Abstract
The main goal of this study was to develop methods for quality control of naftifine hydrochloride in solution and cream forms, focusing on “Quantitative Determination” and “Related Impurities.” New, precise, accurate, and environmentally friendly high performance liquid chromatography (HPLC) methods were developed for the determination of naftifine hydrochloride and its impurities. “Quantitative determination” was performed using a diode array detector at 254 nm with an isocratic mobile phase (1.154 g of ammonium acetate R dissolved in 300 mL of water R, followed by the addition of 0.2 mL of glacial acetic acid R, mixed well) and methanol (30:70). The chromatographic columns Gemini C18 and Luna C18 were used. “Related impurities” were separated at 270 nm using a gradient mobile phase consisting of 10 M sodium octanesulfonate, 0.4 g/L disodium hydrogen phosphate anhydrous solution (pH 6.5), acetonitrile, and the Synergi Hydro-RP chromatographic column. The developed method, validated according to ICH guidelines, showed run times of 55 min for impurity analysis and 6 min for active ingredient determination. The methods were successfully applied to the quality control of the solution and cream.
1. Introduction
The role of antifungal agents, such as naftifine hydrochloride, in the treatment of various dermatological conditions is widely recognized in modern pharmaceutical practice [1]. Naftifine hydrochloride is a well-established antifungal agent primarily used in the treatment of skin infections caused by dermatophytes, such as athlete’s foot, ringworm, and jock itch. It is a broad-spectrum antifungal that works by inhibiting the synthesis of ergosterol, a vital component of fungal cell membranes. This action leads to the disruption of the fungal cell membrane and subsequent cell death. Naftifine hydrochloride has a low systemic absorption when applied topically, making it an ideal candidate for local treatment, minimizing systemic side effects [2].
According to current clinical guidelines and dermatological treatment recommendations, naftifine hydrochloride is indicated for topical therapy of superficial fungal infections, including tinea pedis, tinea corporis, and tinea cruris, and is commonly used as a first-line treatment option. It is recommended in the form of creams, gels, or solutions for once-daily application, depending on the formulation and severity of infection. The favorable safety profile and high local efficacy of naftifine hydrochloride support its widespread clinical use in outpatient dermatological practice.
Given the widespread use of topical antifungal medications, ensuring the quality, safety, and effectiveness of formulations containing naftifine hydrochloride is of paramount importance. Quality control methods for these products must be precise and reliable to confirm both the active ingredient content and the absence of harmful impurities, ensuring therapeutic efficacy while minimizing risks to patients.
Many existing methods face challenges related to sensitivity, specificity, time consumption. Therefore, there is a growing need for the development and optimization of analytical techniques for the quality control of these compounds in pharmaceutical products, ensuring that the methods used are efficient, reliable, and environmentally friendly. The optimization of such methods is crucial for improving the accuracy and speed of analysis, reducing the cost of quality control, and ensuring the safety and efficacy of the pharmaceutical products.
In particular, currently available compendial methods for naftifine hydrochloride either lack sufficient selectivity for comprehensive impurity profiling, are not suitable for complex matrices such as creams, or require long analysis times and high solvent consumption. These limitations highlight the need for a unified, robust, and environmentally responsible analytical approach applicable to multiple dosage forms.
The US Pharmacopoeia (USP) [3] monograph for naftifine hydrochloride includes a normal-phase high performance liquid chromatography (HPLC) method for its assay. However, this method has a relatively long runtime for a single-component analysis, making it inefficient for routine use. In addition to the extended analysis time, the procedure is resource-intensive: mobile phase equilibration takes approximately 12 h, and column equilibration requires around 4 h. Such a demanding protocol significantly increases time and resource consumption, raises the risk of operational errors, and may negatively affect the accuracy of results. Therefore, alternative analytical methods for the determination of naftifine and impurities should be considered, aiming to achieve higher accuracy, more efficient resource utilization, and reduced analysis time.
For the determination of related substances, the USP monograph [3] also utilizes the same normal-phase HPLC method. However, this approach presents a significant limitation, as the method is unable to detect all potential degradation products of naftifine. The lack of comprehensive impurity profiling compromises the reliability of the method, especially in the context of stability studies and quality control. Therefore, there is a clear need to develop a new, more selective and robust analytical procedure capable of identifying and quantifying a broader range of naftifine-related impurities and degradation products.
In contrast, the Chinese Pharmacopoeia [4] proposes a more favorable reversed-phase HPLC method for the assay of naftifine hydrochloride. However, it does not specify the type or dimensions of the chromatographic column, which necessitates additional method verification and optimization prior to implementation. For the determination of related substances, the Chinese Pharmacopoeia recommends using the same procedure as for the assay. Nevertheless, this approach is insufficient for the comprehensive identification of all degradation products of naftifine hydrochloride. As a result, the method requires substantial refinement to ensure accurate impurity profiling and suitability for stability testing.
Although published data on naftifine hydrochloride are scarce, a literature search revealed a broader range of analytical studies dedicated to terbinafine, a structurally and pharmacologically related allylamine antifungal agent [5,6,7,8,9,10]. Both compounds share a similar mechanism of action, inhibiting squalene epoxidase in the fungal ergosterol biosynthesis pathway, and exhibit comparable physicochemical properties, such as lipophilicity and susceptibility to oxidative degradation. These similarities suggest that analytical strategies applied for terbinafine could provide a useful foundation for developing methods for naftifine hydrochloride. However, due to differences in formulation matrices, impurity profiles, and regulatory requirements, the reported terbinafine methodologies required adaptation to our specific experimental conditions. Consequently, there was a clear need to design and validate novel, optimized procedures tailored to the quantitative determination and impurity profiling of naftifine hydrochloride.
Recently, Shinde et al. (2025) reported an RP-HPLC method for the quantitative estimation of naftifine hydrochloride in formulated products [11]. While this study provides a useful approach for assay determination, it primarily focuses on the main active ingredient and does not provide a detailed analysis of related impurities or degradation products. This limitation further highlights the need for a tailored analytical procedure capable of comprehensive impurity profiling in both solution and cream dosage forms.
The aim of this study was to develop analytical methods for the quality control of naftifine hydrochloride in solution and cream dosage forms, with a focus on the parameters “Quantitative determination” and “Related substances”. The present study introduces a novel reversed-phase HPLC method intended for routine application in quality control laboratories. The proposed approach ensures faster, more accurate, and resource-efficient analysis, addressing the limitations of existing pharmacopoeial methods and meeting the practical requirements of industrial pharmaceutical testing.
2. Materials and Methods
2.1. Chemicals and Reagents
Naftifine hydrochloride solution and cream were purchased from a local pharmacy. Reference standards of the active pharmaceutical ingredient naftifine hydrochloride (molecular formula C21H21N·HCl, molecular weight 323.86 g/mol, purity ≥ 99.0% by HPLC, USP #cat. 1450404), as well as its specified impurities—N-Methyl-1-naphthalenemethylamine hydrochloride (molecular formula C10H7CH2NHCH3·HCl, molecular weight 207.70 g/mol, purity ≥ 97.5% by HPLC, Sigma #cat. 262315, Boston, MA, USA) and trans-cinnamaldehyde (molecular formula C6H5CH=CHCHO, molecular weight 132.16 g/mol, purity ≥ 99.0% by HPLC, Sigma #cat. 239968, Boston, MA, USA).
For the preparation of placebo solutions, the excipients used were: propylene glycol (Sigma, Boston, MA, USA), ethanol 96% (Ukrspirt, Chernihiv, Ukraine), and purified water. For placebo cream, the excipients were: isopropyl myristate (BASF, Ludwigshafen, Germany), cetostearyl alcohol (BASF, Ludwigshafen, Germany), cetyl palmitate (Croda Singapore, Singapore), sorbitan laurate (Seppic, Paris, France), polysorbate 60 (Seppic, Paris, France), benzyl alcohol (Merck, Darmstadt, Germany), sodium hydroxide (Merck, Darmstadt, Germany), and purified water. Placebo formulations were prepared by mixing the excipients in the same proportions as in the corresponding commercial products, without the active pharmaceutical ingredient, ensuring matrix composition identical to the test formulations.
All reagents labeled with “R” (e.g., Acetonitrile R, Na2HPO4 R) are of analytical grade and comply with the purity requirements specified in the European Pharmacopoeia. This notation indicates that the reagents are suitable for analytical and pharmaceutical purposes.
The chemicals used—ammonium acetate, glacial acetic acid, methanol, acetonitrile, sodium octanesulfonate, and phosphoric acid—were of gradient grade and purchased from Merck (Darmstadt, Germany). Demineralized water used for the analyses was produced in-house by Stilman and had a conductivity of less than 0.5 µS/cm.
The following HPLC columns were used: Gemini C18 50 mm × 4.6 mm, 3 µm (#cat. 00B-4439-E0), Luna C18 50 mm × 4.6 mm, 3 µm (#cat. 00B-4251-E0) and Synergi Hydro-RP 250 mm × 4.6 mm, 4 µm (#cat. 00G-4375-E0). The used columns were purchased from Phenomenex, 411 Madrid Avenue, Torrance, CA 90501-1430, USA. In this research, the Agilent 1200 Infinity with a diode array detector was used (Agilent Technologies, Inc., Santa Clara, CA, USA).
Chromatographic data acquisition and processing were performed using Agilent OpenLAB CDS software 2.8 (Agilent Technologies, Santa Clara, CA, USA). Peak integration and calculation of peak areas were carried out automatically, with manual review where necessary.
Statistical evaluation of validation parameters, including precision, accuracy, linearity, and robustness, was performed using Microsoft Excel 2024 (Microsoft Corp., USA). Mean values, standard deviations (SD), and relative standard deviations (RSD, %) were calculated for replicate measurements.
The following additional instrumental equipment was used: analytical balance Mettler Toledo XPE-205 (Mettler-Toledo International Inc., Greifensee, Switzerland), pH meter Mettler Toledo Seven Easy (Mettler-Toledo International Inc., Greifensee, Switzerland) and US bath Elmasonic P (Elma Schmidbauer GmbH, Singen, Germany).
The syringe filters used for sample preparation included regenerated nylon (NY) 0.2 µm (AF0-1207-52) and 0.45 µm (AF0-1107-52), as well as polytetrafluoroethylene (PTFE) 0.2 µm (AF0-1202-52) and 0.45 µm (AF0-1102-52) filters purchased from Phenomenex. Regenerated cellulose (RC) 0.45 µm syringe filters (CH4525) were obtained from Choice.
2.2. Sample Preparation and Chromatographic Conditions
2.2.1. Sample Preparation and Chromatographic Conditions for Determination of Impurities of Naftifine Hydrochloride in Solution and Cream
Buffer solution. 0.4 g of disodium hydrogen phosphate, anhydrous R and 2.34 g of sodium octanesulfonate R were dissolved in 1000 mL of water for chromatography R, and the pH was adjusted to 6.5 with phosphoric acid.
Mobile phase A. A mixture of buffer solution and acetonitrile R in a ratio of 80:20 (v/v).
Mobile phase B. A mixture of buffer solution and acetonitrile R in a ratio of 5:95 (v/v).
Diluent. Acetonitrile R.
Preparation of test solution (cream dosage form). An accurately weighed amount of cream equivalent to 400 µg/mL of naftifine hydrochloride was dissolved in the diluent. The mixture was sonicated in an ultrasonic bath for 15 min at 45 °C. After cooling to room temperature, the solution was diluted with the same diluent to the final volume, mixed thoroughly, and filtered through a regenerated nylon (NY) syringe filter with a pore size of 0.2 µm, discarding the first 5 mL of filtrate.
Preparation of test solution (solution dosage form). A solution containing 400 µg/mL of naftifine hydrochloride in the diluent was prepared.
Preparation of reference solution. A solution was prepared containing 2 µg/mL of the reference standards: naftifine hydrochloride, N-Methyl-1-naphthalenemethylamine hydrochloride, and trans-cinnamaldehyde, in the diluent.
Chromatographic conditions. Chromatography was carried out on a liquid chromatograph equipped with a spectrophotometric detector under the following conditions: a 250 mm × 4.6 mm octadecylsilyl column, 4 µm (Synergi Hydro RP); a flow rate of 1.0 mL/min; detection at 270 nm; a column temperature: 40 °C; injection volume: 15 μL and elution mode: gradient elution was performed according to the program (Table 1):

Table 1.
Gradient mode for the HPLC method for analysis of impurities in solution and cream.
The system suitability for the reference solution:
Relative standard deviation (RSD) of trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and naftifine hydrochloride peak areas from three replicate injections of reference solution: not more than 2.9%;
Resolution factor: not less than 5.0 between the peaks corresponding to N-Methyl-1-naphthalenemethylamine and trans-cinnamaldehyde;
Limits for impurities:
N-Methyl-1-naphthalenemethylamine hydrochloride: not more than 0.5%.
Trans-cinnamaldehyde: not more than 0.5%.
Unspecified impurity: not more than 0.5%.
Total impurities: not more than 1.0%.
2.2.2. Sample Preparation and Chromatographic Conditions for Quantitative Determination of Naftifine Hydrochloride in Solution and Cream
Mobile phase. A mixture of ammonium acetate solution and methanol R in a ratio of 30:70 (v/v). The ammonium acetate solution was prepared by dissolving 1.154 g of ammonium acetate R in 300 mL of water for chromatography R, followed by the addition of 0.2 mL of glacial acetic acid R and thorough mixing.
Diluent. Methanol R.
Preparation of test solution (cream dosage form). An accurately weighed amount of the cream equivalent to 80 µg/mL of naftifine hydrochloride was transferred into a volumetric flask and dissolved in the diluent. The mixture was sonicated in an ultrasonic bath for approximately 5 min at 45 °C until complete dissolution of the cream base. After cooling to room temperature, the solution was diluted to volume with the same diluent and mixed thoroughly. The resulting solution was filtered through a 0.45 µm membrane filter, discarding the first 5 mL of the filtrate prior to injection.
Preparation of test solution (solution dosage form). A solution containing 80 µg/mL of naftifine hydrochloride in the diluent was prepared.
Preparation of reference solution. A solution containing 80 µg/mL of the reference standard naftifine hydrochloride in the diluent was prepared.
Chromatographic conditions. Chromatography was performed using a liquid chromatograph equipped with a spectrophotometric detector under the following conditions: a 50 mm × 4.6 mm octadecylsilyl column, 3 µm (Gemini C18 or Luna C18); flow rate: 1.0 mL/min; detection at 254 nm; column temperature: 25 °C; injection volume: 10 μL; and isocratic elution.
The system suitability for the reference solution:
Symmetry factor: from 0.8 to 1.8 for the peak corresponding to naftifine hydrochloride;
Relative standard deviation (RSD): not more than 1.0% for the peak corresponding to naftifine hydrochloride, based on three replicate injections;
Theoretical plates: at least 2000 for the peak corresponding to naftifine hydrochloride.
The content of naftifine hydrochloride should be between 9.5 and 10.5 mg/mL in solution, and between 9.5 and 10.5 mg/g in cream.
2.3. Validation of HPLC Method
All validation activities were performed in accordance with the requirements of the ICH Validation of Analytical Procedures: Text and Methodology, Q2 (R2) [12].
2.3.1. Validation of HPLC Method for the Determination of Impurities of Naftifine Hydrochloride in Solution and Cream
To evaluate specificity, it was verified that there were no peaks of naftifine hydrochloride and its specified impurities with the peaks of the diluent and other components of the drug product.
To assess linearity, eight solutions of the analytes were prepared in the diluent, covering the following concentration range: 0.19–2.80 µg/mL for trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and naftifine hydrochloride, which corresponds to an impurity content of 0.05–0.7%.
For the accuracy study, nine model solutions were prepared at three concentration levels (three replicates per level). Each contained a known amount of added impurities to the test solution. Additionally, three parallel test solutions were prepared at the same levels without the addition of impurities. The solutions were prepared separately for each of the two dosage forms.
Precision was assessed at the levels of repeatability and intermediate precision. Under repeatability conditions, six replicate test solutions were prepared for each of the two dosage forms. Under intermediate precision conditions, twelve replicate test solutions were prepared for each of the dosage forms.
Additionally, robustness of the impurity method was evaluated by introducing small deliberate variations in chromatographic conditions, including column temperature (±5 °C), flow rate (±0.1 mL/min), mobile phase composition, and the use of columns from different batch/serial numbers. The method demonstrated consistent performance under these variations, confirming its suitability for routine quality control.
2.3.2. Validation of HPLC Method for the Quantitative Determination of Naftifine Hydrochloride in Solution and Cream
To evaluate specificity, it was confirmed that there was no interference between the peaks of naftifine hydrochloride and those of the solvent mixture or other components of the drug.
Linearity was assessed using seven solutions in the concentration range of 57 µg/mL to 107 µg/mL.
The accuracy of the method was evaluated using nine model solutions containing placebo and the target analyte within the following concentration ranges: for solution: 64 µg/mL to 95 µg/mL (80–120%), for cream: 72 µg/mL to 97 µg/mL (90–121%).
Precision was assessed at the levels of repeatability and intermediate precision. Under repeatability conditions, six replicate test solutions were prepared for each of the two dosage forms. Under intermediate precision conditions, twelve replicate test solutions were prepared for each of the dosage forms.
Robustness of the quantitative method was similarly assessed by varying the column temperature (±5 °C), flow rate (±0.1 mL/min), mobile phase composition, and using columns from different batch/serial numbers. The method maintained acceptable accuracy and precision under these conditions, demonstrating reliability for routine analysis.
3. Results
3.1. HPLC Method Development
The first step in developing methods for the quality control of Naftifine solution and cream is the creation of an HPLC method suitable for routine use in quality control laboratories, in accordance with the requirements for “Quantitative Determination” and “Impurities”.
3.1.1. HPLC Method Development for the Determination of Impurities in Solution and Cream
A gradient RP-HPLC method was developed to improve the separation of structurally similar naftifine-related impurities. During method development, conditions initially optimized for assay determination were evaluated for impurity analysis; however, key impurities (N-Methyl-1-naphthalenemethylamine hydrochloride and trans-cinnamaldehyde) showed insufficient retention and co-eluted with placebo components near the void volume (k′ ≈ 0). Subsequent optimization of gradient conditions and organic solvent content did not provide adequate selectivity, necessitating further modification of chromatographic parameters to achieve satisfactory impurity resolution.
To improve retention and resolution, chromatographic columns with lengths of 50 mm, 150 mm, and 250 mm were systematically evaluated during column screening in an attempt to delay the elution of the interfering peaks. Columns with a length of 300 mm were not investigated, as satisfactory separation of the target impurities was already achieved using a 250 mm column. Moreover, extending the column length beyond 250 mm was expected to significantly increase analysis time, which would contradict the objective of developing a rapid and practical method suitable for routine quality control. Nevertheless, the use of a 250 mm column alone did not result in effective separation from placebo components. Subsequently, more potent ion-pairing reagents—such as sodium octanesulfonate (10 mM and 20 mM) and sodium dodecyl sulfate—were evaluated, in combination with acetonitrile as the organic modifier to maintain diluent compatibility and avoid adverse effects during chromatographic separation. Although stronger ion-pairing reagents enhanced retention, they did not achieve baseline separation nor sufficient selectivity between the impurities and interfering peaks.
During column screening with different stationary phases, it was observed that the peak of N-Methyl-1-naphthalenemethylamine hydrochloride frequently exhibited poor chromatographic behavior, including peak splitting or unacceptable asymmetry (tailing factor > 2.5). This behavior is likely related to the basic nature of the analyte and its strong secondary interactions with residual silanol groups on the stationary phase.
Given these limitations, the focus shifted toward pH modulation of the mobile phase. Sodium octanesulfonate was selected over sodium dodecyl sulfate due to its lower foaming tendency and better handling properties. The initial pH of a 10 mM sodium octanesulfonate solution was approximately 4.5. To increase pH and shift analyte elution, disodium hydrogen phosphate was added at concentrations of 0.2, 0.4, and 0.6 g/L, with final pH adjustments made to 7.0, 6.5, 6.0, 5.5, and 5.0 using phosphoric acid.
Although the Synergi Hydro-RP column does not provide an ideal peak shape for N-Methyl-1-naphthalenemethylamine hydrochloride, it demonstrated the best overall performance among the tested columns. Under optimized conditions and during robustness studies, peak symmetry for this impurity ranged from 1.4 to 1.8, which meets method acceptance criteria and ensures adequate accuracy and precision. Therefore, this column was selected as the most suitable compromise between chromatographic performance, robustness, and practical applicability at the current stage of method development.
The optimal chromatographic performance—defined by resolution, peak symmetry, and efficiency—was achieved using a Synergi Hydro-RP column (250 × 4.6 mm, 4 µm), which provided suitable retention for polar impurities while ensuring compatibility with aqueous mobile phases.
The results of the pH optimization study (Table 2) demonstrated that a buffer pH of 6.5 provided the best compromise between separation, symmetry, and efficiency. At this pH, satisfactory resolution was observed between naftifine hydrochloride and both impurities, while minimizing co-elution with placebo constituents. Therefore, pH 6.5 was selected for the final method.

Table 2.
Effect of buffer pH on separation, peak symmetry, efficiency, and retention times.
An important consideration during method development was the need to unify the analytical procedure for both solution and cream dosage forms of naftifine hydrochloride. Therefore, diluent selection was guided not only by chromatographic compatibility but also by the ability to effectively dissolve the cream matrix. Between the two diluents tested—acetonitrile and methanol—methanol initially demonstrated superior solubilizing capacity for the cream base. However, during accuracy evaluation, the test solution prepared in methanol exhibited chemical instability. Specifically, significant degradation of trans-cinnamaldehyde was observed, with a decrease of approximately 5% after the first injection and an additional 1–3% loss upon subsequent injections. Moreover, a new unidentified degradation product emerged at a retention time of approximately 13 min. In contrast, acetonitrile provided a stable environment for cinnamaldehyde, making it the preferred diluent for the final method.
Various ratios of buffer solution and organic component in mobile phases A and B were evaluated as part of the ongoing method development to improve impurity separation. In parallel, attempts were made to shorten the chromatographic run time by modifying the gradient program. The final composition of the mobile phases, which ensured optimal chromatographic performance on two columns from different batches, was selected based on experimental results and is presented in Table 1.
The representative chromatogram of the reference solution obtained under the optimized conditions is shown in Figure 1, where the observed peaks appear in the following order: first, trans-cinnamaldehyde; second, N-Methyl-1-naphthalenemethylamine hydrochloride; and finally, the main peak of naftifine hydrochloride. This sequence confirms the identity and purity of the standards and the ability of the HPLC method to separate the active ingredient from its specified impurities. In contrast, the chromatogram of the test solution is presented in Figure 2, which includes the main peak of naftifine hydrochloride, peaks of its impurities in the same order, and additional placebo peaks arising from the cream matrix. These observations illustrate the ability of the developed method to distinguish between the active ingredient, its impurities, and the excipient peaks in the test samples.
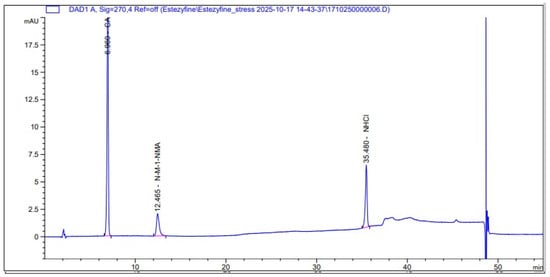
Figure 1.
Chromatogram of reference solution obtained using gradient elution with phosphate-sulfonate buffer (pH 6.5) and acetonitrile, at a flow rate of 1.0 mL/min and column temperature of 40 °C, on a Synergi Hydro RP column (250 × 4.6 mm, 4 µm), used for the determination of related impurities in naftifine hydrochloride.
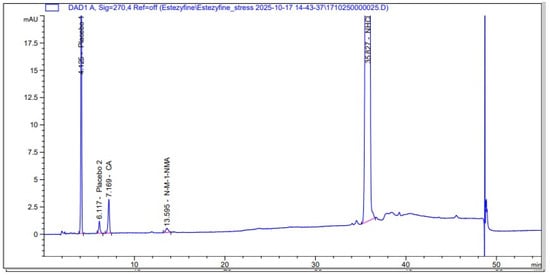
Figure 2.
Chromatogram of test solution obtained using gradient elution with phosphate-sulfonate buffer (pH 6.5) and acetonitrile, at a flow rate of 1.0 mL/min and column temperature of 40 °C, on a Synergi Hydro RP column (250 × 4.6 mm, 4 µm), used for the determination of related impurities in naftifine hydrochloride.
3.1.2. HPLC Method Development for the Quantitative Determination of Naftifine Hydrochloride in Solution and Cream
A reliable and environmentally friendly HPLC method was successfully developed for the determination of naftifine hydrochloride in pharmaceutical dosage forms. The method utilized an isocratic elution system with mobile phase (a solution of ammonium acetate: dissolve 1.154 g of ammonium acetate R in 300 mL of water R, add 0.2 mL of glacial acetic acid R, and mix thoroughly)—methanol (30:70). The following diluents were tested:
- Methanol
- Mobile phase (a solution of ammonium acetate: dissolve 1.154 g of ammonium acetate R in 300 mL of water for chromatography R, add 0.2 mL of glacial acetic acid R, and mix thoroughly)—methanol (30:70)
The results of the study are presented in Table 3.

Table 3.
Comparison of methanol and mobile phase as diluents.
Methanol was selected as the diluent because:
- It provides better peak symmetry.
- The chromatographic column efficiency exceeds 2000 theoretical plates.
- Methanol offers visibly better solubility of the cream base, which is advantageous for unifying the methodology for both products.
During sample preparation of the solution form, no precipitate was observed. However, for Naftifine, 1% cream, the test solution required filtration to remove the cream base, which should not be present in the analytical sample. To ensure the reliability and accuracy of the results, various types of filters were tested. For each filter type, the first 5 mL of the filtrate was discarded.
The results of the study are presented in Table 4.

Table 4.
Evaluation of different filters for the sample preparation of the cream.
The results of the study indicate a good recovery rate and effective filtration across all tested filter types. This demonstrates that each of the evaluated filters is capable of providing the necessary level of sample purification.
As the literature [4] specifies only the type of chromatographic column (packed with octadecylsilyl silica gel, C18), various chromatographic columns from different manufacturers and with different dimensions were tested experimentally. The results are presented in Table 5.

Table 5.
Comparison of various C18 columns to select the optimal one.
Based on the obtained results, the Gemini C18 (50 × 4.6 mm, 3 µm) and Luna C18 (50 × 4.6 mm, 3 µm) columns were selected, as they demonstrated the highest number of theoretical plates for the naftifine hydrochloride peak and provided the best peak symmetry.
The retention times for naftifine hydrochloride were 4.8 min. An injection volume of 10 μL was used, and the optimal detection wavelength of 254 nm provided the highest sensitivity with no interference from the diluent peak. The flow rate of 1.0 mL/min was confirmed to deliver efficient separation within a reasonable runtime, ensuring the method’s practicality for routine analysis (Figure 3).
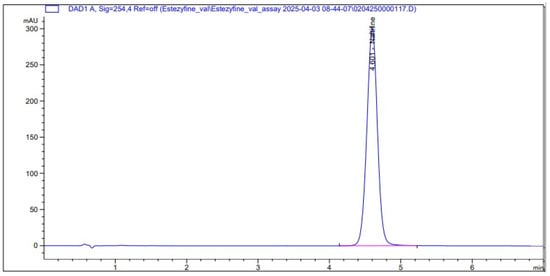
Figure 3.
Chromatogram obtained using mobile phase consisting of a solution of ammonium acetate: dissolve 1.154 g of ammonium acetate R in 300 mL of water for chromatography R, add 0.2 mL of glacial acetic acid R—methanol (30:70), at a flow rate of 1.0 mL/min and temperature 25 °C, used for the analysis on an Luna C18 (50 × 4.6 mm, 3 µm) column.
3.2. Method Validation
The procedure was validated in compliance with the standards in accordance with the International Conference on Harmonization (ICH) [12].
3.2.1. Results of Validation of HPLC Method for the Determination of Impurities in Solution and Cream
To confirm the efficiency of the method, the following parameters were studied: specificity; linearity in the range of application; accuracy; precision; and limit of quantification (LOQ) [12]. The formulas that were used for the calculations are given in Appendix A. The summary of the validation results is given in Appendix B, Table A1 and Appendix C, Table A2.
The separation of impurities and other components in the spiking sample was appropriate. No interference was obtained among the trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and naftifine hydrochloride peaks and the matrix peaks.
The linear regression parameters were calculated in accordance with the recommendations of the ICH Validation of Analytical Procedures: Text and Methodology, Q2 (R2) [12]. Excellent linearity of the method for the analysis of impurities of trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and naftifine hydrochloride was confirmed by the obtained correlation coefficient (Table 6, Appendix B, Table A1, Appendix C, Table A2).

Table 6.
Linear regression parameters.
The parameters accuracy and precision were evaluated using the spiking method for trans-cinnamaldehyde and N-Methyl-1-naphthalenemethylamine hydrochloride with the test solution, with 12 replicates analyzed for the solution and 12 replicates for the cream. Recovery and RSD were assessed for six replicates analyzed by Analyst 1 and six replicates analyzed by Analyst 2, for both the solution and the cream, including the differences in results between the two analysts. The results are presented in Table 7 and Table 8.

Table 7.
Calculations for the parameter “accuracy”.

Table 8.
Calculations for the parameter “precision”.
The limit of quantitation (LOQ) of the method for the determination of trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and each unspecified impurity was established at 0.10%, meeting the acceptability criterion of LOQ ≤ 0.1%.
To calculate the content of trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and each unspecified impurity, the reference standards of trans-cinnamaldehyde, N-Methyl-1-naphthalenemethylamine hydrochloride, and naftifine hydrochloride were used.
3.2.2. Results of Validation of HPLC Method for the Quantitative Determination of Naftifine Hydrochloride in Solution and Cream
To assess the performance of the developed HPLC method, key validation parameters were evaluated, including specificity, linearity within the application range, accuracy, and precision, in accordance with ICH guidelines Q2(R2) “Validation of Analytical Procedures: Text and Methodology” [12]. The formulas used for calculations are presented in Appendix A, and the detailed validation results are provided in Appendix C, Table A2.
Linear regression parameters were calculated over the working concentration range of naftifine hydrochloride. The method demonstrated excellent linearity, with a correlation coefficient (r) of 0.99954, indicating a nearly perfect linear relationship (Table 9, Appendix D, Table A3, Appendix E, Table A4).

Table 9.
Linear regression parameters for the calibration curve.
The accuracy of the method was confirmed by recovery studies, which showed individual recovery values between 99.6% and 101.3% for the solution and between 99.5% and 100.8% for the cream. Mean recovery values were 100.2% in both cases, fully meeting the acceptance criteria (Table 10).

Table 10.
Accuracy of naftifine hydrochloride in solution.
Precision was assessed by repeatability and intermediate precision studies involving two analysts. For both dosage forms, the relative standard deviation (RSD) values were below the acceptance thresholds (≤1.0% for 6 parallels and ≤1.5% for 12 parallels). The difference between analysts’ results did not exceed 2.0%, confirming reproducibility of the method (Table 11).

Table 11.
Precision of naftifine hydrochloride in solution.
3.3. Streamlined HPLC Method for Naftifine Hydrochloride: Comparison with USP
To address the limitations of the official USP method for the quantitative determination of naftifine hydrochloride, a novel HPLC procedure was developed. The compendial method requires extensive equilibration times, including at least 12 h for mobile phase stabilization and approximately 4 h for column saturation, making it time-consuming and resource-intensive. In contrast, the newly developed method significantly reduces the overall analysis time, with the main peak eluting at approximately 4 min. This streamlined approach enhances laboratory efficiency while maintaining high analytical performance. A comparative overview of key procedural parameters is presented in Table 12.

Table 12.
Comparison between the Proposed HPLC Method and the USP Method for Naftifine Hydrochloride Determination.
Compared to the USP method, the proposed HPLC method significantly reduces both the analysis time and solvent consumption. The mobile phase does not require prolonged stabilization (12 h in the USP method), and the column can be equilibrated in less than 30 min instead of the 4 h recommended by USP. The total chromatographic run time is shortened to less than 7 min, greatly improving throughput and making the method ideal for routine quality control laboratories with high sample volumes.
3.4. Greenness Profile of the Developed Methods
To assess the environmental impact of the proposed methods, the greenness metrics were applied [13,14,15]. For the HPLC method developed for the analysis of naftifine hydrochloride in solution and cream, the total MOGAPI scores reached 82, and for determining impurities in solution and cream, they reached 80). In addition, AGREE greenness metrics were applied and the AGREE score was 0.79. The obtained values indicate an excellent green analysis, which is a great advantage of the technique. Analyzing Table 13, we can notice that the score MOGAPI was 82. The highest score is 82 in our developed method and there were no red operations. In contrast, the USP method showed red operations indicating lower environmental friendliness.

Table 13.
Comparison of the greenness profiles between the proposed and reported methods for determining naftifine hydrochloride in solution and cream.
4. Discussion
During the development of our methods, we prioritized on optimization of analytical conditions to ensure selectivity, simplicity, and efficiency—key requirements for routine pharmaceutical quality control. Special attention was paid to the selection of chromatographic parameters, including the choice of column, mobile phase composition and instrumental settings, in order to obtain reproducible results while minimizing analysis time [16,17].
For the quantitative determination of naftifine hydrochloride, the Gemini C18 and Luna C18 columns (50 mm × 4.6 mm, 3 µm) were selected due to their optimal balance between analytical efficiency and speed. This column provided favorable peak symmetry and significantly reduced run time, making it suitable for fast and reliable analysis. The mobile phase consisted of a mixture 1.154 g of ammonium acetate R in 300 mL of water R, add 0.2 mL of glacial acetic acid R (thoroughly mixed) and methanol in a 30:70 ratio. This simplified approach to method optimization reduced the need for additional solvents, aligning with current trends in sustainable and cost-effective analytical chemistry.
During method development, various alternative chromatographic conditions were systematically tested. Columns of different lengths (50, 150, 250 mm) and multiple batch/serial numbers were evaluated, while longer columns (300 mm) were not tested as the 250 mm column already provided sufficient separation and longer columns would have increased analysis time. Several mobile phase compositions and gradient programs were explored, and in some cases, impurity peaks, particularly N-Methyl-1-naphthalenemethylamine, exhibited poor symmetry or co-elution with placebo peaks. The Synergi Hydro-RP column, while not providing ideal peak symmetry for all impurities, offered the best compromise between resolution, reproducibility, and robustness under small variations in flow rate, temperature, and mobile phase composition.
For the determination of impurities, the HPLC employed a Synergi Hydro-RP column (250 mm × 4.6 mm, 4 µm) and a mobile phase composed of a buffer and acetonitrile mixtures. The buffer was prepared by dissolving 0.4 g of disodium hydrogen phosphate, anhydrous R and 2.34 g of sodium octanesulfonate R in 1000 mL of water for chromatography R, with pH adjusted to 6.5 using phosphoric acid. Mobile phase A consisted of buffer–acetonitrile R in an 80:20 (v/v) ratio and mobile phase B in a 5:95 (v/v) ratio. Although this method is not rapid, it enables accurate detection of all impurities—unlike the USP method—and uses conventional reversed-phase HPLC instead of UPLC, which is not available at all pharmaceutical manufacturing sites.
The developed methods allow for the determination of impurities within 55 min and quantitative determination of active ingredient in just 6 min, with a total analysis time compared to the official USP method.
Method validation was performed according to ICH guidelines, covering parameters such as accuracy, precision, linearity, specificity, robustness, and system suitability. The methods demonstrated high robustness and consistent performance under minor variations in experimental conditions, confirming their reliability for routine use. All acceptance criteria were met for each validation parameter, ensuring the suitability of methods for pharmaceutical quality control.
The novelty of the developed methods lies in their ability to unify the analysis of both solution and cream dosage forms, simultaneously quantifying the active ingredient and its impurities with high specificity and reproducibility. Compared to previously reported methods, the developed procedures provide faster analysis for the assay, comprehensive impurity profiling, and the use of widely available HPLC equipment, making them accessible for routine pharmaceutical laboratories.
Furthermore, chromatograms of the cream and solution dosage forms were nearly identical due to the unified method, confirming its applicability for both pharmaceutical forms without additional adjustments. The robustness of the method was verified by intentionally varying column temperature (±5 °C), flow rate (±0.1 mL/min), and mobile phase composition, as well as by using columns from different serial numbers, which demonstrated consistent retention times, peak shapes, and resolution for all impurities. Compared with previously reported methods, including USP and methods described in the Chinese pharmacopeia, the present approach offers improved throughput, comprehensive impurity detection, and reduced solvent consumption, highlighting both its practical and environmental advantages.
The implementation of these optimized methods provides several advantages: enhanced analytical throughput, reduced resource consumption, and improved environmental sustainability. By minimizing analysis time and simplifying procedures, the methods are well-suited for routine use in high-throughput manufacturing environments.
In addition to their analytical performance, these methods support green chemistry principles. The reduced consumption of solvents and reagents contributes to lower environmental impact, making these methods not only efficient but also environmentally responsible.
Full procedural details for both the impurity profiling and quantitative determination methods are provided in Appendix A and Appendix B. These methods are adaptable and may be applied to a wide range of pharmaceutical products with similar compositions.
5. Conclusions
Validated and robust HPLC methods were successfully developed for the quantitative determination of naftifine hydrochloride and the identification of its related impurities in pharmaceutical formulations (solution and cream). These methods fully comply with ICH guidelines, demonstrating high specificity, accuracy, linearity, precision, and reproducibility across the studied range.
The developed methods allow fast and reliable analysis, with assay completion in 6 min and impurity profiling within 55 min, significantly improving throughput compared to the USP method.
These methods also support green chemistry principles, with reduced solvent and reagent consumption and excellent ecological profiles (MOGAPI scores 80–82, AGREE score 0.79), highlighting their environmental sustainability.
In summary, the developed HPLC methods provide a fast, reliable, and environmentally responsible solution for the quality control of naftifine hydrochloride in cream and solution, and are adaptable for similar pharmaceutical products.
Author Contributions
Conceptualization, Y.K., O.H. and L.L.; methodology, Y.K., O.H. and L.L.; investigation, O.H.; resources, Y.K., O.H. and L.L.; data curation, Y.K., O.H., K.T. and L.L.; writing—original draft preparation, O.H. and L.L.; writing—review and editing, Y.K., O.H. and L.L.; investigation, Y.K., O.H. and L.L.; supervision, Y.K. and L.L.; project administration, Y.K., O.H. and L.L.; funding acquisition, Y.K., O.H. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from JSC Farmak, (63 Kyrylivska Street, 04080 Kyiv, Ukraine).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that this study received funding from JSC Farmak. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Appendix A
Formulas for calculations
The difference between the retention times for the peaks of naftifine hydrochloride, N-Methyl-1-naphthalenemethylamine hydrochloride, and trans-cinnamaldehyde on the chromatograms of the reference solution and the tested solution was calculated using the following formula:
where
is the retention time of naftifine hydrochloride, N-methyl-1-naphthylmethylamine hydrochloride, and trans-cinnamaldehyde on the chromatogram of the reference solution;
is the retention time of naftifine hydrochloride, N-methyl-1-naphthylmethylamine hydrochloride, and trans-cinnamaldehyde on the chromatogram of the test solution.
To verify the accuracy of solution preparation, a reference solution and a control solution were prepared. Response factors for each solution were calculated using the following formula:
where
is the concentration of the analyte in the respective solution, µg/mL;
is the mean peak area of the analyte.
The relative response factor was calculated using the formula:
Concentration values in normalized coordinates were calculated using the following formula:
The value of the response of the device in normalized coordinates was calculated according to the following formula:
The “found/put” ratio was calculated using the following formula:
Coefficient b was calculated using the following formula:
where
m is the number of model solutions.
Coefficient a was calculated using the following formula:
Correlation coefficient r was calculated using the following formula:
The concentration of N-methyl-1-naphthylmethylamine hydrochloride and trans-cinnamaldehyde in the tested solutions with additives was calculated using the following formula (“found”):
where
C01 is the concentration of N-methyl-1-naphthylmethylamine hydrochloride and trans-cinnamaldehyde in the reference solution;
is the peak area of N-methyl-1-naphthylmethylamine hydrochloride and trans-cinnamaldehyde in the test solution;
S01 is the peak area of N-methyl-1-naphthylmethylamine hydrochloride and trans-cinnamaldehyde in the reference solution.
The “found”/“put” ratio (in percent) was calculated using the following formula:
The limit of quantification (LOQ) were calculated using the following formula:
where
is the standard deviation of the response;
is the slope of the calibration curve.
Appendix B

Table A1.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Solution” according to the indicator “Impurities”.
Table A1.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Solution” according to the indicator “Impurities”.
| Investigation | Parameter | Eligibility Criteria | Evaluation | ||
|---|---|---|---|---|---|
| Specificity | Peak areas on the chromatogram of the blank/placebo solution that may interfere with the NHCl peak or its specified impurities | ≤0.01% | CA | Not detected | |
| MNHCl | Not detected | ||||
| NHCl | Not detected | ||||
| System suitability | Resolution factor between CA and MNHCl peaks calculated from the chromatograms of the reference solution | ≥5.0 | ≥12.0 | ||
| RSD of CA, MNHCl, and NHCl peaks area from three replicate injections of reference solution | ≤2.9% | CA | 0.00% | ||
| MNHCl | 1.67% | ||||
| NHCl | 0.21% | ||||
| Tailing factor of the for the CA, MNHCl, and NHCl peaks | 0.8–1.8 | CA | ≤1.1 | ||
| MNHCl | ≤1.7 | ||||
| NHCl | ≤1.1 | ||||
| Signal-to-noise ratio for CA, MNHCl, and NHCl peaks from the chromatograms of the reference solution | ≥50 | CA | ≤668.7 | ||
| MNHCl | ≤63.0 | ||||
| NHCl | ≤169.4 | ||||
| Linearity (Range) | Verified concentration range of calibration model | 0.10–0.60% | CA | 0.05–0.70% | |
| MNHCl | 0.05–0.72% | ||||
| NHCl | 0.05–0.70% | ||||
| Correlation coefficient | ≥0.990 | CA | 0.9997 | ||
| MNHCl | 0.9987 | ||||
| NHCl | 0.9988 | ||||
| Absolute value of intercept as a percentage of nominal concentration | ≤10.0% | CA | 0.53% | ||
| MNHCl | 2.52% | ||||
| NHCl | 3.31% | ||||
| Limit of Quantitation | Calculated limit of quantitation | ≤0.10% | CA | 0.05% | |
| MNHCl | 0.10% | ||||
| NHCl | 0.05% | ||||
| Individual recovery values | ≤0.10% | 70.0–130.0% | CA | 99.15% | |
| MNHCl | 114.41% | ||||
| NHCl | 82.58% | ||||
| 0.10% | 75.0–125.0% | CA | 99.18% | ||
| MNHCl | 98.22% | ||||
| NHCl | 95.46% | ||||
| RSD between parallel injections | ≤0.10% | ≤20.0% | CA | 0.23% | |
| MNHCl | 12.44% | ||||
| NHCl | 3.56% | ||||
| 0.10% | ≤18.0% | CA | 0.19% | ||
| MNHCl | 2.26% | ||||
| NHCl | 3.28% | ||||
| Signal-to-noise ratio | ≤0.10% 0.10% | ≥10 ≥10 | CA | ≥234.6 | |
| MNHCl | ≥20.5 | ||||
| NHCl | ≥63.9 | ||||
| Accuracy | Individual recovery values | ≤0.25% | 75.0–125.0% | CA | 98.01–100.15% |
| MNHCl | 92.80–102.56% | ||||
| >0.25% | 92.5–107.5% | CA | 97.06–101.47% | ||
| MNHCl | 92.70–99.84% | ||||
| Mean recovery value | ≤0.25% | 82.0–118.0% | CA | 99.05% | |
| MNHCl | 98.85% | ||||
| >0.25% | 95.0–105.0% | CA | 100.08% | ||
| MNHCl | 96.42% | ||||
| Precision | RSD for 6 parallel determinations for analyst A | >0.25% | ≤7.5% | CA | 0.49% |
| MNHCl | 1.32% | ||||
| RSD for 6 parallel determinations for analyst B | >0.25% | ≤7.5% | CA | 1.55% | |
| MNHCl | 2.80% | ||||
| RSD for 12 parallel determinations | >0.25% | ≤11.0% | CA | 3.28% | |
| MNHCl | 3.66% | ||||
| Difference between mean results of two analysts | >0.25% | ≤7.5% | CA | 4.17% | |
| MNHCl | 6.32% | ||||
| Solution Stability | Change in peak area after 24 h at room temperature | >0.25% | 95.0–105.0% | CA | 100.25% |
| MNHCl | 99.15% | ||||
| Robustness | Change in peak area ratio (test/reference) under altered and nominal conditions | >0.25% | 95.0–105.0% | CA | 98.97–100.47% |
| MNHCl | 98.40–103.32% | ||||
Appendix C

Table A2.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Cream” according to the indicator “Impurities”.
Table A2.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Cream” according to the indicator “Impurities”.
| Investigation | Parameter | Eligibility Criteria | Evaluation | ||
|---|---|---|---|---|---|
| Specificity | Peak areas on the chromatogram of the blank/placebo solution that may interfere with the NHCl peak or its specified impurities | ≤0.01% | CA | Not detected | |
| MNHCl | Not detected | ||||
| NHCl | Not detected | ||||
| System suitability | Resolution factor between CA and MNHCl peaks calculated from the chromatograms of the reference solution | ≥5.0 | ≥12.0 | ||
| RSD of CA, MNHCl, and NHCl peaks area from three replicate injections of reference solution | ≤2.9% | CA | 0.00% | ||
| MNHCl | 1.67% | ||||
| NHCl | 0.21% | ||||
| Tailing factor of the for the CA, MNHCl, and NHCl peaks | 0.8–1.8 | CA | ≤1.1 | ||
| MNHCl | ≤1.7 | ||||
| NHCl | ≤1.1 | ||||
| Signal-to-noise ratio for CA, MNHCl, and NHCl peaks from the chromatograms of the reference solution | ≥50 | CA | ≤668.7 | ||
| MNHCl | ≤63.0 | ||||
| NHCl | ≤169.4 | ||||
| Linearity (Range) | Verified concentration range of calibration model | 0.10–0.60% | CA | 0.05–0.70% | |
| MNHCl | 0.05–0.72% | ||||
| NHCl | 0.05–0.70% | ||||
| Correlation coefficient | ≥0.990 | CA | 0.9997 | ||
| MNHCl | 0.9987 | ||||
| NHCl | 0.9988 | ||||
| Absolute value of intercept as a percentage of nominal concentration | ≤10.0% | CA | 0.53% | ||
| MNHCl | 2.52% | ||||
| NHCl | 3.31% | ||||
| Limit of Quantitation | Calculated limit of quantitation | ≤0.10% | CA | 0.05% | |
| MNHCl | 0.10% | ||||
| NHCl | 0.05% | ||||
| Individual recovery values | ≤0.10% | 70.0–130.0% | CA | 99.15% | |
| MNHCl | 114.41% | ||||
| NHCl | 82.58% | ||||
| 0.10% | 75.0–125.0% | CA | 99.18% | ||
| MNHCl | 98.22% | ||||
| NHCl | 95.46% | ||||
| RSD between parallel injections | ≤0.10% | ≤20.0% | CA | 0.23% | |
| MNHCl | 12.44% | ||||
| NHCl | 3.56% | ||||
| 0.10% | ≤18.0% | CA | 0.19% | ||
| MNHCl | 2.26% | ||||
| NHCl | 3.28% | ||||
| Signal-to-noise ratio | ≤0.10% 0.10% | ≥10 ≥10 | CA | ≥234.6 | |
| MNHCl | ≥20.5 | ||||
| NHCl | ≥63.9 | ||||
| Accuracy | Individual recovery values | ≤0.25% | 75.0–125.0% | CA | 96.78–98.00% |
| MNHCl | 97.46–98.74% | ||||
| >0.25% | 92.5–107.5% | CA | 96.63–99.94% | ||
| MNHCl | 99.43–100.25% | ||||
| Mean recovery value | ≤0.25% | 82.0–118.0% | CA | 97.51% | |
| MNHCl | 98.12% | ||||
| >0.25% | 95.0–105.0% | CA | 98.27% | ||
| MNHCl | 99.92% | ||||
| Precision | RSD for 6 parallel determinations for analyst A | ≤0.25% | ≤25.0% | CA | 1.44% |
| MNHCl | 0.96% | ||||
| RSD for 6 parallel determinations for analyst B | ≤0.25% | ≤25.0% | CA | 1.08% | |
| MNHCl | 5.3% | ||||
| RSD for 12 parallel determinations | ≤0.25% | ≤35.0% | CA | 2.83% | |
| MNHCl | 13.12% | ||||
| Difference between mean results of two analysts | ≤0.25% | ≤25.0% | CA | 0.05% | |
| MNHCl | 16.37% | ||||
| Solution Stability | Change in peak area after 24 h at room temperature | >0.25% | 95.0–105.0% | CA | 100.28% |
| MNHCl | 99.56% | ||||
| Robustness | Change in peak area ratio (test/reference) under altered and nominal conditions | >0.25% | 95.0–105.0% | CA | 99.43–101.31% |
| MNHCl | 98.20–102.68% | ||||
Appendix D

Table A3.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Solution” according to the indicator “Naftifine Hydrochloride”.
Table A3.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Solution” according to the indicator “Naftifine Hydrochloride”.
| Investigation | Parameter | Eligibility Criteria | Evaluation |
|---|---|---|---|
| Specificity | Peak area on blank/placebo chromatograms potentially interfering with naftifine peak | ≤0.2% | Not detected |
| Difference in retention times of naftifine peak in test and reference chromatograms | ≤2% | ≤0.02% | |
| System suitability | RSD of naftifine peak area from three replicate injections of reference solution | ≤1.0% | ≤0.3% |
| Tailing factor of the naftifine peak | 0.8–1.8 | 1.0–1.1 | |
| Column efficiency (theoretical plates) for the naftifine peak | ≥2000 | ≥4731 | |
| Linearity (Range) | Verified concentration range of calibration model | 7.6–12.6 mg/mL | 7.0–13.3 mg/mL |
| Correlation coefficient | ≥0.998 | 1.000 | |
| Absolute value of intercept as a percentage of nominal concentration | ≤1.0% | 0.03% | |
| Accuracy | Individual recovery values | 98.0–102.0% | 99.6–101.3% |
| Mean recovery value | 99.0–101.0% | 100.2% | |
| Precision | RSD for 6 parallel determinations | ≤1.0% | ≤0.6% |
| RSD for 12 parallel determinations | ≤1.5% | 0.6% | |
| Difference between mean results of two analysts | ≤2.0% | 0.6% | |
| Solution Stability | Change in peak area after 47 h at room temperature | 99.0–101.0% | 99.3–100.7% |
| Robustness | Change in peak area ratio (test/reference) under altered and nominal conditions | 99.0–101.0% | 99.8–100.3% |
Appendix E

Table A4.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Cream” according to the indicator “Naftifine Hydrochloride”.
Table A4.
Summary of validation results of the quality control method for “Naftifine Hydrochloride Cream” according to the indicator “Naftifine Hydrochloride”.
| Investigation | Parameter | Eligibility Criteria | Evaluation |
|---|---|---|---|
| Specificity | Peak area on blank/placebo chromatograms potentially interfering with naftifine peak | ≤0.2% | Not detected |
| Difference in retention times of naftifine peak in test and reference chromatograms | ≤2% | ≤0.1% | |
| System suitability | RSD of naftifine peak area from three replicate injections of reference solution | ≤1.0% | ≤0.3% |
| Tailing factor of the naftifine peak | 0.8–1.8 | 1.0–1.1 | |
| Column efficiency (theoretical plates) for the naftifine peak | ≥2000 | ≥4731 | |
| Linearity (Range) | Verified concentration range of calibration model | 7.6–12.6 mg/mL | 7.0–13.3 mg/mL |
| Correlation coefficient | ≥0.998 | 1.000 | |
| Absolute value of intercept as a percentage of nominal concentration | ≤1.0% | 0.03% | |
| Accuracy | Individual recovery values | 98.0–102.0% | 99.5–100.8% |
| Mean recovery value | 99.0–101.0% | 100.2% | |
| Precision | RSD for 6 parallel determinations | ≤1.0% | ≤0.7% |
| RSD for 12 parallel determinations | ≤1.5% | 0.8% | |
| Difference between mean results of two analysts | ≤2.0% | 1.0% | |
| Solution Stability | Change in peak area after 47 h at room temperature | 99.0–101.0% | 99.4–100.7% |
| Change in peak area after 24 h at room temperature | 99.0–101.0% | 99.2–99.7% | |
| Robustness | Change in peak area ratio (test/reference) under altered and nominal conditions | 99.0–101.0% | 99.8–100.9% |
References
- Dogra, S.; Sahni, K.; Singh, S. Newer Topical Treatments in Skin and Nail Dermatophyte Infections. Indian Dermatol. Online J. 2018, 9, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Camp, W.L.; Elewski, B.E. Advances in Topical and Systemic Antifungals. Dermatol. Clin. 2007, 25, 165–183. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeia. The National Formulary; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2024; Available online: https://www.uspnf.com (accessed on 25 September 2025).
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2015th ed.; China Medical Science Press: Beijing, China, 2020; p. 5906. [Google Scholar]
- Gokhale, V.M.; Kulkarni, V.M. Understanding the Antifungal Activity of Terbinafine Analogues Using Quantitative Structure–Activity Relationship (QSAR) Models. Bioorganic Med. Chem. 2000, 8, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Patel, H.D. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Terbinafine Hydrochloride and Mometasone Furoate in Combined Dosage Form. J. Chil. Chem. Soc. 2016, 61, 2958–2962. [Google Scholar] [CrossRef]
- Patel Krupa, K.A. Validated RP-HPLC Method For Determination of Terbinafine Hydrochloride in Pharmaceutical Solid Dosage Form. IJPT 2012, 4, 4663–4669. [Google Scholar]
- Matysová, L.; Solich, P.; Marek, P.; Havlikova, L.; Nováková, L.; Šícha, J. Separation and determination of terbinafine and its four impurities of similar structure using simple RP-HPLC method. Talanta 2006, 68, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Misro, L.; Boini, T.; Maurya, R.; Radhakrishnan, T.; Rohith, K.S.; Kumar, V.; Sharma, P.; Singh, A.; Singh, R.; Srikanth, N.; et al. Analytical method development and validation for simultaneous estimation of seven markers in polyherbal formulation JKC by using RP-HPLC. Futur. J. Pharm. Sci. 2024, 10, 92. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Patil, J.K.; Jain, V.H.; Pawar, S.P. Development and Validation of The Uvspectrophotometric and RP-HPLC Method for Simultenious Estimation of Itraconazole and Terbinafine Hydrochloride in Bulk and in Formulation. World J. Pharm. Pharm. Sci. 2019, 8, 675–702. [Google Scholar]
- Shinde, K.S.; Jangme, C.M.; Patil, A.R. RP-HPLC method for quantitative estimation of naftifine hydrochloride in formulated products. J. Appl. Pharm. Res. 2025, 13, 177–186. [Google Scholar] [CrossRef]
- ICH Validation of Analytical Procedures: Text and Methodology, Q2 (R2), November 2023. Available online: https://www.ich.org/page/quality-guidelines (accessed on 25 September 2025).
- Gałuszka, A.; Migaszewski, Z.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. R. Soc. Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed. 2024. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition (accessed on 25 September 2025).
- Snyder, R.L.; Kirckland, J.; Dolan, W.J. Introduction to Modern Liquid Chromatography, 3rd ed.; John Willey & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.