Abstract
This study addresses the growing consumer demand for effective and sustainable hair care solutions by evaluating a novel bioactive crosslink repair complex designed to restore chemically damaged hair. The complex comprises itaconic acid, arginine, D-panthenol, and polysaccharides from linseed and chia, which work synergistically to promote fiber crosslinking, protein restructuring, and cuticle barrier restoration. The complex was incorporated into two formulations: a bleaching mixture as a protective agent and a leave-in conditioner as a repair treatment for chemically damaged hair. The protective efficacy was assessed through tensile strength measurements, differential scanning calorimetry, combability tests, shine evaluation, and scanning electron microscopy. The repair potential was evaluated using differential scanning calorimetry and tensile strength analysis. Results demonstrated that incorporating the complex into the bleaching mixture significantly enhanced break stress, denaturation enthalpy, shine, and combability, while maintaining improved cuticle alignment. The hair repair evaluation showed that post-treatment application of the complex successfully restored hair tensile strength and denaturation. These findings confirm the dual functionality of Bioactive Crosslink Repair Complex as both a protective and reparative agent, highlighting synergistic mechanisms in preventing and reversing chemical damage to hair fibers. This bioactive approach offers a promising alternative for hair care formulations targeting chemically treated hair.
1. Introduction
In recent years, growing individual awareness of health and well-being has reshaped consumer behavior, leading to increased interest in personal care products that support physiological integrity and promote measurable benefits [1,2]. Within this context, hair care has emerged as a central element of self-care routines, extending beyond basic hygiene to encompass aspects of identity, self-esteem, and overall health [3,4]. This shift has driven the need for greater consumer education concerning hair biology, treatment mechanisms, and product efficacy, moving beyond conventional categorizations based solely on hair types and styling preferences.
The increasing interest in hair care products is evidenced by global search trends, where hair-related inquiries dominate the beauty segment. This highlights not only the relevance of hair care in consumer routines but also the growing scientific and industrial focus on developing innovative formulation systems that combine performance with sustainability [5]. Consequently, the formulation of hair products now demands a deeper understanding of fiber structure, damage mechanisms, and the efficacy of repair agents [6].
The hair fiber consists of three main layers: the cuticle, the cortex, and, when present, the medulla. The cuticle is the outermost layer and acts as the first line of defense, protecting the underlying cortex and contributing to visual properties such as shine [7,8]. This layer is coated by a thin lipid film primarily composed of 18-methyleicosanoic acid (18-MEA), which imparts hydrophobicity to the fiber and forms a protective barrier against external aggressors and chemical treatments [7]. Beneath the cuticle, the cortex constitutes the majority of the hair mass and is responsible for its strength, elasticity, and natural color due to its keratin filaments and matrix, melanin pigments, and inter- and intramolecular interactions [9].
Hair fiber quality is widely recognized as a visible marker of health and plays a vital role in psychological well-being and self-esteem [3,10]. Key attributes associated with healthy hair, such as shine, softness, and tensile strength, are often compromised by cosmetic procedures, particularly chemical transformations [6,11]. Hair bleaching is one of the most aggressive treatments among these. It involves the oxidative degradation of melanin pigments within the cortex, using alkaline hydrogen peroxide systems that penetrate the cuticle and initiate the breakdown of eumelanin and pheomelanin. While effective for lightening, this process leads to collateral damage in the hair protein matrix, especially affecting cysteine residues and disulfide bonds, resulting in weakened structure, increased porosity, and a compromised barrier function [12,13].
Repairing and protecting the hair fiber from such damage is crucial to preserving its strength and integrity. The degradation of amino acids, disruption of disulfide bridges, and loss of hydrogen and ionic interactions collectively contribute to the fragility of bleached hair, demanding targeted strategies for damage prevention and restoration [13]. However, current repair approaches typically focus on single mechanisms or isolated structural components, such as protein and lipid restoration, bonding agents, deep conditioning, or the deposition of film-forming agents on the hair surface [6,13]. Isolated, these strategies generally provide partial improvements rather than achieving meaningful structural recovery [6]. In contrast, the integration of synergistic mechanisms may offer a more comprehensive and promising pathway for restoring the integrity of bleached hair.
In this context, the objective of the present study was to investigate the efficacy of a novel ingredient, uniquely composed of itaconic acid, arginine, D-panthenol, and polysaccharides extracted from linseed and chia seeds. This innovative multifunctional system was specifically designed to act through synergistic fiber crosslinking and reinforcement mechanisms, simultaneously targeting protein restructuring, barrier restoration, and structural integrity enhancement in chemically treated hair. Given its composition and the crosslinking potential of the system, this ingredient will be referred to as bioactive crosslink repair complex (BCRC) for the purposes of this study.
Therefore, this study aimed to expand current strategies for bleached hair treatment by evaluating a multifunctional bio-based complex that addresses both protection and repair mechanisms in hair care formulations.
2. Materials and Methods
2.1. Materials
The disodium EDTA (Trade Name: EDTA-2NA) was acquired from Indústria Química Anastácio S/A (São Paulo, SP, Brazil). The cetrimonium chloride (Trade name: Quatercap C-1650) was obtained from Capuani do Brasil S/A (Tiete, SP, Brazil). The glycerin (Trade name: Oxipurity 1000 U GC) was acquired from Oxiteno SA Indústria e Comércio (Camaçari, BA, Brazil). The cetearyl alcohol (Trade name: Álcool Ceto Estearílico G 16/18) was obtained from Indústria Química Anastácio S/A (São Paulo, SP, Brazil). The cetyl alcohol (Trade name: Alkonat 1698 P) was acquired from Oxiteno SA Indústria e Comércio (Camaçari, BA, Brazil). The ceteareth-20 (Trade name: Alkonat CE 200 F) was obtained from M. Cassab Com. e Ind. Ltda. (Jarinu, SP, Brazil). Citric acid (Trade name: Ácido Cítrico Anidro Fino Granular) was acquired from Nishi Ingredientes Ltda. (Hortolândia, SP, Brazil). The BCRC active (INCI Name: water (and) itaconic acid (and) arginine (and) panthenol (and) Linum usitatissimum (linseed) seed extract (and) Salvia hispanica seed extract (and) polyglyceryl-10 laurate) and xylityl sesquicaprylate (Trade name: Hebeatol Plus; INCI Name: Xylityl Sesquicaprylate) were supplied by Chemyunion Ltda. (Sorocaba, SP, Brazil).
2.2. Active Ingredient Composition
The active ingredient evaluated in this study (BCRC) was a complex formed through a crosslinking strategy involving an unsaturated dicarboxylic acid (itaconic acid), an amino acid (arginine), provitamin B5 (D-panthenol), and polysaccharides derived from Linum usitatissimum (linseed) and Salvia hispanica (chia) seeds. The formulation also includes polyglyceryl-10 laurate as a solubilizing agent in an aqueous base. The name of this ingredient, according to the International Nomenclature of Cosmetic Ingredients (INCI), is water (and) itaconic acid (and) arginine (and) Linum usitatissimum (linseed) seed extract (and) Salvia hispanica seed extract (and) polyglyceryl-10 laurate. The BCRC was supplied by Chemyunion (Sorocaba, SP, Brazil).
2.3. Studied Formulation
A leave-in conditioner formulation was developed for hair repair.
The formulation was based on water (up to 100.00%), disodium EDTA (0.10%), cetrimonium chloride (0.80%), glycerin (1.00%), cetearyl alcohol (3.00%), cetyl alcohol (0.70%), ceteareth-20 (0.30%), citric acid (0.10%), BCRC active (5.00%) (Chemyunion, Brazil), and xylityl sesquicaprylate (0.50%) (Chemyunion, Sorocaba, SP, Brazil).
2.4. Experimental Analysis
2.4.1. Pre-Treatment Protocol
Virgin human hair tresses, naturally straight and dark brown, were obtained from International Hair Importers (New York, NY, USA), a certified supplier of standardized hair materials for cosmetic testing. The selected fibers had no prior exposure to any chemical treatments such as coloring, bleaching, or straightening, ensuring the structural integrity necessary for baseline analysis. All tresses were visually inspected, manually aligned, and trimmed to uniform lengths before the experiments. The hair tresses were washed using a shampoo containing 8.1% Sodium Lauryl Ether Sulfate, massaged for 1 min, then thoroughly rinsed and dried with a blow-dryer set at medium temperature.
2.4.2. Hair Treatment Protocol—Protective Study
After the pre-treatment, the hair tresses were bleached with a standardized mixture of 30 g of bleaching powder and 60 g of 40-volume hydrogen peroxide. 7.5 g of the BCRC was incorporated into this mixture as a protective agent for the test group. The bleaching formulation was evenly applied along the length of the hair tresses, which were subsequently wrapped in aluminum foil and left to process for 45 min at room temperature (25 °C). After processing, the tresses were rinsed thoroughly with water, washed again using the same sodium lauryl ether sulfate-based shampoo, and dried under identical conditions. The bleaching procedure was repeated as necessary until a statistically significant reduction in mechanical resistance was observed (p < 0.05) in relation to untreated virgin hair. This criterion was used to guarantee the chemically damaged state for experimental analysis.
The study was designed with two experimental conditions: (i) bleached hair (control group) and (ii) bleached hair with BCRC (test group). To evaluate the protective effects of the active ingredient, a combination of instrumental techniques was employed, including tensile strength testing, thermal denaturation analysis via differential scanning calorimetry (DSC), morphological examination using scanning electron microscopy (SEM), and functional assessments of combing force and shine surface.
Following the bleaching phase, all samples were conditioned for 24 h in a controlled environment (22 ± 2 °C; 50 ± 5% relative humidity) and maintained under these conditions throughout the testing procedures.
2.4.3. Hair Treatment Protocol—Repair Study
To evaluate the reparative effect of the leave-in formulation, hair tresses were subjected to bleaching using a mixture composed of 30 g of bleaching powder and 60 g of 40-volume hydrogen peroxide. The bleaching formulation was evenly distributed along the hair surface, and each tress was wrapped in aluminum foil and processed for 45 min at room temperature (25 °C). After each cycle, the hair was rinsed with water and dried under the same controlled conditions. Upon completion of the bleaching cycles, the fibers were again washed with the same standard shampoo to remove residual chemicals and to standardize baseline conditions for the repair study.
Following this preparation, the tresses were divided into two experimental groups: (i) untreated bleached hair (control); (ii) bleached hair treated with a leave-in formulation containing 5.0% of the BCRC.
The formulation was applied uniformly along the entire length of the hair tresses and left to dry naturally under controlled ambient conditions (22 ± 2 °C and 50 ± 5% relative humidity), without rinsing. For the repair study, the tensile strength and differential scanning calorimetry were analyzed.
2.5. Mechanical, Thermal, and Performance Analyses
2.5.1. Tensile Strength
Hair fiber diameters were first measured using the FDAS-770 system (Dia-Stron Ltd., Andover, UK), followed by tensile strength evaluation with the MTT-686 Automated Miniature Tensile Tester (Dia-Stron Ltd., Andover, UK). For each group, 50 ± 5 individual hair fibers were randomly selected and analyzed under controlled environmental conditions (22 ± 2 °C; 50 ± 5% RH). The maximum stress (gf/µm2) required to break each fiber was recorded, and mean values were statistically compared between groups to determine mechanical resistance preservation [14].
2.5.2. Differential Scanning Calorimetry (DSC)
For the thermal analysis, the hair tresses were equilibrated for 24 h at 22 ± 2 °C and 60 ± 5% relative humidity. Samples (~2.0 mg per replicate, five replicates per group) were finely chopped and placed into partially sealed aluminum pans. Differential scanning calorimetry (DSC) was performed using a DSC-60 Plus (Shimadzu Corporation®, Kyoto, Japan) under dynamic nitrogen flow (50 mL/min), with a heating rate of 10 °C/min over a range of 25–300 °C. Denaturation enthalpy values were calculated from the DSC curves using LabSolutions TA software (v1.01), allowing comparison of denaturation enthalpy between the untreated control and BCRC-treated hair tresses.
2.5.3. Combability of the Hair Tresses
The combability analysis was carried out using the texturometer Texture Analyzer (Stable Micro Systems®, Godalming, UK) equipped with a hair combing rig. The equipment determines the required force for a comb to pass through the hair tress. A decrease in this force indicates more detangled hair and, therefore, an improvement in combability. For this analysis, each group was composed of three tresses, and twenty measurements per tress were performed.
2.5.4. Hair Shine Analysis
Hair shine assessment was performed using the SAMBA Hair System (Bossa Nova Technologies, Los Angeles, CA, USA). The equipment captures two parallel images of the hair tresses, regarding their specular and diffuse images. The specular image is formed only by the light reflected by the hair surfaces, while the diffuse image is composed of the scattered light coming from inside the hair. The luster parameter was calculated according to the Reich-Robbins formula. As the combability analysis, each group was composed of three hair tresses. Six measurements per tress were carried out [14].
2.5.5. Scanning Electron Microscopy (SEM)
The cuticular surface of the hair was analyzed through scanning electron microscopy. This test consists of a non-destructive method that allows a high-resolution visualization of cuticle pattern and hair shape [15]. The SEM images were taken using a Jeol 6460 LV Scanning Electron Microscope (JEOL Ltd., Tokyo, Japan) [14].
2.6. Molecular Dynamics Simulation
A two-keratin filament structure was built using a keratin 3D structure obtained from the Protein Data Bank [16], PDB ID 6JFV [17]. All the lysine and cysteine side chains were modified to reflect the reaction of itaconic acid with their primary amine and thiol reactive groups, respectively. This assembly was placed in an orthorhombic SPC water box with some of the water molecules replaced by free arginine residues. After a short minimization step, a 10 ms NPT simulation was performed at a temperature of 300 K and ambient pressure. The polar interactions between the compounds and the hair fiber were qualitatively assessed, providing insights into the mechanisms by which these compounds may influence hair properties [18].
2.7. Statistical Analysis
Statistical significance between the groups was determined using an unpaired Student’s t-test. Differences were considered statistically significant at p < 0.05. GraphPad Prism software (version 9.0.0, Boston, MA, USA) was used for data analysis and graph generation.
3. Results
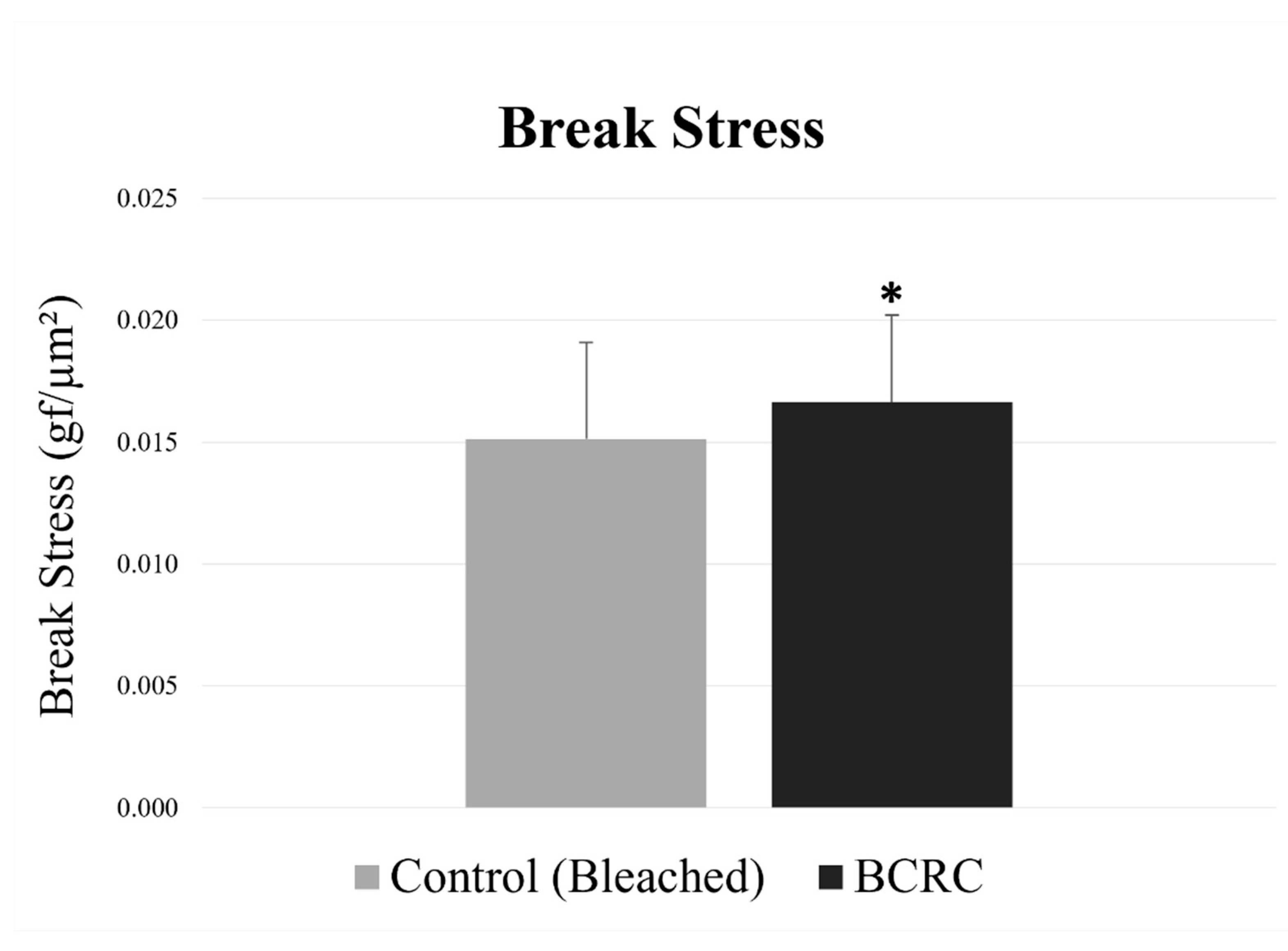
To evaluate the protective study of BCRC, break stress values were measured to quantify the mechanical resistance of hair fibers after bleaching. As shown in Figure 1, the BCRC-treated group showed a higher mean break stress compared to the bleached control group (p < 0.05).
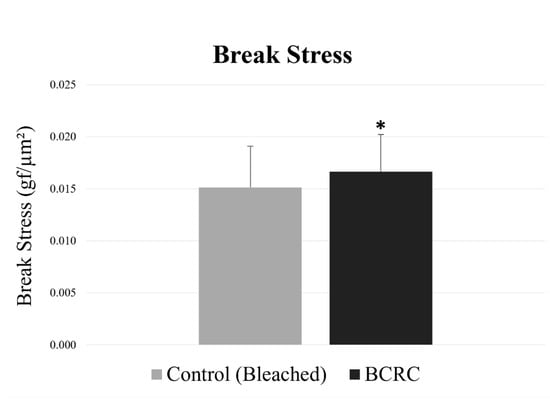
Figure 1.
Break stress (gf/µm2) of bleached hair fibers with and without BCRC treatment. Data represent mean ± standard deviation (n = 50). * Statistically significant difference compared to the bleached control group (p < 0.05).
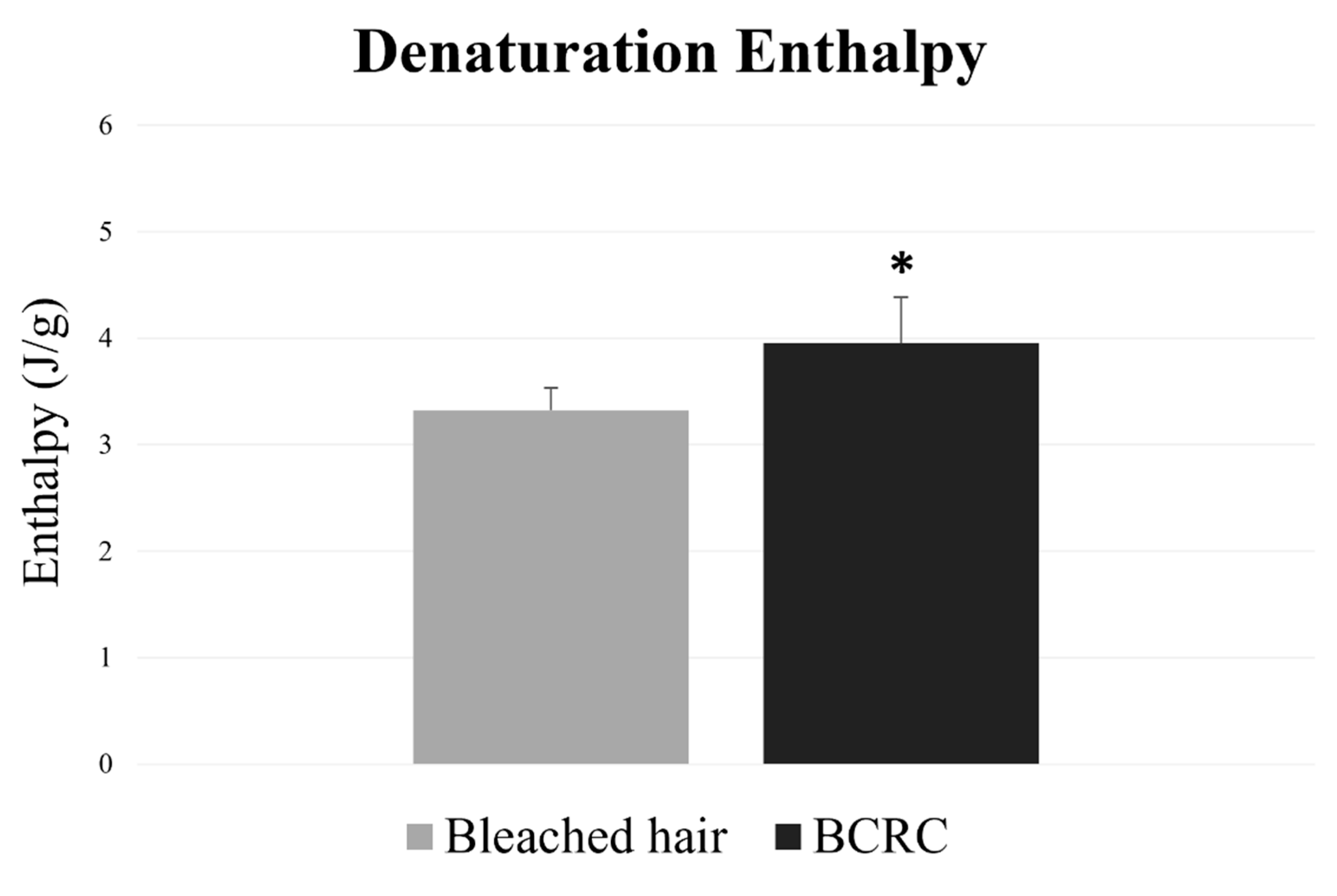
The DSC curve results showed that hair fibers treated with BCRC had significantly higher values of denaturation enthalpy (p < 0.05) compared to the bleached control group (Figure 2), indicating protection of protein integrity.
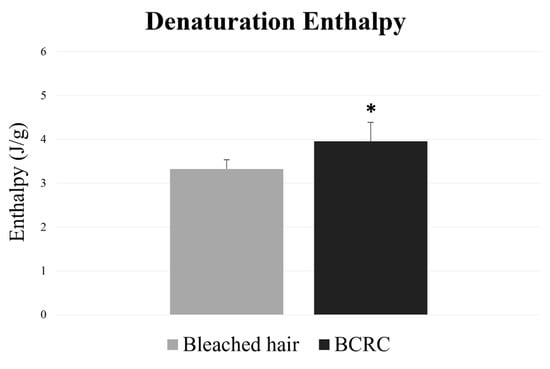
Figure 2.
Denaturation enthalpy (J/g) of bleached hair fibers with and without BCRC treatment. Data represent mean ± standard deviation (n = 5). * Statistically significant difference compared to the bleached control group (p < 0.05).
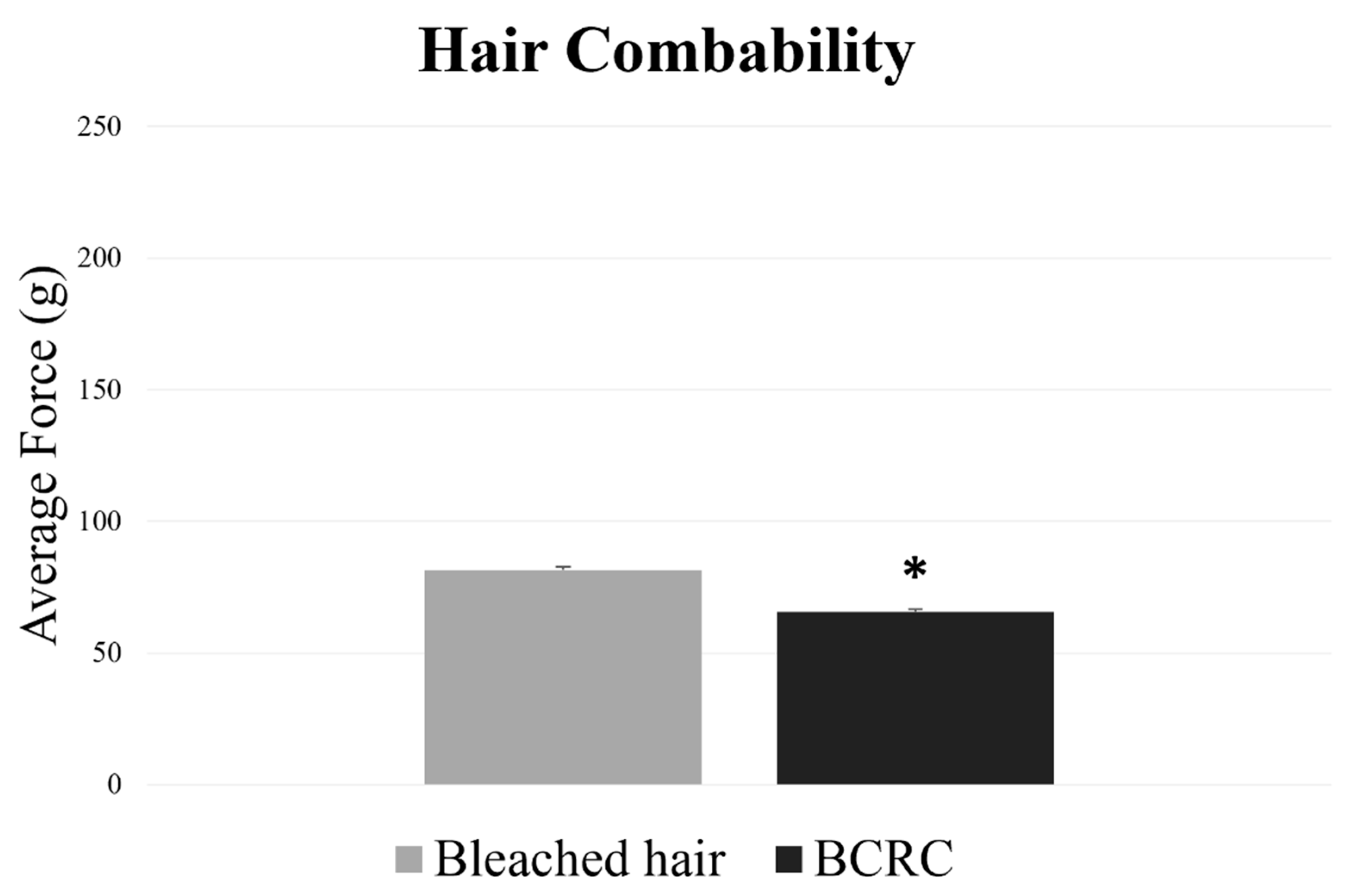
Following the structural and thermal analyses, hair combability was evaluated to assess surface-level performance. As shown in Figure 3, fibers treated with BCRC required a lower average force to be combed compared to the bleached control group.
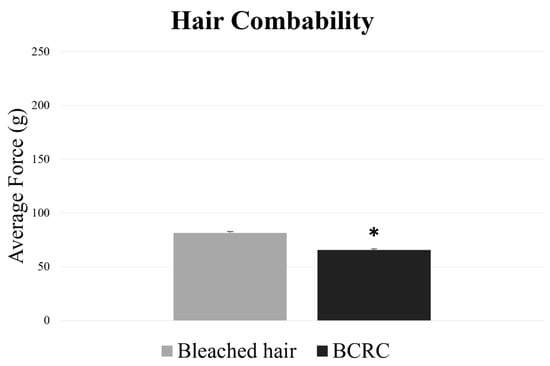
Figure 3.
Average combing force (g) required to detangle bleached hair fibers with and without BCRC treatment. Data represent mean ± standard deviation (n = 60). * Statistically significant difference compared to the bleached control group (p < 0.05).
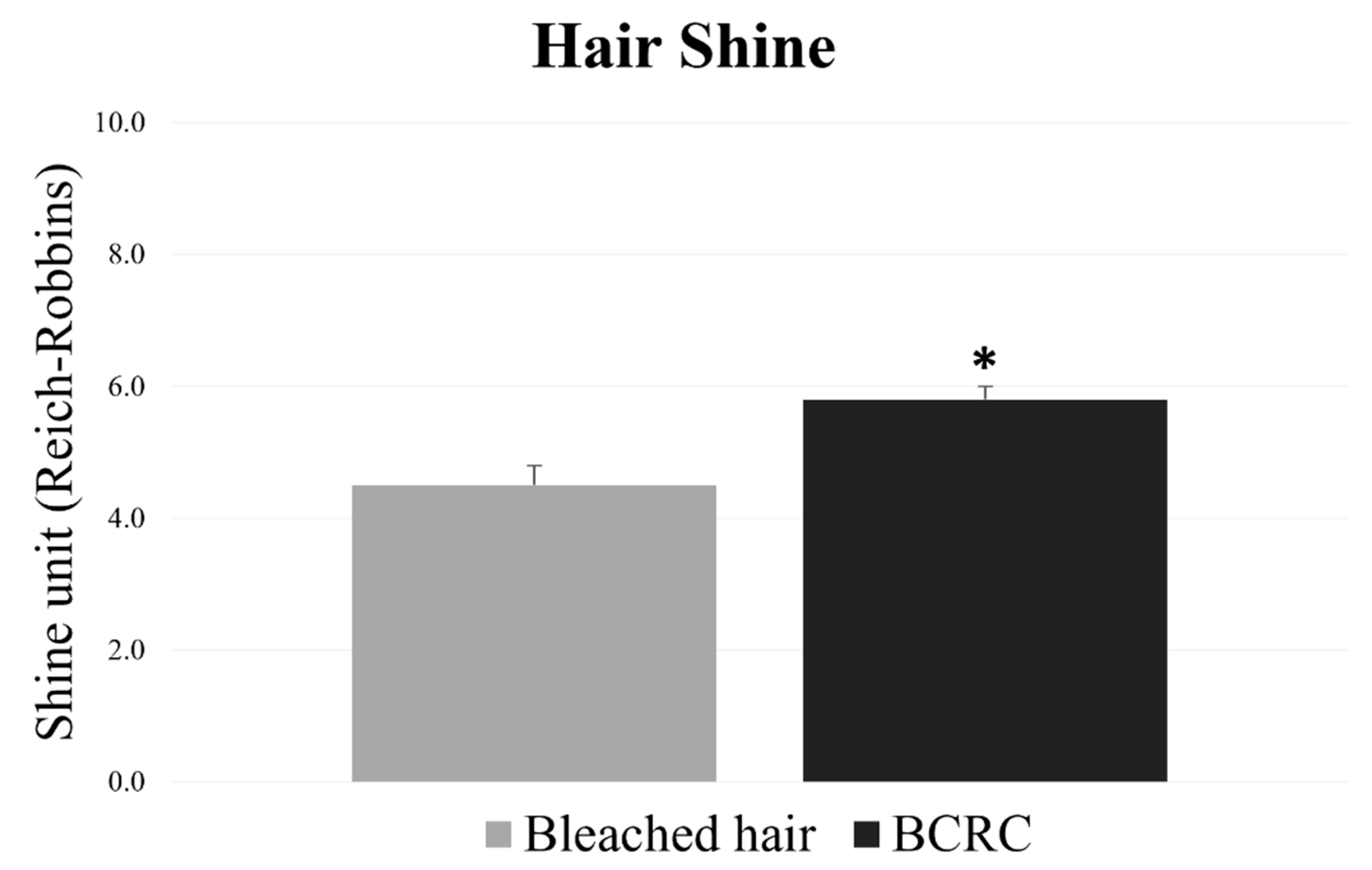
In parallel, surface shine was measured using reflectance-based image analysis. BCRC-treated fibers exhibited higher shine values, relative to the untreated bleached group (Figure 4). The improvement in surface luster further supports the preservation of cuticle alignment. For both parameters, the differences between groups were statistically significant (p < 0.05).
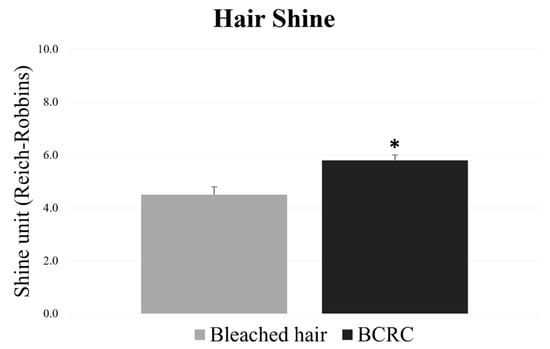
Figure 4.
Shine values (Reich–Robbins units) of bleached hair fibers with and without BCRC treatment. Data represent mean ± standard deviation (n = 18). * Statistically significant difference compared to the bleached control group (p < 0.05).
To complement the instrumental evaluations, SEM was used to visualize the surface morphology of the hair fibers. As shown in Figure 5, virgin hair exhibited an aligned, compact cuticle structure, while bleached hair displayed clear signs of surface damage, including lifted and eroded cuticle layers. In contrast, fibers bleached with BCRC maintained a more continuous and organized cuticular arrangement, with fewer disruptions observed along the fiber surface.

Figure 5.
SEM images of hair fiber surfaces: (a) virgin hair; (b) bleached hair; (c) bleached hair with BCRC.
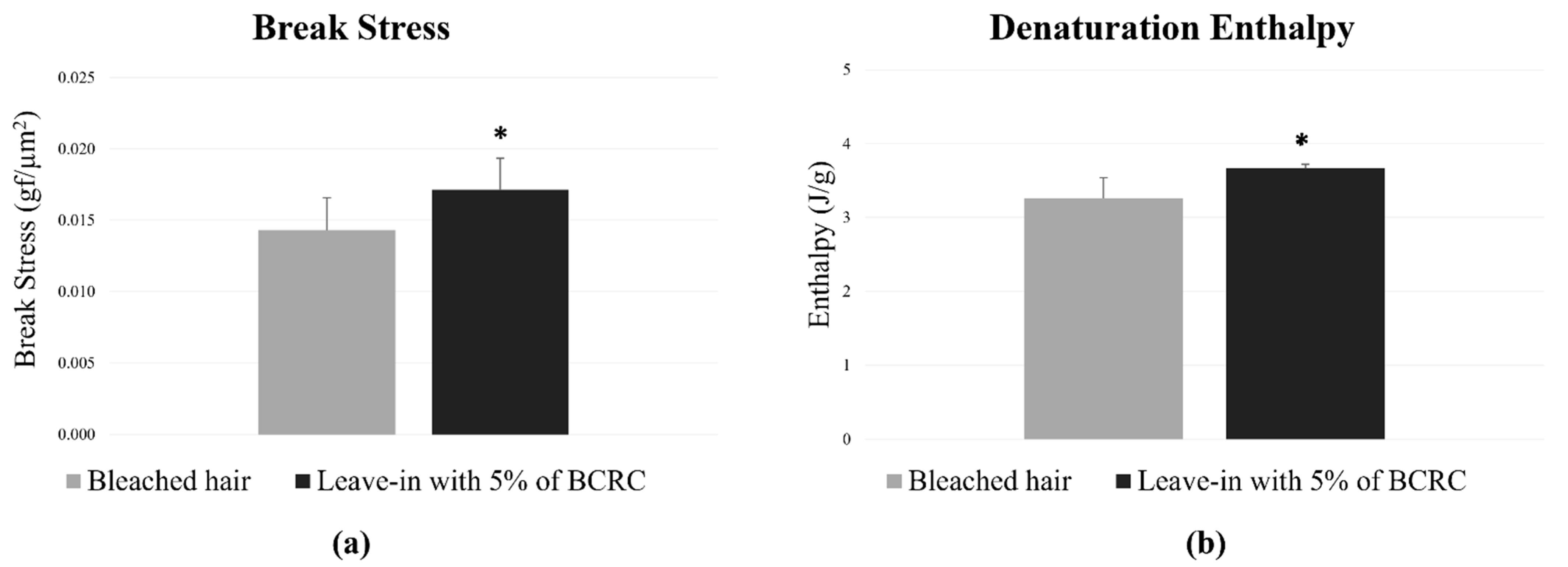
As for the repair study following the application of the leave-in treatments, mechanical strength analysis showed that the leave-in with 5% of BCRC increased in 19.58% the tensile strength compared to the bleached control group. Relative to the virgin hair group, the test formulation restored up to 92% of the original tensile strength, indicating high recovery of fiber resistance following chemical bleaching. Regarding the DSC analysis, the hair tresses treated with the test leave-in formulation containing 5.0% of BCRC exhibited a 12.12% increase in denaturation enthalpy compared to the bleached control group (Figure 6). This result indicates improved structural integrity of hair proteins following chemical damage.
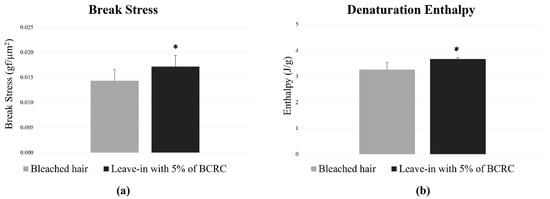
Figure 6.
(a) Break stress (gf/µm2) and (b) denaturation enthalpy (J/g) of bleached hair fibers treated or not with leave-in formulation containing 5% BCRC. Data represent mean ± standard deviation (n = 25 for break stress; n = 3 for enthalpy analysis). * Statistically significant difference compared to the bleached control group (p < 0.05).
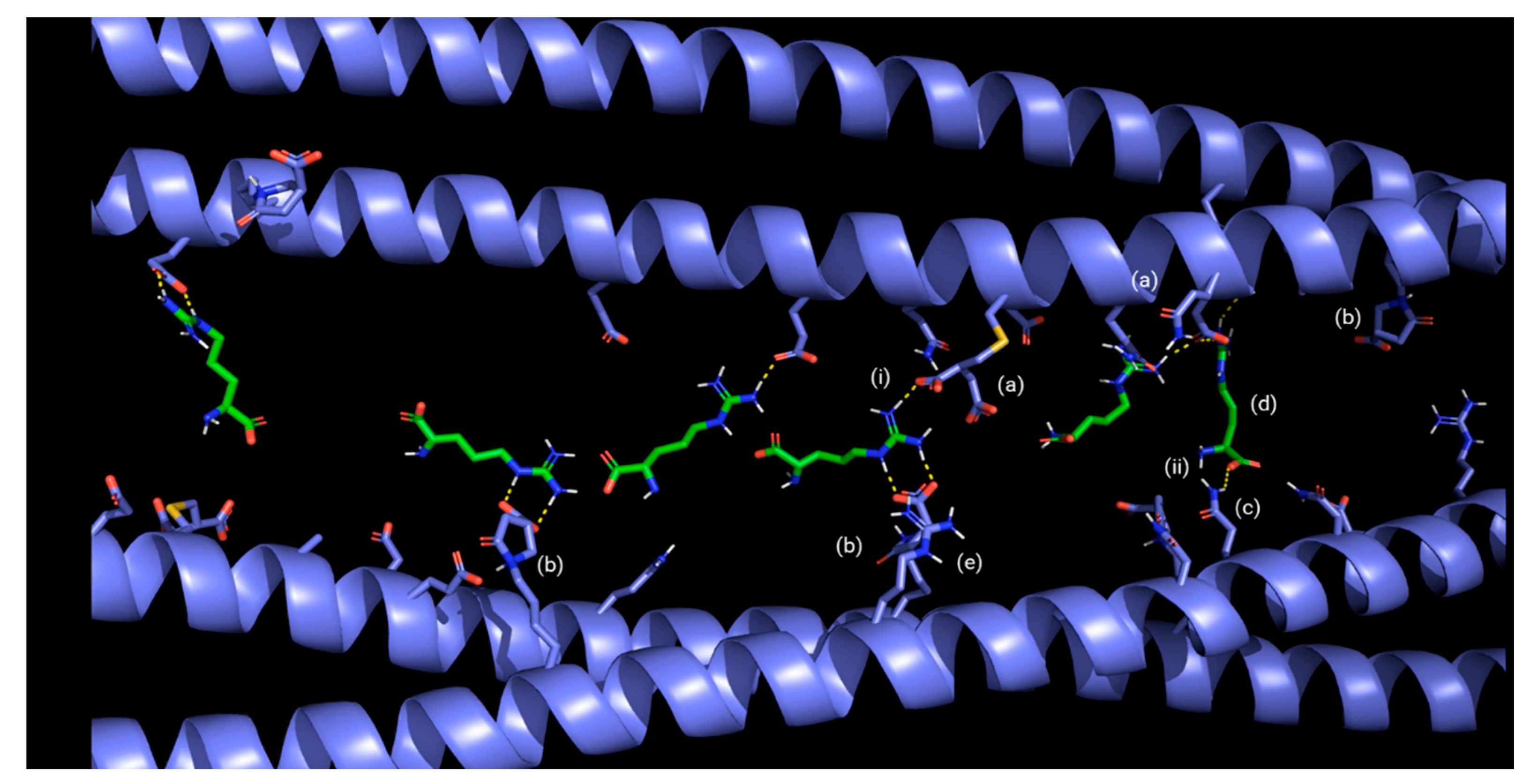
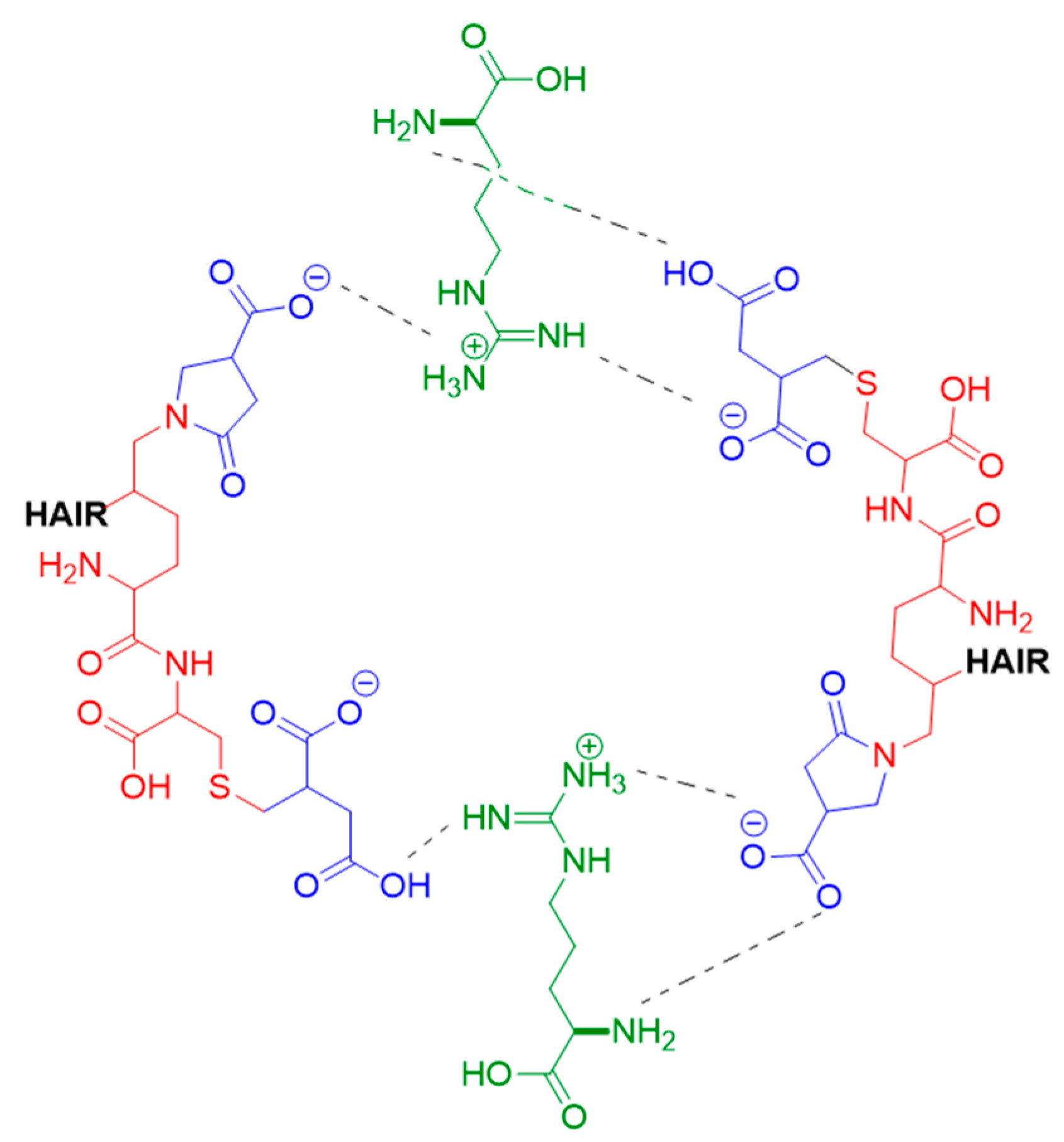
Finally, the simulations showed that the arginine present in BCRC strongly interacts with keratin residues, establishing ionic interactions, hydrogen bonds, and dipole–dipole interactions that act as additional anchoring points within the network (Figure 7 and Figure 8); the formation of crosslinks between keratin filaments could mechanically stabilize the hair fiber.
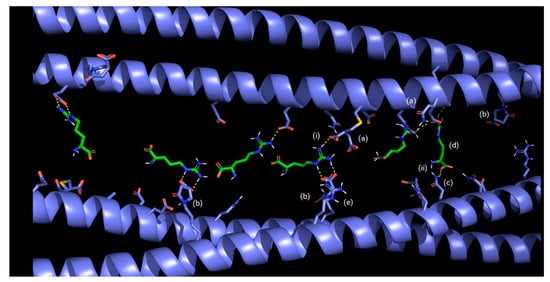
Figure 7.
Schematic representation of the mechanism of action of BCRC, illustrating crosslinking interactions between modified α-keratin chains, arginine, and itaconic acid: (a) itaconic acid added to a thiol group of keratin; (b) itaconic acid added to the primary amine group of keratin; (c) keratin asparagine; (d) in green color: arginine from product; (e) in violet color: arginine from keratin; (i) ion–dipole interaction; (ii) hydrogen bond.
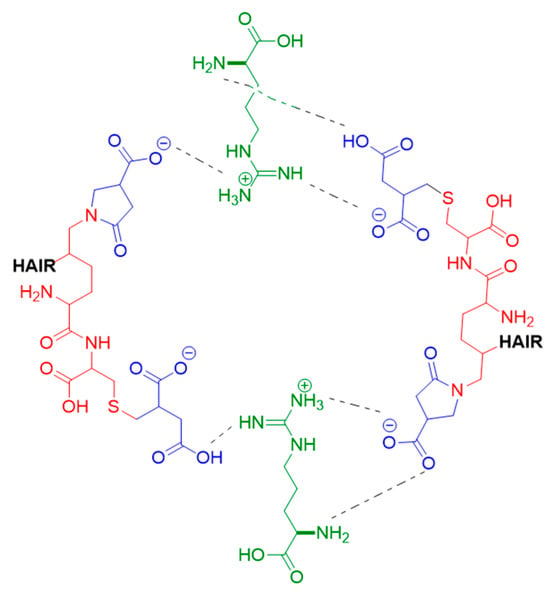
Figure 8.
Schematic representation of the crosslinking interactions between modified α-keratin chains and arginine in the presence of itaconic acid. Blue represents itaconic acid, green represents arginine, and red represents amino acids from hair keratin.
4. Discussion
Chemical bleaching is a very aggressive procedure for human hair, known to cause irreversible damage to both its internal protein structure and external cuticle layers [14]. This damage results from oxidative degradation of amino acids and disruption of disulfide bonds, leading to weakened mechanical properties, increased porosity, and diminished aesthetic attributes such as shine and combability [13]. In accordance with these reports, the bleached control group in the present study showed reduced break stress, lower denaturation enthalpy, impaired surface performance, and clear cuticle disruption under SEM analysis. This finding validates the establishment of a chemically damaged model that is suitable for evaluating protective and repair strategies.
In this context, the addition of BCRC in the bleaching process significantly reduced (p < 0.05) the structural damage typically induced by oxidative treatment, as shown by the increased tensile strength and denaturation enthalpy. These protective effects were associated with enhanced combability and shine, suggesting that the complex maintained, at least to a certain extent, the internal protein architecture and the external cuticular organization of the hair fiber. This protective effect can be attributed to the molecular mechanisms of the active compound, which involve both covalent and non-covalent interactions with the hair fiber.
Itaconic acid (2-methylidenebutanedioic acid) is an organic acid obtained through a sustainable fermentation process based on corn residues [19]. This acid has been thoroughly studied since it is a chemical building block, due to its α,β-unsaturated dicarboxylic structure, which also qualifies it as an effective Michael acceptor. This reactivity arises from a conjugated carbon–carbon double bond adjacent to an electron-withdrawing carboxylic group, a structural motif known to favor nucleophilic addition under mild conditions [20]. The inherent electrophilicity of the β-carbon, enhanced through resonance stabilization, renders this position particularly susceptible to 1,4-conjugate addition reactions with nucleophilic species, including the thiol (-SH) and amine (-NH2) functionalities prevalent throughout the keratin protein matrix [20].
This covalent interaction mechanism proves especially significant in the context of chemically bleached hair, where oxidative damage processes expose reactive cysteine (-SH) [21] and lysine (-NH2) residues within the protein structure. The potential for itaconic acid to act as a Michael acceptor arises from its α,β-unsaturated carboxylic structure [22], which enables 1,4-conjugate addition with nucleophilic groups such as amines and thiols. Such molecular interactions facilitate the formation of crosslinks throughout the keratin network, potentially contributing to the restoration and stabilization of protein architecture in chemically compromised hair fibers, as suggested by the molecular dynamics simulation, consistent with the observed increases in tensile strength and denaturation enthalpy in BCRC-treated hair fibers.
Beyond the mechanistic interpretation, it is important to contextualize these findings in the context of existing hair repair strategies. Recent research has investigated various methodologies for reinforcing chemically damaged fibers, including the use of dicarboxylic acids such as citric acid [6], Michael-acceptor crosslinkers [20,21], protein- or peptide-based systems [9], and bio-based conditioning agents and film-forming polymers [11,14,23,24,25,26]. These technologies have been shown to enhance specific parameters, such as tensile strength, elasticity, or surface conditioning. However, the majority of these technologies are designed as either standalone repair treatments applied after chemical damage or as single-mechanism systems focused predominantly on covalent crosslinking or on surface conditioning. In contrast, BCRC integrates multiple mechanisms within a single bio-based complex. These mechanisms include covalent network reinforcement via itaconic acid, non-covalent interactions mediated by arginine, and film-forming/surface conditioning provided by plant polysaccharides and D-panthenol. BCRC is effective both when added directly to the bleaching mixture (protection) and when used as a leave-in treatment on already bleached hair (repair). This dual-use, multi-mechanism profile differentiates BCRC from more conventional repair agents and may help explain the concomitant gains observed in mechanical, thermal, and surface parameters in the present study.
Moreover, molecular dynamics simulation suggests that arginine in the BCRC ingredient is key to the formation of a highly dense crosslinked network within the hair fiber (Figure 8). It interacts with itaconic acid moieties and keratin side chains via hydrogen bonds, ionic bonds, ion–dipole forces, and dipole–dipole interactions. Among these, the ion–dipole interactions are exceptionally strong, and they play the dominant role in boosting the hair’s mechanical strength. In addition, arginine can interact with anionic regions of the hair surface and with carboxylate groups present in the protein matrix, acting as an auxiliary bridge between the itaconic acid-based network and negatively charged sites along the fiber [23]. Collectively, these intermolecular interactions drive the formation of additional non-covalent (hydrogen bonding and ionic interactions) crosslinkages among keratin macrofibrils in the hair cortex. This enhanced crosslinking density increases the modulus, tensile strength, and overall structural stability of the keratin matrix.
The BCRC in the bleaching formulation also promotes non-covalent stabilization of the hair fiber through the presence of arginine. This cationic amino acid establishes electrostatic interactions with anionic protein residues, while its carboxylic group (-COOH) simultaneously participates in hydrogen bonding networks with the itaconic acid matrix [23]. These interactions may enhance inter-fibrillar cohesion, thereby contributing to the mechanical integrity observed in the hair tress after the bleaching process and after being treated with the leave-in formulation.
Both these mechanisms can explain the increase in the tensile strength and the improved denaturation enthalpy analysis.
Complementing the internal reinforcement, the BCRC complex also includes film-forming agents, notably plant-derived polysaccharides [24,25,27] from linseed (Linum usitatissimum) and chia (Salvia hispanica) seeds, along with provitamin B5 (D-panthenol). The natural polysaccharide complex can adhere to the hair fiber to form flexible and resistant films that provide structural support and moisture retention [26,28]. D-panthenol brings additional benefits due to its humectant properties and ability to improve hair combability, reduce surface roughness, and enhance shine [29,30]. These film-forming agents create hydrating protective barriers over the hair surface, which help seal cuticular damage and restore damaged hair structure. The synergistic action of these compounds likely accounts for the improved sensorial properties, including increased shine, enhanced combability, and reduced combing force observed in the treated samples. The qualitative SEM analysis performed in this study is in line with these interpretations, since BCRC-treated fibers showed more continuous cuticle coverage and fewer lifted scales than the bleached control group.
As for the repair study, the tensile strength analysis showed that BCRC-treated fibers had significantly (p < 0.05) higher tensile resistance values than untreated bleached controls. Consistently, DSC measurements revealed higher denaturation enthalpy values, indicating improved structural integrity within the fiber inner region. Since denaturation enthalpy is closely related to the degradation of keratin intermediate filaments and matrix pyrolysis [31], these findings suggest that the active ingredient BCRC can protect the structure and the crosslink bonds, but also, when used after treatment, may promote the formation of new ones. This combined effect accounts for the observed increases in both tensile strength and denaturation enthalpy.
In addition, the leave-in formulation containing 5.0% BCRC restored up to 92% of the original tensile strength of virgin hair, indicating that the active ingredient can partially reverse the damage caused by bleaching. These results, combined with the protective effects observed when BCRC is present in the bleaching mixture, support the concept of a dual mode of action, in which BCRC acts not only as a protective agent that reduces damage during bleaching but also as a repair agent that reinforces and reorganizes the structure of already compromised hair fibers.
Although this study evaluated a single concentration use of BCRC in a chemically bleached hair model, future studies could investigate a broader concentration range, assess performance under in vivo or long-term use conditions, and extend the application to other types of damaged hair fibers.
5. Conclusions
The findings demonstrate that BCRC can be effectively used both as a protective and a restorative agent for chemically damaged hair. By enhancing crosslink density, the treatment not only prevents structural weakening during bleaching but also promotes the repair of already compromised fibers. Acting within both the cortex and cuticle, BCRC improves mechanical strength while also enhancing surface properties of the hair fiber. Moreover, the results highlight that combining multiple protective mechanisms to safeguard bleached hair represents a promising direction for advancing hair care strategies.
Author Contributions
F.B.C.J., A.M.G., G.F.D.O., M.R.R., C.R.P., E.K., and W.M.: Study conceptualization. F.B.C.J., A.M.G., G.F.D.O., M.R.R., C.R.P., E.K., W.M., and P.M.B.G.M.C.: Methodology. F.B.C.J., A.M.G., G.F.D.O., M.R.R., C.R.P., E.K., and W.M.: Formal analysis. P.M.B.G.M.C., L.K., R.d.A.Z., A.M.G., G.F.D.O., M.R.R., C.R.P., E.K., W.M., and F.B.C.J.: Writing—original draft preparation. P.M.B.G.M.C., L.K., and R.d.A.Z.: Review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
AI-assisted tools were used only for minor grammar and style edits using Grammarly Pro https://www.grammarly.com/pro (accessed on 20 October 2025). The authors have reviewed and edited the content and take full responsibility for the final version of the manuscript.
Conflicts of Interest
Flavio B Camargo Junior, Alessandra M Goshiyama, Gessica FD Oliveira, Marcos R Rossan, Cleverson R Princival, Edson Katekawa, and Wagner Magalhães are employees of Chemyunion. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BCRC | Bioactive crosslink repair complex |
| DSC | Differential scanning calorimetry |
| SEM | Scanning electron microscopy |
References
- Das, D.; Sarkar, A.; Debroy, A. Impact of COVID-19 on Changing Consumer Behaviour: Lessons from an Emerging Economy. Int. J. Consum. Stud. 2022, 46, 692–715. [Google Scholar] [CrossRef]
- de Morais, I.C.; Nascimento, T.C.d.; Tayt-son, D.B.d.C. Reimagining Beauty: Digital Consumption Practices in a Disrupted World. BAR Braz. Adm. Rev. 2024, 21, e240111. [Google Scholar] [CrossRef]
- Newton-Fenner, A.; Hirst, W.M.; Jones, T.; Scott, M.; Roberts, C.; Smeets, M.A.M.; Shen, J.; Thomas, A.; Giesbrecht, T. Development of the Hair & Scalp CARE Questionnaire: Measuring the Impact of Hair and Scalp Issues on Psychological Wellbeing in Healthy Populations. Int. J. Cosmet. Sci. 2025, 47, 807–819. [Google Scholar] [CrossRef]
- Udayanga, L.; Subashini, N.; Udugama, M.; Silva, P.; Ranathunge, T. Knowledge, Perceptions, and Consumption Behaviour of Cosmetics among Undergraduates of Sri Lanka: A Descriptive Cross-Sectional Study. Front. Public Health 2024, 11, 1184398. [Google Scholar] [CrossRef]
- Bjelošević Žiberna, M.; Grilc, B.; Gašperlin, M.; Gosenca Matjaž, M. Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability. Gels 2025, 11, 374. [Google Scholar] [CrossRef]
- Zhang, D.; Baghdadli, N.; Greaves, A.J. Reinforcing Chemically Treated Human Hair with Citric Acid. Int. J. Cosmet. Sci. 2025, 47, 411–423. [Google Scholar] [CrossRef]
- Tokunaga, S.; Tanamachi, H.; Ishikawa, K. Degradation of Hair Surface: Importance of 18-MEA and Epicuticle. Cosmetics 2019, 6, 31. [Google Scholar] [CrossRef]
- Nagase, S. Hair Structures Affecting Hair Appearance. Cosmetics 2019, 6, 43. [Google Scholar] [CrossRef]
- Gu, W.; Gu, L.; Tao, N.; Wang, X.; Xu, C. Composite Fish Collagen Peptide-Based Biopolymer Emulsion for Keratin Structure Stabilization and Hair Fiber Repair. Polymers 2025, 17, 907. [Google Scholar] [CrossRef] [PubMed]
- Gabarra, M.A.L.; Favaretto, G.; Martini, A.P.M.; Maia, P.M. Characterization of Aging Hair and Its Influence in Quality of Life. J. Biomed. Biopharm. Res. 2015, 12, 79–89. [Google Scholar] [CrossRef]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Oh, S.H.; Chang, B.S. Effects of Excessive Bleaching on Hair: Comparative Analysis of External Morphology and Internal Microstructure. Appl. Microsc. 2024, 54, 11. [Google Scholar] [CrossRef]
- Martins, E.; Castro, P.; Ribeiro, A.B.; Pereira, C.F.; Casanova, F.; Vilarinho, R.; Moreira, J.; Ramos, Ó.L. Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics 2024, 11, 150. [Google Scholar] [CrossRef]
- Camargo, F.B., Jr.; Minami, M.M.; Rossan, M.R.; Magalhães, W.V.; Porto Ferreira, V.T.; Maia Campos, P.M.B.G. Prevention of Chemically Induced Hair Damage by Means of Treatment Based on Proteins and Polysaccharides. J. Cosmet. Dermatol. 2022, 21, 827–835. [Google Scholar] [CrossRef]
- Koch, S.L.; Tridico, S.R.; Bernard, B.A.; Shriver, M.D.; Jablonski, N.G. The Biology of Human Hair: A Multidisciplinary Review. Am. J. Hum. Biol. 2020, 32, e23316. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, C.H.; Coulombe, P.A.; Leahy, D.J. The Crystal Structure of 2B-2B Complex from Keratins 5 and 14 (C367A Mutant of K14). Worldwide Protein Data Bank [Internet]. 2020. Available online: https://www.rcsb.org/structure/6JFV (accessed on 1 October 2025).
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Kollman (2022). In Amber 2022; University of California: San Francisco, CA, USA, 2022; Available online: https://ambermd.org/doc12/Amber22.pdf (accessed on 11 August 2025).
- Yi, X.; Li, X.; Han, J.; Liu, Z.; Shi, X.; Wen, T.; Zhu, J. Itaconic acid production from corn stover hydrolysates for a newly isolated Aspergillus terreus through adaptive evolution. Bioprocess Biosyst. Eng. 2025, 48, 1069–1087. [Google Scholar] [CrossRef]
- Di Foggia, M.; Boga, C.; Micheletti, G.; Nocentini, B.; Taddei, P. Structural Investigation on Damaged Hair Keratin Treated with α,β-Unsaturated Michael Acceptors Used as Repairing Agents. Int. J. Biol. Macromol. 2021, 167, 620–632. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Chen, T.; Chang, K.; Wang, J. Reconnection of Cysteine in Reduced Hair with Alkylene Dimaleates via Thiol-Michael Click Chemistry. Int. J. Cosmet. Sci. 2024, 46, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Peyrton, J.; Avérous, L. Aza-Michael Reaction as a Greener, Safer, and More Sustainable Approach to Biobased Polyurethane Thermosets. ACS Sustain. Chem. Eng. 2021, 9, 4872–4884. [Google Scholar] [CrossRef]
- Wang, B.; Sun, H.; Gao, J.; Kang, L.; Wan, H.; Wu, Y.; Ma, H.; Teng, Z.; Xu, X.; Geng, L.; et al. Synergistic Enhancement of Surface Deposition and Moisturizing Performance of Trehalose and N2-(2,3-Dihydroxypropyl)Arginine Hydrochloride through Hydrogen Bonding Interactions. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 136979. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Leite, M.G.A.; Maia Campos, P.M.B.G. Film-Forming Properties of Topical Formulations for Skin and Hair: In Vivo and In Vitro Studies Using Biophysical and Imaging Techniques. AAPS PharmSciTech 2022, 24, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, N.; Guo, S.; Liu, X.; Liu, C.; Ai, M. Unlocking the Potential of Keratin: A Comprehensive Exploration from Extraction and Structural Properties to Cross-Disciplinary Applications. J. Agric. Food Chem. 2025, 73, 1014–1037. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Kakuda, L.; Souza, C.R.F. Film-Forming, Moisturizing, and Sensory Properties of a Cosmetic Formulation Containing Tara Gum and Brazilian Berry Extracts. AAPS PharmSciTech 2024, 25, 71. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A.; Sheikh, F.A. Flaxseed (Linum usitatissimum) Mucilage: A Versatile Stimuli–Responsive Functional Biomaterial for Pharmaceuticals and Healthcare. Int. J. Biol. Macromol. 2024, 278, 134817. [Google Scholar] [CrossRef]
- Crowther, J.M. Understanding Humectant Behaviour through Their Water-holding Properties. Int. J. Cosmet. Sci. 2021, 43, 601–609. [Google Scholar] [CrossRef]
- Weiss, C.L.; Fairchild, M.R.; Stanton, B.; Nshime, B.S.; Parkanzky, P.D. Innovative Method for the Analysis of Dexpanthenol in Hair Care Products. J. AOAC Int. 2019, 102, 633–637. [Google Scholar] [CrossRef]
- Popescu, C.; Gummer, C. DSC of Human Hair: A Tool for Claim Support or Incorrect Data Analysis? Int. J. Cosmet. Sci. 2016, 38, 433–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.