Abstract
This study investigates the development and evaluation of anti-photoaging creams enriched with natural extracts from avocado, apple, and kiwi by-products, with and without nanobubbles (NBs), focusing on their antioxidant, photoprotective, anti-inflammatory, and antiplatelet properties. Extract-containing creams showed significantly higher antioxidant capacity, particularly in the ferric reducing antioxidant power (FRAP) assay (S: 710.4 ± 344.3, NB: 566.3 ± 185.0, X: 202.8 ± 145.6 μmol TE/g DW at production; S: 631.7 ± 277.8, NB: 1019.3 ± 574.0, X: 449.8 ± 43.9 μmol TE/g DW after 1 month; p < 0.05), indicating up to a 250% improvement compared to the base cream and stable antioxidant activity during storage. The sun protection factor (SPF) increased in extract-containing creams after storage (8.7 ± 0.8 → 9.5 ± 0.6; p < 0.05). Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) with Strat-M® membranes revealed enhanced penetration of active compounds in enriched creams, while NBs did not significantly change absorption profiles. Platelet aggregation assays showed markedly lower half maximal inhibitory concentration (IC50) values in extract-enriched creams compared to the base cream for both the platelet-activating factor (PAF) pathway (S: 300.0 ± 42.0, NB: 258.0 ± 31.0 vs. X: 685.0 ± 35.0; after 1 month S: 325.0 ± 50.0, NB: 275.0 ± 42.0 vs. X: 885.0 ± 112.0; p < 0.05) and the adenosine diphosphate (ADP) pathway (S: 450.0 ± 65.0, NB: 400.0 ± 31.0 vs. X: 880.0 ± 58.0; after 1 month S: 470.0 ± 52.0, NB: 412.0 ± 42.0 vs. X: 1102.0 ± 125.0; p < 0.05). In silico analysis was also performed to demonstrate the ligand/protein complex with the strongest affinity to the PAF receptor. Overall, these findings highlight the potential of fruit by-products as sustainable, multifunctional cosmetic ingredients supporting circular economy principles.
1. Introduction
The need for sustainable development makes the transition to a circular economy model more important than ever, where the utilization of agri-food by-products plays a central role [1,2]. By-products from fruits, such as avocado [3,4], apple [5,6], and kiwi [7,8], which are rich in organic bioactives and especially in phenolic compounds, are often discarded, contributing to environmental issues with a release of unpleasant odors due to the persistence of these compounds against biodegradation. Their valorization for the production of natural extracts with antioxidant and anti-inflammatory properties could contribute both to environmental protection and the development of innovative bio-functional cosmetics and food products with increased added value and health-promoting properties [1,9,10,11].
Avocado by-products, including the kernel and peel, are particularly rich in phenolic bioactive compounds, polar lipids, carotenoids, and tocopherols, which have been reported to exhibit strong antioxidant, anti-inflammatory, and photoprotective activities in skin cells [3]. Apple by-products, such as the core (including calyx, seeds, and stem), contain high concentrations of fibers, polysaccharides, and polar phenolic compounds, which have been shown to protect dermal fibroblasts against oxidative stress and modulate inflammatory responses [5,6]. Kiwi peel and seeds are rich in vitamins, polyphenols, and antioxidant molecules that contribute to strengthening the skin barrier and regulating UV-induced inflammation and inflammatory signaling [7,8]. Previous studies have demonstrated the efficacy of avocado, apple, and kiwi by-product extracts in topical formulations, reporting improvements in skin hydration, elasticity, and protection against oxidative and UV-induced damage [3,5,7]. These findings highlight their potential as multifunctional, sustainable ingredients for advanced cosmetic products.
The high phenolic and carotenoid content of avocado, apple, and kiwi by-products contributes to strong antioxidant activity, helping to neutralize reactive oxygen species (ROS), a major factor in premature skin aging and oxidative damage [12]. By reducing oxidative stress, these compounds help maintain the integrity and health of skin cells [13].
The natural antioxidant and phenolic compounds in the extracts also help reduce photoaging by limiting damage from ultraviolet (UV) radiation. They can absorb or reflect part of the UV radiation and attenuate the associated inflammatory and oxidative responses, thereby enhancing the skin’s photoprotective capacity [1,9,13,14].
Oxidative stress and chronic inflammation are two interconnected pathological mechanisms involved in the onset and progression of many chronic diseases. While inflammation is a normal defensive response of the body, its prolonged duration can lead to damaging effects on tissues, propagating the onset and development of inflammation-related chronic disorders, including melanoma and skin cancer [15].
PAF is a lipid mediator with potent pro-inflammatory activity, produced under inflammatory and oxidative stress conditions. Among other inflammatory cellular responses, PAF also promotes platelet aggregation and microvascular dysfunction and, together with other platelet agonists like ADP, amplifies inflammatory and thrombotic responses [11,12]. PAF and related inflammatory mediators are implicated in skin microcirculation, erythema, and photo-inflammation in tumor onset, development, and metastatic processes, including those of skin tumors and melanoma [16]. Thus, downregulating platelet aggregation via inhibiting the PAF/ADP pathways may indicate potential for reducing UV-induced inflammatory skin damage [16], extending the concept of “cosmeceutical” activity. Accordingly, natural compounds such as phenolic compounds and polar lipids, found in plant extracts, have demonstrated the ability to inhibit platelet aggregation induced by either PAF or ADP [4,5,6,8,16]. The close interplay between inflammation, oxidative stress, and platelet activation highlights the importance of developing natural bioactive substances with multifunctional properties—anti-inflammatory, antioxidant, and antiplatelet—which can be effectively incorporated into cosmetic formulations for enhanced protective and revitalizing effects on the skin [15,16].
The ability of these active compounds to penetrate the skin, due to their low molecular weight and amphiphilic nature, is critical for their effective action in deeper skin layers [16]. Altogether, using such a natural blend supports the development of cosmetic products with enhanced protective and revitalizing properties, addressing modern demands for sustainable and effective solutions [17].
In the context of enhancing the effectiveness of cosmetic formulations based on natural extracts, the use of NBs has emerged as a promising technological approach for the transdermal delivery of active compounds. NBs are spherical gas cavities with diameters smaller than 200 nm, characterized by an exceptionally large surface area and remarkable stability in aqueous solutions [18,19]. These features make NBs ideal carriers for the gradual release of bioactive substances and for improving their bioavailability. Moreover, their ability to penetrate deeper skin layers without the need for aggressive chemical enhancers or invasive techniques makes them highly suitable for cosmetic applications [20].
Although their exact mechanism of action has not been fully elucidated, preliminary findings indicate that NBs can enhance the transport of active ingredients into the skin, improving both penetration and performance of enriched formulations. In this study, our working hypothesis was that NBs, due to their exceptionally high surface area and stability, could facilitate the deeper penetration of both hydrophilic and amphiphilic bioactive compounds, thereby enhancing antioxidant, anti-inflammatory, and photoprotective efficacy in topical formulations. The use of NB-enriched water without the need for complex chemical processes or additional agents aligns with the principles of green chemistry and sustainable innovation [19,21]. Therefore, their incorporation into cosmetic products represents a modern, gentle, and technologically advanced strategy that can significantly improve both the biological efficacy and safety of the final products.
The present study aims to develop and evaluate anti-photoaging cosmetic formulations enriched with natural extracts derived from fruit by-products—specifically from avocado, apple, and kiwi. These by-products, although often discarded, are rich in bioactive compounds with well-documented beneficial effects on skin health and function. The innovation of the study lies both in the synergistic use of the three extracts as a unified botanical complex and in the incorporation of NBs as carriers to optimize the transdermal absorption of the active ingredients.
Τhe research focuses on the systematic investigation of key properties of the formulations, such as antioxidant, photoprotective, anti-inflammatory, and antiplatelet activity, as well as the skin permeation capacity of the bioactive. At the same time, the study highlights the relevance of this approach within the framework of the circular economy, by valorizing agri-food residues for the development of innovative, environmentally friendly cosmetic products with high added value.
2. Materials and Methods
2.1. Materials, Reagents, and Instruments
The extracts used were derived from avocado, apple, and kiwi by-products, as analyzed in previous studies [4,5,7], and were obtained through drying of the by-products, ethanol–water maceration, filtration, and rotary evaporation, following our previously validated methods. The other raw materials for the preparation of the cosmetic cream were procured from certified distributors of cosmetic-grade raw materials. All reagents, as well as solvents and phenolic and lipid standards, were purchased from Sigma Aldrich (St. Louis, MI, USA).
Additionally, NBs were produced through hydrodynamic cavitation using a Venturi system. The liquid and gas flow rates were controlled via an in house developed mass flow controller, allowing the formation of a stable, high-density NB solution [19].
UV-Vis spectroscopy analysis was performed with an LLG- uniSPEC 2 spectrophotometer (lab Logistics Group GmbH, Meckenheim, Germany), while ATR-FTIR spectroscopic analysis was performed with a Perkin Elmer Frontier ATR/FT-NIR/MIR spectrometer (Perkin Elmer, Waltham, MA, USA). Samples were centrifuged using an Eppendorf 5702R centrifuge (Eppendorf Ltd., Stevenage, UK).
For the evaluation of antiplatelet activity, concentrated platelet-rich plasma (hPRP) was used, which was prepared by centrifugation of blood samples. Centrifugations were performed on an Eppendorf 5702R (Eppendorf Ltd., Stevenage, UK).
Antiplatelet activity assays were performed using the Chronolog-490 platelet aggregation tholometer (Chronolog, Havertown, PA, USA), in conjunction with the accompanying AGGRO/LINK®8 software. PAF and bovine serum albumin (BSA) standards from Sigma Aldrich were used to study PAF pathway inhibition, while ADP was supplied by Chronolog (Havertown, PA, USA).
2.2. Cosmetic Formulations
The cosmetic cream was prepared as an oil-in-water (O/W) emulsion through emulsification of the aqueous and fatty phases, following a process of thermal mixing and gradual addition of active ingredients. Nine cream samples were prepared: three without extract (control cream X), three with extract (S), and three with extracts and NBs. The procedure for the preparation of the samples without an extract followed the basic methodology. In the S samples, the mixture of avocado, apple, and kiwi extracts was additionally incorporated at the stage of adding the active ingredients. Finally, in the samples with NBs, the deionized water of the aqueous phase was completely replaced with natural air-based NBs-enriched deionized water.
The process began with the preparation of the aqueous phase. Deionized water (64.5%) and disodium EDTA (0.1%) were added to a beaker under continuous stirring. In a separate beaker, xanthan gum (0.4%) was incorporated into glycerol (6.0%) and stirred until completely dissolved. This mixture was added to the aqueous phase under vigorous stirring, forming a homogeneous gel, which was heated to 70 °C.
At the same time, the fatty phase was prepared, which included cetearyl alcohol (5.0%), almond oil (5.0%), and wheat germ oil (5.0%), which were selected for their skin-conditioning properties and favorable fatty acid profiles, as well as a mixture of Cetearyl olivate and sorbitan olivate (2.5%), chosen as a natural emulsifier system with good skin compatibility. The fatty ingredients were heated in a separate vessel at 70 °C until fully homogenized. When both phases reached the same temperature, the fatty phase was added to the aqueous phase under vigorous stirring and homogenization. Stirring was then maintained at a controlled speed until the emulsion cooled to room temperature.
In the next step, the active ingredients were added, including vitamin E (0.5%) and sodium hyaluronate (0.1%) for their synergistic antioxidant and moisturizing effects, zinc oxide (10.0%) as a physical UV filter, and a mixture of phenoxyethanol and caprylyl glycol (0.9%). For the samples containing extract (S), a mixture of avocado, apple, and kiwi extracts was added in a ratio of 1:5:10. This ratio was established based on preliminary in-house antioxidant screening of individual extracts and adjusted according to their total phenolic content to achieve balanced contributions from all three sources. The mixture contained 10.2 mg of avocado by-product extract, 52 mg of apple by-product extract, and 106 mg of kiwi by-product extract (Table 1). Finally, the homogeneity of the product was checked, and the pH was adjusted to the desired range (5.0–5.5) using the appropriate buffer.

Table 1.
Composition of the cream formulation: ingredients, functions, and concentrations (% w/w).
This methodology ensured the stability and homogeneous distribution of the active ingredients in the creams, allowing for comparison among samples without extracts, with extracts, and NBs.
The cosmetic formulations were subjected to appropriate pre-treatment in order to perform the analysis. In particular, an extract of 1 g of each cream sample was prepared in 10 g of ethanol. The mixture was subjected to sonication for 5 min in order to enhance the extraction of the bioactive ingredients [21]. This was followed by filtration to remove insoluble residues. The filtrate obtained was either used as is or further diluted (1 mL of filtrate in 10 mL of ethanol), depending on the requirements of each analytical method.
2.3. Total Antioxidant Activity (TAA)
The antioxidant activity of the samples was evaluated by two different methods: the 2,2-diphenyl-1-picrylhydrazyl radical binding (DPPH) method and the antioxidant capacity method (FRAP). The procedure was performed as previously described [4,5,6,8].
The antioxidant activity of the samples was evaluated by the DPPH radical binding method. For this analysis, 0.2 mL of ethanol, 0.8 mL of Tris-HCl buffer (pH 7.4), and 1 mL of DPPH solution were added to each sample. Vortex stirring was performed between successive additions of the reagents to ensure homogeneous mixing of the solutions. The samples were incubated at room temperature for 30 min, and then their absorbance at 517 nm was recorded. The percentage of inhibition (%) of the DPPH radical was calculated according to the following equation:
where A1 is the absorbance of the control solution and A2 is the absorbance of the test sample.
Inhibition (%) = (A1 − A2) × 100/A1,
Subsequently, the IC50 value, i.e., the concentration of extract required to neutralize 50% of the DPPH radical, was calculated. The antioxidant activity was expressed as Trolox equivalent (TEAC), which was calculated by the following equation:
TEAC = IC50 of Trolox (μg/L)/IC50 of sample (μg/L).
All measurements were performed on triplicate samples, and results were expressed as mean ± standard deviation.
For FRAP determination, 3 mL of FRAP solution was transferred to each sample, followed by vortex stirring. The solutions were incubated in the dark at ambient temperatures for 15 min, and immediately afterwards their absorbance at 593 nm was measured. Trolox was used as a standard. The concentration of Trolox was chosen under the conditions of an absorbance value ranging from 0.2 to 0.8 for the standard curve. The results were expressed as μmol TE/g DW, according to the following formula:
where c is the Trolox concentration (μmol/mL) of the corresponding standard curve of the diluted sample, V is the sample volume (mL), t is the dilution factor, and m is the dry weight of the sample (g).
FRAP (μmol TE/g DW) = c × V × t/m,
2.4. Measurement of Sun Protection—SPF Calculation
The estimation of the SPF was performed by UV-Vis spectroscopy, recording the absorption of the samples at wavelengths from 290 to 320 nm, every 5 nm. To prepare the samples, 1 mL of cream was diluted in 10 mL of ethanol, and the solution was subjected to ultrasound for 5 min to ensure homogeneous particle distribution, followed by filtration. Measurements were performed on a UV-Vis spectrophotometer using 1 cm quartz cells, with ethanol used as a blank sample. SPF was calculated using the Mansur equation:
where CF is a correction factor (=10), EE(λ) is the spectrum of erythematogenic effect, i.e., the effectiveness of UVB radiation in causing redness and inflammatory reaction in the skin; I(λ) is the intensity spectrum of solar radiation; and Abs(λ) is the absorbance of the cream at the respective wavelengths.
SPF = CF × ∑ EE(λ × I(λ) × ABS (λ),
As described in the literature [22], the constant values of the coefficients are shown in Table 2.

Table 2.
Parameters used to calculate the SPF [19].
2.5. ATR-FTIR Analysis
This method involves passing a beam of infrared radiation through a high refractive index crystal, while a small portion of the radiation is absorbed by the sample in contact with the crystal. The absorption of the radiation causes vibrations in the chemical bonds of the sample, creating a spectrum that is recorded and analyzed. This spectrum provides information about the chemical composition of the sample, such as bond types, functional groups, and molecular orientation. The ATR technique is particularly useful for the analysis of materials without special preparation, as it requires a minimal amount of sample and is non-destructive. In the present study, a small amount of cosmetic cream was used and applied to a Strat-M® synthetic membrane. The ATR-FTIR spectroscopy technique (Perkin Elmer Frontier ATR/FT-NIR/MIR spectrometer) was applied for the analysis in the wavenumber range 4000–600 cm−1 [23]. The Strat-M® membrane was used as an alternative model of the Stratum Corneum (SC) (Figure 1), allowing the simulation of transdermal absorption of the active ingredients. Spectroscopic analyses were performed on both the smooth and rough sides of the membranes.
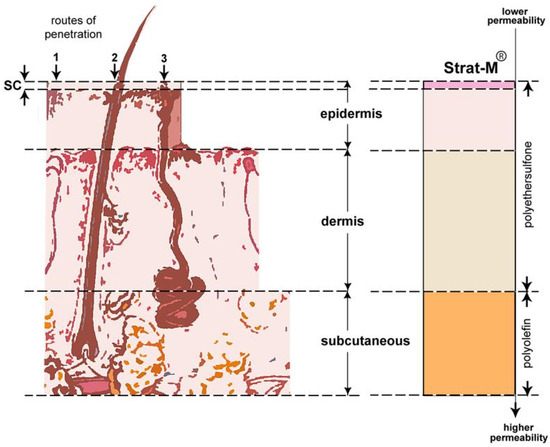
Figure 1.
Regions of human skin versus Strat-M® membrane. Left (regions of human skin): upper layer epidermis with penetration pathways (1) stratum corneum (SC), (2) hair follicles, and (3) sweat glands; middle layer dermis; lower layer of subcutaneous adipose tissue. Right (Strat-M®) upper layer made of polyether sulfone with lower permeability; lower layer made of polyolefin with higher permeability [19].
The edge of the crystal was adjusted to touch the membrane. As soon as they come into contact, a green line appears on the force meter, and the applied force is increased through the rotating tower until the spectrum displayed on the work surface is stabilized.
2.6. Antiplatelet and Anti-Inflammatory Properties
The antiplatelet and anti-inflammatory activity of the extracts was evaluated by inhibiting the activation and accumulation of human platelets. Tests were performed against the inflammatory and thrombotic mediator PAF, as well as the agonist ADP. The method was performed as previously described [4,5,6,8]. For this evaluation, an in vitro study of platelet aggregation was performed using platelet-rich plasma (PRP) and platelet-poor plasma (PPP) isolated from human blood samples, which were obtained from healthy adult volunteers at the General Hospital of Kavala (Ethics approval code ΔΠΘ/ΕHΔΕ/7690/70). The effect of the products was examined before and after incubation of platelets with different concentrations of the tested products to determine their possible inhibitory effect on platelet activation.
The study was performed using the Light Transmission Aggregometry (LTA) method, which is based on the change in optical permeability of PRP as an indication of platelet accumulation. Tests were performed with a Chrono-log 490 aggregometer, where platelet aggregation curves were recorded after the addition of PAF and ADP agonists, both in the presence and absence of products. The antiplatelet and anti-inflammatory activity of the products was quantified by means of the IC50 value (semi-inhibitory concentration), which represents the concentration of product required to reduce platelet aggregation by 50%. IC50 values were used to estimate the inhibitory effect of creams with and without extracts. Lower IC50 values indicate a stronger inhibitory effect, whereas higher values are associated with reduced efficacy. This approach allows for the evaluation of the anti-inflammatory and antithrombotic potential of different formulations.
2.7. Statistical Analysis
The Kolmogorov–Smirnov test was applied to assess whether the parameters follow a normal distribution or not. Parameters that showed a normal distribution were compared by ANOVA, while those that did not meet this condition were analyzed by non-parametric techniques using the Kruskal–Wallis test. A difference between parameter values was considered statistically significant when the p-value was less than 0.05. The data were analyzed using a statistical software package (IBM-SPSS Statistics 26 for Windows, SPSS Inc., Chicago, IL, USA).
2.8. Molecular Docking Process
Molecular docking has emerged as a useful tool in the discovery and study of active substances, as it enables the rapid collection and prediction of interactions between the selected active substances under study and the target proteins [24]. The principle of CB-Dock2 software is based on calculating the curvature of the surface of the selected target protein to identify potential binding sites and then grouping the curvature data into clusters. The clusters constitute the candidate binding cavities. The cavities are then detected and classified. Usually, as in the present analysis, the 5 best clusters are selected and investigated for docking. Then, for each cavity, its geometric center and dimensions are calculated in order to cover it adequately without unnecessary space by adjusting the size of the docking box. After the ligand is introduced, docking is performed for all cavities, and the different binding positions (poses) are calculated, which are then re-ranked, and the optimal one is selected based on the binding affinity. Protein–ligand binding is widely used to predict the binding modes and affinities of ligands and to investigate and discover drugs using computers. The binding site is represented as a cubic box, which defines the boundaries of the sampling space. In most cases, the binding sites are unknown. While blind docking is less accurate than normal docking, it is valuable for discovering unexpected interactions in unrecognized binding modes. CB-Dock2 is an extremely useful tool, as it offers an interactive 3D visualization of blind docking and protein cavity scanning results and is available free of charge [25].
2.8.1. Ligand Preparation
Three representative ligands were selected (Table 3) from the PubChem Compound Database, and their 3D structure was stored as a Spatial Data File (.SDF) for each fruit extract (apple, avocado, and kiwi). The selected ligands were Phloretin from apple, Rutin from kiwi, and Avocatin B from avocado.

Table 3.
The three candidates and most frequently occurring phytochemicals selected for docking analysis and the fruits in which they are found, according to [3,5,7].
2.8.2. Protein Target Selection
The human Platelet-Activating Factor Receptor (PAFR, UniProt ID: P25105) was selected as the receptor for performing the docking analysis, as it was also the target protein of the experimental analysis and plays a central role in platelet activation and inflammation. Its 3D structure was retrieved from the AlphaFold Protein Structure Database (PSD) as a PDB file (Figure 2).
Figure 2.
Three-dimensional structure of human Platelet-Activating Factor Receptor (AlphaFold Protein Structure Database).
2.8.3. Molecular Docking of Ligands with the Protein Receptor
After selecting the candidate ligands and receptors, docking analysis was performed for each of the three substitutes using the CB-Dock2 (cavity-detection-guided blind docking) tool. Usually, as in the present analysis, the 5 best clusters are selected and investigated for docking. Then, for each cavity, its geometric center and dimensions are calculated in order to cover it adequately without unnecessary space, by adjusting the size of the docking box. After the ligand is introduced, docking is performed for all cavities, and the different binding positions (poses) are calculated, which are then re-ranked, and the optimal one is selected based on the binding affinity.
3. Results and Discussion
3.1. Antioxidant Activity Results
3.1.1. Antioxidant Capacity According to the DPPH Assay
DPPH determination was applied to evaluate the antioxidant activity of cosmetic creams by testing the ability of their ingredients to neutralize DPPH free radicals. During this process, the color of the solution changes from purple to yellow, which can be detected by spectrophotometric analysis. The more pronounced the color change, the greater the antioxidant capacity of the tested substance [26].
In the present study, all three samples of cosmetic creams, without extracts, with extracts, and with NBs were evaluated at two time periods: on the day of production and after one month (30 days) of storage. The results of the DPPH test are presented in Table 4.

Table 4.
Comparison of the antioxidant capacity of cosmetic creams without extracts, with extracts, and with NBs by the DPPH method before and after 30 days of storage.
Initially, cream X showed a higher value of DPPH antioxidant activity compared to cream S; however, the difference was not statistically significant. Even after one month of storage, the antioxidant activity of cream X decreased (34.0 ± 11.4), while cream S, on the contrary, showed a slight increase (34.9 ± 18.4), outperforming the base cream. However, the difference was not statistically significant. These results indicate that the presence of natural bioactive agents from extracts of apple, kiwi, and avocado by-products in the cream did not bring about a change in the antioxidant activity of the base cream, but a generally better stability of its antioxidant capacity over time. This result also comes in accordance with other extracts from these sources that were rich in phenolic antioxidants [27,28,29]. The NB sample initially exhibited the lowest TEAC value and, although it showed an upward trend after one month, it still remained the lowest among all samples, without a statistically significant difference (24.9 ± 11.5).
The addition of natural extracts of avocado, kiwi, and apple to the cosmetic creams S and NB was expected to enhance their antioxidant activity, due to the high content of polyphenols and carotenoids in the extracts, which have been shown to have a strong antioxidant potential [3,5,7]. Carotenoids neutralize free radicals through physical quenching and chemical reactions, while polyphenols act as potent electron donors, inhibiting oxidative reactions [30,31,32,33]. However, the DPPH value recorded for the enriched cream in the present study did not differ significantly compared to the base cream, suggesting that the complexity of these natural extracts may have led to interactions with the lipids in the cream, altering their immediate availability for reaction with the DPPH radical.
After one month of storage, the antioxidant capacity of cream X had a decreasing trend, probably due to increased lipid oxidation and the absence of additional antioxidant components. In contrast, cream S and NB showed a tendency to maintain and enhance their antioxidant activity, which may be attributed to the stabilizing effect of the natural extracts, as shown in previous studies for extracts from these fruits in cosmetic products [27,28,29]. Polyphenols and carotenoids probably slowed down the oxidative processes, contributing to the maintenance of the overall antioxidant capacity of the product, as also observed in other studies for such natural bioactives in cosmetic applications [32,33].
3.1.2. Antioxidant Capacity According to the FRAP Assay
The FRAP (Ferric Reducing Antioxidant Power) assay was used to evaluate the antioxidant capacity of cosmetic creams, those without extracts, those containing extracts, and NBs. The analysis is based on the ability of the antioxidant components to reduce Fe3+ ions to Fe2+ ions, allowing quantification of the overall reducing activity of the samples under consideration [4,5,6,8].
The results of the FRAP analysis confirm the high antioxidant capacity of the extracts, as the creams containing extracts showed significantly higher FRAP values compared to the creams without extracts, as shown in Table 5. This result also comes in accordance with the ones previously observed in other studies where extracts from these fruits were also utilized as functional ingredients for several cosmetic applications [27,34,35,36].

Table 5.
Comparison of the antioxidant capacity of cosmetic creams without extracts, with extracts, and with NBs by the FRAP method before and after 30 days of storage.
Specifically, the FRAP value of the antioxidant activity of cream S on the day of production was 710.4 ± 344.3, and that of cream NB was 566.3 ± 185.0, both significantly higher and statistically different compared to cream X (202.8 ± 145.6). After one month of storage, the values stabilized across all three samples, with the extract-containing cream (S) and the NB cream maintaining higher antioxidant activity (631.7 ± 277.8 and 1019.3 ± 574.0, respectively) compared to cream X (449.8 ± 43.9).
The retention of antioxidant activity observed in both creams after one month of storage can be attributed to the stabilization of antioxidant compounds in the product [33]. However, cream S showed higher reducing capacity, suggesting that the polyphenolic and carotenoid components of the extracts contributed to additional protection against oxidative degradation, maintaining the antioxidant efficacy of the product over time.
The FRAP (Ferric Reducing Antioxidant Power) method differs from the DPPH test, which evaluates the ability to neutralize free radicals through electron or proton transfer to stable synthetic radicals. In contrast, FRAP determines the reductive capacity of samples, i.e., their ability to donate electrons and reduce Fe3+ (trivalent iron) ions to Fe2+ (divalent iron) [4,5,6,8,26]. The FRAP mechanism is a purely electron transfer process rather than a mixed SET and HAT mechanism. When used in combination with other methods, it can be very useful in distinguishing the dominant mechanisms of different antioxidants. Moreover, it has been argued that the ability to reduce iron has little relationship to the radical quenching processes (H transfer) mediated by most antioxidants. However, oxidation or reduction of radicals to ions still stops radical chains, and reducing power reflects the ability of compounds to modulate redox tone in plasma and tissues [37].
This reflects the process more realistically under physiological conditions in vivo, as antioxidant activity in the human body is not limited to free radical neutralization but also includes the maintenance of redox balance, which is crucial for biomolecule stability and proper cell function. Therefore, the FRAP method provides a reliable estimate of the overall reducing capacity of antioxidant compounds contained in cosmetic creams, revealing their potential role in maintaining skin health and protecting against oxidative stress.
Thus, the higher antioxidant capacity observed in the FRAP determination for the creams with extracts suggests their enhanced antioxidant activity under conditions closer to the in vivo environment. Extracts appear to act protectively, providing stable and long-lasting antioxidant activity, which may contribute to maintaining skin health and improving the resistance of cosmetic products against oxidation.
3.2. Measurement of Sun Protection Action—SPF Calculation
The Table 6 shows the sun protection factor (SPF) measurements of cosmetic creams, both during production and after one month of storage. In particular, the cream without extract (X) initially showed a higher SPF (9.5 ± 0.6) compared to the cream with extract (S) (8.7 ± 0.8). However, after one month, the SPF of cream X decreased (8.7 ± 0.8), while in contrast, the SPF of cream S increased (9.5 ± 0.6). Moreover, the SPF of NB cream was higher than that of X, and it could also be maintained at the same level (9.5 ± 1.0).

Table 6.
Comparison of sunscreen action of cosmetic creams without extracts, with extracts, and with NBs by calculating the sun protection factor SPF, before and after 30 days of storage.
The observed SPF reduction in the cream without extract may result from the degradation of the sunscreen filters or the natural deterioration of the ingredients during storage. On the contrary, the increase in SPF in the cream with extract suggests a possible stabilizing effect of the natural antioxidants contained in the avocado, apple, and kiwi by-products’ extracts, which probably contributed to maintaining or enhancing the protective capacity against UV radiation.
Avocado oil and extracts have shown potential for anti-UV protection in cosmetics due to their rich content of antioxidants, vitamins, polyphenols, and fatty acids [3,4], which offer photoprotective benefits by absorbing UV radiation and acting as antioxidants to combat skin damage. Research supports avocado’s use in sunscreens and skincare, with studies demonstrating its efficacy as a natural sunscreen agent [38,39,40] However, its SPF may be low on its own, as also found in the present study, which indicates that it often requires combination with other ingredients for significant UV protection.
Similarly, even though apple extracts have been proposed as ingredients that induce photoprotection [5], low SPF values between 0.51 and 0.90 have also been previously observed for cosmetic emulsions containing different concentrations of hydroalcoholic apple extracts (10–40%) [41]. Again, it is suggested that apple extracts also require the addition of other ingredients in cosmetic anti-UV formulations for achieving a significant increase in the SPF, in order to maximize their photoprotective benefits. Kiwi has also been suggested to possess several natural antioxidants with anti-aging benefits and anti-UV protection [42], but again, no results on the increase in SPF by such kiwi bioactives have been studied so far.
Overall, the comparison of the previously reported results for the SPF in cosmetic products containing extracts from these fruits with those obtained from the present study further indicates that the bioactives of apple and avocado extracts present in the S cream seem to enhance and preserve its SPF values over time, in contrast with the base cream that did not contain such extracts.
Independent of the observed changes in SPF, plant-based compounds such as flavonoids can absorb UVA and UVB rays and possess antioxidant, anticarcinogenic, and anti-inflammatory effects that contribute to photoprotection [43]. Apart from flavonoids, other natural products such as certain vegetable oils, carotenoids, stilbenes, and ferulic acid also have UV-absorbing properties. Thus, it is also highly possible that the presence of carotenoids in these extracts and in the S cream, while absent from the X cream, also contributes to the preservation and enhancement of the SPF value only in the S cream, since most carotenoids have absorption peaks in the visible range, but their conjugated system enables them to also absorb in shorter wavelengths, including UV light [32,43].
In addition, phenolic bioactives that are present in high concentrations in extracts from these fruits, such as phloretin, rutin, and especially flavonoids like quercetin, possess UV-absorbing properties (primarily UVA radiation) [44], thereby directly preventing the UV-induced formation of ROS and direct DNA damage [43]. Moreover, such bioactives possess potent antioxidant activities (i.e., they inhibit lipid peroxidation, and bind transition metal ions to form inert chelate complexes) [43], as well as anti-inflammatory and antithrombotic properties [44], which along with their antioxidant protection could also contribute to indirect protective cellular responses against UV-induced damage (i.e., they reduce the UV-induced oxidative stress and its associated malondialdehyde production, and also prevent the decrease in activities of antioxidant enzymes like glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase after UVA irradiation). Furthermore, their ability to enhance in synergy the antioxidant and anti-inflammatory activities of other compounds that do not possess direct anti-UV effects [44] further suggests that their presence in cosmetic applications seems to enhance the anti-UV capabilities of the overall formulation and thus the overall sun protection of these products [43].
As for NBs, it appears that they can improve the stability of sunscreen filters, protecting them from degradation and maintaining their effectiveness over time.
3.3. ATR-FTIR Analysis of Cosmetic Creams on Synthetic Membrane
ATR-FTIR analysis was used to find the bioactive components and study the absorption of cosmetic creams through the Strat-M® synthetic membrane, which simulates transdermal absorption and is used as an alternative model of the Stratum Corneum (SC).
For the experimental procedure, all three types of cosmetic cream were applied: one without extracts (X), one with extracts (S), and one with extracts and NBs. Each cream was applied on the smooth side of the membrane, and measurements were performed on both sides (smooth and rough) at different times after application: immediately after (0 min), 30 min, and 90 min. The experiment was repeated after one month of storage of the creams, with additional application on the smooth side of the same membranes.
In addition, an unused Strat-M® membrane was used as a reference to compare the spectra and detect possible differences resulting from the absorption of the cream components. While Strat-M® provides a reproducible and standardized in vitro model, there are no published data directly comparing its performance with that of porcine skin, which remains the recommended choice for assessing dermal absorption due to its similarity to human skin in terms of permeability, thickness, and lipid composition [27]. Full validation of Strat-M® for cosmetic ingredient permeability assessment requires understanding its similarities to porcine skin and establishing equivalence in key parameters such as permeability coefficient, flux, and penetration depth of active ingredients [28].
Figure 3a,b show the FTIR spectra of Strat-M membranes from the smooth and rough sides, respectively, exposed to the cream without extract immediately after application, while Figure 3c,d show the spectra after one month of cream storage. Similarly, Figure 4a,b represent the FTIR spectra of Strat-M membranes exposed to the cream with extract immediately after production, while Figure 4c,d show the spectra after one month of storage. Finally, Figure 5a,b show the spectra of Strat-M membranes from the smooth and rough side of the membrane, respectively, exposed to the cream with extract and NBs immediately after production, while Figure 5c,d show the spectra after one month of storage. The spectra of the clean membranes (STRAT_M_CLEAN) were used as a reference, allowing comparison with the treated membranes.
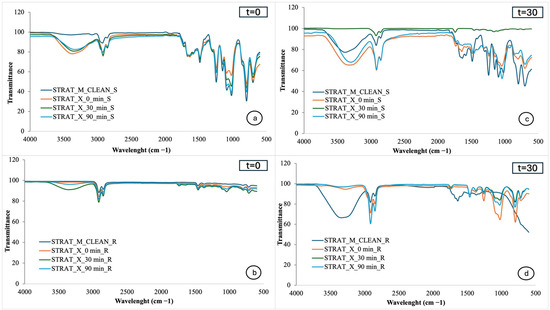
Figure 3.
FTIR spectra of Strat-M® with cosmetic cream without extracts: (a) smooth side, time 0; (b) rough side, time 0; (c) smooth side, after one month; (d) rough side, after one month. Absorbance vs. wavenumber (cm−1).
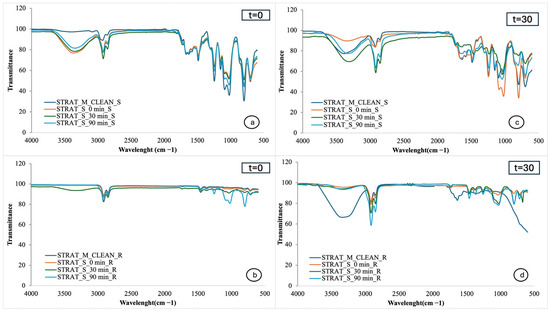
Figure 4.
FTIR spectra of Strat-M® with cosmetic extracts: (a) smooth side, time 0; (b) rough side, time 0; (c) smooth side, after one month; (d) rough side, after one month. Absorbance vs. wavenumber (cm−1).
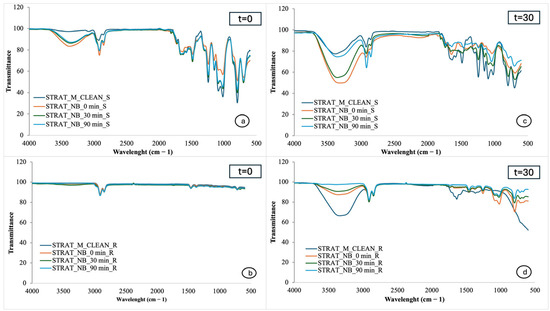
Figure 5.
FTIR spectra of Strat-M® with cosmetic cream with extracts and NBs: (a) smooth side, time 0; (b) rough side, time 0; (c) smooth side, after one month; (d) rough side, after one month. Absorbance vs. wavenumber (cm−1).
In the spectra of the smooth side of the Strat-M® membrane (Figure 3a,c), the peaks appear broadly similar to those of the clean membrane (STRAT_M_CLEAN_S), suggesting limited structural changes on the membrane surface. However, there is a distinct enhancement of the peak in the 3330–3370 cm−1 region in the cream-exposed samples, which is associated with vibrations of the -OH groups and suggests hydration of the membrane [4]. This change becomes more noticeable over time, especially after 30 and 90 min, and after one month of storage, which is an indication of gradual absorption of cream components from the smooth membrane surface. This absorption is linked to the presence of hydrophilic components, which enhance the hydration of the membrane.
However, the discrepancy observed in the 30 min spectrum in Figure 3c may be due to the selection of an unrepresentative membrane spot during measurement. Possible microdefects or surface irregularities on the surface of this particular section may have affected absorption or contact with the ATR crystal, leading to a different spectrum recording compared to the other samples.
Figure 3b shows the FTIR spectra of the rough side of the Strat-M membrane immediately after preparation of the cream without extracts (0, 30, and 90 min). The spectra show very little variation compared to the clean sample (STRAT_M_CLEAN_R), suggesting that the cream does not significantly affect the structure of the membrane, i.e., it does not appear to penetrate deeply into the deeper layers of the epidermis simulated by the rough side of Strat-M®.
Figure 3d shows the FTIR spectra of the rough side of the Strat-M® membrane after one month of storage of the cream without extracts. In contrast to Figure 3b, noticeable changes in the peaks of the spectra are observed. In particular, there is an enhancement of the peak at 3370 cm−1, which is attributed to the hydroxyl (-OH) groups and is associated with the presence of phenolic compounds or water-soluble moisturizing agents such as glycerol and sodium hyaluronate [4]. The 2920–2850 cm−1 region shows enhancement of the peaks, which are attributed to asymmetric and symmetric vibrations of the alkyl bonds CH2 and CH3 [4]. These vibrations are indicative of the presence of polar lipids, such as the vegetable oils present in the cream composition, in particular wheat oil and almond oil. Finally, in the 1500–1000 cm−1 region, peaks appear that are attributed to C-O and C-O-C bonds associated with ethers and esters. These bonds are associated with the presence of esterified polar lipids, as well as phenolic components [4].
In Figure 4a, the peaks appear broadly similar to those of the pure membrane. However, there is an enhancement of the peak in the 3330–3370 cm−1 region, which is associated with the tensor vibrations of the hydroxyl (-OH) groups and is attributed to increased hydration of the membrane [4]. This hydration is probably due to both the presence of phenolic compounds present in avocado, apple, and kiwi extracts (such as gallic acid, tannins, and flavonoids) and to water-soluble hydrating components of the cream base, such as glycerol [4].
Figure 4c shows that the spectral changes become more pronounced after 1 month of storage of the creams. The peak in the 3330–3370 cm−1 region remains enhanced, confirming the preservation of the hydration provided by the cream even after 1 month of storage. At the same time, the CH bond regions (2920–2850 cm−1), associated with the presence of lipids, carotenoids, and fatty acids, and the 1500–1000 cm−1 region, attributed to C-O and C-O-C bonds, characteristic of esterified polar lipids and phenolic compounds, are enhanced [4].
An important difference is also observed in the spectra of the smooth side of the membrane, where the cream without extract and the cream with extract were applied. On the day of preparation, the spectra of the two samples show only slight differences, with the extract-containing cream showing slightly more intense peaks, indicating that some components of the extract are already beginning to interact with the membrane. However, after a month of storage, the difference between them becomes more pronounced. The cream with extracts seems to cause more hydration of the membrane and leave more ingredients on the surface, compared to the cream without extracts. This is probably due to the presence of phenolic and lipid compounds contained in the plant extracts (avocado, apple, kiwi), which enhance the chemical action of the cream. Also, the more pronounced effect after a month can be explained by the gradual stabilization and activation of these components within the composition of the cream.
Figure 4b shows the FTIR spectra of the rough side of the S Strat-M® membrane after the production of the extracted cream. Gradual enhancement of several peaks is observed over time, suggesting that the cream components are absorbed into the membrane. After 90 min, an enhanced peak appears around 780 cm−1, which is associated with CH aromatic compound vibrations, indicating the presence of phenolic compounds from the extracts. Significant enhancement is also observed in the 860–1050 cm−1 region, associated with C-O and C-O-C bonds, characteristic of polar lipids and phenolic compounds [4]. These changes reveal that the action of the extracts is not limited to the application surface, but continues in depth as cream components reach the rough side of the membrane, thus enhancing overall hydration and penetration.
Figure 4d shows the FTIR spectra of the rough side of the Strat-M® membrane after application of the extract cream and storage of the cream for one month. As the application had been performed on the smooth side, this spectrum captures the penetration of the ingredients towards the deeper layers of the membrane. It is observed that the peak in the 3370 cm−1 region, associated with vibrations of hydroxyl (-OH) groups and attributed to hydration, appears more pronounced in the clean membrane. This phenomenon may be due to the fact that the same membrane was used during the initial application of the cream, so that previous absorption or loss of moisture may have affected the signal intensity. Overall, the spectrum shows that the active components of the extracts are retained after storage, remaining able to be absorbed and reaching a depth within the membrane.
Significant differences are observed in the FTIR spectra of the rough side of the Strat-M membrane between the cream with extracts and the cream without extracts. The membrane exposed to the cream with extracts shows stronger peaks in regions associated with phenolic and lipid components. In particular, a stronger spectral response appears in specific regions, indicating the presence of flavonoids, polyphenols, and polar lipids from the extracts. In contrast, the membrane exposed to the cream without extracts shows a milder spectral behavior, with fewer or weaker peaks in the same regions, indicating less penetration of active compounds.
Even though it has been proposed that high concentrations of catechins are usually maintained in the stratum corneum, while only 10% has been measured to penetrate the subcutaneous skin [42], our results suggest that the rich in phenolic extracts contribute to a stronger and deeper absorption of active ingredients through the membrane, which may also be related to the presence of other amphiphilic compounds in the extracts that facilitate this penetration, such as the polar lipid bioactives that have been detected in this and previous studies for these extracts [4,5,6,8].
In Figure 5a, which corresponds to the smooth side of the Strat-M® membrane immediately after application of the cream, the recorded peaks appear largely similar to those of the clean membrane (STRAT_M_CLEAN), suggesting that the formula does not cause direct structural changes in the membrane [4].
However, there is an enhancement of the peak in the 3330–3370 cm−1 region, which is associated with vibrations of hydroxyl groups (-OH) and is attributed to increased hydration of the membrane. This hydration is mainly due to the bioactive compounds of the plant extracts [4].
Figure 5c shows that the spectral changes become more pronounced after one month of storage of the cream, which indicates an increased interaction of the active ingredients with the Strat-M membrane. The enhancement of the peaks, especially in the region of 3300–3400 cm−1, is associated with increased hydration [4].
In Figure 5b, corresponding to the rough side of the film immediately after cream preparation, the spectral fingerprint shows little variation compared to that of the clean film. The limited intensity of the characteristic peaks suggests that the hydration or the action of the active ingredients did not extend in depth, remaining mainly in the surface layer of the membrane [4].
In Figure 5d, concerning the rough side of the membrane after one month of storage, there is an enhancement of the peak in the region 3330–3370 cm−1, indicating retention of hydration and possible penetration of active ingredients into the deeper layers. The findings indicate that the active components of the extracts remain stable and capable of being absorbed even after storage [4].
According to the data, in the 3200–3500 cm−1 (O–H) region, cream X exhibits moderate peaks, indicating stable hydrophilic activity, while cream NB shows strong peaks that gradually decrease from 0 to 90 min, displaying behavior similar to phenolic compounds found in cream S.
In the 2800–3000 cm−1 (C–H sp2) region, both samples initially exhibit similar permeability of polyunsaturated fatty acids and polar lipids, with increased intensity compared to the baseline. However, after 90 min, the NB sample shows improved permeability. In the 1700–1800 cm−1 (C=O) region, both samples show intense peaks, indicating strong retention of carbonyl groups, with small differences in intensities. Finally, in the 1000–1200 cm−1 (C–O) region, cream X exhibits greater intensity at the peaks, indicating better interaction with polar compounds compared to NB.
Data from infrared spectroscopy highlighted the contribution of NBs and extracts to the retention of active ingredients on the skin surface. Improved absorption and retention of these ingredients prevent their rapid evaporation or degradation, thereby enhancing the overall performance of the cosmetic product.
However, the comparison of the FTIR spectra of the formulations containing extracts, with those combining extracts and NBs, showed that both types presented similar absorption patterns. The incorporation of NBs did not lead to significant differences in the spectral behavior or penetration of the active ingredients compared to the formulations containing extracts alone. The data suggest that NBs do not substantially affect the chemical interaction of the product with the membrane, maintaining similar levels of hydration and absorption.
3.4. Evaluation of Antiplatelet and Anti-Inflammatory Properties
The biological activities of the cosmetic creams were evaluated through their anti-inflammatory and antiplatelet activity, studying the inhibition of human platelet activation and accumulation induced by the inflammatory and thrombotic mediator PAF, as well as by the classical platelet agonist ADP. The results of the IC50 (semi-inhibitory concentration) assays against the PAF pathway are presented in Figure 6, demonstrating the differences in bioactivity between creams that did not contain extracts (X), those that did (S), and those that contained extracts and NBs (NB).
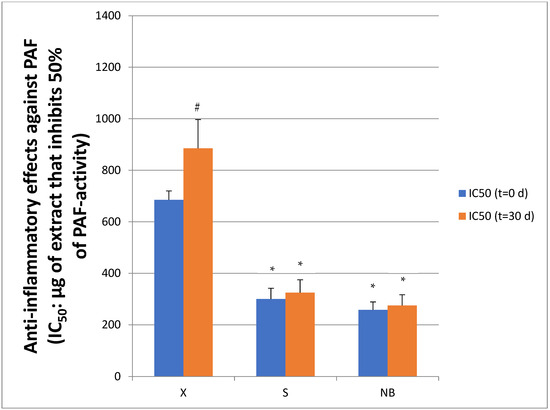
Figure 6.
Inhibitory effects of creams with (S) and without (X) extracts, and with extracts and NBs (NB) against the PAF pathway in human platelets. Results are expressed as IC50 values (half maximum inhibitory concentration), in platelet-rich plasma (hPRP), both initially (time t = 0 d) and after 1 month (t = 30 d) of storage in the fridge. Lower IC50 values indicate stronger anti-inflammatory activity through inhibition of PAF-induced inflammatory activation. Data are presented as mean ± standard deviation. * indicates statistically significant lower IC50 values of creams S and NB versus the control cream X at both t = 0 and t = 30 days. # indicates statistically significant increase in IC50 value of the X cream at t = 30 d versus t = 0 d.
PAF is a phospholipid molecule that regulates important biological processes such as inflammation and blood clotting. Under normal conditions, it helps to regulate cellular communication and the immune response. However, when its levels are increased excessively, it can trigger chronic inflammatory responses, contributing to the development of diseases such as cardiovascular disease and cancer. PAF acts through its receptor (PAF-R), affecting platelet function, vascular permeability, and the production of inflammatory substances. In cases where its production is uncontrolled, it can lead to increased inflammation and thrombosis, exacerbating pathological conditions [16]. The contribution of PAF is also important in cutaneous inflammation, both acute and chronic, such as those from UV-induced melanoma cancers [16]. This implication of thrombo-inflammatory complications is the springboard for targeting its inflammatory pathway for prevention and/or treatment in skin diseases as well [16]. In this direction, the contribution of the presence of natural bioactives in cosmetic products, including those from apple, avocado, and kiwi, seems to be beneficial [3,5,7].
Regarding the cosmetic creams containing extracts, cream S and cream NB showed a significantly lower IC50 value (300.0 ± 42.0 μg/mL, 258.0 ± 31.0 μg/mL) than cream X (p = 0.003), indicating a stronger inhibitory effect against PAF, as the lower the IC50 value, the more effective the inhibition of the thrombophilic mediator. In contrast, cream X, which did not contain extracts, showed a significantly higher IC50 value (685.0 ± 35.0 μg/mL), indicating reduced anti-inflammatory activity.
After one month of storage, the anti-inflammatory activity of cream X was further reduced, as shown by the increase in IC50 value (885.0 ± 112.0 μg/mL). In contrast, cream S showed little change (IC50 = 325.0 ± 50.0 μg/mL), as did cream NB (IC50 = 275.0 ± 42.0 μg/mL), largely retaining its original anti-inflammatory activity. These results suggest that the presence of natural extracts not only enhanced the initial activity of the cream but also contributed to the long-term stability of its anti-inflammatory property during storage.
Moreover, these data also suggest that the addition of extracts to cosmetic formulas may enhance their anti-inflammatory properties, thereby reducing excessive platelet activation and possibly the risk of thrombotic events. It has also been previously reported that topical application of PAF inhibitors (antagonists) and/or agonists for the PAF Receptor (PAFR) can suppress acute and chronic inflammation and possess cancer chemopreventive activities in the skin [16,45]. Thus, the anti-PAF compounds contained in the extracts and S and NB creams of the present study appear to exhibit a protective effect against the inflammatory response associated with PAF activity, making these natural raw materials interesting for possible use in cosmetic and nutricosmetic applications. Furthermore, NBs appear to facilitate the incorporation and bioavailability of the bioactives from these extracts, thus enhancing their effectiveness.
Regarding the antiplatelet effects of the cosmetic creams, Figure 7 shows the IC50 values of the S and X samples against the ADP-induced classical thrombotic platelet aggregation pathway. Specifically, the IC50 values of the samples were evaluated to assess their ability to inhibit platelet aggregation.
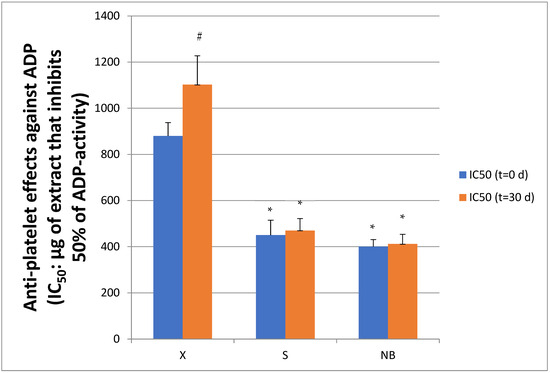
Figure 7.
Inhibitory effects of creams with (S) and without (X) extracts, and with extracts and NBs (NB) against the ADP pathway in human platelets. Results are expressed as IC50 values (half maximum inhibitory concentration), in platelet-rich plasma (hPRP), both initially (Time t = 0 d) and after 1 month (t = 30 d) of storage in the fridge. Lower IC50 values indicate stronger anti-inflammatory activity through inhibition of ADP-induced inflammatory activation. Data are presented as mean ± standard deviation. * indicates statistically significant lower IC50 values of creams S and NB versus the control cream X at both t = 0 and t = 30 days. # indicates statistically significant increase in IC50 value of the X cream at t = 30 d versus t = 0 d.
ADP is an important regulator of platelet activation, as it binds to specific receptors on their cell membrane, such as P2Y1 and P2Y12, which belong to the family of G protein-coupled receptors (GPCRs). This binding triggers a chain reaction that enhances platelet aggregation, stimulating the clotting process. Furthermore, platelet activation via ADP leads to structural changes in the cells and the release of substances that further enhance platelet aggregation, thus contributing to clot formation and stabilization [11].
Similarly, to the results of the PAF route, cream S and cream NB, containing extracts, showed a lower IC50 value (450.0 ± 65.0 μg/mL and 400.0 ± 31.0 μg/mL, respectively) than cream X (880.0 ± 58.0 μg/mL) (p = 0.007), indicating a stronger antiplatelet effect. After one month of storage, a significant increase in IC50 was observed in cream X (1102.0 ± 125.0 μg/mL), indicating a further decrease in its antiplatelet capacity. In contrast, cream S largely retained its bioactivity, as its IC50 value (470.0 ± 52.0 μg/mL) showed only a slight change, as did the NB cream (IC50 = 412.0 ± 42.0 μg/mL). These results indicate that adding natural extracts enhanced the antiplatelet activity of cream S while maintaining its stability during storage, thereby making it more effective in inhibiting the ADP pathway compared with the cream without extracts. Moreover, the NBs did not appear to significantly enhance the biodegradation and absorption of the extracts, suggesting that their role in the specific formulation may be limited or may require further optimization.
Overall, the results show that creams containing natural extracts exhibited stronger antiplatelet activity compared to creams without extracts, with cream S and cream NB showing higher biological activity in both pathways (PAF and ADP). After one month of storage, cream S and cream NB largely retained their antiplatelet activity, whereas cream X showed a significant decrease in efficacy, as indicated by the increase in IC50 value.
Cream S and cream NB showed stronger activity against the PAF pathway compared to the ADP pathway, suggesting that the bioactive compounds in the extracts may be more effective in regulating this pathway. This difference may be due to the presence of polar and amphiphilic compounds, which probably inhibit the binding of PAF to its receptor, thus reducing the inflammatory and thrombotic response. Furthermore, these two creams retained their antiplatelet activity after storage, suggesting that natural extracts contribute not only to the immediate bioactivity of the product but also to maintaining its stability over time.
Research in antiplatelet cosmetics containing naturally derived compounds from sustainable sources, like those in avocado, apple, and kiwi, seems to provide new avenues for such products. The prevention of blood clots and the reduction in redness, among their anti-inflammatory properties, can substantially improve conditions like facial redness. Moreover, the use of platelet-rich plasma (PRP), which is recognized as a safe and effective therapy for regenerative skin healing and rejuvenation, utilizes autologous blood enriched with various growth factors [46]. This may also be beneficially affected by the presence of such bioactives in the skin, as they are able to reduce the PRP reactivity induced by the thrombo-inflammatory stimuli of PAF and/or ADP (i.e., in cases of rupture of cells, the presence of small undetected injuries and/or immune reactions against PRP). Thus, this may facilitate the inhibition of possible formation of PRP clots that would make PRP ineffective for skin rejuvenation and may preserve PRP effectiveness for such skin benefits observed. The goal is to develop safe, effective cosmetic ingredients that can offer skin benefits beyond traditional anti-aging, though more clinical research is needed to validate these findings in humans for cosmetic applications.
3.5. Results of Molecular Docking Analysis
After collecting the candidate ligands and receptors, three docking analyses were performed, one for each substitute, using the CB-Dock2 (cavity-detection-guided blind docking) tool. Usually, as in the present analysis, the five best clusters are selected and investigated for docking. Then, for each cavity, its geometric center and dimensions are calculated in order to cover it adequately without unnecessary space, by adjusting the size of the docking box. After the ligand is introduced, docking is performed for all cavities, and the different binding positions (poses) are calculated, which are then re-ranked, and the optimal one is selected based on the binding affinity.
PAFR is a receptor coupled to a G protein that is linked to PAF [16,47]. Clinical and preclinical studies highlight PAF and PAFR’s detrimental role: they play an important pathophysiological role in various mechanisms, such as bronchial asthma, endotoxin shock, central nervous system disorders, and skin diseases and disorders [48].
Targeting PAFR with inhibitors offers a remarkable strategy for mitigating and treating inflammatory reactions and oxidative stress, especially when using natural bioactives with few to no side effects [16,49]. Blocking of PAFR by PAF-inhibitors (antagonist for PAFR) interrupts the downstream signaling pathways, such as MAPK and NF-κB, which are involved in PAF-induced cytokine production and associated inflammatory stimuli [16].
Therefore, in silico studies aimed at revealing the anti-PAF antagonistic effects of natural bioactives present in such extracts are of great importance for unveiling the anti-inflammatory and protective properties of cosmetic formulation products containing such extracts and bioactives for skin health.
Apple, avocado, and kiwi by-products and their extracts are rich in flavonoids like quercetin, but also in other types of phenolics, with the most abundant being rutin (kiwi) and phloretin (apples) [3,5,7]. Quercetin is a natural flavonoid found in high concentrations in apples, avocados, and kiwis, which, apart from its general antiplatelet properties, has also recently been found to potently inhibit PAF activity directly and thus to reduce inflammation and platelet activation by blocking PAFR activity [44].
Such anti-inflammatory effects have been observed for other main phytochemicals from apples and kiwis, such as phloretin and rutin, too. By being the most abundant phenolic bioactives in these fruits and their extracts, the molecular docking properties of these three bioactives on PAFR were further evaluated.
3.5.1. Docking of Phloretin with PAFR
While specific antiplatelet-activating factor (PAF) research for phloretin is not readily available in the provided results, studies indicate phloretin exhibits anti-inflammatory effects by modulating various pathways relevant to PAF-induced inflammation, including NF-κB, MAPK, and TLR2/1 signaling [50]. It also reduces the PAF-induced production of pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), and inflammatory cytokines like IL-6 and TNF-α; thus, it can influence other cellular processes indirectly related to PAF-associated inflammatory responses [50].
Nevertheless, this is the first time it has been reported that phloretin seems to be directly associated with PAFR, since the optimal docking score (vina score) from the binding of phloretin to this receptor was equal to −8.8 kcal/mol (Table 7), indicating strong thermodynamic binding to PAFR (Figure 8). The negative and low value of ΔG indicates a strong binding affinity of the protein–ligand complex [51].

Table 7.
Results obtained from docking analysis.
Figure 8.
Representation of the binding of the selected active substance to the target protein, as shown by docking in the CB-Dock2 software.
The binding cavity, 6073 Å3, indicates that phloretin occupies a large proportion of the active site of the receptor and binds strongly within the internal cavity of PAFR. In this way, it seems that phloretin exerts effective inhibitory effects, and the high score explains its anti-inflammatory and antioxidant properties and may be the reason why creams containing the extract inhibited the PAF pathway for a prolonged time, due to strong interaction and binding to a protected internal active site.
The 2D diagram shows the interactions of the phloretin molecule with the receptor, highlighting the conventional hydrogen bonds of phloretin with amino acids, such as Tyr22(A), Tyr77(A), Tyr102(A), Tyr177(A), and Gln252(A), as well as van der Waals interactions with Ile191(A), Thr101(A), His275(A) and others presented in the 2D diagram of Figure 9. The participation of phloretin’s aromatic rings in pi–pi stacking interactions (Phe97(A), Trp73(A)) and pi–pi T-shaped contacts is also evident. All of these interactions, and in particular the aromatic ring of the molecule, probably contribute to its orientation in PAFR (Figure 10 and Figure 11).
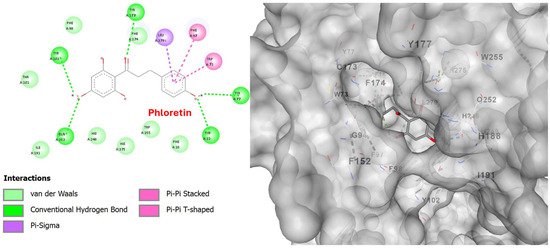
Figure 9.
Two-dimensional interaction diagram (left) and three-dimensional binding pose (right) of phloretin docked to the PAFR active site. The 2D diagram highlights hydrogen bonds (dashed green lines) between phloretin and residues Tyr22(A), Tyr77(A), Tyr102(A), Tyr177(A), and Gln252(A). Other interactions, such as Pi-sigma, pi–pi stacked, and van der Waals, are also visible. The 3D structure shows phloretin positioned within the PAFR binding pocket.
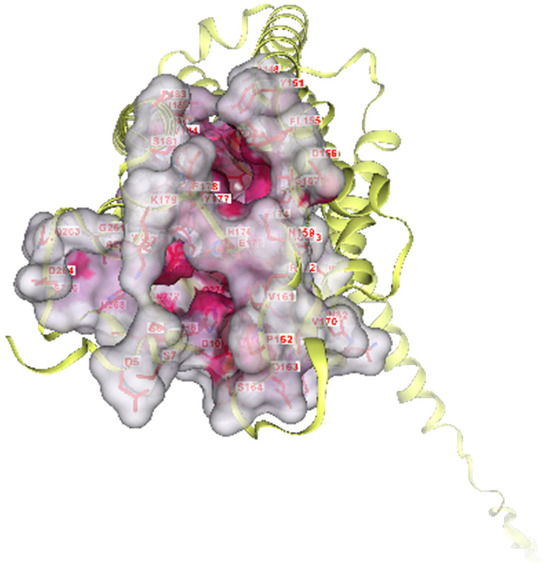
Figure 10.
Surface representation of PAFR, where the predicted binding pockets detected through pocket scanning analysis in CB-Dock are highlighted. The receptor is depicted in yellow as a ribbon and is overlaid by its molecular surface (gray cavity), while the binding regions are depicted in pink, highlighting the hydrophilic surfaces suitable for ligand binding. The key amino acids that form the binding site are also labeled. The depicted cavities were used for blind molecular docking of candidate ligands.
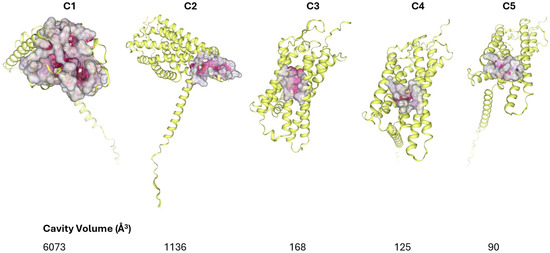
Figure 11.
The detected curvatures resulting from scanning the surface of the PAFR. The five optimal ones were found (C1, C2, C3, C4, C5). These positions constitute the binding sites of the ligands.
3.5.2. Docking of Rutin with PAFR
Rutin, a potent bioactive phytochemical abundant in kiwi and its extracts, can act as an anti-PAF compound by inhibiting its production and effects, particularly in inflammatory conditions like cigarette smoke-induced COPD in mouse models and gastric ulceration in animal studies, suggesting a PAF-inhibitory anti-inflammatory activity for this phenolic bioactive [52].
With respect to its molecular docking on the PAF receptor, this is the first study investigating such a binding. Results show that rutin also obtained a high score of −7.9 kcal/mol (Table 7), exhibiting a strong interaction with the C2 binding pocket of PAFR (1136 Å3). As a large polyhydroxylated molecule, the results are impressive but justified by the fact that rutin has the ability to form multiple hydrogen bonds. This is clearly visible in the 2D diagram (Figure 12). It is clear that rutin participates in a large and rich set of interactions with the active site of the receptor. It forms hydrogen bonds with amino acids such as Arg115(A), Ala118(A), Glu225(A), and Lys298(A), and other interactions such as pi–sigma, van der Waals, and others, which are presented in detail in the figure. These interactions explain why rutin interacts with the peripheral residues of the PAF receptor without deep internal binding, as observed with smaller ligands. Its binding to a position with a larger cavity volume (C1), apart from size, is probably also prevented by electrostatic overlaps, which are those with the red dotted lines in the 2D diagram (unfavorable positive–positive, unfavorable donor–donor), with the amino acids Arg229(A) and Arg115(A). Electrostatic repulsion is created because the two amino acids carry a positive charge and because of their binding with rutin. This must be the reason why rutin exhibits weaker anti-PAF activity compared to other flavonoids.
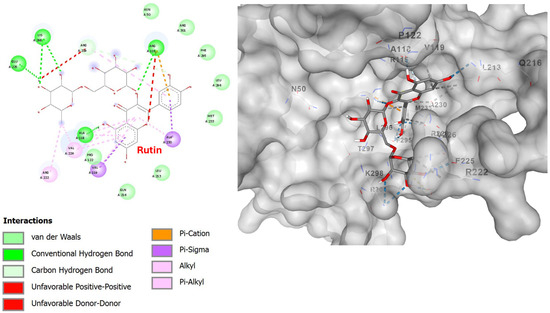
Figure 12.
Two-dimensional interaction diagram (left) and three-dimensional binding pose (right) of rutin docked to the PAFR active site. The 2D diagram highlights hydrogen bonds (dashed green lines) between rutin and residues Arg115(A), Ala118(A), Glu225(A), and Lys298(A). Other interactions, such as pi–sigma, pi–pi stacked, and van der Waals, are also visible. The 3D structure shows rutin positioned within the PAFR binding pocket.
3.5.3. Docking of Quercetin with PAFR
Recent research on the anti-inflammatory benefits of quercetin shows that it possesses significant direct anti-PAF activities, primarily acting as an inhibitor of PAFR [44], or by modulating PAF-related pathways such as Lyn kinase and calcium influx [53].
With respect to its molecular docking on PAFR, quercetin yielded the optimal score of −9.0 kcal/mol (Table 7), indicating that it has the strongest binding to the C1 binding site of PAFR, targeting the same active site as phloretin (6073 Å3 cavity). This obviously suggests that the two substituents compete with each other and, since they are both contained in the extract, they work together for greater and optimal inhibition of the most active region of PAFR. The observed better affinity of quercetin seems to be associated with its additional -OH groups, which increase hydrogen bonds with the receptor and thus improve affinity.
The interactions of quercetin with PAFR and the hydrogen bonds are shown in detail in the 2D diagram in Figure 13. Hydrogen bonds are formed with the amino acids Arg115(A), Ala118(A), Glu225(A), and Lys298(A), pi–pi stacked with Phe174(A), Trp73(A), pi–sigma with Leu279(A), and other interactions that are visible in the 2D diagram.
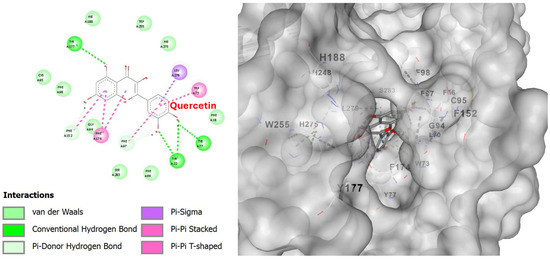
Figure 13.
Two-dimensional interaction diagram (left) and three-dimensional binding pose (right) of quercetin docked to the PAFR active site. The 2D diagram highlights hydrogen bonds (dashed green lines) between rutin and residues Arg115(A), Ala118(A), Glu225(A), and Lys298(A). Other interactions, such as pi–sigma, pi–pi stacked, and van der Waals, are also visible. The 3D structure shows rutin positioned within the PAFR binding pocket.
Studies demonstrate that apart from such an anti-PAF direct effect observed in human platelets [44], quercetin can further reduce inflammatory mediators, cytokine production, and mast cell degranulation, which are critical components of allergic responses and inflammation driven by PAF [54,55]. Quercetin’s ability to target PAF-mediated pathways makes it a potential therapeutic agent for various inflammatory conditions [56], including skin inflammation and associated cancers [16]. Subsequently, its incorporation as a bioactive ingredient in cosmetic applications, by utilizing extracts from quercetin-rich fruit by-products, such as those used in the present study, further enhances the anti-inflammatory protection that these products may provide for skin health.
3.6. Organoleptic Properties and Stability
Creams containing the extract mixture exhibited a light greenish hue and a subtle fruity aroma, attributable to the natural pigments and volatile compounds present in the fruit by-product extracts. No adverse changes in texture were observed compared to the control formulations. Informal stability checks over a one-month storage period at room temperature revealed no signs of phase separation, changes in color or odor, or rancidity. These observations indicate that the incorporation of the extracts, at the tested concentrations, did not compromise the organoleptic quality or the short-term stability of the creams.
4. Discussion
The results demonstrated that these extracts contain bioactive compounds with strong antioxidant potential and significant inhibitory activity against platelet activation, induced both by the inflammatory mediator PAF and the classical agonist ADP. Creams containing the extract blend exhibited lower IC50 values against both agonists compared to base creams, indicating a notable enhancement of anti-inflammatory and antithrombotic activity. This effect is particularly important, as the inflammatory mediator PAF and the classical agonist ADP are key factors in platelet activation, a process associated with impaired microcirculation, oxidative stress, and the amplification of local inflammation. It should also be stressed that the platelet aggregometry assay applied to this study is a fast and reliable test to assess the anti-PAF activity of a compound, rather than making expensive and multiparametric analyses in other cell–culture/animal models. In case a compound shows an anti-PAF effect on platelets through its interaction with the PAF-receptor (as shown by the molecular docking results too), this compound can actually inhibit PAF in all body cells where the PAF-receptor exists, including skin cells and fibroblasts, as well as in leukocytes, endothelial, and epithelial cells of the skin microvessels.
Moreover, excessive platelet activation in the skin microvessels can contribute to vascular dysfunction, redness, and delayed wound healing. PAF—specifically related to its pathway—and inflammatory mediators are implicated in skin microcirculation, erythema, photoinflammation, and UV damage, even in melanoma and other skin cancers. Thus, inhibiting platelet aggregation via PAF/ADP pathways may indicate potential for reducing UV-induced and inflammatory skin damage, extending the concept of “cosmeceutical” activity. Therefore, topical formulations that inhibit the actions of PAF and ADP can improve capillary blood flow, reduce inflammatory responses, and support the repair and revitalization of the skin, particularly after exposure to ultraviolet radiation or other irritating factors, with anti-tumor benefits against skin cancers [16,45].
Regarding the overall antioxidant activity, it was observed that in the DPPH assay, no significant improvement was detected in the cream containing the extracts. This may be due to a possible interaction between the polyphenols and the lipid components of the cream matrix, which reduces the immediate availability of antioxidants to neutralize DPPH radicals. However, the FRAP values, which better reflect the redox potential of the components, showed a significant improvement, indicating that the overall antioxidant capacity was enhanced, even if this was not reflected in the specific DPPH method.
Moreover, ATR-FTIR analysis showed that the enriched formulations exhibited increased absorption of active compounds through synthetic membranes, suggesting improved transdermal penetration of the bioactive molecules, which was more profound in the creams containing both the extracts and the NBs. The addition of the extracts also contributed to the retention of SPF protection after storage, indicating enhanced product stability and protection against oxidation and degradation over time. A key finding of this study was that in nearly all measurements, the formulations containing the extracts showed improved or at least stable performance after one month of storage. This indicates that the addition of natural extracts contributed not only to improved initial bioactivity but also to its maintenance or even enhancement over time. Nevertheless, it should also be stressed that the specific extracts used were obtained from the by-products of avocado, kiwi, and apple by conventional extraction methodology, which may imply that different results could be obtained in case of the use of green extracts from these agri-food by-products, leading to more ex vivo and in vivo testing of these creams.
Moreover, compared to the extracts alone, the presence of nanobubbles (NBs) did not produce a notable effect on bioavailability, photoprotection, or overall bioactivity in the in vitro assays. Nevertheless, the rationale for their inclusion was to investigate their potential as a green technology for enhancing the transdermal delivery of bioactives without the use of chemical penetration enhancers. While their contribution to biological endpoints appeared limited, FTIR analysis indicated improved absorption and retention of active compounds through synthetic membranes, suggesting enhanced transdermal penetration. This implies that although the immediate bioactivity was not significantly improved, NB-assisted formulations may still offer advantages in terms of stability and delivery that warrant further optimization. These aspects were clarified in the revised discussion, where claims regarding NB contribution were carefully tempered.
The in silico study yielded important data supporting and explaining for the first time the inhibitory potential of the flavonoids (phloretin, rutin, and quercetin) against PAFR, by moderating inflammation, blocking active sites, and thus the entire receptor. Through careful molecular docking with the CB-Dock tool and subsequent analysis with BIOVIA Discovery Studio, the binding affinity and size of the occupied cavities for each ligand were interpreted and characterized approximately. The in silico analysis provides an indicative molecular interpretation of the above observation, as well as the greater stability of creams with extract and anti-inflammatory action. This stability comes from the interactions under study of the selected phytochemicals, as well as from others contained in the extract. Depending on their chemical structure, these compounds interact similarly to PAFR.
Among the selected substances, quercetin was the one that presented the most optimal vina score (−9.0 kcal/mol), which is a very high value and strong binding affinity. This and kaempferol cover an internal and deep active center of the receptor, with various interactions.
When a drug interacts deeply and penetrates inside a receptor, it acts as an orthosteric inhibitor, i.e., it effectively competes with the natural activator—PAF in this case—at its normal binding site, preventing its binding to a greater extent. Compared to allosteric drugs, orthosteric drugs bind to the active site, competing strongly with the natural substrate of the receptor and, if their affinity is high, successfully blocking the site. In fact, most commercially available drugs are orthosteric [57]. Rutin, despite its ability to form a sufficient network of hydrogen bonds, due to interfering interactions, had a lower vina score (−7.9), which is not disappointing.
The computational findings were consistent with our in vitro data, in which the lowest IC50 values were observed in creams formulated with polyphenolic extract from the by-products of the three fruits.
In addition, the application of other types of NBs (i.e., of CO2 or N2) in such cosmetic applications seems also to be of interest, as these types of NBs may affect the anti-photoaging properties of these formulations too. Thus, the combination of each type of NBs with natural extracts may warrant further investigation for their potential in improving product stability or delivery in more specialized formulations. These outcomes are in line with the high total phenolic and polyphenolic content of the extracts used, along with the presence of carotenoids and polar lipids, which seem to play a critical role in the bioactivity of the final formulations. The evaluation of both the chemical composition and functional performance confirms the essential role of natural extracts in enhancing the efficacy and stability of cosmetic products.
Additionally, the extraction process uses low-cost by-products and ethanol–water solvent, making it economically viable at scale. NBs’ incorporation adds minimal cost if integrated into industrial mixing lines. Next steps include extended stability testing, consumer sensory panels, and regulatory compliance assessment.
5. Limitations
This study has certain limitations that should be acknowledged. All biological assays, including antioxidant, photoprotective, anti-inflammatory, and antiplatelet evaluations, were performed in vitro, which may not fully replicate the complex physiological environment of human skin in vivo. The sample size of biological replicates was limited, and the tested concentrations were specific to the current formulations, potentially restricting the generalizability of the findings. Only short-term effects were assessed, without evaluation of long-term stability, safety, or sustained efficacy. The penetration and bioavailability of the active compounds were inferred from indirect analytical methods, without direct in vivo skin absorption studies. Future research should include extended stability assessments and well-designed clinical trials to confirm the observed effects and validate the potential of these fruit by-product extracts, with or without nanobubbles, in cosmetic applications.
6. Conclusions
In this study, innovative cosmetic formulations were developed and evaluated, enriched with natural extracts obtained from avocado, apple, and kiwi by-products, combined with nanobubbles (NBs). The results demonstrated strong antioxidant, photoprotective, anti-inflammatory, and antiplatelet properties, with extract-enriched creams showing enhanced bioactivity and stability over time. Although NBs did not markedly improve in vitro performance, they enhanced the penetration of active compounds, suggesting potential in vivo benefits.
In our current formulation, the overall impact of NBs on in vitro endpoints such as antioxidant activity, SPF, and anti-photoaging efficacy was modest. This limited contribution may be attributed to several factors: (i) the inherently high bioavailability of the low-molecular-weight active compounds already present in the extracts, (ii) possible NB collapse during the emulsification process, and (iii) the suboptimal concentration of NBs incorporated into the formulations. Nevertheless, FTIR analysis indicated improved retention and absorption of bioactive compounds, suggesting that NBs may still play a role in enhancing transdermal penetration. These findings imply that, although immediate biological outcomes were not dramatically improved, NB-assisted systems could offer advantages in stability and delivery that require further optimization. Future studies will, therefore, explore different NB gases (e.g., CO2, N2) and optimized process parameters to maximize NB stability and delivery efficiency.
These findings highlight the dual value of the proposed approach: (i) scientific innovation, by utilizing fruit by-products as sustainable, high-value ingredients in topical cosmetics; and (ii) environmental impact, by promoting circular economy principles through the valorization of agri-food waste. The integration of natural extracts with NBs offers a promising, eco-friendly strategy for the cosmetics industry, enabling the development of stable, effective, and skin–health-promoting products. Further in vivo studies and long-term evaluations are warranted to confirm the observed effects and optimize the formulations for commercial applications.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; software, all authors; validation, A.T.; formal analysis, O.I.T., N.-I.K., A.O., K.P., and A.T.; investigation, A.T. and K.P.; resources, A.M. and A.T.; data curation, O.I.T., N.-I.K., V.P., K.P., and A.T.; writing—original draft preparation, O.I.T., N.-I.K., K.P., and A.T.; writing—review and editing, A.O., V.P., S.L., R.I.K., A.M., and A.T.; visualization, A.T.; supervision, A.T. and A.M.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In this study, the evaluation of the anti-inflammatory and antithrombotic properties of these natural bioactives was performed in vitro using human platelet-rich plasma obtained from blood samples of healthy donors/volunteers. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Democritus University of Thrace (protocol code ΔΠΘ/ΕHΔΕ/7690/70, 27 September 2024).
Informed Consent Statement
Informed consent was obtained from all healthy blood donors involved in the study.
Data Availability Statement
All data are contained within the article. Any further information concerning raw data or the article can be provided by the authors upon request.
Acknowledgments
The authors would like to thank the School of Chemistry of the Faculty of Sciences of the Democritus University of Thrace and the General Hospital of Kavala for their continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsiapali, O.I.; Ayfantopoulou, E.; Tzourouni, A.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Unveiling the Utilization of Grape and Winery By-Products in Cosmetics with Health Promoting Properties. Appl. Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. [Google Scholar] [CrossRef]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Marra, A.; Manousakis, V.; Koutis, N.; Zervas, G.P.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. In Vitro Antioxidant, Antithrombotic and Anti-Inflammatory Activities of the Amphiphilic Bioactives Extracted from Avocado and Its By-Products. Antioxidants 2025, 14, 146. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. In Vitro Antioxidant, Antithrombotic and Anti-Inflammatory Properties of the Amphiphilic Bioactives from Greek Organic Starking Apple Juice and Its By-Products (Apple Pomace). Appl. Sci. 2025, 15, 2807. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Chrysikopoulou, V.; Rampaouni, A.; Plakidis, C.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage. Antioxidants 2025, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Moysidou, A.M.; Cheimpeloglou, K.; Koutra, S.I.; Finos, M.A.; Ofrydopoulou, A.; Tsoupras, A. A Comprehensive Review on the Antioxidant and Anti-Inflammatory Bioactives of Kiwi and Its By-Products for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5990. [Google Scholar] [CrossRef]
- Moysidou, A.M.; Cheimpeloglou, K.; Koutra, S.I.; Manousakis, V.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. In Vitro Antioxidant, Antithrombotic and Anti-Inflammatory Activities of Bioactive Metabolites Extracted from Kiwi and Its By-Products. Metabolites 2025, 15, 400. [Google Scholar] [CrossRef]
- Kontaxi, N.-I.; Panoutsopoulou, E.; Ofrydopolou, A.; Tsoupras, A. Anti-Inflammatory Benefits of Grape Pomace and Tomato Bioactives as Ingredients in Sun Oils against UV Radiation for Skin Protection. Appl. Sci. 2024, 14, 6236. [Google Scholar] [CrossRef]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Makris, D.P. Beverage Industry By-Products as Bio-Resources of Functional Compounds. Beverages 2023, 9, 48. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Kurzawa, M.; Sionkowska, A.; Grisel, M. Antioxidant Activity of Plant-Derived Colorants for Potential Cosmetic Application. Cosmetics 2022, 9, 81. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. Front. Biosci. (Landmark Ed) 2024, 29, 345. [Google Scholar] [CrossRef]
- Habachi, E.; Rebey, I.B.; Dakhlaoui, S.; Hammami, M.; Sawsen, S.; Msaada, K.; Merah, O.; Bourgou, S. Arbutus Unedo: Innovative Source of Antioxidant, Anti-Inflammatory and Anti-Tyrosinase Phenolics for Novel Cosmeceuticals. Cosmetics 2022, 9, 143. [Google Scholar] [CrossRef]
- Park, Y.; Shin, S.; Shukla, N.; Kim, K.; Park, M.-H. Effects of Nanobubbles in Dermal Delivery of Drugs and Cosmetics. Nanomaterials 2022, 12, 3286. [Google Scholar] [CrossRef]
- Mitropoulos, A.C.; Pappa, C.; Kosheleva, R.I.; Kyzas, G.Z. The Effect of Nanobubbles on Transdermal Applications. Nanomaterials 2023, 13, 2600. [Google Scholar] [CrossRef]
- Anastasiou, E.A.; Ayfantopoulou, E.; Lykartsi, E.E.; Papadopoulou, S.N.; Toganidou, I.T.; Tsiapali, O.I.; Tzourouni, A.; Veneti-kidou, M.G.; Tsoupras, A.B.; Koumentakou, I.; et al. Nanostructured Materials in Industrial Applications, Personal Care, and Health Care: A Cosmetic Approach. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 978-0-12-803581-8. [Google Scholar]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of In Vivo and In Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Vordos, N.; Giannakopoulos, S.; Vansant, E.F.; Kalaitzis, C.; Nolan, J.W.; Bandekas, D.V.; Karavasilis, I.; Mitropoulos, A.C.; Touloupidis, S. Small-Angle X-Ray Scattering (SAXS) and Nitrogen Porosimetry (NP): Two Novel Techniques for the Evaluation of Urinary Stone Hardness. Int. Urol. Nephrol. 2018, 50, 1779–1785. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Munguira, E.B.; Juan, C.A.; Lobo, F.; Pérez-Lebeña, E.; Pérez De La Lastra, J.M. In Silico Exploration of Natural Antioxidants for Sepsis Drug Discovery. Molecules 2025, 30, 2288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Grimm, M.; Dai, W.; Hou, M.; Xiao, Z.-X.; Cao, Y. CB-Dock: A Web Server for Cavity Detection-Guided Protein–Ligand Blind Docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial Activity of Phenolic-Rich Extracts from Avocado Fruit Waste: Influence on the Colloidal and Oxidative Stability of Emulsions and Nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Ramanauskienė, K.; Janulis, V. Release of Apple Extract and Phenolic Compounds from Five Semi-Solid Formulations. In Proceedings of the Scientific Collection «InterConf+»: Proceedings of the 3rd International Scientific and Practical Conference «Scientific Goals and Purposes in XXI Century», Seattle, WA, USA, 19–20 July 2022; Svoboda, A., Granko, M., Eds.; ProQuest LLC.; Scientific Publishing Center «InterConf»: Seattle, WA, USA, 2022. [Google Scholar]
- Gomes, S.M.; Miranda, R.; Santos, L. Sustainable Cosmetics: Valorisation of Kiwi (Actinidia Deliciosa) By-Products by Their Incorporation into a Moisturising Cream. Sustainability 2023, 15, 14059. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef]
- Adamantidi, T.; Lafara, M.-P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Intern. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Tarigan, C.; Pramastya, H.; Insanu, M.; Fidrianny, I. Syzygium Samarangense: Review of Phytochemical Compounds and Pharmacological Activities. Biointerface Res. Appl. Chem. 2021, 12, 2084–2107. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Scognamiglio, M.; Monaco, P. Identification and Assessment of Antioxidant Capacity of Phytochemicals from Kiwi Fruits. J. Agric. Food Chem. 2009, 57, 4148–4155. [Google Scholar] [CrossRef]
- Quirin, K.W. Specialty Fatty Oils for Healthy Skin. Cosmet. Sci. Technol. 2009. [Google Scholar]
- Proestos, C.; Komaitis, M. Antioxidant Capacity of Hops. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 467–474. ISBN 978-0-12-373891-2. [Google Scholar]
- Dewi, R.H.T.M.; Sholihah, N.; Nofitasari, R.; Adhityasmara, D.; Shabrina, A. The Potential of Avocado Oil for Topical Use: A Narrative Review. J. Ilmu Farm. Dan. Farm. Klin. 2024, 21, 106–114. [Google Scholar] [CrossRef]
- Shabrina, A.; Firdausi, Z.M.; Poerba, A.T.; Setyani, D.A.; Heroweti, J. Sunscreen Effectivity and Physical Characterization of Avocado Oil in Nanoemulsion Using Isopropyl Myristate Variations. Pharmaciana 2024, 14, 69. [Google Scholar] [CrossRef]
- Fares, M.M.; Radaydeh, S.K.; AlAmeen, H.M. Green Tannins /Avocado Oil Composites; Suncare and Skincare Materials. Arab. J. Chem. 2023, 16, 104764. [Google Scholar] [CrossRef]
- Opriş, O.; Lung, I.; Soran, M.-L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P.; Sturza, R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules 2022, 27, 1679. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Quantification of Skin Penetration of Antioxidants of Varying Lipophilicity. Int. J. Cosmet. Sci. 2013, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The Potential of Natural Compounds in UV Protection Products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulou, V.; Rampaouni, A.; Koutsia, E.; Ofrydopoulou, A.; Mittas, N.; Tsoupras, A. Anti-Inflammatory, Antithrombotic and Antioxidant Efficacy and Synergy of a High-Dose Vitamin C Supplement Enriched with a Low Dose of Bioflavonoids; In Vitro Assessment and In Vivo Evaluation Through a Clinical Study in Healthy Subjects. Nutrients 2025, 17, 2643. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Rezania, S.; Ocana, J.A.; DaSilva-Arnold, S.C.; Bradish, J.R.; Richey, J.D.; Warren, S.J.; Rashid, B.; Travers, J.B.; Konger, R.L. Topical Application of a Platelet Activating Factor Receptor Agonist Suppresses Phorbol Ester-Induced Acute and Chronic Inflammation and Has Cancer Chemopreventive Activity in Mouse Skin. PLoS ONE 2014, 9, e111608. [Google Scholar] [CrossRef]
- Phoebe, L.K.W.; Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Wu, R.; Wong, S.; Wan, J.; Yi, K. Use of Platelet Rich Plasma for Skin Rejuvenation. Ski. Res. Technol. 2024, 30, e13714. [Google Scholar] [CrossRef]
- Ishii, S.; Nagase, T.; Shimizu, T. Platelet-Activating Factor Receptor. Prostaglandins Other Lipid Mediat. 2002, 68–69, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.B.; Rohan, J.G.; Sahu, R.P. New Insights Into the Pathologic Roles of the Platelet-Activating Factor System. Front. Endocrinol. 2021, 12, 624132. [Google Scholar] [CrossRef]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical Basis of Anti-Cancer-Effects of Phloretin—A Natural Dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Kiran, P.C.; Bulbule, S.R.; Hanumanthappa, R.; Nanjaiah, H.; Devaraju, K.S. Does Protein Kinases Exhibit Binding Affinity with Antipsychotic Drugs?—A Molecular Docking Approach. J. Appl. Bioinform. Comput. Biol. 2023, 12, 1–6. [Google Scholar]
- Chen, J.; Su, J.; Dong, L.; Bai, Z.; Liu, Y.; Bao, H.; Lu, Y.; Wu, Y. Rutin Ameliorates Cigarette Smoke Mediated Lung Inflammation in a Mouse Model of Chronic Obstructive Pulmonary Disease via Regulating the Levels of Tumor Necrosis Factor-α, Interleukin-8 and PAF. Arch. Med. Sci. 2020, 21, 258–265. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T.; et al. Quercetin as a Lyn Kinase Inhibitor Inhibits IgE-Mediated Allergic Conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef] [PubMed]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the Potential Effect on Allergic Diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Chaudhary, M.; Mandotra, S.K.; Tuli, H.S.; Chauhan, R.; Joshi, N.C.; Kaur, D.; Dufossé, L.; Chauhan, A. Anti-Inflammatory Potential of Quercetin: From Chemistry and Mechanistic Insight to Nanoformulations. Curr. Res. Pharmacol. Drug Discov. 2025, 8, 100217. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.-J. The Different Ways through Which Specificity Works in Orthosteric and Allosteric Drugs. CPD 2012, 18, 1311–1316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).