Metabolic Covariance Connectivity of Posterior Cingulate Cortex Associated with Depression Symptomatology Level in Healthy Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. PET Data Acquisition and Preprocessing
2.4. Covariance Connectivity Analysis
2.5. Statistical Analysis
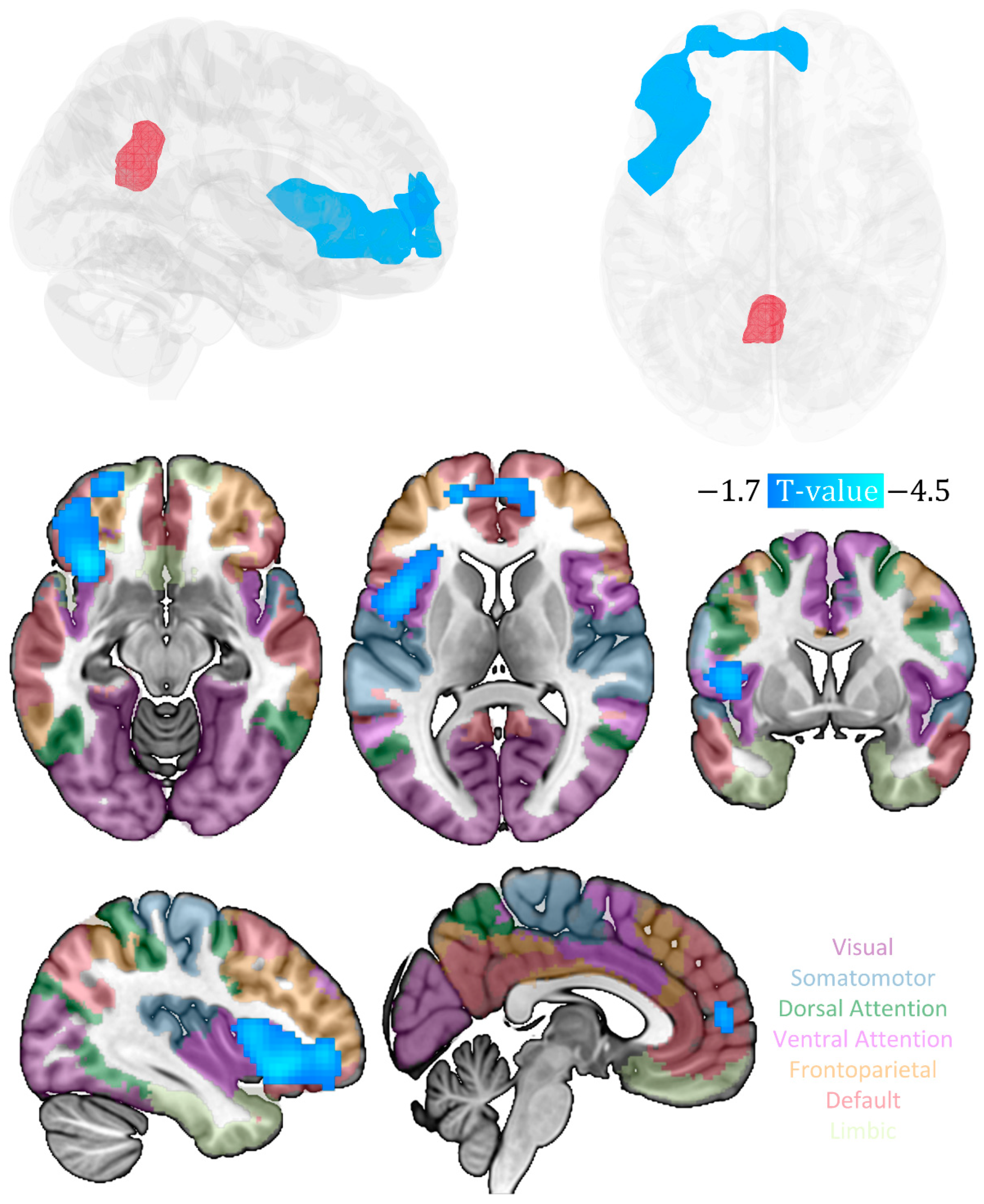
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.J.; Pratt, L.A.; Hughes, J.P. Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS Data Brief 2018, 1–8. [Google Scholar]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). 2015. Available online: http://www.samhsa.gov/data/ (accessed on 16 March 2022).
- Association, A.C.H. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2019; American College Health Association: Silver Spring, MD, USA, 2019. [Google Scholar]
- Jaracz, J.; Rybakowski, J. Studies of cerebral blood flow in metabolism in depression using positron emission tomography (PET). Psychiatr. Pol. 2002, 36, 617–628. [Google Scholar]
- Drevets, W.C. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001, 11, 240–249. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Wei, K.; Xue, H.-l.; Guan, Y.-h.; Zuo, C.-t.; Ge, J.-j.; Zhang, H.-y.; Liu, B.-j.; Cao, Y.-x.; Dong, J.-c.; Du, Y.-j. Analysis of glucose metabolism of 18F-FDG in major depression patients using PET imaging: Correlation of salivary cortisol and α-amylase. Neurosci. Lett. 2016, 629, 52–57. [Google Scholar] [CrossRef]
- Drevets, W.C.; Bogers, W.; Raichle, M.E. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002, 12, 527–544. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Brody, A.L.; Saxena, S.; Mandelkern, M.A.; Fairbanks, L.A.; Ho, M.L.; Baxter, L.R., Jr. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 2001, 50, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Brannan, S.K.; Tekell, J.L.; Silva, J.A.; Mahurin, R.K.; McGinnis, S.; Jerabek, P.A. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol. Psychiatry 2000, 48, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Goldapple, K.; Segal, Z.; Garson, C.; Lau, M.; Bieling, P.; Kennedy, S.; Mayberg, H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry 2004, 61, 34–41. [Google Scholar] [CrossRef]
- Maddock, R.J.; Garrett, A.S.; Buonocore, M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003, 18, 30–41. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Lau, W.K.; Wei, X.; Liu, Y.; Huang, R.; Zhang, R. Hyperconnectivity between the posterior cingulate and middle frontal and temporal gyrus in depression: Based on functional connectivity meta-analyses. Brain Imaging Behav. 2022, 16, 1538–1551. [Google Scholar] [CrossRef]
- Yang, R.; Gao, C.; Wu, X.; Yang, J.; Li, S.; Cheng, H. Decreased functional connectivity to posterior cingulate cortex in major depressive disorder. Psychiatry Res. Neuroimaging 2016, 255, 15–23. [Google Scholar] [CrossRef]
- Bertocci, M.A.; Afriyie-Agyemang, Y.; Rozovsky, R.; Iyengar, S.; Stiffler, R.; Aslam, H.A.; Bebko, G.; Phillips, M.L. Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples. Mol. Psychiatry 2023, 28, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Xiao, J.; Liao, J.; Zhong, M.; Wang, W.; Yao, S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry 2012, 71, 611–617. [Google Scholar] [CrossRef]
- Jacobs, R.H.; Jenkins, L.M.; Gabriel, L.B.; Barba, A.; Ryan, K.A.; Weisenbach, S.L.; Verges, A.; Baker, A.M.; Peters, A.T.; Crane, N.A. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS ONE 2014, 9, e104366. [Google Scholar] [CrossRef]
- Ely, B.A.; Xu, J.; Goodman, W.K.; Lapidus, K.A.; Gabbay, V.; Stern, E.R. Resting-state functional connectivity of the human habenula in healthy individuals: Associations with subclinical depression. Hum. Brain Mapp. 2016, 37, 2369–2384. [Google Scholar] [CrossRef]
- Philippi, C.L.; Motzkin, J.C.; Pujara, M.S.; Koenigs, M. Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. J. Psychiatr. Res. 2015, 71, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Benschop, L.; Poppa, T.; Medani, T.; Shahabi, H.; Baeken, C.; Leahy, R.M.; Pizzagalli, D.A.; Vanderhasselt, M.-A. Electrophysiological scarring in remitted depressed patients: Elevated EEG functional connectivity between the posterior cingulate cortex and the subgenual prefrontal cortex as a neural marker for rumination. J. Affect. Disord. 2021, 281, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.; Deckersbach, T.; Dougherty, D.D.; Hooley, J.M. The default mode network and rumination in individuals at risk for depression. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad032. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Chan, F.H.; Zheng, M.M.Z.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef]
- Liu, T.T. Reprint of ‘Noise contributions to the fMRI signal: An overview’. Neuroimage 2017, 154, 4–14. [Google Scholar] [CrossRef]
- Ward, P.G.; Orchard, E.R.; Oldham, S.; Arnatkevičiūtė, A.; Sforazzini, F.; Fornito, A.; Storey, E.; Egan, G.F.; Jamadar, S.D. Individual differences in haemoglobin concentration influence BOLD fMRI functional connectivity and its correlation with cognition. NeuroImage 2020, 221, 117196. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Horwitz, B.; Duara, R.; Rapoport, S.I. Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. J. Cereb. Blood Flow Metab. 1984, 4, 484–499. [Google Scholar] [CrossRef]
- Moeller, J.; Strother, S.; Sidtis, J.; Rottenberg, D. Scaled subprofile model: A statistical approach to the analysis of functional patterns in positron emission tomographic data. J. Cereb. Blood Flow Metab. 1987, 7, 649–658. [Google Scholar] [CrossRef]
- Di, X.; Biswal, B.B.; Alzheimer’s Disease Neuroimaging Initiative. Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain Connect. 2012, 2, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Eidelberg, D. Metabolic brain networks in neurodegenerative disorders: A functional imaging approach. Trends Neurosci. 2009, 32, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, M.; Eidelberg, D. Metabolic brain networks in translational neurology: Concepts and applications. Ann. Neurol. 2012, 72, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-R.; Baeken, C. Individual interregional perfusion between the left dorsolateral prefrontal cortex stimulation targets and the subgenual anterior cortex predicts response and remission to aiTBS treatment in medication-resistant depression: The influence of behavioral inhibition. Brain Stimul. 2022, 15, 182–189. [Google Scholar] [PubMed]
- Wu, G.-R.; Baeken, C. The left ventrolateral prefrontal cortex as a more optimal target for accelerated rTMS treatment protocols for depression? Brain Stimul. 2023, 16, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-R.; Baeken, C. Precision targeting in prediction for rTMS clinical outcome in depression: What about sgACC lateralization, metabolic connectivity, and the potential role of the cerebellum? Eur. Arch. Psychiatry Clin. Neurosci. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Wu, G.-R.; Baeken, C. Lateralized subgenual ACC metabolic connectivity patterns in refractory melancholic depression: Does it matter? Cereb. Cortex 2023, 33, 3490–3497. [Google Scholar] [CrossRef]
- Jamadar, S.D.; Ward, P.G.; Close, T.G.; Fornito, A.; Premaratne, M.; O’Brien, K.; Stäb, D.; Chen, Z.; Shah, N.J.; Egan, G.F. Simultaneous BOLD-fMRI and constant infusion FDG-PET data of the resting human brain. Sci. Data 2020, 7, 363. [Google Scholar] [CrossRef]
- Eaton, W.W.; Muntaner, C.; Smith, C.; Tien, A.; Ybarra, M. Center for epidemiologic studies depression scale: Review and revision. In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2004. [Google Scholar]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Thomas Yeo, B.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Mah, L.; Zarate, C.A., Jr.; Singh, J.; Duan, Y.-F.; Luckenbaugh, D.A.; Manji, H.K.; Drevets, W.C. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol. Psychiatry 2007, 61, 765–775. [Google Scholar] [CrossRef]
- Drevets, W.C. Prefrontal cortical-amygdalar metabolism in major depression. Ann. N. Y. Acad. Sci. 1999, 877, 614–637. [Google Scholar] [CrossRef]
- Videbech, P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: A critical review. Acta Psychiatr. Scand. 2000, 101, 11–20. [Google Scholar] [CrossRef]
- Drevets, W.C. Neuroimaging studies of mood disorders. Biol. Psychiatry 2000, 48, 813–829. [Google Scholar] [CrossRef]
- Yakushev, I.; Chételat, G.; Fischer, F.U.; Landeau, B.; Bastin, C.; Scheurich, A.; Perrotin, A.; Bahri, M.A.; Drzezga, A.; Eustache, F. Metabolic and structural connectivity within the default mode network relates to working memory performance in young healthy adults. Neuroimage 2013, 79, 184–190. [Google Scholar] [CrossRef]
- Drevets, W.C.; Videen, T.O.; Price, J.L.; Preskorn, S.H.; Carmichael, S.T.; Raichle, M.E. A functional anatomical study of unipolar depression. J. Neurosci. 1992, 12, 3628–3641. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Kelley, M.E.; McGrath, C.L.; Craighead, W.E.; Mayberg, H.S. Preliminary findings supporting insula metabolic activity as a predictor of outcome to psychotherapy and medication treatments for depression. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 237–239. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, D.; Ban, M.; Kong, L.; Xiao, Q.; Yuan, F.; Zhu, X. Regional metabolic heterogeneity in anterior cingulate cortex in major depressive disorder: A multi-voxel 1H magnetic resonance spectroscopy study. J. Affect. Disord. 2022, 318, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zuo, C.; Zhang, H.; Jiao, F.; Zhang, B.; Tang, W.; Geng, D.; Guan, Y.; Shi, S. Regional cerebral metabolism alterations affect resting-state functional connectivity in major depressive disorder. Quant. Imaging Med. Surg. 2018, 8, 910–924. [Google Scholar] [CrossRef]
- Ge, R.; Hassel, S.; Arnott, S.R.; Davis, A.D.; Harris, J.K.; Zamyadi, M.; Milev, R.; Frey, B.N.; Strother, S.C.; Müller, D.J. Structural covariance pattern abnormalities of insula in major depressive disorder: A CAN-BIND study report. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110194. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Cui, Q.; Chen, Y.; He, Z.; Sheng, W.; Tang, Q.; Yang, Y.; Luo, W.; Yu, Y.; Chen, J. Insular-associated causal network of structural covariance evaluating progressive gray matter changes in major depressive disorder. Cereb. Cortex 2023, 33, 831–843. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Ainsworth, M.; Wu, Z.; Browncross, H.; Mitchell, A.S.; Bell, A.H.; Buckley, M.J. Frontopolar cortex shapes brain network structure across prefrontal and posterior cingulate cortex. Prog. Neurobiol. 2022, 217, 102314. [Google Scholar] [CrossRef]
- Heo, E.-H.; Choi, K.-S.; Yu, J.-C.; Nam, J.-A. Validation of the center for epidemiological studies depression scale among Korean adolescents. Psychiatry Investig. 2018, 15, 124. [Google Scholar] [CrossRef]
- Hwang, J.; Egorova, N.; Yang, X.; Zhang, W.; Chen, J.; Yang, X.; Hu, L.; Sun, S.; Tu, Y.; Kong, J. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry 2015, 5, e683. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.G.; Clasen, P.; Stice, E.; Schnyer, D. Depression symptoms and cognitive control of emotion cues: A functional magnetic resonance imaging study. Neuroscience 2010, 167, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Di, X.; Lei, H.; Yang, J.; Xiao, J.; Wang, X.; Yao, S.; Rao, H. Imbalanced spontaneous brain activity in orbitofrontal-insular circuits in individuals with cognitive vulnerability to depression. J. Affect. Disord. 2016, 198, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, L.; Yuan, X.; Zhang, L.; He, Y.; Yao, S.; Wang, J.; Xiao, J. Regional homogeneity and functional connectivity patterns in major depressive disorder, cognitive vulnerability to depression and healthy subjects. J. Affect. Disord. 2018, 235, 229–235. [Google Scholar] [CrossRef]
- Strikwerda-Brown, C.; Davey, C.G.; Whittle, S.; Allen, N.B.; Byrne, M.L.; Schwartz, O.S.; Simmons, J.G.; Dwyer, D.; Harrison, B.J. Mapping the relationship between subgenual cingulate cortex functional connectivity and depressive symptoms across adolescence. Soc. Cogn. Affect. Neurosci. 2015, 10, 961–968. [Google Scholar] [CrossRef]
- Schultz, D.H.; Ito, T.; Solomyak, L.I.; Chen, R.H.; Mill, R.D.; Anticevic, A.; Cole, M.W. Global connectivity of the fronto-parietal cognitive control network is related to depression symptoms in the general population. Netw. Neurosci. 2018, 3, 107–123. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Kang, M.S.; Lew, Y.; Van Der Feen, J.; Aguirre, B.; Clegg, R.; Goer, F.; Esposito, E.; Auerbach, R.P.; Hutchison, R.M. Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: A preliminary resting-state co-activation pattern analysis. Neuropsychopharmacology 2019, 44, 1604–1612. [Google Scholar] [CrossRef]
- Baxter, L.R.; Schwartz, J.M.; Phelps, M.E.; Mazziotta, J.C.; Guze, B.H.; Selin, C.E.; Gerner, R.H.; Sumida, R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry 1989, 46, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Martinot, J.-L.; Hardy, P.; Feline, A.; Huret, J.-D.; Mazoyer, B.; Attar-Levy, D.; Pappata, S.; Syrota, A. Left prefrontal glucose hypometabolism in the depressed state: A confirmation. Am. J. Psychiatry 1990, 147, 1313–1317. [Google Scholar]
- Fransson, P.; Marrelec, G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 2008, 42, 1178–1184. [Google Scholar] [CrossRef]
- Goldstein-Piekarski, A.N.; Staveland, B.R.; Ball, T.M.; Yesavage, J.; Korgaonkar, M.S.; Williams, L.M. Intrinsic functional connectivity predicts remission on antidepressants: A randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.; Zhu, X.; Tan, Y.; Zhong, Y. Aberrant connectivity within the default mode network in first-episode, treatment-naive major depressive disorder. J. Affect. Disord. 2015, 183, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wu, X.; Gong, R.; Yang, R.; Wang, X.; Zhu, W.; Lin, P. Sub-regional anterior cingulate cortex functional connectivity revealed default network subsystem dysfunction in patients with major depressive disorder. Psychol. Med. 2021, 51, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.G.; Peltier, S.; Nee, D.E.; Kross, E.; Deldin, P.J.; Jonides, J. Depression, rumination and the default network. Soc. Cogn. Affect. Neurosci. 2011, 6, 548–555. [Google Scholar] [CrossRef]
- Rzepa, E.; McCabe, C. Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. J. Psychopharmacol. 2018, 32, 1067–1074. [Google Scholar] [CrossRef]
- Manoliu, A.; Meng, C.; Brandl, F.; Doll, A.; Tahmasian, M.; Scherr, M.; Schwerthöffer, D.; Zimmer, C.; Förstl, H.; Bäuml, J. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2014, 7, 930. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, L.; Xie, F.; Guo, X.; Zhang, J.; Yao, L.; Wu, X. The altered triple networks interaction in depression under resting state based on graph theory. BioMed Res. Int. 2015, 2015, 386326. [Google Scholar] [CrossRef]
- Guha, A.; Yee, C.M.; Heller, W.; Miller, G.A. Alterations in the default mode-salience network circuit provide a potential mechanism supporting negativity bias in depression. Psychophysiology 2021, 58, e13918. [Google Scholar] [CrossRef]
- Chand, G.B.; Wu, J.; Hajjar, I.; Qiu, D. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017, 7, 401–412. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Yin, S.; Ren, W.; He, R.; Li, J. The fronto-insular cortex causally mediates the default-mode and central-executive networks to contribute to individual cognitive performance in healthy elderly. Hum. Brain Mapp. 2018, 39, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
| Female | Male | p-Value | |
|---|---|---|---|
| Count | 20 | 7 | 0.012 1 |
| Age (years) | 19.2 ± 1.196 | 20.14 ± 1.676 | 0.118 2 |
| Education (years) | 14.55 ± 1.468 | 15.29 ± 1.496 | 0.267 2 |
| CESD-R | 10.15 ± 8.952 | 6.14 ± 6.04 | 0.285 2 |
| Cluster | Anatomical Region | Cluster Size (Voxel) | Peak MNI Coordinates (x, y, z) | Peak t-Value (df = 22) | Cluster Level PFWE-corr |
|---|---|---|---|---|---|
| 1 | 974 | 0.008 | |||
| inferior frontal gyrus | 161 | −33, 24, −6 | −4.47 | ||
| Insula | 158 | −33, 21, −9 | −4.33 | ||
| middle frontal gyrus | 84 | −39, 45, −3 | −3.8 | ||
| medial prefrontal cortex | 72 | 12, 57, 3 | −3.43 | ||
| anterior cingulate | 11 | 6, 54, 9 | −3.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Baeken, C.; Wu, G.-R. Metabolic Covariance Connectivity of Posterior Cingulate Cortex Associated with Depression Symptomatology Level in Healthy Young Adults. Metabolites 2023, 13, 920. https://doi.org/10.3390/metabo13080920
Wang Z, Baeken C, Wu G-R. Metabolic Covariance Connectivity of Posterior Cingulate Cortex Associated with Depression Symptomatology Level in Healthy Young Adults. Metabolites. 2023; 13(8):920. https://doi.org/10.3390/metabo13080920
Chicago/Turabian StyleWang, Zhixin, Chris Baeken, and Guo-Rong Wu. 2023. "Metabolic Covariance Connectivity of Posterior Cingulate Cortex Associated with Depression Symptomatology Level in Healthy Young Adults" Metabolites 13, no. 8: 920. https://doi.org/10.3390/metabo13080920
APA StyleWang, Z., Baeken, C., & Wu, G.-R. (2023). Metabolic Covariance Connectivity of Posterior Cingulate Cortex Associated with Depression Symptomatology Level in Healthy Young Adults. Metabolites, 13(8), 920. https://doi.org/10.3390/metabo13080920







