Abstract
Radiotherapy (RT) is a standard treatment for cervical cancer, but its efficacy is often limited by tumor hypoxia and low radiosensitivity. Radiosensitizers that enhance RT without dose escalation are therefore of clinical interest. Alpha-mangostin (AM), a xanthone from Garcinia mangostana, exhibits anticancer and ROS-inducing properties. This study evaluated whether AM enhances radiosensitivity in vitro. Cytotoxicity (0–35 µM) was assessed by the MTT assay, and radiation sensitivity (0–6 Gy) was assessed by clonogenic survival. γ-H2AX immunofluorescence, cell cycle distribution, apoptosis induction, and clonogenic survival assessments were used to investigate the radiosensitization effect. AM showed dose-dependent cytotoxicity in HeLa cells at an inhibitory concentration 20 (IC20) of 13.67 µM while sparing fibroblasts. The radiation lethal dose 20 (LD20) was 1.4 Gy. However, combination treatment used AM at 12 µM (IC14) combined with 2 Gy (LD30) irradiation to avoid 50% cell death. AM enhanced G2/M arrest by 21.10% (p < 0.01) versus controls. In combination treatment, AM significantly increased γ-H2AX-positive cells to 48.2% (p < 0.0001), elevated apoptosis to 39.48% (p < 0.0001), and decreased clonogenic survival to 28% (p < 0.0001) compared with control. A combination index of about 0.9 indicated synergism. Therefore, AM effectively radiosensitized HeLa cells via increased DNA double-strand breaks and G2/M arrest.
1. Introduction
Cervical cancer is one of the most common cancers among females worldwide, with over half a million new cases reported annually in Thailand [1]. Surgery or radiation has been used for early-stage diseases with equivalent treatment outcomes. For locally advanced cervical cancer, radiation therapy remains the primary and often the only curative treatment option [2]. However, cervical cancers frequently experience hypoxia due to their rapid growth and insufficient blood supply [3,4]. Hypoxia compromises both the induction and repair of radiation-mediated DNA damage, enabling tumor cells to evade cytotoxic effects, survive, and proliferate under low-oxygen conditions [5]. To overcome this resistance, higher radiation doses are often required, but dose escalation inevitably increases the risk of adverse reactions in surrounding normal tissues [6].
To address this limitation, combining radiotherapy with novel therapeutic strategies, such as radiosensitizers that selectively sensitize tumor cells, represents a promising approach to maximize treatment efficacy while minimizing toxicity. Radiosensitizers are agents that increase the susceptibility of tumor cells to radiation, enabling tumor control at lower radiation doses while minimizing normal tissue damage [7]. In recent years, natural compounds have emerged as attractive candidates for radiosensitizers due to their diverse biological activities, lower systemic toxicity, and favorable safety profiles [8]. Several plant-derived compounds, such as curcumin, plumbagin, and resveratrol, have been shown to enhance radiosensitivity in various cancer models through modulation of reactive oxygen species (ROS) production, inhibition of pro-survival signaling pathways, and induction of apoptosis [9,10,11].
Alpha-mangostin (AM) is a prenylated xanthone isolated from the pericarp of Garcinia mangostana, widely used in modern pharmaceutical and cosmetic industries [12]. AM exhibits broad-spectrum anticancer activities, including pro-apoptotic, anti-proliferative, and anti-metastatic effects [13,14,15]. Previous studies have demonstrated that AM can induce mitochondrial dysfunction, increase intracellular ROS levels, and activate caspase-dependent apoptosis in multiple cancer cell types, including breast, prostate, and cervical cancers [13,14,15]. In addition, AM has been shown to induce arrest at the G2/M phase in Hela cells [13], a stage that is recognized as the most radiosensitive phase of the cell cycle [16]. Despite these findings, to our knowledge, no prior studies have systematically evaluated the radiosensitizing effect of AM in combination with radiation.
Although the anticancer properties of AM are well documented, its role as a radiosensitizer in cervical cancer remains poorly understood. The present study therefore aimed to investigate the radiosensitizing effects of AM in HeLa cervical cancer cells. Specifically, we evaluated the combined effects of AM and photon beam irradiation on cell viability, DNA double-strand break (DSBs) formation, apoptosis induction, cell cycle distribution, and clonogenic survival. This approach allowed us to determine whether AM can potentiate radiation-induced cytotoxicity and to identify potential mechanisms underlying its radiosensitizing activity.
2. Materials and Methods
2.1. Cell Lines and Cell Culture
HeLa cervical cancer cells were kindly provided by Assoc. Prof. Dr. Nipaporn Ngernyuang, and CCD fibroblast cells were kindly provided by Assoc. Prof. Dr. Kanchana Usuwanthim. Each cell type was cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco; ThermoFisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin-streptomycin (Gibco). Cultures were maintained at 37 °C in a humidified incubator containing 5% CO2 and 95% air. Cells were subculture every 3–4 days, and only exponentially growing cells were used for subsequent experiments.
2.2. Cytotoxicity Evaluation and Determine Inhibitory Concentration 20 (IC20)
HeLa cells and fibroblasts were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated overnight. Cells were treated with increasing concentrations of AM (0–35 µM) for 24 h. Three replicates were used for each tested condition. Dimethyl sulfoxide (DMSO; 0.2%, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) served as a vehicle control. Following treatment, MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Thermo Fisher Scientific Inc., Waltham, MA, USA) was added at a final concentration of 0.5 mg/mL in serum-free medium (100 µL/well) and incubated for 4 h at 37 °C in the dark. Formazan crystals were dissolved in DMSO, and absorbance was measured at 570 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). The IC20 defined as the AM concentration at which 80% of cells survive was obtained by interpolation of the dose–response curves in Hela cell lines, which used in combination experiment [17].
2.3. Radiation Lethal Dose 20 (LD20) Evaluation
Radiation lethal dose was determined using a clonogenic assay. Briefly, HeLa cells were seeded at 500–3000 cells/well in 6-well plates and incubated overnight. Cells were irradiated at 0, 1, 2, 4, or 6 Gy. Following irradiation, the medium was replaced with fresh DMEM, and cells were allowed to grow for 10 days. Colonies were fixed with methanol, stained with 0.01% crystal violet (Loba Chemie, Mumbai, Maharashtra, India) for 45 min, and counted. Colonies containing more than 50 cells were scored to calculate plating efficiency (PE) and survival fraction (SF). Survival curves were generated by fitting the data to a linear–quadratic (LQ) model using GraphPad Prism version 10.5 (GraphPad Software Inc., Boston, MA, USA). The LD20 defined as the radiation dose at which 80% of cells survive was obtained by interpolation of the dose–response curves in Hela cell lines, which used further in combination experiments [17].
2.4. Combination Treatment
For combination experiments, HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h. HeLa cells were pretreated with AM at a concentration at the IC20 value to combined with a radiation dose at the LD20 value or equal to the standard 2 Gy clinical fraction dose if LD20 greater than 2 Gy. This ensured that the combination treatment did not reduce viability by more than 50%, allowing the radiosensitizing effect of AM to be assessed independently of high baseline cytotoxicity [17]. We calculated the combination index (CI) described previously [18], to determine if the interaction between radiation and drug is synergistic according to the formula: CI = (d1/Dx1) + (d2/Dx2) whereas Dx1: dose of radiation alone at which 28% of cells survive; d1: dose of radiation in AM treated cells at which 28% of cells survive; Dx2: Concentration of AM alone at which 28% of cells survive; d2: Concentration of AM in irradiated cells at which 28% of cells survive. A CI bellow 1 implies supra-additive effect and a true synergism between the two agents.
2.5. Photon Irradiation Setup
HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h. Cells were pretreated with or without AM for 24 h prior to irradiation. Photon beam irradiation was performed using a 6 MV linear accelerator (Varian Clinac 2100C, Varian Medical Systems, Palo Alto, CA, USA) at the Faculty of Medicine, Naresuan University. Single doses of LD20 Gy or 2 Gy were delivered at room temperature using a solid water phantom (30 × 30 × 15 cm3, Varian) to simulate human tissue. The source-to-axis distance (SAD) was 100 cm, and 24-well plate was irradiated vertically in a 15 × 20 cm2 field, positioned at the isocenter within a bolus phantom at a depth of 3 cm [17].
2.6. Immunofluorescence for γ-H2AX
HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h, then pretreated with or without AM (IC20) for 24 h. After AM pretreatment, cells were irradiated with photon beam at LD20 Gy and γ-H2AX immunofluorescence staining was performed at 1 h post-irradiation. Cells were washed with PBS, fixed in 4% paraformaldehyde for 30 min, permeabilized in 0.5% Triton X-100/PBS for 7 min, and blocked with 2% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS for 1 h. Immunostaining was performed using a mouse monoclonal anti–phospho-Histone H2AX (Ser139) antibody (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 1:300 dilution overnight at 4 °C. After three washes in PBS, Alexa Fluor 488–conjugated goat anti-mouse IgG (Gibco) was applied at 1:400 dilution for 1 h at room temperature. Nuclei were counterstained with DAPI (Gibco), and images were acquired using a Carl Zeiss fluorescence microscope (Zeiss, Jena, Germany). Mean γ-H2AX fluorescence intensity per nucleus was quantified using ImageJ software version 1.53t (NIH, Bethesda, MD, USA) [17,19]. All experiments were performed in triplicate.
2.7. Cell Cycle Analysis
Cell cycle distributions were analyzed by assessing the cellular DNA content. HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h, then pretreated with or without AM (IC20) for 24 h. Cells were subsequently irradiated with a photon beam at LD20 Gy and incubated for an additional 24 h. Following treatment, cells were harvested, fixed in 70% ethanol (RCI Labscan, Bangkok, Thailand), and stored at −20 °C overnight. Fixed cells were stained with the Muse® Cell Cycle Kit (EMD Millipore Corp., Billerica, MA, USA) according to the manufacturer’s protocol for 30 min at room temperature in the dark. DNA content was analyzed using a Muse® Cell Analyzer (EMD Millipore Corp., Burlington, MA, USA) with the Cell Cycle software module, and phase distributions (G0/G1, S, and G2/M) were quantified using the GuavaSoft 2.7 software (EMD Millipore Corp., Burlington, MA, USA) [17].
2.8. Apoptosis Assay
HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h, then pretreated with or without AM (IC20) for 24 h. Cells were subsequently irradiated with a photon beam at LD20 Gy and incubated for an additional 24 h. Following treatment, cells were harvested, and quantitative analysis of live, early apoptotic, late apoptotic, and dead cells was performed using the Muse® Cell Analyzer (EMD Millipore Corp., Burlington, MA, USA) with the Annexin V and Dead Cell software module (EMD Millipore Corp., Burlington, MA, USA) [17].
2.9. Colony Formation Assay
HeLa cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 24 h, then pretreated with or without AM (IC20) for 24 h. Cells were subsequently irradiated with a photon beam at LD20 Gy. After irradiation, cells were reseeded and incubated for 10 days to allow colony formation. Colonies were counted from each triplicate sample and presented as the mean ± standard deviation (SD). Colonies were then fixed with methanol and stained with 0.05% crystal violet (Loba Chemie, Mumbai, Maharashtra, India) for 45 min. Colonies containing more than 50 cells were counted to calculate plating efficiency (PE) as the ratio of the number of colonies to the number of cells seeded in the non-irradiated control group. The survival fraction (SF) was determined as the mean number of colonies divided by the product of the number of cells seeded and the PE. Survival curves were generated by fitting the data to the linear–quadratic (LQ) model using GraphPad Prism version 10.5 (GraphPad Software, Inc., Boston, MA, USA) [17].
2.10. Statistical Analysis
All experiments were conducted in biological at least triplicates. Results are expressed as mean ± standard deviation (SD). Statistical comparisons were performed using one-way ANOVA. A p-value < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 10.5 (GraphPad Software, Inc., Boston, MA, USA).
3. Results
3.1. Cytotoxicity Assessment and Determination of IC20 and LD20 Values of Alpha-Mangostin and Radiation in Hela Cells
MTT assays were conducted to assess the cytotoxic effects of AM on HeLa cervical cancer cells and normal fibroblasts after 24 h of treatment with increasing concentrations (0–35 µM) (Figure 1B,C). In HeLa cells, AM induced a clear dose-dependent reduction in viability, decreasing from 98% at 5 µM to 6.38% at 35 µM. The IC20 value, calculated from the dose–response curve, was approximately 13.67 µM (Figure 1B). By contrast, fibroblasts displayed much higher tolerance, maintaining over 85% viability up to 25 µM, with noticeable reductions only at greater than 30 µM (41% and 40% viability for 30 and 35 µM, respectively) (Figure 1C). These findings indicate that AM exhibits greater cytotoxicity toward cancer cells than normal fibroblasts at equal concentrations, supporting the use of low, sub-toxic concentrations in combination with radiotherapy.
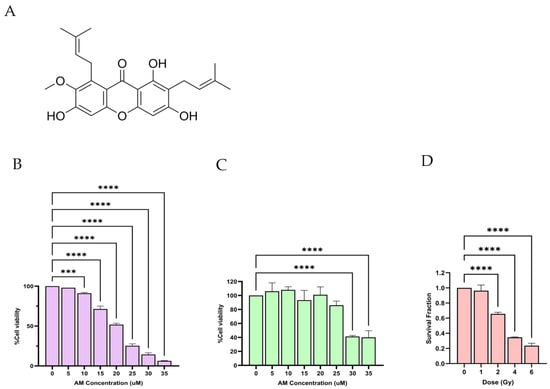
Figure 1.
Determination of IC20 of AM and LD20 of radiation in HeLa cells. (A) Chemical structure of AM. (B) Cytotoxicity of AM in HeLa cells after 24 h treatment with increasing concentrations (0–35 µM), as determined by the MTT assay. Cell viability decreased in a dose-dependent manner, with the IC20 value calculated to be approximately 13.67 µM, which interpolated by using equation: y . (C) Cytotoxicity of AM in normal human fibroblasts under the same conditions, showing fibroblast viability remained >85% at concentrations up to 20 µM, indicating selective toxicity of AM toward cancer cells at equivalent concentrations. (D) Clonogenic survival of HeLa cells following exposure to single doses of ionizing radiation (0–6 Gy) for 10 days. SF were calculated by clonogenic assay. LQ model was used to interpolating the suitable radiation dose which SF . The LD20 was determined to be approximately 1.4 Gy. Data are presented as mean ± SD from three independent experiments. Statistical significance: *** p < 0.001, **** p < 0.0001.
Radiation sensitivity was evaluated using a clonogenic survival assay in HeLa cells exposed to single radiation doses of 0, 1, 2, 4, or 6 Gy (Figure 1D). Survival fractions were 0.96, 0.66, 0.35, and 0.23 for 1, 2, 4, and 6 Gy, respectively. From the survival curve, the LD20 was estimated to be 1.4 Gy; however, since the conventional clinical fractionated radiotherapy dose (2 Gy) [20] that is about LD30, this dose was chosen for our study. To compensate for the increased radiation dose, the AM concentration was reduced from 13.67 µM to 12 µM. Importantly, combining AM (12 µM) with 2 Gy irradiation did not induce more than 50% cell death, enabling the evaluation of the radiosensitizing potential of AM without excessive baseline cytotoxicity from radiation alone.
3.2. Alpha-Mangostin Induced DNA Damage Response Assessed by γ-H2AX Staining
To investigate whether AM potentiates radiation-induced DNA damage, we assessed γ-H2AX foci formation by immunofluorescence staining (Figure 2). Quantitative analysis revealed that AM alone induced a modest but significant increase in γ-H2AX intensity compared with the control (12.26% vs. 0.67%, p < 0.001). Irradiation at 2 Gy markedly elevated γ-H2AX expression (25.94%, p < 0.0001 vs. control). Notably, the combination of AM and 2 Gy irradiation resulted in a pronounced increase in γ-H2AX intensity (48.20%, p < 0.0001 vs. 2 Gy alone), representing nearly a twofold enhancement relative to radiation alone. Representative immunofluorescence images as show in Figure 2B supported these findings, showing sparse γ-H2AX foci in untreated cells, a slight increase with AM treatment, moderate foci accumulation following 2 Gy irradiation, and a substantial increase in γ-H2AX foci when AM was combined with irradiation. These results indicate that AM significantly potentiates radiation-induced DSBs and impairs their repair, consistent with its proposed role as a radiosensitizer.
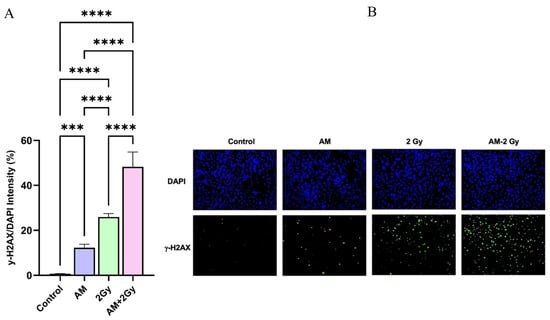
Figure 2.
Effect of AM on radiation-induced DSBs in HeLa cells. (A) Quantification of γ-H2AX fluorescence intensity following treatment with AM 12 µM, 2 Gy irradiation, or their combination. Data are presented as mean ± SD (n = 3). Statistical significance: *** p < 0.001, **** p < 0.0001. (B) Representative immunofluorescence images of γ-H2AX positive (green) and DAPI-stained nuclei (blue) for each treatment condition (×20). AM in combination with 2 Gy markedly increased γ-H2AX positive compared to either treatment alone.
3.3. Effects of Alpha-Mangostin and Ionizing Radiation on Cell Cycle Distribution in HeLa Cells
To investigate whether AM modulates cell cycle progression and contributes to radiosensitization, cell cycle distribution was analyzed by flow cytometry. As shown in Figure 3A, in the control group, the majority of cell were in the G0/G1 phase (71.22%), with smaller proportions in the S phase (13.86%) and G2/M phase (14.18%). In treatment with AM or 2 Gy irradiation alone significantly decreased the proportion of HeLa cells in the G0/G1 phase compared with the control group (60.76% and 59.62%, respectively, p < 0.05–0.001). In contrast, both treatments increased the percentage of cells in the G2/M phase, consistent with cell cycle arrest. Notably, the combination of AM with irradiation resulted in the most pronounced effect, elevating the G2/M fraction to 26.66% compared with 14.18% in controls (p < 0.001). No significant differences were observed among groups in the S-phase population. These results demonstrate that AM induces G2/M phase arrest in HeLa cells and enhances radiation-induced accumulation in this radiosensitive phase. This mechanistic effect suggests that AM potentiates radiosensitivity by delaying cell cycle progression at the G2/M checkpoint, thereby preventing DNA repair prior to mitotic entry.
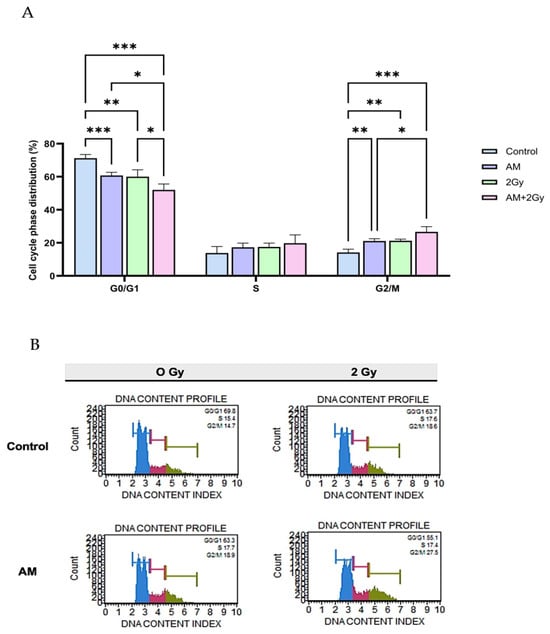
Figure 3.
Effects of AM and ionizing radiation on cell cycle distribution in HeLa cells. (A) Quantitative analysis of cell cycle phase distribution following treatments: control, AM (5 µg/mL), 2 Gy irradiation, and AM + 2 Gy combination. Data are presented as mean ± SD from three independent experiments. Statistical significance: * p < 0.05, ** p < 0.01, *** p < 0.001. (B) Representative DNA content histograms for each treatment, clearly marked with G0/G1 (blue), S (red), and G2/M (green) phases for better visualization. Cells were exposed to 0 Gy or 2 Gy radiation with/without AM pretreatment. The combination treatment resulted in the highest G2/M accumulation and the lowest G0/G1 proportion compared with other groups.
3.4. Combined Alpha-Mangostin and Radiation Treatment Enhances Apoptosis in HeLa Cells
The pro-apoptotic effects of AM in combination with ionizing radiation were evaluated in HeLa cells using Annexin V/PI staining and flow cytometric analysis (Figure 4A,B). Quantitative analysis (Figure 4A) revealed that AM treatment alone significantly increased apoptosis to 21.88% compared with the control (4.02%, p < 0.001). Irradiation with 2 Gy alone induced a modest increase in apoptosis (7.16%, p < 0.01 vs. control). Notably, the combined treatment of AM with 2 Gy irradiation resulted in a marked elevation of apoptosis (39.48%, p < 0.0001 vs. either single treatment), representing the highest apoptotic fraction among all groups. Representative flow cytometry plots (Figure 4B) corroborated these findings, showing increased populations of early and late apoptotic cells in the combination group compared with AM or radiation alone. These results indicate that AM strongly enhances radiation-induced apoptosis in HeLa cells, supporting its role as a potential radiosensitizer.
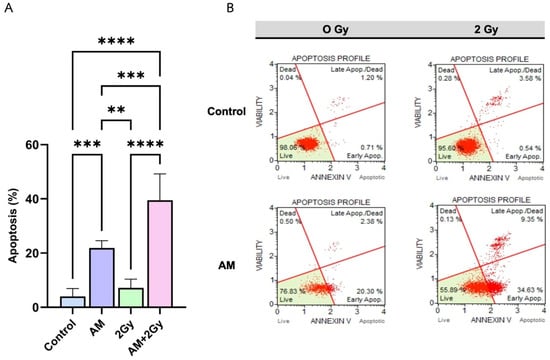
Figure 4.
(A) Quantification of apoptotic cells (% cell appearance) measured by Annexin V/Dead Cell assay 24 h after treatment with control, AM 5 µg/mL, 2 Gy irradiation, or the combination (AM + 2 Gy). Data are presented as mean ± SD (n = 5). Statistical significance: ** p < 0.01, *** p < 0.001, **** p < 0.0001. (B) Representative dot plots showing Annexin V vs. viability staining profiles for each treatment condition, with live cells (lower left quadrant), early apoptotic cells (lower right), late apoptotic/dead cells (upper right), and necrotic cells (upper left). AM + 2 Gy markedly increased both early and late apoptotic populations compared to single treatments.
3.5. Radiosensitizing Effect in Clonogenic Assay
The clonogenic assay was performed to evaluate the radiosensitizing potential of AM in HeLa cells (Figure 5A,B). Treatment with AM 5 µg/mL alone slightly reduced colony formation to 79% compared with the untreated control (p < 0.01), whereas 2 Gy irradiation alone further decreased survival to 62% of control levels (p < 0.05 vs. control). Notably, the combination of AM with 2 Gy irradiation led to a marked reduction in clonogenic survival, with only 28% of colonies remaining relative to the control (p < 0.0001 vs. irradiation alone). Representative colony formation images (Figure 5B) confirmed these findings, showing visibly fewer and smaller colonies in the combination group compared with either single treatment. These results strongly indicate that AM enhances the cytotoxic effects of photon irradiation, thereby functioning as a potential radiosensitizer in cervical cancer cells.
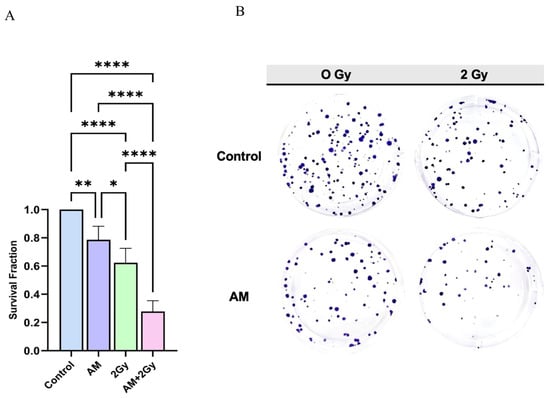
Figure 5.
(A) Quantification of clonogenic survival fraction in HeLa cells treated with control, AM, 2 Gy irradiation, or the combination (AM + 2 Gy). Data are presented as mean ± SD (n = 5). Statistical significance: * p < 0.05, ** p < 0.01, **** p < 0.0001. (B) Representative images of crystal violet–stained colonies formed after 10 days of growth under each treatment condition. The combination of AM with 2 Gy irradiation resulted in markedly fewer colonies compared with either treatment alone.
4. Discussion
AM, a prenylated xanthone isolated from the pericarp of Garcinia mangostana, has been widely reported for its anticancer activity; however, its potential role as a radiosensitizer has not been previously investigated. In the present study, AM exhibited dose-dependent cytotoxicity against HeLa cervical cancer cells (IC20 = 13.67 µM) while sparing normal fibroblasts (>85% viability at concentrations up to 25 µM) (Figure 1B,C), indicating selective cytotoxicity at low concentrations. This cytotoxicity is consistent with previous reports showing that AM inhibits mitochondrial respiratory complexes I–V, disrupts electron transport, and generates superoxide anions, resulting in elevated intracellular reactive oxygen species (ROS) [21,22]. The consequent reduction in mitochondrial membrane potential promotes cytochrome c release and activates the caspase-9/caspase-3 apoptotic cascade [21,22]. Therefore, AM-induced cytotoxicity primarily results from oxidative stress and mitochondrial dysfunction rather than direct DNA interaction. This mechanism is particularly relevant to radiosensitization, as radiation-induced ROS contribute to indirect DNA damage and may act synergistically with AM.
Radiosensitizers enhance tumor cell sensitivity to radiation through several mechanisms, including increased DNA damage, inhibition of DNA repair, modulation of the cell cycle, and alleviation of hypoxia [23]. These mechanisms enable effective tumor control at lower radiation doses while minimizing injury to normal tissues [7]. To our knowledge, this is the first study to demonstrate that AM enhances the radiosensitivity of HeLa cells to X-rays in vitro. To reflect clinically relevant fractionated doses and to preserve sufficient surviving cells for clonogenic evaluation, we selected AM at 12 µM (IC14) combined with 2 Gy irradiation (LD30), rather than the IC20-LD20 pair. This optimization prevented excessive cytotoxicity that might obscure whether the combined effects were additive or synergistic, thereby providing a more accurate assessment of radiosensitization. Thus, instead of the standard IC50 and LD50 parameters typically used in cytotoxicity assays, this approach was better suited for radiosensitization analysis, where post-irradiation survival is essential for interpretation.
To determine whether ROS accumulation contributed to AM-mediated radiosensitization, γ-H2AX was used as a biomarker of DSBs and repair kinetics [16,24,25,26,27,28,29]. AM alone modestly increased γ-H2AX expression compared with the control, whereas the combination of AM with 2 Gy markedly elevated γ-H2AX intensity beyond irradiation alone (Figure 2B), indicating delayed repair and sustained DNA damage. The persistence of γ-H2AX at 1 h post-irradiation—when most DSB repair is typically completed within 30–60 min [19]—further supports impairment of DNA repair, in agreement with prior findings for xanthone derivatives [30] and corresponds with the increased apoptosis observed in the combination group. As AM induces mitochondrial dysfunction and excessive ROS production, these ROS likely act synergistically with ionizing radiation to exacerbate DNA damage [31]. Because AM with 2 Gy treatment produced dense, overlapping γ-H2AX foci, signal intensity was used as a quantitative readout, providing a more reliable indicator of accumulated DSBs under these conditions [17,19]. Nonetheless, focus counting and time-course analyses should be incorporated in future studies to confirm these findings.
After DNA damage, cells often undergo cycle arrest to allow repair, and if repair fails, apoptosis is triggered. As cells in the G2/M phase are the most radiosensitive [16], induction of G2/M arrest is a recognized mechanism of radiosensitization. Both AM and irradiation alone significantly increased the proportion of cells in G2/M compared with controls; the combination further increased this fraction, although not significantly beyond irradiation alone. Importantly, the AM with 2 Gy showed a significantly lower G0/G1 fraction than irradiation alone, indicating a reduced viable population. This pattern corresponds with the γ-H2AX and apoptosis results (Figure 2 and Figure 4), which demonstrated that AM with 2 Gy induced higher levels of DNA damage and apoptosis than radiation alone. These observations suggest that AM inhibits DNA repair and promotes apoptosis at 24 h post-irradiation, explaining why G2/M accumulation did not rise further despite enhanced cell death. The increased apoptosis is likely mediated through the intrinsic mitochondrial pathway, consistent with AM’s known mechanism of cytochrome c release and caspase-9/caspase-3 activation. Such effects parallel those of other plant-derived radiosensitizers, including gingerol, genistein, curcumin, and plumbagin, which increase radiosensitivity by inducing DNA damage, G2/M arrest, and apoptosis [2,10,32,33,34].
Clonogenic survival assays confirmed the radiosensitizing potential of AM. The combination of AM with 2 Gy irradiation reduced clonogenic survival to 28% compared with the untreated control (Figure 5), and the calculated combination index (CI) < 1 indicated a synergistic interaction with supra-additive effects [18]. Notably, this radiosensitization was achieved at non-cytotoxic concentrations of AM and at clinically relevant radiation doses (2 Gy), emphasizing its translational relevance. When considering the radiation enhancement ratio at a SF of 28%, cells treated without AM would require approximately 5 Gy to reach the same level of cell killing—equivalent to a 2.5-fold increase in radiation dose. This finding underscores AM’s strong radiosensitizing efficacy, suggesting that it can achieve comparable tumor cell control at substantially lower radiation doses. Given that curative radiotherapy for cervical cancer typically delivers approximately 50 Gy in 1.8–2 Gy fractions [35], AM represents a promising natural radiosensitizer that could enhance tumor control while minimizing normal tissue toxicity.
Although this study provides clear in vitro evidence for the radiosensitizing effects of AM, several limitations should be acknowledged. The experiments were conducted using only one cervical cancer cell line (HeLa) and a single radiation dose (2 Gy). While this setup establishes proof-of-concept, in vitro systems cannot fully reproduce the complexity of the tumor microenvironment, drug metabolism, or host immune interactions. In addition, clonogenic survival was evaluated primarily at one radiation dose, which, although clinically relevant, limits the ability to generate a full dose–response curve. Assessing multiple radiation doses and plotting complete survival curves would provide a more rigorous measure of radiosensitivity and clarify the mechanistic effects of AM on DNA damage and repair. Therefore, future studies should include multi-dose clonogenic assays, additional cervical cancer cell lines (e.g., SiHa and CaSki), and in vivo validation to confirm AM’s pharmacokinetics, tumor selectivity, and therapeutic efficacy under clinically relevant conditions. Such studies will be essential to establish the translational potential of AM as a natural radiosensitizer for cervical cancer therapy.
5. Conclusions
AM significantly enhances the radiosensitivity of HeLa cervical cancer cells through multiple interrelated mechanisms. AM potentiates radiation-induced DSBs and delays their repair, as evidenced by the elevated and sustained γ-H2AX expression. It also induces G2/M phase arrest—the most radiosensitive stage of the cell cycle—and promotes apoptosis activation, leading to a marked reduction in clonogenic survival. These findings indicate that AM exerts a synergistic effect with ionizing radiation by impairing DNA repair, modulating the cell cycle, and activating apoptotic pathways. Given its cytotoxicity toward malignant cells and low toxicity to normal fibroblasts, AM represents a promising natural radiosensitizer for cervical cancer radiotherapy. Nonetheless, further in vivo and mechanistic studies are required to confirm its pharmacological properties, tumor specificity, and therapeutic potential under clinically relevant conditions.
Author Contributions
Conceptualization, P.A., A.P. and C.S.; methodology, P.A., A.P. and C.S.; investigation, P.A. and C.S.; formal analysis, P.A., A.P. and C.S.; validation, P.A., A.P. and C.S.; writing—original draft preparation, P.A., A.P. and C.S.; writing—review and editing, C.S.; supervision, C.S.; project administration, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the University Income Fund, Naresuan University, grant number R2567C014.
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
Acknowledgments
The authors sincerely thank the Faculty of Allied Health Sciences at Naresuan University for generously providing the facilities and institutional support needed for the experimental work. We also appreciate the Department of Radiology in the Faculty of Medicine at Naresuan University for granting access to the LINAC machine. Our gratitude extends to Orawan Kumcharoenkun (Radiologist) and Kamonchanok Nobphuek (Medical physicist) for their invaluable technical assistance with the irradiation setup.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, X.; Ma, J.; Zheng, Y.; Wang, Q.; Wang, Y.; Shang, H. Human papillomavirus 16 E6 contributes HIF-1α induced Warburg effect by attenuating the VHL-HIF-1α interaction. Int. J. Mol. Sci. 2014, 15, 7974–7986. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Megadhana, W.; Winata, I.G.; Widiyanti, E.; Lawu, A. Role of Oxygenation Factor Hypoxia-inducible Factor-1α (HIF-1α) as Prognostic Indicators in Cervical Cancer. J. South Asian Fed. Obstet. Gynaecol. 2023, 15, 490–496. [Google Scholar] [CrossRef]
- Olcina, M.M.; Kim, R.; Giaccia, A.J. The Role of Hypoxia in Radiation Response. In Strategies to Enhance the Therapeutic Ratio of Radiation as a Cancer Treatment; Anscher, M.S., Valerie, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–42. [Google Scholar]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef]
- da Costa Araldi, I.C.; Bordin, F.P.R.; Cadoná, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.; Bauermann, L.F. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem. Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Nair, R.R.; Srinivas, P.; Srinivas, G.; Pillai, M.R. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol. Carcinog. 2008, 47, 22–33. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef]
- Saraswathy, S.U.P.; Lalitha, L.C.P.; Rahim, S.; Gopinath, C.; Haleema, S.; SarojiniAmma, S.; Aboul-Enein, H.Y. A Review on Synthetic and Pharmacological Potential of Compounds Isolated from Garcinia mangostana Linn. Phytomed. Plus 2022, 2, 100253. [Google Scholar] [CrossRef]
- El Habbash, A.I.; Mohd Hashim, N.; Ibrahim, M.Y.; Yahayu, M.; Omer, F.A.E.; Abd Rahman, M.; Nordin, N.; Lian, G.E.C. In vitro assessment of anti-proliferative effect induced by α-mangostin from Cratoxylum arborescens on HeLa cells. PeerJ 2017, 5, e3460. [Google Scholar] [CrossRef]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer 2014, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Dukaew, N.; Konishi, T.; Chairatvit, K.; Autsavapromporn, N.; Soonthornchareonnon, N.; Wongnoppavich, A. Enhancement of Radiosensitivity by Eurycomalactone in Human NSCLC Cells Through G2/M Cell Cycle Arrest and Delayed DNA Double-Strand Break Repair. Oncol. Res. 2020, 28, 161–175. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.J.; Vasireddy, R.S.; Tang, M.M.; Georgiadis, G.T.; El-Osta, A.; Karagiannis, T.C. Quantification of H2AX foci in response to ionising radiation. J. Vis. Exp. 2010, 38, 1957. [Google Scholar] [CrossRef]
- Royce, T.J.; Lee, D.H.; Keum, N.; Permpalung, N.; Chiew, C.J.; Epstein, S.; Pluchino, K.M.; D’Amico, A.V. Conventional Versus Hypofractionated Radiation Therapy for Localized Prostate Cancer: A Meta-analysis of Randomized Noninferiority Trials. Eur. Urol. Focus 2019, 5, 577–584. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Medina-Campos, O.N.; Sciutto, E.; Fragoso, G.; Pedraza-Chaverri, J. α-Mangostin induces oxidative damage, mitochondrial dysfunction, and apoptosis in a triple-negative breast cancer model. Phytother. Res. 2023, 37, 3394–3407. [Google Scholar] [CrossRef]
- Lee, C.H.; Ying, T.H.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Chou, R.H.; Hsieh, Y.H. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Celeste, A.; Fernandez-Capetillo, O.; Kruhlak, M.J.; Pilch, D.R.; Staudt, D.W.; Lee, A.; Bonner, R.F.; Bonner, W.M.; Nussenzweig, A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell Biol. 2003, 5, 675–679. [Google Scholar] [CrossRef]
- Mladenov, E.; Magin, S.; Soni, A.; Iliakis, G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front. Oncol. 2013, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Pilch, D.R.; Redon, C.; Bonner, W.M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2003, 2, 233–235. [Google Scholar] [CrossRef]
- Ward, I.M.; Minn, K.; Jorda, K.G.; Chen, J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J. Biol. Chem. 2003, 278, 19579–19582. [Google Scholar] [CrossRef] [PubMed]
- West, M.H.; Bonner, W.M. Histone 2A, a heteromorphous family of eight protein species. Biochemistry 1980, 19, 3238–3245. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, X.; Yang, K.; Feng, S.; Zhang, Y.; Dong, J.; Liu, Z.; Qiao, X. Synthesis and biological evaluation of xanthone derivatives as anti-cancer agents targeting topoisomerase II and DNA. Med. Chem. Res. 2022, 31, 720–734. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.J.; Zhang, J.F.; Dai, W.J.; Li, R.L.; Lu, Z.Y.; Duan, J.L.; Li, J.P.; Chen, X.P.; Fan, J.P.; et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget 2017, 8, 50747–50760. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein enhances the radiosensitivity of breast cancer cells via G2/M cell cycle arrest and apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; Luo, L.; Zhang, Q.; Gao, C.; Zhuang, X.; Yuan, S.; Qiao, T. [6]-Gingerol enhances the radiosensitivity of gastric cancer via G2/M phase arrest and apoptosis induction. Oncol. Rep. 2018, 39, 2252–2260. [Google Scholar] [CrossRef]
- Fu, Q.; Li, W.; Zuo, J.; Yang, X.; Xu, Y.; Huang, M.; An, J.; Jia, S.; Wu, L. A feasibility study of a modified treatment strategy combined external beam radiation therapy and brachytherapy for cervical cancer. J. Appl. Clin. Med. Phys. 2022, 23, e13621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).