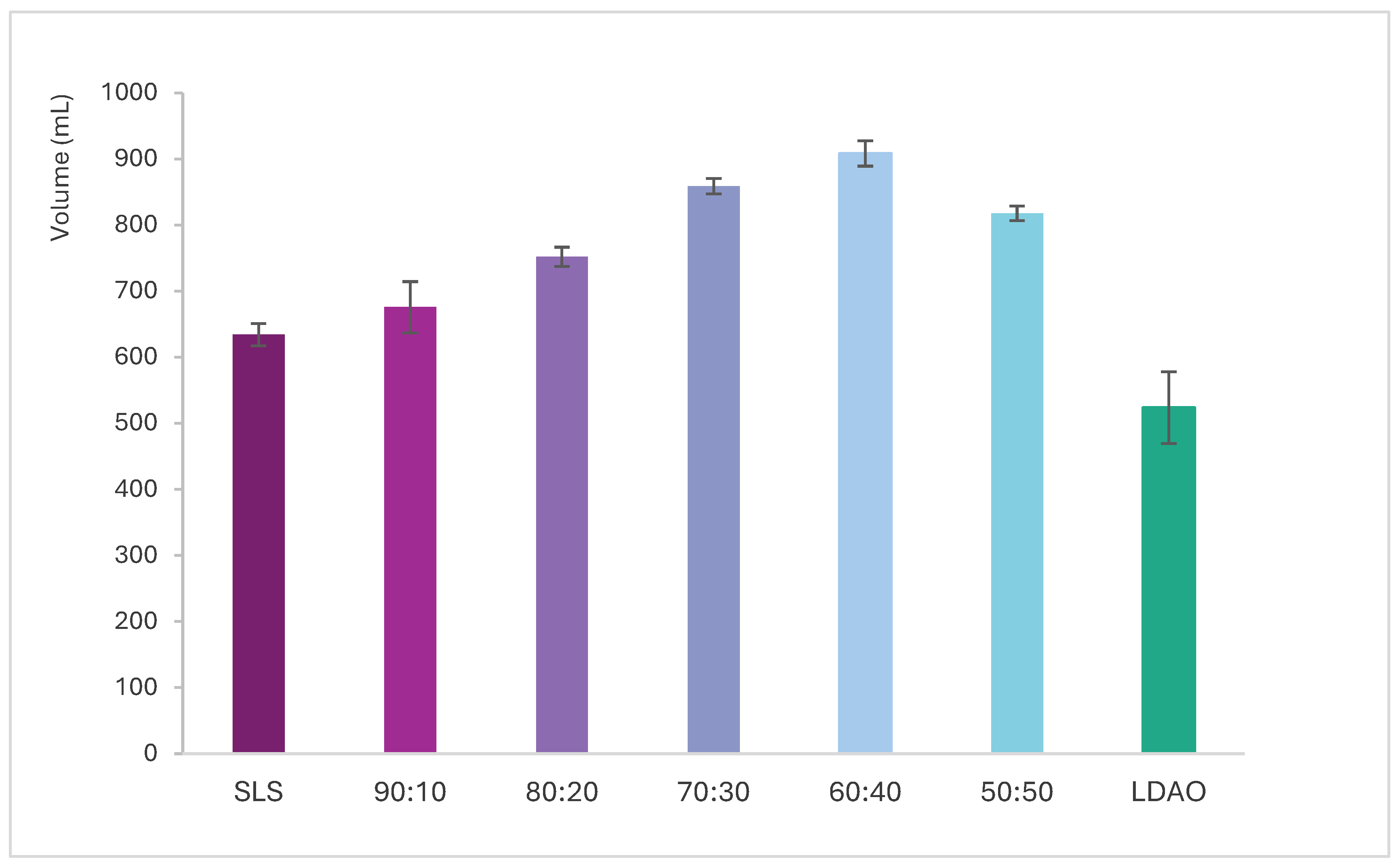Abstract
Surfactants are commonly employed in cleaning, cosmetic, and pharmaceutical formulations due to their ability to lower surface tension and facilitate the formation of emulsions, foams, and dispersions. Recent research highlights the advantages of synergistic interactions between anionic and nonionic surfactants to improve overall performance. In this study, the physicochemical properties and performance of binary mixtures of the anionic surfactant sodium lauryl sulfate (SLS) and the amphoteric surfactant lauryl dimethyl amine oxide (LDAO) at varying ratios (100% SLS, 90:10, 80:20, 70:30, 60:40, and 50:50) were investigated. Key parameters analysed included critical micelle concentration (CMC), surface tension (γ), foam volume, and potential irritability, assessed via the Zein test. The results revealed a clear synergistic effect between SLS and LDAO: all mixtures showed reduced CMC and minimum surface tension compared to the individual surfactants, while exhibiting enhanced foam volume and stability. Regarding irritability, increasing LDAO content consistently led to decreased protein denaturation, indicating lower irritancy levels. Furthermore, the results obtained in the Zein test confirmed that mixtures induced less protein denaturation than the sum of their individual surfactant components, with formulations ranging from moderately to non-irritating. The results obtained indicate that the more stable mixed micelle systems (SLS + LDAO) might improve the performance of cleaning formulations (γ, CMC, foam) while reducing the irritability.
1. Introduction
Surfactants are amphipathic molecules consisting of a nonpolar (hydrophobic) part and a polar (hydrophilic) part. This dual structure allows them to interact simultaneously with polar solvents such as water and nonpolar substances like oils, lipids, or hydrophobic surfaces [1]. As a result, surfactants play a key role in solubilizing immiscible phases and stabilizing interfaces. The balance between the hydrophilic and lipophilic parts—known as the hydrophilic–lipophilic balance (HLB)—is a fundamental parameter that influences critical properties of the system, such as adsorption at interfaces, emulsification, and the formation of aggregates like micelles [2,3]. These compounds are widely used in cleaning products, cosmetics, pharmaceuticals, and food products due to the characteristics, which allow the formation of emulsions, foams, and dispersions [2,4,5].
Surfactant adsorption is driven by the reduction of interfacial tension, which increases with surfactant concentration at the interface. On the other hand, surfactants in aqueous solution tend to aggregate to form micelles. The driving force for micelle formation is the reduction of contact between the hydrophobic part of the molecule and water, thus reducing the free energy of the system [6]. Micelle formation is a key property that defines surfactant behaviour in solution, along with the critical micelle concentration (CMC), defined as the minimum concentration at which surfactant molecules begin to form these micelles. This parameter is key because it directly affects properties such as solubilization, foaming, and detergency [2,3,4].
Foam generation is one of the most valued properties in surfactant-based formulations, especially in household and personal care products [7]. Foam not only contributes to the mechanical removal of dirt and oils but also plays a psychological role in consumer perception of product efficacy [4,8]. However, excessive or unstable foam can hinder rinsability and reduce user satisfaction. Moreover, the relationship between foam performance and skin tolerance is complex: while high foam volume is often associated with strong detergency, it may also correlate with increased skin irritation, particularly in formulations containing high concentrations of anionic surfactants [9].
Modern cleaning formulations contain mixtures of surfactants, generally anionic, amphoteric, and/or nonionic. Anionic surfactants are usually the main surfactant due to their high cleaning power and foaming capacity, although they have drawbacks such as high irritancy and sensitivity to hard water [10]. Mixtures of anionic and nonionic (or amphoteric) surfactants, such as the SLS + LDAO mixture, offer various benefits, such as reducing the irritating potential of the formula or increasing the stability of the foam generated [4,9,11].
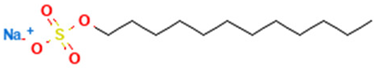
Sodium lauryl sulfate (SLS) is an anionic surfactant naturally sourced from coconut and/or palm kernel oil. It is widely used in personal care products due to its foaming, cleansing, and emulsifying properties. Its ability to generate foam makes it ideal for shampoos, shower gels, or dishwashing liquids. However, its ability to strip the skin of its natural oils gives it a high potential for skin irritation, especially in sensitive skin or at high concentrations [12,13].
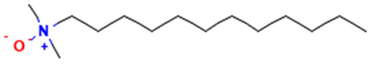
Amine-oxide-based surfactants such as lauryl dimethylamine oxide (LDAO) possess several valuable properties, including enhancing and stabilizing foam in blends with other amphoteric or anionic surfactants and thickening due to their strong dipolar moment, which structures the surfactant phase [14,15]. They improve compatibility with skin and hair, reducing the irritation caused by anionic surfactants such as SLS [2]. These characteristics make them suitable for use in a variety of industrial, household, and cosmetic products including detergents, dishwashing liquids, shampoos, and hair conditioners [16].
There are several previous studies that suggest a synergistic interaction in mixtures of anionic surfactants with nonionic or amphoteric surfactants [9,17,18,19,20,21], with “synergy” being understood as the joint action of two or more elements that results in a superior effect or an improvement in the individual effects of each of them [22]. These synergies are mainly due to the formation of more stable mixed micelles, thanks to the ability of nonionic (or amphoteric) surfactants to increase the distance between the hydrophilic heads of anionic surfactants, thereby reducing electrostatic repulsive forces [23], as well as the reduction of monomers in the medium. The formation of more stable mixed micelles and, consequently, the reduction in the CMC has beneficial effects on efficacy characteristics such as foam generation and stability or cleaning ability, as well as reducing skin irritation [2,24,25].
The irritant potential of anionic surfactants like SLS is a well-documented concern. The SLS penetrates the stratum corneum, disrupting lipids and proteins, which increases the transepidermal water loss and compromises barrier function. This severity of irritation varies with individual factors such as stratum corneum thickness and baseline TEWL, explaining differences in skin response [26]. This adverse effect has driven the development of milder formulations and the use of synergistic systems, such as the SLS + LDAO system, as a strategy to minimize aggressiveness without sacrificing performance, especially in the development of manual dishwashing formulas, which come into direct contact with the skin [12].
Although numerous studies have explored the synergistic effects between anionic and nonionic or amphoteric surfactants—particularly in relation to their irritant potential using various analytical techniques [8,27,28,29,30,31]—few have specifically examined how the ratio between anionic and nonionic components influences skin irritation [8]. Understanding this relationship is essential for optimizing detergent formulations that balance performance with mildness. Moreover, the underlying physicochemical mechanisms driving these synergistic interactions remain insufficiently characterized, underscoring the need for further investigation into how each surfactant contributes to key functional properties.
To address this gap, the present study not only evaluates the physicochemical behaviour of SLS + LDAO mixtures but also quantifies the degree of synergy in terms of irritant potential. This is achieved by comparing the Zein (Zn) solubilization values of the mixtures against the cumulative values derived from the individual surfactants at equivalent concentrations, thereby providing a more precise assessment of their combined effect. In this study, the synergistic effect of SLS + LDAO mixtures at different ratios (90:10, 80:20, 70:30, 60:40, and 50:50) was evaluated. To this end, the surface tension and CMC values of the different mixtures, as well as those of the individual surfactants, were obtained, the foam generation and foam decay of each of them were evaluated, and finally, the irritant profile of these mixtures was studied by applying the Zein test. To determine the SLS + LDAO ratio that offers maximum synergy, in addition to evaluating the dissolved Zein of the different mixtures, the accumulated Zein value was also obtained by adding the contribution of each individual surfactant at the same concentration as in the mixture.
The research concludes that synergism is favourable in all the solutions analysed, which have been able to lower their binary mixture CMC and the minimum surface tension compared to that of the individual surfactants. Moreover, regarding protein denaturation, the Zein solubilization caused by the mixtures of surfactants is significantly lower than the cumulative score of Zein solubilization by each of the surfactants separately tested. These findings confirm a synergistic interaction between the surfactants, resulting in reduced protein denaturation.
2. Materials and Methods
2.1. Materials
The surfactants used in this study were sodium lauryl sulfate (SLS), provided by Grupo ADI (Terrassa, Spain), and lauryl dimethylamine oxide (LDAO), provided by KAO Corporation S.A. (Barcelona, Spain), both with 30% active matter content (Table 1). Stock solutions containing 5% active matter were prepared for each SLS + LDAO ratio (90:10, 80:20, 70:30, 60:40, and 50:50), as well as for the individual surfactants. Working solutions were obtained by diluting the stock preparations to the specific concentrations required for each experimental protocol. Purified Zein from ThermoFisher Scientific (Waltham, MA, USA) was used. Solution pH was adjusted using 99% citric acid and sodium hydroxide (analytical grade), both supplied by Sigma-Aldrich (St. Louis, MO, USA) and used as 30% aqueous solutions (Table 1).

Table 1.
Characteristics of the surfactants used in the study.
2.2. Methods
2.2.1. Surface Tension
The surface tension of the solutions was determined using an Attension Theta Flex optical tensiometer (Biolin Scientific, Västra Frölunda, Sweden) with the Pendant Drop method [32]. For each measurement, 200 μL of the corresponding solution was dispensed at the micropipette tip. Experimental parameters such as drop size and dispensing rate were adjusted according to the expected surface tension value. In general, larger drop volumes and lower dispensing rates were used for solutions with high surface tension, whereas smaller drops and higher dispensing rates were employed for solutions with lower surface tension. All measurements were performed at a controlled room temperature of 22 °C, and once the required drop volume was reached, surface tension was measured for five seconds. The instrument recorded images at a frequency of 17 frames per second (fps), and the average surface tension value (expressed in mN/m) was obtained from the data collected during this interval (17 frames/s × 5 s). Two surface tension measurements were carried out per sample.
2.2.2. Critical Micelle Concentration
To determine the critical micelle concentration (CMC), serial dilutions of each surfactant and their mixtures were prepared within a concentration range of 0.1 to 0.005%. For SLS, the range was extended up to 0.25% since a higher CMC was expected [2]. Surface tension was measured as previously described, and the data obtained were plotted as surface tension versus concentration.
The CMC was estimated graphically by fitting two linear segments to the curve—one before and one after the inflection point—and identifying their intersection. In addition to the CMC, other parameters related to surfactant adsorption at the interface were extracted from the surface tension vs. concentration curve. These included the effectiveness of adsorption [2], defined as the minimum surface tension achieved (lowest γ), and the efficiency of adsorption C1–2, defined as the surfactant concentration required to reduce surface tension to a target value (31 mN/m in this study) [2]. Finally, in order to test the synergistic interaction between SLS and LDAO, the theoretical CMC was obtained using Clint’s equation (CMCM,Clint) [29], which represents an ideal model of micellization. According to Clint’s model, the CMC of the mixture is obtained from the CMC of the individual ingredients (CMC1 and CMC2) and their molar fractions (α1 and α2) (Equation (1)).
2.2.3. Protein Denaturation
Zein is a corn-derived protein that mimics the denaturation behaviour of skin keratinocytes and is insoluble in aqueous solutions unless disrupted by surfactants. The Zein test is a widely accepted method for estimating the irritant potential of surfactants, based on the amount of protein solubilized upon denaturation [9].
An adaptation of the original Zein test [33] was carried out, with quantification of the dissolved Zein using the Kjeldahl method. The results are expressed as mg N/100 mL of solution (Zein number). Surfactant solutions were prepared at 1% active matter with the pH adjusted to 8 ± 0.2. A suspension of 2 g of Zein in 40 mL of surfactant solution was stirred at 300 rpm for 1 h at room temperature. Insoluble Zein was then removed by centrifugation for 15 min at 4000 rpm (Bench top centrifuge ScanSpeed 1248) and subsequently filtered under vacuum through a Whatman filter (8 μm pore). Finally, the amount of dissolved Zein was analysed by quantifying the nitrogen in solution using the Kjeldahl method [33]. Two replicates per sample have been carried out.
The higher the Zein number, the more protein has been denatured, and therefore, the more irritant the tested solution is. The method includes classification of the surfactant’s irritant potential based on the Zein number obtained, as shown in Table 2.

Table 2.
Classification of surfactant irritant potential based on Zein number [34].
The Zein method was used to quantify the irritant potential of the binary SLS + LDAO mixtures. Additionally, the presence of a synergistic effect in irritancy was evaluated by analysing the surfactants individually at the concentrations present in the binary mixtures and then summing the values to obtain the cumulative Zein number. The synergistic effect was subsequently quantified by comparing the Zein number of the mixture to the cumulative Zein number, expressed as a % increase (Equation (2)). This approach enables identification of the SLS + LDAO ratio that yields the highest synergistic reduction in irritant potential.
2.3. Foam Generation and Stability
Foam generation and stability were evaluated using a SITA Foam Tester R-2000 (Dresden, Germany). Surfactant mixtures were analysed in solution at 0.1% active matter. Foam was generated by performing 20 agitation cycles of 10 s each at 1500 rpm, with the volume recorded after each cycle. To assess the stability of the generated foam, volume measurements were taken every 20 s for 5 min after the final agitation. All measurements were performed in triplicate.
3. Results and Discussion
3.1. Determination of Surface Tension and CMC
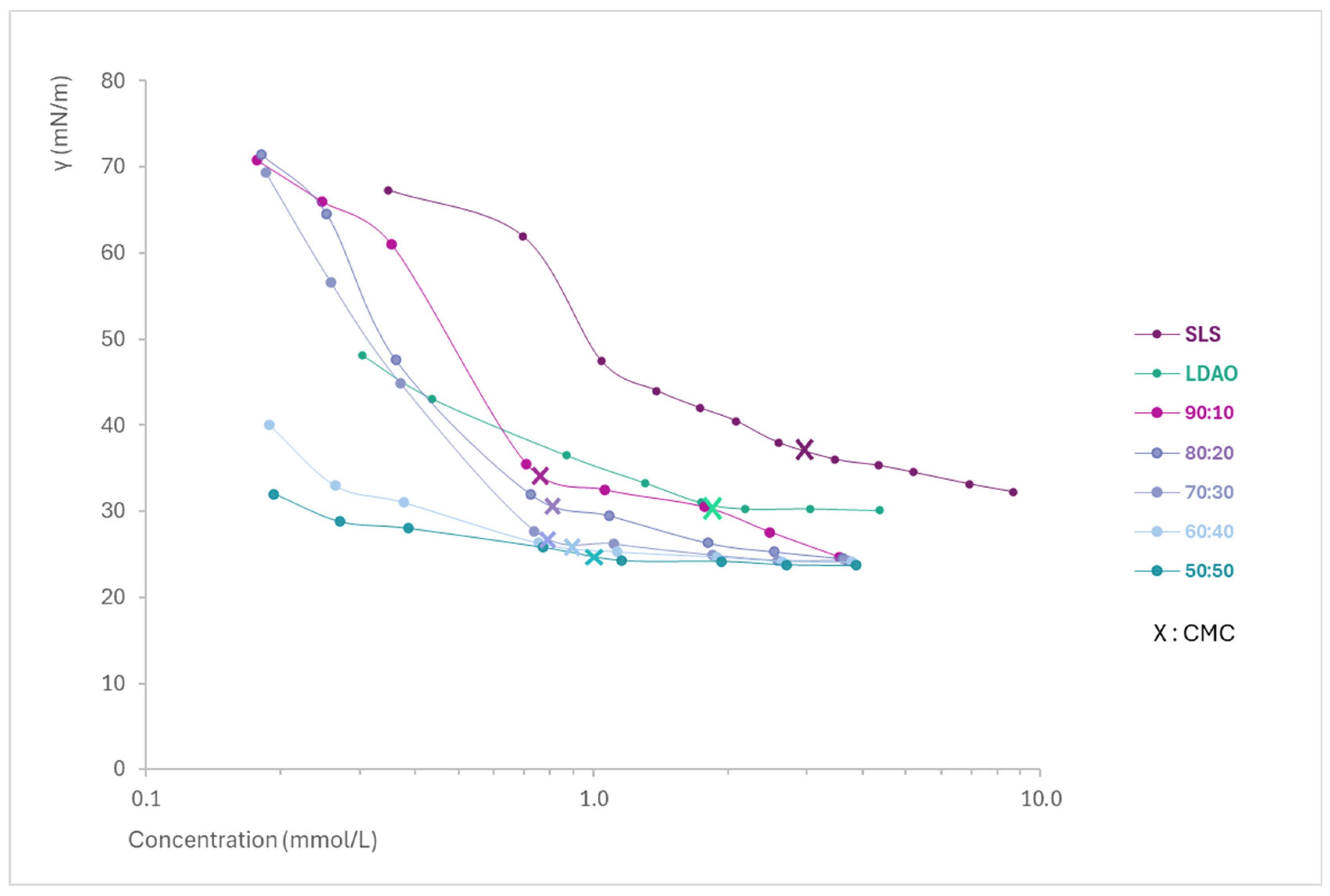
By representing the concentration (logarithmic scale) versus the surface tension in solutions of the mixtures of each surfactant and the pure ingredients (Figure 1), the Critical Micelle Concentration (CMC) and the minimum surface tension of each of them were determined. From Figure 1, it can be easily observed how the SLS + LDAO mixtures are able to reach a lower surface tension, as well as a lower CMC (indicated with an X) compared to individual surfactants.
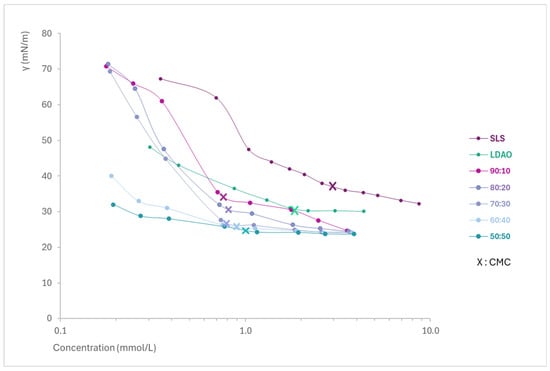
Figure 1.
Surface tension profiles of SLS, LDAO, and their binary mixtures as a function of the concentration.
The results (Table 3) show the CMC, CMCM,Clint, lowest γ, and C1–2 (concentration required to reduce surface tension to 31 mN/m). SLS has a much higher CMC than LDAO and its mixtures, as expected for an anionic surfactant. This is because for anionic surfactants, higher concentrations are necessary to overcome the electrostatic repulsion between the head groups during aggregation [2]. In addition, all mixtures have a lower CMC than pure LDAO and further reduce the minimum surface tension compared to either of the surfactants alone.

Table 3.
CMC, CMCM,Clint, minimum surface tension (lowest γ), and surfactant concentration required to reach 31 mN/m (C1–2) for SLS, LDAO, and their binary mixtures.
The results obtained allow us to conclude that there is a strong synergistic interaction among SLS and LDAO, resulting in a clear benefit in terms of the most important interfacial phenomena, which are the generation of mixed micelles in liquid solutions (micellization) and the reduction of the interfacial tension, compared to the values obtained for the individual surfactants. The synergy between SLS and LDAO allows the obtention of mixed micelles in solution at a concentration much lower than the CMC of the individual ingredients, even at a very low percentage of LDAO (90:10). The CMCM,Clint, obtained using the equation of Clint, which represents ideal micellization, gives much higher CMC values than the ones obtained experimentally, probing the synergy of the mixtures.
Regarding the efficacy on the γ reduction, all the mixtures of SLS + LDAO assessed improve the values of the individual surfactants, with efficacy increasing in mixtures richer in LDAO and reaching the smallest γ for the 50:50 mixture (23.7 mN/m). On the other hand, the efficiency in surface tension reduction has been assessed to determine the surfactant concentration required to achieve a surface tension of 31 mN/m. In this case, as for the lowest surface tension, all the SLS + LDAO mixtures show a synergistic effect with lower surfactant concentration than for the individual surfactants, with the highest degree of synergy observed in the 50:50 mixture (0.217 mmol/L).
The synergistic behavior in CMC reduction can be explained by assuming that in the mixed micelles, the hydrophilic part of the LDAO molecule coils around the charged head groups of the anionic surfactant, screening the electrostatic repulsions and thus favoring micelle formation [2,17]. A similar mechanism may explain the enhanced γ reduction, as nonionic or amphoteric surfactants exhibit greater adsorption efficiency due to the absence of electrostatic repulsion, which otherwise increases the free energy required for head group transfer from the bulk phase to the interface [32].
Similar synergistic phenomena have been reported in other anionic/nonionic systems [17,19,35]. For example, Kotsi et al. [18] proved that mixtures of nonionic tristyrylphenol ethoxylate and an anionic sodium dodecyl benzene sulfonate significantly lowered the CMC of each surfactant alone. Their results were attributed to cooperative interactions between the hydrophobic chains and the ethoxylated head groups, leading to improved molecular packing at the air–water interface and the formation of mixed micelles with more thermodynamic stability.
3.2. Protein Denaturation
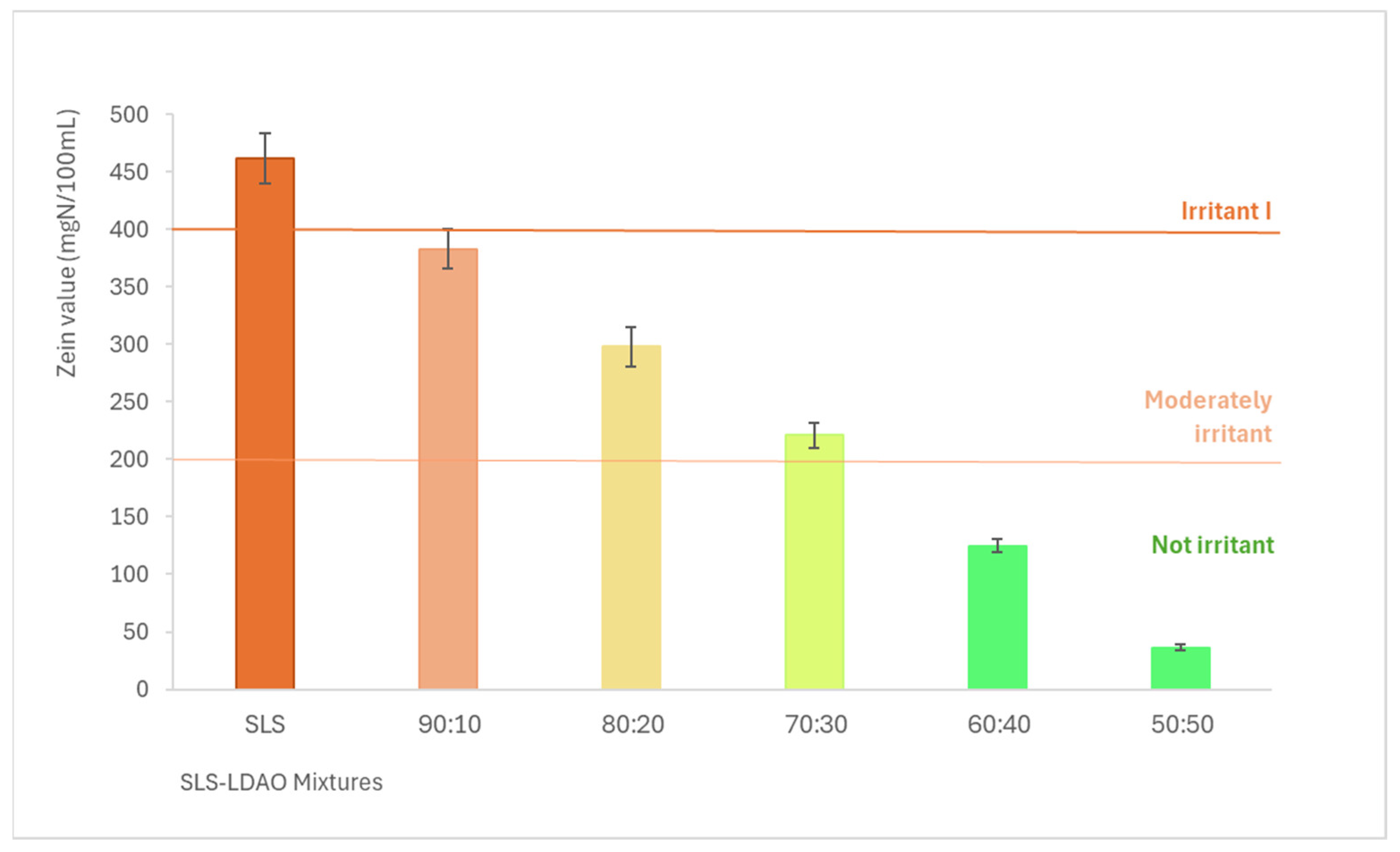
The results obtained regarding protein denaturation are shown in Figure 2, where the Zein number is expressed in mgN/100 mL of solution. According to these results, the higher the proportion of LDAO in the mixture, the lower the irritant potential. The value obtained for SLS corresponds to a type I irritant according to the Zein method classification scale. The 90:10, 80:20, and 70:30 mixtures would be classified as moderately irritating and the 60:40 and 50:50 mixtures as non-irritating.
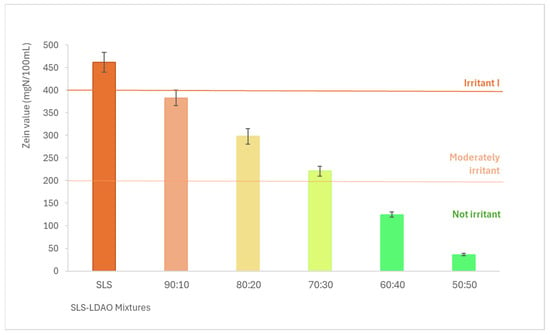
Figure 2.
Zein number (mgN/100 mL) for SLS and SLS + LDAO binary mixtures.
This study demonstrates that using surfactant mixtures of different natures such as anionic/nonionic or anionic/amphoteric is one of the simplest options to reduce the dermal irritation caused by anionic surfactants due to the synergistic interaction achieved by these mixtures [20,23,36,37]. This way, it is possible to drastically reduce the irritant potential of SLS (type I irritant) to obtain moderately irritating (90:10, 80:20 and 70:30) or non-irritating mixtures (60:40 and 50:50).
As it has been widely demonstrated in the literature, the application of surfactant mixtures of different nature, such as SLS + LDAO, allows the formation of bigger and more stable micelles, in comparison to anionic surfactant micelles [2,3]. Micelles formed in solution by anionic surfactants are relatively small in size, which can promote their penetration into the epidermis [23]. Moreover, the strong electrostatic repulsion of the hydrophilic group in the micelle-forming molecules has an influence reducing the stability leading to its breakdown into monomers. The use of a nonionic or amphoteric surfactant leads to weakening of the electrostatic repulsion, and so micelle stabilization occurs.
On the other hand, as previously discussed, the formation of mixed micelles significantly reduces the CMC (from 2.913 mmol/L for SLS to 0.738 mmol/L for 90:10 mixture) and so the concentration of free monomers in the solution. According to the literature, skin irritation is mainly due to the presence of surfactant monomers in aqueous solutions since they are able to easily penetrate the stratum corneum (because of their small size) to cause protein denaturation or lipid disruption [23]. These theories are confirmed by several studies where the irritant potential of anionic/nonionic mixtures has been assessed, and the results correlated with the CMC values determined for the mixtures [20,36].
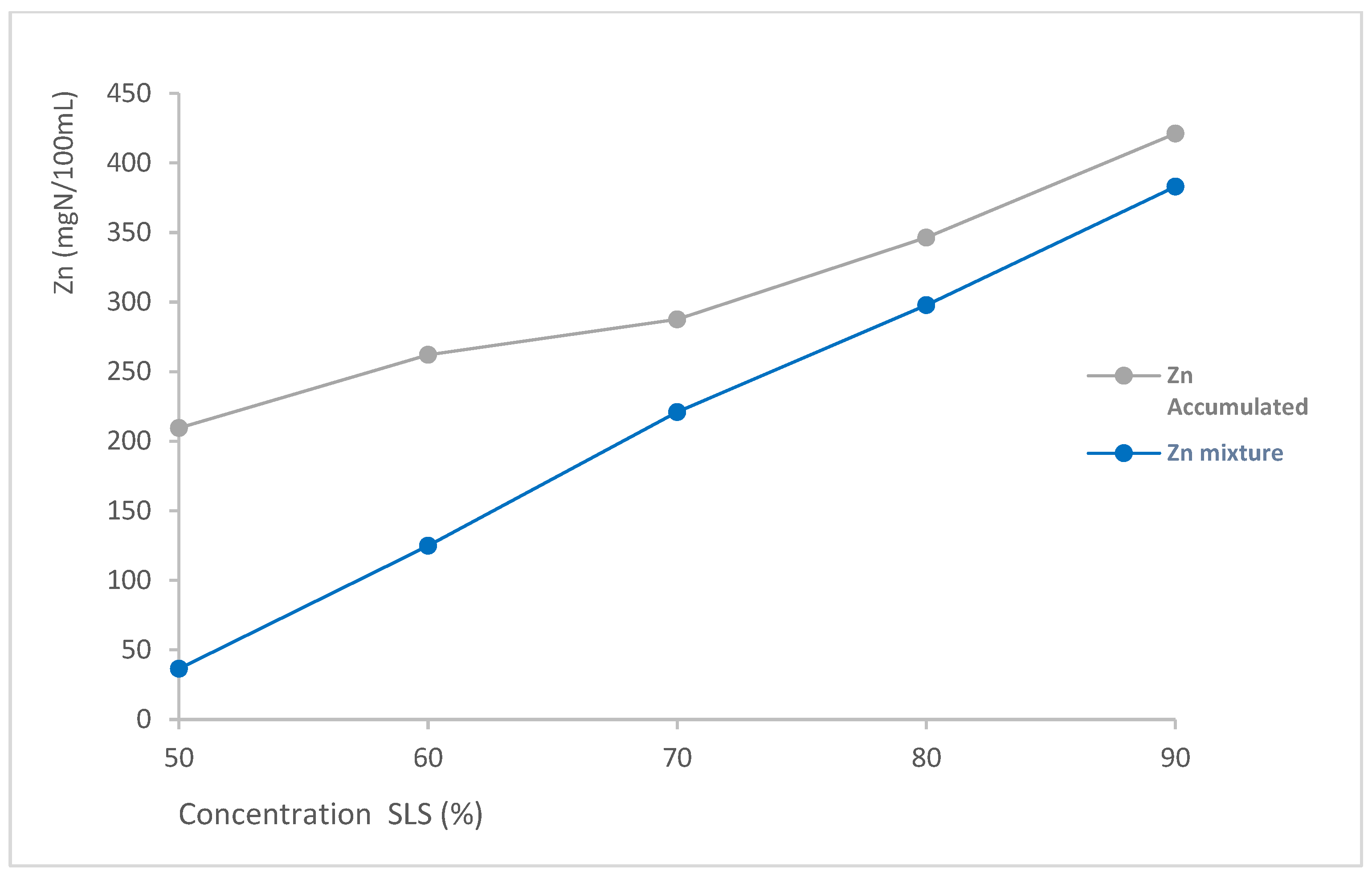
To quantify the synergistic effect that occurs in surfactant mixtures, Table 4 shows the Zein number for each individual surfactant at the concentration found in the mixtures (Znindividual), the sum of the contribution of each ingredient (Znaccumulated), and the comparison with the experimentally obtained Zein value of the mixture (Znmixture). Likewise, the percentage increase in irritant potential has been calculated by considering the accumulated effect vs. the Zein value obtained for the mixture. The percentage increase serves as a metric to quantify the synergistic reduction in protein denaturation. Figure 3 shows both curves (Zn Accumulated and Zn mixture) vs. SLS concentration.

Table 4.
Quantification of the synergistic effect in SLS + LDAO mixtures based on the Zein number for the mixtures and the Zein accumulated summing the contribution of each surfactant at the same concentration.
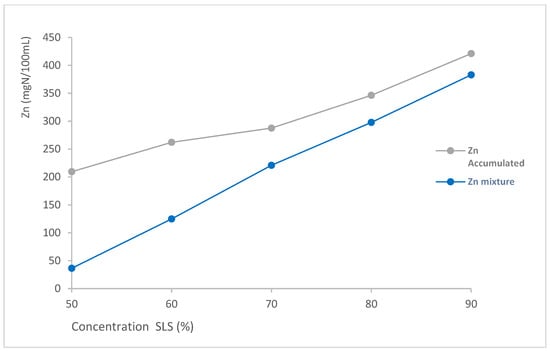
Figure 3.
Comparison of accumulated Zn and experimental (Zn mixture) for SLS + LDAO mixtures as a function of the SLS concentration.
As can be observed in Table 4 and Figure 3, the denaturation of Zein caused by surfactant mixtures is significantly lower than the accumulated value of Zein when adding the values of the individual ingredients for all the ratios analysed. This effect is specially observed for the 50:50 mixture, where Znaccumulated is 209.5 mgN/100 mL, while Zn mixture is just 36.5 mgN/100 mL.
These results demonstrate the existence of a synergistic effect in the SLS + LDAO mixture, resulting in a reduction in the protein denaturation potential of the mixture. Through the calculated % increase, it can also be seen that this synergistic effect is more pronounced for the mixture with a higher proportion of LDAO (474.9%), demonstrating its regulatory effect on the potential irritation of surfactant mixtures. This reduction in the irritant potential of the mixture compared to the accumulated potential of the individual surfactants has already been demonstrated in different mixtures of surfactants in commercial laundry detergents [27]. However, the study of the synergy obtained as a function of the ratio of each surfactant in binary mixtures provides added value since no previous literature has been found where similar studies have been carried out.
With this assessment, it can be concluded that dermal irritation does not have to be proportional to the surfactant concentration. Due to the synergies that might take place on surfactant mixtures, especially on those of different natures such as anionic–nonionic and anionic–amphoteric, it is possible to achieve a lower dermal irritation while increasing the total surfactant concentration [27].
3.3. Foam Generation and Stability
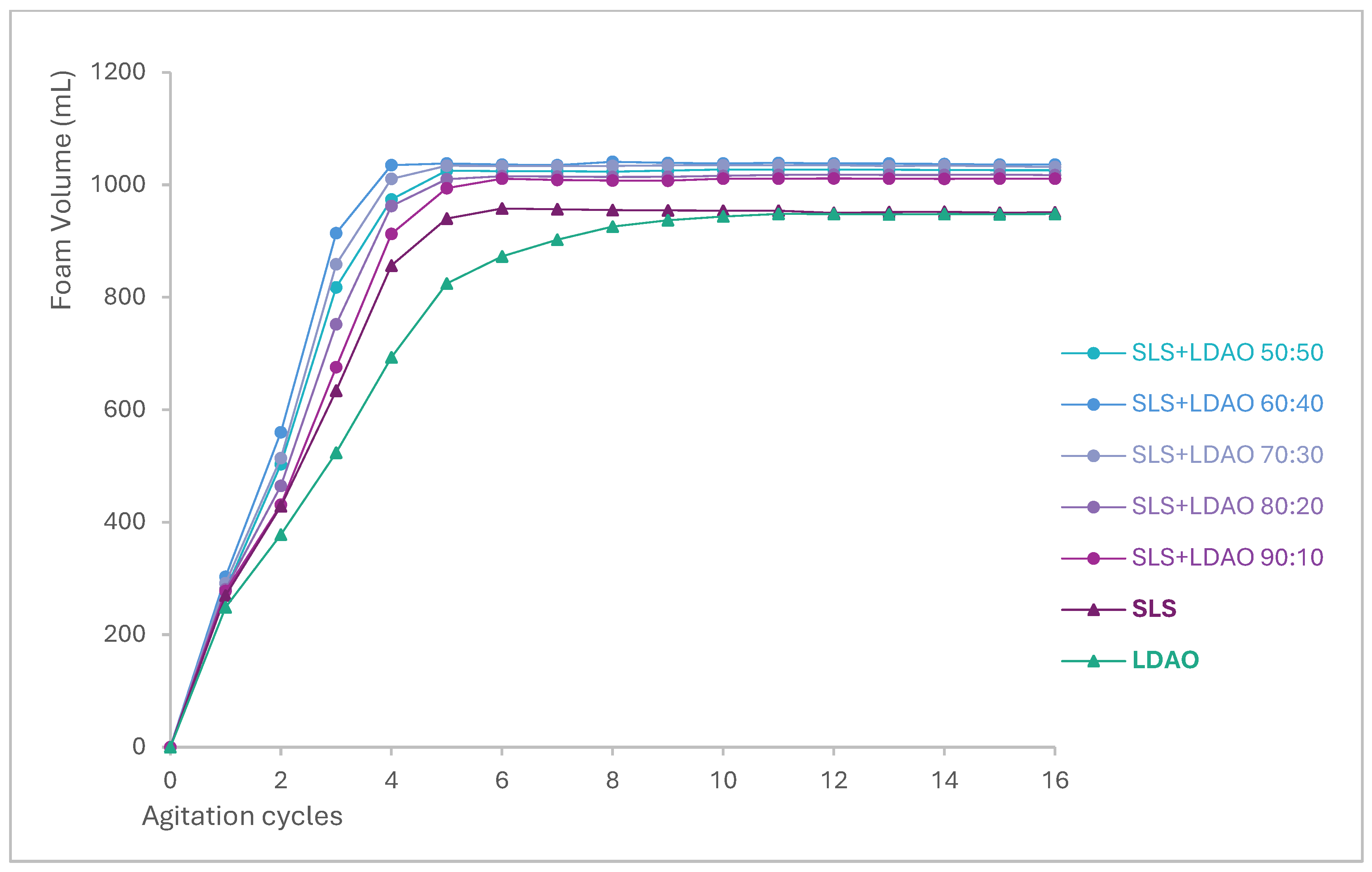
Figure 4 illustrates the evolution of the foam volume as a function of agitation cycles for each SLS + LDAO mixture and the individual surfactants. The results indicate that increasing the proportion of LDAO accelerates foam generation, with the highest volume observed for the 60:40 mixture. All binary mixtures outperform the individual surfactants in both generation speed and total foam volume, confirming a synergistic effect in foam formation. This enhancement highlights the potential of SLS + LDAO mixtures for use in hand dishwashing formulations.
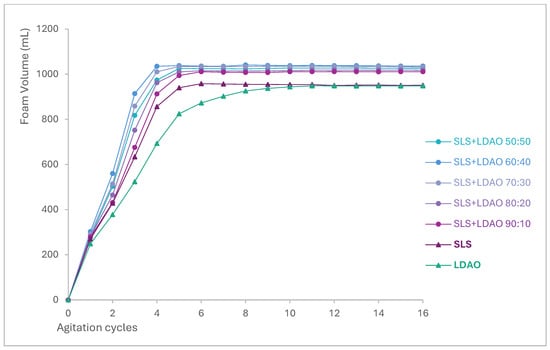
Figure 4.
Foam volume evolution during agitation cycles for SLS, LDAO, and their binary mixtures.
The most pronounced differences in foam volume are observed between the second and sixth agitation cycles. Figure 5 presents the foam volume generated after three agitation cycles, plotted against the percentage of SLS in the mixture, as this cycle showed the greatest variation among formulations. Additionally, Figure 6 includes foam stability curves, showing foam volume recorded every 20 s over a 5 min period following the final agitation.
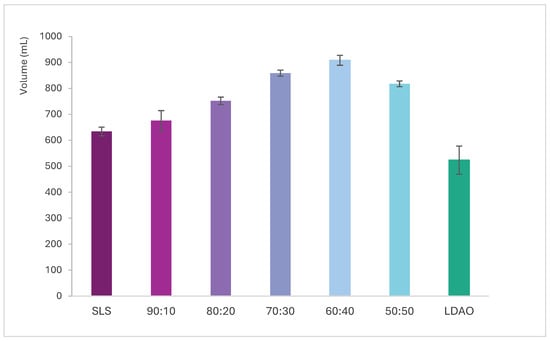
Figure 5.
Volume of foam generated vs. SLS + LDAO ratio after 3 shaking cycles.
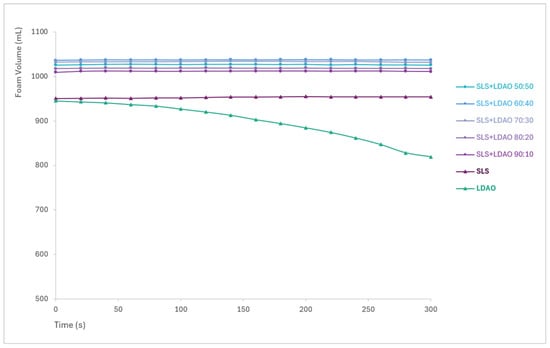
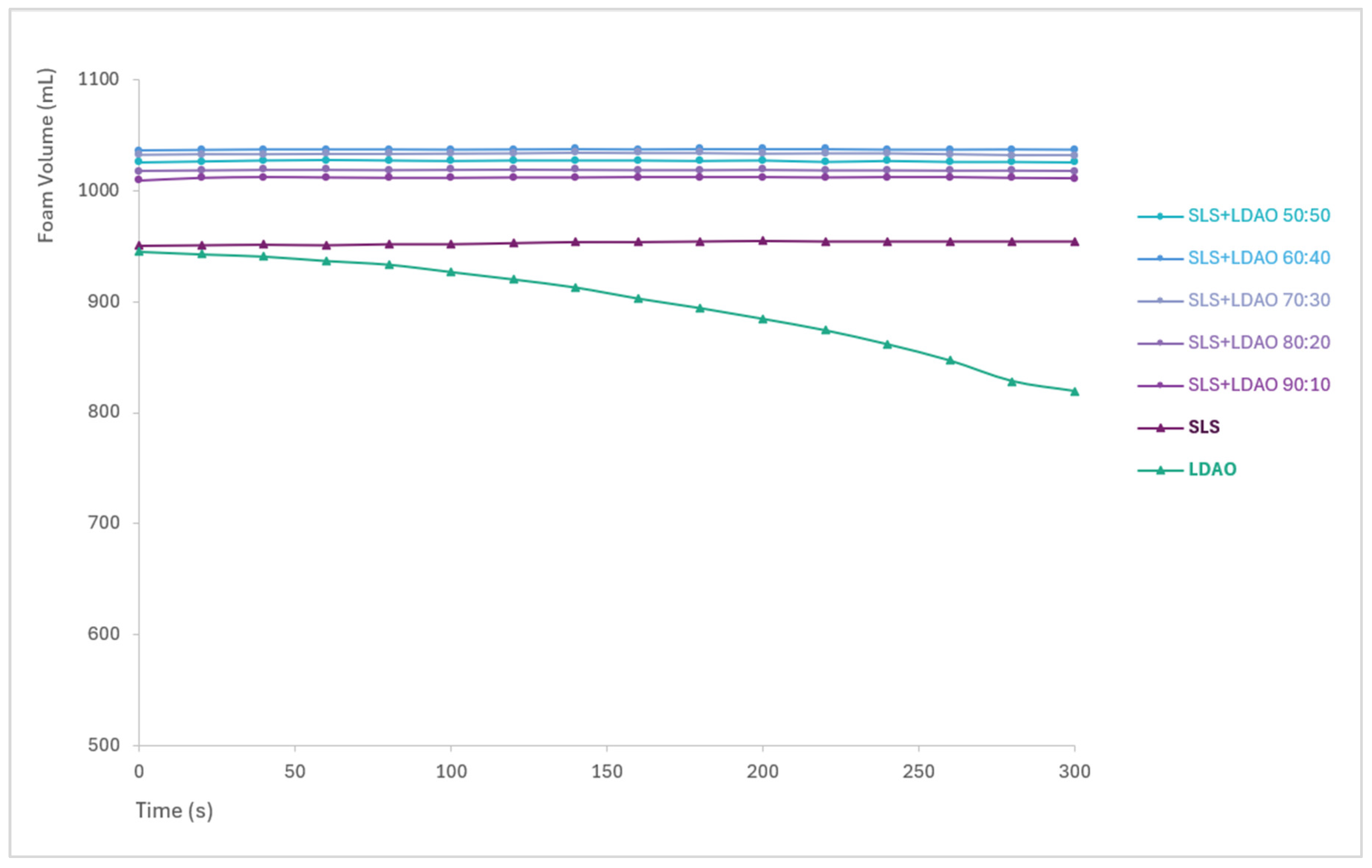
Figure 6.
Foam stability over time for SLS, LDAO, and their binary mixtures.
Foam generated by SLS and its mixtures remained stable throughout the 5 min observation period, with no significant volume loss. In the case of LDAO, the foam generated is not as stable, with a clear decrease in volume. Although LDAO alone exhibits lower foam generation and stability, it can be used as a co-surfactant to improve the foaming properties of SLS, with the improvement offered being proportional to the % of LDAO.
In foaming, as in other surface properties, correlations between surfactant structure and foaming in aqueous solution require a distinction between the efficiency of the surfactant (concentration required to produce a significant amount of foam) and effectiveness (maximum foam height obtained with the surfactant solution regardless of its concentration). Foam height typically increases with surfactant concentration up to the CMC, beyond which no further increase is observed. Thus, the CMC of a surfactant is a good measure of its efficiency as a foaming agent; the lower the CMC, the more efficient the surfactant as a foamer. On the other hand, the effectiveness of a surfactant as a foaming agent depends on the effectiveness in reducing the surface tension of the foaming solution [2]. Regarding stability, a mixed-surfactant system has greater foam stability than a single one; this is because the dispersion of two different types of surfactants together in a continuous phase increases surface viscosity and minimizes the liquid drainage rate [29].
In the study carried out, thanks to the synergistic interactions among SLS and LDAO, a reduction in the CMC as well as in the minimum surface tension is achieved for all the mixtures analyzed; consequently, foam height and foam stability are considerably improved for all the mixtures, compared to the individual surfactants. These synergies in foaming and foaming stability in surfactant mixtures of different natures have been proven in previous studies, as well as their relationship with interfacial parameters such as the CMC [19,38].
4. Conclusions
This study demonstrates that binary mixtures of sodium lauryl sulfate (SLS) and lauryl dimethylamine oxide (LDAO) exhibit a pronounced synergistic effect that enhances key physicochemical and functional properties relevant to surfactant-based formulations. The combination of these anionic and amphoteric surfactants led to a significant reduction in critical micelle concentration (achieving 0.755 mmol/L in 70:30 mixture compared to 2.913 mmol/L in SLS) and minimum surface tension (γ), indicating improved micellization efficiency and interfacial activity compared to the individual surfactants. Foam generation and stability were also markedly improved in all SLS + LDAO mixtures, with the 60:40 ratio showing the highest foam volume and persistence (908.3 mL compared to 634 mL in SLS). These enhancements are particularly relevant for applications such as cosmetics and dishwashing liquids, where both performance and user perception are critical. Importantly, the Zein test revealed that the irritant potential of the mixtures was substantially lower than that of SLS alone, obtaining non-irritant mixtures (<200 mgN/100 mL) at 60:40 and 50:50 ratios. The reduction in protein denaturation was not only proportional to the LDAO content but also significantly lower than the cumulative irritancy expected from the individual surfactants. This confirms the presence of a synergistic effect, which also resulted in a 474.9% increase between the Zein accumulated and the Zein mixture for the 50:50 ratio. This finding underscores the potential of mixed surfactant systems to mitigate skin irritation without compromising performance. Overall, the results highlight the value of combining surfactants of different natures—anionic and amphoteric—in optimizing formulation performance. The study also provides a quantitative framework for evaluating synergy in terms of irritant potential, offering a useful tool for the rational design of milder and more effective cleaning products.
Author Contributions
Conceptualization, E.H., F.R. and M.L.; methodology, E.H. and C.C.; validation, M.L. and F.R.; formal analysis, E.H. and C.C.; investigation, E.H. and C.C.; data curation, E.H. and C.C.; writing—original draft preparation, E.H. and C.C.; writing—review and editing, F.R. and M.L.; visualization, E.H., C.C., F.R. and M.L.; supervision, E.H., F.R. and M.L.; project administration, E.H.; funding acquisition, E.H. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the Regional Ministry of Innovation, Industry, Trade, and Tourism of the Valencian Regional Government, through the Valencian Institute of Competitiveness and Innovation (IVACE). DIFU+ID (Plan ANE AITEX 2026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank Rosana Martínez (AITEX, Department of Microbiology) for her work in conducting the Kjeldahl method used to quantify the Zein dissolved.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| SLS | Sodium Lauryl Sulfate |
| LDAO | Lauryl Dimethyl Amine Oxide |
| CMC | Critical Micelle Concentration |
| HLB | Hydrophilic–Lipophilic Balance |
| INCI | International Nomenclature of Cosmetics Ingredients |
| Zn | Zein |
| TEWL | Transepidermal Water Loss |
References
- Kaur, J.; Farzeen, R.; Singh, M.; Thakur, N.; Lal, M.; Upadhyaya, S.K.; Walia, Y.K.; Kishore, K. Overview of Surfactants, Properties, Types, and Role in Chemistry. In Advances in Surfactant Biosensor and Sensor Technologies; Manjunatha, J.G., Ed.; Springer: Cham, Switzerland, 2024; pp. 1–23. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Myers, D. Surfactant Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lai, K.-Y. Liquid Detergents; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Aisha; Batool, I.; Iftekhar, S.; Taj, M.B.; Carabineiro, S.A.C.; Ahmad, F.; Khan, M.I.; Shanableh, A.; Alshater, H. Wetting the Surface: A Deep Dive into Chemistry and Applications of Surfactants. Clean. Chem. Eng. 2025, 11, 100197. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants: Principles and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Luengo, G.S.; Aubrun, O.; Restagno, F. Foams in Cosmetics: New Trends in Detergency, Friction, Coatings. Curr. Opin. Colloid Interface Sci. 2025, 77, 101906. [Google Scholar] [CrossRef]
- Klein, K. Evaluating Shampoo Foam. Cosmet. Toilet. 2004, 119, 67–72. [Google Scholar]
- Blagojević, S.M.; Pejić, N.D.; Blagojević, S.N. Synergism and Physicochemical Properties of Anionic/Amphoteric Surfactant Mixtures with Nonionic Surfactant of Amine Oxide Type. Russ. J. Phys. Chem. A 2017, 91, 2690–2695. [Google Scholar] [CrossRef]
- Salager, J.L. Surfactants—Types and Uses; Lab. FIRP, Booklet 300A; Laboratorio FIRP: Mérida, Venezuela, 2019. [Google Scholar]
- Ranji, H.; Babajanzadeh, B.; Sherizadeh, S. Detergents and surfactants: A brief review. Open Access J. Sci. 2019, 3, 94–99. [Google Scholar] [CrossRef]
- Salomon, G.; Giordano-Labadie, F. Surfactant Irritations and Allergies. Eur. J. Dermatol. 2022, 32, 677–681. [Google Scholar] [CrossRef]
- Horita, K.; Horita, D.; Tomita, H.; Yasoshima, M.; Yagami, A.; Matsunaga, K. Effects of Different Base Agents on Prediction of Skin Irritation by Sodium Lauryl Sulfate Using Patch Testing and Repeated Application Test. Toxicology 2017, 382, 10–15. [Google Scholar] [CrossRef]
- Singh, S.K.; Bajpai, M.; Tyagi, V.K. Amine Oxides: A Review. J. Oleo Sci. 2006, 55, 99–119. [Google Scholar] [CrossRef]
- Zhang, H.; Miller, C.A.; Garrett, P.R.; Raney, K.H. Lauryl Alcohol and Amine Oxide as Foam Stabilizers in the Presence of Hardness and Oily Soil. J. Surfactants Deterg. 2005, 8, 99–107. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Fernández-Serrano, M.; Fernández-Arteaga, A. Aerobic Biodegradation of Amphoteric Amine-Oxide-Based Surfactants: Effect of Molecular Structure, Initial Surfactant Concentration and pH. Chemosphere 2017, 171, 324–331. [Google Scholar] [CrossRef]
- Joshi, T.; Mata, J.; Bahadur, P. Micellization and Interaction of Anionic and Nonionic Mixed Surfactant Systems in Water. Colloids Surf. Physicochem. Eng. Asp. 2005, 260, 209–215. [Google Scholar] [CrossRef]
- Kotsi, K.; Dong, T.; Kobayashi, T.; Robbie, I.M.; Striolo, A.; Angeli, P. Synergistic Effects between a Non-Ionic and an Anionic Surfactant on the Micellization Process and the Adsorption at Liquid/Air Surfaces. Soft Matter 2024, 20, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, N.; Adib, B.; Malihi, F.B. Synergism and Performance Optimization in Liquid Detergents Containing Binary Mixtures of Anionic–Nonionic, and Anionic–Cationic Surfactants. J. Surfactants Deterg. 2013, 16, 115–121. [Google Scholar] [CrossRef]
- Vilaplana, J.; Lecha, M.; Trullas, C.; Coll, J.; Comelles, F.; Romaguera, C.; Pelejero, C. A Physicochemical Approach to Minimize the Irritant Capacity of Anionic Surfactants. Exog. Dermatol. 2002, 1, 22–26. [Google Scholar] [CrossRef]
- Grady, B.P. Surfactant Mixtures: A Short Review. J. Surfactants Deterg. 2023, 26, 237–250. [Google Scholar] [CrossRef]
- Abdel-Rahem, R. Synergism in Mixed Anionic–Amphoteric Surfactant Solutions: Influence of Anionic Surfactant Chain Length. Tenside Surfactants Deterg. 2009, 46, 298–305. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions Between Surfactants and the Skin—Theory and Practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- García Martín, J.F.; Herrera-Márquez, O.; Vicaria, J.M.; Jurado, E. Synergistic Effect on Wettability of Mixtures of Amine Oxides, Alkylpolyglucosides, and Ethoxylated Fatty Alcohols. J. Surfactants Deterg. 2014, 17, 1035–1042. [Google Scholar] [CrossRef]
- Alves, L.; Magalhães, S.; Esteves, C.; Sebastião, M.; Antunes, F. Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems. J 2024, 7, 169–182. [Google Scholar] [CrossRef]
- de Jongh, C.M.; Jakasa, I.; Verberk, M.M.; Kezic, S. Variation in Barrier Impairment and Inflammation of Human Skin as Determined by Sodium Lauryl Sulphate Penetration Rate. Br. J. Dermatol. 2006, 154, 651–657. [Google Scholar] [CrossRef]
- Paye, M.; Block, C.; Hamaide, N.; Hüttmann, G.-E.; Kirkwood, S.; Lally, C.; Lloyd, P.H.; Makela, P.; Razenberg, H.; Young, R. Antagonisms Between Surfactants: The Case of Laundry Detergents. Tenside Surfactants Deterg. 2006, 43, 290–294. [Google Scholar] [CrossRef]
- Löffler, H.; Happle, R. Profile of Irritant Patch Testing with Detergents: Sodium Lauryl Sulfate, Sodium Laureth Sulfate and Alkyl Polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar Shah, S.; Chakraborty, G.; Bhattarai, A.; De, R. Synergistic and Antagonistic Effects in Micellization of Mixed Surfactants. J. Mol. Liq. 2022, 368, 120678. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Chen, D.; Ma, L.; Ji, L.; Han, C.; Zhu, L.; Jiang, J. Foaming and Emulsification Synergies in Skin Mild and Environmentally Friendly Detergents Based on Gleditsia sinensis Saponins. J. Mol. Liq. 2025, 417, 126649. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Lobato-Guarnido, I.; Fernández-Serrano, M. Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas Putida and Marine Microalgae Phaeodactylum tricornutum. Toxics 2023, 11, 344. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of Surface and Interfacial Tension Using Pendant Drop Tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef]
- DB-ALM Protocol n° 26. The Zein Test: Skin Irritation and Corrosivity; European Commission, Joint Research Centre. Available online: http://data.europa.eu/89h/b7597ada-148d-4560-9079-ab0a5539cad3 (accessed on 21 November 2025).
- Lechuga, M.; Avila-Sierra, A.; Lobato-Guarnido, I.; García-López, A.I.; Ríos, F.; Fernández-Serrano, M. Mitigating the Skin Irritation Potential of Mixtures of Anionic and Non-Ionic Surfactants by Incorporating Low-Toxicity Silica Nanoparticles. J. Mol. Liq. 2023, 383, 122021. [Google Scholar] [CrossRef]
- Muherei, M.; Junin, R. Equilibrium Adsorption Isotherms of Anionic, Nonionic Surfactants and Their Mixtures to Shale and Sandstone. Mod. Appl. Sci. 2009, 3, 158–167. [Google Scholar] [CrossRef]
- Paye, M. Mechanism of Skin Irritation by Surfactants and Anti-Irritants for Surfactant-Based Products. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A., Paye, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 353–366. [Google Scholar] [CrossRef]
- Lechuga, M.; García, P.A.; García-López, A.I.; Tapia-Navarro, C.; Ríos, F. Development of a Predictive Classification Model for Surfactant-Induced Skin Irritation. ACS Omega 2025, 10, 55868–55878. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic Effect of Mixed Surfactant Systems on Foam Behavior and Surface Tension. J. Surfactants Deterg. 2013, 16, 621–630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.