Abstract
This study evaluated the thermal properties, crystallinity, particle size, morphology, and in vivo local inflammation and persistence of two poly-L-lactic acid (PLLA) injectable implants, Sculptra® (PLLA-SCA) and GANA V® (PLLA-GA). PLLA-SCA and PLLA-GA underwent differential scanning calorimetry and X-ray powder diffraction to evaluate their thermal properties and degree of crystallinity. X-ray powder diffraction spectra displayed a sharper, more intense peak for PLLA-GA than PLLA-SCA, with smaller peaks on either side of the main peak of PLLA-GA but not PLLA-SCA. Differential scanning calorimetry thermograms indicated three thermal events for both PLLA-SCA and PLLA-GA. For PLLA-SCA, the first two events occurred between 65 °C and 90 °C, and the third event occurred at 165 °C. For PLLA-GA all three events occurred between 156 °C and 169 °C. Heating samples to 120 °C and cooling to room temperature prior to differential scanning calorimetry resulted in no thermal events being observed between 65–90 °C with either product, while three events were observed with PLLA-GA and one event with PLLA-SCA between 156 °C and 169 °C. The median volume distribution diameter was 46.4 µm for PLLA-SCA and 31.7 µm for PLLA-GA. Scanning electron microscopy showed PLLA-GA particles were irregular in shape, had no sharp edges and had a wrinkled and crimped surface, while PLLA-SCA particles displayed plate-like shapes and had smoother surfaces. In vivo inflammatory reactivity scores indicated a slight reaction for PLLA-SCA at all time points (3.7 ± 1.1, 6.1 ± 1.6, 5.7 ± 1.2 and 6.2 ± 1.2 at 2, 12, 26 and 52 weeks, respectively), while for PLLA-GA, a moderate reaction was observed at 12 and 26 weeks (2.9 ± 1.5, 10.1 ± 1.0, 9.4 ± 0.7 and 7.1 ± 1.3 at 2, 12, 26 and 52 weeks, respectively). PLLA-SCA and PLLA-GA had similar persistence scores at 2, 12 and 26 weeks, while at 52 weeks the score was markedly higher for PLLA-SCA versus PLLA-GA (1.9 ± 0.2 versus 0.7 ± 0.2). In conclusion, PLLA-SCA is more amorphous than PLLA-GA. The single melting point of PLLA-SCA contrasts with the broader spectrum of melting points for PLLA-GA suggests a more homogenous formulation of PLLA-SCA. This, and its less crystalline structure, result in the slower degradation rate and more sustained biological response of PLLA-SCA compared with PLLA-GA. The physiochemical properties of PLLAs affect the biological response in clinical practice and should be taken into consideration when selecting a PLLA treatment for aesthetic use.
1. Introduction
Aesthetic injectables have been used extensively for many years in cosmetic dermatology to address a number of medical and aesthetic concerns. Over recent years there has been increasing interest among dermatologists and cosmetic specialists in the biocompatible and biodegradable substance poly-L-lactic acid (PLLA) [1,2]. Across all PLLA injectables (or biostimulators) currently available, Sculptra® (PLLA-SCA) was the first available, is the most widely studied and has been approved for facial aesthetic uses since 1999 in Europe; since 2004, it has been used in the United States for the restoration and correction of the signs associated with HIV-associated lipoatrophy (including fat loss of the limbs, buttocks, and face) and since 2009 for correction of nasolabial folds and other facial wrinkles. Other PLLA injectables have only recently become available in a very limited range of markets.
Injection of PLLA-SCA stimulates the expression of genes associated with collagen and elastin formation, inflammation and extracellular matrix remodelling [3,4,5,6,7]. The subclinical inflammatory response leads to encapsulation of the injected microparticles, followed by stimulation of collagen production [3,5,8,9]. Directly after injection of PLLA-SCA, protein adsorption occurs onto the surface of PLLA, followed by infiltration by neutrophils and macrophages. The suspension of the microparticles leads to distension, which creates the appearance of immediate volumization; this effect is temporary, resolving within several hours to a few days. Within three weeks of injection, the microparticles are encapsulated, and at one-month post-injection they are surrounded by mast cells, mononuclear macrophages, foreign body cells and lymphocytes. At three months, the inflammatory response wanes, while collagen production increases. After six months, the inflammatory response has returned to baseline while collagen production continues to increase. Up to 8–24 months post-injection, significant increases in type I collagen are found around the periphery of the PLLA-SCA microparticles as collagenesis continues [3,8].
Poly lactic acid injectable implants include poly D-lactic acid (PDLA); PLLA; and an equal ratio of PDLA and PLLA, called poly D,L-lactic acid (PDLLA). In PDLLA, the ratio of L- and D-units plays a key role in the physicochemical properties and degradation profile [10]. Here, we focus on injectable implants utilizing PLLA that comprise a mixture of PLLA microparticles and other components (carboxymethylcellulose [a viscosity modifier] and mannitol [an inert binding agent with low hygroscopicity]). Despite relatively similar formulations, differences are often observed between PLLA injectables in everyday clinical practice, including ease of injection and the occurrence of complications or adverse events. Studies have indicated that the clinical effectiveness and safety of various dermal biostimulators are closely linked to the physicochemical characteristics of the PLLA microparticles contained within them [8,11,12].
The degradation of PLLA, which is via hydrolysis to lactic acid, an endogenous compound found in humans and other living organisms, is dependent on a range of factors, including stereochemistry, molecular weight, crystallinity, particle size, microparticle morphology and pH [13,14,15]. The degradation profile of the PLLA injectable determines the rate of lactic acid formation, which can affect the activity of fibroblasts in the synthesis of collagen [15]. Hence, the physicochemical characteristics of a PLLA injectable can both directly and indirectly influence its clinical performance.
The crystallinity of PLLA is thought to play an important role in its clinical performance. PLLA samples that are less crystalline and more amorphous in structure can preserve mechanical strength for longer [15], while less crystallinity usually results in faster degradation [16].
Therefore, while some published data are available on the physicochemical properties of some commercially available poly lactic acid-based injectables [15,17,18,19,20], this study evaluated the thermal properties, crystallinity, particle size and morphology, and in vivo local inflammation and persistence of two commercially available PLLA injectables—(Sculptra®, Galderma, Sweden, PLLA-SCA) and GANA V® (GCS Co., Seoul, Republic of Korea, PLLA-GA)—with a focus on properties that might affect the subclinical inflammatory response which stimulates prolonged collagen production in the skin.
2. Materials & Methods
2.1. Sample Preparation
The two commercially available PLLA materials (approximately 800 mg/sample), PLLA-SCA and PLLA-GA, were washed with water, captured onto a 0.22 µm filter, and rinsed with isopropyl alcohol to remove water and dried to constant weight over silica gel in a desiccator to remove excipients.
2.2. Differential Scanning Calorimetry
The thermal properties and the degree of crystallinity were studied using differential scanning calorimetry analysis performed on a Mettler DSC822e (Mettler Toledo, Greifensee, Switzerland) equipped with a Thermo Haake EK45/MT cooler (Thermo Fisher, Waltham, MA, USA). Samples were weighed into a 40 μL Al-cup, which was then closed with a pierced lid. For each PLLA evaluated, two replicates were prepared and analysed.
Samples were scanned from 25 to 300 °C in a nitrogen atmosphere with a heating rate of 10 °C/min. To gain further understanding on the nature of the thermal events occurring in the samples, additional samples were heated to 120 °C, allowed to cool to room temperature and then analysed by heating from 25 to 300 °C at a heating rate of 10 °C/min.
2.3. X-Ray Powder Diffraction
Samples were spread onto a Zero Background Holder using a spatula. These were then mounted in stainless steel holders and placed in the X-ray powder diffractometer. Measurements were performed at room temperature (approximately 22 °C) on a PANalytical X’Pert PRO diffractometer (Malverna Panalytica, Malvern, UK), equipped with a Cu, long fine focus X-ray tube and a PIXcel detector (Malverna Panalytica, Malvern, UK). Automatic divergence- and anti-scatter slits were used together with 0.02 rad soller slits and a Ni-filter. The scan length was 17 min. To increase the randomness of the samples, they were spun during the analysis. The samples were analysed between 2–40° in 2-theta using 255 detector channels.
2.4. Particle Size and Morphology
Particle size distribution was determined by means of laser diffraction using a MasterSizer 3000 (Malvern Instruments, Malvern, UK). Values of 1.465 and 0.010 were used for the refractive index and absorbance of the PLLA particles, respectively, and the refractive index of the continuous ethanol phase was set to 1.36. Measurements were performed using a “small volume cell” (total volume ~8 mL) rather than the standard large volume recirculation cell (total volume ~400 mL) as this allowed a reduction in the total amount of PLLA particles needed. Prior to being added to the instrument measuring cell, approximately 2 mg of the sample was dispersed in a glass vial containing 8 mL of ethanol. This vial was sonicated for 30 s after which the contents were immediately transferred into the small volume measuring cell, which contained an internal stirrer set to 800 rpm. Reported values are an average of three measurements.
Morphology was evaluated using low-vacuum scanning electron microscopy (SEM) involving a Quanta 250 FEG ESEM (FEI Oxford Instruments, Oxford, UK) coupled to a X-Max 50 mm2 EDS (Oxford Instruments, Oxford, UK). Samples to be analysed were placed on specimen stubs and imaging was performed in low-vacuum mode using a 5 kV beam and a large field detector.
2.5. In Vivo Local Inflammatory Tissue Response (Reactivity) and Persistence of Response
Adult male New Zealand White rabbits (each 2.7–3.3 kg at selection; NAMSA, Northwood, OH, USA) received subcutaneous implants containing 0.2 mL of either PLLA-SCA, PLLA-GA (both reconstituted as per manufacturer’s instructions) or negative control on the dorsal surface. On the day of implantation, each rabbit was injected subcutaneously with 0.02 mg/kg buprenorphine. For general anaesthesia, each rabbit was injected intramuscularly with a mixture of ketamine hydrochloride and xylazine (34 mg/kg + 5 mg/kg) at a dose volume of 0.6 mL/kg. After the anaesthetic had taken effect, the surgical site was scrubbed, wiped with 70% isopropyl alcohol, and painted with an antiseptic such as povidone iodine. Rabbits were implanted by the trocar method and placed on an inhaled anaesthetic for a short duration to obtain an appropriate depth of anaesthesia.
An incision was made through the skin and parallel to the vertebral column for each test product and negative control site. The incisions were placed at appropriately spaced intervals and a location marker was implanted with each test product site. A stylet was placed in the hub of a location marker loaded needle and the needle was inserted into the subcutaneous tissue ventrally through the skin incision. While holding the needle in place, the stylet was pushed slightly to place the location marker. To implant the test products, only the stylet was removed and the pre-loaded syringe containing the test article was attached and 0.2 mL was injected. The syringe and needle were then removed. To implant the negative controls, a stylet was placed in the hub of a negative control (high-density polyethylene; HDPE)-loaded needle and the needle was inserted into the subcutaneous tissue ventrally through the incision. While holding the needle in place, the stylet was pushed slightly to place the HDPE in the implant pocket. The skin was closed with tissue glue.
To aid in the recovery from the anaesthetic, each rabbit received one 0.3 mL injection of atipamezole (Antisedan®) intramuscularly. Rabbits were monitored for recovery from the anaesthetic and returned to their respective cages. Another dose of buprenorphine was administered at the end of the day.
Animals were individually housed in stainless steel or plastic suspended cages at a room temperature of 16–22 °C and a relative humidity of 30–70%. The light cycle was controlled using an automatic timer (12 h light, 12 h dark), a commercially available rabbit feed was provided daily, and water was provided ad libitum.
The study was approved by the NAMSA Northwood Division Institutional Animal Care and Use Committee (IACUC approval #23-019) prior to it being conducted and was compliant with animal welfare guidelines. NAMSA maintains an approved Animal Welfare Assurance on file with the National Institutes of Health, Office for Laboratory Animal Welfare. The conditions conformed to NAMSA Standard Operating Procedures, the study was conducted based on ISO 10993-6, standard veterinary medical care was provided throughout the study and all associates involved in the study were appropriately qualified and trained.
Animals were observed daily for general health and body weights were measured monthly and at termination. Designated animals were terminated by an intravenous injection of a sodium pentobarbital-based drug at 4 days (baseline), and 2, 12, 26 and 52 weeks. The subcutaneous tissue was examined and the area around each implant was dissected free; the implant sites and regional lymph nodes were collected, fixed in 10% neutral buffered formalin, and processed for histopathology to evaluate local reaction and article degradation. Evidence of a local tissue inflammatory response was determined using a microscopic reactivity scoring scale (Table 1) and compared with the negative control at each time interval.

Table 1.
Microscopic reactivity scoring scale.
As a result of the significance of inflammatory cell infiltrates and necrosis, the scores for polymorphonuclear cells, lymphocytes, plasma cells, macrophages, giant cells and necrosis were multiplied by ‘2’ to give additional weighting to these observations (Table 1).
To determine a reactivity score, values were totalled, and an average score for each treatment and the negative control was calculated. The average score for the negative control was subtracted from the average score of each treatment, and the reactivity score assigned based on the following scale:
Minimal or no reaction (0.0–2.9);
Slight reaction (3.0–8.9);
Moderate reaction (9.0–15.0);
Severe reaction (≥15.1).
Microscopic persistence (degradation) was scored using the following grading scale:
0: No microspheres/vehicle observed;
1: Minimal amount (approximately < 10% compared to baseline) of microspheres/vehicle visible;
2: Partial amount (approximately 10–90% compared to baseline) of microspheres/vehicle visible;
3: Microspheres/vehicle appear similar to baseline.
3. Results
3.1. Crystallinity and Thermal Properties
The X-ray powder diffraction spectra and differential scanning calorimetry thermograms of the PLLA-SCA and PLLA-GA samples are shown in Figure 1 and Figure 2 and Figure 3, respectively. Individual The X-ray powder diffraction spectra displayed a sharper and more intense peak for PLLA-GA compared with the spectra from PLLA-SCA. There were also additional, smaller peaks observed either side of the main peak, which are more obvious on the right-hand side, in the diffractogram of PLLA-GA that were absent with PLLA-SCA (Figure 1).
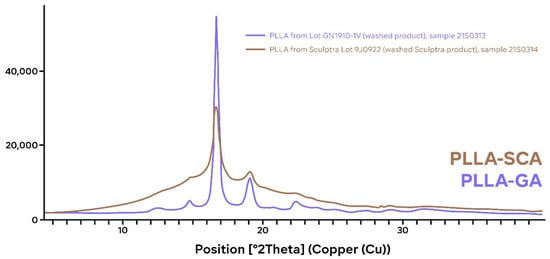
Figure 1.
Individual X-ray powder diffraction spectra of PLLA-SCA (brown) and PLLA-GA (blue).
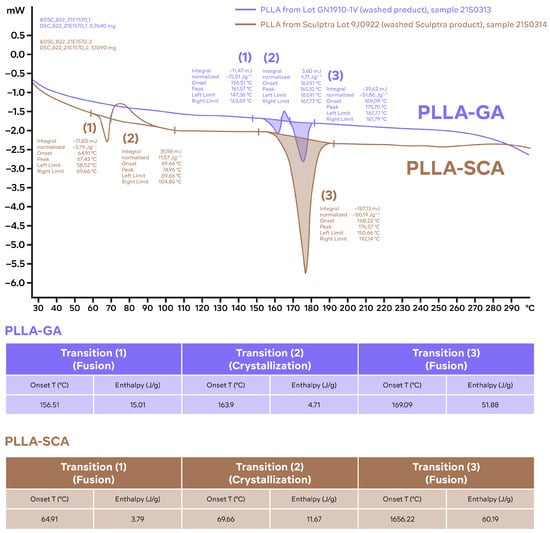
Figure 2.
Individual differential scanning calorimetry thermograms of samples of PLLA-SCA and PLLA-GA. Thermograms obtained with the standard program: 25–300 °C; 10 °C/min. The characteristic parameters (peak temperature and enthalpies) of the different thermal events identified are included in tables for reference (results from single measurements). T: temperature.
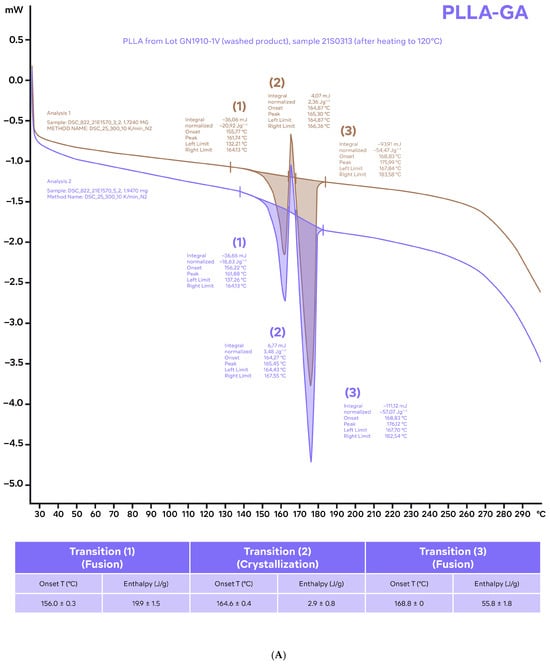
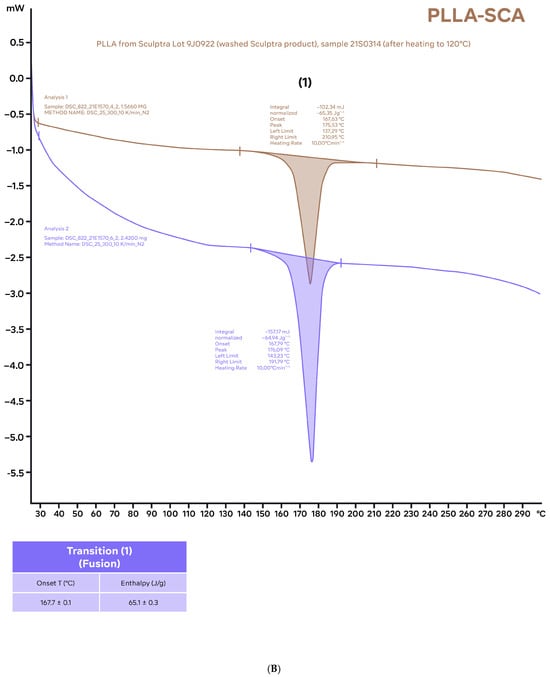
Figure 3.
Differential scanning calorimetry thermograms of samples of PLLA-GA (A) and PLLA-SCA (B) using the standard program (25–300 °C; 10 °C/min) after the samples were heated to 120 °C and allowed to cool to room temperature before analysis. The characteristic parameters (onset temperature and enthalpies) of the different thermal events identified (average values of two independent measurements) are included in tables for reference.
The individual differential scanning calorimetry thermograms indicated that there were three thermal events occurring for both the PLLA-SCA and PLLA-GA samples (Figure 2). For PLLA-SCA, the first two thermal events occurred between 65 and 90 °C and the third thermal event occurred at 165 °C, while for the PLLA-GA sample all three thermal events occurred between 156 °C and 169 °C (Figure 2).
Heating the PLLA-SCA and PLLA-GA samples to 120 °C and allowing them to cool to room temperature prior to performing differential scanning calorimetry thermograms resulted in no thermal events being observed in the temperature range 65–90 °C with either product (Figure 3). In the temperature range 156 °C and 169 °C, three thermal events were observed with PLLA-GA and one thermal event was observed with PLLA-SCA (Figure 3), which were consistent with those observed with the two products without the initial heating–cooling cycle (Figure 2).
3.2. Particle Size and Morphology
The particle size distribution data indicated that PLLA-SCA particles have larger diameters than those of PLLA-GA, with a median volume distribution drop diameter [D(0.5)] of 46.4 vs. 31.7 µm (Figure 4). In both PLLA-SCA and PLLA-GA, the distribution consisted of a large volume fraction of “big” particles and a smaller volume fraction of smaller ones (Figure 4).
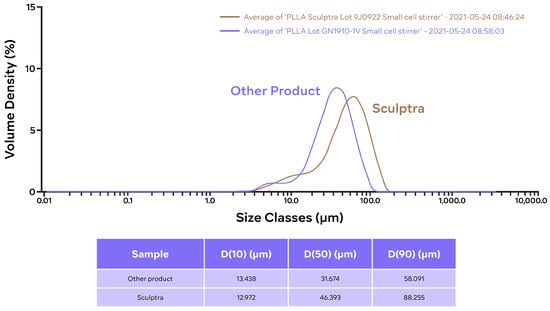
Figure 4.
Particle size (volume) distribution of PLLA-SCA (Lot 9J0922) and PLLA-GA (Lot GN1910-1V) particles.
The size distribution data for PLLA-SCA and PLLA-GA are consistent with the SEM images (Figure 5). These images showed that the two PLLA products displayed differences in morphology. The particles for PLLA-GA were irregular in shape, had no sharp edges and had a surface that appears very wrinkled and crimped, while the PLLA-SCA particles, while also irregular in shape, displayed flake or plate-like shapes and had a smoother surface (Figure 5).
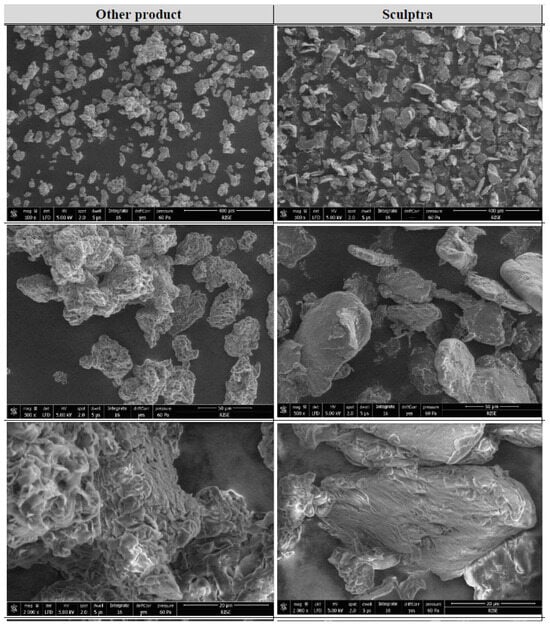
Figure 5.
Low-vacuum SEM images of PLLA-SCA (right) and PLLA-GA (left) at different magnifications (×100; ×500 and ×2000).
3.3. In Vivo Local Inflammatory Tissue Response (Reactivity) and Persistence Score
The differences in average reactivity scores between test and negative control groups indicated a slight reaction for PLLA-SCA at all time points evaluated (reactivity scores of 3.7 ± 1.1, 6.1 ± 1.6, 5.7 ± 1.2 and 6.2 ± 1.2 at 2, 12, 26 and 52 weeks, respectively), while for PLLA-GA a moderate reaction was observed at 12 and 26 weeks (reactivity score of 2.9 ± 1.5, 10.1 ± 1.0, 9.4 ± 0.7 and 7.1 ± 1.3 at 2, 12, 26 and 52 weeks, respectively) (Figure 6).
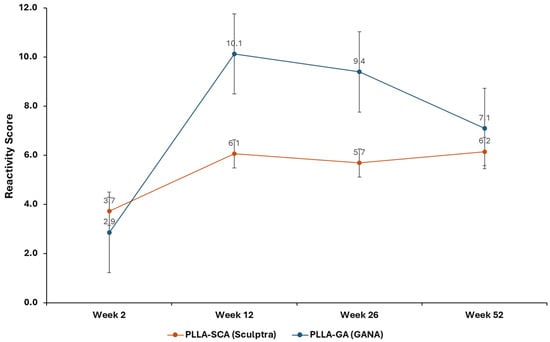
Figure 6.
Reactivity scores for PLLA-SCA and PLLA-GA vs. negative control at all time points.
PLLA-SCA and PLLA-GA had similar average persistence scores (ranging from 1.9 ± 0.1 to 2.4 ± 0.3) at 2, 12 and 26 weeks, while the score at 52 weeks was markedly higher for PLLA-SCA (1.9 ± 0.2) compared with PLLA-GA (0.7 ± 0.2) (Figure 7).
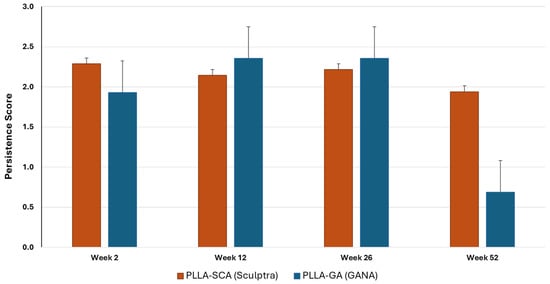
Figure 7.
In vivo persistence scores for PLLA-SCA and PLLA-GA at all time points.
Histological examination of the harvested samples revealed no evidence of PLLA-SCA, PLLA-GA or any degradation products in the draining lymph nodes over 52 weeks.
4. Discussion
There are clear differences in the X-ray powder diffraction spectra and differential scanning calorimetry thermograms of PLLA-SCA and PLLA-GA. It is well established that PLLA can crystallize and this analysis of the X-ray powder diffraction spectra of the two PLLA products indicates that PLLA-SCA is more amorphous (less crystalline) than PLLA-GA. This difference in the crystallinity of the two PLLA products has also been reported in a separate comparative study that included poly-D,L-lactic acid products [15].
Analysis of thermal events using differential scanning calorimetry suggests differences between PLLA-SCA and PLLA-GA. When the two products had not undergone an initial heating–cooling cycle, the analysis demonstrated the presence of thermal events in the PLLA-SCA sample in the temperature range 65–90 °C that were absent in the PLLA-GA sample. However, these thermal events seen with PLLA-SCA were absent once the sample had undergone prior heating, suggesting they were the result of an irreversible process. Sedush et al. [15] also reported the loss of thermal events in the temperature range 65–90 °C with PLLA-SCA after being heated prior to differential scanning calorimetry. While PLLA-SCA and PLLA-GA particles had a melting transition at around 165 °C, PLLA-GA also exhibited a melting transition at 157 °C. This transition resulted in the number of smaller peaks observed in the X-ray powder diffraction diffractogram of PLLA-GA, which supports the presence of a different, potentially more crystalline structure. The presence of several melting points with PLLA-GA was also observed by Sedush et al. [15] who went on to calculate degrees of crystallinity of 64% and 72% for PLLA-SCA and PLLA-GA, respectively.
Crystallinity plays an important role in polymer performance. PLLA is a semicrystalline polyester with slow crystallisation rate and low crystallinity [15]. PLLA samples that are less crystalline and more amorphous in structure are able to preserve mechanical strength for longer than more crystalline samples under both in vitro and in vivo degradation conditions [15]. Hence, the less crystalline (more amorphous) structure of PLLA-SCA is likely to preserve mechanical strength for longer. Although less crystallinity usually results in faster degradation, PLLA-SCA has demonstrated a slower degradation rate than the more crystalline PLLA-GA [15]. Degradation of PLLA is, in part, via hydrolytic cleavage, which can take place on the surface or within the structure (surface and bulk erosion, respectively) [16]. The faster degradation rate of PLLA-GA could, therefore, be linked to a higher surface area as a result of smaller particles, a rougher surface and/or larger pores within the surface structure of the PLLA-GA. Sedush et al. [15] also reported a faster degradation rate with PLLA-GA compared with PLLA-SCA, which is in agreement with the reduced persistence observed here with PLLA-GA versus PLLA-SCA at 52 weeks. In addition, this faster degradation with PLLA-GA could lead to the increased in vivo local inflammatory response (reactivity) observed with the product compared with PLLA-SCA, leading to an earlier inflammatory response and earlier generation of fibrous capsules and resulting in a weaker effect of maintaining soft tissue volume. The greater inflammatory response observed with PLLA-GA may contribute towards the increase in adverse events reported clinically [21,22]. Furthermore, the faster degradation rate of PLLA-GA could lead to increased levels of lactic acid, the main degradation product, creating a more toxic local environment, as well as reducing the duration of clinical effect [15]. Hence, microspheres with a slower degradation rate, such as PLLA-SCA are considered better for applications in aesthetic medicine to reduce the number and/or frequency of retreatments. The lower crystallinity and slower degradation rate observed with PLLA-SCA translates into clinical practice with improvement in the appearance of wrinkles for up to 25 months after treatment [23,24], and well beyond 12 months across various body regions in key clinical studies (for International Consensus, see Reference [25]).
Using laser diffraction and scanning electron microscopy, the median volume distribution drop diameter was slightly higher for PLLA-SCA compared with PLLA-GA (46.4 vs. 31.7 µm), and while both PLLA-SCA and PLLA-GA particles were irregular in shape, PLLA-GA particles had a surface that appears very wrinkled and crimped whereas PLLA-SCA particles had a smoother surface. These findings are in line with those of Sedush et al. [15] who reported PLLA-GA particles to have an average diameter of 42 ± 22 µm and to be irregular in shape with visible pores, while PLLA-SCA particles had an average diameter of 52 ± 29 µm and an irregular smooth, flat, plate-like shape. Sedush et al. [15] also indicated that a smooth regular morphology might be more favourable as particles such as those seen with PLLA-GA that have rough surfaces and irregular shapes can cause a foreign body granuloma as a dominant characteristic of the long-term biological response. The presence of visible pores in the structure could also explain the reduced persistence (and faster degradation) observed here with PLLA-GA versus PLLA-SCA at 52 weeks as these pores allow fluid access to the inner areas of the structure which facilitates decomposition from within (bulk erosion) [15].
The presence of additional peaks on either side of the main peak in the X-ray powder diffraction spectra of PLLA-GA might be the result of several melting points with PLLA-GA, which were absent with PLLA-SCA. This would suggest that PLLA-GA comprises either a heterogenous mix of polymer isoforms or a mix of crystal forms that result in uneven melting of the product. In contrast, PLLA-SCA has a single peak in the diffractogram suggesting it comprises a homogenous formulation. This could indicate that PLLA-SCA is a purer formulation compared with PLLA-GA, which could potentially have implications on clinical post-treatment adverse events. In head-to-head comparative studies, approximately 19% more injection-related adverse events were reported with PLLA-GA than PLLA-SCA, and there was at least a two-fold higher incidence of lumps/bumps (7.3% vs. 3.6%) and at least a four-fold higher incidence of induration/firmness (16.4% vs. 3.6%) for PLLA-GA vs. PLLA-SCA [21,22].
In conclusion, the single melting point observed with PLLA-SCA suggests the presence of a homogenous formulation that when combined with the more amorphous (less crystalline) structure, could result in the increased persistence (slower rate of degradation) and more sustained biological response compared with PLLA-GA. Furthermore, the smooth, flat, plate-like shape of PLLA-SCA particles reported here and elsewhere [15] may contribute to a more beneficial clinical response as particles with rough surfaces and irregular shapes (e.g., PLLA-GA) can cause a foreign body granuloma as a dominant characteristic of the long-term biological response. In addition, this could contribute to the higher incidence of lumps/bumps and induration/firmness observed with PLLA-GA compared with PLLA-SCA. The differences in physiochemical properties of the two commercially available PLLAs reported here are likely to impact the biological response observed in clinical practice and should be taken into consideration when selecting a PLLA treatment for aesthetic use.
Author Contributions
Conceptualization, L.A., P.M. and Å.Ö.; Methodology, P.M., Å.Ö., B.L. and L.L.; Investigation, P.M., Å.Ö., B.L. and L.L.; Writing—Original Draft Preparation, L.A., P.M. and Å.Ö.; Writing—Review & Editing, L.A., A.H., S.G.F., M.S., K.B., S.B.A., K.T.-B., P.M., C.M., Å.Ö., B.L., L.L., E.N. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by an unrestricted educational grant from Galderma SA, Zug, Switzerland. The authors declare that no financial support was received for the research, authorship, and/or publication of this article. Editorial assistance for this article was provided by Inizio Evoke Comms, and funded by Galderma SA, Zug, Switzerland.
Institutional Review Board Statement
This study did not involve humans so ethical approval was not required.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to acknowledge Amitava Mukhopadhyay, Senior Biostatistician at Galderma, for providing statistical support.
Conflicts of Interest
Luiz Avelar is a speaker, trainer, consultant, and clinical trial investigator for Galderma. Alessandra Haddad is a speaker, trainer, consultant, and clinical trial investigator for Galderma. Sabrina G. Fabi is a consultant, investigator, and speaker for Galderma, Allergan, Merz and Revance. Michael Somenek is a speaker, trainer, clinical trial investigator and consultant for Galderma and Merz. Shino Bay Aguilera is a speaker and trainer for Galderma, Allergan, Merz, Prollenium, Solta, Revision, SkinCeuticals, Dp Derm, Beneve. Katie Beleznay is a consultant, speaker, and investigator for Galderma, Allergan and L’Oréal. Kathlyn Taylor-Barnes is a trainer and consultant for Galderma, a consultant for Candela, and an investigator for Evolus. Peter Morgan, Cheri Mao, Åke Öhrlund, Björn Lundgren, Lian Leng, Edwige Nicodeme and Daniel Bråsäter are employees of Galderma. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Upadhya, P.N. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Vleggaar, D.; Fitzgerald, R.; Lorenc, Z.P. Composition and Mechanism of Action of Poly-L-Lactic Acid in Soft Tissue Augmentation. J. Drugs Dermatol. 2014, 13, s29–s31. [Google Scholar]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Nonhoff, M.; Puetzler, J.; Hasselmann, J.; Fobker, M.; Gosheger, G.; Schulze, M. The Potential for Foreign Body Reaction of Implanted Poly-L-Lactic Acid: A Systematic Review. Polymers 2024, 16, 817. [Google Scholar] [CrossRef]
- Waibel, J.; Nguyen, T.Q.; Le, J.H.T.D.; Ziegler, M.; Widgerow, A.; Meckfessel, M.; Bråsäter, D. A randomized, comparative study describing the gene signatures of Poly-L-Lactic Acid (PLLA-SCA) and Calcium Hydroxylapaptite (CaHA) in the treatment of nasolabial folds. IMCAS World Congress. 2024, 91, AB110. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, C. Poly-L-lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the TGF-β/Smad pathway. J. Cosmet. Dermatol. 2023, 22, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, 13–17. [Google Scholar] [CrossRef]
- Cabral, L.R.B.; Teixeira, L.N.; Gimenez, R.P.; Demasi, A.P.D.; de Brito Junior, R.B.; de Araújo, V.C.; Martinez, E.F. Effect of hyaluronic acid and poly-L-lactic acid dermal fillers on collagen synthesis: An in vitro and in vivo study. Clin. Cosmet. Investig. Dermatol. 2020, 13, 701–710. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications —A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Duan, L.; Feng, X.; Xu, W. Superiority of poly(L-lactic acid) microspheres as dermal fillers. Chin. Chem. Lett. 2021, 32, 577–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Luo, Q.; Chen, J.; Zhao, N.; Gao, W.; Pu, Y.; He, B.; Xie, J. In vivo inducing collagen regeneration of biodegradable polymer microspheres. Regen. Biomater. 2021, 8, rbab042. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation mechanisms and acceleration strategies of poly (lactic acid) scaffold for bone regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Sedush, N.G.; Kalinin, K.T.; Azarkevich, P.N.; Gorskaya, A.A. Physicochemical Characteristics and Hydrolytic Degradation of Polylactic Acid Dermal Fillers: A Comparative Study. Cosmetics 2023, 10, 110. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, J.Y.; Yang, D.Y.; Lee, S.H.; Kim, J.Y.; Kang, M. Efficacy and safety of poly-D,L-lactic acid microspheres as subdermal fillers in animals. Plast. Aesthetic Res. 2019, 6, 16. [Google Scholar] [CrossRef]
- Yang, D.Y.; Ko, K.; Lee, S.H.; Lee, W.K. A Comparison of the Efficacy and Safety Between Hyaluronic Acid and Polylactic Acid Filler Injection in Penile Augmentation: A Multicenter, Patient/Evaluator-Blinded, Randomized Trial. J. Sex. Med. 2019, 16, 577–585. [Google Scholar] [CrossRef]
- Christen, M.O. Collagen Stimulators in Body Applications: A Review Focused on Poly-L-Lactic Acid (PLLA). Clin. Cosmet. Investig. Dermatol. 2022, 15, 997–1019. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.H.; Kim, H.M.; Batsukh, S.; Sung, M.J.; Lim, T.H.; Lee, M.H.; Son, K.H.; Byun, K. Poly-L-Lactic Acid Fillers Improved Dermal Collagen Synthesis by Modulating M2 Macrophage Polarization in Aged Animal Skin. Cells 2023, 12, 1320. [Google Scholar] [CrossRef]
- Han, W.Y.; Kim, H.J.; Kwon, R.; Kang, S.M.; Yon, D.K. Safety and Efficacy of Poly-L-Lactic Acid Filler (Gana V vs. Sculptra) Injection for Correction of the Nasolabial Fold: A Double-Blind, Non-Inferiority, Randomized, Split-Face Controlled Trial. Aesthetic Plast. Surg. 2023, 47, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Fabi, S.; Bråsäter, D. Letter to the Editor on: “Safety and Efficacy of Poly-L-Lactic Acid Filler (Gana V vs. Sculptra) Injection for Correction of the Nasolabial Fold: A Double-Blind, Non-Inferiority, Randomized, Split-Face Controlled Trial”. Aesthetic Plast. Surg. 2025, 49, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Narins, R.S.; Baumann, L.; Brandt, F.S.; Fagienet, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. A randomized study of the efficacy and safety of injectable poly-L-lactic acid versus human-based collagen implant in the treatment of nasolabial fold wrinkles. J. Am. Acad. Dermatol. 2010, 62, 448–462. [Google Scholar] [CrossRef]
- Brandt, F.S.; Cazzaniga, A.; Baumann, L.; Fagien, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. Investigator global evaluations of efficacy of injectable poly-L-lactic acid versus human collagen in the correction of nasolabial fold wrinkles. Aesthet. Surg. J. 2011, 31, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Avelar, L.; Fabi, S.G.; Sarubi, J.; Somenek, M.; Coimbra, D.D.; Palm, M.; Durairaj, K.K.; Somji, M.; Vasconcelos-Berg, R.; et al. Injectable Poly-L-Lactic Acid for Body Aesthetic Treatments: An International Consensus on Evidence Assessment and Practical Recommendations. Aesthet. Plast. Surg. 2025, 49, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.