1. Introduction
The skin is the largest organ of the human body and is in direct contact with the external environment. It is made of several layers, each with its own cellular composition. Due to its constant renewal, the skin is an organ with high turnover, and relies heavily on adenosine triphosphate (ATP) produced by mitochondria during oxidative phosphorylation [
1].
The epidermis, the upper layer of the skin, is composed of precursor cells, differentiating into keratinocytes and cornified keratinocytes as the epidermis regenerates. When the keratinocytes are terminally differentiated, they are filled with keratin, denucleate and die in the stratum corneum, the outermost layer of the skin, and are desquamated. The dermis, located below the epidermis, is principally composed of fibroblasts producing extracellular matrix (ECM) proteins such as collagen and elastin. These two proteins are the main components of the ECM, a structural support network involved in a variety of processes such as cell migration and wound healing [
2]. In addition, the dermis is responsible for the delivery of nutrients to the skin via blood vessels [
3]. The epidermal–dermal junction lies at the interface between the dermis and the epidermis. Below the dermis is the hypodermis, a layer rich in fat cells [
4]. With age, the function and structure of the skin are altered, making it appear drier, thinner, and finely wrinkled. At the tissue level, the epidermis and the dermis are modified, with the most prominent changes occurring in the ECM [
5].
ECM remodeling during skin aging is primarily due to oxidative stress and mitochondrial dysfunction. Mitochondria are the main organelles affected by chronological aging and exposure to ultraviolet (UV) light [
5]. Reactive oxygen species (ROS) are natural by-products of oxidative phosphorylation, the process during which ATP is synthesized in the mitochondria. At a molecular level, during aging, endogenously produced ROS such as superoxide anion, singlet oxygen, and peroxides activate the mitogen-activated protein kinase (MAPK) family and, through a cascade of events, upregulate the expression of matrix metalloproteinases (MMPs). MMPs are a family of endopeptidases that can degrade ECM proteins, therefore regulating collagen and elastin homeostasis. Additionally, ROS inhibit the transforming growth factor beta (TGF-ß) signaling pathway in fibroblasts, which causes a decrease in collagen synthesis [
5].
When ECM–fibroblast interactions are impaired, fibroblasts become senescent, producing higher levels of ROS. Mitochondrial DNA can also be damaged by ROS, in turn altering the oxidative phosphorylation process and mitochondrial efficiency [
1,
6]. The two events lead to a vicious circle of ROS damage and mitochondrial dysfunction. In 2001, cytoglobin (CYGB) was the fourth globin to be discovered in vertebrates, after hemoglobin, myoglobin, and neuroglobin. Contrary to the other globins, it is expressed in a large range of tissues [
7,
8]. CYGB reversibly binds O
2 via the Fe
2+ ion of the heme group [
9,
10,
11,
12] and is upregulated in hypoxia to stimulate the transport of oxygen to the mitochondria through a hypoxia-inducible factor-1 (HIF-1)-dependent pathway [
13,
14,
15].
Although its function remains unclear, CYGB has been suggested to play a role in collagen synthesis [
14], linking it to the ECM. CYGB has peroxidatic activity, consuming hydrogen peroxide and lipid peroxides [
10,
16]. In addition, CYGB was reported to protect cells from death during oxidative stress [
17] and to be involved in nitric oxide (NO) homeostasis, strongly suggesting the role of this protein in cell protection [
18]. Although more investigation is needed to understand the physiological role of CYGB, overall, this protein seems to be involved in oxygen homeostasis and redox monitoring.
Tropaeolum majus (nasturtium), which belongs to the Tropaeolaceae family, a member of the Brassicales order, has long been used in traditional medicine for its anti-inflammatory and antiseptic properties (PMID: 7617765; 30279303; 32566105). A recent study has identified a chlorophyll-derived compound in T. majus called Pyro-phylloxanthobili, belonging to a family of bilins, which exhibits bioactivity against ROS (PMID: 38843802). Substances of this family are found to stabilize with heme proteins in eukaryotes (PMID: 27959940; 34382282).
Given the pivotal role of ROS in skin aging and due to the presumed action of CYGB against ROS, the objective of this study was to evaluate in vitro whether a T. majus extract upregulating the expression of CYGB had a protective effect on various markers of skin aging, such as mitochondrial function, ROS production, and ECM composition. Senescent cells accumulate in human tissues and organs during aging and can be recognized and eliminated by various cell types of the immune system, including macrophages, neutrophils, CD4+ T cells and natural killer (NK) cells. Natural killer cell activity is oxygen-dependent. Therefore, we hypothesized that a plant extract such as that of T. majus, which boosts cytoglobin and oxygen delivery, can restore NK cell cytotoxicity, which decreases with age.
2. Materials and Methods
2.1. Preparation and Characterization of Tropaeolum majus
T. majus extract enriched in arabinogalactanes was prepared by Silab (Silab, Saint-Viance, France), as described in this patent (FR2965483).
2.2. Skin Sample Procurement: Skin Sample and Cell Culture
Primary skin cell cultures were derived from an abdominal skin biopsy of a healthy donor undergoing plastic surgery. Normal human skin samples were obtained from the surgical discard of anonymous healthy patients in accordance with ethical guidelines (French Bioethics law of 2004) and declared to the French research ministry (Declaration no. DC-2025-7230), delivered to LVMH Recherche. Approval was not required as per the local legislation where the study was conducted. This study used strains obtained from deidentified human samples. Ethical guidelines (French Bioethics law of 2004) did not require the study to be reviewed or approved by an ethics committee because the samples are the surgical discard of anonymous healthy patients.
2.3. Culture and Treatments of Isolated Keratinocytes and Fibroblast
Keratinocytes were cultured in Keratinocyte Serum-Free Medium (KSFM, Gibco, Waltham, MA, USA), enhanced with 5 ng/mL human recombinant EGF (Gibco, MA, USA), 50 µg/mL bovine pituitary extract (Gibco, Waltham, MA, USA), and 100 µg/mL Primocin® (InvivoGen, San Diego, CA, USA). Fibroblasts were cultured in DMEM 1 g/L glucose (Lonza, Basel, Switzerland), supplemented with 10% FBS (Gibco, Waltham, MA, USA), 2 mM L-Glutamine (Lonza, Basel, Switzerland), and 100 µg/mL Primocin® (InvivoGen, San Diego, CA, USA). All cultures were maintained at 37 °C in a 5% CO2 atmosphere. T. majus extract was directly diluted into the culture medium.
2.4. mRNA Synthesis and Transfection
The CYGB cDNA sequence was retrieved from Ensembl (ENST00000589342.1) and synthesized by Twist Bioscience with specific homologous sequences for in-fusion cloning. With slight modifications, the Takara IVTpro (BspQI) mRNA Synthesis System (TaKaRa) was used to prepare the template plasmid for subsequent mRNA synthesis. In brief, 200 ng of the CYGB and 500 ng of the FLuc (TaKaRa) in vitro transcription templates were BspQI-linearized, purified, and used for mRNA synthesis. CleanCap AG (TriLink) was used as a 5′capping analog. mRNA was purified using the Monarch RNA Cleanup Kit (NEB), assessed for mRNA integrity with TapeStation 4200 (Agilent, Santa Clara, CA, USA) using RNA ScreenTape (Agilent), and quantified using NanoDrop2000c (Thermo Fisher Scientific, Waltham, MA, USA). In total, 500 ng of CYGB or FLuc mRNAs was reverse-transfected into 1.5 × 105 cells with Lipofectamine MessengerMAX (Invitrogen, Waltham, MA USA), following the manufacturer’s instructions. At 24 h post transfection, cells were harvested for flow cytometry analysis.
2.5. Cellular and Mitochondrial ROS Analysis Using Flow Cytometry and Confocal Microscopy
Cellular and mitochondrial ROS levels were determined using CellROX Deep Red (Invitrogen), or MitoBright ROS Deep Red (Dojindo), according to the manufacturer’s instructions. In brief, cells were incubated in a 37 °C, 5% CO2 incubator with culture media containing CellROX Deep Red (5 μM) or MitoBright ROS Deep Red (10 μM) for 30 min. Cells were then harvested with TrypLE-Express (Gibco) and subjected to flow cytometry analysis using BD FACSymphony A5 SE (BD Biosciences, Herlev, Denmark). Over 1 × 104 singlet events were analyzed in each of the samples. The raw data were analyzed using FlowJo 10 (BD Biosciences), and the relative ROS level was normalized with R675 intensity for CellROX Deep Red and R680 for MitoBright ROS Deep Red for cells transfected with the control FLuc mRNA. A two-tailed unpaired Student’s t-test was used for testing the statistical significance.
Normal human keratinocytes were seeded in culture medium and incubated at 37 °C in an atmosphere containing 5% CO2. Irradiations were conducted with an LED simulator emitting blue light at 415 nm (Omnilux Clear U) for 45 min at an intensity of 450 ± 50 Lux. After irradiation, the cells were or were not treated with 0.25% v/v T. majus extract. After the last irradiation, the cells were recovered and incubated for 30 min at 37 °C with a CM-H2DCFDA probe. Visualization was performed with an LSM 700 confocal microscope (Zeiss) coupled to a Zen Blue image analysis system (Zeiss, Oberkochen, Germany). The production of ROS was proportional to the intensity of green fluorescence in cells. The images were quantitatively analyzed using Matlab® software, version R2012b (MathWorks), and the results are expressed as the fluorescence intensity/number of cells (AU).
2.6. Proliferation Assessment for Keratinocytes and Fibroblasts
Proliferation assay was monitored in real-time using the IncuCyte® Live-Cell Analysis System (Sartorius, Aubagne, France). Primary human keratinocytes and fibroblasts were seeded in 96-well flat-bottom microplates (Corning, Clayton, Australia) at a density of 3000 cells per well in 100 µL of complete growth medium. The seeding density was optimized through preliminary experiments to ensure logarithmic growth throughout the observation period. Each experimental condition was performed in triplicate, with cell-free medium wells serving as background controls. Following seeding, the plates were allowed to equilibrate at room temperature for 30 min to ensure uniform cell distribution before being placed in the IncuCyte® system. Image acquisition and cell proliferation analysis was monitored using the IncuCyte® integrated analysis software (IncuCyte® S3). Phase-contrast images were acquired every 2 h over a 96 h period using a 10× objective lens, with four images captured per well.
2.7. Measurement of Keratinocyte Respiration and Quantification of ATP Production
Mitochondrial respiration was evaluated using a Seahorse extracellular flux analyzer (Agilent, les Ulis, France). 25 × 103 cells were seeded per well in a cell culture microplate. After 24 h, the culture medium was replaced with XF assay medium, and the following compounds were sequentially injected: 1.5 μM oligomycin A, 1 μM FCCP, and 0.5 μM antimycin/rotenone (Sigma-Aldrich, Saint Quentin Fallavier, France). The resulting data were normalized against the total protein content per well, as quantified using a DC Protein Assay Reagent (Sigma-Aldrich, Saint Quentin Fallavier, France).
2.8. Immunohistochemical Analysis of Fibroblasts
Cytoglobin detection: Fibroblasts are treated with 0.25% v/v plant extract; after 24 h, the medium was changed and replaced with 50 mM cobalt and 0.25% v/v T. majus extract for 24 h, and cytoglobin was labeled with an anti-cytoglobin rabbit polyclonal antibody. Visualization was performed with an IX 70 microscope (Olympus) coupled to an image analysis system (NIS-Elements AR, Nikon, Tokyo, Japan). Fluorescence intensity was proportional to the synthesis of cytoglobin. Semi-quantitative analysis of the images was carried out using Matlab® software, version R2009a (MathWorks).
Type I procollagen was labeled with a rat monoclonal anti-type I procollagen antibody (Merck Millipore, Burlington, MA, USA; 1/100 dilution, 90 min incubation) followed by an Alexa Fluor® 488 donkey anti-rat secondary antibody (Invitrogen, MA, USA; 1/1000 dilution, 60 min incubation). Elastin was labeled with a rabbit polyclonal anti-elastin primary antibody (Abcam, Cambridge, UK; 1/2000 dilution, 90 min incubation) followed by an Alexa Fluor® 488 donkey anti-rat secondary antibody (Invitrogen, Waltham, MA, USA; 1/1000 dilution, 60 min incubation). For all immunolabeling procedures, nuclei were counterstained with DAPI, and slides were mounted using Fluoromount-GTM (Electron Microscopy Sciences, Hatfield, PA, USA). The slides were examined using an Axiovert 200M microscope (Zeiss, Oberkochen, Germany). Pictures were taken with an EXI blue camera (Teledyne, Tucson, AZ, USA) and analyzed using Volocity® acquisition software (PerkinElmer, MA, USA); we considered five photos per condition, each of which were performed in duplicate (n = 10).
2.9. Bulk RNA-seq Sample Preparation
Bulk RNA-seq experiments, RNA library preparations, and sequencing were performed by GENEWIZ, Inc/Azenta US, Inc. (South Plainfield, NJ, USA). Total RNA (1mg) was extracted from three biological replicates per condition of keratinocytes, obtained from three different donors, using the Direct-zol RNA MiniPrep Kit (Zymo Research R2053). RNA concentration was determined using a Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA), and RNA integrity was assessed using an Agilent TapeStation 4200 (Agilent Technologies, Palo Alto, CA, USA). RNA sequencing libraries were prepared using the NEBNext Ultra II RNA Library Prep Kit for Illumina and the NEBNext Poly(A) mRNA Magnetic Isolation Module, following the manufacturer’s protocols (NEB, Ipswich, MA, USA). Briefly, mRNAs were enriched using Oligod(T) beads and fragmented at 94 °C for 15 min. First- and second-strand cDNA synthesis was then performed. cDNA fragments underwent end-repair and adenylation at the 3′ ends. Universal adapters were ligated to the cDNA fragments, followed by index addition and library enrichment via PCR with a limited number of cycles. The resulting sequencing library was validated using an Agilent TapeStation (Agilent Technologies, Palo Alto, CA, USA) and quantified using a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA) and quantitative PCR (KAPA Biosystems, Wilmington, MA, USA). Following clustering on a flow cell, sequencing libraries were loaded onto an Illumina NovaSeq X Plus instrument according to the manufacturer’s instructions. Samples were sequenced using a 2 × 150 bp Paired-End (PE) configuration, targeting approximately 30 million reads per sample. Image analysis and base calling were conducted using the control software. Raw sequence data (.bcl files) were converted into fastq files and demultiplexed using Illumina’s bcl2fastq 2.20 software, allowing for one mismatch in index sequence identification.
2.10. Bulk RNA-seq Data Analysis
Sequence reads were trimmed using Trimmomatic v.0.36 to remove potential adapter sequences and low-quality nucleotides. The trimmed reads were then aligned to the Aedes_aegypti_ERCC reference genome (Ensembl) using the STAR aligner v.2.5.2b, a splice aligner that identifies and incorporates splice junctions to improve read sequence alignment. The resulting mapping .bam files were used to generate hit counts for genes and exons. Unique gene hit counts were calculated using the Counts feature of the Subread package v.1.5.2. Only unique reads mapping within exon regions were counted and used for subsequent analysis. For differential gene expression analysis, read counts were normalized using the DESeq2 v.1.16.1 software package to account for factors such as variations in sequencing yield between samples. Sample similarities were evaluated through heatmap and principal component analysis. A Wald test, implemented in the DESeq2 package (v.1.16.1), was used to assess changes in gene expression. Genes with an adjusted p-value < 0.05 and an absolute log2-fold change > 1 were considered statistically significant differentially expressed genes. Global transcriptional changes across samples were visualized using a volcano plot, with upregulated and downregulated genes highlighted in red and blue, respectively.
DEXSeq differential exon usage test: To estimate the expression levels of alternatively spliced transcripts, splice variant hit counts were extracted from the RNA-seq reads mapped to the genome. Differentially spliced genes were identified in groups with more than one sample by testing for significant differences in read counts on exons (and junctions) of the genes using DEXSeq. For groups with only one sample, the exon hit count tables were provided.
2.11. PBMC Isolation from Leukoreduction System (LRS) Chambers
Human LRS Chambers from two female donors (age 45 and age 51) were purchased from Vitalant (Auburn, CA, USA). Peripheral blood mononuclear cells (PBMCs) were isolated as described below. Blood was first diluted with an equal volume of 2%FBS/DPBS. Then, 12 mL of Ficoll-Paque PLUS density gradient media was gently added to the diluted blood. Gradient centrifugation was carried out by centrifuging the diluted blood with Ficoll for 30 min at 2000 rpm with no break at room temperature. After centrifugation, the plasma layer was carefully removed, and the buffy coat containing enriched PBMCs was collected. The purified PBMCs were resuspended in 15 mL of complete RPMI medium, counted, and cryopreserved in 10% DMSO/FBS.
2.12. K-562 Cell Culture and Labeling with CellTrace Violet
K-562 cells serve as the target cells to activate the killing activity of natural killer (NK) cells due to their reduced expression of HLA class I and increased expression of ligands for activating NK receptors. K-562 cells were purchased from ATCC. For the NK cytotoxicity assay, the K-562 cells were labeled with CellTrace Violet as described below. One million K-562 cells were washed once with D-PBS and resuspended in 1 mL of D-PBS, before being incubated with CellTrace Violet reagent (0.1 nM) for 20 min in the dark at 37 °C. After incubation, the CellTrace Violet reagent was quenched by adding 10 mL of complete RPMI medium and incubating for 5 min in the dark at 37 °C. The labeled K-562 cells were then counted and prepared for NK cytotoxicity assay.
2.13. NK Characterization and CD107a Degranulation Assay
To evaluate the effects of treatment on the activation and degranulation of NK cells, K-562 cells (2 × 5 105/well) were added to PBMCs along with CD107a antibody (1 μL/well) and incubated for 1 h. Afterwards, Monensin (2 μM) was added to the cell culture and incubated for an additional 2 h to preserve the cytokine signals produced during NK activation. After incubation, the cells were spun down (1400 rpm for 5 min) to remove culture medium and resuspended in 150 μL/well of 2% FBS/D-PBS for staining with flow cytometry antibodies. The cells were first stained with Live/Dead Blue reagent (0.1 μL/well) for 10 min in the dark at room temperature. Surface markers were stained with an antibody cocktail for 15 min in the dark at 4 °C, and the cells were washed once with 2% FBS/D-PBS and fixed with 150 μL/well of 1x fixation buffer for 30 min in the dark at 4 °C for intracellular staining. After fixation, they were washed once with 200 μL/well of 1× permeabilization buffer resuspended in 100 μL of permeabilization buffer, and stained with an antibody cocktail (ki-67 and IFNG) for intracellular staining for 20 min in the dark at 4 °C. The cells were then washed once with 1× permeabilization buffer, resuspended in 150 μL/well of 2% FBS/D-PBS, and analyzed using a Cytek Aurora flow cytometer.
2.14. FACS Data Analysis
Spectral unmixing of FCS files was performed using SpectroFlo software (SpectroFlo 11), while compensation and further analysis were performed using FlowJo software (FlowJo 11). A heatmap was plotted using the ComplexHeatmap package (v2.14.0), and correlation network analysis was conducted using the corrr package (v0.4.4) in R.
2.15. Statistical Analysis
Following verification of data normality using the Shapiro–Wilk test (p < 0.05), statistical differences were determined using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test, or using Student’s t-test. Results are presented as mean ± standard error of the mean (SEM). Statistical significance was defined as p ≤ 0.05, and high statistical significance as p ≤ 0.01.
3. Results
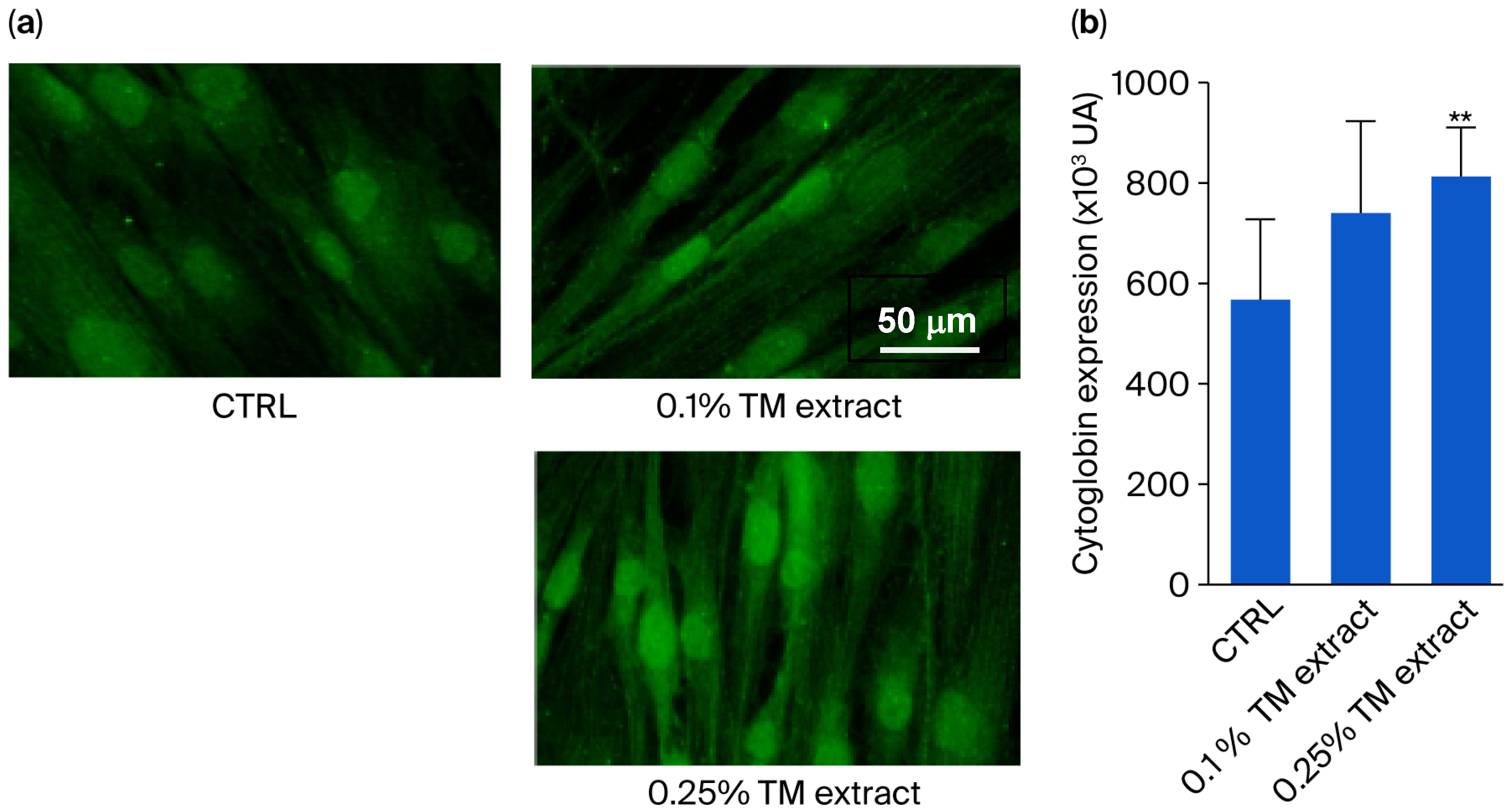
The
T. majus extract was added at 0.1%
v/
v and 0.25%
v/
v concentrations to aged human fibroblasts (64-year-old), cultured under hypoxic conditions, selected due to their optimal cytoglobin expression levels [
12]. The addition of the extract at both concentrations increased the synthesis of cytoglobin in a dose-dependent manner, by 22% for the 0.10%
v/
v extract and by 56% for the 0.25%
v/
v extract (
Figure 1).
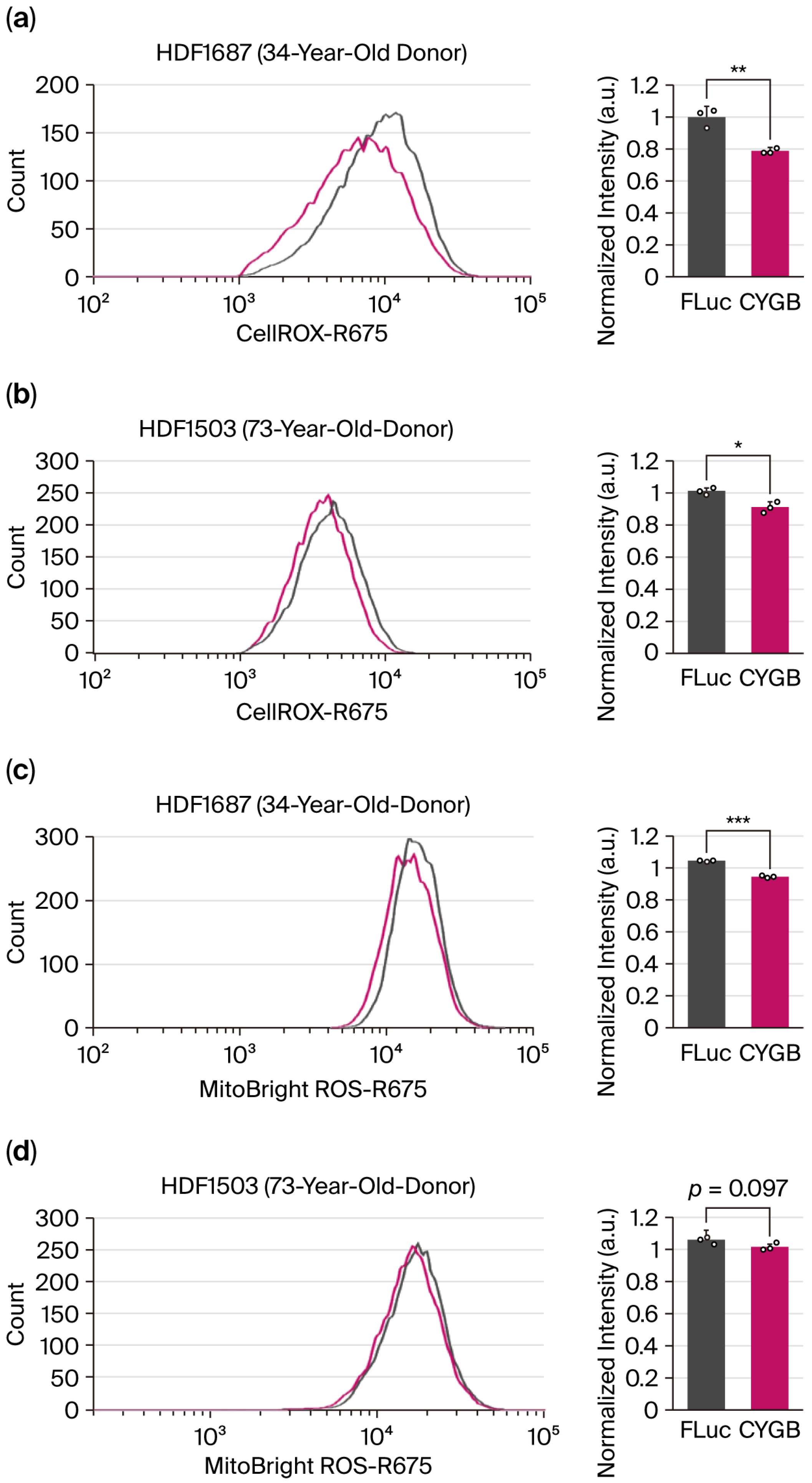
It has been reported that CYGB may have a potent superoxide dismutase (SOD) function in cells (PMID: 34930834). To investigate this effect, we synthesized CYGB mRNAs and delivered them into two human dermal fibroblast (HDF) cell lines derived from donors of different ages. Cells were grown under normoxic conditions to minimize the expression of endogenous CYGB. Transfections of CYGB mRNA to cells reduced cellular ROS in both fibroblast cell lines (
Figure 2a,b). We then tested whether CYGB could also contribute to quenching mitochondrial ROS utilizing the specific dye MitoBright ROS Deep Red. Notably, the transfection of CYGB mRNA reduced mitochondrial ROS levels in fibroblast cells from a 34-year-old donor; however, this effect was not significant in cells from a 73-year-old donor (
Figure 2c,d). Overall, our results suggest that a high level of CYGB may have beneficial effects in countering ROS in both cytosolic and mitochondrial environments, although age-related differences and cell line dependence should be further investigated.
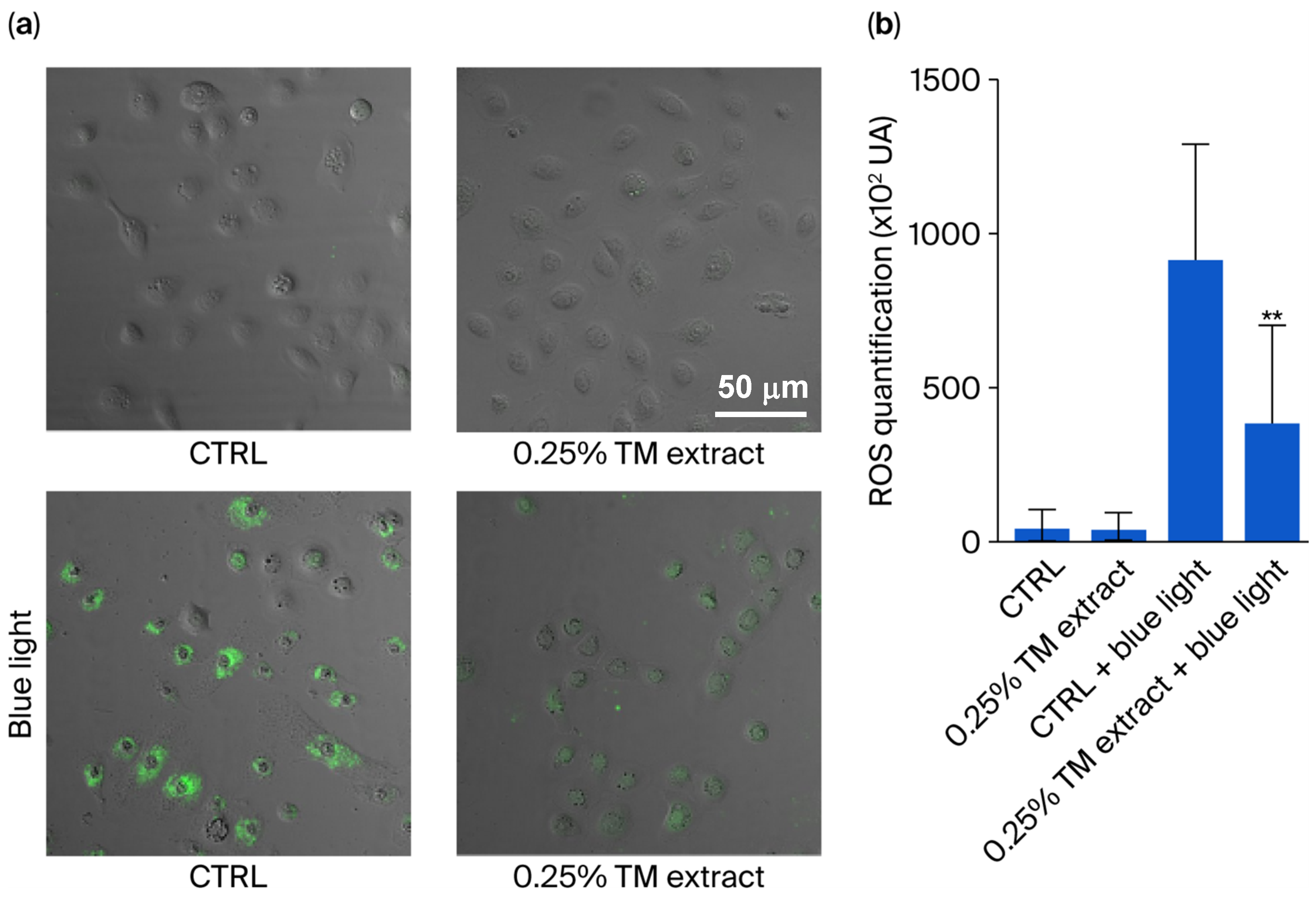
Considering the reduction in ROS levels detected in fibroblasts overexpressing cytoglobin,
T. majus extract’s stimulation of cytoglobin production was tested on keratinocytes, the main cell type present in the epidermis. Cells were grown at 1% oxygen for 48 h to induce cytoglobin and then treated with the 0.5%
v/
v T. majus extract at for 24 h under normoxic conditions. The treatment with the extract slightly reduced ROS levels in controls (
Figure 3). Then, control keratinocytes were exposed to blue light for 45 min, causing a significant 17-fold increase in ROS concentration. When these irradiated keratinocytes were treated with the extract after irradiation, ROS levels were decreased by 57% (
Figure 3).
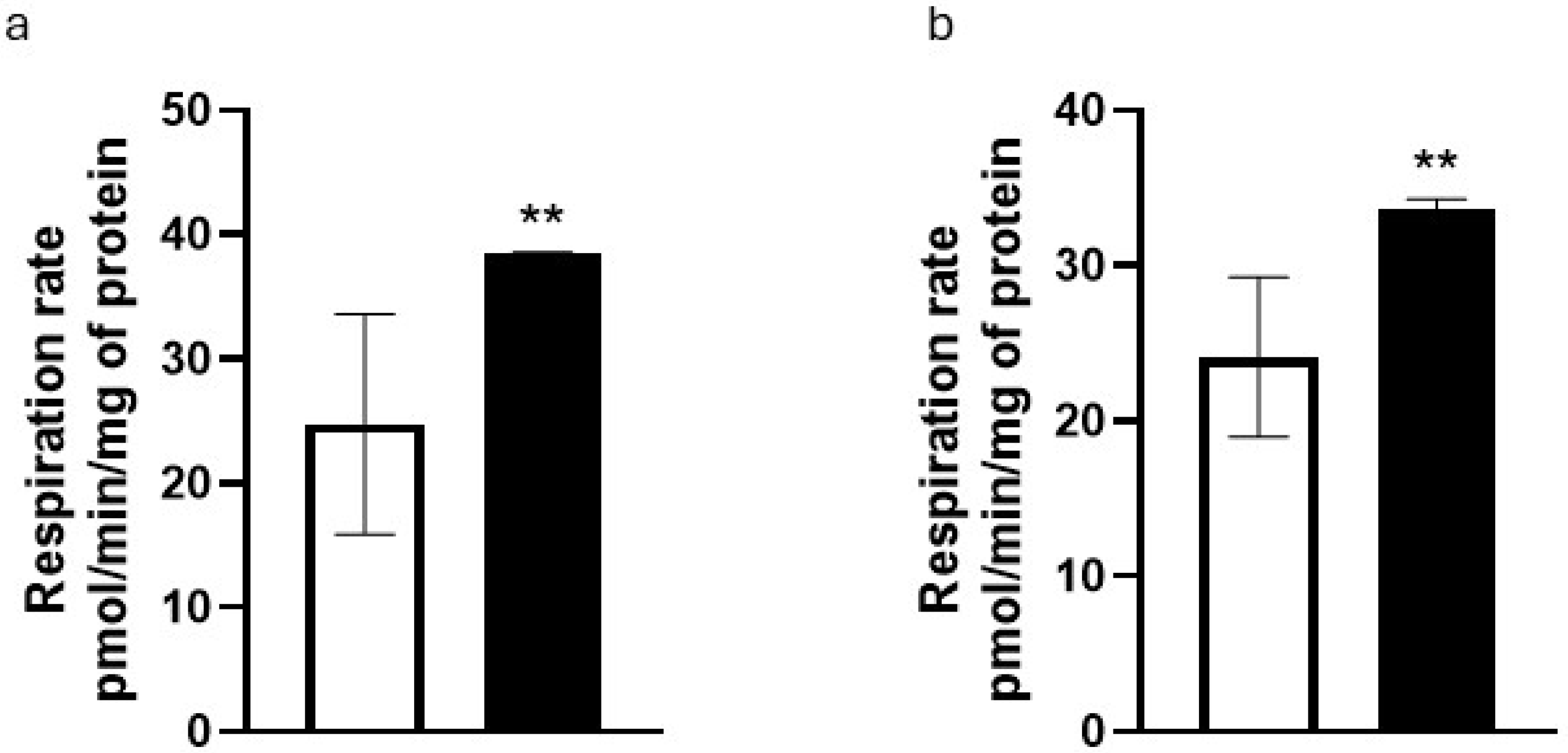
After observing a decrease in ROS concentrations in both fibroblasts and keratinocytes treated with the
T. majus extract, the mitochondrial activity was measured in NHKs. When cells were treated with the extract at a 0.5%
v/
v concentration, we detected a 57% increase in respiratory rate (
Figure 4a) and a 40% increase in the production of ATP by mitochondria (
Figure 4b).
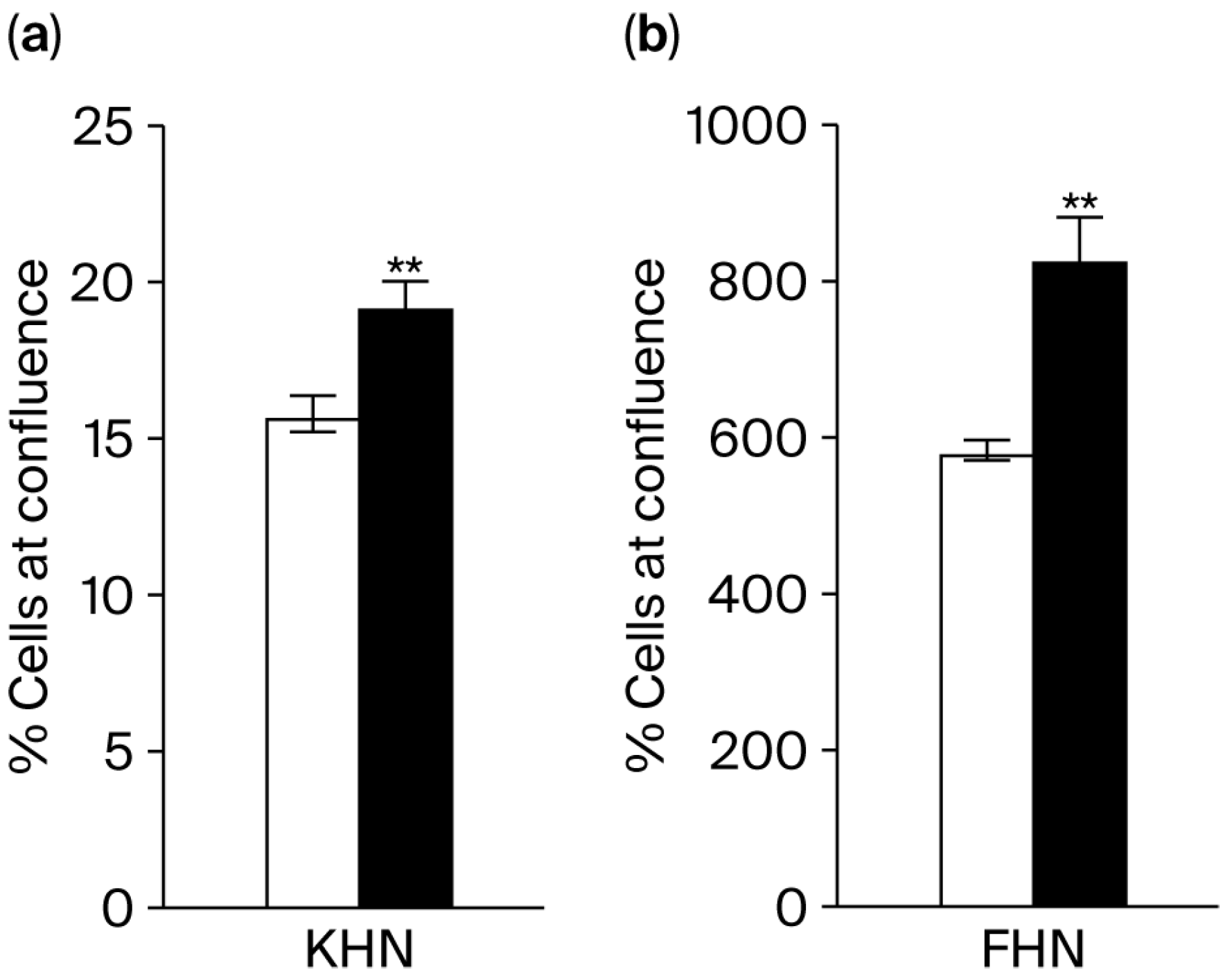
The proliferation rates of keratinocytes and HDFs were subsequently measured by assessing their confluence levels. When the extract was added to the culture, there was a 22% increase in keratinocyte proliferation (
Figure 5a) and a 42% increase in HDF proliferation (
Figure 5b).
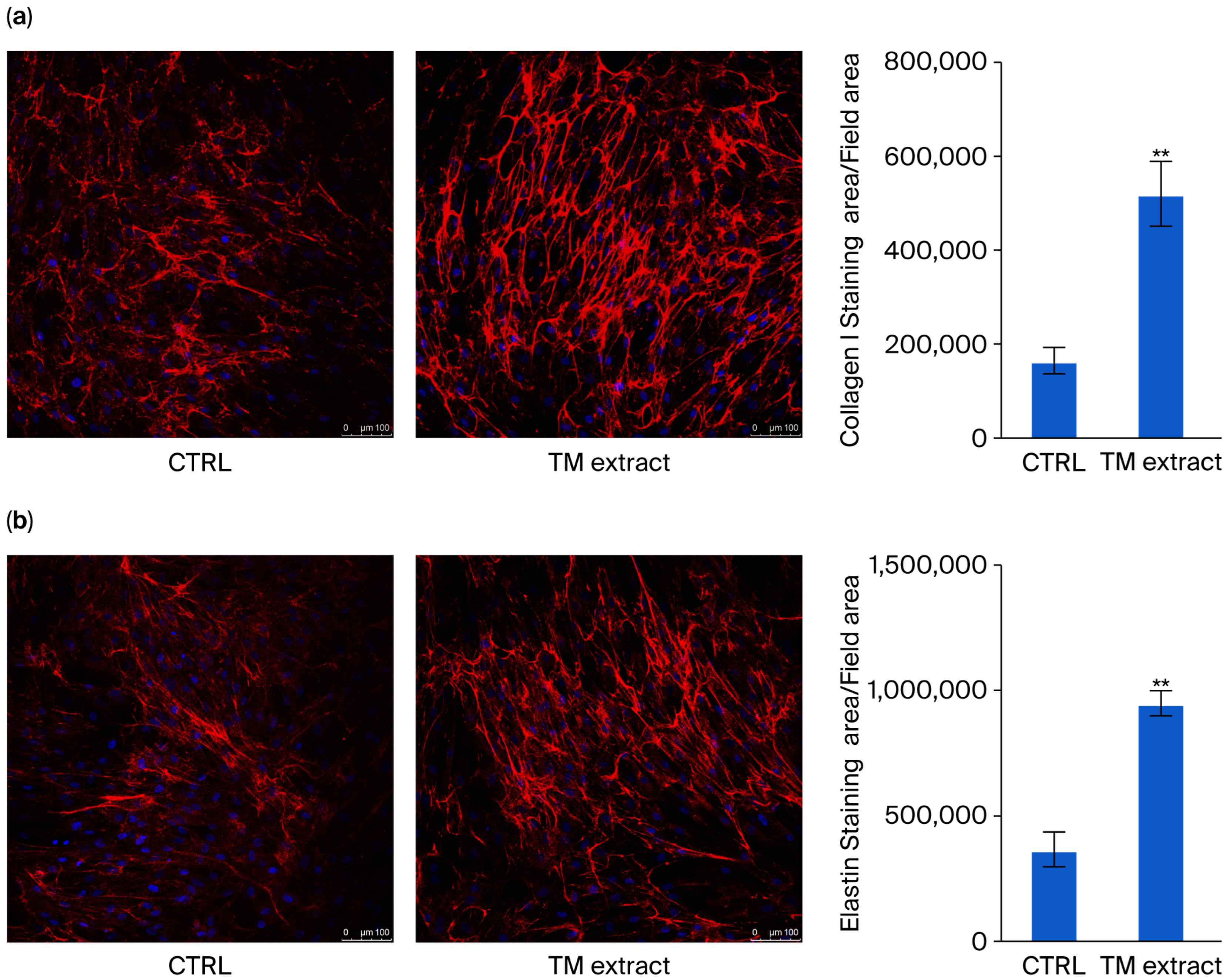
As the ECM composition in collagen and elastin is severely affected by ROS [
2], the production of collagen and elastin was evaluated in HDFs. When the extract was added to the cell culture (0.5%
v/
v), there was a 215% increase in collagen levels (
Figure 6a) and a 158% increase in elastin concentrations (
Figure 5b).
To evaluate the transcriptomic impact of
T. majus extract on epidermal cells, RNA-seq analysis was conducted on primary keratinocytes isolated from young (neonatal) and elderly donors (66 years old) in biological independent triplicate. For each age group, gene expression was compared between untreated cells and those treated with the TM extract (0.5%
v/
v) (
Figure 7a,b). In young keratinocytes, treatment resulted in 30 differentially expressed genes (DEGs), including 5 upregulated and 25 downregulated genes (
Figure 7c), while in aged keratinocytes, 40 DEGs were identified (30 upregulated, 10 downregulated) (
Figure 7d). Interestingly, a comparison of the DEGs from both age groups showed a 25% overlap, suggesting partially shared molecular responses to the treatment. Among the commonly regulated genes was
LCE, a family involved in terminal differentiation and cornified envelope formation. The observed reduction in
LCE expression may indicate a shift away from terminal differentiation toward earlier stages of keratinocyte maturation and renewal. This suggests that the extract may promote basal keratinocyte proliferation and regeneration, reducing the demand for late-stage barrier protein synthesis and potentially enhancing epidermal repair capacity.
The involvement of TM extract in keratinocyte-related pathways was confirmed by gene ontology analysis, which revealed that differentially expressed genes were predominantly involved in keratinization and keratinocyte differentiation, with similar enrichment in pathways related to epidermal differentiation and cornification (
Table 1).
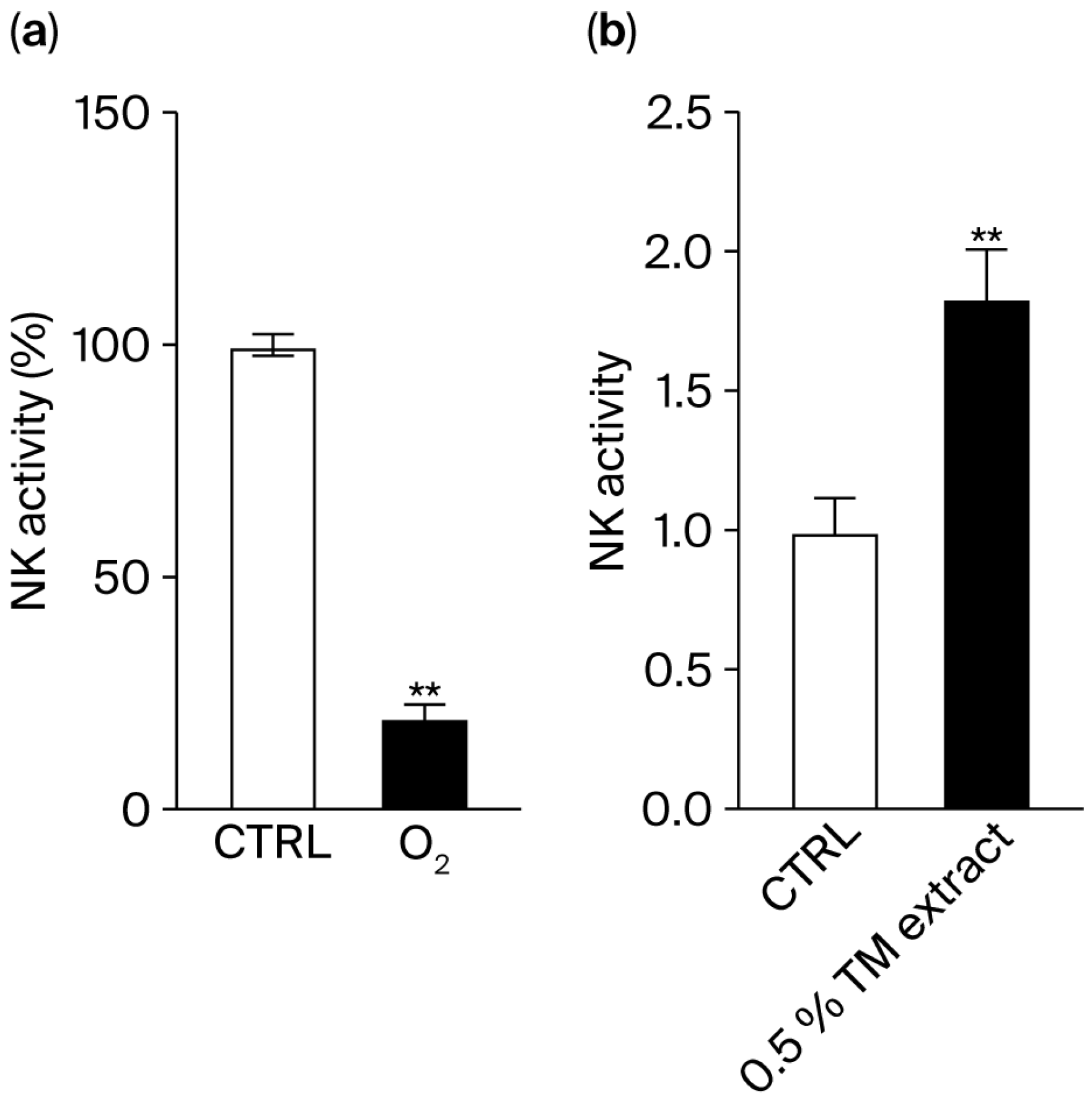
Natural killer (NK) cells are immune cells that can directly attack and eliminate their targets, such as tumoral cells and senescent cells. The release of perforins by NK cells was measured using a degranulation assay. When NK cells were incubated with target cells (K562 cells) in hypoxic conditions, an 80% decrease in their activity was observed (
Figure 8a), indicating that oxygen is essential for natural killer (NK) cell function. Within tumors, hypoxic conditions limit NK cell activity, reducing their ability to kill cancer cells. Therefore, we hypothesized that a plant extract such as that of
T. majus, which boosts cytoglobin and oxygen delivery, can restore NK cell cytotoxicity.
When the extract (0.5%
v/
v) was added to the culture in hypoxia, we observed an 84% increase in NK cell activity (
Figure 8b).
4. Discussion
Our results showed that the treatment of dermal fibroblasts and keratinocytes with a T. majus extract reduced ROS, potentially by stimulating an increase in CYGB protein. Fibroblasts treated with the T. majus extract showed an increase in CYGB expression. The overexpression of CYGB in dermal fibroblasts was associated with a decrease in cytosolic and mitochondrial ROS concentration. The treatment of cells with the extract improved several pathways altered by skin aging, as well as leading to a decrease in ROS levels in keratinocytes, improving the efficiency of mitochondria, and stimulating keratinocyte proliferation and the synthesis of elastin and collagen. In addition, the treatment with the extract caused a downregulation of genes involved in the control of the cornified envelope of keratinocytes and keratinocyte differentiation, and an increase in the activity of NK cells.
Chronological aging leads to ROS production and mitochondrial dysfunction. This, in turns, leads to reduced collagen synthesis and elastin degradation in the dermis [
1,
6]. Our data showed that the overexpression of CYGB in fibroblasts, and the treatment with an extract stimulating the production of CYGB in keratinocytes, had beneficial effects in countering ROS. This is in line with previous studies showing that CYGB can trap ROS [
18,
19]. In a follow-up study, it will be of significant interest to further evaluate the effect of CYGB overexpression in aged cells. In addition, we demonstrated that the extract improved mitochondrial efficiency by increasing the basal respiration rate and the production of ATP. This could help reduce the cellular effects of aging, as mitochondria are the main organelles affected by aging, with a decline in mitochondrial function and increased ROS production [
1,
20]. This is particularly important for the skin, as this organ relies heavily on mitochondria to produce the energy necessary for its constant renewal. Previous studies using a mouse model of mitochondrial DNA depletion have shown that the restoration of mitochondrial function leads to wrinkle reversal, highlighting the central role of mitochondria in skin aging [
20,
21]. Therefore, the extract may protect against and reverse signs of skin aging through a reduction in ROS levels and the improvement in mitochondrial function.
Our data also showed that the extract stimulated the synthesis of collagen and elastin, two major components of the ECM. The ECM, which plays a key role in maintaining dermal structure and elasticity, is severely affected by aging. The activation of matrix metalloproteinases (MMPs) by ROS stimulates collagen fragmentation and degradation, as well as elastin degradation. This diminishes fibroblast–ECM interactions, leading to a loss of structure, the appearance of wrinkles, and a reduction in skin elasticity, which are the main signs of skin aging [
6,
22,
23]. Therefore, the extract may contribute to the maintenance of the ECM during aging. In a further study, it would be interesting to measure the levels of MMPs after treatment with the extract to confirm if the increase in elastin and collagen observed here is mediated by a reduction in MMP levels.
As CYGB was reported to play a role in ROS defense [
17,
18], and the
T. majus extract increased the synthesis of CYGB, the actions of the extract described here on mitochondrial function and ROS are likely to be mediated by the increase in CYGB levels.
Our data showed a reduction in the expression of several genes involved in the regulation of keratinocyte differentiation and of the cornified envelope, which replaces the plasma membrane in differentiating keratinocytes.
Several of these genes are also involved in the protection against ROS. Firstly, an in vitro experiment showed that keratinocyte differentiation resulted in increased activity of ROS-detoxifying enzymes [
24]. ROS levels were reduced in fibroblasts and keratinocytes treated with the extract; therefore, the ROS-detoxifying activity of keratinocyte differentiation may not need to be stimulated.
GDF15, another gene downregulated by the treatment with the extract, is a marker of aging and senescence [
25,
26]. Its expression is induced by ROS [
27] and mitochondrial dysfunction [
28]. Here, as mitochondrial efficiency was increased and ROS levels were decreased following treatment with the extract, the expression of
GDF15 might not need to be upregulated. The reduction in
GDF15 gene expression may also indicate a decrease in the cellular effects of aging after treatment with the
T. majus extract. Finally, LCE3 members have been hypothesized to function as barrier repair proteins. Their expression is driven by pro-inflammatory cytokines [
29]. The expression of the
LCE3 gene was downregulated in young and old fibroblasts treated with the extract. As previously seen in cells treated with the extract, ROS concentration was decreased, preventing a ROS-induced inflammatory response; therefore,
LCE3 expression might not need to be upregulated.
In addition, the decrease in expression of genes involved in the regulation of the cornified envelope, keratinocyte differentiation, and the cross-linking of the cornified protein envelope might indicate a shift from the terminal differentiation stage of keratinocytes to earlier stages of epidermal turnover and regeneration, therefore reducing the need for cornified envelope formation. To confirm this hypothesis, it would be interesting to investigate the expression of genes involved in early stages of epidermal turnover.
Our data also indicated that the extract stimulated the activity of NK cells. NK cells are lymphocytes of the innate immune system able to clear senescent cells [
30]. Therefore, the
T. majus extract might induce senescent fibroblast clearance by stimulating NK cell activity in skin. This could be tested in a following study by measuring the activity of NK cells incubated with senescent fibroblasts, after treatment with the extract.