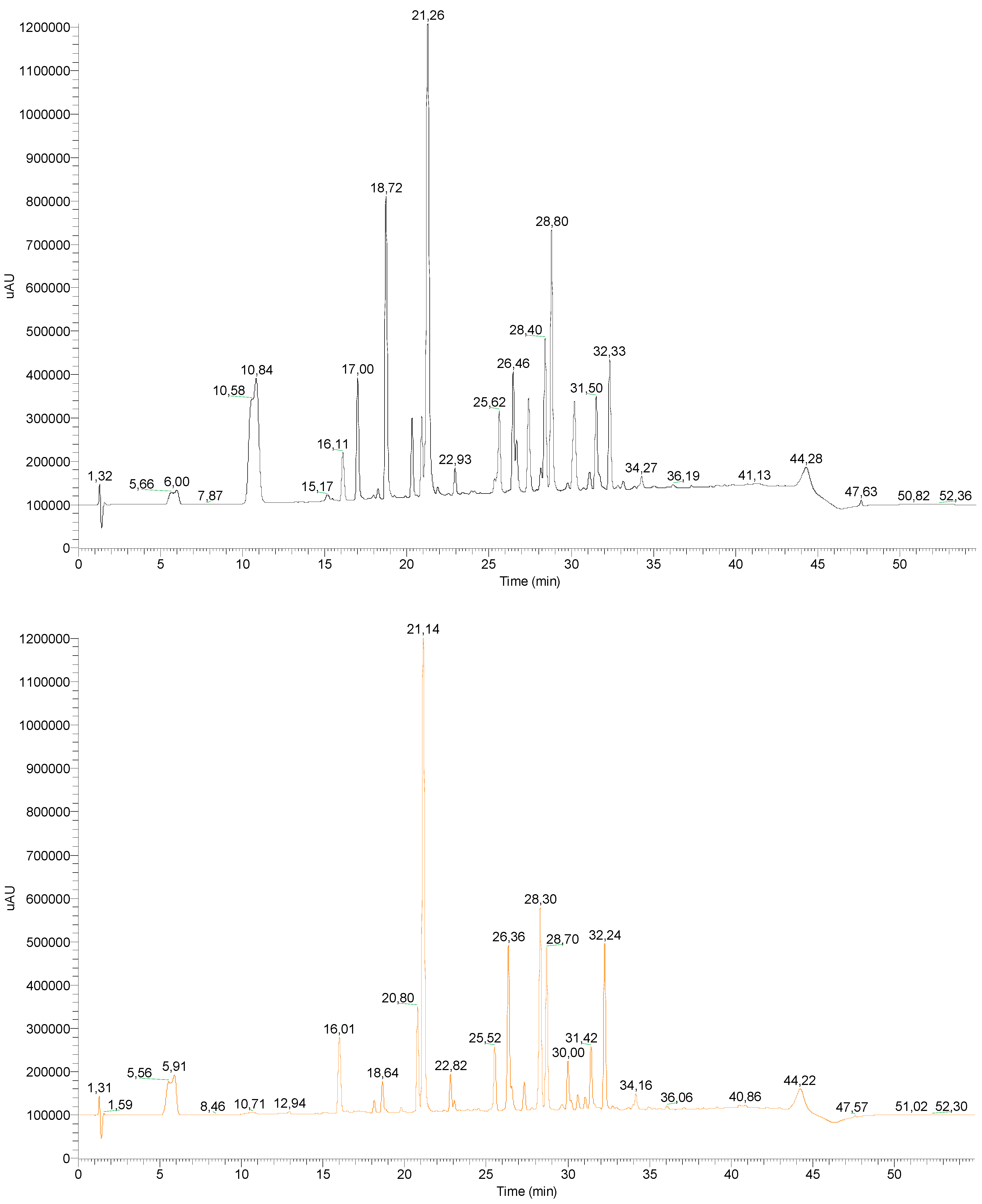1. Introduction
Oakmoss is a lichen of the species
Evernia prunastri, and is different to tree moss, which is mostly obtained from lichens belonging to
Evernia furfuracea [
1,
2]. Dry oakmoss can be extracted by organic solvents such as hexane to yield an odorant resinoid. Upon treatment with hot ethanol and further cooling, insoluble waxes precipitate, and an absolute is obtained after filtration and ethanol removal by distillation. The use of oakmoss absolute in perfumery was popularized by the fragrance
Chypre (Guerlain, Paris, France, 1840), and has become one the main ingredients constitutive of the ‘chypre’ and ‘fougere’ accords. Symbiotic organisms, such as lichens, develop secondary metabolites for which the biosynthetic origin is not always known with accuracy [
3]. Common metabolites include mono- and polyphenolics [
4], dibenzofurans [
5], quinones/anthraquinones, and xanthones. The chemical composition of oakmoss absolute has been the subject of several analytical studies [
6,
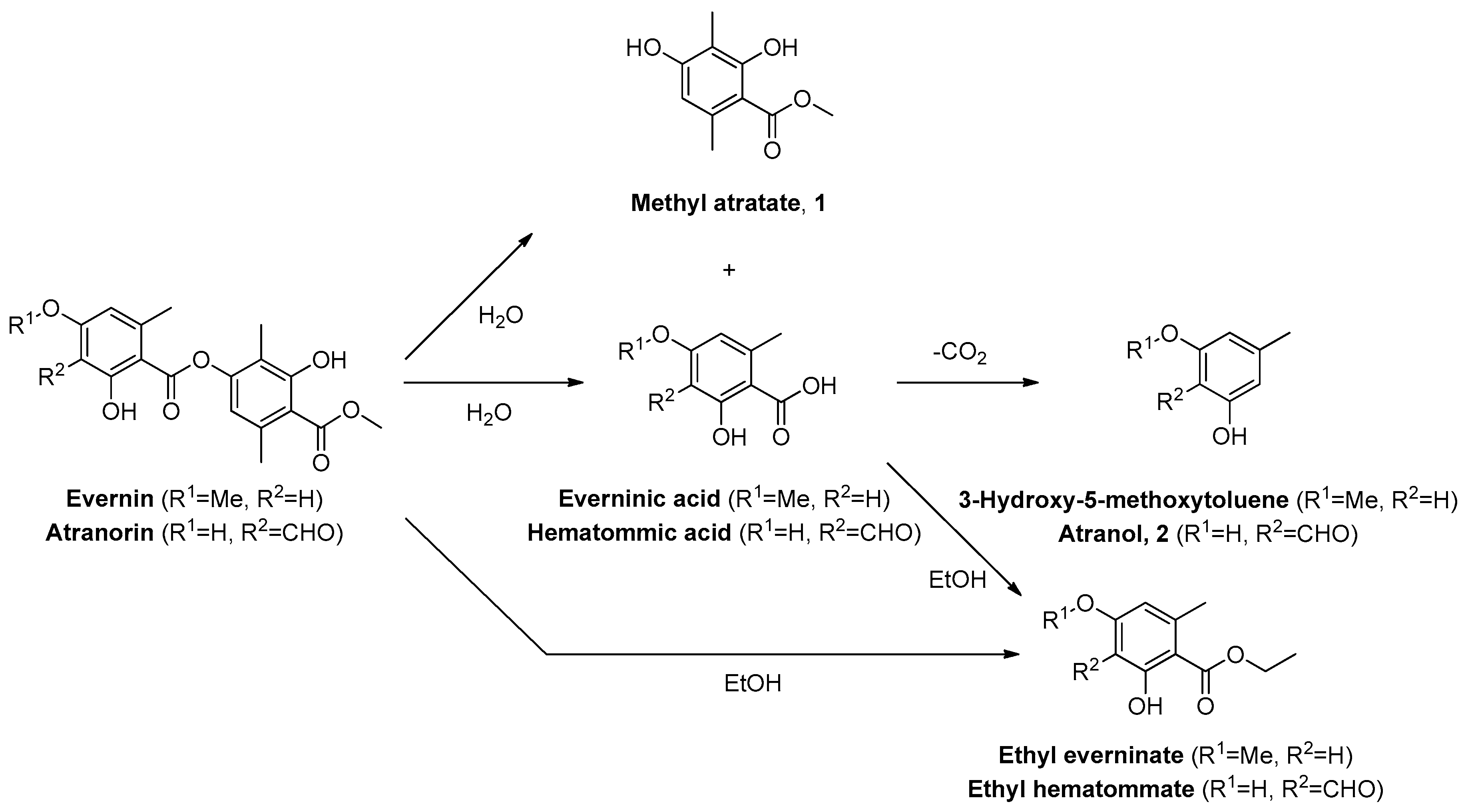
7]. It is mostly constituted of monoaromatic compounds and their dimers, called depsides. These are typically formed by an ester link between two monoaromatic units. Depsides are not supposed to confer an odor to the extract, but monoaromatic compounds such as methyl atratate
1, formed upon hydrolysis or transesterification by EtOH of some depsides, are considered odor impact constituents of oakmoss absolute (
Figure 1). Unfortunately, toxic atranol
2 and chloroatranol
3, responsible for allergic contact dermatitis, are also formed upon solvolytic cleavage of depsides [
8].
Complex natural products obtained by extraction techniques are mixtures of dozens of individual compounds encompassing a relatively large number of chemical families.
Evernia prunastri (L.) extracts may thus contain more than 170 individual chemicals [
2] including 14 depsides, such as atranorin and evernic acid; 18 monoaromatic compounds, such as methyl atratate
1, atranol
2, and ethyl orsellinate; 9 chlorinated monoaromatic compounds, such as chloroatranol
3 and methyl chloroatratate; 25 triterpenes, or steroids, such as hopanoid derivatives [
9]; and usnic acid, which is characteristic of
Evernia prunastri (L.) [
5]. However, being insoluble in EtOH, usnic acid is generally present in the resinoid but not in the absolute. Volatile compounds, including those formed upon depsides’ degradation, are also present [
10].
Although oakmoss absolute has been used for decades, it is known to contain undesirable molecules such as atranol
2 and chloroatranol
3, which are severe allergens by contact [
11,
12,
13,
14,
15]. Sensitization and elicitation in allergic contact dermatitis are complicated cellular and molecular processes [
16], and the issue of skin irritation has been taken very seriously by perfume manufacturers and regulatory bodies. In 1999, the Scientific Committee on Consumer Safety (SCCS) of the European Community issued a list of 26 allergens. Manufacturers were thus required to identify these ingredients individually in cosmetic and fragrance products containing them in more 0.01% in rinsed products and 0.001% in non-rinsed products [
17]. The list was expanded to 82 allergens in 2012 [
18]. In the particular case of atranol
2 and chloroatranol
3, which are not simple allergens but severe irritation elicitors, IFRA recommendations require their presence to be limited to 100 ppm in oakmoss extracts used in perfumery [
19], and SCCS recommends banning these compounds for cosmetic products. Various studies on the frequency of oakmoss absolute contact allergy in large panels in European countries show, on average, 1% frequency in the general population and 2% in unselected dermatitis patients, upon exposure to 2% oakmoss absolute solutions in petrolatum [
20]. The European Union (EU) published Commission Regulation 2017/1410 on 2 August 2017 prohibiting the use of atranol and chloroatranol in cosmetic products. From 23 August 2019, cosmetic products containing these substances shall not be placed on the Union market, and from 23 August 2021, cosmetic products containing these substances shall not be made available on the Union market.
Considering both the importance of oakmoss absolute in fine perfumery and the deleterious effect of atranols, various processes, of varying efficacies, have been evaluated to remove them, resulting in low-atranol or atranol-free products that could eventually be used in formulas compliant with the latest regulation. However, the body of knowledge on the subject available in the scientific literature and in patent databases is very limited, possibly because it can be more profitable for knowledge to remain confidential than for patented technology to be made public. There are two broad approaches to the removal of atranol and related compounds from a natural complex mixture such as oakmoss absolute. The first is based on specific physicochemical interactions, while the second involves the reactivity of aromatic aldehydes. Thus, combined preparative chromatographic methods (including column chromatography, GPC, HPTLC, and HPLC) [
21], are known methods used to remove atranol from oakmoss absolute. Distillation techniques, in particular molecular distillation, which is already used for discoloration purposes in the case of oakmoss absolute [
2], might also be considered for atranol removal, but to the best of our knowledge, this is not documented in the literature. Techniques based on molecular approaches are mostly aimed at converting aromatic aldehyde moieties into their corresponding benzyl alcohols. This limits their ability to form imines with skin proteins, which triggers the immune response [
22]. Protocols involving catalytic hydrogenation [
21]; reaction with hydrides, such as sodium and lithium borohydrides [
23]; or alkaline treatment (mostly for hematommates and atranorin) [
21], have thus been used. Imination reactions with water-soluble amino acids, such as leucine or lysine, have also been used to facilitate the removal of atranol and other aldehydes by liquid–liquid extraction [
24,
25]. The trapping of allergens contained in natural complex substances using aminoalkyl resins has been reported, but was not applied to atranol nor other oakmoss absolute allergens [
26,
27]. Selective filtration through molecular imprinting polymers has been developed to remove safrole from nutmeg oil, but this approach has not been applied to oakmoss extracts neither [
28].
These methodologies generally suffer from a lack of selectivity, resulting in an alteration of the olfactory quality of the modified extract; excessive consumption of energy, resources and manpower; or weak practical applicability.
We have recently been using enzymatic approaches to specifically modify the chemical composition of natural complex mixtures, such as essential oils, in order to improve their properties [
29,
30,
31,
32,
33]. In particular, we have described a procedure which involves use of horseradish peroxidase (HRP) to selectively convert 2-methoxy-4-(2-propenyl)-phenol (eugenol) into insoluble dimeric material, followed by simple separation of the mixture by filtration [
34]. Regardless of the allergenic potentials of atranorin, evernic acid, perlatolic acid, divaricatic acid, and fumarprotocetraric acid, we have been interested in developing an enzymatic procedure based on peroxidases to selectively remove atranol
2 and chloroatranol
3 from oakmoss extracts, considering that they remain strong contact allergens and that only these two compounds are formally targeted by health agencies’ decisions and IFRA recommendations. Our strategy consisted of the use of HRP (EC 1.11.1.7) in the presence of H
2O
2 to catalyze the dimerization of atranols and facilitate their separation from the absolute.
2. Results and Discussion
Atranol
2 was synthesised by formylation of commercially available orcinol
4. Our initial attempts to perform the direct formylation by the action of POCl
3 and DMF following the standard procedure led to a regioisomer of
2 by formylation in
ortho position relative to the methyl group. However, upon protection of the hydroxyl functions by methoxymethyl groups,
ortho-lithiation could be performed efficiently, and the target atranol
2 was obtained in 85% isolated yield (39% in four steps from
4) [
35]. Alternate synthesis should be performed upon methylation/formylation/demethylation by BBr
3 [
36]. With this starting material, we started our study by testing HRP on pure
2 at pH 7 and 8, with and without cosolvent, to ensure substrate solubilisation (
Table 1) [
34,
37,
38]. The use of a cosolvent miscible with water was likely to modify the conformation of the enzyme, and therefore aspects of its activity, such as how it accommodated substrates. However, at a low 2%
v/
v, we considered this effect limited. The purpose of the solvent was to ensure substrate solubilisation.
None of these conditions allowed the conversion of
2, which was recovered quantitatively in almost all cases. In order to activate the phenolic functions of
2, the enzymatic reaction was performed in carbonate buffer at pH 9 (20 mM). Surprisingly, 5-methylpyrogallol
5 was the only observed product, albeit in low yields (
Table 2).
The product
5 was characterised by
1H and
13C NMR as well as MS (see
supplementary materials). It is likely that at such basic pH, the aromatic aldehyde substrate underwent a Dakin oxidation by H
2O
2 to the corresponding phenol. A series of control experiments was performed to confirm this result (
Table 3).
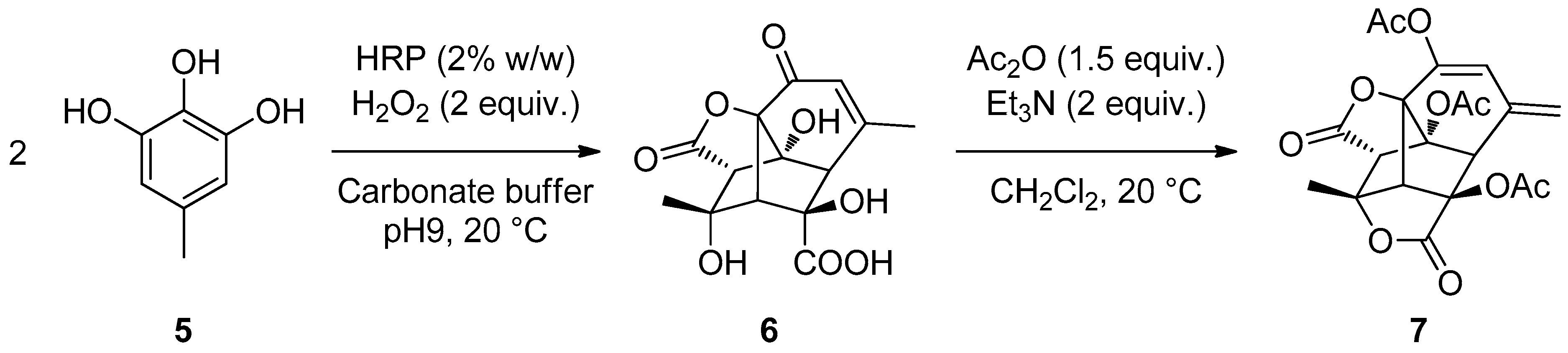
In the absence of H2O2, 2 was recovered unchanged upon extraction with AcOEt, either with or without HRP, used in 1% w/w (entries 1, 2). In the presence of H2O2 but without HRP, 5 was obtained in 84% isolated yield upon extraction with AcOEt (entry 3). Surprisingly, in the presence of HRP (1% w/w) and H2O2 (2 equiv.), only 2% of 5 was isolated by extraction with AcOEt. Evaporation of the aqueous phase, however, led to the isolation of a hydrosoluble dimer 6 isolated in 75% yield.
Upon optimisation of the amounts of H
2O
2 and HRP, the isolated yield of
6 could be increased to 82%. When
5, prepared independently, was submitted to the same reaction conditions, the dimer
6 was formed similarly (
Scheme 1). A mechanistic rationale to account for this unusual product formation has been described in a separate publication [
35]. It was thus established that
5 could be converted into a water-soluble dimeric product
6 in an unprecedented and surprising way. The structure of this original product was determined by
1H and
13C NMR spectroscopy, and in particular COSY, HMBC, and HMQC, and MS. It was further confirmed by formation of its triacetylated product, which occurred together with a lactonization of the 3-hydroxyacid moiety, acetylation of the two hydroxyl groups, and acetylation of the dienol form of the α,β-unsaturated ketone through enolisation on the methyl group. Additional NMR analysis and HRMS on the triacetylated product
7 confirmed the proposed structure.
Following a Dakin oxidation of 2 to 5 occurring in the presence of H2O2 at pH 9, an additional oxidation step led to an ortho-quinone intermediate, possibly dependent on HRP catalysis since in the absence of the enzyme, 5 was isolated in good yield. This activated species then engaged in a fast reaction with a second molecule of 5 in a 1,6-conjugate addition/enol addition to an oxocarbenium ion intermediate, to yield a bicyclo[2.2.2]octane core fused with a cyclohexadiene. This dimeric adduct then underwent an epoxidation of the double bond by peroxylate ions, easily formed in the reaction medium. Upon internal nucleophilic attack of the enol towards the epoxide, and subsequent transannulation reaction, the final product 6 was formed.
Following our results on pure
2, we next turned our attention to crude oakmoss absolute for the HRP-based enzymatic treatment. Oakmoss absolute (100 mg) was dissolved in a carbonate buffer at pH 9 (20 mM, 110 mL) under sonication for 2 h. The reaction was initiated with the addition of HRP (1 mg) and H
2O
2 (as a concentrated aqueous solution; several equivalents were tested). After two different reaction times, the reaction mixture was extracted with AcOEt, the modified absolute was analysed by HPLC-UV, and residual
2 was specifically titrated by HPLC-MS (external calibration method) and compared with the title in the untreated oakmoss absolute used in this study (43,000 ppm) [
14,
39]. The results are summarized in
Table 4.
Residual
2 below the regulatory threshold value of 100 ppm could be obtained when more than 2 equivalents of H
2O
2 were used. With 4 equivalents of H
2O
2, and upon 4 h of reaction, a residual title as low as 7 ppm was measured. The reaction could be performed at the gram scale and yielded, after 2 h of reaction, a modified oakmoss absolute containing 60 ppm of atranol and in which chloroatranol was not detected (HPLC-MS, SIM mode). To assess the overall effect of the enzymatic reaction on the entire oakmoss absolute, HPLC-UV remained useful. The chemical composition globally remained the same, even if some compounds showed slight variation; by comparing chromatograms before and after enzymatic treatment, no significant mass losses were observed (
Figure 2). For example, orsellinic acid (10.8 min) and everninic acid (18.7 min) were simply washed out by liquid–liquid extraction during the work-up. The most odorant compound, methyl atratate, eluting at 21.2 min (confirmed by injection of an authentic sample), was unchanged under our conditions.
The olfactory quality of the modified oakmoss absolute was assessed by sensory analysis following the triangular testing methodology. Three identical vials containing two samples of oakmoss absolute (samples 1 and 2) and one sample of modified oakmoss absolute (sample 3), as solutions in EtOH (0.5%
w/
w), were submitted to a panel of 56 persons who were separately asked to identify the modified sample within the three samples. Statistical distribution will therefore be 33% for each sample, and a test should be used to determine whether our results fall in this statistical distribution or not. The following formulas were used, with
n1–3 being the value one should exceed to be sure that the result is not the statistical distribution for a given level of confidence, and
N the number of panelists:
| For 95% of confidence | For 98% of confidence | For 99.9% of confidence |
| + 0.5 | + 0.6 | + 0.8 |
For a panel of 56 persons, n1 = 25.9, n2 = 28.0, and n3 = 30.9. Scores obtained during the triangular testing for samples 1, 2, and 3 were 18, 16, and 22, respectively. A score of 22 means that even at the lowest level of confidence, the panel was not able to identify the modified sample.
3. Materials and Methods
1H NMR and 13C NMR spectra were recorded on Bruker (Billerica, MA, USA) AC Avance spectrometers (200 and 400 MHz). 1H NMR spectra are reported as follows: chemical shifts in ppm (δ) relative to the chemical shift of TMS at 0 ppm, multiplicities (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and br = broad), coupling constants (Hz), and integration. 13C NMR spectra chemical shifts are reported in ppm (δ) relative to CDCl3 at 77.16 ppm. Identity was assessed by comparison with data from authentic samples or literature data.
Column chromatography was carried out on silica gel (spherical, neutral, 63–200 µm, Geduran Si 60, Merck KGaA, Darmstadt, Germany).
GC/MS analyses were carried out using a Shimadzu QP2010 gas chromatograph (conditions: carrier gas, He; injector and detector temperatures, 250 °C; injected volume, 0.5 μL; split ratio, 1/100; pressure, 180 kPa; SLB-5ms capillary column (thickness: 0.25 mm, length: 30 m, inside diameter: 0.25 mm); temperature program, 60–315 °C at 10 °C·min−1 and 10 min at 315 °C) coupled to a mass selective detector. Mass spectra were obtained by electron ionization at 70 eV, m/z 35–400, source temperature 250 °C; only the most abundant ions are given.
HPLC analyses were carried out using an Agilent 1100 chromatograph equipped with a UV photodiode array (PDA) detector (Agilent, Santa Clara, CA, USA) and a mass analyzer Thermo LCQ advantage (Thermo, Waltham, MA, USA), ionization by electrospray, and a quadrupole mass detector. Chromatographic conditions: water/ACN 85/15 for 5 min, then 100% ACN for 45 min and 5 min at 0/100 at a 0.3 mL/min flow rate, column Marcherey–Nagel Nucleodur C18, 150 × 2.0 mm, 3 µm particle size, 10 µL injection volume.
High resolution mass spectrometry (HRMS) was performed at ERINI platform (Grasse, France) using a Waters UPLC coupled with a Waters Xevo G2 QTOF spectrometer (Waters, Milford, MA, USA).
Enantioselective SFC was performed on a Jasco Extrema apparatus equipped with Daicel ChiralPak IA (Daicel, Osaka, Japan) column, coupled with a dual wavelength 190 to 600 nm UV-4070/75 detector. Pressure: 150 bars; flow: 4 mL/min; MeOH: 15%; wavelength: 245 nm.
Polarimetry was performed on an Anton Paar MCP150 (Anton Paar, Graz, Austria) polarimeter at 20 °C in MeOH/H2O 10:6 v/v. Wavelength: 589 nm.
Materials: Dimethylformamide (DMF), tetrahydrofuran (THF), methanol (MeOH), ethanol (EtOH), and cyclohexane (CHX) were purchased from Sigma-Aldrich and dried and/or distilled according to conventional procedures. Orcinol, POCl3, MOMCl, NaH, n-BuLi, AcCl, Na2CO3, NaHCO3, H2O2 (30% w/w in water), and HRP (peroxidase from horseradish, EC 1.11.1.7) were purchased from Sigma-Aldrich (Saint-Louis, MO, USA) and used as received. Oakmoss absolute was a kind gift from IFF-LMR naturals (Grasse, France).
Synthesis of 1,3-di(methoxymethoxy)-5-methylbenzene: Orcinol 4 (0.8 g, 6.5 mmol) was dissolved in freshly dried and distilled DMF (40 mL). To this solution was added NaH (0.58 g as a 60% dispersion in mineral oil, 14.5 mmol). The mixture was stirred under a nitrogen atmosphere at 0 °C in a round-bottomed flask equipped with a condenser and a bubbler, allowing us to monitor H2 evolution. After 15 min, MOM-Cl (1.10 mL, 14.5 mmol) was added and the mixture stirred over 18 h. Water was then carefully added (30 mL) and the resulting mixture extracted with Et2O (5 × 30 mL). Organic layers were then pooled and washed with a 2 M aqueous NaOH solution (3 × 20 mL) and brine (20 mL). After drying over MgSO4, filtration, and solvent removal, a yellow oil was obtained, which was submitted to column chromatography over silica gel (petroleum ether/EtOAc 9:1) to yield the MOM-protected orcinol as a colorless liquid (1.13 g, 95%). Rf 0.7 (petroleum ether/EtOAc 8:2). 1H NMR (CDCl3, 200 MHz): δ ppm 6.6–6.5 (m, 3H, ArH), 5.15 (s, 4H), 3.48 (s, 6H), 2.32 (s, 3H). 13C NMR (CDCl3, 50 MHz): δ ppm 158.2 (C), 140.3 (C), 110.4 (CH), 102.1 (CH), 94.4 (CH), 55.9 (OCH3), 21.7 (CH3). MS (EI) m/z: 212 (8), 182 (1), 152 (3), 136 (2), 123 (1), 108 (2), 91 (1), 77 (2), 45 (100).
Synthesis of 1,3-di(methoxymethoxy)-4-methylbenzaldehyde: 1,3-di(methoxymethoxy)-5-methylbenzene (0.95 g, 4.5 mmol) was dissolved in freshly distilled THF (50 mL) and stirred under a nitrogen atmosphere at 0 °C while n-butyllithium was added dropwise (3.4 mL as a 1.6 M solution in hexane, 5.4 mmol). The mixture was stirred over 1.5 h and allowed to warm to room temperature, and the reaction was quenched with DMF (0.7 mL, 9 mmol). The resulting mixture was washed with water (50 mL) and extracted with Et2O (4 × 30 mL). Organic layers were pooled and washed with water (40 mL) and brine (40 mL), dried over MgSO4, filtrated, and concentrated in vacuo. The resulting yellow oil was submitted to column chromatography over silica gel (petroleum ether/EtOAc 95:5) to yield a yellow solid (0.81 g, 75%). 1H NMR (CDCl3, 200 MHz): δ ppm 10.40 (s, 1H, CHO), 6.58 (s, 2H, ArH), 5.17 (s, 4H), 3.42 (s, 6H) 2.26 (s, 3H). 13C NMR: (CDCl3, 50 MHz): δ ppm 188.7 (CHO), 159.4 (C), 147.3 (C), 113.7 (CH), 109.3 (CH), 94.6 (CH), 56.3 (OCH3), 22.5 (CH3). MS (EI) m/z: 240 (2), 209 (2), 195 (3), 179 (4), 178 (10), 165 (3), 164 (4), 136 (2), 77 (2), 46 (3), 45 (100).
Synthesis of atranol 2: 1,3-di(methoxymethoxy)-4-methylbenzaldehyde (0.315 g, 1.3 mmol) was dissolved in 30 mL of MeOH at room temperature under a nitrogen atmosphere. Acetyl chloride was then added dropwise (60 µL, 0.9 mmol). The mixture was stirred for 20 h and concentrated in vacuo. An aqueous HCl solution was then added (30 mL at 0.1 M) and the resulting mixture extracted with EtOAc (3 × 150 mL). Organic layers were pooled and dried over MgSO4, filtrated, and concentrated in vacuo. The resulting light yellow oil was submitted to column chromatography over silica gel (petroleum ether/EtOAc 8:2), and atranol 2 was obtained as a light yellow oil (0.164 g, 90%). 1H NMR (acetone-d6, 200 MHz): δ ppm 10.71 (s, 2H, OH), 10.26 (s, 1H, CHO), 6.25 (s, 2H, ArH), 2.23 (s, 3H). 13C NMR (acetone-d6, 50 MHz): δ ppm 194.2 (CHO), 163.1 (C), 151.6 (C), 109.3 (C), 108.4 (CH), 22.4 (CH3). MS (EI) m/z: 152 (84), 151 (100), 134 (6), 123 (4), 106 (16), 95 (9), 77 (14), 69 (6), 67 (11), 55 (12).
Synthesis of 5-methylpyrogallol 5: Atranol 2 (0.152 g, 1 mmol) and sodium percarbonate (0.236 g, 1.5 mmol) were dissolved in THF/water 3:7 mixture (5 mL). The reaction mixture was then stirred at room temperature for 2 h. After completion of the reaction, aqueous 0.1 M HCl solution was added (5 mL) and the mixture extracted with EtOAc (2 × 10 mL). Organic layers were pooled and dried over MgSO4, filtrated, and concentrated in vacuo. An orange solid was obtained (0.119 g, 85%). 1H NMR (acetone-d6, 200 MHz): δ ppm 7.64 (s, 2H, OH), 7.02 (s, 1H, OH), 6.20 (s, 2H, ArH), 2.10 (s, 3H). 13C NMR (acetone-d6, 50 MHz): δ ppm 146.3 (C), 131.0 (C), 129.2 (C), 108.5 (CH), 20.9 (CH3). MS m/z: 140 (100), 139 (34), 134 (6), 123 (11), 122 (19), 121 (9), 94 (35), 77 (4), 66 (37), 65 (21), 55 (6), 53 (17).
Dimerization of atranol into 6: HRP (124 U/mg, 4 mg) was dissolved in 65 mL of pH 9 carbonate buffer (20 mM) containing atranol (200 mg, 1.32 mmol). The reaction flask was covered with aluminum foil to avoid peroxide decomposition. Reaction was initiated by the slow addition of a 30% aqueous H2O2 solution at 0.1 mL/h to ensure the final addition of 2 equivalents of hydrogen peroxide (0.264 mL) with respect to atranol. After 6 h at room temperature, an aqueous HCl solution (0.1 M) was added until pH 4 was reached. This aqueous layer was extracted with ethyl acetate (3 × 70 mL). The aqueous phases were concentrated by rotary evaporation to give 6 as a white powder (305 mg, 75%). 1H NMR (D2O, 400 MHz): δ ppm 6.37 (t, 1H), 3.97 (d, 1H), 3.37 (s, H), 2.68 (s, 2H), 2.62 (s, H), 2.14 (s, 3H), 1.79 (s, 3H). 13C RMN (D2O, 100 MHz): δ ppm 197.2 (C), 178.0 (C), 171.5 (C), 164.1 (C), 127.1 (CH), 88.7 (C), 87.1 (C), 85.7 (C), 77.7 (C), 60.1 (CH), 54.4 (CH), 52.9 (CH), 25.7 (CH3), 23.6 (CH3). MS (ESI): [M − H]− ion was observed at m/z 309, [2M − H]+ at m/z 619, [2M − 2H + Na]+ at m/z 641. α(20 °C, 589 nm) = −0.002° ± 0.000 (triplicates).
Acetylated dimer 7: To a solution of dimer 6 (0.100 g, 0.32 mmol) in freshly distilled dichloromethane (2 mL) were added Et3N (0.28 mL, 2.1 mmol) and Ac2O (0.2 mL, 2.1 mmol). The mixture was stirred overnight at room temperature under a nitrogen atmosphere. After concentration in vacuo, water was added (10 mL) and the resulting solution extracted with EtOAc (2 × 10 mL). The organic layers were pooled, dried over MgSO4, filtrated, and concentrated in vacuo. A colorless oil was obtained (0.12 g, 90%). 1H NMR (CDCl3, 400 MHz): δ ppm 6.12 (s, 1H), 5.26 (s, 1H), 5.16 (s, 1H), 3.88 (s, 1H), 3.86 (s, 1H), 3.62 (s, 1H), 2.23 (s, 3H), 2.10 (s, 3H), 2.05 (s, 3H), 1.67 (s, 3H). 13C NMR (CDCl3, 100 MHz): δ ppm 170.9 (CO), 169.8 (CO), 169.7 (CO), 169.5 (CO), 169.1 (CO), 140.6 (C), 134.3 (C), 120.9 (C), 88.6 (C), 87.3 (C), 80.9 (C), 77.7 (C), 58.6 (CH), 55.5 (CH), 50.6 (CH), 23.2 (CH3), 20.9 (CH3), 20.7 (CH3), 20.7 (CH3), 20,5 (CH3). MS (EI) m/z: 418 (1), 376 (20), 334 (7), 290 (2), 273 (3), 266 (1), 246 (2), 231 (3), 214 (6), 182 (23), 175 (3), 162 (6), 141 (28), 140 (100), 91 (1), 77 (2), 69 (2), 43 (100). HRMS 419.0950, calculated for [M.H]+ C20H19O10 419.0978, ∆ = −6.7 ppm; 377.0859, calculated for [M(− CH3CO + H).H]+ C18H17O9 377.0873, ∆ = −3.7 ppm; 335.0754, calculated for [M(− 2CH3CO + 2H).H]+ C16H15O8 335.0767, ∆ = −3.9 ppm.
HRP-catalyzed removal of atranol 2 (4.3%) and chloroatranol 3 (2.3%) from oakmoss absolute at the milligram scale: Oakmoss absolute (250 mg) was dissolved in 270 mL of pH 9 carbonate buffer (20 mM). After sonication of the reaction mixture over 2 h, HRP (124 U/mg, 2 mg) was added. The reaction flask was covered with an aluminum foil, and 2 equivalents of 30% aqueous H2O2 solution (20 μL) were added. The agitation was maintained for 2 h at room temperature. Extraction by ethyl acetate allowed the recovery of the modified absolute (231 mg, 92%) after drying over magnesium sulfate, filtration, and solvent removal by rotary evaporation. HPLC-UV-MS and GC-MS analysis of the modified oakmoss absolute confirmed the disappearance of atranol 2 and chloroatranol 3.
HRP-catalyzed removal of atranol 2 (4.3%) and chloroatranol 3 (2.3%) from oakmoss absolute at the gram scale: Oakmoss absolute (1.2 g) was dissolved in 1.3 L of pH 9 carbonate buffer (20 mM). After sonication of the reaction mixture during 2 h, HRP (124 U/mg, 10 mg) was added. The reaction flask was covered with an aluminum foil, and 2 equivalents of 30% aqueous H2O2 solution (97 µL) were added. The agitation was maintained for 2 h at room temperature. Extraction by ethyl acetate allowed the recovery of the modified absolute (1.1 g, 91%) after drying over magnesium sulfate, filtration, and solvent removal by rotary evaporation. HPLC-UV-MS and GC-MS analysis of the modified oakmoss absolute confirmed the disappearance of atranol 2 and chloroatranol 3.