mounting frequency, testosterone levels, sperm count, motility, and morphology, alongside histological and physiological changes in reproductive organs. Furthermore, the beneficial effects of these extracts on body weight and antioxidant status have also been observed. These studies offer valuable insights into the potential impacts of the phenolic compounds and flavonoids present in DPP on sexual parameters and reproduction [45,48,49]. However, it is important to note that these studies have not fully elucidated the precise mechanisms through which these biomolecules enhance reproductive characteristics. The positive results observed with both aqueous and ethanolic extracts of Date Palm Pollen (DPP) strongly suggest potent biological activity within its polar fraction. These extracts, each targeting distinct solubility profiles, reveal the presence of bioactive polar compounds like phenolic acids and flavonoids. These constituents, known for their affinity to cellular components such as enzymes, receptors, and signaling pathways crucial for reproduction, are often associated with robust biological effects. The combined effectiveness of both extracts implies a significant contribution of polar molecules, likely concentrated in these fractions, to the observed improvements in reproductive health parameters. Further research exploring the specific polar compounds present in DPP and their precise mechanisms of action is essential to elucidate their pivotal roles in promoting reproductive function.
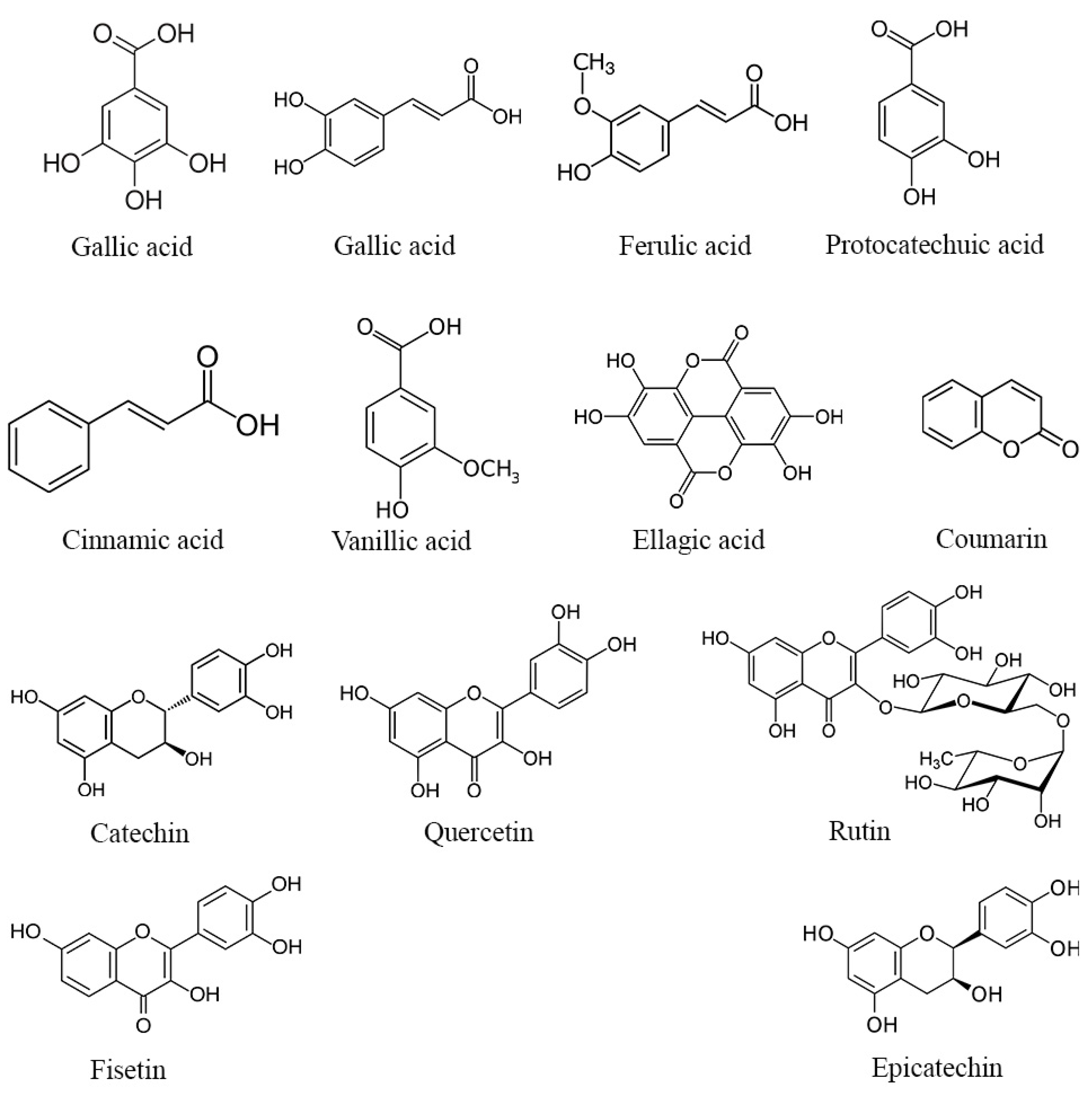
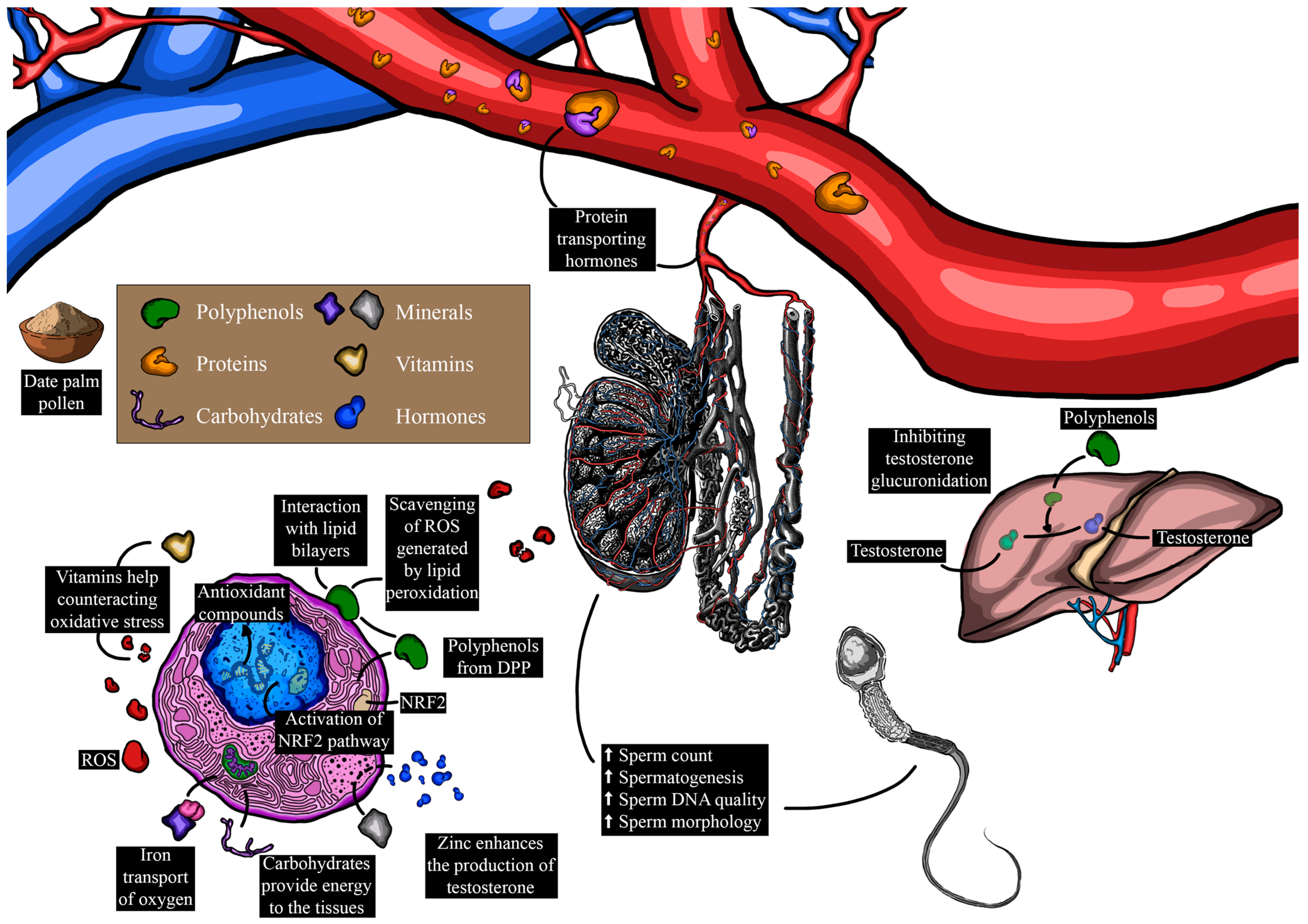
While the complete mechanisms underlying the potential enhancement of reproductive parameters by phenolic compounds and flavonoids in DPP remain incompletely understood, several hypotheses warrant consideration. It is plausible that these bioactive molecules might exert their effects through the modulation of hormonal pathways, including the potential influence on testosterone, luteinizing hormone, and estradiol levels, all of which play pivotal roles in reproductive function. For example, Jenkinson et al. [50] reported that phenolic compounds present in red wine can elevate testosterone levels by inhibiting its glucuronidation and subsequently reducing its urinary excretion. Furthermore, the antioxidant properties of phenolic compounds and flavonoids could potentially contribute to the reduction in oxidative stress within the reproductive system, thereby promoting healthier testicular architecture and function [51]. Several phenolic compounds possess both hydrophilic and lipophilic properties, rendering them amphiphilic in nature [52]. This unique property allows them to attach to cell membrane surfaces, potentially forming lipid bilayers and interacting with hydrophobic lipid chains. Through these interactions, polyphenols such as the gallic, α-coumaric, and ellagic acids (structures shown in Figure 2) can scavenge free radicals by H-atom transfer, resulting from lipid peroxidation, thereby safeguarding cell membranes and their contents from oxidative damage [53]. Moreover, it has been reported that these phenolic compounds enhance the antioxidant defense system by activating the extracellular signal-regulated kinase/nuclear transcription factor–erythroid 2-related factor 2 (ERK/Nrf2) pathways [54,55]. This activation leads to an upsurge in the expression of antioxidant enzymes, thereby contributing to the observed antioxidant activity displayed by certain phenolic compounds [55].
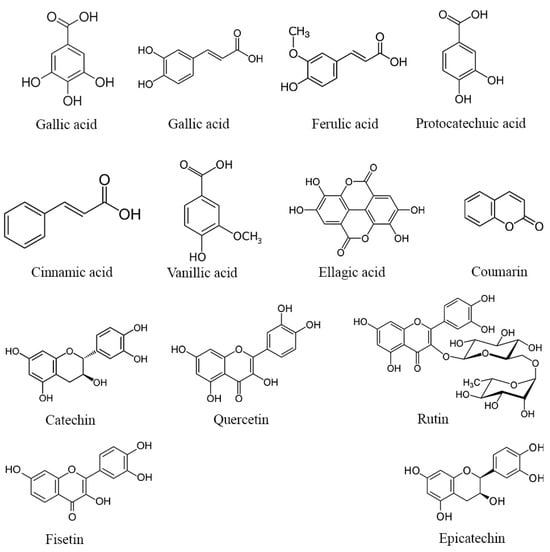
Figure 2.
Structure of the main phenolic compounds found in DPP.

Table 2.
Main phenolic compounds found in DPP.
Table 2.
Main phenolic compounds found in DPP.
| Main Phenolic Compounds | Origin of DPP | References |
|---|---|---|
| Gallic acid, protocatechuic acid, chlorogenic acid, vanillic acid, caffeic acid, ferulic acid, alpha-coumaric acid, ellagic acid, cinnamic acid, salycilic acid, pyrogallol, catechin, catechol, epicatechein, caffeine, coumarin, reversetrol, narengin, hesperidin, rutin, quercetrin, rosmarinic, quercetin, naringenin, hesperitin, kaempferol, rhamnetin, apigenin, acacetin | Aswan governorate, northern Egypt | Ibrahim et al. [38] |
| Gallic acid, caffeic acid, ferulic acid, cinnamic acid, catechin, rutin, quercetin | Tata oasis region, south-east of Morocco | Salhi et al. [11] |
| Protocatechuic acid, vanillic acid, ellagic acid, rutin, quercetin, fisetin | Kerkennah and Tozeur regions, east and south-east of Tunisia | Daoud et al. [43] |
| Gallic acid, vanillic acid, caffeic acid, catechin, epicatechein, coumarin, rutin, quercetin | Biskra, south-east of Algeria | Benouamane et al. [56] |
| Chlorogenic acid, caffeic acid, cinnamic acid, catechin, rutin, quercetin, kaempferol, apigenin | Sharkia governorate, northern Egypt | Abdallah et al. [57] |
| Gallic acid, protocatechuic acid, chlorogenic acid, vanillic acid, caffeic acid, ferulic acid, alpha-coumaric acid, benzoic acid, ellagic acid, cinnamic acid, salycilic acid, pyrogallol, catechin, catechol, epicatechein, caffeine, coumarin, reversetrol, narengin, hesperidin, rutin, quercetrin, quercetin, hesperitin, kaempferol, rhamnetin, apigenin, acacetin | Riyadh, central Saudi Arabia | Abou Zeid et al. [58] |
| Gallic acid, chlorogenic acid, caffeic acid, catechin, rutin, quercetin | Alexandria, Egypt | El-Kholy et al. [9] |
3.1.2. Hormones
As early as 1947, Hassan and El Wafa [59], reported that the water-soluble form of the non-saponified fraction of the oil extracted from DPP exhibited similar cornification of the vaginal smear in rats, comparable to those injected with pure estradiol dipropionate. Consequently, these authors suggested the presence of biologically estrogenic substances within DPP. Previous investigations have also unveiled the presence of estrogenic materials in DPP extracts. Indeed, gonadotropic molecules derived from DPP were initially extracted by Soliman and Soliman [60], who highlighted the presence of a suitable Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) -like in pollen grain extracts, inducing reproductive actions in rats. Subsequently, similar studies were carried out by El-Ridi et al. [61] and, more recently, by Otify et al. [62]. Additionally, Mahran et al. [63] isolated a steroidal saponin glycoside and a glycoprotein demonstrating gonadotrophic activity. They also confirmed the presence of estrone through thin-layer chromatography. However, to date, no study has succeeded in fully purifying and identifying gonadotropic hormones from DPP.
3.2. Proteins and Amino Acids
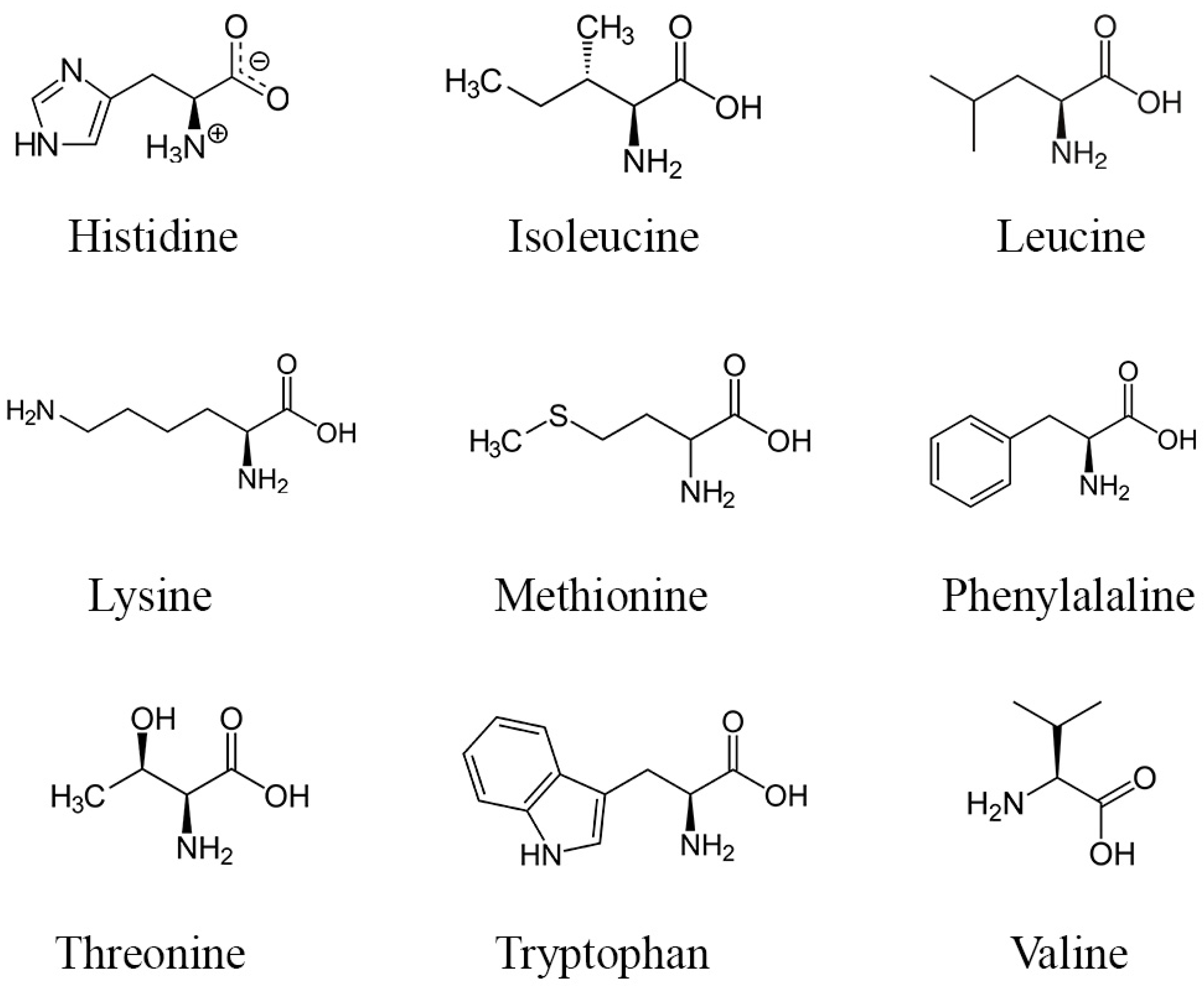
The protein content of DPP has exhibited a substantial range, varying from 15.81% to 38.18% [35,64] (Table 3). This considerable variability in protein content across studies can primarily be attributed to factors such as genotype, geographic origin, soil type, and climate conditions under which date palms are cultivated. For instance, Bacha et al. [64] investigated protein contents of 13 cultivars of date palms grown in Riyadh, Saudi Arabia, revealing protein contents ranging from 15.81% to 18.02%. Similarly, in Iraq, a study focusing on DPP of two cultivars demonstrated that the Ghannamy Ahmar pollen displayed a higher crude protein content of 27.24% compared to the Samasmi pollen, which contained 23.42% [39]. Furthermore, various studies conducted in Giza, Egypt, on the protein content in DPP of the El Hayani variety showed contents ranging between 30% and 33%, highlighting consistent protein richness [9,10,24,65]. Conversely, an investigation carried out in the Sohag governorate on the same variety demonstrated an even higher protein content, reaching 36% [66]. Similar results were obtained in Moroccan DPP, which contains about 35% of crude proteins [11]. The highest protein content was recorded in Deglet Nour DPP collected from Sfax, Tunisia, with 38.18% [35]. Despite the variations among studies, the protein content in DPP remains notably higher compared to date fruit, which does not exceed 6.5% [67,68]. DPP has also been described as a rich source of essential amino acids, particularly arginine (0.15–5.17%), valine (1.81–5.16%), histidine (1.61–2.53%), isoleucine (1.49–4.37%), leucine (3.43–8.35%), lysine (2.95–7.73%), methionine (0.11–2.37%), phenylalanine (1.63–4.25%), and threonine (1.72–4.70%) [10,35].
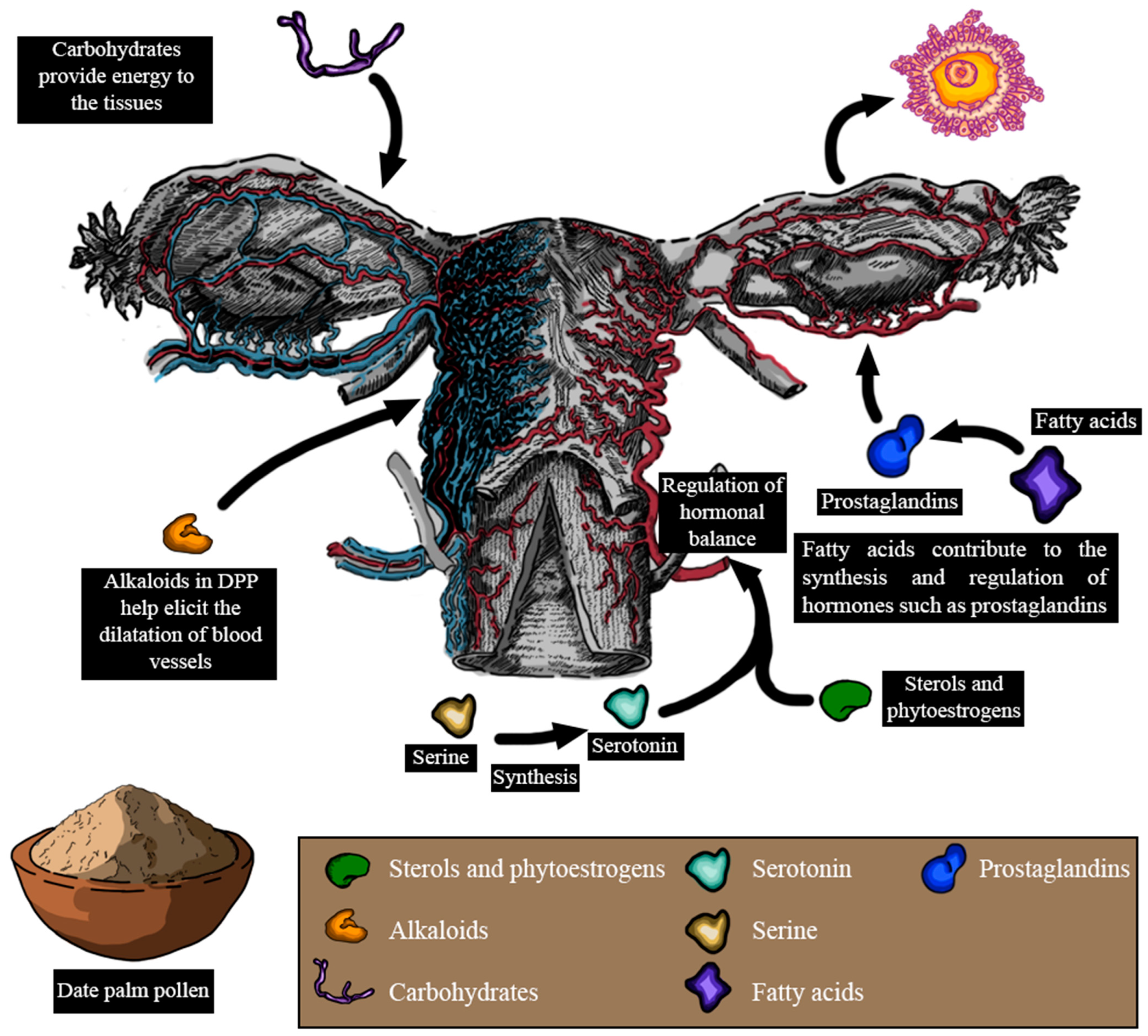
Proteins and amino acids present in DPP may potentially impact fertility, yet further research is necessary to delineate their precise mechanisms as current knowledge remains incomplete. Nevertheless, amino acids and proteins found in DPP might enhance fertility through various mechanisms. Amino acids serve as the fundamental constituents of proteins, crucial for the synthesis of sexual hormones, facilitating the conversion of precursor molecules into active hormones via specialized proteins known as enzymes [69]. Sexual hormones, such as testosterone and estrogen, typically bind to carrier proteins for stable transportation to target tissues [70]. Upon reaching these target tissues, these hormones interact with specific receptor proteins on cells, instigating gene expression alterations and eliciting cellular responses by initiating reactions [71]. Hormone production is regulated by feedback mechanisms involving proteins to maintain a delicate balance [72]. Moreover, research has indicated that specific amino acids, including arginine (molecular structures in Figure 3), can enhance sperm quality and function by reducing heat and oxidative stress without adverse effects [73,74,75].
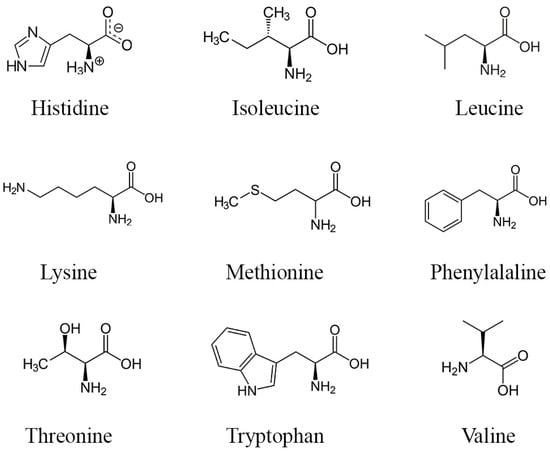
Figure 3.
Structure of the main essential amino acids found in DPP.

Table 3.
Amino acid composition of DPP.
Table 3.
Amino acid composition of DPP.
| Amino Acids | 3 Letter Code | 1 Letter Code | DPP | DPP | DPP | DPP | DPP |
|---|---|---|---|---|---|---|---|
| Alanine | ala | A | 2.14–8.36 | 2.61 | 6.48 | 2.71 | 2.43–8.27 |
| Arginine | arg | R | 1.18–1.73 | 1.61 | 5.77 | 1.42 | 1.18–1.74 |
| Aspartic acid | asp | D | 4.48–3.13 | 3.55 | 10.41 | 1.53 | 3.23–4.70 |
| Cysteine | cys | C | N/A | 0.42 | 1.11 | 0.91 | N/A |
| Glutamine | gln | Q | 2.58–4.66 | 1.74 | 13.23 | 2.23 | 3.91–4.71 |
| Glycine | gly | G | 8.46–7.81 | 2.24 | 5 | 1.81 | 4.40–8.19 |
| Histidine | his | H | 1.14–1.64 | 1.61 | 2.53 | 1.89 | 0.82–1.84 |
| Isoleucine | ile | I | 2.38–1.54 | 1.49 | 4.37 | 1.51 | 1.39–2.57 |
| Leucine | leu | L | 3.65–2.21 | 3.34 | 8.35 | 3.51 | 1.99–3.72 |
| Lysine | lys | K | 1.92–3.21 | 2.95 | 7.73 | 2.81 | 2.87–3.77 |
| Methionine | met | M | 0.41–6.64 | 0.11 | 2.37 | 0.14 | 0.50–0.80 |
| Phenylalaline | phe | F | 1.19–1.87 | 1.63 | 4.25 | 1.73 | 1.15–1.90 |
| Proline | pro | P | 1.53–2.04 | 0.28 | N/A | N/A | 1.73–2.28 |
| Serine | ser | S | 0–1.88 | 1.89 | 5.74 | 0.45 | 0.72–1.92 |
| Threonine | thr | T | 0.55–1.46 | 1.72 | 4.7 | 1.66 | 0.62–1.91 |
| Tryptophan | trp | W | N/A | N/A | N/A | N/A | N/A |
| Tyrosine | tyr | Y | 0.01–0.16 | 1.55 | 3.46 | 0.82 | 0.37–1.53 |
| Valine | val | V | 2.77–1.88 | 1.81 | 5.16 | 1.92 | 1.24–2.80 |
| References | Bishr and Desoukey [13] | H. M. Hassan [10] | Sebii et al. [35] | Basuny et al. [36] | Aly [76] |
3.3. Carbohydrates and Fatty Acids
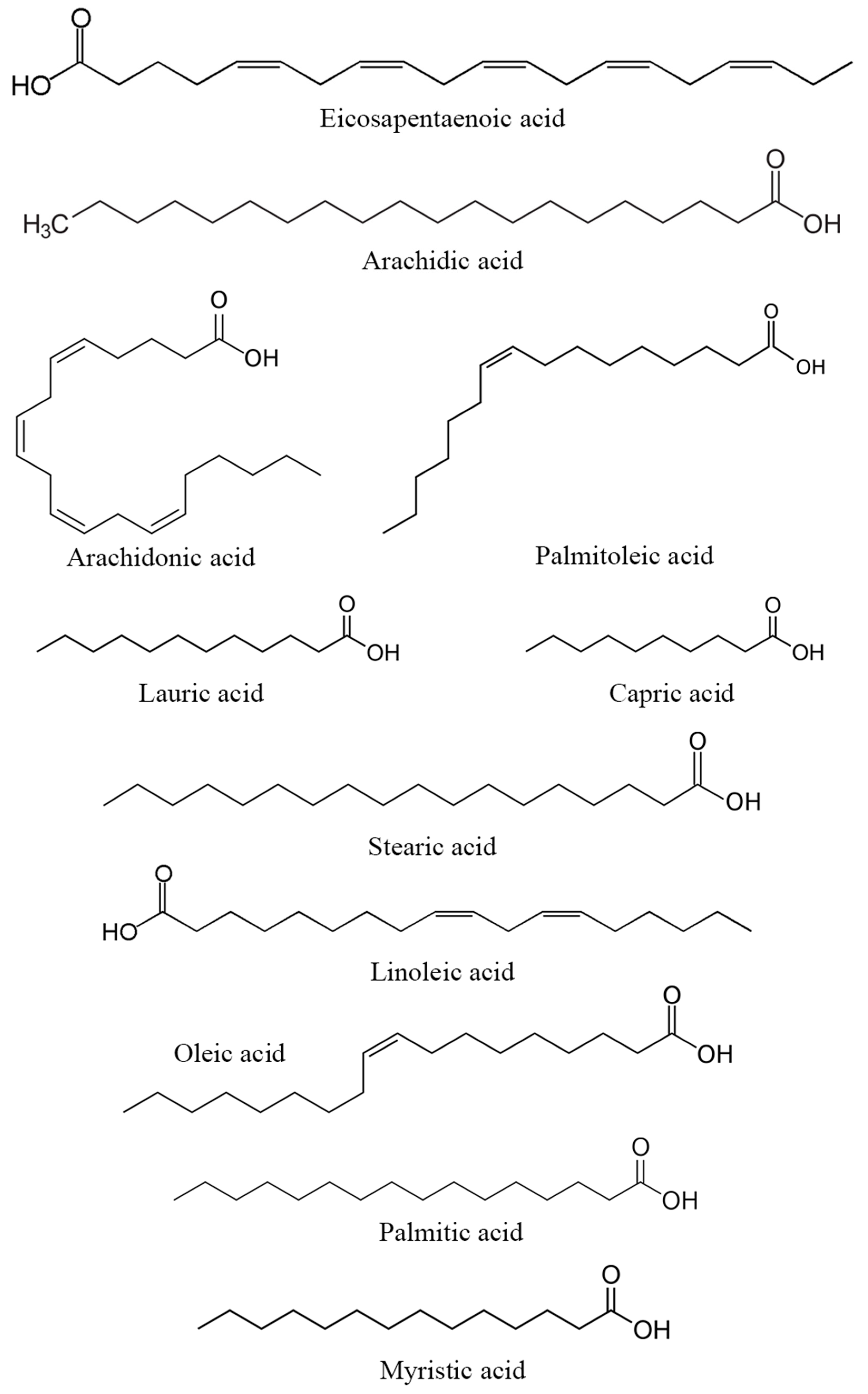
Several studies have examined the carbohydrate content in DPP across different varieties and regions (Table 4). The highest percentage was observed in Moroccan DPP at 26.51% [11], followed by the Ghannamy Ahmar variety grown in Iraq, which reached a percentage of 26.25% [37]. Another study reported that Iraqi Khikri and Samsami DPP contain 16.27% and 22.78% of carbohydrates, respectively [39]. Additional research conducted on the El-Hayani variety cultivated in Egypt reported that carbohydrate levels vary between 13.41% and 17.10% [9,10,24]. Regarding fatty acids, a study carried out in Egypt on DPP from the El-Hayani variety identified 13 fatty acids, with palmitic acid (34.45%) and linoleic acid (14.24%) having the highest concentrations [10]. Additionally, oleic, linolenic, stearic, margaric, behenic, arachidonic, and lignoceric acids were also detected at high levels in Egyptian and Moroccan DPP [9,11,77]. Furthermore, palmitic, oleic, and linoleic acids were the major fatty acids identified in the Khikri and Samsami varieties of Iraqi DPP [39].

Table 4.
Fatty acid composition of DPP.
Similar to proteins and amino acids, the specific contribution of carbohydrates and fatty acids from DPP to reproduction has not been extensively investigated. However, these compounds constitute a part of the nutritional content of DPP and could potentially indirectly influence reproductive processes. Carbohydrates serve as an energy source for reproductive tissues through the bonds between carbon and hydrogen atoms (fatty acid structure highlighted in Figure 4). [78], while fatty acids play a crucial role in the structure and function of cell membranes [79]. Essential fatty acids, including omega-3 and omega-6, participate in physiological processes that may potentially impact reproduction. Certain types of fatty acids contribute to the synthesis and regulation of hormones, including prostaglandins, which play a role in vital processes such as ovulation and implantation. While their roles are plausible, the scientific studies directly linking DPP carbohydrates and fatty acids to human reproductive processes are currently limited.
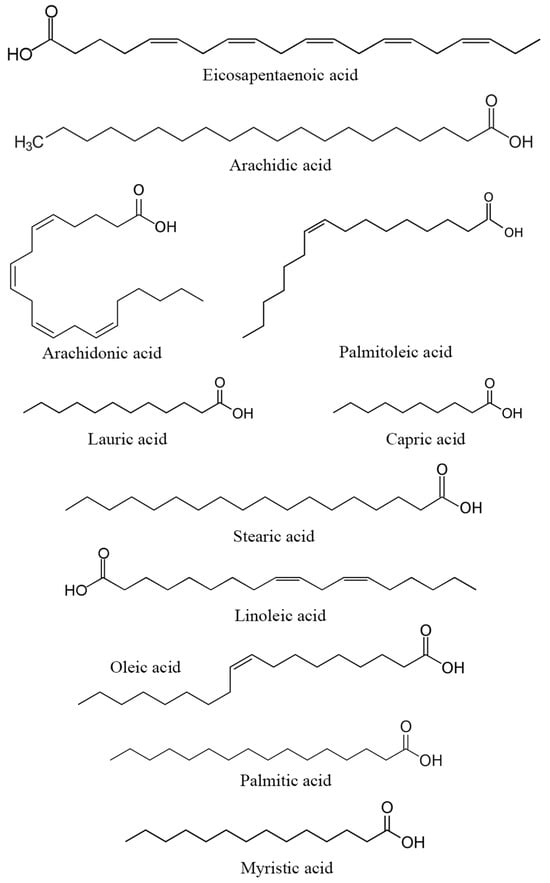
Figure 4.
Structure of the main fatty acids found in DPP.
3.4. Minerals and Vitamins
The DPP of the Egyptian variety El Hayani was found to contain significant amounts of minerals, including zinc (125–309 mg/100 g), calcium (60.5–560 mg/100 g), potassium (160–750 mg/100 g), selenium (305 mg/100 g), magnesium (130–318 mg/100 g), iron (241–226 mg/100 g), molybdenum (302 mg/100 g), copper (319.6 mg/100 g), manganese (170–310 mg/100g), and cobalt (305 mg/100g) [9,10,24,65,66]. However, variations observed between these studies could be attributed to factors such as the collection site and specific soil conditions at each location, as noted above for other compounds [80]. Moreover, DPP from the El-Ghannmi Ahmar cultivated in Iraq has demonstrated even higher mineral contents compared to Egyptian DPP. Specifically, the Iraqi DPP revealed elevated levels of potassium (7350 mg/100 g), magnesium (1960 mg/100 g), calcium (1080 mg/100 g), iron (8500 mg/100 g), and copper (365 mg/100 g), in comparison to Egyptian DPP [37] (Table 5).

Table 5.
Mineral and elemental composition of DPP.
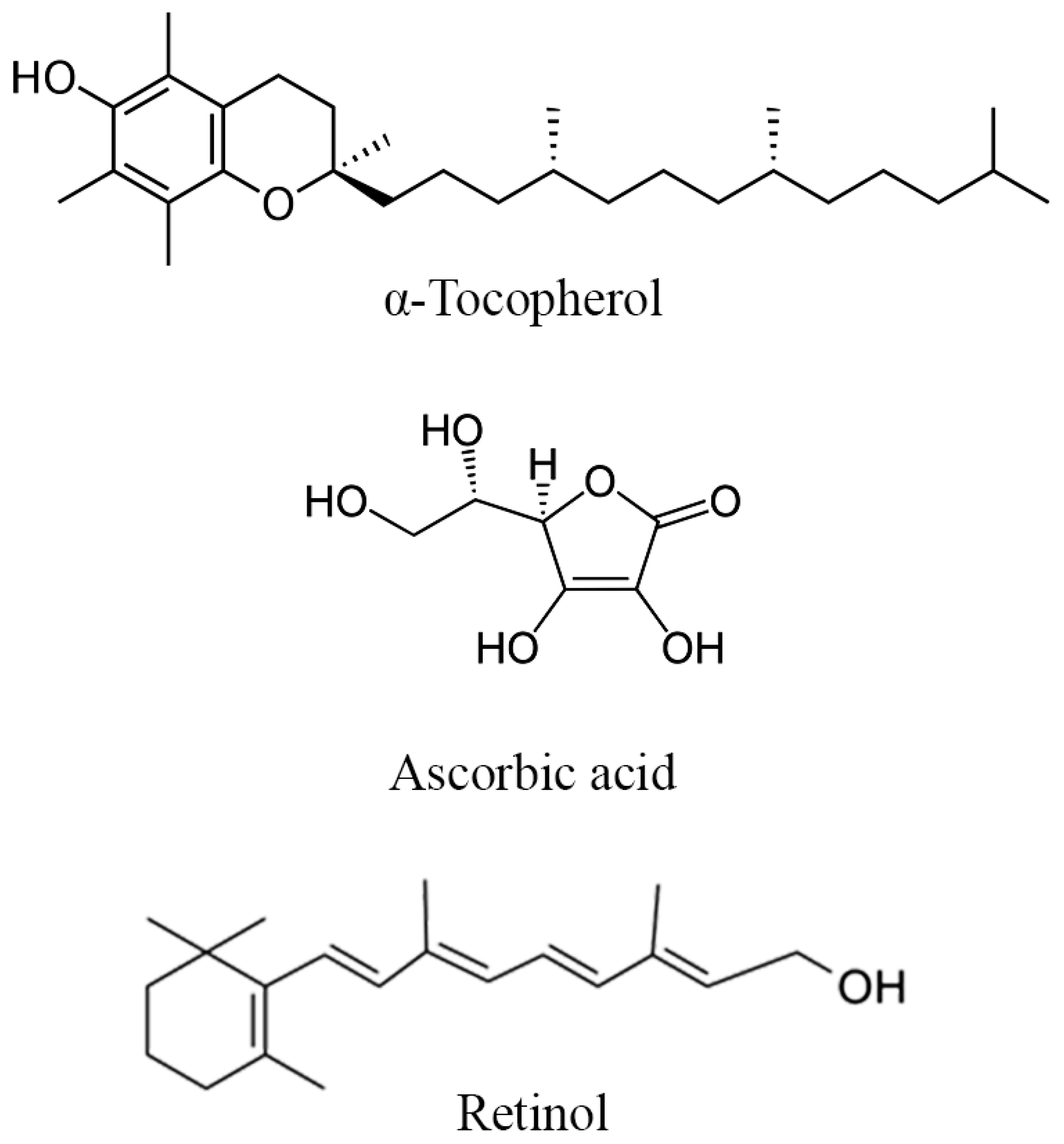
Regarding vitamin content, a study focused on the Egyptian El-Hayani DPP indicated the presence of substantial levels of several vitamins, including high amounts of vitamins A (7708.33 IU/100 g), E (3030.92 IU/100 g), and C (89.09 mg/10 g). Another Egyptian study, involving four DPP varieties treated similarly and collected from comparable soil and climate conditions during the same period Bishr and Yehia, [13] revealed high amounts of vitamins B2 (260 mg/g) and B12 (2316 mg/g) in El Hayani, followed by Zaghlol (B2: 193 mg/g; B12: 182 mg/g), Amhat (B2: 15 mg/g; B12: 43 mg/g), and Sewy (B2: 10 mg/g; B12: 14 mg/g). Additionally, the Ahmat variety contained the highest amount of vitamin B1 (60 mg/g), followed by Sewy (44 mg/g), El Hayani (13 mg/g), and Zaghlol (11 mg/g) (Table 6).

Table 6.
Vitamin composition of DPP.
The potential mechanism behind the plausible improvement of reproductive parameters by minerals and vitamins in DPP could be related to their roles in cellular function, antioxidant defense, and optimization of hormonal balance. For instance, zinc is necessary for the production and secretion of testosterone from the Leydig cells [81], enhancing desire and general sexual health, ovulation, and fertilization [82]. Calcium and magnesium levels influence the smooth muscle spasms that occur during ejaculation, potentially enhancing sexual function [83,84,85]. Iron contributes to oxygen transport and energy metabolism [86], factors that can indirectly impact reproductive functions. Antioxidant vitamins like A, E, and C are particularly crucial in counteracting oxidative stress through the release of electrons to free radicals (Structure of vitamins in DPP highlighted in Figure 5), which can have detrimental effects on sperm quality and reproductive organs [87,88,89]. By neutralizing free radicals, these vitamins can protect sperm from DNA damage, improve motility, and enhance overall fertility [90].
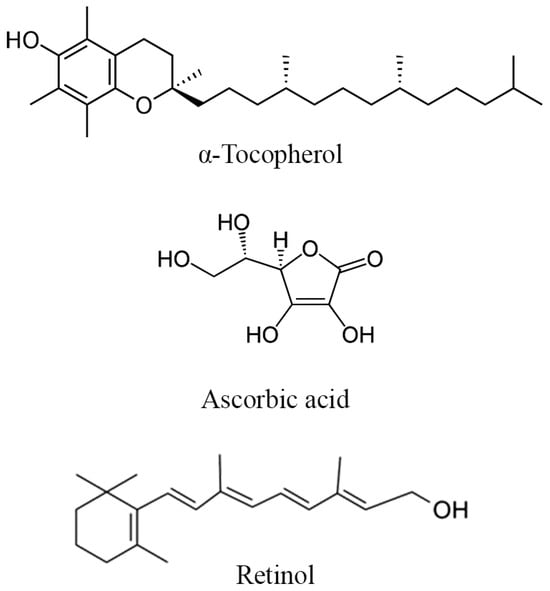
Figure 5.
Structure of the main vitamins found in DPP.
4. Effect of Date Palm Pollen on Human Reproduction
4.1. Traditional Utilization of Date Palm Pollen to Enhance Human Fertility
In historical contexts, pollen formulations have been widely distributed globally, serving as food additives and supplements to enhance overall dietary consumption [65,91]. Additionally, small quantities of DPP are selectively harvested for direct human consumption. For instance, pollen candies, a combination of pollen with honey or molasses and chocolate, have been marketed as a healthful food product in the United States of America [92]. Furthermore, reports indicate that both pollen-infused candy and capsules containing pollen collected from bees’ hind legs as they return to their hives are linked to numerous health benefits [92]. This practice finds proponents notably in Sweden and England [93]. Moreover, early Egyptians and ancient Chinese recognized the rejuvenating properties of DPP, often referred to as the “fountain of youth”, using this botanical substance as a therapeutic treatment for revitalization [10]. The investigation of various date palm varieties with medicinal properties from 17 oases in southern Algeria has revealed that pollen from Ghardaïa, Biskra, and Adrar is commonly employed in traditional medicine to address male and female fertility issues [94]. Traditional practices suggest combining this pollen with bee honey for both genders. In women, a popular method involves consuming a mixture of pollen powder and honey daily before breakfast, particularly during the ovulation period. Another technique includes blending pollen with herbal extracts and applying it to a sanitary towel during the fertile phase to promote ovulation and uterine health. This approach is believed to assist with uterine cleansing, lubrication, and fertility [94]. The use of DPP to enhance human fertility is also widespread in Morocco and Tunisia [95]. In these countries, it is consumed directly or in combination with pure honey and/or royal jelly. This convergence of traditional indigenous knowledge and modern studies underscores the significance of DPP in the realm of human reproduction.
In response to global fertility challenges, clinical studies are increasingly dedicated to examining the effects of DPP on human fertility [49,96,97]. These studies aim to evaluate how this product might influence fertility and reproductive health. While the current research may not primarily focus on deciphering the specific mechanisms, their exploration contributes to a deeper understanding of the potential impact of DPP on human fertility. By merging traditional knowledge and scientific research, these studies offer hope of providing valuable insights that could aid in addressing significant fertility issues faced by numerous individuals worldwide.
4.2. Therapeutic Effects of Date Palm Pollen on Male Fertility: In Vivo Assay
4.2.1. Male Sexual Desire Disorders
Infertility, affecting approximately 15% of couples, represents a pressing challenge with broad societal and health implications [1]. Male infertility is a complex issue that impacts a substantial number of individuals, with often elusive causes [98]. This intricate matter encompasses various factors, including physiological aspects such as secondary hypogonadism [99], genetic factors like Y chromosome microdeletions [46,100], behavioral contributors such as smoking and medication use [101], environmental influences like exposure to toxic substances [102], and socio-demographic variables including age and profession [103]. Despite the prevalence and complexity of male infertility, the field has lacked empirically recommended, evidence-based pharmaceutical interventions, leading to a reliance on traditional pharmaceutical approaches assessed through clinical trials. Within this context, the potential therapeutic efficacy of DPP has emerged as a promising avenue. Table 7 and Table 8 and Figure 6 summarize the primary pharmacological effects of DPP on male reproductive parameters. Male sexuality is widely recognized as a complex mechanism, and sexual dysfunction represents a highly prevalent issue. It is characterized by disturbances in sexual behavior and sensation, encompassing problems such as erectile dysfunction, difficulties with intercourse, and diminished libido [104]. Various physiological systems, including the nervous, cardiovascular, endocrine, and reproductive systems, contribute to maintaining normal sexual function [105,106,107]. Disruption of these systems or psychosocial aspects can lead to sexual dysfunction [108]. Epidemiological research has established connections between male sexual dysfunction and various disorders, notably type II diabetes [109] and cardiovascular diseases [110]. In the realm of reproductive medicine, limited therapies have prompted researchers to explore natural products as potential treatments [111,112,113]. Of these, DPP has garnered considerable attention. It has been traditionally used as an aphrodisiac for patients with sexual dysfunctions [5], and recent clinical studies have delved into its pharmacological effects on enhancing sexual function. In this vein, Al-Sanafi et al. [114] reported that the co-administration of 500 mg of pollen powder and 100 mg of zinc sulfate capsules twice daily for 3 months promoted sexual desire in infertile men. Similarly, Marbeen et al. [115] found that the consumption of 500 mg of DPP powder twice daily for 3 months yielded similar results in infertile men. Furthermore, a double-blind controlled clinical trial demonstrated that the daily administration of 300 mg of DPP powder capsule over 30 days improved male sexual function [97]. Specifically, this dose of DPP significantly enhanced erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction [97]. In a recent double-blind controlled clinical trial conducted on Iranian men after coronary artery bypass graft (CABG) with sexual dysfunctions, the consumption of 3 g of DPP powder twice a day for two months significantly increased their International Index of Erectile Function (IIEF) and Hurlbert Index of Sexual Desire (HISD) scores (from 23.21 to 46.57 and from 59.39 to 64.45, respectively) over time [116]. This study suggested that DPP has a cardiotonic effect due to its polyphenol contents, primarily involved in vasodilation through nitric oxide production [116], which translates into penile erection [117]. Moreover, the beneficial effects of DPP on sexual function and behavior may also be attributed to its androgenic properties [41]. Androgens, including testosterone, play a crucial role in regulating the magnitude of the penile erectile response, venous outflow from cavernous spaces, and sexual desire [101,118]. Additionally, DPP contains significant amounts of steroids, flavonoids, saponins, and lipids, which stimulate endogenous testosterone levels by raising the level of LH [119]. DPP also contains alkaloids that play a crucial role in inhibiting erectile dysfunction-related enzymes (arginase and PDE-5), leading to penile erection [120].
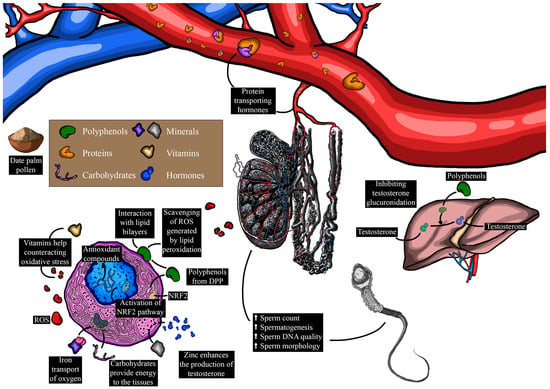
Figure 6.
Influence of date palm pollen components on male reproduction and the underlying mechanisms (ROS: reactive species; Nrf2: nuclear factor erythroid 2-related factor 2).
4.2.2. Sperm Quality and Hormonal Levels
Semen quality stands as a major predictor of male fertility outcome [121]. Consequently, male infertility may arise from the gradual decline in sperm quality, influenced by a variety of factors, spanning from genetic mutations to lifestyle choices, medical illnesses, or medications [122]. Rasekh et al. [123] investigated the effects of the same dosage administered to a group of 40 infertile men through gelatinous capsules every two days instead of daily, revealing a significant increase in sperm count, motility, and morphology after two months of treatment. Marbeen et al. [115] also noted a substantial rise in sperm count and active sperm motility in infertile patients treated with 500 mg of DPP twice daily for 3 months. Similarly, Al-Sanafi et al. [114] reported that the coadministration of the same dose of DPP (500 mg) and 100 mg of zinc sulfate capsules twice daily for 3 months increased serum LH, FSH, and testosterone levels, while improving sperm count and active sperm motility. In an Iranian case report study involving a man with idiopathic severe oligoasthenoteratozoospermia, normal morphology, total motility, progressive motility, and sperm concentration impressively increased [124]. The results from these studies illustrate the ameliorative effects of DPP not only on semen quality parameters but also on the reproductive system as a whole. These beneficial effects have been attributed to the abundance of phenolic compounds in DPP [3,41], known for their antioxidant activities. In this vein, a recent study by Falahati et al. [47] indicated that the consumption of 400 mg/kg of body weight over 74 days in infertile men significantly improved semen volume, count, and morphology by reducing reactive oxygen species (ROS) and increasing the expression of antioxidant genes, such as peroxiredoxin-1 (PRDX1) and peroxiredoxin-6 (PRDX6). This finding may support the notion that phenolic compounds present in DPP could induce the expression of nuclear factor-erythroid factor 2 (Nrf2) [125,126,127], a transcription factor regulating the expression of peroxiredoxin antioxidant genes [128]. Furthermore, a controlled clinical trial demonstrated that the administration of 400 mg/kg of gelatinous capsules daily for 30 days by infertile men increased the expressions of Nrf2, glutathione peroxidase (GPx4), superoxide dismutase (SOD2), and catalase (CAT) genes [129]. The study emphasized that this increase in antioxidant genes was positively correlated with semen quality [129].
Despite the low oxygen tensions characterizing the testicular microenvironment, the testicular tissue remains vulnerable to oxidative stress. Consequently, improved serum levels of testosterone rely on enhanced testicular antioxidant activity, achieved through the activation of the testicular endocrine and antioxidant systems [130]. The testes heavily depend on major reactive oxygen species (ROS) processing enzymes, as well as small molecular weight antioxidant factors, including minerals such as zinc and copper, and vitamins C and E. DPP, abundant in these biomolecules, provides zinc and copper, recognized as cofactors for free radical scavenging enzymes like superoxide dismutase (SOD), and protectors of sulfhydryl groups. Zinc is also known to impede lipid peroxidation by displacing transition metals like iron and copper from catalytic sites. Vitamin E, a potent lipophilic antioxidant, is crucial for maintaining mammalian spermatogenesis [131]. It is found in particularly high concentrations in Sertoli cells and pachytene spermatocytes, and to a lesser extent in round spermatids [132]. Vitamin C (ascorbic acid) contributes to supporting spermatogenesis, at least partially through its ability to reduce α-tocopherol and maintain this antioxidant in an active state. Vitamin C is itself kept in a reduced state by a GSH-dependent dehydroascorbate reductase, which is abundant in the testes [133]. Deficiencies in vitamins C or E result in a state of oxidative stress in the testes, disrupting both spermatogenesis and testosterone production [131]. Vitamin E has also been found to suppress lipid peroxidation in testicular microsomes and mitochondria [122,125]. DPP, rich in vitamin C and E, as well as caffeic acid, which has been shown to decrease MDA value and increase GPx activity in the testes, could explain its beneficial effect on the antioxidant system, leading to improved testosterone synthesis. In a recent study, Karimi et al. [49] reported that a daily dose of 6 g of DPP dry powder in two separate doses (3 g every 12 h) administered orally to 30 eligible men for three months significantly increased serum testosterone levels (from 5.31 ± 0.40 ng/mL to 6.88 ± 0.71 ng/mL). Steroidal components also play a vital role in regulating the renewal of spermatogenic cells and male reproductive tissues that possess estrogen receptors [126,127]. Moreover, it has been reported that saponins present in DPP stimulate Leydig cells, consequently increasing testosterone production [3,128], a crucial component in spermatogenesis [134].

Table 7.
Main in vivo experimental studies highlighting potential effects of date palm pollen (DPP) on different aspects of reproductive parameters *.
Table 7.
Main in vivo experimental studies highlighting potential effects of date palm pollen (DPP) on different aspects of reproductive parameters *.
| Test Group | Form of DPP | Type of Administration | Doses of DPP | Time | Results | Reference |
|---|---|---|---|---|---|---|
| Male rats | Aqueous extract | Intraperitoneal injection | 140 and 350 mg/kg BW twice a day | 120 min | ↑ Mount, intromission and ejaculation frequency; ↑ ejaculation latency; ↑ post-ejaculatory interval; ↑ index of libido; ↑ blood levels of testosterone and estradiol; ↑ penile erection; ↓ mount and intromission latency | Abedi et al. (2012) [44] |
| 140 mg/kg BW/day | 70 min | ↑ Mount and intromission frequency; ↑ ejaculation latency; ↑ release of dopamine levels; ↑ penile erection; ↓ ejaculation frequency; ↓ mount and intromission latency | Abedi et al. (2014) [119] | |||
| Aqueous extract | Oral administration | 100 mg/kg BW/day | 4 weeks | ↑ Testicular histological architecture and integrity; ↑ testis weight; ↑ serum testosterone, LH and FSH levels; ↑ sperm count, motility and viability; ↑ sexual desire; ↓ testicular nitric oxide levels; ↓ malondialdehyde levels | Mohamed et al. (2018) [45] | |
| Ethanolic extract | 150 mg/kg | 56 days | ↑ Body, epididymis, prostate gland, seminal vesicle and testis weight; ↑ serum LH, testosterone and estradiol levels; ↑ testicular antioxidant status; ↑ sperm count and motility; ↑ DNA integrity; ↓ pro-apoptotic markers expression; ↓ DNA damage | El-Kashlan et al. (2015) [46] | ||
| Aqueous extract | 120 mg/kg BW/day | 35 days | ↑ Serum testosterone levels; ↑ body weight; ↑ Johansen score | Iftikhar et al. (2011) [100] | ||
| ↑ Body and testis weight; ↑ serum and intratesticular testosterone levels | Yasir et al. (2014) [111] | |||||
| Aqueous extract | 120, 240 and 360 mg/kg BW/day | 35 days | ↑ Sperm count and motility; ↑ seminiferous tubules diameter; ↑ germinal cell layer thickness; ↑ Leydig and spermatogonia cells; ↑ serum testosterone, LH and estradiol levels; immotile sperm; ↓ sperm abnormality | (Mehraban et al. (2014) [48] | ||
| Female mice | Aqueous extract | n.d. | 100, 200, 400 mg/kg BW | n.d. | ↑ Testosterone, estrogen and progesterone levels; ↑ number of antral and secondary follicles | Hosseini et al. (2014) [135] |
| Female rats | DPP water suspension | Oral administration | 150 mg/kg BW/day | 6 weeks | ↑ Serum FSH and LH levels | Hammed et al. (2012) [136] |
| Ethanolic extract | Oral administration | 100 mg/kg BW/day | 28 days | ↑ GSH, FSH and LH levels | Jiheel and Arrak (2015) [137] |
* BW: body weight; LH: luteinizing hormone; FSH: follicle-stimulating hormone; GSH: glutathione reduced; ↑: increase; ↓: decrease; n.d: not determined.

Table 8.
Pharmacological effects oral administration of date palm pollen (DPP) powder on different aspects of men reproductive parameters *.
Table 8.
Pharmacological effects oral administration of date palm pollen (DPP) powder on different aspects of men reproductive parameters *.
| Test Group | Doses of DPP | Time | Results | Reference |
|---|---|---|---|---|
| Infertile men | 500 mg twice daily | 3 months | ↑ Sexual desire; ↑ sperm count; ↑ active sperm motility; ↑ serum testosterone, LH and FSH levels | Al-sanafi et al. (2023) [114] |
| 500 mg twice daily | 3 months | ↑ Sexual desire; ↑ sperm count; ↑ active sperm motility; ↑ serum testosterone, LH and FSH levels; ↑ intercourse rate | Marbeen et al. (2005) [115] | |
| 400 mg/kg BW | 74 days | ↑ Semen volume, count and morphology. | Falahati et al. (2023) [47] | |
| 120 mg/kg BW every 2 days | 2 months | ↑ Sperm count, motility and morphology | Rasekh et al. (2015) [123] | |
| 300 mg per day | 30 days | ↑ Erectile function; ↑ orgasm sexual desire; ↑ intercourse satisfaction | Jahromi et al. (2022) [97] | |
| Men | 3g twice a day | 2 months | ↑ Vasodilatation; ↑ IIEF; ↑ HISD | Hooshang et al. (2022) [116] |
| 3 g every 12 h | 3 months | ↑ Serum testosterone | Karimi et al. (2023) [49] | |
| Man: case report | 3 g every 12 h | 3 months | ↑ Sperm morphology, total and progressive motility and concentration | Karimi et al. (2018) [124] |
* BW: body weight; LH: luteinizing hormone; FSH: follicle-stimulating hormone; IIEF: International Index of Erectile Function; HISD: Hurlbert Index of Sexual Desire; ↑: increase; ↓: decrease.
4.3. Therapeutic Effects of Date Palm Pollen on Female Fertility: In Vivo Assays
Despite the limited number of studies evaluating the effect of DPP on female reproduction, the year 2017 marked the initiation of several clinical trials (Table 9). Sadeghi et al. [138] observed that the administration of DPP capsules (350 mg daily) for 35 days improved vaginal lubrication and reduced dyspareunia in postmenopausal women compared to the control group receiving a starch placebo. Additionally, another trial demonstrated that 300 mg of DPP supplementation for 35 days in non-menopausal women improved the lubrication and desire domains of the Female Sexual Function Index, without significantly affecting arousal, orgasm, satisfaction, and pain compared to the control group receiving 300 mg of the starch capsule [113]. Another study reported that the oral administration of the same dose increased arousal, orgasm, lubrication, pain during intercourse, and satisfaction in women [97]. Moreover, Yosefzadeh et al. [112] showed that this dose improved orgasm in postmenopausal women without affecting sexual satisfaction. However, Loripoor et al. [25] reported that the daily administration of 300 mg of DPP aqueous extract for 4 weeks did not significantly reduce the score of sexual dysfunctions in postmenopausal women. In all these studies, the same dose of pollen was used for the same duration; however, variations in the date palm genotypes used in each study and the dissimilar composition of pollen from various date palms, particularly in terms of amino acids [139], may contribute to the differences in these results. Moreover, the results obtained by Loripoor et al. [25] may also be explained by the possible absence of bioactive compounds in the aqueous extract of DPP that are implicated in the improvement of sexual dysfunction. El-Wahed et al. [96] also demonstrated that daily consumption of 3 g of DPP for 3 months improved sex hormone levels in women with polycystic ovarian syndrome, leading to lower estrogen and LH levels, higher progesterone and FSH levels, and a cumulative impact on ovulation.

Table 9.
Pharmacological effects of the oral administration of DPP on different aspects of women reproductive parameters *.
According to these studies, it has become evident that DPP could improve lubrication, desire domains, sexual hormone levels, and ovulation, as well as reduce dyspareunia (Figure 7). However, the mechanisms through which DPP elicits these effects remain unclear. The enhancement in lubrication and desire found after DPP supplementation might be due to several factors. DPP contains sterols and phytoestrogens, which have been suggested to regulate hormonal balance and potentially influence sexual desire [96]. Moreover, the vitamins and amino acids found within DPP could indirectly support libido by promoting overall health and boosting energy levels. L-arginine is a naturally occurring amino acid that plays a pivotal role in circulation and sexual function [140]. It serves as a precursor to nitric oxide (NO) and acts as a crucial mediator in this process. Through the action of nitric oxide synthase (NOS), L-arginine is converted into NO, increasing its level and cyclic guanosine monophosphate (cGMP). This biochemical cascade ultimately influences circulation and sexual function [141]. Serine, another amino acid found in DPP, could be involved in the synthesis of serotonin, a neurotransmitter known to play a pivotal role in regulating sexual desire [142]. Additionally, the carbohydrates present in DPP could provide a quick energy boost, potentially enhancing interest in sexual activities. Gauthaman and Ganesan reported that alkaloids present in DPP possess ergogenic properties that are capable of eliciting the dilation of blood vessels. Hence, by promoting vasodilation, DPP could potentially contribute to improving vaginal blood circulation, leading to enhanced lubrication and comfort [143]. In addition, psychological variables and interactions with the vaginal flora, as well as DPP’s historical usage as an aphrodisiac, might all play a role.
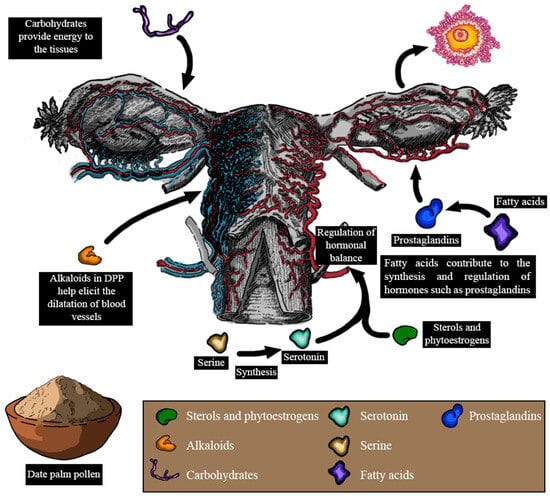
Figure 7.
Pharmacological effects of date palm pollen on female reproduction and the plausible mechanisms implicated in some of these effects (LH: luteinizing hormone; FSH: follicle-stimulating hormone).
4.4. Regulatory Imperatives, Toxicological Effects, and Research Directions for Date Palm Pollen in Fertility Enhancement
Despite the promising therapeutic potential of DPP in addressing human fertility concerns, its broader application necessitates thorough toxicological assessments to identify potential adverse effects and establish safe dose thresholds. In rats and mice, it was reported that the dose of DPP affects reproduction parameters. For instance, Abedi et al. [44] indicated that 140 mg/kg of aqueous DPP extract improved rat sexual behavior more effectively than 350 mg/kg, with the latter causing adverse effects like diarrhea. Additionally, Mehraban et al. [48] observed the dose-dependent effects of oral DPP administration (120, 240, and 360 mg/kg) on reproductive parameters in rats. While the 120 and 240 mg/kg doses showed significant enhancements in reproductive metrics, the higher dose of 360 mg/kg did not yield substantial differences compared to controls. However, in human, the majority of studies have focused on assessing the impact of DPP on fertility using a single dosage [45,46,100,111]. Therefore, comprehensive studies elucidating its toxicological profile, including acute and chronic toxicity assessments, mutagenicity, reproductive toxicity, and potential interactions with medications, remain critical gaps in current knowledge. The establishment of regulatory frameworks is imperative, encompassing standardized production methods, quality control measures, and robust labeling standards to ensure product safety, efficacy, and proper consumer information. Future research avenues should emphasize long-term safety evaluations across diverse populations, exploring mechanistic insights into DPP’s effects on reproductive systems. Large-scale clinical trials and epidemiological studies are essential to validate its efficacy and safety, considering variations in demographic profiles and potential susceptibilities. Moreover, conducting comprehensive toxicological profiling will aid in delineating the exact mechanisms of action, facilitating the development of informed guidelines for its judicious use in addressing fertility concerns.
5. Conclusions
DPP presents a versatile natural resource abundant in secondary metabolites, proteins, amino acids, carbohydrates, fatty acids, minerals, and vitamins. Its capacity to positively impact reproductive health is promising, as its bioactive components may influence hormonal pathways and enhance fertility. The traditional use of DPP to enhance human fertility, combined with recent clinical research, emphasizes its potential therapeutic effects on both male and female reproductive parameters. DPP shows promise in improving sexual function, hormone levels, and semen quality. While variations in study outcomes do exist, the historical significance of DPP and ongoing research offer valuable insights into addressing fertility issues and promoting reproductive health. This comprehensive understanding underscores the importance of further exploration and application of DPP’s potential in the realm of human reproduction.
Funding
This research was funded by Académie de recherche et d’enseignement supérieur grant number N° CPO 4-8192-C0 ARES PRD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the agreement established between INRA Morocco and University of Namur, Belgium, in the framework of the Project N° CPO 4-8192-C0 ARES PRD. entitled «Improving practices and knowledge sharing among small ruminant breeders in Morocco», for supporting financially this research. The authors also wish to thank Jalal Kassout for his critical review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UCLA Health Infertility. Available online: https://www.uclahealth.org/medical-services/obgyn/conditions-treated/infertility (accessed on 26 August 2023).
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic Potential of Date Palm against Human Infertility: A Review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef]
- Moshfegh, F.; Baharara, J.; Namvar, F.; Zafar-Balanezhad, S.; Amini, E.; Jafarzadeh, L. Effects of Date Palm Pollen on Fertility and Development of Reproductive System in Female Balb/C Mice. J. Herbmed Pharmacol. 2015, 5, 23–28. [Google Scholar]
- Tahvilzadeh, M.; Hajimahmoodi, M.; Rahimi, R. The Role of Date Palm (Phoenix dactylifera L.) Pollen in Fertility: A Comprehensive Review of Current Evidence. J. Evid. Based Complement. Altern. Med. 2016, 21, 320–324. [Google Scholar] [CrossRef]
- Abdi, F.; Roozbeh, N.; Mortazavian, A.M. Effects of Date Palm Pollen on Fertility: Research Proposal for a Systematic Review. BMC Res. Notes 2017, 10, 363. [Google Scholar] [CrossRef]
- Chao, C.T.; Krueger, R.R. The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. Horts 2007, 42, 1077–1082. [Google Scholar] [CrossRef]
- Abbas, F.A.; Ateya, A.-M. Estradiol, Esteriol, Estrone and Novel Flavonoids from Date Palm Pollen. Aust. J. Basic Appl. Sci. 2011, 5, 606–614. [Google Scholar]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of Date Palm Pollen (Phoenix dactylifera L.) Encapsulation, Impact on the Nutritional and Functional Properties of Fortified Yoghurt. PLoS ONE 2019, 14, e0222789. [Google Scholar] [CrossRef]
- Hassan, H.M.M. Chemical Composition and Nutritional Value of Palm Pollen Grains. Glob. J. Biotech. Biochem. 2011, 6, 1–7. [Google Scholar]
- Salhi, S.; Chentouf, M.; Harrak, H.; Rahim, A.; Çakir, C.; Çam, D.; Öztürk, M.; Hamidallah, N.; Cabaraux, J.F.; El Amiri, B. Assessment of Physicochemical Parameters, Bioactive Compounds, Biological Activities, and Nutritional Value of the Most Two Commercialized Pollen Types of Date Palm (Phoenix dactylifera L.) in Morocco. Food Sci. Technol. Int. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Salomón-Torres, R.; Krueger, R.; García-Vázquez, J.P.; Villa-Angulo, R.; Villa-Angulo, C.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Samaniego-Sandoval, L. Date Palm Pollen: Features, Production, Extraction and Pollination Methods. Agronomy 2021, 11, 504. [Google Scholar] [CrossRef]
- Bishr, M.; Desoukey, S.Y. Comparative Study of the Nutritional Value of Four Types of Egyptian Palm Pollens. J. Pharm. Nutr. Sci. 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Ahmed, W.; Feyissa, T.; Tesfaye, K.; Farrakh, S. Genetic Diversity and Population Structure of Date Palms (Phoenix dactylifera L.) in Ethiopia Using Microsatellite Markers. J. Genet. Eng. Biotechnol. 2021, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Tengberg, M. Beginnings and Early History of Date Palm Garden Cultivation in the Middle East. J. Arid. Environ. 2012, 86, 139–147. [Google Scholar] [CrossRef]
- Ghazzawy, H.S.; Gouda, M.M.; Awad, N.S.; Al-Harbi, N.A.; Alqahtani, M.M.; Abdel-Salam, M.M.; Abdein, M.A.; Al-Sobeai, S.M.; Hamad, A.A.; Alsberi, H.M. Potential Bioactivity of Phoenix dactylifera Fruits, Leaves, and Seeds against Prostate and Pancreatic Cancer Cells. Front. Nutr. 2022, 9, 998929. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, S.; Ibrahim, H.; Darwish, L.; Farag, M.; El-Habbak, A.H.; El-Kashif, E. Effect of Environmental Conditions on Date Palm Fiber Composites. In Date Palm Fiber Composites; Composites Science and Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 287–320. ISBN 9789811593383. [Google Scholar]
- INRA Atlas du Palmier Dattier au Maroc; INRA: Paris, France, 2011.
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The Impact of Date Palm Fruits and Their Component Polyphenols, on Gut Microbial Ecology, Bacterial Metabolites and Colon Cancer Cell Proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix dactylifera L.)—A Review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Midani, M.; Elseify, L.A.; Hamouda, T.; Hassanin, A.H. Chapter 15—Comparison of Coconut Coir and Date Palm Coir (Sheath Fiber) and Their Composites. In Coir Fiber and Its Composites; Jawaid, M., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2022; pp. 331–345. ISBN 978-0-443-15186-6. [Google Scholar]
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date Fruit (Phoenix dactylifera L.): An Underutilized Food Seeking Industrial Valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Sedra, M. Date Palm Status and Perspective in Morocco. In Date Palm Genetic Resources and Utilization: Volume 1: Africa and the Americas; Springer: Dordrecht, The Netherlands, 2015; pp. 257–323. [Google Scholar] [CrossRef]
- Abdel-Shaheed, M.; Abdalla, E.; Khalil, A.; El-Hadidy, E. Effect of Egyptian Date Palm Pollen (Phoenix dactylifera L.) and Its Hydroethanolic Extracts on Serum Glucose and Lipid Profiles in Induced Diabetic Rats. Food Nutr. Sci. 2021, 12, 147–161. [Google Scholar]
- Loripoor, M.; Esmaeili, F.; Vazirinejad, R.; Dan, S. The Effect of Palm Pollen Extract on Sexual Disorders in Postmenopausal Women: A Randomized Clinical Trial. Int. J. Community Based Nurs. Midwifery 2023, 11, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bahmanpour, S.; Panjeh, S.M.; Talaei, T.; Vojdani, Z.; Poust, P.A.; Zareei, S.; Ghaemian, M. Effect of Phoenix dactylifera Pollen on Sperm Parameters and Reproductive System of Adult Male Rats. Iran. J. Med. Sci. 2006, 31, 208–212. [Google Scholar]
- Metwaly, M.; Dkhil, M.; Al-Quraishy, S. Anti-Coccidial and Anti-Apoptotic Activities of Palm Pollen Grains on Eimeria Papillata-Induced Infection in Mice. Biologia 2014, 69, 254–259. [Google Scholar] [CrossRef]
- Nasser, R.A.; Salem, M.Z.M.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical Analysis of Different Parts of Date Palm (Phoenix dactylifera L.) Using Ultimate, Proximate and Thermo-Gravimetric Techniques for Energy Production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- García-González, C.; Salomón-Torres, R.; Montero-Alpírez, G.; Chávez-Velasco, D.; Ortiz-Uribe, N.; Ruiz-Ortiz, N.S.; Coronado-Ortega, M.A.; Curiel-Alvarez, M.A. Effect of Pollen Sources on Yield Oil Extraction and Fatty Acid Profile of the Date Seed (Phoenix dactylifera L.) Cultivar Medjool from Mexico. Grasas Y Aceites 2019, 70, e315. [Google Scholar] [CrossRef]
- Beroual, M.; Trache, D.; Mehelli, O.; Boumaza, L.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Effect of the Delignification Process on the Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Extracted from Date Palm Fronds. Waste Biomass Valorization 2021, 12, 2779–2793. [Google Scholar] [CrossRef]
- Al-Abbasi, H.H.; Mahdi, A.S.; Washam, A.F.; Al-Wazeer, A.A. Role of Date Palm Pollen on Heifer’s Puberty and Maturity in Iraq. Arch. Razi Inst. 2023, 78, 241–247. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of Secondary Metabolites Using Tissue Culture-Based Biotechnological Applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Gullon, B.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Phoenix dactylifera Products in Human Health—A Review. Trends Food Sci. Technol. 2020, 105, 238–250. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A.M.; Danthine, S.M.; Blecker, C.; Attia, H.; Besbes, S. Physico-Chemical, Surface and Thermal Properties of Date Palm Pollen as a Novel Nutritive Ingredient. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 84–91. [Google Scholar] [CrossRef]
- Basuny, A.M.M.; Arafat, S.; Soliman, H. Chemical Analysis of Olive and Palm Pollen: Antioxidant and Antimicrobial Activation Properties. Wudpecker J. Food Technol. 2013, 1, 14–21. [Google Scholar]
- Al-Samarai, A.H.; Al-Salihi, F.G.; Al-Samarai, R.R. Phytochemical Constituents and Nutrient Evaluation of Date Palm (Phoenix dactylifera, L.) Pollen Grains. Tikrit J. Pure Sci. 2018, 21, 56–62. [Google Scholar] [CrossRef]
- Ibrahim, F.Y.; Khalil, M.M.; Nezam EL Din, A.A.M.M.; Atieya, K.M. Studies on Biological Effect of Some Selected Foods (Un-Pollinated Siwi Date, Date Palm Pollen and Doum Fruit). J. Food Dairy Sci. 2017, 8, 461–468. [Google Scholar] [CrossRef]
- Alanber, L. Estimation of The Content of Lipids And Fatty Acids in Pollen of Phoenixdactylifera (Date Palm) from Basrah, Iraq. Rev. Boliv. De Química 2017, 34, 9–13. [Google Scholar]
- Abed El Azim, M.H.M. Identification Phenolic and Biological Activities of Methanolic Extract of Date Palm Pollen (Phoenix dactylifera). J. Microb. Biochem. Technol. 2015, 7, 47–50. [Google Scholar] [CrossRef]
- Waly, M.I. Health Benefits and Nutritional Aspects of Date Palm Pollen. Can. J. Clin. Nutr. 2020, 8, 1–3. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of Polyphenol Composition, Antioxidant and Antimicrobial Properties of Various Extracts of Date Palm Pollen (DPP) from Two Tunisian Cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef]
- Abedi, A.; Karimian, S.M.; Roudsari, H.R.S. The Effect of Aqueous Extract of Phoenix dactylifera Pollen Grain on Sexual Behavior of Male Rats. J. Physiol. Pharmacol. Adv. 2012, 2, 235–242. [Google Scholar]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative Effects of Bee Pollen and Date Palm Pollen on the Glycemic State and Male Sexual Dysfunctions in Streptozotocin-Induced Diabetic Wistar Rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef]
- El-Kashlan, A.M.; Nooh, M.M.; Hassan, W.A.; Rizk, S.M. Therapeutic Potential of Date Palm Pollen for Testicular Dysfunction Induced by Thyroid Disorders in Male Rats. PLoS ONE 2015, 10, e0139493. [Google Scholar] [CrossRef]
- Falahati, A.M.; Fallahi, S.; Allamehzadeh, Z.; Izadi Raieni, M.; Malekzadeh, K. Effects of Date Palm Pollen Supplementations on The Expression of PRDX1 and PRDX6 Genes in Infertile Men: A Controlled Clinical Trial. Int. J. Fertil. Steril. 2023, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, F.; Jafari, M.; Toori, M.A.; Sadeghi, H.; Joodi, B.; Mostafazade, M.; Sadeghi, H. Effects of Date Palm Pollen (Phoenix dactylifera L.) and Astragalus Ovinus on Sperm Parameters and Sex Hormones in Adult Male Rats. Iran. J. Reprod. Med. 2014, 12, 705. [Google Scholar] [PubMed]
- Karimi, F.Z.; Salehian, M.; Hosseini, H.; Norouzi, Z.; Afiat, M. The Effect of the Medicinal Plants on Sexual Function in Postmenopausal Women in Iran: A Systematic Review of Clinical Trials. J. Sabzevar Univ. Med. Sci. 2023, 30, 144–162. [Google Scholar]
- Jenkinson, C.; Petroczi, A.; Naughton, D.P. Red Wine and Component Flavonoids Inhibit UGT2B17 in Vitro. Nutr. J. 2012, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Yockell-Lelievre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The Effects of Dietary Polyphenols on Reproductive Health and Early Development. Hum. Reprod. Update 2015, 21, 228–248. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative Properties of Phenolic Compounds and Their Effect on Oxidative Stress Induced by Severe Physical Exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, Nitric Oxide, Oxidants, and Dietary Plant Polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Yang, S.Y.; Pyo, M.C.; Nam, M.H.; Lee, K.W. ERK/Nrf2 Pathway Activation by Caffeic Acid in HepG2 Cells Alleviates Its Hepatocellular Damage Caused by t-Butylhydroperoxide-Induced Oxidative Stress. BMC Complement. Altern. Med. 2019, 19, 139. [Google Scholar] [CrossRef]
- Benouamane, O.; Vergara-Barberan, M.; Benaziza, A.; Garcia-Alvarez-Coque, M.C.; Simó-Alfonso, E.; China, B.; Lerma-Garcia, M.J. Characterization of Different Cultivars of Algerian Date Palm (Phoenix dactylifera L.) Leaves and Pollen by Comprehensive Two-Dimensional Liquid Chromatography of Phenolic Compounds Extracted with Different Solvents. Microchem. J. 2022, 182, 107874. [Google Scholar] [CrossRef]
- Abdallah, W.E.; Awad, H.M.; AbdelMohsen, M.M. Phytochemical Composition, Antioxidant and Antitumor Activities of Some Date Palm Pollen Extracts. Egypt. J. Chem. 2023, 66, 425–434. [Google Scholar] [CrossRef]
- Abou Zeid, H.M.; Shiha, M.A.; Shehata, A.A. Comparative Study of Pollen Grains Morphology and Phytochemical Constituents of Some Saudi Arabian Date Palm (Phoenix dactylifera L.) Cultivars. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2800–2809. [Google Scholar] [CrossRef]
- Hassan, A.; El Wafa, M.H.A. An Œstrogenic Substance in Pollen-Grains of Date Palm Tree Phœnix dactylifera L., Palmæ. Nature 1947, 159, 409–410. [Google Scholar] [CrossRef]
- Soliman, F.A.; Soliman, L. The Gonadotrophic Activity of Date Palm Pollen Grains. Experientia 1957, 13, 411–412. [Google Scholar] [CrossRef]
- El-Ridi, M.S.; El Mofty, A.; Khalifa, K.; Soliman, L. Gonadotrophs Hormones in Pollen Grains of the Date Palm. Z. Naturforschung B 1960, 15, 45–49. [Google Scholar] [CrossRef]
- Otify, A.M.; Hammam, A.M.M.; Aly Farag, M. Phoenix dactylifera L. Date Tree Pollen Fertility Effects on Female Rats in Relation to Its UPLC-MS Profile via a Biochemometric Approach. Steroids 2021, 173, 108888. [Google Scholar] [CrossRef]
- Mahran, G.H.; Abdel–Wahab, S.M.; Attia, A.M. A Phytochemical Study of Date Palm Pollen. Planta Med. 1976, 29, 171–175. [Google Scholar] [CrossRef]
- Bacha, M.A.; Ali, M.A.; Farahat, F.A. Chemical Composition of Pollen Grains of Some Date Palm Males Grown in Riyadh, Saudi Arabia. Arab. Gulf J. Sci. Res. 1997, 15, 783–803. [Google Scholar]
- Shahin, F.M. Utilization of Date Palm Pollen as Natural Source for Producing Function Bakery Product. Egypt. J. Agric. Res. 2014, 92, 1457–1470. [Google Scholar] [CrossRef]
- Saleh, M.; Kokoszyński, D.; Mousa, M.A.A.; Abuoghaba, A.A.K. Effect of Date Palm Pollen Supplementation on the Egg Production, Ovarian Follicles Development, Hematological Variables and Hormonal Profile of Laying Hens. Animals 2021, 11, 69. [Google Scholar] [CrossRef]
- Chaira, N.; Mrabet, A.; Ferchichi, A.L.I. Evaluation of Antioxidant Activity, Phenolics, Sugar and Mineral Contents in Date Palm Fruits. J. Food Biochem. 2009, 33, 390–403. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Adekoya, A.E.; Idowu, S. Dates Palm Fruits: A Review of Their Nutritional Components, Bioactivities and Functional Food Applications. AIMS Agric. Food. 2020, 5, 734–755. [Google Scholar] [CrossRef]
- Handgraaf, S.; Philippe, J. The Role of Sexual Hormones on the Enteroinsular Axis. Endocr. Rev. 2019, 40, 1152–1162. [Google Scholar] [CrossRef]
- Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JMBR Plus 2021, 5, e10418. [Google Scholar] [CrossRef]
- Hansberg-Pastor, V.; González-Arenas, A.; Piña-Medina, A.G.; Camacho-Arroyo, I. Sex Hormones Regulate Cytoskeletal Proteins Involved in Brain Plasticity. Front. Psychiatry 2015, 6, 165. [Google Scholar] [CrossRef]
- Lynch, S.; Boyett, J.E.; Smith, M.R.; Giordano-Mooga, S. Sex Hormone Regulation of Proteins Modulating Mitochondrial Metabolism, Dynamics and Inter-Organellar Cross Talk in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 8, 610516. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Gao, A.; Khawar, M.B.; Gao, F.; Li, W. Food-Derived High Arginine Peptides Promote Spermatogenesis Recovery in Busulfan Treated Mice. Front. Cell Dev. Biol. 2021, 9, 791471. [Google Scholar] [CrossRef]
- Chen, J.Q.; Li, Y.S.; Li, Z.J.; Lu, H.X.; Zhu, P.Q.; Li, C.M. Dietary L-Arginine Supplementation Improves Semen Quality and Libido of Boars under High Ambient Temperature. Animal 2018, 12, 1611–1620. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.S.H. Evaluation of Pollen Grains Germination, Viability and Chemical Composition of Some Date Palm Males. Middle East. J. Agric. Res. 2018, 7, 235–247. [Google Scholar]
- Abdallah, E.M. Preliminary Screening for Antibacterial Properties of the Male Flowers of Phoenix dactylifera. South Asian J. Res. Microbiol. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Ma, X.; Wu, L.; Wang, Y.; Han, S.; El-Dalatony, M.M.; Feng, F.; Tao, Z.; Yu, L.; Wang, Y. Diet and Human Reproductive System: Insight of Omics Approaches. Food Sci. Nutr. 2022, 10, 1368–1384. [Google Scholar] [CrossRef]
- Collodel, G.; Castellini, C.; Lee, J.C.-Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell. Longev. 2020, 2020, e7038124. [Google Scholar] [CrossRef]
- El-Yazal, S.A.S.; El-Yazal, M.A.S. Determination of Five Mineral Element Contents in Pollen Grains of Different Seedling Date Palm (Phoenix dactylifera L.) Male Trees Grown in Fayoum Governorate, Egypt. J. Hortic. Plant Res. 2019, 7, 16–25. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc Is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of Zinc in Female Reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Bader, A.A.; Dashti, H.; Oriowo, M.A. Magnesium in Human Semen: Possible Role in Premature Ejaculation. Arch. Androl. 2001, 46, 59–66. [Google Scholar] [CrossRef]
- Hull, E.M.; Dominguez, J.M. Male Sexual Behavior. In Hormones, Brain and Behavior; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 3–137. [Google Scholar]
- Souza, I.L.L.D.; Ferreira, E.D.S.; Vasconcelos, L.H.C.; Cavalcante, F.D.A.; Silva, B.A.D. Erectile Dysfunction: Key Role of Cavernous Smooth Muscle Cells. Front. Pharmacol. 2022, 13, 895044. [Google Scholar] [CrossRef]
- Hirota, K. An Intimate Crosstalk between Iron Homeostasis and Oxygen Metabolism Regulated by the Hypoxia-Inducible Factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in Reproduction and Development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef]
- Mohd Mutalip, S.S.; Ab-Rahim, S.; Rajikin, M.H. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants 2018, 7, 22. [Google Scholar] [CrossRef]
- Vašková, J.; Klepcová, Z.; Špaková, I.; Urdzík, P.; Štofilová, J.; Bertková, I.; Kl’oc, M.; Rabajdová, M. The Importance of Natural Antioxidants in Female Reproduction. Antioxidants 2023, 12, 907. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirsalehian, A.; Bahador, A. Prevalence of Urogenital Mycoplasmas in Iran and Their Effects on Fertility Potential: A Systematic Review and Meta-Analysis. Iran. J. Public. Health 2016, 45, 409–422. [Google Scholar]
- Kroyer, G.; Hegedus, N. Evaluation of Bioactive Properties of Pollen Extracts as Functional Dietary Food Supplement. Innov. Food Sci. Emerg. Technol. 2001, 2, 171–174. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology Biochemistry Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Binding, G.P. About Pollen; Springer: London, UK, 1971. [Google Scholar]
- Selmani, C.; Chabane, D.; Bouguedoura, N. Ethnobotanical Survey of Phoenix dactylifera L. Pollen Used for the Treatment of Infertility Problems in Algerian Oases. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 175–186. [Google Scholar] [CrossRef]
- Guerradi, M.; Outlioua, K.; Hamdouni, N. Rôle de La Femme Dans La Gestion de La Diversité Génétique Du Palmier Dattier Dans Les Oasis Du Maghreb. Rev. Régions Arid. Numéro Spécial 2004, 869–873. [Google Scholar]
- El-Wahed, A.; Mahfouz, R.; Nasr, A.A.A. Effects of Date Palm Pollen on Women with the Polycystic Ovarian Syndrome. Egypt. J. Hosp. Med. 2022, 89, 4622–4625. [Google Scholar] [CrossRef]
- Jahromi, A.R.; Mosallanezhad, Z.; Hosini, F.S.; Jamali, S.; Sharifi, N. The Effect of Date Palm on Sexual Function in Infertile Couples: A Double-Blind Controlled Clinical Trial. BMC Res. Notes 2022, 15, 55. [Google Scholar] [CrossRef]
- Mirzaei, M.; Namiranian, N.; Bagheri-Fahraji, B.; Gholami, S. Infertility and Physical Activity: A Cross-Sectional Study of Women Living in Yazd Aged 20–49 Yr, 2014–2015. Int. J. Reprod Biomed. 2020, 18, 795. [Google Scholar] [CrossRef]
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of Male Infertility: A 9-Year Prospective Monocentre Study on 1737 Patients with Reduced Total Sperm Counts. Hum. Reprod. 2017, 32, 18–31. [Google Scholar] [CrossRef]
- Iftikhar, S.; Bashir, A.; Anwar, M.S.; Mastoi, S.M.; Shahzad, M. Effect of Date Palm Pollen (DPP) on Serum Testosterone Levels in Prepubertal Albino Rats. Pak. J. Med. Health Sci. 2011, 5, 639–644. [Google Scholar]
- Durairajanayagam, D. Lifestyle Causes of Male Infertility. Arab. J. Urol. 2019, 16, 10–20. [Google Scholar] [CrossRef]
- Sengupta, P.; Nwagha, U.; Dutta, S.; Krajewska-Kulak, E.; Izuka, E. Evidence for Decreasing Sperm Count in African Population from 1965 to 2015. Afr. Health Sci. 2017, 17, 418–427. [Google Scholar] [CrossRef]
- Ugwuja, E.I.; Ugwu, N.C.; Ejikeme, B.N. Prevalence of Low Sperm Count and Abnormal Semen Parameters in Male Partners of Women Consulting at Infertility Clinic in Abakaliki, Nigeria. Afr. J. Reprod. Health 2008, 12, 67–73. [Google Scholar]
- Rösing, D.; Klebingat, K.J.; Berberich, H.J.; Bosinski, H.A.G.; Loewit, K.; Beier, K.M. Male Sexual Dysfunction: Diagnosis and Treatment from a Sexological and Interdisciplinary Perspective. Dtsch. Arztebl. Int. 2009, 106, 821–828. [Google Scholar] [CrossRef]
- Spasovska Trajanovska, A.; Vujovic, V.; Ignjatova, L.; Janikevik Ivanovska, D.; Chibishev, A. Sexual Dysfunction as a Side Effect of Hyperprolactinemia in Methadone Maintenance Therapy. Med. Arh. 2013, 67, 48–50. [Google Scholar] [CrossRef]
- Gerra, G.; Manfredini, M.; Somaini, L.; Maremmani, I.; Leonardi, C.; Donnini, C. Sexual Dysfunction in Men Receiving Methadone Maintenance Treatment: Clinical History and Psychobiological Correlates. Eur. Addict. Res. 2016, 22, 163–175. [Google Scholar] [CrossRef]
- Ujah, G.A.; Nna, V.U.; Agah, M.I.; Omue, L.O.; Leku, C.B.; Osim, E.E. Effect of Quercetin on Cadmium Chloride-Induced Impairments in Sexual Behaviour and Steroidogenesis in Male Wistar Rats. Andrologia 2018, 50, e12866. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Huang, D.; Li, Y.; Ma, C.; Shi, M.; Su, B.; Shi, G. Male Sexual Dysfunction: A Review of Literature on Its Pathological Mechanisms, Potential Risk Factors, and Herbal Drug Intervention. Biomed. Pharmacother. 2019, 112, 108585. [Google Scholar] [CrossRef]
- Weinberg, A.E.; Eisenberg, M.; Patel, C.J.; Chertow, G.M.; Leppert, J.T. Diabetes Severity, Metabolic Syndrome, and the Risk of Erectile Dysfunction. J. Sex. Med. 2013, 10, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary Intake of Heme Iron and Risk of Cardiovascular Disease: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef]
- Yasir, A.; Nasir, M.; Munazza, A.; Muhammad, T.; Fan, Z.; Di-Jie, L.; Chen, Z.; Chong, Y.; Peng, S.; Ai-Rong, Q. Effect of Date Palm Pollen on Serum Testosterone and Intra-Testicular Environment in Male Albino Rats. Afr. J. Pharm. Pharmacol. 2014, 8, 793–800. [Google Scholar] [CrossRef][Green Version]
- Yosefzadeh, S.; Sadeghi, S.; Rakhshandeh, H.; Dadghar, S.; Mazloum, S.R. The Effect of Date Palm Pollen Capsule on Orgasm and Sexual Satisfaction in Menopausal Women: A Double-Blind Controlled Clinical Trial. Iran. J. Obstet. Gynecol. Infertil. 2017, 20, 43–51. [Google Scholar] [CrossRef]
- Salmani, R.; Nasiri, K.; Javadzadeh, Y.; Salmani, R.; Clark, C.C.T.; Aghamohammadi, V. Effect of Date Palm Pollen Supplementation on Female Sexual Function in Non-Menopausal Women: A Double Blind Randomized Clinical Trial. Chin. Herb. Med. 2022, 14, 643–648. [Google Scholar] [CrossRef]
- Al-Sanafi, A.E.; Bahaaldean, E.F.; Marbeem, M.I.; Marbut, M.M. The Effect of Date Palm Pollen & Zinc Sulphate in the Treatment of Human Male Infertility. Tikrit J. Pure Sci. 2023, 2, 31–34. [Google Scholar] [CrossRef]
- Marbeen, M.I.; Al-Snafi, A.E.; Marbut, M.M.; Allahwerdy, I.Y. The Probable Therapeutic Effects of Date Palm Pollen in the Treatment of Male Infertility. Tikrit J. Pure Sci. 2005, 1, 30–35. [Google Scholar] [CrossRef]
- Hooshang, H.; Farahani, A.V.; Rezaeizadeh, H.; Forouzannia, S.K.; Alaeddini, F.; Ashraf, H.; Karimi, M. Efficacy of Date Palm Pollen in the Male Sexual Dysfunction after Coronary Artery Bypass Graft: A Randomized, Double-Blind, Clinical Trial. Evid. Based Complement. Altern. Med. 2022, 2022, 5032681. [Google Scholar] [CrossRef]
- Sullivan, M.E.; Thompson, C.S.; Dashwood, M.R.; Khan, M.A.; Jeremy, J.Y.; Morgan, R.J.; Mikhailidis, D.P. Nitric Oxide and Penile Erection: Is Erectile Dysfunction Another Manifestation of Vascular Disease? Cardiovasc. Res. 1999, 43, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Foresta, C. New Genetic Markers for Male Infertility. Curr. Opin. Gynecol. Obstet. 2014, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Abedi, A.; Karimian, S.M.; Parviz, M.; Mohammadi, P.; Roudsari, H.R.S. Effect of Aqueous Extract of Phoenix dactylifera Pollen on Dopamine System of Nucleus Accumbens in Male Rats. Neurosci. J. 2014, 5, 49–59. [Google Scholar] [CrossRef]
- Omojokun, O.S.; Famurewa, A.J.; Jaiyeoba, O.A.; Oboh, G.; Agbebi, O.J. Alkaloid Extracts from Bitter Leaf (Vernonia amygdalina) and Black Nightshade (Solanum nigrum) Inhibit Phosphodiesterase-5, Arginase Activities and Oxidative Stress in Rats Penile Tissue. J. Food Biochem. 2019, 43, e12889. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Impact of Environmental Factors on Human Semen Quality and Male Fertility: A Narrative Review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Fainberg, J.; Kashanian, J.A. Recent Advances in Understanding and Managing Male Infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, A.; Jashni, H.K.; Rahmanian, K.; Jahromi, A.S. Effect of Palm Pollen on Sperm Parameters of Infertile Man. Pak. J. Biol. Sci. 2015, 18, 196–199. [Google Scholar] [CrossRef]
- Karimi, M.; Asbagh, F.A.; Rahimi, R.; Safavi, M.; Pourmand, G.; Hoseini, F.S.; Mirzaei, M. Improvement of Semen Parameters in a Man With Idiopathic Severe Oligoasthenoteratozoospermia Using Date Palm Pollen (Phoenix dactylifera L.) Based on Iranian Traditional Medicine: A Case Report. Iran. Red. Crescent Med. J. 2018, 20, e62969. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Pharm. Sci. 2015, 2015, e823539. [Google Scholar] [CrossRef]
- Alahyane, A.; Harrak, H.; Ayour, J.; Elateri, I.; Ait-Oubahou, A.; Benichou, M. Bioactive Compounds and Antioxidant Activity of Seventeen Moroccan Date Varieties and Clones (Phoenix dactylifera L.). S. Afr. J. Bot. 2018, 121, 402–409. [Google Scholar] [CrossRef]
- Abdelkhalek, F.; Said, M.; El Nabtity, S.; Darwish, W. Nutritive Value, and Pharmacological Effects of Dates (Phoenix dactylifera L.): A Mini Review. Zag. Vet. J. 2022, 50, 73–86. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin Induces the Expression of Peroxiredoxins 3 and 5 via the Nrf2/NRF1 Transcription Pathway. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1055–1063. [Google Scholar] [CrossRef]
- Fallahi, S.; Rajaei, M.; Hesam, M.J.; Koolivand, M.; Malekzadeh, K. The Effect of Phoenix dactylifera Pollen on the Expression of NRF2, SOD2, CAT, and GPX4 Genes, and Sperm Parameters of Fertile and Infertile Men: A Controlled Clinical Trial. Int. J. Reprod. Biomed. 2021, 19, 545–558. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; El-Maddawy, Z.K.; El-Sayed, Y.S. Therapeutic Effects of Date Palm (Phoenix dactylifera L.) Pollen Extract on Cadmium-Induced Testicular Toxicity. Andrologia 2013, 45, 369–378. [Google Scholar] [CrossRef]
- Johnson, F.C.; Sinclair, H.M. The Antioxidant Vitamins. Crit. Rev. Food Sci. Nutr. 1979, 11, 217–309. [Google Scholar] [CrossRef]
- Yoganathan, T.; Eskild, W.; Hansson, V. Investigation of Detoxification Capacity of Rat Testicular Germ Cells and Sertoli Cells. Free Radic. Biol. Med. 1989, 7, 355–359. [Google Scholar] [CrossRef]
- Paolicchi, A.; Pezzini, A.; Saviozzi, M.; Piaggi, S.; Andreuccetti, M.; Chieli, E.; Malvaldi, G.; Casini, A.F. Localization of a GSH-Dependent Dehydroascorbate Reductase in Rat Tissues and Subcellular Fractions. Arch. Biochem. Biophys. 1996, 333, 489–495. [Google Scholar] [CrossRef]
- Di Guardo, F.; Vloeberghs, V.; Bardhi, E.; Blockeel, C.; Verheyen, G.; Tournaye, H.; Drakopoulos, P. Low Testosterone and Semen Parameters in Male Partners of Infertile Couples Undergoing Ivf with a Total Sperm Count Greater than 5 Million. J. Clin. Med. 2020, 9, 3824. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Mehrabani, D.; Razavi, F. Effect of Palm Pollen Extract on Sexual Hormone Levels and Follicle Numbers in Adult Female BALB/c Mice. Intern. Med. Today 2014, 20, 139–143. [Google Scholar]
- Hammed, M.S.; Arrak, J.K.; Al-Khafaji, N.J.; Hassan, A.A. Effect of Date Palm Pollen Suspension on Ovarian Function and Fertility in Adult Female Rats Exposed to Lead Acetate. Diyala J. Med. 2012, 3, 90–96. [Google Scholar]
- Jiheel, M.J.; Arrak, J.K. Effect of Different Doses of Ethanolic Extract of Date Palm Pollen Grains on Serum Gonadotropin and Total Glutathione in Mature Female Rats. Kufa J. For. Vet. Med. Sci. 2015, 6, 109–119. [Google Scholar] [CrossRef]
- Sadeghi, S.; Yosefzadeh, S.; Rakhshandeh, H.; Dadghar, S.; Mazloum, S.R. The Impact of Date Palm Pollen Capsule on Vaginal Iubrication and Dyspareunia In Menopausal Woman. J. Kebidanan 2018, 6, 1399. [Google Scholar]
- Abdel-Sattar, M.; Mohamed, Y.I. Pollen Viability of Date Palm from Different Sources in Relation to Its Chemical Composition. Alex. J. Agric. Sci. 2017, 62, 149–155. [Google Scholar]
- Ito, T.Y.; Trant, A.S.; Polan, M.L. A Double-Blind Placebo-Controlled Study of ArginMax, a Nutritional Supplement for Enhancement of Female Sexual Function. J. Sex Marital Ther. 2001, 27, 541–549. [Google Scholar] [CrossRef]
- Cieri-Hutcherson, N.E.; Jaenecke, A.; Bahia, A.; Lucas, D.; Oluloro, A.; Stimmel, L.; Hutcherson, T.C. Systematic Review of L-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy 2021, 9, 71. [Google Scholar] [CrossRef]
- Croft, H.A. Understanding the Role of Serotonin in Female Hypoactive Sexual Desire Disorder and Treatment Options. J. Sex Med. 2017, 14, 1575–1584. [Google Scholar] [CrossRef]
- Gauthaman, K.; Ganesan, A.P. The Hormonal Effects of Tribulus terrestri and Its Role in the Management of Male Erectile Dysfunction–an Evaluation Using Primates, Rabbit and Rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).