Abstract
Ataxia-telangiectasia is an autosomal recessive disorder that usually manifests in childhood due to mutations in the Ataxia-Telangiectasia Mutated (ATM) gene. It is believed that there is an association between this gene mutation/polymorphism and cancer risk, including breast, lung, and pancreatic cancers. We report a rare case of a 69-year-old woman who developed three different primary cancers, including non-small cell lung cancer (NSCLC) in both lungs and pancreatic adenocarcinoma, and was later found to have a rarely reported variant mutation in the ATM gene, namely Exon 39, c.5644 C > T. We hypothesize that the ATM gene, c.5644 C > T mutation could be a plausible contributor in the pathogenesis of these three cancers. This hypothesis has yet to be validated by larger studies that focus on a mechanistic approach involving DNA repair genes such as the ATM. More importantly, this paves the way to developing new patient-specific targeted therapies and inaugurating precision medicine as a cornerstone in cancer therapeutics.
1. Introduction
Ataxia-telangiectasia is an autosomal recessive disorder that is caused by a defective Ataxia-Telangiectasia Mutated (ATM) gene [1]. Heterozygous mutations of the ATM gene have been associated with increased risk of developing malignancies through defective DNA-repairing mechanisms [2,3]. It is believed that there is an established association between ATM gene mutation/polymorphism and increased risk of breast cancer [4]. Studies have demonstrated that some variants of ATM are linked to an increased risk of breast cancer development and a worse prognosis [4,5]. Association with lung cancer, especially non-small cell lung cancer (NSCLC), and pancreatic cancer is becoming more established with many reported mutation variants in the literature for both cancers [6,7]. However, this relationship has yet to be fully understood, given the genetic complexity and the involvement of many other genes that harbor pathogenic variants in the pathogenesis of these types of cancers [8]. Over 1000 mutation variants, pathogenic and non-pathogenic, have been reported to involve the ATM gene [9].
In this case report, we discuss a rare case of a 69-year-old woman who developed three different primary cancers, including non-small cell lung cancer (adenocarcinoma) in both lungs and pancreatic adenocarcinoma. This patient was found to have a rarely reported variant mutation in the ATM gene, the Exon 39, c.5644C > T. This variant creates a premature translational stop signal (p.Arg1882*) that prompts loss of function in the ATM gene. Such mutation variants are known to be pathogenic. The c.5644C > T gene has been briefly mentioned in two studies by Coutinho et al. [2] and Buzin et al. [3]; this type of nonsense mutation causes a truncated, dysfunctional ATM protein. We hypothesize that the ATM gene c.5644C > T could be a plausible contributor in the pathogenesis of these three cancers. This case report was conducted and reported in accordance with the Surgical Case Reports (SCARE) guidelines for reporting case reports.
2. Case Presentation
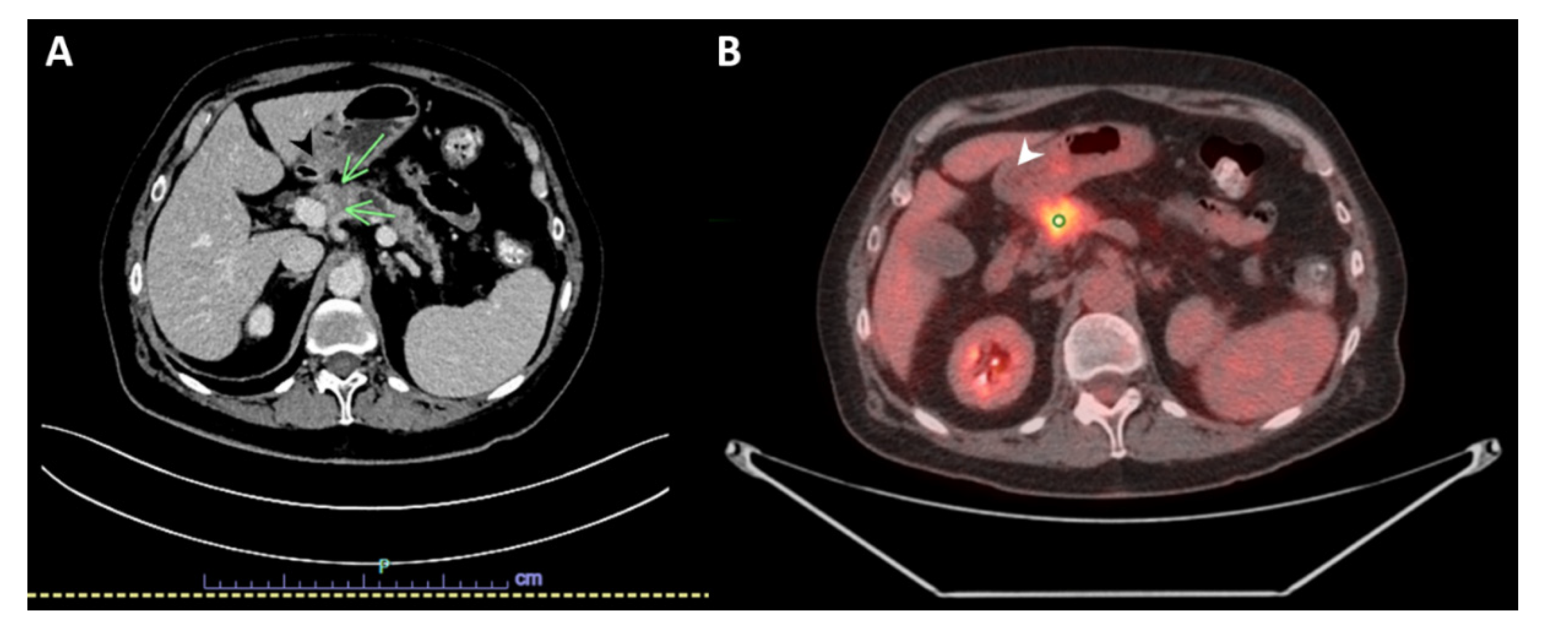
A 69-year-old woman with a history of primary left lung adenocarcinoma (confirmed and supported by immunohistochemical (IHC) stains) in 2014 that was treated with post left upper lobe resection and Cisplatin-based chemotherapy (treated at another institution), and contralateral primary lung adenocarcinoma in 2016 (confirmed and supported by IHC stains as well), which was treated at another institution with a right wedge resection, presented to our institution with abdominal discomfort. A computed tomography (CT) scan of the abdomen and pelvis with IV contrast demonstrated a hypoenhancing pancreatic mass centered in the pancreatic neck, measuring 2.7 cm in greatest dimension, with a dilatation of the upstream main pancreatic duct (up to 7 mm in diameter) and atrophy of the upstream pancreatic parenchyma that was consistent with pancreatic malignancy. The mass appeared to be abutting the splenoportal confluence with mild to moderate stenosis of the splenic vein, moderate stenosis of the superior mesenteric vein, and mild stenosis of the main portal vein at the confluence (Figure 1A). An abdominal positron emission tomography (PET)/CT scan was performed in April 2021, and showed a mildly enlarged pancreas with a 2.7 cm heterogeneous, hypoechoic mass in the head of the pancreas with abnormal hypermetabolic uptake in the SUV MAX 9.3. Multiple subcentimeter peripancreatic lymph nodes were seen without abnormal hypermetabolic uptake (Figure 1B).
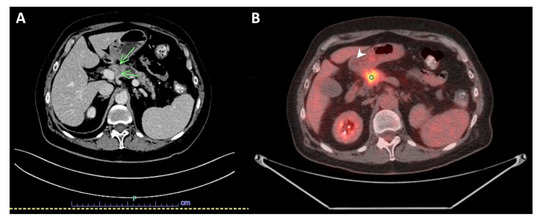
Figure 1.
Imaging studies. (A) Computed tomography (CT) scan with IV contrast of the abdomen and pelvis showing a hypoenhancing pancreatic mass centered in the pancreatic neck, measuring 2.7 × 2 cm (green arrows). (B) Positron emission tomography (PET)/CT scan of the abdomen demonstrating mildly enlarged pancreas with a 2.7 cm heterogeneous, hypoechoic mass in the head of the pancreas with abnormal hypermetabolic uptake in the SUV MAX 9.3 (white arrow).
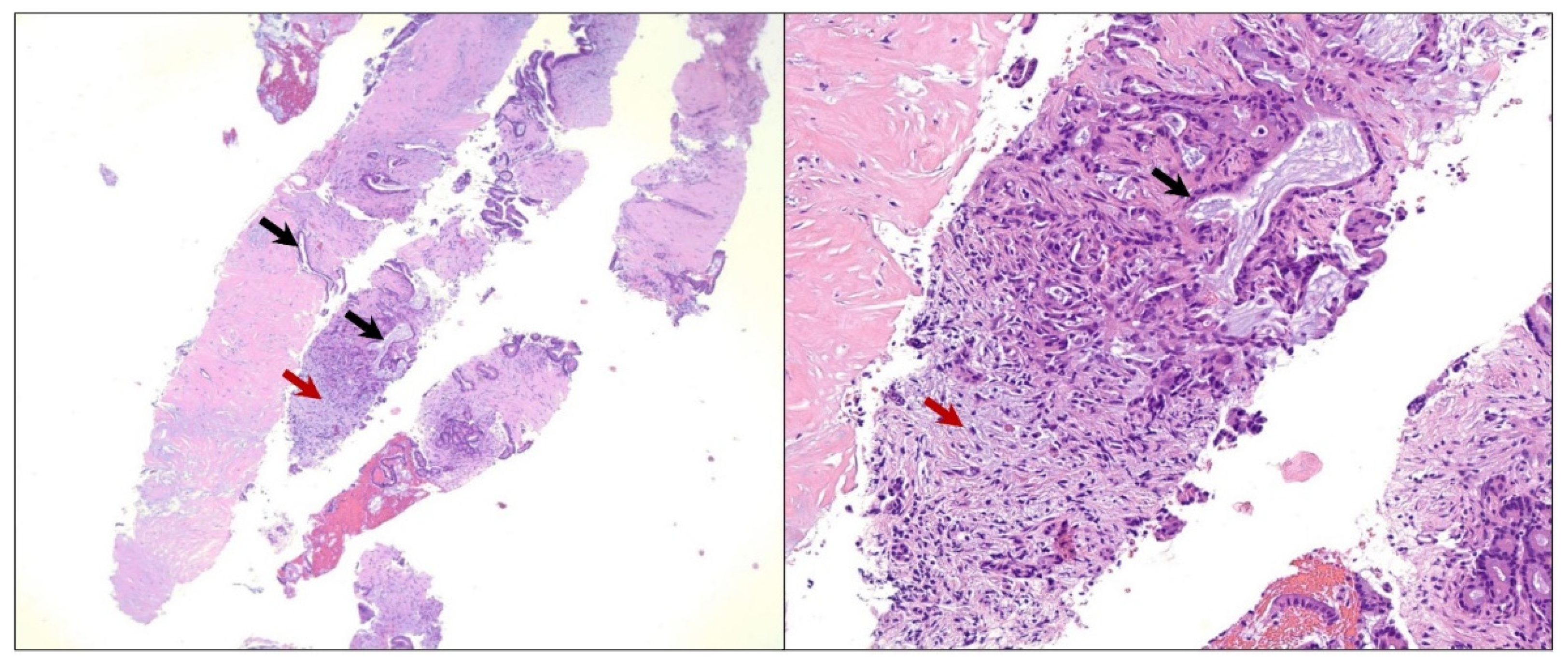
The patient underwent an upper gastroesophageal endoscopy (EGD) with endoscopic ultrasound (EUS), showing an approximately 3 cm hypoechoic mass extending from the neck to the body of the pancreas. An EUS-guided fine needle aspiration (FNA) was performed at an outside institution, and histopathologic examination revealed neoplastic epithelial cells forming glands that were infiltrating the pancreatic stroma (Figure 2). The neoplastic cells showed marked cytologic atypia, a high nuclear-cytoplasmic ratio, and nuclear pleomorphism. A diagnosis of primary moderately differentiated adenocarcinoma in the head of the pancreas was made.
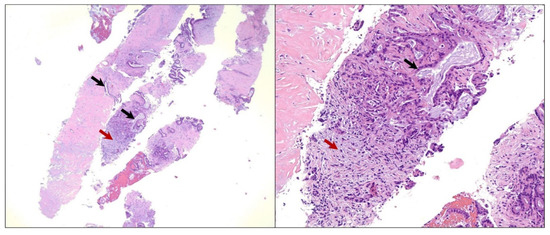
Figure 2.
Microscopic image of the pancreatic fine needle aspiration specimen. Hematoxylin and eosin (H&E) staining showing neoplastic epithelial cells forming glands that are infiltrating the pancreatic stroma (black arrows). Also, a desmoplastic stromal reaction is seen (red arrows). The neoplastic cells show marked cytologic atypia, a high nuclear-cytoplasmic ratio, and nuclear pleomorphism, yielding a diagnosis of moderately differentiated adenocarcinoma in the head of the pancreas. Low-power magnification (40× objective) is shown in the panel to the left and high-power magnification (200× objective) is shown in the panel to the right.
Initial laboratory results, including complete blood count, chemistry profile, liver enzymes, and liver function tests, were within normal reference ranges. The cancer antigen 125 (CA-125) was normal at 12 u/mL (standard range 0.0–38.1 u/mL), the carbohydrate antigen 19-9 (CA19-9) was elevated at 257 u/mL (standard range 0–35 u/mL), and the carcinoembryonic antigen (CEA) was borderline elevated at 4.9 ng/mL (standard range 0–4.7 ng/mL).
Genetic analysis testing of the patient’s blood was performed through an INVITAE multi-cancer panel (Invitae Corp., San Francisco, CA, USA). The Invitae Multi-Cancer Panel analyzes 84 genes associated with genetic disorders and hereditary cancers across major organ systems, including: breast and gynecologic (breast, ovarian, uterine), gastrointestinal (colorectal, gastric, pancreatic), endocrine (thyroid, paraganglioma/pheochromocytoma, parathyroid, pituitary), genitourinary (renal/urinary tract, prostate), skin (melanoma, basal cell carcinoma), brain/nervous system, sarcoma, and hematologic (myelodysplastic syndrome/leukemia). Results revealed one pathogenic variant identified in the ATM gene. This gene is associated with autosomal dominant predisposition to certain cancers and autosomal recessive ataxia-telangiectasia. The results are summarized in Table 1, showing mutation in the ATM gene Exon 39, c.5644C > T (p.Arg1882*) variant.

Table 1.
Results of the genetic analysis testing of the patient’s blood performed through INVITAE multi-cancer panel.
In addition, Guardant360® CDx testing (Guardant Health, Redwood City, CA, USA) was performed on the patient’s blood to provide tumor mutation profiling for her advanced solid malignancies. Guardant360® CDx sequences 74 cancer-associated genes to identify somatic alterations. Cell-free DNA (cDNA) is extracted from plasma, enriched for targeted regions, and sequenced using the Illumina platform and hg19 as the reference genome. All exons are sequenced in some genes; only clinically significant exons are sequenced in other genes. The types of genomic alterations detected by Guardant360 include single nucleotide variants, gene amplifications, fusions, short insertions/deletions (the longest detected was 70 base pairs), and splice site disrupting events. Moreover, microsatellite instability (MS) is assessed for all cancer types by evaluating somatic changes in the length of repetitive sequences on the Guardant360 panel. Guardant360® CDx is a qualitative next generation sequencing (NGS)-based in vitro diagnostic device that uses targeted high throughput hybridization-based capture technology for the detection of single nucleotide variants (SNVs), insertions and deletions (indels) in 55 genes, copy number amplifications (CNAs) in 2 genes, and fusions in 4 genes. Guardant360 CDx utilizes circulating cell-free DNA (cDNA) from the plasma of peripheral whole blood collected in Streck Cell-Free DNA Blood Collection Tubes (BCTs). The test is intended to be used as a companion diagnostic to identify non-small cell lung cancer (NSCLC) patients who may benefit from treatment with the targeted therapy in accordance with the approved therapeutic product labeling. The patient’s results revealed two detected somatic alterations, as shown in Table 2, one of which was the ATM gene (R1882* alteration), which was consistent with INVITAE multi-cancer panel’s results.

Table 2.
Results of the genetic analysis testing of the patient’s blood performed through Guardant360® CDx testing.
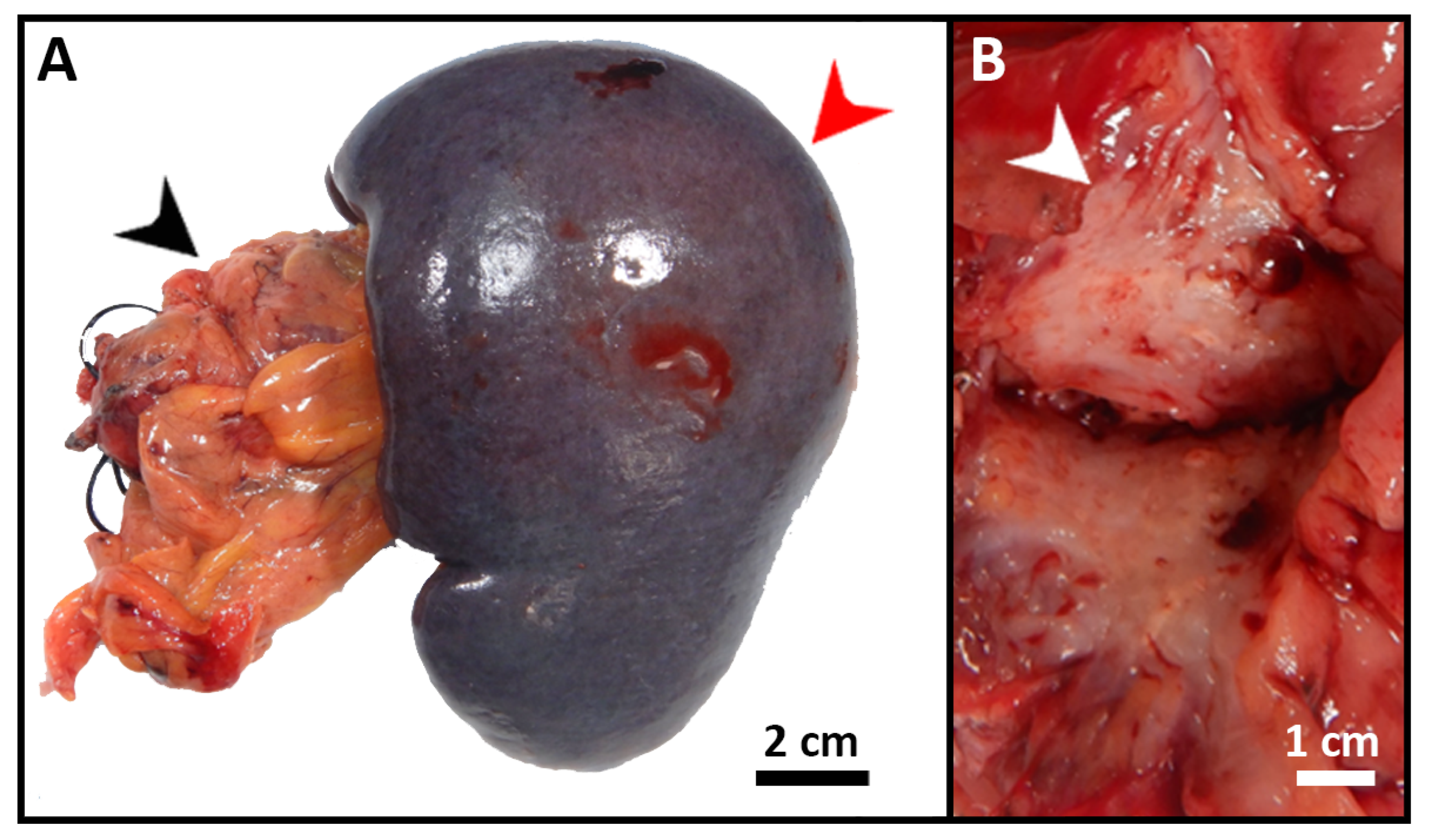
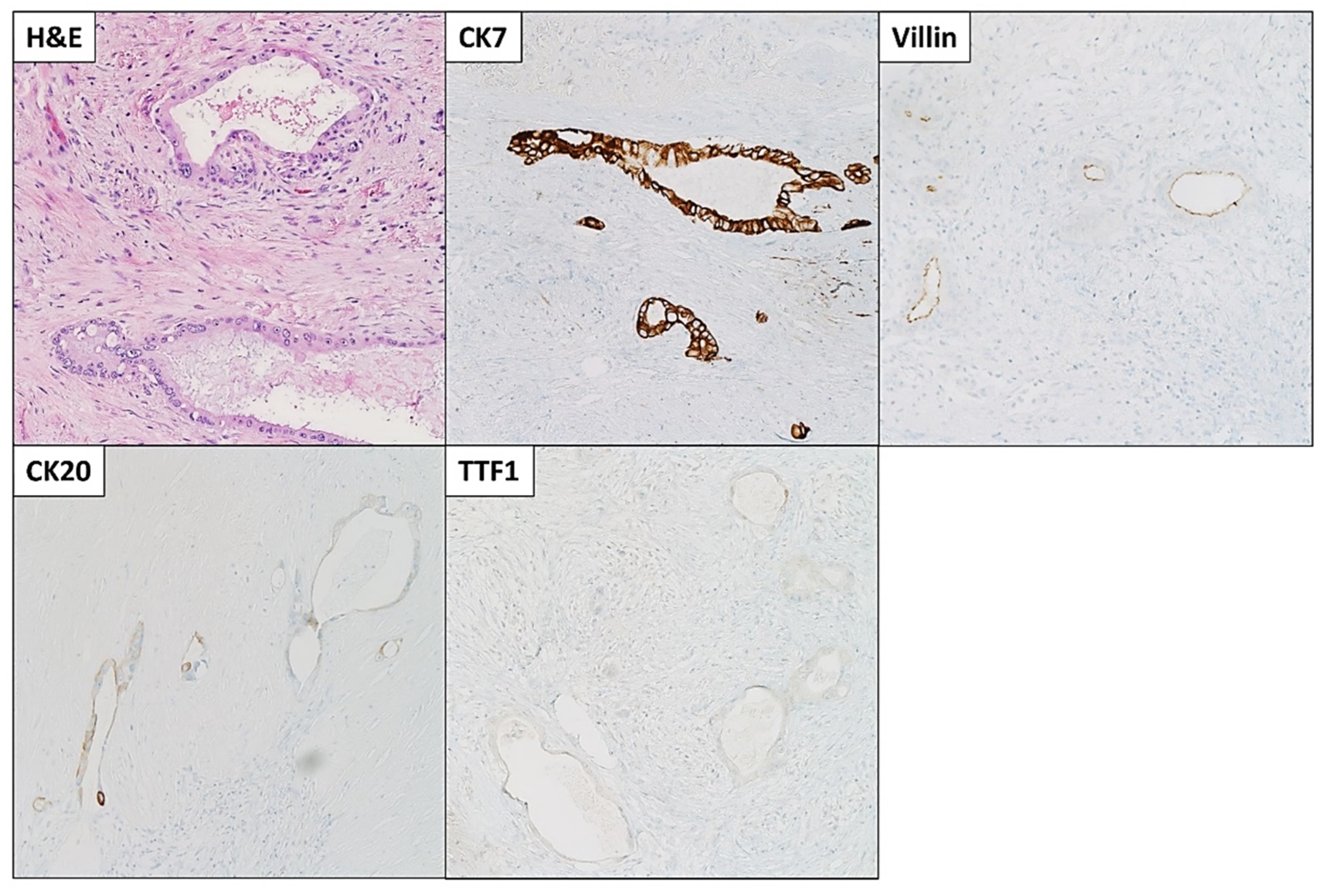
The patient was started on FOLFIRINOX (Leucovorin, 5-Fluorouracil (5-FU), Irinotecan, and Oxaliplatin) as a neoadjuvant therapy and completed six cycles. She also received a total of 45 Gray (Gy) adjuvant radiotherapies. After chemoradiation, she was referred to surgical oncology and underwent staging laparoscopy, extended distal pancreatectomy and splenectomy, distal gastrectomy, left hepatectomy and cholecystectomy with patch repair of portal vein, and celiac to right hepatic arterial bypass (Figure 3A). A 3 cm firm mass with a white-to-yellow indurated cut surface was found extending throughout the body and tail of the pancreas (Figure 3B). Histopathologic examination confirmed the biopsy’s diagnosis of pancreatic moderately differentiated ductal adenocarcinoma. Immunohistochemical (IHC) stains were positive for cytokeratin (CK)7, CK20 (weak), and villin. TTF-1 was negative, which supported the diagnosis (Figure 4).
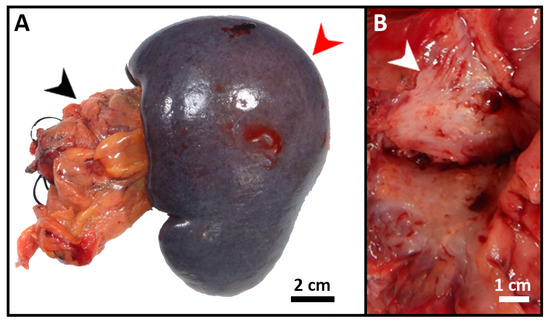
Figure 3.
Gross images of the resected pancreatic tumor. (A) Segment of distal pancreas (black arrow) with attached spleen (red arrow) and attached hilar adipose tissue. (B) Cut section through the tail of pancreas revealed a 3 cm firm mass (white arrow) with a white-to-yellow indurated cut surface.
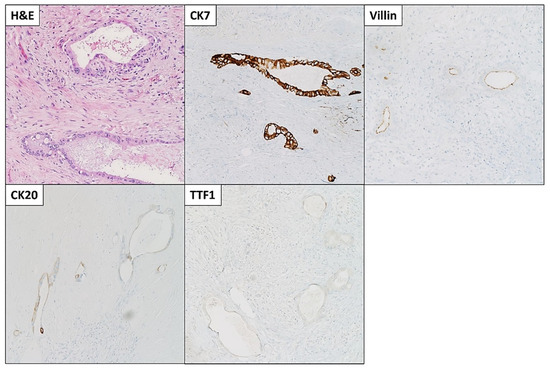
Figure 4.
Microscopic images and immunohistochemical (IHC) stains of the pancreatic moderately differentiated ductal adenocarcinoma. Tumor cells were positive for cytokeratin (CK)7, CK20 (weak), and villin. TTF1 was negative. Microscopic images were examined at 200× objective.
The tumor was confined to the pancreas with negative surgical margins. The treatment effect was evident where extensive parenchymal atrophy with associated fibrosis were appreciated. Residual cancer with evident tumor regression was seen, but more than single cells or rare small groups of cancer cells, which are referred to as partial response (score 2) according to the College of American Pathologists (CAP) cancer protocol. All 32 excised lymph nodes were negative for carcinoma (0/32). Also, the splenectomy, distal gastrectomy, left hepatectomy, and cholecystectomy specimens were free of carcinoma. Therefore, the pTNM pathologic stage (American Joint Committee on Cancer (AJCC) 8th Edition) given was ypT2N0M0.
The patient’s postoperative course was complicated by duodenal stump leak and sepsis, for which the patient had a washout. The patient had a worsening liver ischemia, due to hepatic artery thrombosis and pneumonia, with subsequent respiratory failure that required intubation. She ultimately expired 11 days after the surgery.
3. Discussion
The ATM gene is located on human chromosome 11q22-q23 and consists of 66 exons that span 150 kb of genomic DNA [10]. It encodes a PI3K-related serine/threonine protein kinase that plays a pivotal role in double-strand DNA break repairs. Genes that are involved in repairing double-strand DNA breaks, such as BRCA1/2, ATM, PALB2, CHEK1/2, RAD51, and ATR, can sometimes carry germline or somatic mutations that hinder the normal function of these genes [11,12].
As a master regulator of DNA damage and repair, the ATM gene is responsible for both homologous repair (HR) and non-homologous end joining (NHEJ) [13]. Either a germline or an induced mutation (i.e., radiotherapy induced) can alter the function of the ATM gene initiating carcinogenesis [14].
The ATM gene is associated with autosomal dominant predisposition to breast, pancreatic [15], and possibly prostate cancer [16,17], in addition to autosomal recessive ataxia-telangiectasia (A-T). There is also preliminary evidence suggesting that ATM is associated with autosomal dominant predisposition to other cancer types, including stomach [18], ovarian [19], bladder [20], and colon [21]. The pathogenic variant of the ATM gene identified in our patient has been observed in individuals with ataxia-telangiectasia and breast cancer [2,3,22,23,24]. Importantly, the ATM R1882* (c.5644C > T) alteration that was detected in our patient’s sample was at an allele fraction that was suspicious for its germline origin. This variant may lead to the loss of functional protein, and similar variants have been associated with hereditary predisposition to cancer. However, the germline versus somatic origin of this finding cannot be confirmed.
The JAK2 V617F mutation in the patient’s sample was an extremely rare mutation in the patient’s stated diagnosis, but is very common in myeloproliferative neoplasms such as polycythemia vera (>95% of cases) and essential thrombocythemia (approximately 50% of cases), which could raise the possibility of an incidental finding of a second myeloproliferative neoplasm [25,26]. However, the patient’s normal complete blood count, with differential and lacking signs and symptoms of a myeloproliferative neoplasm, makes this possibility unlikely.
In our case report, we hypothesize that this rarely reported variant mutation in the ATM gene is possibly linked to the pathogenesis of both NSCLC and pancreatic cancer. The ATM gene is highly polymorphic, and mutations within this gene increase the risk of breast cancer. Over the past 10 years, it became more evident that pancreatic cancer and lung cancer are also associated with pathogenic ATM mutations [6,7]. This presumable association is possibly related to the advancement in gene analysis and next-generation sequencing (NGS) that is allowing more gene variants to be discovered in the appropriate clinical setting.
Multiple studies demonstrated the relationship between pancreatic cancer and pathogenic ATM gene variants [7,11]. A study by Klavanian et al. revealed an association between harboring pathogenic ATM gene mutations and increased family history of pancreatic cancers [27]. The study showed that among 114 patients with identified ATM mutations, 22 of them (19.3%) had a family history of pancreatic cancer. Besides, two of the tested families had the high-penetrance ATM mutation c.7271T > C (p.V2424G) [27]. Furthermore, Roberts et al. found that four variants of the ATM genes c.3214G > GT; p.E1072X, c.6095G > GA; p.R2032K, IVS41-1G > GT, and c.3801delG were identified in 166 pancreatic cancer patients, whereas these variants were not identified in the control group [28].
Heterozygous ATM mutations can be associated with breast cancer, especially the missense mutation ATM c.7271T > G (p.Val2424Gly) [12]. Whether these mutations are associated with pancreatic cancer or not has yet to be determined. However, we are adding here a new mutation variant that could be linked to the pathogenesis of both lung and pancreatic cancer. To the best of our knowledge, there are no studies delineating an association between the presence of the mutation variant we discovered in our patient and the occurrence of pancreatic cancer.
Besides the newly discovered pancreatic cancer, our patient had a history of primary non-small cell lung cancer in both lungs. The lungs are the most commonly exposed organ to environmental DNA-damaging agents, and more research is showing that genetic heterogeneity (either somatic or germline) is implicated in the pathogenesis of lung cancer [29,30,31]. Among the various DNA damage response and repair (DDR) genes, the ATM gene remains one of the leading genes in that sphere, but that comes at a cost of harboring many somatic and germline mutations. Up to 34% of patients with pancreatic ductal adenocarcinoma have germline mutations in the ATM gene [7]. The tumor suppressor gene TP53 and proto-oncogenes, such as KRAS, EGFR, and ALK, are well studied in the pathogenesis of lung cancer [32,33,34,35]. In addition to those genes, more studies are demonstrating a correlation between homologous repair (HR) genes, including BRCA1, BRCA2 and ATM, with non-small cell lung cancer [36].
Based on a study by Scheffler et al., 11.9% of 1078 lung cancer patients with KRAS mutations had concomitant mutations in 12 different locations in the ATM gene [37]. This suggests a role for the latter gene in lung cancer. Henceforth, ATM gene mutations are presumably linked to both pancreatic and lung cancer, especially NSCLC. Nevertheless, it is still unclear whether the ATM c.5644C > T variant is indeed linked to the pathogenesis of the aforementioned cancers that our patient had. More research is warranted to decipher the exact role of this gene mutation variant in this cancer association.
4. Conclusions
In the setting of complex disorders such as pancreatic and lung cancers, each of which harbor various genetic aberrations and molecular signature mutations, common hits could instigate a role in the pathogenesis of each cancer. The fact that more than one cancer occurred concomitantly in our patient raises the question whether a single nonsense mutation might have driven a carcinogenic pathway of those cancers, this being the ATM c.5644C > T variant in our case. Indeed, our hypothesis has yet to be validated by larger studies that focus on a mechanistic approach involving DNA repair genes such as the ATM. More importantly, this paves the way to developing new patient-specific targeted therapies and inaugurating precision medicine as a cornerstone in cancer therapeutics.
Author Contributions
Conceptualization, A.A.A.; methodology, A.A.A. and H.F.B.; investigation, A.A.A., H.F.B., M.K.E. and E.H.M.; resources, A.A.A.; data curation, A.A.A., H.F.B. and M.K.E.; writing—original draft preparation, A.A.A. and H.F.B.; writing—review and editing, H.F.B., M.K.E., E.H.M., A.M.M. and M.C.; visualization, A.M.M. and M.C.; supervision, M.C.; project administration, A.A.A. and M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the protocols approved by the Institutional Review Board of Mount Sinai Medical Center of Florida (protocol code FWA00000176).
Informed Consent Statement
Patient consent was waived since the patient passed away.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all members of the Arkadi M. Rywlin Department of Pathology and Laboratory Medicine and Department of Internal Medicine, at the Mount Sinai Medical Center of Florida (Miami Beach, FL, USA) for their help with this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, G.; Mitui, M.; Campbell, C.; Costa Carvalho, B.T.; Nahas, S.; Sun, X.; Huo, Y.; Lai, C.H.; Thorstenson, Y.; Tanouye, R.; et al. Five haplotypes account for fifty-five percent of ATM mutations in Brazilian patients with ataxia telangiectasia: Seven new mutations. Am. J. Med. Genet. A 2004, 126a, 33–40. [Google Scholar] [CrossRef]
- Buzin, C.H.; Gatti, R.A.; Nguyen, V.Q.; Wen, C.Y.; Mitui, M.; Sanal, O.; Chen, J.S.; Nozari, G.; Mengos, A.; Li, X.; et al. Comprehensive scanning of the ATM gene with DOVAM-S. Hum. Mutat. 2003, 21, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Stucci, L.S.; Internò, V.; Tucci, M.; Perrone, M.; Mannavola, F.; Palmirotta, R.; Porta, C. The ATM Gene in breast cancer: Its relevance in clinical practice. Genes 2021, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Rotman, G.; Shiloh, Y. ATM: From gene to function. Hum. Mol. Genet. 1998, 7, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Tong, X.; Ma, Y.; Liu, S.; Yang, L.; Yang, X.; Yang, X.; Bai, M.; Fan, H. Association between ATM gene polymorphisms, lung cancer susceptibility and radiation-induced pneumonitis: A meta-analysis. BMC Pulm. Med. 2017, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Schultz, C.W.; Azimi-Sadjadi, A.; Brody, J.R.; Pishvaian, M.J. ATM dysfunction in pancreatic adenocarcinoma and associated therapeutic implications. Mol. Cancer Ther. 2019, 18, 1899–1908. [Google Scholar] [CrossRef]
- Shen, L.; Yin, Z.H.; Wan, Y.; Zhang, Y.; Li, K.; Zhou, B.S. Association between ATM polymorphisms and cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 5719–5725. [Google Scholar] [CrossRef]
- Ye, F.; Chai, W.; Yang, M.; Xie, M.; Yang, L. Ataxia-telangiectasia with a novel ATM gene mutation and Burkitt leukemia: A case report. Mol. Clin. Oncol. 2018, 9, 493–498. [Google Scholar] [CrossRef]
- McKinnon, P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 2012, 7, 303–321. [Google Scholar] [CrossRef]
- Martino, C.; Pandya, D.; Lee, R.; Levy, G.; Lo, T.; Lobo, S.; Frank, R.C. ATM-Mutated pancreatic cancer: Clinical and molecular response to gemcitabine/nab-paclitaxel after genome-based therapy resistance. Pancreas 2020, 49, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. Jama 2018, 319, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Jeggo, P.A. ATM’s role in the repair of DNA double-strand breaks. Genes 2021, 12, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Zhao, Y.; He, S.; Zhao, R.; Song, Y.; Cheng, J.; Gong, Y.; Xie, J.; Wang, Y.; et al. Caspase-3 knockout attenuates radiation-induced tumor repopulation via impairing the ATM/p53/Cox-2/PGE(2) pathway in non-small cell lung cancer. Aging 2020, 12, 21758–21776. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Bamlet, W.R.; Moore, R.M.; Nandakumar, K.; Eckloff, B.W.; Lee, Y.K.; Petersen, G.M.; McWilliams, R.R.; Couch, F.J. Prevalence of Pathogenic Mutations in Cancer Predisposition Genes among Pancreatic Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2016, 25, 207–211. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Pilié, P.G.; Johnson, A.M.; Hanson, K.L.; Dayno, M.E.; Kapron, A.L.; Stoffel, E.M.; Cooney, K.A. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer 2017, 123, 3925–3932. [Google Scholar] [CrossRef]
- Sriramulu, S.; Ramachandran, M.; Subramanian, S.; Kannan, R.; Gopinath, M.; Sollano, J.; Bissi, L.; Banerjee, A.; Marotta, F.; Pathak, S. A review on role of ATM gene in hereditary transfer of colorectal cancer. Acta Biomed. 2019, 89, 463–469. [Google Scholar] [CrossRef]
- Lilyquist, J.; LaDuca, H.; Polley, E.; Davis, B.T.; Shimelis, H.; Hu, C.; Hart, S.N.; Dolinsky, J.S.; Couch, F.J.; Goldgar, D.E. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol. Oncol. 2017, 147, 375–380. [Google Scholar] [CrossRef]
- van Os, N.J.; Roeleveld, N.; Weemaes, C.M.; Jongmans, M.C.; Janssens, G.O.; Taylor, A.M.; Hoogerbrugge, N.; Willemsen, M.A. Health risks for ataxia-telangiectasia mutated heterozygotes: A systematic review, meta-analysis and evidence-based guideline. Clin. Genet. 2016, 90, 105–117. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef]
- Jeddane, L.; Ailal, F.; Dubois-d’Enghien, C.; Abidi, O.; Benhsaien, I.; Kili, A.; Chaouki, S.; Kriouile, Y.; El Hafidi, N.; Fadil, H.; et al. Molecular defects in moroccan patients with ataxia-telangiectasia. Neuromol. Med. 2013, 15, 288–294. [Google Scholar] [CrossRef]
- Mitui, M.; Campbell, C.; Coutinho, G.; Sun, X.; Lai, C.H.; Thorstenson, Y.; Castellvi-Bel, S.; Fernandez, L.; Monros, E.; Carvalho, B.T.; et al. Independent mutational events are rare in the ATM gene: Haplotype prescreening enhances mutation detection rate. Hum. Mutat. 2003, 22, 43–50. [Google Scholar] [CrossRef]
- Micol, R.; Ben Slama, L.; Suarez, F.; Le Mignot, L.; Beauté, J.; Mahlaoui, N.; Dubois d’Enghien, C.; Laugé, A.; Hall, J.; Couturier, J.; et al. Morbidity and mortality from ataxia-telangiectasia are associated with ATM genotype. J. Allergy Clin. Immunol. 2011, 128, 382–389. [Google Scholar] [CrossRef]
- Tefferi, A.; Vardiman, J.W. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008, 22, 14–22. [Google Scholar] [CrossRef]
- Nielsen, C.; Bojesen, S.E.; Nordestgaard, B.G.; Kofoed, K.F.; Birgens, H.S. JAK2V617F somatic mutation in the general population: Myeloproliferative neoplasm development and progression rate. Haematologica 2014, 99, 1448–1455. [Google Scholar] [CrossRef]
- Klavanian, J.; Zakalik, D.; Konde, A.S.; Rangarajan, T. ATM mutation carriers and family history of pancreatic cancer. J. Clin. Oncol. 2020, 38, 1529. [Google Scholar] [CrossRef]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, S.-H.; Weng, M.-w.; Wang, H.-T.; Huang, W.C.; Lepor, H.; Wu, X.-R.; Chen, L.-C.; Tang, M.-s. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Daouk, R.; Hassane, M.; Bahmad, H.F.; Sinjab, A.; Fujimoto, J.; Abou-Kheir, W.; Kadara, H. Genome-Wide and phenotypic evaluation of stem cell progenitors derived from Gprc5a-deficient murine lung adenocarcinoma with somatic kras mutations. Front. Oncol. 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Greulich, H. The genomics of lung adenocarcinoma: Opportunities for targeted therapies. Genes Cancer 2010, 1, 1200–1210. [Google Scholar] [CrossRef]
- Scoccianti, C.; Vesin, A.; Martel, G.; Olivier, M.; Brambilla, E.; Timsit, J.F.; Tavecchio, L.; Brambilla, C.; Field, J.K.; Hainaut, P. Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: The EUELC cohort. Eur. Respir. J. 2012, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Aran, V.; Zalis, M.; Montella, T.; de Sousa, C.A.M.; Ferrari, B.L.; Gil Ferreira, C. Evaluation of KRAS concomitant mutations in advanced lung adenocarcinoma patients. Medicina 2021, 57, 1039. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Savic Prince, S.; Rothschild, S.I. Targeted therapy in advanced and metastatic non-small cell lung cancer. an update on treatment of the most important actionable oncogenic driver alterations. Cancers 2021, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Weng, X.; Xu, D.; Cai, S.; Lou, H.; Ding, L. Non-small cell lung cancer cells with deficiencies in homologous recombination genes are sensitive to PARP inhibitors. Biochem. Biophys. Res. Commun. 2020, 522, 121–126. [Google Scholar] [CrossRef]
- Scheffler, M.; Ihle, M.A.; Hein, R.; Merkelbach-Bruse, S.; Scheel, A.H.; Siemanowski, J.; Brägelmann, J.; Kron, A.; Abedpour, N.; Ueckeroth, F.; et al. K-ras mutation subtypes in NSCLC and associated co-occuring mutations in other oncogenic pathways. J. Thorac. Oncol. 2019, 14, 606–616. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).