Resting Metabolic Rate and Substrate Utilization during Energy and Protein Availability in Male and Female Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
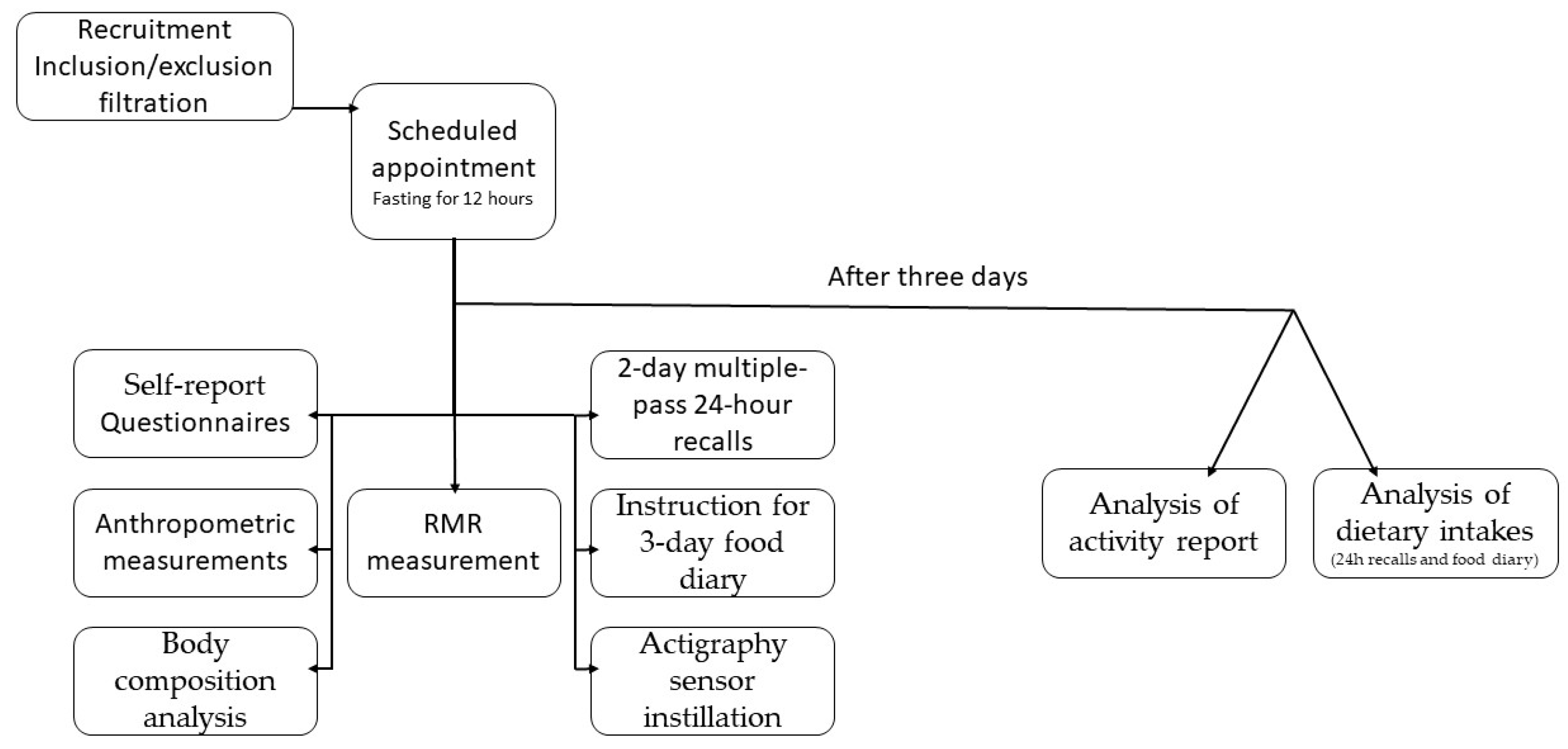
2.2. Procedures
2.2.1. Self-Report Questionnaires
2.2.2. Anthropometric Measurements
2.2.3. Body Composition Analysis
2.2.4. Dietary Analysis
2.2.5. Measuring the RMR
2.2.6. Estimated Activity–Energy Expenditure Monitoring
2.2.7. Energy and Protein Availability
2.3. Statistical Analysis
3. Results
3.1. Anthropometric and Body Composition Characters of the Development Sample
3.2. Dietary and Macronutrient Intake in the Study Sample
3.3. Resting Energy Expenditure and Activity–Energy Expenditure Results
3.4. Energy and Protein Availability
3.5. Correlation of Energy and Protein Availability with RMR and Substrate Utilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westerterp, K. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, A.; Marcolin, G.; Paoli, A. Non-exercise activity thermogenesis in the workplace: The office is on fire. Front. Public Health 2022, 10, 1024856. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Eberhardt, N.L.; Jensen, M.D. Role of non-exercise activity thermogenesis in resistance to fat gain in humans. Science 1999, 283, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Schofield, K.L.; Thorpe, H.; Sims, S.T. Resting metabolic rate prediction equations and the validity to assess energy deficiency in the athlete population. Exp. Physiol. 2019, 104, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand the female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Kuikman, M.A.; Burke, L.M. Low Energy Availability in Athletes: Understanding Undereating and Its Concerns. Nutr. Today 2023, 58, 51–57. [Google Scholar] [CrossRef]
- Otis, C.L.; Drinkwater, B.; Johnson, M.; Loucks, A.; Wilmore, J. ACSM position stand: The female athlete triad. Med. Sci. Sport Exerc. 1997, 29, i–ix. [Google Scholar] [CrossRef]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073–1098. [Google Scholar] [CrossRef]
- Nattiv, A.; De Souza, M.J.; Koltun, K.J.; Misra, M.; Kussman, A.; Williams, N.I.; Barrack, M.T.; Kraus, E.; Joy, E.; Fredericson, M. The Male Athlete Triad—A Consensus Statement From the Female and Male Athlete Triad Coalition Part 1: Definition and Scientific Basis. Clin. J. Sport Med. 2021, 31, 335–348. [Google Scholar] [CrossRef]
- Franca, P.A.; Gonçalves Lima, C.K.; Oliveira, T.M.; Ferreira, T.J.; Da Silva, R.R.; Loureiro, L.L.; Pierucci, A.P. Effectiveness of current protein recommendations in adolescent athletes on a low-carbon diet. Front. Nutr. 2022, 9, 1016409. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the academy of nutrition and dietetics, dietitians of canada, and the american college of sports medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Pettinger, M.; Thomson, C.A.; Mossavar-Rahmani, Y.; Thomas, F.; Qi, L.; Huang, Y. An exploratory study of respiratory quotient calibration and association with postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2374–2383. [Google Scholar] [CrossRef]
- Staal, S.; Sjödin, A.; Fahrenholtz, I.; Bonnesen, K.; Melin, A.K. Low RMRratio as a surrogate marker for energy deficiency, the choice of predictive equation vital for correctly identifying male and female ballet dancers at risk. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 412–418. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, B.; Skouby, S.; Møller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjödin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef]
- Araujo, C.G.; Scharhag, J. Athlete: A working definition for medical and health sciences research. Scand. J. Med. Sci. Sports 2016, 26, 4–7. [Google Scholar] [CrossRef]
- Kinoshita, N.; Uchiyama, E.; Ishikawa-Takata, K.; Yamada, Y.; Okuyama, K. Association of energy availability with resting metabolic rates in competitive female teenage runners: A cross-sectional study. J. Int. Soc. Sports Nutr. 2021, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.M.; Olmstead, M.P.; Polivy, J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983, 2, 15–34. [Google Scholar] [CrossRef]
- Abulmeaty, M.M.A.; Almajwal, A.M.; ElSadek, M.F.; Aldisi, D.; Al-Momani, M.; Alquraishii, M.; Razak, S.; Almadani, N.K.; Almutawa, D.A. Dietary and lifestyle determinants of the lifetime cardiovascular risk during early adulthood. Prog. Nutr. 2019, 21, 297–306. [Google Scholar]
- Abulmeaty, M.M.A.; Almajwal, A.M.; Almadani, N.K.; Aldosari, M.S.; Alnajim, A.A.; Ali, S.B.; Hassan, H.M.; Elkatawy, H.A. Anthropometric and central obesity indices as predictors of long-term cardiometabolic risk among Saudi young men and women. Saudi Med. J. 2017, 38, 372–380. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Kim, G.S.; Park, J.S.; Kim, E.K. Accuracy of predictive equations for resting metabolic rate in Korean athletic and non-athletic adolescents. Nutr. Res. Pract. 2015, 9, 370–378. [Google Scholar] [CrossRef]
- Burke, L.M. Dietary Assessment Methods for the Athlete: Pros and Cons of Different Methods. Sports Sci. Exch. 2015, 28, 150. [Google Scholar]
- Moss, K.; Kreutzer, A.; Graybeal, A.J.; Zhang, Y.; Braun-Trocchio, R.; Porter, R.R.; Shah, M. Nutrient Adequacy in Endurance Athletes. Int. J. Environ. Res. Public Health 2023, 20, 5469. [Google Scholar] [CrossRef]
- Peric, R.; Meucci, M.; Nikolovski, Z. Fat Utilization During High-Intensity Exercise: When Does It End? Sports Med. Open 2016, 2, 35. [Google Scholar] [CrossRef]
- Birnbaumer, P.; Dietz, P.; Watson, E.D.; Mukoma, G.; Müller, A.; Sattler, M.C.; Jaunig, J.; van Poppel, M.N.M.; Hofmann, P. Absolute Accelerometer-Based Intensity Prescription Compared to Physiological Variables in Pregnant and Nonpregnant Women. Int. J. Environ. Res. Public Health 2020, 17, 5651. [Google Scholar] [CrossRef]
- Clemente, F.M.; Nikolaidis, P.T.; Martins, F.M.L.; Mendes, R.S. Weekly physical activity patterns of university students: Are athletes more active than non-athletes? SpringerPlus 2016, 5, 1808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Erdman, K.A.; Burke, L.M. American college of sports medicine joint position statement. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Veligekas, P.; Selima, E.; Christofi, E.; Pafili, Z. Elite high jumpers exhibit inadequate nutrient intakes. J. Phys. Educ. Sport 2013, 13, 330–337. [Google Scholar]
- Nascimento, M.V.S.; do Villa-Nova, T.M.S.; Silva, D.G.; da Nascimento, V.T.; Mendes-Netto, R.S. Nutrient and food inadequacies among athletes: Gender comparisons. J. Phys. Educ. 2016, 27, 2758. [Google Scholar] [CrossRef][Green Version]
- de Borja, C.; Holtzman, B.; McCall, L.M.; Carson, T.L.; Moretti, L.J.; Farnsworth, N.; Ackerman, K.A. Specific dietary practices in female athletes and their association with positive screening for disordered eating. J. Eat. Disord. 2021, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Bratland-Sanda, S.; Sundgot-Borgen, J. Eating disorders in athletes: Overview of prevalence, risk factors and recommendations for prevention and treatment. Eur. J. Sport Sci. 2013, 13, 499–508. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Phillips, S.M.; Van Loon, L.J. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29 (Suppl. S1), S29–S38. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Brown, S.R. A Systematic Review of Dietary Protein During Caloric Restriction in Resistance Trained Lean Athletes: A Case for Higher Intakes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 127–138. [Google Scholar] [CrossRef]
- Butterfield, G.E. Whole-body protein utilization in humans. Med. Sci. Sports Exerc. 1987, 19 (Suppl. S5), S157–S165. [Google Scholar] [CrossRef]
- Gomez-Arbelaez, D.; Crujeiras, A.B.; Castro, A.I.; Martinez-Olmos, M.A.; Canton, A.; Ordoñez-Mayan, L.; Sajoux, I.; Galban, C.; Bellido, D.; Casanueva, F.F. Resting metabolic rate of obese patients under very low calorie ketogenic diet. Nutr. Metab. 2018, 15, 18. [Google Scholar] [CrossRef]
- Sterringer, T.; Larson-Meyer, D.E. RMR Ratio as a Surrogate Marker for Low Energy Availability. Curr. Nutr. Rep. 2022, 11, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.C.; Williams, N.I.; Scheid, J.L.; Toombs, R.J.; De Souza, M.J. The Association of a High Drive for Thinness With Energy Deficiency and Severe Menstrual Disturbances: Confirmation in a Large Population of Exercising Women. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, S.; Ghorbaninejad, P.; Shahinfar, H.; Ebaditabar, M.; Babaei, N.; Davarzani, S.; Djafarian, K.; Shab-Bidar, S. The low-carbohydrate-diet score is associated with resting metabolic rate: An epidemiologic study among Iranian adults. J. Diabetes Metab. Disord. 2021, 20, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.J.; Yokum, S.; Stice, E. Low energy intake plus low energy expenditure (low energy flux), not energy surfeit, predicts future body fat gain. Am. J. Clin. Nutr. 2016, 103, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Selles, A.; Galvan-Alvarez, V.; Martinez-Canton, M.; Garcia-Gonzalez, E.; Morales-Alamo, D.; Santana, A.; Gonzalez-Henriquez, J.J.; Dorado, C.; Calbet, J.A.L.; Martin-Rincon, M. Fast regulation of the NF-κB signaling pathway in human skeletal muscle revealed by high-intensity exercise and ischemia at exhaustion: Role of oxygenation and metabolite accumulation. Redox Biol. 2022, 55, 102398. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Kilding, A.E.; Stewart, T.; Plews, D.J. Factors Influencing Substrate Oxidation During Submaximal Cycling: A Modelling Analysis. Sports Med. 2022, 52, 2775–2795. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Sample (n = 60) Mean ± SD | Female Athletes (n = 21) Mean ± SD | Male Athletes (n = 39) Mean ± SD | p-Value |
|---|---|---|---|---|
| Age (years) | 26.83 ± 7.12 | 28.90 ± 7.02 | 25.71 ± 7.00 | 0.099 |
| Weight (kg) | 70.95 ± 14.64 | 61.80 ± 9.59 | 75.87 ± 14.61 | <0.001 |
| Height (cm) | 168.73 ± 7.76 | 160.81 ± 4.25 | 173.00 ± 5.53 | <0.001 |
| Body mass index (kg/m2) | 24.80 ± 4.14 | 23.84 ± 3.24 | 25.32 ± 4.51 | 0.190 |
| Dominant Average hand grip (kg) | 35.80 ± 11.87 | 23.98 ± 4.94 | 41.85 ± 9.57 | <0.001 |
| Non-dominant Average hand grip (kg) | 33.93 ± 11.04 | 22.58 ± 4.80 | 39.90 ± 8.35 | <0.001 |
| Waist circumference (cm) | 76.95 ± 9.88 | 72.65 ± 9.39 | 79.15 ± 9.50 | 0.015 |
| Hip circumference (cm) | 100.29 ± 9.81 | 98.98 ± 11.72 | 100.96 ± 8.77 | 0.466 |
| Waist hip ratio | 0.77 ± 0.07 | 0.74 ± 0.06 | 0.78 ± 0.06 | 0.009 |
| Estimated Percent body fat (%) | 20.82 ± 7.84 | 28.21 ± 6.05 | 16.84 ± 5.44 | <0.001 |
| Estimated Fat mass (kg) | 14.93 ± 6.61 | 17.86 ± 6.06 | 13.34 ± 6.42 | 0.010 |
| Estimated Fat-mass index (kg/m2) | 5.30 ± 2.42 | 6.87 ± 2.21 | 4.46 ± 2.11 | <0.001 |
| Estimated Fat-free mass (kg) | 56.01 ± 11.95 | 43.89 ± 4.65 | 62.54 ± 9.24 | <0.001 |
| Estimated Fat-free mass index (kg/m2) | 19.49 ± 2.96 | 16.96 ± 1.53 | 20.86 ± 2.63 | <0.001 |
| Estimated Muscle mass (kg) | 53.00 ± 11.96 | 41.67 ± 4.40 | 59.09 ± 10.15 | <0.001 |
| Estimated Total body water (kg) | 41.01 ± 8.74 | 32.13 ± 3.40 | 45.79 ± 6.74 | <0.001 |
| Variables | Total Sample (n = 60) Mean ± SD | Female Athletes (n = 21) Mean ± SD | Male Athletes (n = 39) Mean ± SD | p-Value |
|---|---|---|---|---|
| Average daily energy intake (Kcal/day) | 1911.00 ± 690.86 | 1919.45 ± 694.34 | 1906.43 ± 698.03 | 0.099 |
| Average daily protein intake (g/day) | 99.90 ± 39.11 | 91.66 ± 33.44 | 104.34 ± 41.58 | 0.234 |
| Average daily CHO intake (g/day) | 222.44 ± 96.77 | 211.94 ± 95.55 | 228.10 ± 98.18 | 0.542 |
| Average daily fiber intake (g/day) | 13.87 ± 7.52 | 16.01 ± 9.68 | 12.72 ± 5.88 | 0.106 |
| Average daily total sugar intake (g/day) | 77.82 ± 49.87 | 67.61 ± 39.29 | 83.31 ± 54.41 | 0.248 |
| Average daily added sugars (g/day) | 27.75 ± 34.15 | 15.30 ± 17.92 | 34.86 ± 38.86 | 0.037 |
| Average daily fat intake (g/day) | 71.69 ± 37.27 | 81.60 ± 39.43 | 66.35 ± 35.43 | 0.132 |
| Average daily sat. Fat intake (g/day) | 25.00 ± 16.99 | 28.25 ± 18.66 | 23.24 ± 15.98 | 0.280 |
| Average daily MUF intake (g/day) | 14.56 ± 9.59 | 15.38 ± 12.46 | 14.13 ± 7.88 | 0.641 |
| Average daily PUF intake (g/day) | 8.10 ± 5.27 | 9.05 ± 6.72 | 7.61 ± 4.37 | 0.326 |
| Average daily trans fat intake (g/day) | 0.43 ± 0.60 | 0.23 ± 0.31 | 0.54 ± 0.69 | 0.061 |
| Variables | Total Sample (n = 60) Mean ± SD | Female Athletes (n = 21) Mean ± SD | Male Athletes (n = 39) Mean ± SD | p-Value |
|---|---|---|---|---|
| Measured RMR (kcal/day) | 1786.73 ± 362.02 | 1503.19 ± 188.49 | 1939.41 ± 341.22 | <0.001 |
| Percent of utilized fat (%) | 55.28 ± 23.00 | 51.70 ± 24.13 | 57.21 ± 22.46 | 0.382 |
| Percent of utilized carbohydrates (%) | 25.22 ± 23.65 | 25.57 ± 25.50 | 25.04 ± 22.94 | 0.935 |
| Percent of utilized protein (%) | 19.65 ± 4.13 | 23.16 ± 3.77 | 17.76 ± 2.92 | <0.001 |
| Estimated average AEE/day (kcal/day) | 1288.41 ± 532.77 | 1153.93 ± 421.69 | 1360.83 ± 575.96 | 0.153 |
| Estimated average AEE/hour (kcal/h) | 73.90 ± 27.18 | 62.79 ± 22.75 | 79.87 ± 27.74 | 0.019 |
| Metabolic equivalent (METs) | 1.45 ± 0.17 | 1.38 ± 0.17 | 1.48 ± 0.17 | 0.030 |
| % of time in light activity (%) | 77.62 ± 6.52 | 77.17 ± 8.38 | 77.87 ± 5.38 | 0.695 |
| % of time in moderate activity (%) | 22.27 ± 6.79 | 22.59 ± 8.86 | 22.10 ± 5.49 | 0.791 |
| % of time in vigorous activity (%) | 0.07 ± 0.33 | 0.14 ± 0.47 | 0.04 ± 0.22 | 0.244 |
| % of time in very vigorous activity (%) | 0.04 ± 0.21 | 0.10 ± 0.35 | 0.00 ± 0.00 | 0.068 |
| Count of steps (step/day) | 42,800.13 ± 24,064.55 | 55,2796.8 ± 30,062.1 | 37,417.3 ± 18,399.0 | 0.017 |
| Steps per min (step/min) | 10.06 ± 3.51 | 10.34 ± 4.28 | 9.90 ± 3.06 | 0.650 |
| Variables | Total Sample (n = 60) Mean ± SD | Female Athletes (n = 21) Mean ± SD | Male Athletes (n = 39) Mean ± SD | p-Value |
|---|---|---|---|---|
| Energy intake (Kcal/kg eFFM/day) | 35.43 ± 14.47 | 43.79 ± 14.94 | 30.92 ± 12.17 | 0.001 |
| Estimated AEE (Kcal/kg eFFM/day) | 23.16 ± 8.30 | 26.11 ± 8.64 | 21.57 ± 7.77 | 0.043 |
| Energy availability (Kcal/kg eFFM/day) | 13.81 ± 15.09 | 18.85 ± 16.65 | 11.09 ± 13.63 | 0.057 |
| Protein availability (g/kg/day) | 1.40 ± 0.44 | 1.46 ± 0.40 | 1.37 ± 0.46 | 0.446 |
| Protein availability (g/kg eFFM/day) | 1.79 ± 0.62 | 2.06 ± 0.65 | 1.65 ± 0.56 | 0.012 |
| Variables | Female Athletes (n = 21) % within Variable (% within the Group) | Male Athletes (n = 39) % within Variable (% within the Group) | p-Value |
|---|---|---|---|
| Energy availability | 0.080 | ||
| Sufficient energy availability | 62.5 (32.8) | 37.5 (7.7) | |
| Low energy availability | 30.8 (76.2) | 69.2 (92.3) | |
| Protein availability | 0.705 | ||
| Sufficient protein availability | 36.6 (71.4) | 63.4 (66.7) | |
| Low protein availability | 31.6 (28.6) | 68.4 (33.3) | |
| Sports | 0.127 | ||
| Bodybuilding | 22.2 (9.5) | 77.8 (17.9) | |
| Powerlifting | 33.3 (9.5) | 66.7 (10.3) | |
| Spinning | 100.0 (4.8) | 0.0 (0.0) | |
| Basketball | 100 (2.5) | 0 (0) | |
| CrossFit | 0.0 (0.0) | 100.0 (12.8) | |
| Martial arts | 100 (4.8) | 0.0 (0.0) | |
| Tennis | 100 (4.8) | 0.0 (0.0) | |
| Football | 39.1 (42.9) | 60.9 (35.9) | |
| Weight-lifting | 100.0 (9.5) | 0.0 (0.0) | |
| Beach volleyball | 0 (0) | 100.0 (2.6) | |
| Karate | 0 (0) | 100.0 (2.6) | |
| Cycling | 22.2 (9.5) | 77.8 (17.9) | |
| Judo | 100 (4.8) | 0.0 (0.0) | |
| Sport type | 0.740 | ||
| Individual sport | 33.3 (57.1) | 66.7 (61.5) | |
| Team sport | 37.5 (42.9) | 62.5 (38.5) |
| Variables | Energy Availability | p-Value | Protein Availability | p-Value | ||
|---|---|---|---|---|---|---|
| Sufficient EA (n = 8) Mean ± SD | Low EA (n = 52) Mean ± SD | Sufficient PA (n = 41) Mean ± SD | Low PA (n = 19) Mean ± SD | |||
| Age (year) | 25.00 ± 9.00 | 27.12 ± 6.85 | 0.439 | 27.56 ± 7.52 | 25.26 ± 6.05 | 0.248 |
| Gender (female %) | 62.50 | 30.80 | 0.080 | 36.6 | 31.6 | 0.705 |
| EA (Kcal/kg eFFM/day) | 45.53 ± 12.52 | 8.93 ± 7.72 | <0.001 | 17.48 ± 16.14 | 5.89 ± 8.39 | 0.005 |
| PA (g/kg/day) | 1.78 ± 0.34 | 1.34 ± 0.43 | 0.008 | 1.62 ± 0.32 | 0.91 ± 0.20 | <0.001 |
| RMR (Kcal/day) | 1538.38 ± 210.86 | 1824.94 ± 366.54 | 0.036 | 1800.24 ± 397.84 | 1757.58 ± 276.68 | 0.675 |
| Fat utilization (%) | 43.55 ± 25.26 | 57.08 ± 22.35 | 0.122 | 58.52 ± 21.40 | 48.29 ± 25.32 | 0.110 |
| CHO utilization (%) | 34.29 ± 26.91 | 23.83 ± 23.08 | 0.248 | 21.97 ± 21.65 | 32.26 ± 26.74 | 0.132 |
| Protein utilization (%) | 22.16 ± 3.63 | 19.26 ± 4.09 | 0.064 | 19.74 ± 4.67 | 19.45 ± 2.70 | 0.118 |
| AEE (Kcal/day) | 886.62 ± 336.66 | 1350.23 ± 532.53 | 0.021 | 1323.03 ± 574.44 | 1213.71 ± 434.11 | 0.805 |
| Variables | Energy Availability | Protein Availability | Gender | ||||
|---|---|---|---|---|---|---|---|
| Sufficient EA (n = 8) | Low EA (n = 52) | Sufficient PA (n = 41) | Low PA (n = 19) | Females (n = 21) | Males (n = 39) | ||
| RMR (Kcal/day) | r | 0.501 | 0.308 * | 0.032 | −0.194 | −0.058 | 0.084 |
| p-Value | 0.206 | 0.027 | 0.844 | 0.426 | 0.801 | 0.612 | |
| Fat utilization (%) | r | 0.135 | 0.312 * | −0.158 | 0.279 | 0.178 | 0.001 |
| p-Value | 0.749 | 0.024 | 0.323 | 0.248 | 0.441 | 0.998 | |
| CHO utilization (%) | r | −0.066 | −0.246 | 0.179 | −0.281 | −0.172 | 0.020 |
| p-Value | 0.876 | 0.079 | 0.262 | 0.244 | 0.457 | 0.902 | |
| Protein utilization (%) | r | −0.450 | −0.263 | −0.096 | 0.170 | 0.095 | −0.159 |
| p-Value | 0.289 | 0.059 | 0.551 | 0.488 | 0.684 | 0.335 | |
| Variables | Energy Availability | Protein Availability | Gender | ||||
|---|---|---|---|---|---|---|---|
| Sufficient EA (n = 8) | Low EA (n = 52) | Sufficient PA (n = 41) | Low PA (n = 19) | Females (n = 21) | Males (n = 39) | ||
| RMR (Kcal/day) | r | 0.477 | 0.355 * | 0.451 ** | −0.094 | 0.105 | 0.319 * |
| p-Value | 0.232 | 0.010 | 0.003 | 0.701 | 0.650 | 0.048 | |
| Fat utilization (%) | r | 0.121 | 0.253 | 0.111 | 0.020 | 0.048 | 0.259 |
| p-Value | 0.776 | 0.071 | 0.490 | 0.934 | 0.835 | 0.111 | |
| CHO utilization (%) | r | −0.050 | −0.188 | −0.009 | −0.027 | −0.032 | −0.208 |
| p-Value | 0.907 | 0.182 | 0.954 | 0.911 | 0.889 | 0.204 | |
| Protein utilization (%) | r | −0.473 | −0.263 | −0.420 ** | 0.080 | 0.115 | −0.358 * |
| p-Value | 0.263 | 0.060 | 0.006 | 0.746 | 0.621 | 0.025 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abulmeaty, M.M.A.; Almajwal, A.; Elsayed, M.; Hassan, H.; Alsager, T.; Aldossari, Z. Resting Metabolic Rate and Substrate Utilization during Energy and Protein Availability in Male and Female Athletes. Metabolites 2024, 14, 167. https://doi.org/10.3390/metabo14030167
Abulmeaty MMA, Almajwal A, Elsayed M, Hassan H, Alsager T, Aldossari Z. Resting Metabolic Rate and Substrate Utilization during Energy and Protein Availability in Male and Female Athletes. Metabolites. 2024; 14(3):167. https://doi.org/10.3390/metabo14030167
Chicago/Turabian StyleAbulmeaty, Mahmoud M. A., Ali Almajwal, Mervat Elsayed, Heba Hassan, Thamer Alsager, and Zaid Aldossari. 2024. "Resting Metabolic Rate and Substrate Utilization during Energy and Protein Availability in Male and Female Athletes" Metabolites 14, no. 3: 167. https://doi.org/10.3390/metabo14030167
APA StyleAbulmeaty, M. M. A., Almajwal, A., Elsayed, M., Hassan, H., Alsager, T., & Aldossari, Z. (2024). Resting Metabolic Rate and Substrate Utilization during Energy and Protein Availability in Male and Female Athletes. Metabolites, 14(3), 167. https://doi.org/10.3390/metabo14030167







