Abstract
The γδ T cells belong to a subgroup of T cells known as non-conventional T cells due to their limited T cell receptor (TCR) repertoire and ability to recognize non-peptide antigens. They play a crucial role in combating infections and tumors. Vγ9Vδ2 T cells are typically activated by molecules containing diphosphate groups, collectively known as phosphoantigens (pAgs), through a non-canonical mechanism which involves the intracellular domain of butyrofilin (BTN)3A1 protein. However, no FDA-approved drugs have yet been shown to activate them, and the underlying cellular mechanisms remain unknown. In this study, we combined high-throughput virtual screening of an FDA-approved drug database with in vitro cellular assays to identify potential γδ T cells activators. Our findings demonstrate that Nitazoxanide (NTZ) and Tinidazole induce moderate elicited a statistically significant increase in interferon (IFN)-γ production of Vγ9Vδ2 T cells by their probably interaction with the pAg binding site of BTN3A1. Additionally, NTZ induces expression of CD107a, but only at the highest concentrations tested and promotes the upregulation of HLA-DR in total PBMCs and CD14+ monocytes. Blocking BTN3A with a specific antibody led to a marked reduction in all NTZ-induced activations. This work identifies NTZ as a previously unrecognized activator of γδ T cells, highlighting its immunomodulatory potential beyond its known clinical uses. These findings broaden our understanding of γδ T cells pharmacology and suggest new opportunities for drug repurposing and the design of novel chemical scaffolds. Further mechanistic studies will be essential to fully define how NTZ engages the BTN3A–γδ T cells axis.
1. Introduction
γδ T cells are classified as non-conventional T cells because they possess a restricted T cell receptor (TCR) repertoire and are capable of recognize non-peptide antigens [1]. They serve as rapid responders with a limited but functionally diverse set of receptors [2]. γδTCR is generated through RAG-mediated recombination of V (variable), D (diversity), and J (joining) gene segments. In humans, only a few Vγ and Vδ lineages are available, and their pairing is not random and follows tissue-specific patterns [3,4]. For instance, Vγ9 pairs with Vδ2, forming the predominant subset in peripheral blood, while Vδ1 T cells are abundant in peripheral tissues such as the skin, intestine, liver, and mucosal surfaces [4].
The pairing of Vγ and Vδ chains in the TCR influences γδ T cell activation, which may be major complex histocompatibility (MHC)-dependent or MHC-independent [5]. For example, the Vδ1 TCR repertoire can recognize lipids presented by CD1d, a mechanism analogous to the canonical activation pathway of natural killer T (NKT) cells [6,7]. In addition, Vδ1 and Vδ3 TCRs can directly recognize the MHC class I-related protein 1 (MR1), independently of antigen presentation, a mechanism otherwise characteristic of mucosal-associated invariant T (MAIT) cells [8,9]. By contrast, within the Vγ9Vδ2 T cells subset, the TCR interacts with the butyrophilin (BTN) proteins BTN3A1 and BTN2A1, which are expressed in multiple cell types, including antigen-presenting cells (APCs) [10].
Activation of the Vγ9Vδ2 T cells subset is the most extensively characterized and depends on engagement of the BTN3A1 and BTN2A1 proteins [10]. Strong activation occurs only when these receptors undergo conformational changes induced by small phosphate molecules, called phosphoantigens (pAgs), binding to the intracellular B30.2 domain of BTN3A1 [10]. The best characterized pAgs are isopentenyl pyrophosphate (IPP), an intermediate of the endogenous mevalonate pathway that accumulates in tumor cells, and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), a metabolite produced in the bacterial and parasitic MEP pathway [11]. Recognition of BTN3A1 and BTN2A1 by the Vγ9Vδ2 TCR, triggered by phosphoantigen binding, leads to potent Th1 and Th17 responses with inflammatory and cytotoxic functions, enabling these cells to play a crucial role in controlling infections and cancer [11].
The tetrameric arrangement, pAgs-stabilized, between BTN2A1 and BTN3A1 leads to γδ TCR activation, as demonstrated by recent cryo-EM studies [12]. When the tetrameric complex consists of a BTN2A1 homodimer and a BTN3A1-containing dimer (either a heterodimer with BTN3A2 or BTN3A3, or a BTN3A1 homodimer), this arrangement stabilizes interactions with the TCR’s complementarity determining regions (CDRs). However, BTN3A1 homodimers form less stable complexes, resulting in weaker activation [12].
Apart from pAgs, the only commercial drugs known to activate Vγ9Vδ2 T cells are bisphosphonates, including zoledronic acid (Zol), alendronic acid, and pamidronic acid [13,14,15]. These compounds are clinically used for the treatment of osteoporosis and other bone-related diseases. However, their activation mechanism differs from that of pAgs because they do not interact directly with the B30.2 intracellular domain of BTN3A1. Instead, they inhibit farnesyl diphosphate synthase (FDPS), blocking the conversion of IPP in the mevalonate pathway and thereby promoting its intracellular accumulation [14].
The design of novel Vγ9Vδ2 T cell agonists often relies on chemically modifying the HMBPP scaffold, most commonly through hydroxyl group substitutions, which significantly enhance Vγ9Vδ2 T cells activation [15,16,17]. Specifically, pivaloyloxymethyl substitutions induce more rapid and potent IFN-γ production, together with enhanced cytotoxicity, compared to HMBPP [16].
Therefore, we conducted high-throughput virtual screening of a subset of the Food and Drug Administration (FDA)-approved drugs, targeting the B30.2 intracellular domain of BTN3A1, with the aim of discovering novel Vγ9Vδ2 T cells activators structurally distinct from canonical pAgs. Our findings demonstrate that some approved drugs exhibit moderate stimulatory activity toward Vγ9Vδ2 T cells, thereby paving the way for the design of new therapeutic agents.
2. Materials and Methods
2.1. High-Throughput Virtual Screening
The detailed in silico methodology is provided in the Supplementary Information. Briefly, a receptor-based virtual screening approach was employed, focusing on the HMBPP-binding site within the intracellular region of BTN3A1 (PDB ID: 5ZXK). The initial screening carried out against a library of approximately 2812 optimized molecules using deMon2k (see Supplementary Material SI), representing a randomly select subset of approved drugs sourced from the PubChem database, containing up to ~12,000 structures.
The binding site was defined as a 10 Å radius sphere centered around the key residues known to be involved in pAg recognition. The docking protocol was rigorously validated by redocking HMBPP into the defined binding pocket to confirm the pose prediction accuracy. To enhance the hit rate and ensure the selection of compounds with high predictive affinity and proper interaction geometry, a consensus scoring strategy was developed, integrating the three primary criteria: binding free energy (ΔG; using Autodock 4.2.6 program suite), center of mass distance (CMD), and ligand–receptor contact distance (LRCD), a parameter assessing similarity in the interactions between the ligands and the protein receptor relative to the interactions in a reference crystallographic complex (see Figure 1a) [18].
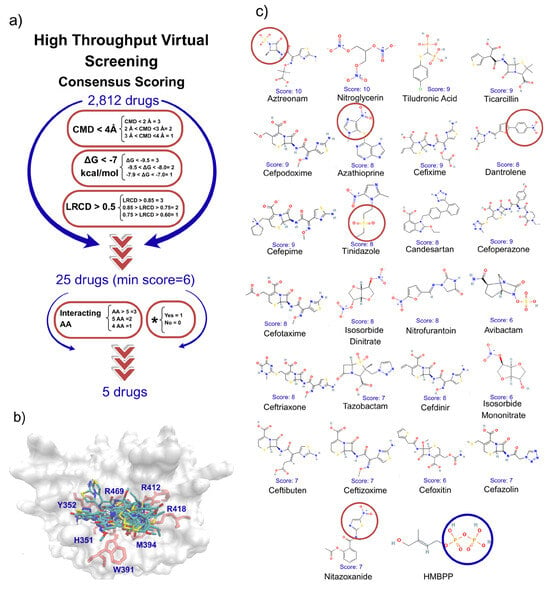
Figure 1.
High-throughput virtual drug screens and drug selection for in vitro assays. (a) Drug selection strategy based on multiple virtual screens. From a database of 2812 FDA-approved drugs, we scored compounds that met all parameters: CMD, ΔG, and LRCD, considered collectively. The top 25 ranked drugs were further evaluated based on their docking interactions with six key residues—Arg412, Arg418, Arg469, Lys393, His351, Tyr352, and His378—and through a literature search for any reported immune-related effects (*). (b) Superimposition of the top 25 drugs and HMBPP onto the pAg binding site of the B30.2 domain of BTN3A1, shown in a front view. Seven key interacting residues are highlighted in red. (c) Chemical structures of the top 25 FDA-approved drugs. The five compounds selected for in vitro assays are highlighted with red circles around their chemical scaffolds, which, according to docking analysis, interact with Arg residues at the pAg binding site, where HMBPP binds via its diphosphate group.
A weighted scoring system was implemented for these parameters (Figure 1a). This approach was utilized to filter the initial hits down to 25 top-ranked drugs. The final set of 25 compounds was subjected to a secondary filtering step based on two additional, biologically relevant criteria: number of interactions with Amino Acids and immunoregulatory effect. The drugs with the better cumulative scores and information from this final filtering step were selected for further experimental validation.
2.2. Donors and PBMC Isolation
The protocol for this study was approved by the Institutional Research Ethics Committee of the Escuela Superior de Medicina in Mexico City (register number: ESM-CEI-02/16-02-23). All donors signed an informed consent letter prior to participating in the study. Peripheral Blood Mononuclear Cells (PBMCs) from buffy coats of healthy donors were obtained using a Ficoll Paque (Cytiva) density gradient.
2.3. Cell Viability and BTN3A Expression Assays
A total of 1 × 106 PBMCs were cultured in RPMI-1640 medium (Gibco, Waltham, MA, USA) supplemented with 10% of fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin (Gibco). For viability assays, cells were stimulated with Nitazoxanide (NTZ, Supleco, Bellefonte, PA, USA) at various concentrations (1, 10, 100, and 200 µM) for 4 h. Viability was then determined by staining with 5 µL of 7-amino-actinomycin D (7-AAD, Biolegend, San Diego, CA, USA) followed by flow cytometry analysis. For BTN3A expression, a separate set of PBMCs were stained with a PE anti-human BTN3A antibody (Miltenyi Biotech, Bergisch Gladbach, Germany) for 20 min at 4 °C. The cells were then fixed with 1% formaldehyde (Sigma Aldrich, Burlington, MA, USA) and analyzed using flow cytometry.
2.4. Stimulation of PBMCs in the Presence of Drugs and NTZ Dose–Response Curves
For initial activation assays, 1 × 106 PBMCs were cultured in 24-well plates with supplemented RPMI-1640 medium and stimulated with 10 µM HMBPP (Sigma Aldrich), 60 µM aztreonam (Sigma Aldrich), 50 µM azathioprine (Sigma Aldrich), 16 µM dantrolene (Sigma Aldrich), 60 µM NTZ, or 80 µM tinidazole (Sigma Aldrich), according to plasma concentrations reported in the literature. One hour after stimulation, 5 µg/mL Brefeldin A (BD Biosciences, Franklin Lakes, NJ, USA) was added, and cells were incubated for 3 h at 37 °C and 5% CO2. Following incubation, cells were collected for membrane and intracellular staining with antibodies.
For NTZ dose–response curves, 1 × 106 PBMCs were cultured and stimulated with varying concentrations of NTZ. For CD107a expression, cells were stimulated with 50, 100, 150, or 200 µM NTZ, including a non-stimulated control (NS). For IFN-γ production, cells were stimulated with 15, 30, 60, or 100 µM NTZ, also including an NS condition.
For competition assays, PBMCs were co-incubated with 60 µM NTZ—identified as the optimal concentration for IFN-γ secretion—and 10 µM HMBPP.
For BTN3A blockade assays, 10 µg/mL of purified anti-human BTN3A (clone 103.2#, Cell Sciences, Newburyport, MA, USA) was added to PBMCs and incubated for 2 h at 37 °C and 5% CO2. After incubation, cells were stimulated with either 100 µM NTZ or 10 µM HMBPP.
2.5. Isolation and Stimulation of Vγ9Vδ2 T Cells and Monocytes via Cell Sorting
For the isolation of Vγ9Vδ2 T cells and monocytes, 8 × 106 PBMCs from healthy donors were stained with PE-Cy7 anti-human CD3 (BD Biosciences) and APC anti-human TCRVδ2 (Miltenyi Biotech), and Pacific Blue anti-human CD14 (BD Biosciences), respectively, and sorted by fluorescence-activated cell sorting using a FACS Aria II cell sorter (BD Biosciences, San Jose, CA, USA). Sorted cells were cultured in round-bottom polystyrene tubes (Falcon, Corning, NY, USA) with supplemented RPMI-1640 culture medium and incubated for 18 h at 37 °C and 5% CO2.
Subsequently, monocytes were pulsed with 10 µM HMBPP and/or 60 µM NTZ for 1 h to evaluate fast stimulatory activity. After incubation, monocytes were washed with 1X phosphate saline buffer (PBS) to remove residual stimuli, and Vγ9Vδ2 T cells were added at a 1:3 monocyte-to-T cell ratio. Brefeldin A (5 µg/mL) was added, and tubes were briefly centrifuged at 1300 RPM for 1 min to ensure cell–cell contact. The co-culture was then incubated at 37 °C and 5% CO2 for 3 h before intracellular antibody staining.
For stimulation of Vγ9Vδ2 T cells in the absence of monocytes, stimuli were not removed, and all conditions remained constant throughout the 4 h evaluation period.
2.6. Membrane and Intracellular Staining with Antibodies, Flow Cytometry Acquisition and Analysis
PBMCs or sorted cells, treated as previously described, were incubated with phenotype antibodies—APC-Cy7 anti-human CD3 and APC anti-human TCRVδ2—as well as antibodies for membrane activation markers: BV605 anti-human CD107a (BD Biosciences) or FITC anti-human CD69 (Biolegend). Incubations were carried out for 20 min at 4 °C in the dark, and after cells were washed. Membrane stains were fixed with 1% formaldehyde and stored until acquisition on the cytometer.
For intracellular staining, cells were subsequently permeabilized with 1X BD Permeabilizing Solution 2 (BD Biosciences) for 10 min at room temperature in the dark and washed. Then, cells were incubated with PE anti-human IFN-γ (BD Biosciences) for 20 min at room temperature in the dark and washed again. Finally, the cells were fixed with 1% formaldehyde and stored at 4 °C in the dark until acquisition on the cytometer.
Within 72 h, 50,000 or 2000 single events of PBMCs or sorted cells, respectively, were acquired using a BD FACS ARIA II flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo™ V10 software (FlowJo™, Ashland, OR, USA).
2.7. CD69 and HLA-DR Overexpression in the Presence of NTZ
For HLA-DR overexpression, a total of 2 × 106 PBMCs were cultured in 24-well plates using supplemented RPMI-1640 medium and stimulated with 10 µM HMBPP or NTZ at different concentrations (NS, 30, 60, and 100 µM) for 36 h. After incubation, cells were collected and stained with PerCP anti-human HLA-DR (BD Biosciences) for 20 min at 4 °C in the dark, and after cells were washed.
For the BTN3A block condition, a total of 1 × 106 PBMCs were cultured in round-bottom polystyrene tubes with supplemented RPMI-1640 medium and 10 µg/mL of purified anti-human BTN3A (clone 103.2#, Cell Sciences) was added and incubated for 2 h at 37 °C and 5% CO2. Following this, cells were stimulated with 60 µM NTZ or 10 µM HMBPP and incubated at 37 °C and 5% CO2 for 36 h. After incubation, PBMCs were washed and stained with PerCP anti-human HLA-DR and Pacific Blue anti-human CD14. Finally, cells were fixed with 1% formaldehyde and stored at 4 °C in the dark until acquisition on the cytometer.
For CD69 overexpression, 1 × 106 PBMCs were cultured in round-bottom polystyrene tubes with supplemented RPMI-1640 medium. Cells were stimulated with 100 µM NTZ or 10 µM HMBPP and incubated at 37 °C and 5% CO2 for 4, 24, and 72 h. After each incubation period, cells were washed and prepared for membrane staining with antibodies.
2.8. Statistical Analysis
Data were analyzed using GraphPad Prism 10 software. A paired t-test was applied for the analysis of BTN3A expression. Repeated measures (RM) one-way ANOVA was used to evaluate cell viability, PBMC stimulation, BTN3A blockade, and CD69 and HLA-DR upregulation. The Friedman test was employed for the analysis of NTZ dose–response curves and stimulation assays with sorted cells.
Results are presented as mean ± SD in scatter and bar plots. Statistically significant p-values are indicated as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
3. Results
3.1. Selection of Drugs with High Affinity to the BTN3A1 Domain
We conducted in silico virtual screening of ~2812 approved drugs from the PubChem database to identify candidates capable of binding the B30.2 domain of BTN3A1 (Figure 1a). Compounds were ranked using consensus scoring, and only those with a score above 6 were pre-selected. From these, five candidates were chosen for in vitro analysis based on their ability to mimic HMBPP interactions, their reported immunological properties, and their representation across different pharmacological classes to avoid redundancy (Figure 1b,c). A ranking of the 25 best scored molecules is presented in Supplementary Table S2.
3.2. Determination of the Expression of BTN3A in Immune Cells and the Effect of Selected Drugs in the Activation of Vγ9Vδ2 T Cells
BTN3A levels were assessed prior to in vitro activation in monocytes and lymphocytes isolated from PBMCs of healthy volunteers. These cell populations were identified based on size and granularity to rule out the possibility that differences in BTN3A expression might influence Vγ9Vδ2 T cells activation (Supplementary Figure S1a). We observed that BTN3A expression in monocytes is constitutive and consistent across donors, whereas lymphocyte exhibited lower expression and greater variability between donors (Supplementary Table S1). Both dantrolene and NTZ induced a modest increase in CD107a expression during Vγ9Vδ2 T cells activation in PBMCs from healthy donors; however, neither induced a statistically significant difference at the tested concentration (Figure 2a). In contrast, stimulation with dantrolene, tinidazole, and NTZ led to a modest increase in IFN-γ production (Figure 2b), with only tinidazole and NTZ reaching statistical significance difference compared to the non-stimulated cells.
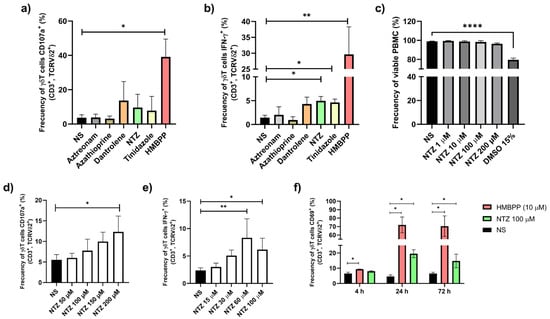
Figure 2.
Selection of NTZ based on Vγ9Vδ2 T cell activation in PBMCS from healthy donors compared with other candidate drugs. Bar graphs show the % of (a) CD107a+ and (b) IFN-γ+ Vγ9Vδ2 T cells from PBMC cultures stimulated during 4 h with different concentrations of selected drugs. HMBPP was included as a positive (N = 6; * p < 0.05, ** p < 0.01; Friedman test). (c) NTZ at concentrations ranging from 1 to 200 µM did not affect cell viability, as assessed by 7-AAD staining (N = 4; **** p < 0.0001; RM one-way ANOVA). (d) CD107a surface expression and (e) intracellular IFN-γ production by Vγ9Vδ2 T cells in response to increasing NTZ concentrations (N = 4; * p < 0.05, ** p < 0.01; RM one-way ANOVA and Friedman test, respectively). (f) CD69 up-regulation on T cells stimulated with NTZ or HMBPP at 4, 24, and 72 h (N = 3; * p < 0.05; RM one-way ANOVA). Results are presented as mean ± SD in bar plots.
3.3. NTZ Promotes the Activation of Vγ9Vδ2 T Cells When Incubated In Vitro
The cytotoxicity of NTZ was assessed at concentrations of 1, 10, 100, and 200 µM using 7-AAD staining in PBMCs from healthy donors. No loss of cell viability was detected after 4 h of incubation (Figure 2c). Vγ9Vδ2 T cells were treated with increasing concentrations of NTZ, and activation was subsequently assessed by CD107a expression and IFN-γ production, both of which exhibited a clear dose-dependent pattern (Figure 2d,e). Maximal Vγ9Vδ2 T cells activation occurred at 24 h, defined by CD69 upregulation (Figure 2f).
3.4. Co-Incubation of NTZ with HMBPP Dampers the Production of IFN-γ in Vγ9Vδ2 T Cells When Incubated In Vitro
We compared the IFN-γ production induced by NTZ with that induced by the canonical pAg HMBPP and investigated whether there was a competitive effect of the presence of both molecules in a PBMC culture. Co-stimulation with NTZ and HMBPP did not enhance IFN-γ production. On the contrary, it decreased compared to HMBPP alone, suggesting potential competition between the two molecules for the B30.2 domain of BTN3A1 (Figure 3a,b). To confirm that the activating effect induced by NTZ is specific to Vγ9Vδ2 T-cells, we analyzed the remaining CD3+ cells and found no statistically significant difference in IFN-γ production among groups (Figure 3c,d).
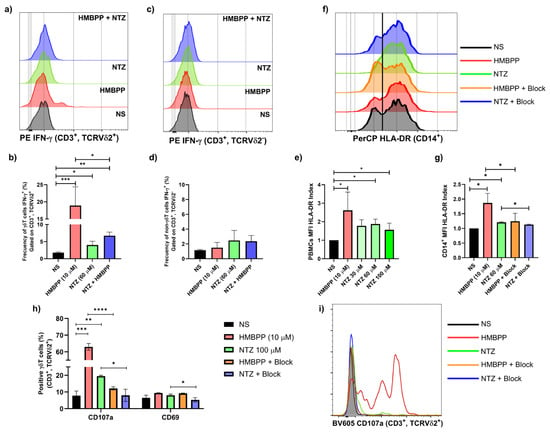
Figure 3.
NTZ-mediated activation of Vγ9Vδ2 T cells and PBMCs. (a,b) Representative histograms and bar graphs show the effect of NTZ at 60 µM and HMBPP at 10 µM exert opposing effects on Vγ9Vδ2 T cells from healthy donors (N = 3; * p < 0.05, ** p < 0.01, *** p < 0.001; Friedman test). (c,d) Total CD3+ cells did not show activation, demonstrating that the phenomenon occurs only in the selected phenotype (N = 3). (e) Upregulation of HLA-DR in total PBMCs exposure to increase NTZ concentrations (N = 3; * p < 0.05; RM-one-way ANOVA). (f,g) Overexpression of HLA-DR in CD14+ monocytes was reduced by BTN3A blockade with a blocking antibody (clone 103.2#) (N = 3; * p < 0.05; RM-one-way ANOVA). (h,i) CD107a and CD69 activation markers also were reduced by BTN3A blockade with a blocking antibody (clone 103.2#) (N = 3; * p < 0.05, ** p < 0.01, *** p < 0.001 **** p < 0.0001; RM one-way ANOVA). Results are presented as mean ± SD in bar plots.
Additionally, to further demonstrate that NTZ mediates BTN3A-dependent Vγ9Vδ2 TCR activation, we added a BTN3A blocking antibody (clone 103.2#). This antibody significantly reduced the upregulation of the activation markers CD107 and CD69 in Vγ9Vδ2 T cells (Figure 3h,i).
3.5. Total PBMCs Stimulated with NTZ Promote Upregulation of HLA-DR
Consistent with the observed IFN-γ production in Vγ9Vδ2 T cells, NTZ treatment of PBMCs led to HLA-DR upregulation after 36 h of incubation (Figure 3e). In the monocytes (CD14+), this effect was significantly reduced by the addition of a blocking BTNA3 antibody, suggesting that HLA-DR expression is mediated through IFN-γ-dependent activation (Figure 3f,g).
3.6. NTZ Promotes the Production of IFN-γ in Sorted Vγ9Vδ2 T Cells from Healthy Donors in the Presence of Monocytes
Sorted Monocyte and Vγ9Vδ2 T cells from PBMCs of healthy donors representing more purified in vitro environment were incubated in the presence of NTZ, resulting in a significant increase in IFN-γ production in this purified in vitro system (Figure 4b,d). Interestingly, self-weak activation of Vγ9Vδ2 T cells was observed in the presence of HMBPP but not NTZ, possibly reflecting its partial agonist activity (Figure 4c,d).
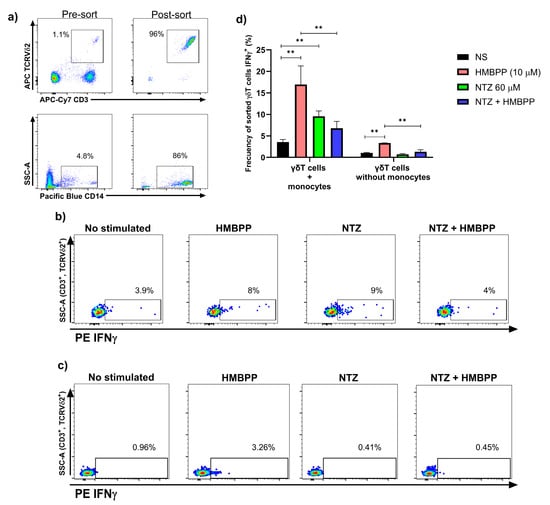
Figure 4.
Co-incubation of NTZ and HMBPP with sorted Vγ9Vδ2 T cells with or without monocytes shows a pronounced inhibitory effect. (a) Vγ9Vδ2 T cells and monocytes were sorted from PBMCs of healthy donors. (b, left d) representative dot plots and bar charts show the strong inhibition of NTZ on HMBPP-induced activation in sorted Vγ9Vδ2 T cells with monocytes (N = 3; ** p < 0.01; Friedman test). (c, right d) representative dot plots and bar charts show the complete inhibition of NTZ on weak HMBPP-induced self-activation in sorted Vγ9Vδ2 T cells without monocytes. NTZ did not show self-activation in Vγ9Vδ2 T cells (N = 3; ** p < 0.01; RM one-way ANOVA). Results are presented as mean ± SD in bar plots.
A key finding was the pronounced decrease in IFN-γ production when NTZ and HMBPP were combined, with Vγ9Vδ2 T cells—both in the presence and absence of monocytes—showing significantly lower responses compared to HMBPP alone (Figure 4b–d). These results suggest competition between the two molecules.
3.7. Molecular Docking Reveals Putative Interactions Between NTZ and Its Competition over HMBPP in the B30.2 Domain of BTN3A1
Molecular docking analyses reveal distinct interaction patterns for HMBPP and NTZ within the B30.2 domain of BTN3A1, a highly basic pocket defined by residues such as Arg412, Arg418, Arg469, His351, Tyr352, and Trp391 (Figure 5a). HMBPP exhibits strong ionic anchoring: its diphosphate group drives binding by engaging three arginine residues within the positively charged cleft, while an additional hydrogen bond between its hydroxyl group and His351 provides further stabilization. These multiple, short-range, charge-driven interactions form a highly stable, multi-point tether. In contrast, NTZ lacks the diphosphate moiety and instead relies on its 2-amino-5-nitrothiazole group, which contributes several electronegative atoms acting as hydrogen bond acceptors (Figure 5b). The longer interaction distances and reliance on non-ionic forces, rather than phosphate-mediated charge complementarity, suggest a less stable association. This structural distinction likely underlies the observed differences in binding affinity and biological potency.
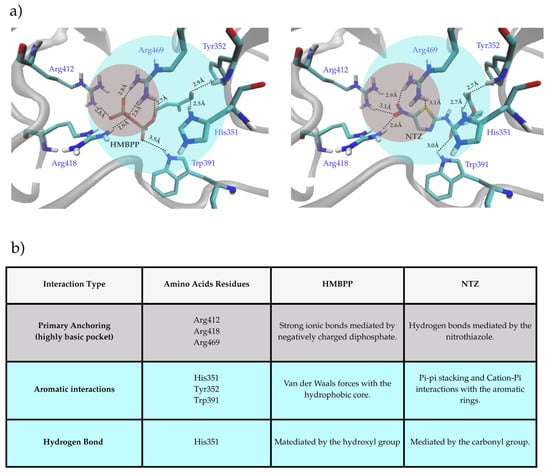
Figure 5.
(a) Stick representations showing the predicted binding modes of HMBPP (left) and NTZ (right) within the BTN3A1 intracellular B30.2 binding pocket. Key interacting residues are labeled (Arg412, Arg418, Arg469, His351, Tyr352, Trp391). The gray circle highlights the cluster of highly basic arginine residues, and the blue circle highlights the cluster of aromatic residues. (b) Summary table of interaction types for HMBPP and NTZ, listing participating residues and their characteristics, grouped into primary anchoring, aromatic interactions, and hydrogen bonding.
4. Discussion
To the best of our knowledge, this is the first report demonstrating the direct immunomodulatory effects of NTZ and tinidazole—two broad-spectrum antiparasitic agents—on human Vγ9Vδ2 T cells. Importantly, these findings show that chemical scaffolds distinct from canonical pAgs and bisphosphonates can stimulate this non-conventional T cells subset, expanding the current repertoire of known Vγ9Vδ2 T cell agonists.
Using in silico screening of the FDA-approved drug library, we identified NTZ as a candidate with predicted affinity for the intracellular B30.2 domain of BTN3A1. Although its docking score was lower than other compounds, NTZ was prioritized due to its well-documented immunomodulatory properties in the literature.
Docking studies revealed that NTZ reproduced the hydrogen-bonding pattern observed in the crystallographic complex of the reference activator HMBPP [19], providing a plausible explanation for its stimulatory effects despite lacking the diphosphate group required for strong ionic interactions. Competitive in vitro assays confirmed this mechanism: NTZ reduced HMBPP-driven activation by ~50% in short incubations, suggesting competition for the same binding site. Blocking experiments further confirmed that NTZ activation in Vγ9Vδ2 cells is TCR mediated. Although a direct physical interaction between NTZ and BTN3A1 has not yet been demonstrated, our results support the involvement of BTN3A1 as the underlying mechanism and argue against alternative pathways such as IPP accumulation.
Functionally, NTZ induced rapid cytokine production and up-regulation of activation markers such as CD69, CD107a in Vγ9Vδ2 T cells and HLA-DR in monocytes, indicating both immunostimulatory and cytotoxic potential. Interestingly, NTZ failed to activate Vγ9Vδ2 T cells in the absence of monocytes, whereas HMBPP elicited weak but significant autologous activation. This suggests that NTZ functions as a partial agonist, which is insufficient to overcome the lower BTN3A1 expression in lymphocytes or potential differences in the BTN2A1-BTN3A activator–tetramer composition. Nevertheless, the addition of NTZ abolished HMBPP-driven self-activation, reinforcing the idea of direct competition at the BTN3A1 binding site. In addition, previous studies have observed that “bulky” HMBPP analogs act as partial agonists of Vγ9Vδ2 T cells, but exhibit inhibitory effects in the presence of HMBPP [20].
From a pharmacological standpoint, NTZ holds clear advantages over HMBPP. While HMBPP is a potent natural agonist, its unfavorable drug-like properties (high polarity, poor membrane permeability, and lack of oral bioavailability) hamper its therapeutic development. In contrast, NTZ complies fully with Lipinski’s rules, offering oral bioavailability, membrane permeability, and a well-established safety profile. These features, juxtaposed with the limitations of HMBPP, make NTZ a promising scaffold for further drugs development or repurposing.
While tinidazole has been implicated in severe cutaneous adverse drug reactions [21], our findings also align with previous reports of NTZ’s immunological effects in disease contexts. NTZ has been shown to suppress T cell proliferation and cytokine production in diabetes [22], COVID-19 [23], and viral infections [24,25,26]. Here, we extend its profile to include the capacity to activate Vγ9Vδ2 T cells. This dual role underscores the complexity of NTZ’s immunomodulatory activity and highlights the need for a specific context of evaluation.
Looking forward, the chemical scaffold of NTZ provides a tractable starting point for rational design of novel Vγ9Vδ2 T cell agonists or antagonists. Its active metabolite tizoxanide, which replaces a ketone with a hydroxyl group, exemplifies how subtle modifications may improve activity. Further structural optimization—guided by crystallographic studies of BTN3A1 in complex with NTZ—could yield new small molecules with enhanced potency while retaining favorable pharmacological properties.
In summary, NTZ represents the first drug-like scaffold distinct from phosphoantigens capable of activating Vγ9Vδ2 T cells. These results not only broaden our understanding of NTZ’s immunomodulatory capacity but also lay the foundation for the development of novel γδ T cell agonists with therapeutic potential in infection, cancer, and immune modulation.
5. Conclusions
NTZ provides the first drug-like scaffold, juxtaposed to HMBPP, capable of activating Vγ9Vδ2 T cells and paving the way for the design of novel γδ T cells immunomodulators.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/scipharm93040053/s1. References [18,19,27,28,29] are cited in the Supplementary Materials File.
Author Contributions
Conceptualization, Á.D.C.-J., O.R.-C. and J.L.C.-F.; methodology, Á.D.C.-J., O.R.-C., A.A.G.-N., M.A.R.-C., J.B.C.-S., D.P.R.-R., G.B.-A. and M.A.M.-E.; formal analysis, Á.D.C.-J., O.R.-C., A.A.G.-N. and J.B.C.-S.; investigation, Á.D.C.-J., O.R.-C., A.A.G.-N. and J.L.C.-F.; resources, J.L.C.-F.; data curation, Á.D.C.-J., O.R.-C., M.A.R.-C. and C.Z.G.-C.; writing—original draft preparation, Á.D.C.-J., O.R.-C., A.A.G.-N., I.P.T.-A. and J.L.C.-F.; writing—review and editing, Á.D.C.-J., O.R.-C., M.A.R.-C., I.P.T.-A., D.J.N., M.A.M.-E. and J.L.C.-F.; visualization, Á.D.C.-J., O.R.-C. and J.L.C.-F.; supervision, J.L.C.-F.; project administration, J.L.C.-F.; funding acquisition, J.L.C.-F., Á.D.C.-J. and O.R.-C. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by federal funding CONACYT FORDECYT-PRONACES/514873/2020 for J.L.C.-F. We thank the Secretaría de Ciencia, Humanidades, Tenología e Innovación (SECIHTI) for PhD support 4035453 awarded to Ángel Daniel Campos-Juárez (CVU: 1232064). SECIHTI also supports Mónica Adriana Rodríguez-Cadena (CVU: 1175034).
Institutional Review Board Statement
The study was approved by the Ethics Committee of Escuela Superior de Medicina (ESM-CEI-02/16-02-23) on 16 February 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Access to the data will be granted upon request, provided a justification is submitted.
Acknowledgments
We also Thank to Raúl Flores-Mejía and Elizabeth Gonzáles for the operational and regulatory facilities for obtaining the buffy-coat sample from healthy donors. The authors gratefully acknowledge the computing time granted by LANCAD and SECIHTI on the supercomputer Yoltla/Miztli/Xiuhcoatl at LSVP UAM-Iztapalapa/DGTIC UNAM/CGSTIC Cinvestav. Á.D.C.-J. extends special thanks to M.N.M.-A. for their constant orientation and invaluable support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mayassi, T.; Barreiro, L.B.; Rossjohn, J.; Jabri, B. A multilayered immune system through the lens of unconventional T cells. Nature 2021, 595, 501–510. [Google Scholar] [CrossRef]
- Kurioka, A.; Klenerman, P. Aging unconventionally: γδ T cells, iNKT cells, and MAIT cells in aging. Semin. Immunol. 2023, 69, 101816. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, J.L.; Benyamine, A.; Pasero, C.; Olive, D. New insights into the regulation of γδ T cells by BTN3A and other BTN/BTNL in tumor immunity. Front. Immunol. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, G.; Zhang, J.; Wu, X.; Chen, X. The dual roles of human γδ T cells: Anti-tumor or tumor-promoting. Front. Immunol. 2021, 11, 619954. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Ding, Y.P.; Tanaka, Y.; Shen, L.W.; Wei, C.H.; Minato, N.; Zhang, W. γδ T cells and their potential for immunotherapy. Int. J. Biol. Sci. 2014, 10, 119–135. [Google Scholar] [CrossRef]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar]
- Le Nours, J.; Gherardin, N.A.; Ramarathinam, S.H.; Awad, W.; Wiede, F.; Gully, B.S.; Khandokar, Y.; Praveena, T.; Wubben, J.M.; Sandow, J.J.; et al. A class of γδ T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 2019, 366, 1522–1527. [Google Scholar] [CrossRef]
- Rice, M.T.; von Borstel, A.; Chevour, P.; Awad, W.; Howson, L.J.; Littler, D.R.; Gherardin, N.A.; Le Nours, J.; Giles, E.M.; Berry, R.; et al. Recognition of the antigen-presenting molecule MR1 by a Vδ3+ γδ T cell receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2110288118. [Google Scholar]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef]
- Qi, C.; Wang, Y.; Li, P.; Zhao, J. Gamma delta T cells and their pathogenic role in psoriasis. Front. Immunol. 2021, 12, 627139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, W.; Zheng, J.; Bai, Y.; Tian, X.; Huang, T.; Lu, Z.; Dong, D.; Zhang, A.; Guo, C.; et al. Phosphoantigen-induced inside-out stabilization of butyrophilin receptor complexes drives dimerization-dependent γδ TCR activation. Immunity 2025, 58, 1646–1659.e5. [Google Scholar] [CrossRef] [PubMed]
- Sugie, T.; Murata-Hirai, K.; Iwasaki, M.; Morita, C.T.; Li, W.; Okamura, H.; Minato, N.; Toi, M.; Tanaka, Y. Zoledronic acid-induced expansion of γδ T cells from early-stage breast cancer patients: Effect of IL-18 on helper NK cells. Cancer Immunol. Immunother. 2013, 62, 677–687. [Google Scholar] [CrossRef]
- Hodgins, N.O.; Wang, J.T.W.; Al-Jamal, K.T. Nanotechnology-based carriers for nitrogen-containing bisphosphonates delivery as sensitizers of γδ T cells for anticancer immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 143–160. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weißinger, F.; Wilhelm, M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- Hsiao, C.H.C.; Wiemer, A.J. A power law function describes the time- and dose-dependency of Vγ9Vδ2 T cell activation by phosphoantigens. Biochem. Pharmacol. 2018, 158, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sharif, M.; James, E.; Dismorr, J.O.; Tucker, J.H.; Willcox, B.E.; Mehellou, Y. Phosphonodiamidate prodrugs of phosphoantigens (ProPAgens) exhibit potent Vγ9/Vδ2 T cell activation and eradication of cancer cells. RSC Med. Chem. 2024, 15, 2399–2411. [Google Scholar] [CrossRef]
- Gómez-Castro, C.Z.; López-Martínez, M.; Hernández-Pineda, J.; Trujillo-Ferrara, J.G.; Padilla-Martínez, I.I. Profiling the interaction of 1-phenylbenzimidazoles to cyclooxygenases. J. Mol. Recognit. 2019, 32, e2801. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Yuan, L.; Zhou, X.; Duan, J.; Xiao, H.; Cai, N.; Han, S.; Ma, X.; Liu, W.; et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vγ9Vδ2 T cell activation. Immunity 2019, 50, 1043–1053.e5. [Google Scholar] [CrossRef]
- Singh, R.; Rani, S.; Jin, Y.; Hsiao, C.C.; Wiemer, A.J. Synthesis and evaluation of (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate analogs as competitive partial agonists of butyrophilin 3A1. Eur. J. Med. Chem. 2024, 276, 116673. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, L.; Zou, Y.; Wei, H.; Zhou, Y.; Guo, X.; Li, Q.; Ye, Y.; Zhang, L. Antibiotic-induced severe cutaneous adverse reactions: A single-center retrospective study over ten years. Front. Immunol. 2024, 15, 1415830. [Google Scholar]
- Castillo-Salazar, M.; Sánchez-Muñoz, F.; Springall del Villar, R.; Navarrete-Vázquez, G.; Hernández-DiazCouder, A.; Mojica-Cardoso, C.; García-Jiménez, S.; Toledano-Jaimes, C.; Bernal-Fernández, G. Nitazoxanide exerts immunomodulatory effects on peripheral blood mononuclear cells from type 2 diabetes patients. Biomolecules 2021, 11, 1817. [Google Scholar] [CrossRef]
- Blum, V.F.; Cimerman, S.; Hunter, J.R.; Tierno, P.; Lacerda, A.; Soeiro, A.; Cardoso, F.; Bellei, N.C.; Maricato, J.; Mantovani, N.; et al. Nitazoxanide superiority to placebo to treat moderate COVID-19—A pilot proof of concept randomized double-blind clinical trial. EClinicalMedicine 2021, 37, 100981. [Google Scholar] [CrossRef]
- Amorosa, V.K.; Luetkemeyer, A.; Kang, M.; Johnson, V.A.; Umbleja, T.; Haas, D.W.; Yesmin, S.; Bardin, M.C.; Chung, R.T.; Alston-Smith, B.; et al. Addition of nitazoxanide to PEG-IFN and ribavirin to improve HCV treatment response in HIV-1 and HCV genotype 1 coinfected persons naïve to HCV therapy: Results of the ACTG A5269 trial. HIV Clin. Trials 2013, 14, 274–282. [Google Scholar] [CrossRef][Green Version]
- Haffizulla, J.; Hartman, A.; Hoppers, M.; Resnick, H.; Samudrala, S.; Ginocchio, C.; Bardin, M.; Rossignol, J.F. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: A double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2014, 14, 609–618. [Google Scholar]
- Rossignol, J.F.; Kabil, S.M.; El-Gohary, Y.; Elfert, A.; Keeffe, E.B. Randomized, double-blind, placebo-controlled study of nitazoxanide monotherapy for the treatment of patients with chronic hepatitis C genotype 4. Aliment. Pharmacol. Ther. 2008, 28, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.M.; Geudtner, G.; Alvarez-Ibarra, A.; Calaminici, P.; Casida, M.E.; Carmona-Espíndola, J.; Domínguez, V.D.; Flores-Moreno, R.; Gamboa, G.U.; Goursot, A.; et al. deMon2k, Version 5; The deMon Developers, Cinvestav: Mexico City, Mexico, 2018. [Google Scholar]
- Morris, G.; Goodsell, D.; Halliday, R.; Huey, R.; Hart, W.; Belew, R.; Olson, A. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).