Abstract
Fatty amines are nitrogen-containing organic compounds synthesized from fatty acids, olefins, or alcohols, typically derived from natural oils or petrochemical sources. These molecules generally feature long hydrophobic alkyl chains ranging from C8 to C22 and can be positively charged on the nitrogen atom, which confer pronounced cationic surface activity. This property makes them highly effective as emulsifiers, conditioning agents, antistatic agents, and surfactants, particularly in industrial formulations and personal care products such as shampoos, conditioners, and creams. Historically, the production of fatty amines has relied heavily on petrochemical feedstocks, contributing significantly to greenhouse gas emissions, particularly CO2. In response to growing environmental concerns, there is an increasing shift toward renewable and sustainable raw materials, aligning with the principles of the circular economy. The cosmetics and detergent industries are at the forefront of this transition, actively integrating bio-based ingredients to minimize ecological impact. This review provides a comprehensive overview of the sources, synthetic pathways, and applications of fatty amines. It highlights their functional roles in detergents and cosmetic formulations and explores scientific and technological strategies aimed at enhancing sustainability across the fatty amine supply chain.
1. Introduction
Fatty amines, versatile nitrogen-derivatives of fatty acids, olefins, or alcohols, are sourced from nature, fats and oils, or petrochemicals. Typically, their aliphatic carbon chains range between C8 and C22, either as mixtures or specific lengths and exhibit remarkable cationic surface-active properties. This inherent characteristic allows them to strongly adhere to surfaces through both physical and chemical bonding, effectively altering surface properties. Their surface activity positions them as indispensable commodity chemicals, finding broad application in fabric softening, coating and corrosion inhibition, emulsification, flotation, anti-caking, and crop protection. Within the personal care industry, fatty amines are integral components of shampoos, conditioners, and foaming and wetting agents, underscoring their multifaceted utility [1].
Petrochemical and oleochemical feedstocks have traditionally dominated surfactant production [2]. However, the reliance on petrochemical-derived surfactants is inextricably linked to a substantial surge in CO2 emissions [3], a critical greenhouse gas driving climate change and global warming. Recognizing this environmental burden, the scientific community has increasingly prioritized the exploration of greener synthetic pathways and alternative renewable compounds with comparable properties [4,5]. This shift towards the exploitation of renewable feedstock offers a promising avenue for mitigating environmental impact. Furthermore, the imperative to transition away from finite fossil fuel reserves underscores the urgency to identify sustainable resources, ensuring environmental stewardship for future generations.
Fatty amines and their derivatives—including quaternary ammonium compounds, amine oxides, and ethoxylated amines—play critical roles in detergents and cosmetics [6,7] due to their strong cationic surface activity, conditioning properties, and antimicrobial effects. These compounds are commonly used in shampoos, conditioners, creams, and household cleaners, where their amphiphilic structure enables effective emulsification, foaming, and cleansing.
The use of both petrochemical and oleochemical feedstocks has been central to developing cosmetic formulations within the 20th century [6]. However, as awareness of environmental impacts increases, cosmetic formulations are increasingly incorporating renewable components, with companies communicating their adherence to biocircular economy principles. For instance, L’Oréal’s 2023 sustainability report highlights their commitment to bio-based ingredients and waste reduction through circular economy initiatives [7]. Furthermore, industry associations like Cosmetics Europe are also encouraging the adoption of sustainable practices and renewable resources in formulation design [8]. Moreover, policy frameworks such as the EU Green Deal and REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) are placing increasing regulatory pressure on the chemical industry to reduce hazardous substances, minimize carbon emissions, and promote the use of safer, sustainable alternatives. These drivers further encourage the adoption of green and circular strategies for fatty amine production and use.
Scientific publications further corroborate this trend, showcasing research dedicated to the development of bio-based alternatives to traditional cosmetic ingredients [9].
Nevertheless, conventional synthesis routes for fatty amines—such as the nitrile process and alcohol amination—often rely on fossil-derived inputs and involve energy-intensive, chemically harsh processes. These issues have sparked growing interest in alternative production methods that align with green chemistry principles. In particular, the valorization of agro-industrial waste and the use of enzymatic catalysis offer promising pathways to reduce the environmental footprint of fatty amine production. production.
This review explores the structural characteristics, industrial functions, and synthesis methods of fatty amines with a particular emphasis on their roles in detergents and cosmetics, discussing emerging biocircular strategies to reduce their environmental impact. In doing so, it aims to provide a comprehensive understanding of both current applications and future directions for sustainable development in the detergent and cosmetic sectors.
2. Fatty Amines Properties and Current State Production
2.1. Fatty Amines Properties
Fatty amines include primary, secondary, or tertiary ones, based on with the number of fatty alkyl or methyl chains (where R ranges from C8 to C22) bound to the nitrogen atom (Figure 1). Typically, the nitrogen is connected to a primary carbon, although variants with secondary or tertiary carbons are available on the market [1].
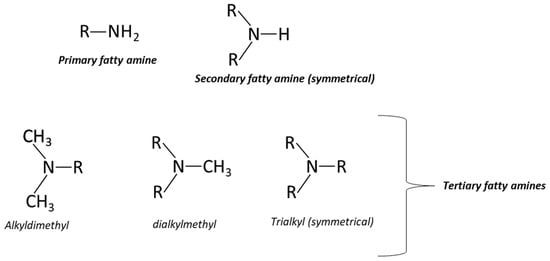
Figure 1.
Primary, secondary and tertiary fatty amines.
Due to the presence of hydrogen atoms bonded to the nitrogen atom, primary and secondary amines exhibit intermolecular hydrogen bonding. This strong secondary inter-action requires a higher energy to disrupt these bonds, resulting in higher boiling points than those of alkanes of similar molar mass. However, alcohols, which form even stronger hydrogen bonds, possess higher boiling points than comparable amines. Conversely, tertiary amines, lacking hydrogen atoms directly bonded to nitrogen, are incapable of intermolecular hydrogen bonding. Consequently, their boiling points are comparable to those of ethers [10].
Fatty amines, in their free base form, exhibit very low solubility in water. However, their salts, such as acetates or hydrochlorides, as well as ethoxylated amines with more than 5 moles of ethylene oxide (EO), are readily dissolved in water. Conversely, most fatty amines and their derivatives exhibit good solubility in organic solvents. For example, commercially available fatty amine acetates are highly soluble in 95% ethanol. It is worth noting that water, though a poor solvent for the base amines themselves, is soluble within them, leading to the formation of hydrates.
The lone pair of electrons on the nitrogen atom imparts the basic behavior to the fatty amines. In aliphatic amines, basicity increases with the number of electron-donating substituents attached to the amino group. This effect enhances the electron density at the nitrogen atom, thereby increasing the availability of the lone pair. Consequently, aliphatic amines exhibit greater basicity than ammonia. Conversely, aromatic amines display lower basicity than those of aliphatic amines due to the mesomeric effect of the aromatic ring, which delocalizes the lone pair and reduces its availability. The relative basicity of amines is typically observed in the following order:
secondary amines > primary amines > tertiary amines > ammonia [11].
2.2. Fatty Amines Production
Fatty acids or esters, which are employed as raw materials for the synthesis of fatty amines, originate from animal or vegetable oils and fats, showing distinct hydrocarbon chain lengths and degrees of unsaturation. However, depending on geographic location and raw material availability, fatty amines can also be synthesized from petrochemical feedstocks via oxoaldehydes, alcohols, or olefins. Nitrile hydrogenation, fatty compound amination, alcohol amination, and N-alkylation represent the prevailing industrial processes for fatty amine production. These synthetic routes involve a variety of reaction steps, including amidation, dehydration, hydrogenation, and dehydrogenation, necessitating the use of multifunctional catalysts.
2.2.1. Nitrile Process
In the traditional fatty nitrile process, triglycerides undergo initial hydrolysis, followed by the catalytic reaction of the resulting fatty acids with ammonia (NH3) in a liquid-phase environment. This reaction is conducted in the presence of metal oxide catalysts, typically alumina or zinc oxide. The main involved chemical reactions are reported in Figure 2.
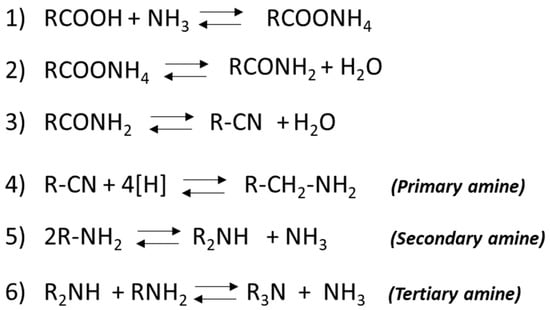
Figure 2.
Main reactions involved in the production of amines by the nitrile route.
All of the aforementioned reactions are equilibrium-limited, so careful control of the reaction conditions is required, to allow the selective fatty amine production. The nitrile formation reaction is typically conducted at temperatures between 280 and 360 °C in the liquid phase for long reaction times. An excess of ammonia (2–4 times the stoichiometric requirement) is introduced, and water is continuously removed from the reactor to shift the equilibrium towards nitrile formation. Subsequently, fatty nitriles undergo hydrogenation, which is generally catalyzed by nickel catalysts, to yield the desired fatty amines. It is important to note that the hydrogenation of nitriles results in a mixture of primary, secondary, and tertiary amines, as illustrated in Figure 3. The use of excess ammonia during hydrogenation inhibits ammonia release, thus suppressing the formation of secondary and tertiary amines. Conversely, the limitation of the ammonia added into the reaction system promotes the formation of secondary amines. In the absence of ammonia, or if any generated ammonia is removed from the system, tertiary amine formation prevails. The choice of the appropriate hydrogenation catalyst significantly influences the selectivity of the process. For example, nickel or cobalt catalysts favor the formation of primary amines, while copper chromite catalysts promote secondary ones [12].
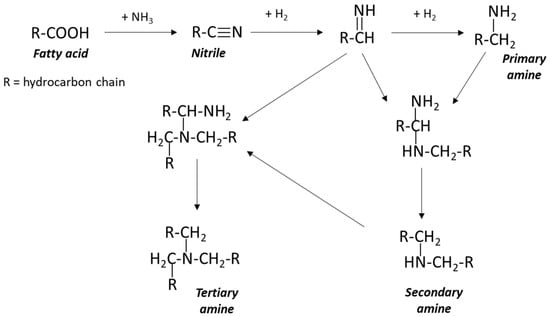
Figure 3.
Scheme of nitrile process for fatty amines synthesis.
While the ‘nitrile route’ is a well-established industrial process with a long history of application (Figure 3), several persistent challenges remain. These include the utilization of toxic heterogeneous metal catalysts, the multi-step nature of the process, the requirement for harsh reaction conditions (elevated pressure and temperature), and the necessity for developing stringent safety protocols due to the use of molecular hydrogen. Furthermore, the inherent limitations in catalyst selectivity [12] often result in undesired mixtures of primary, secondary, and tertiary amines, which can pose significant separation challenges. In batch implementations of the conventional nitrile process, prolonged exposure of fatty acids to high temperatures in the liquid phase leads to the occurrence of various side reactions, such as isomerization, polymerization, Diels-Alder, Piria, and peroxidation. Side reactions are especially common when unsaturated fatty acids are present. In batch reactor systems, the fatty nitriles produced can also undergo hydration, leading to the formation of fatty amides. As a result, the final mixture often contains residual fatty acids, fatty nitriles, fatty amides, and other unwanted byproducts, which lowers the yield of fatty nitriles and complicates the purification process. To improve nitrile yields, researchers have investigated the use of continuous flow reactors filled with catalysts and supplied with fatty acids and ammonia (NH3) [13]. This continuous process is promising for reducing the side reactions, exploiting the better interaction between ammonia and fatty acids, the shorter reaction durations (10–60 min), and the ongoing removal of products.
2.2.2. Amination of Alcohols
An alternative route for the synthesis of fatty amines involves the reaction of fatty alcohols with ammonia, or a low-molecular-weight primary or secondary amine, to produce the corresponding primary, secondary, or tertiary fatty amines, according to the following reaction:
R − OH + NH3 ⇌ R − NH2 + H2O
Fatty alcohols are obtainable from natural sources, fats and oils, or petrochemical feedstocks. The manufacturing processes for fatty alcohols vary, according to the raw material sources, distinguishing between natural and petrochemical ones. By careful tuning of the reaction conditions, high yields of primary, secondary, or tertiary amines can be achieved. Catalysts and reaction conditions play a crucial role in determining the efficiency and outcome of alcohol amination reactions [14]. By careful control of the reaction conditions, high yields of primary, secondary, or tertiary amines can be achieved. Fatty acids are typically the preferred raw material for fatty amine production due to the higher cost associated with fatty alcohols. Because of the reaction pathways leading to primary, secondary, or tertiary amines, the resulting crude product is a mixture of all three amine types. These crude products often possess sufficient purity for direct sale and use as ‘technical’ grade amines. Anyway, if higher purity is required, they can be purified through distillation [15].
2.2.3. Amination of Fatty Compounds and N-Alkylation
Over the past several years, numerous approaches have been explored to enhance the overall performance of fatty amine production. The direct synthesis of fatty amines from triglycerides and hydrogen in the presence of catalysts has been achieved through the N-alkylation of various aniline and alkyl derivatives, yielding the corresponding secondary and tertiary amines (Figure 4). This chemical process entails the introduction of alkyl groups onto a nitrogen atom in amines, resulting in a more highly substituted amine.
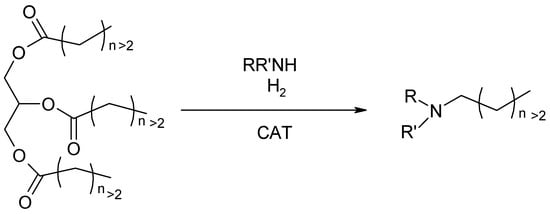
Figure 4.
N-alkylation of triglycerides using hydrogen and amines.
N-alkylation can be accomplished via diverse reactions, including the use of alkylating agents such as alkyl chlorides, alkyl bromides, or epoxides. More recently, the direct reaction of triglycerides with ammonia has been successfully demonstrated, generating the corresponding primary fatty amines and symmetrical secondary ones. These progresses, even if noteworthy, still rely on the use of metal catalysts, harsh reaction conditions, showing selectivity issues [16].
3. Fatty Amines in Detergents
3.1. Detergents Formulations
A detergent encompasses any substance that functions as a cleaning agent, including traditional lye soap, alkaline dishwashing compounds, and solvent cleaners [17]. Nowadays, the term ‘detergent’ is often restricted to cleaning agents containing synthetic surfactants derived from petrochemicals or other sources. Surfactants are the principal components of a detergent but, other compounds can also play an important role to enhance the properties of the final formulation, such as complexing agents, builders, enzymes, alkalis, ion exchangers, foaming stabilizers, anti-redeposition agents, whitener or optical brightener, corrosion inhibitor, viscosity improver, solvents, conditioner or softener. Moreover, the physical appearance of the detergent can also be modified by the addition of dyes, opacifiers and perfumes [18].
Detergent molecules are amphiphilic, possessing two distinct structural components: an extended, hydrophobic hydrocarbon chain and a polar or charged hydrophilic headgroup (Figure 5). Typically, the hydrocarbon chain is a linear, saturated alkane, where hydrophobicity increases with chain length [18]. There is an increasing interest towards the use fatty amines and their derivatives in formulations of detergents for surface cleaning and laundry uses. The use of fatty amines can provide good emulsifying and wetting properties for specific applications, whereas their solubility in the formulation can be improved by adding fatty alcohols [19].
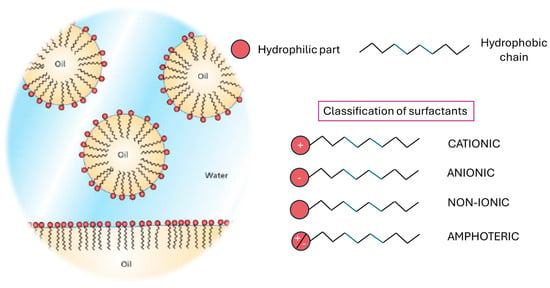
Figure 5.
Schematic representation of oil removal from surfaces via surfactant-enriched water (on the (left)), combined with surfactant structure and classification overview (on the (right)).
Fatty amines and their derivatives come from natural fatty acids and present different chemical behavior which specially depends on the hydrophobic chain. Short or mid chain surfactants normally give to a less foaming detergent since the hydrophobic chain is shorter. On the other side, for a better foaming effect like laundry application for instance, longer chain surfactants are used.
Foaming effect of a detergent is commonly considered to have no cleaning effect. Nevertheless, current research shows that foaming detergents can be used more efficiently for cleaning solid surfaces than non-foaming detergents [20]. In any case, it is important to identify the best combination among all components to achieve the best performances for each application. Remarkably, some computational methods have been developed to design optimal formulations of detergents [19].
3.1.1. Surfactants
A surfactant, an abbreviation for ‘surface-active agent,’ is a compound that reduces the interfacial tension between two liquids, a gas and a liquid, or a liquid and a solid. This property facilitates the formation of stable mixtures, such as emulsions, between immiscible substances like oil and water. A surfactant molecule is amphiphilic, meaning it contains both a water-attracting (hydrophilic) polar head and an oil-attracting (hydrophobic) non-polar tail. The polar head interacts readily with polar substances like water and avoids non-polar ones such as oils and several organic molecules. In contrast, the non-polar tail is drawn to oily substances and repelled by water. This dual affinity causes surfactant molecules to align themselves at the boundary between oil and water. When added to an oi/water mixture, surfactants surround the oil droplets, positioning their hydrophilic heads outward toward the surrounding water phase. This process forms an emulsion, dispersing the oil as micelles or small droplets (Figure 5), thereby enhancing its apparent solubility in water and enabling the removal of oil and dirt during rinsing [21].
Surface-active substances modulate the structure of interfacial layers and influence mass and energy transfer across these boundaries. Surfactants, whether naturally occurring or synthetic, alter the solubility and physicochemical properties of other microconstituents, thereby affecting their accumulation and distribution at phase interfaces. In surfactant molecules, the hydrophobic moiety typically comprises a relatively long aliphatic hydrocarbon chain (from 10 to 20 carbon atoms). The hydrophobic chain in a surfactant can originate from sources such as fatty acids, alkylbenzenes, alcohols, alkylphenols, or polyoxypropylene. The hydrophilic head group may consist of functional groups like sulfonate, sulfate, carboxylate, quaternary ammonium, sucrose, polypeptides, or polyoxyethylene units [17].
Surfactants can be classified according to the molecular structure and the electric charge of the polar moiety, distinguishing between anionic, cationic, non-ionic and am-photeric surfactants.
Anionic surfactants are defined by the presence of a negatively charged hydrophilic head group, typically associated with a positively charged counterion. Upon dissolution in water, these surfactants ionize, releasing the negatively charged head group. This negative charge facilitates binding to positively charged species. It is important to note that clay particles generally exhibit a net negative charge, not positive. Anionic surfactants are known for their ability to generate copious foam and exhibit potent detergency, effectively removing dirt and, potentially, essential epidermal lipids. Examples of anionic surfactant classes include sulfonate salts, alcohol sulfates, linear alkylbenzene sulfonates (LAS), phosphoric acid esters, and carboxylate salts. Linear alkylbenzene sulfonate (LAS) remains a dominant anionic surfactant in industrial applications [22].
Cationic surfactants are characterized by a positively charged hydrophilic head group. This positive charge distinguishes them from anionic surfactants and leads to their strong adsorption onto negatively charged surfaces. Positive charges are considered related to their antimicrobial action because it induces pathogens cell walls disruption [23,24]. Cationic surfactants are less commonly employed in laundry detergents compared to anionic surfactants. This is primarily due to their strong affinity for, and limited desorption from, negatively charged fabrics and soil, which are prevalent under typical conditions. However, this property of strong adsorption is exploited in fabric softeners. The global production of cationic surfactants is estimated to be approximately 500,000 metric tons annually [25]. Their use in fabric softening is well documented [26].
Organic amines—including primary, secondary, and tertiary fatty amines—are widely recognized as key intermediates in the production of cationic, amphoteric, and nonionic surfactants. Among cationic surfactants, fatty amine salts and quaternary ammonium compounds are the most used. Although fatty amines are not cationic surfactants, they are classified as such due to their prevalent use in acidic environments, where they form cationic salts. As previously mentioned, amines are categorized as primary, secondary, or tertiary based on the number of alkyl groups attached to the nitrogen atom (1, 2, or 3, respectively). When the nitrogen atom forms four bonds with carbon atoms, the resulting compound is a quaternary ammonium compound. In quaternary ammonium structures, the nitrogen atom donates a lone pair of electrons to form the fourth bond, thereby acquiring a permanent positive charge. Alkylammonium ions are generated in acidic media through the protonation of the amine. The resulting salt, typically a chloride or bromide, exhibits water solubility due to the solvation of the cationic species [1].
As discussed in Section 2.2, the synthesis of amine or alkylammonium surfactants involves a series of chemical reactions that exhibit varying degrees of selectivity and may not proceed to completion. Consequently, only a fraction of the initial raw material is converted into the desired product, contributing to the generally higher cost of cationic surfactants compared to anionic surfactants, such as sulfonates or sulfates. Therefore, cationic surfactants are reserved for applications where their unique properties, such as a positive charge or bactericidal activity, are indispensable. They serve as antistatic agents in fabric softeners and hair conditioners. In textile manufacturing, they function as dye retarders, competing with dye molecules for binding sites and thereby promoting uniform coloration. Similarly, their corrosion inhibition in acidic environments arises from competition with H+ ions. Ammonium salts and quaternary ammonium compounds are employed as collectors in mineral flotation. Asphalt emulsions for roadway pavement and protective coatings are often stabilized by fatty amine salts (in acidic pH) or quaternary ammonium compounds (in neutral pH). Benzalkonium chloride and alkyltrimethylammonium chloride or bromide are utilized as antiseptic, disinfectant, and sterilizing agents. Furthermore, they are incorporated as additives in nonionic detergent formulations for corrosion inhibition and, in small quantities, in anionic powdered detergents to enhance detergency synergistically [27].
Nonionic surfactants, as their name suggests, lack ionic constituents. Over the past 35 years, nonionic surfactants have significantly expanded their market share, now accounting for approximately 40% of global surfactant production. The nonionic surfactant market is predominantly occupied by ethylene oxide adducts (EOAs) of alkylphenols and fatty alcohols. However, there is a growing trend towards the production of detergent-chain-length fatty alcohols from both natural and petrochemical sources, driven in part by the phase-out of alkylphenol ethoxylates (APEOs) in certain applications. This shift is motivated by environmental concerns, as APEOs exhibit slower biodegradation rates and raise concerns about the toxicity of phenolic residues. Nonionic surfactants are versatile, serving as effective detergents, wetting agents, and emulsifiers. Certain nonionic surfactants also possess desirable foaming properties. These compounds are utilized across a wide range of industries, including textiles, cleaning products, agriculture, polymers, paper production, leather processing, cosmetics, pharmaceuticals, and personal care. Traditionally, the primary feedstocks for the nonionic surfactant sector have been triglycerides of fatty acids, sourced from both animal fats and vegetable oils, encompassing both saturated and unsaturated fatty acids [28]. The most used non-ionic surfactants are ethoxylated linear alcohols, followed by fatty acid esters, ethoxylated alkyl phenols and amine and amide derivatives. Other well-known non-ionic surfactants are alkylpolyglucosides, poly(ethyleneoxide-co-propylene oxide), polyalcohols, ethoxylated polyalcohols and mercaptans and their derivatives.
Amphoteric surfactants possess both anionic and cationic functional groups, enabling them to exhibit either anionic, cationic, or nonionic characteristics depending on the ambient pH. Typically, they feature an amine salt or quaternary ammonium group (cationic) and a carboxylate, sulfonate, or phosphate group (anionic). They are categorized as pH-sensitive, changing their charge with pH, or non-pH-sensitive. pH-sensitive amphoterics behave as anionic surfactants in alkaline conditions, cationic in acidic conditions, and nonionic at neutral pH. These surfactants offer several advantages, including antibacterial properties, low toxicity, hard water resistance, and compatibility with other surfactants. They find applications in detergents, shampoos, and personal care products. For instance, quaternized fatty acid amides glycine and amido propyl betaine, derived from coconut, are used in mild formulations like baby shampoos due to their low irritation. Amphoteric surfactants are often combined with fatty alcohol sulfates to enhance solubility, viscosity, foam stability, and reduce irritation [26].
3.1.2. Surfactants and Other Ingredients for Shampoo
Hair is a fibrous composite biomaterial generated by hair follicles, consisting of proteins, lipids, water, and trace elements such as pigments. Proteins are the main structural component, making up roughly 65% to 95% of hair’s total mass. The predominant protein is keratin—a strong, fibrous molecule primarily built from the amino acids tyrosine, glycine, and cysteine [29].
Hair is made up of two parts [30], the hair follicle, and the hair shaft. The hair follicle is the point from which the hair grows, while the part of the hair seen above the skin is called the hair shaft. This is made up of three main layers:
- Cuticle: This is the outermost layer of the hair shaft, composed of overlapping, scale-like cells that shield the hair and contribute to its visual qualities, such as shine and smoothness.
- Cortex: Located beneath the cuticle, the cortex houses the pigments responsible for hair color and provides strength and elasticity to the hair strand.
- Medulla: This is the innermost core of the hair, running through the center of the strand. Its presence and structure can vary depending on hair type and thickness.
The cortex, which makes up about 90% of the hair’s total mass, consists of elongated cortical cells (Figure 6a). These cells are filled with alpha-helical keratin filaments that run parallel to the length of the hair shaft. These filaments are embedded in a sulfur-rich, amorphous protein matrix, primarily composed of keratin. The cortex is essential for the hair’s mechanical strength, as the keratin filaments are interconnected by covalent disulfide bonds, forming a filamentous network that provides tensile resilience. The helical conformation of the keratin chains contributes to the hair’s elasticity. Additionally, the cortex serves as a reservoir for moisture and houses the pigments responsible for hair color [31].
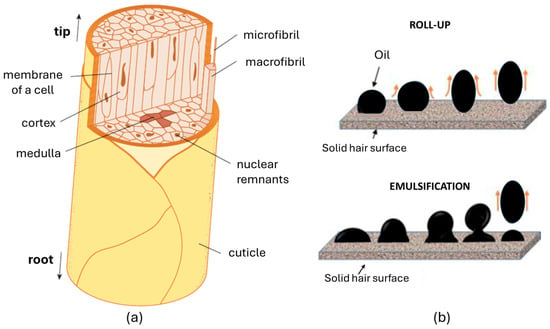
Figure 6.
(a) Structure of hair; (b) Mechanism of oil removal from a surface by roll-up and emulsification.
Hair proximal to the scalp, recently emerged from the hair follicle, exhibits minimal exposure to physical and chemical stressors such as heat styling and hair dyes. These processes can compromise the cuticle layer, diminishing its integrity along the hair shaft from root to tip. The cuticle is naturally coated with sebum, an oil secreted by sebaceous glands near the hair follicle. This sebum provides a protective barrier for the cuticle’s surface and effectively adsorbs environmental grease, dirt, and styling product residues. Consequently, frequent hair washing is necessary to maintain cleanliness. Due to its hydrophobic nature, sebum cannot be effectively removed by water alone; therefore, shampoos are employed to facilitate cleansing [32].
Shampoos are liquid cleansing formulations based on synthetic detergents, specifically designed to remove sebum from the scalp. In some individuals, it may help prevent the development of folliculitis and seborrheic dermatitis when used frequently [33]. Their primary function is to eliminate sebum, sweat, desquamated skin cells, styling product residues, and environmental dirt from hair. While shampoos contribute to hair cleanliness, esthetic enhancement is a secondary objective, primarily addressed by conditioners. Shampoos utilize surfactants, which are amphiphilic molecules. The lipophilic domain interacts with sebum, while the hydrophilic domain interacts with water, facilitating the removal of sebum during rinsing.
Surfactants are central to shampoo formulations, fulfilling a multitude of functions. Their primary role is to detach and remove soils from the hair, but they also contribute to foam generation, viscosity enhancement, suspension of active ingredients, and solubilization of fragrances. Furthermore, surfactants are crucial for the efficacy of cationic polymer-based deposition systems, which deliver active ingredients to the hair and scalp. Finally, careful selection and blending of surfactants are essential to minimize irritation to the skin, hair, and eyes [34].
The detergency of surfactants varies depending on the type of soil present on hair. These soils can be categorized into four primary groups: sebum, desquamated skin cell debris, particulate air pollutants, and hair product residues. The removal of sebum by surfactants is proposed to occur through a combination of four mechanisms: (a) Roll-up; (b) Spontaneous emulsification; (c) Penetration; (d) Solubilization. While evidence supports each of these mechanisms, their relative contributions remain uncertain. It is likely that sebum removal is achieved through the synergistic action of all four processes.
The roll-up mechanism (Figure 6b) proposes that surfactants reduce the interfacial tension at the sebum/water and hair/water interfaces, thereby driving the separation of oil from the hair surface. This reduction in interfacial tension allows for an increase in the surface area of both interfaces, causing sebum lipids to coalesce into spherical droplets and detach. Once detached, these lipid soils remain dispersed in the aqueous surfactant solution, as the surfactant-modified hair surface becomes less attractive for oily material adhesion. While theoretically plausible, the roll-up mechanism is predicated on the assumption that sebum is a freely flowing liquid. However, sebum is typically more viscous and waxier, particularly with age. Furthermore, it has been suggested that the roll-up mechanism may be more effective on damaged hair, which exhibits a more hydrophilic surface.
Spontaneous emulsification, illustrated in Figure 6b and considered an extension of the roll-up mechanism, suggests that lowering the interfacial tension between lipids and water promotes the expansion of the interface. This expansion causes large lipid deposits to form smaller protrusions or ‘buds,’ which then spontaneously break off into emulsified droplets that can be easily removed. This mechanism is particularly relevant for cleaning large lipid accumulations that are too substantial for the basic roll-up process. However, like the roll-up mechanism, it assumes that the lipid soil remains fluid and mobile. The penetration mechanism of detergency, first introduced by Lawrence in 1959, is based on the finding that certain soaps and surfactants can infiltrate insoluble lipid soils. At the interface between the soil and water, they form liquid-crystalline phases. When the system is agitated, this loosened material is dislodged, revealing a new layer of lipid soil beneath. The micelle-based mechanism for removing lipid soils involves the migration of lipid molecules from the contaminated surface into micelles that are adsorbed at the oil/water boundary. This process depends on several kinetic factors: the rate at which micelles adhere to the hair surface, the transfer of lipids into the micelle core, and the release of lipid-loaded micelles into the surrounding solution. This mechanism is particularly notable for its ability to selectively target and remove lipid-based contaminants, as it operates at a molecular level, rather than at bulk level.
Anionic detergents are the most prevalent surfactants used in basic cleansing shampoos due to their exceptional efficacy in removing sebum from the scalp and hair. However, they increase the net negative charge on the hair surface, leading to frizz and increased friction. Consequently, shampoo formulations often incorporate conditioners and anti-frizz agents to mitigate these effects [35].
There are several common detergents in shampoos categorized within the anionic group [32,34]:
- Lauryl sulfates: they are used with water (main component) in most shampoos designed to produce good hair cleansing. Examples of lauryl sulfate detergents include: sodium lauryl sulfate (NaC12H25SO4), triethanolamine lauryl sulfate (C18H41NO7S), and ammonium lauryl sulfate (C12H29NO4S). These compounds are popular primary cleansers because they work well in both hard and soft water, are easily rinsed and produce rich foam. Although they are excellent cleansers, they are not delicate on the hair. A secondary detergent and possible use of a conditioning agent are thus necessary. Lauryl sulfates are commonly used in shampoos for oily hair.
- Laureth sulfates: they are the most used primary detergents in general shampoos designed for normal-to-dry hair. Generally, they produce abundant foam. Examples are: triethanolamine laureth sulfate (C12H25(OCH2CH2)3OSO3H·(HOCH2CH2)3N), sodium laureth sulfate (CH3(CH2)10CH2(OCH2CH2)nOSO3Na), and ammonium laureth sulfate (C12H25(OCH2CH2)nOSO3NH4).
- Sulfonates: Sulfonates—particularly alkylbenzene sulfonates—are extensively used as anionic surfactants in a wide range of cleaning products. They efficiently solubilize and remove dirt and oils. This makes them especially suitable for formulations such as soaps, shampoos, and laundry detergents. They are often used in sulfate-free shampoos. Sulfonate aromatic detergents, specifically those containing alkylbenzene sulfonates (ABS), are generally considered problematic due to their non-biodegradability while some sulfonate detergents, like those with linear alkylbenzene sulfonates (LAS), are biodegradable [36].
- Sarcosines: they are generally not used as primary detergents, because they do not effectively remove sebum from the hair. Nevertheless, they are good conditioners, so they are commonly used as the second (or third) detergent listed on labels reporting the shampoo ingredient list. Sarcosines are used in conditioning and dry hair shampoos. Detergents of this class are generally mentioned on the shampoo label as lauryl sarcosine (C15H29NO3) and sodium lauryl sarcosinate (C15H28NO3Na).
- Sulfosuccinates: they represent a class of very effective detergents, suitable in removing sebum from oily hair. Thus, they are commonly used as secondary surfactant in shampoos for oily hair. Examples of this class are sodium dioctyl sulfosuccinate and disodium oleamine sulfosuccinate.
Cationic surfactants are also very important in shampoo formulations. In fact, anionic surfactants, while effective for sebum and dirt removal, increase negative hair surface charge, causing frizz and friction. Secondary surfactants are added to mitigate these effects and reduce static electricity. The surfactants displaying this property form the basis of conditioners, and they are typically cationic. This mechanism works by neutralizing the negative charge on the hair fiber through the introduction of positively charged agents, while simultaneously lubricating the cuticle to decrease the fiber’s hydrophilicity. These formulations typically include ingredients with antistatic and lubricating properties, which fall into five main categories: polymers, oils, waxes, hydrolyzed amino acids, and cationic compounds. Among these, silicones stand out as the most effective and widely used conditioning agents.
Cationic ingredients are often used into various shampoo formulations alongside anionic surfactants with the aim of counteracting charges and forming a cationic–anionic complex. This complex results in a charge-neutral hydrophobic ingredient. Trimethyl alkylammonium chloride ([R−N(CH3)3]+Cl−), Benzalkonium chloride (C6H5CH2N(CH3)2R+Cl−), and cetrimonium chloride ([C16H33N(CH3)3]+Cl−) are cationic surfactants that are cosmetically acceptable for hair conditioning products [35].
Quaternary Ammonium Compounds (quats), the primary cationic surfactants, enhance conditioner spreadability and provide antimicrobial preservation. However, quats can cause skin and respiratory irritation, with some inducing severe allergies [37]. Certain quats, like benzalkonium chloride, are endocrine disruptors [37]. Furthermore, they pose environmental risks due to aquatic toxicity [38].
Beard et al. [39] investigated the adsorption of long-chain quaternary amine surfactants (alkyl and ester quats) onto human hair, attributing the process to van der Waals, electrostatic (ionic and dipolar), and hydrogen bonding forces. Ionic interactions, highly pH-dependent, are dominated by surface sulfonate groups due to their full ionization at neutral pH. Ester quats, with –C–O–, –C–OH, and –C=O structures, exhibit broader dipolar interactions compared to alkyl quats, which rely primarily on quaternary nitrogen-sulfonate interactions. This multi-site interaction of ester quats allows a single molecule to compensate multiple surface sulfonates, potentially via –OH groups. Alkyl quat adsorption requires migration to sulfonate sites. Van der Waals forces drive alkyl tail adsorption by minimizing system energy through transfer from polar solution to the hair surface.
While shampoo varieties appear numerous, their basic ingredient categories are largely consistent (Figure 7). They are foaming agents, thickeners and opacifiers, sequestering agents, conditioners in shampoo formulations, pH adjusters and specialty additives.
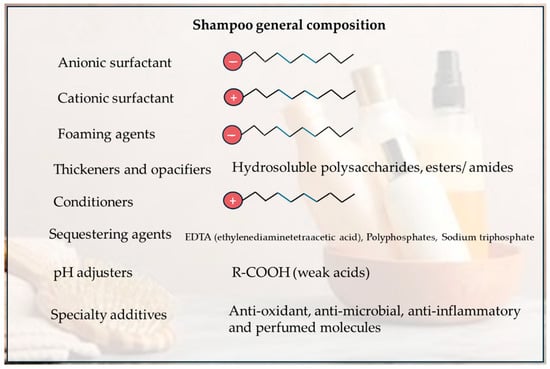
Figure 7.
A scheme summarizing the general composition of a shampoo.
Consumers often equate high foam with superior cleaning, though technically, foam and cleansing efficacy are distinct. Shampoos utilize foaming agents, primarily surfactants, to generate and stabilize air bubbles by reducing surface tension at the air/water interface. Foam stability relies on surfactant concentration at the lamellae. While foam aids detergent spread across hair and scalp, it does not directly clean. Sebum inhibits foam formation, explaining reduced foaming on initial washes. Consequently, corticosteroid shampoos, despite potentially lower foam, can still provide adequate cleaning.
Thickeners enhance shampoo viscosity, influencing consumer perception of quality. Opacifiers create a pearlescent appearance, offering only esthetic benefits, not improved cleaning.
Sequestering agents, crucial shampoo components, chelate calcium and magnesium ions, preventing ‘scum’ formation. This scum, if allowed to form, dulls hair and irritates the scalp, potentially exacerbating seborrheic dermatitis. Therefore, shampoo is preferred over bar soap for hair cleansing.
While shampoos cleanse, over-cleansing leaves hair harsh and unmanageable. To counteract this, conditioners are added, especially in shampoos for dry or treated hair, imparting manageability, antistatic properties and gloss.
Conditioning agents are generally classified into four main types: film-forming polymers, hydrolyzed proteins, cationic surfactants (such as quaternary ammonium compounds), and silicones like dimethicone (C2H6OSi)n and cyclomethicone. Fatty amido-functional tertiary amines are also used and are often available in convenient flake form. These can be neutralized with acids such as citric or lactic acid to produce cationic amine salts, which serve as the primary conditioning agents in products like crème rinses, finishing rinses, and conditioners. A key advantage of these amine salts over quaternary ammonium compounds is their ease of rinsing, which helps prevent surfactant buildup on the hair.
Conditioners lubricate the hair surface and help align the hair fibers, which results in a smoother appearance of the cuticle layer. This effect is particularly beneficial for permed hair, where the cuticle tends to lift. By smoothing and coating the hair, conditioners help protect the underlying cortex from environmental damage. Common conditioning ingredients include hydrolyzed animal proteins, glycerin (C3H8O3), dimethicone, simethicone ((C2H6OSi)n·(SiO2)m), polyvinylpyrrolidone (C6H9NO)n, propylene glycol (C3H8O2), and stearalkonium chloride (C27H50ClN). Protein-based conditioners are occasionally beneficial for damaged hair, as they can temporarily repair split ends—medically known as trichoptilosis—by binding to exposed keratin in the cortex. This property, known as substantivity, allows proteins to hold the split fragments together until the next wash. Regarding the control of pH, shampoo-induced hair damage can be mitigated by preventing alkaline-induced cuticle swelling. pH adjusters, such as glycolic acid, maintain a slightly acidic pH, which is particularly important for protecting chemically treated hair.
The final and arguably most influential category of shampoo ingredients is the specialty additives. These components differentiate one shampoo from another, often serving as the basis for marketing claims. Some of these ingredients offer functional benefits, while others are included simply to align with current trends. For instance, in the 1970s, beer was added to shampoos as a conditioning agent, though such formulations have since disappeared from mainstream markets. In the 1990s, glycolic acid gained popularity, though its role was limited to pH adjustment. More recently, the focus has shifted to incorporating conditioning vitamins like Vitamin B5 (panthenol) [33], along with trendy botanicals such as tea tree oil. It is important to note that shampoo formulations are frequently updated to reflect evolving consumer preferences and marketing strategies [40].
3.2. Fatty Amines and Their Derivatives in Detergents Formulations
Fatty amines may be considered compounds with the structure reported in Figure 8 in which R1 is an alkyl group and R2 and R3 may be alkyl groups or hydrogen in any combination. The unshared pair of electrons allows these molecules to undergo salt formation with acids and exhaustive alkylation with alkyl halides.
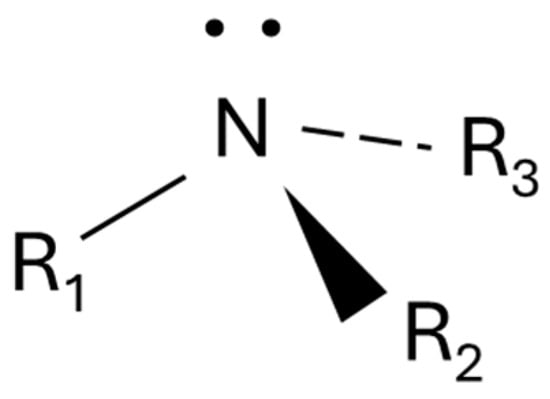
Figure 8.
General structure of a fatty amine.
These amines can be classified into the alkylolamines and the alkyl amines. Alkylolamines available for detergent are mono-, di-, and triethanolamine, and mono-, di-, and tri-isopropanolamines. Moreover, several alkyl-alkylolamines, such as diethyl ethanolamine, morpholine, and diglycolamines, are commercially available. The alkylamines having the general formula, R”-RN-R’, where R” and R’ can be either an aliphatic moiety or hydrogen, are used to neutralize alkyl benzene sulfonic acids or fatty acids for emulsification or detergent purposes [17].
Careful evaluations about the use of alkyl amines as detergents confirm that these compounds work as chemical intermediates. Before their use, amines are typically trans-formed into more stable and functional derivatives, such as ethoxylates, propoxylates, amine oxides (for tertiary amines), or quaternary ammonium compounds. When a free amine is directly added to a formulation, it often reacts in situ with other components, resulting in the formation of an active amine-based derivative, that performs the required function [15]. These amine-derived compounds are particularly attractive, so a specific discussion on these compounds will be provided in the next section.
3.2.1. Amine Oxide
An amine oxide contains an amine group (NH2) and an oxygen atom with a double bond (Figure 9). Generally amine oxides are prepared by adding the amine to 35% solutions of hydrogen peroxide, working for 1 h at 60 °C under vigorous stirring. To prevent the formation of gel, some water must be added, whose amount depends on the kind on used amine. Hydrogen peroxide (H2O2) is the most used oxidizing reagent, but peracids are also considered.
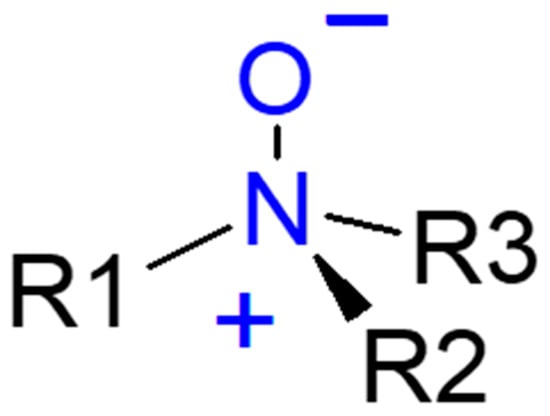
Figure 9.
General structure of amine oxide.
In water-based solutions fatty amine oxides show non-ionic or cationic properties depending on pH. Under neutral or alkaline conditions they are non-ionized hydrates. The main application of dimethyl fatty amine oxides is in food industry cleaning and disinfecting. In the detergent industry, some of the most used amine oxides include:
- Alkyl Dimethyl Amine Oxides (ADAO): These are versatile amine oxides with a range of alkyl chain lengths, typically C10-C16. They are widely used in various detergents due to their excellent cleaning and foaming properties. The interaction of solid alkyl dimethylamine oxide and di-tallow dimethyl ammonium chloride (DTMAC, [(CnH2n+1)2N(CH3)2]Cl) and di-tallow dimethyl ammonium sulfate (DTMAS, [(CnH2n+1)2N(CH3)2]2SO4) quats in different types of fabric softener systems was studied by focusing on their synergistic behavior. Softening, wetting, whiteness retention and static electricity build-up were evaluated for laundry rinses, laundry detergents and dryer sheets. In fabric rinses, combining amine oxide with DTMAC enhanced the wetting of cotton towels due to a synergistic effect. While this synergy was not seen in laundry detergents, formulations with amine oxide still outperformed those with DTMAC alone, offering better cleaning without the blotchy residue often caused by the quaternary salt [41]. Alkyl dimethylamine oxides also showed pronounced antimicrobial activity if used alone or in combination with alkyl betaines [42].
- Coco Amine Oxide: Derived from coconut oil, coco amine oxides are biodegradable and often used in environmentally friendly detergent formulations. They exhibit good surfactant properties and are particularly effective in dishwashing detergents.
- Lauryl Amine Oxide, like C12H25N(CH3)2O Lauryl amine oxides are derived from lauryl alcohol and are commonly used in laundry detergents, household cleaners and foaming agent for skin and hair. They provide excellent cleaning performance and are compatible with other detergent ingredients.
- Behenyl Amine Oxide Behenyl amine oxides, like for instance C22H45N(CH3)2O, are derived from behenic acid and are often used as conditioning agents in hair care products. They provide a soft and silky feel to the hair while also aiding in cleansing.
The effectiveness of these compounds depends on the length of the main alkyl chain and the nitrogen substituents in the amine oxide. For instance, coco alkyl dimethyl amine oxide is a strong foam booster in mild detergents and shampoos, while longer-chain stearyl amine oxides are suitable as hair conditioning agents [43]. Some formulations feature molecules with two amine oxide groups, often modified by replacing the amine hydrogen with ethanol groups. These variants are commonly used as foam boosters in products like bubble baths, hand dishwashing liquids, and baby shampoos [27].
3.2.2. Ethoxylated Fatty Amines
Ethoxylated fatty amines are non-ionic surfactants produced by reacting fatty amines with ethylene oxide, working in the presence of an appropriate catalyst. Their physical form—ranging from liquid to pasty solid—depends on the length of the fatty alkyl chain and the number of ethoxyl groups attached (Figure 10). Ethoxylated fatty amines have both a polar cationic nitrogen atom and a non-ionic polyoxyethylene chain. In this case, the polar character and, more in general, the characteristics of the surfactant molecule, strongly depend on the length of the polyoxyethylene chain. A non-ionic behavior is obtained when the chain is very long (>10 units), while the physical-chemical properties are similar with those of cationic surfactants in the case of medium or short chains.
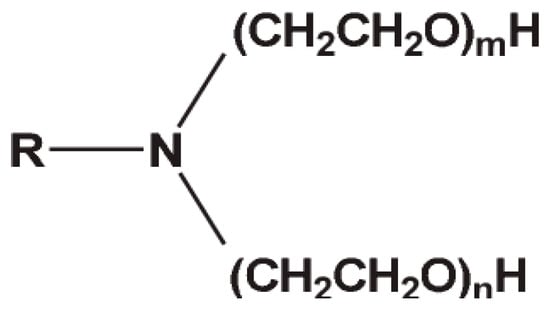
Figure 10.
General structure of polyethoxylated tallow amine. R = tallow chain.
Ethoxylated fatty amines are widely used in both industrial and personal care applications. In cleaners, they act as foam boosters, stabilizers, and wetting agents, while in personal care products like foam baths, shampoos, and aerosol mousses, they enhance foaming performance. In the textile industry, they serve as wetting and dye-leveling agents, and also function as solubilizers in textile processing. Additionally, they are used as anti-corrosion agents in the oil and gas sector and as emulsifiers or adjuvants in agrochemical formulations [44,45,46].
3.2.3. Quaternary Ammonium Compounds
Quaternary ammonium compounds (quats) are organic nitrogen compounds where four carbon atoms are covalently bonded to a central nitrogen atom, forming a positively charged tetrasubstituted ammonium ion paired with a negatively charged anion (Figure 11). This category also includes certain heterocyclic nitrogen compounds with two single bonds and one carbon-nitrogen double bond. Due to their structural flexibility, an extensive variety of higher aliphatic quaternary ammonium salts exist—more than for most other fatty acid derivatives. Their primary appeal lies in their surface activity, making them valuable in both material applications and biological systems.
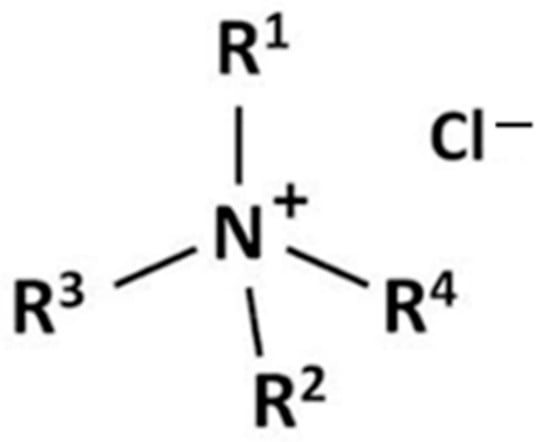
Figure 11.
General structure of a quaternary ammonium compound.
Quaternary ammonium salts are synthesized through exhaustive alkylation of amines, fatty amines, or heterocyclic nitrogen compounds. The specific properties of the final product depend on both the type of amine used and the nature of the alkylating agent, which influence the reaction pathway and product characteristics [47].
Primary and secondary amines are quaternized by methylation with methyl chloride and removal of produced HCl.
R1R2NH + 2CH3Cl → R1R2N+(CH3)2Cl− + HCl ↑
An alternative route can be the reaction between an alkyl bromide and a tertiary amine. This is the most common method to prepare the cetyl-trimethyl-ammonium bromide (CETAB)
C16H33Br + N(CH3)3 → C16H33 − N+(CH3)3Br−
The quaternization of the tertiary amine is carried out with dimethyl or diethylsulfate when a sulfate anion is also required,
R1R2CH3 + (CH3)2SO4 → R1R2N+(CH3)2CH3SO4−
All these synthetic routes result in alkyl ammoniums displaying different alkyl groups [27].
Quaternary ammonium compounds represent the most widely used class of cationic surfactants for several applications [48] including textile conditioning, particularly in laundry detergents and fabric softeners. Among them, distearyl dimethyl ammonium chloride (DSDMAC, C38H80ClN), derived from hydrogenated tallow and composed mainly of C16 and C18 alkyl chains, is the most significant. In Europe, these compounds typically contain chloride as the counterion, while in the U.S., methyl sulfates are also common. The performance of quaternary ammonium compounds is largely influenced by the structure of their substituents—especially chain length, saturation level, and presence of oxygen atoms. Modifications to the saturated tallow chains can impact softening efficiency and formulation pourability. Incorporating hydroxy groups or unsaturated fatty chains improves solubility and enhances the final product properties [49].
Other important quaternary ammonium salts in detergents industry are:
- Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC):(CnH2n+1)N(CH3)2CH2PhCl. ADBAC is widely used in disinfectant and detergent formulations for its broad-spectrum antimicrobial activity against bacteria, viruses, and fungi. It is effective in both acidic and alkaline conditions and is commonly found in household disinfectants and industrial cleaners.
- Dodecyl Dimethyl Ammonium Chloride (DDAC): C22H48ClN. DDAC is another quaternary ammonium salt with strong antimicrobial properties. It is commonly used in healthcare facilities, food processing plants, and institutional settings as a disinfectant and sanitizer. It is effective against a wide range of pathogens, including bacteria, viruses, and fungi.
- Benzalkonium Chloride (BAC): PhCH2(CnH2n+1)N(CH3)2Cl. BAC is a versatile quaternary ammonium salt used in various cleaning and disinfecting products, including detergents, hand sanitizers, and surface disinfectants. It is effective against bacteria, viruses, and algae and is commonly used in both commercial and household settings.
- Cetyl Trimethyl Ammonium Chloride (CTAC): C19H42N(CH3)3Cl. CTAC is often used as a surfactant and antistatic agent in fabric softeners, laundry detergents, and hair care products. It helps to reduce static cling and improve the softness and manageability of fabrics and hair.
- Octyl Decyl Dimethyl Ammonium Chloride (ODDAC): C8H17(C10H21)N(CH3)2CL. ODDAC is commonly used as a surfactant and antimicrobial agent in detergents and disinfectants. It has excellent wetting and emulsifying properties and is effective against a wide range of microorganisms.
- A growing body of literature highlights concerns regarding quats build-up and rinsability, particularly in hair care. Cetyl Trimethyl Ammonium Chloride (CTAC) is used in many products and is generally well tolerated at concentrations between 0.5 and 2.5%, although isolated cases of dermatitis have been reported. Cetylpyridinium chloride (CPC), commonly found in oral care products, has a lower incidence of skin reactions and is often used in milder formulations. Cetyl Trimethyl Ammonium bromide (CTAB), while effective, has been linked to keratinocyte proliferation and sensitization in some studies, suggesting caution with prolonged exposure [12].
3.2.4. Fatty Amines in Fabric and Hair Detergents
As evidenced by the analysis conducted in the preceding sections, fatty amines are fundamental in the detergent industry because of their properties and also those of their derivative compounds. Fatty amines may represent an important example of biobased hydrophobes, but their derivatization (for example to quats) often relies on traditionally nonrenewable chemicals (for instance benzyl chloride, ethylene oxide, dimethyl sulfate, etc.) [5].
Primary (1°) fatty amines are versatile compounds used in a wide range of applications, including cationic surfactants (for textile softeners, dyeing aids, antistatic agents, germicides, and pigment dispersants), amphoteric surfactants (in detergents and shampoos), as well as corrosion inhibitors, asphalt emulsifiers, mold release agents for rubber, anti-caking agents in fertilizers, fuel additives, and sludge inhibitors. They are typically produced by hydrogenating unsaturated fatty nitriles using activated nickel catalysts, with the desired level of olefin retention tailored to their specific end use [50].
Most industrially important aliphatic amines (like alkylamines and diamines), aromatic amines (such as anilines), and amino alcohols (e.g., ethanolamines and propanolamines) are currently derived from fossil resources. For example, ethylenediamine is produced by reacting ammonia (NH3) with 1,2-dichloroethane (DCE), which itself is obtained by chlorinating ethylene. Similarly, ethanolamines are synthesized by reacting ethylene oxide (EO) with excess ammonia. These processes rely on light olefins like ethylene as the carbon source, which are generated from petrol through steam or catalytic cracking—one of the most energy-intensive operations in the chemical industry, responsible for millions of tons of CO2 emissions annually.
Additionally, converting ethylene into intermediates like DCE or EO for subsequent amination is both costly and energy-intensive. Many conventional synthesis routes for amines also present significant drawbacks. For instance, using alkyl halides like DCE can lead to corrosion due to HCl by-product formation and generate large volumes of waste salts. Neutralizing these with NaOH further produces excess NaCl. Ethylene oxide, used in ethanolamine production, is highly toxic and explosive, posing serious handling risks. These challenges highlight the urgent need for more sustainable catalytic processes that align with the 12 principles of green chemistry—emphasizing high atom economy, minimal waste, and the use of safe, renewable feedstocks [51].
Quaternary ammonium salts derive from fatty amines. The primary application of quaternary ammonium compounds is fabric softeners, with a market volume of 40,000 metric tons of 75% active material and an annual growth rate of 4–5%. These products are available in three main forms [52]:
- 3–8 wt % quaternary ammonium compound dispersions for addition to the rinse cycle.
- Quaternary ammonium compound formulations on nonwoven sheets or polyurethane foam, containing a fatty-acid ester transfer agent, for use in clothes dryers.
- Combined detergent, softener, and antistatic formulations for single-cycle use in washing machines.
These products provide fabric softening, antistatic properties, ease of ironing, and odor improvement (via added perfumes). Dimethyl di(hydrogenated tallow) ammonium chlorides or methyl sulfates are the most effective compounds; imidazolinium and amido-amine quaternaries are less effective and can cause fabric yellowing. Recently several studies were carried out regarding innovative biobased softeners for wool or cotton based on fatty acids and aminoacids [53], suggesting that currently industry is searching for these products more sustainable solutions.
The manufacture of organomodified clays represents the second-largest market for quaternary ammonium compounds [54]. In fact organomodified clay are added to drilling mud to improve its lubricity and rheological properties. This application has rapidly grown because of the spreading of well drilling and increased well depths. For this reason, more than 14,000 tons of quaternaries are annually used in the organoclay market [52]. Organoclays are produced through an ion-exchange process, where clay is dispersed in water and mixed with an equivalent amount of a quaternary ammonium compound. The positively charged quaternary ions replace the inorganic cations on the clay surface. The resulting organoclay is then separated, dried, and ground. Common quaternary compounds used include dimethyldi(hydrogenated tallow)ammonium chloride, dimethyl(hydrogenated tallow)benzylammonium chloride, and methyl-di(hydrogenated tallow)benzylammonium chloride. Beyond their use in drilling muds, organoclays also serve as thixotropic agents in paints, coatings, grease additives, foundry additives, cosmetics, resins, and printing inks. Organomodifed clays were also used in the synthesis of polymer [54] and biopolymer [55,56] nanocomposites, thanks to the fact that, during polymer processing by extrusion, the nanometric sheets typical of nanoclays can modify the polymer properties in an extent depending on the composition and achieved morphology. In fact, in general, the desired properties dictate the type of organoclay needed.
Quaternary ammonium compounds (quats) are additionally widely used as bactericides and sanitizers, particularly those derived from benzyl chloride and dimethylalkylamine (alkyl chains C12-C16). These quats are favored over phenols and chlorine-based disinfectants due to their non-irritating, odorless, and long-lasting properties, making them suitable for food processing, restaurants, dairies, and hospitals. Trimethylalkyl quats, like trimethyloctadecylammonium chloride, also exhibit strong germicidal activity. Dialkyldimethyl quats, derived from coconut oil, are effective against anaerobic bacteria, especially sulfate-reducing bacteria found in oil wells, thus preventing corrosion and plugging. Additionally, quats serve as cationic emulsifiers in water-based asphalt emulsions for road construction, offering an environmentally friendly alternative to solvent-based systems and enabling adhesion to negatively charged aggregates [52].
As reported in Section 3.1.2 and Section 3.2, the use of fatty amines derivatives in shampoo formulation is well known and several chemical structures can be selected on the basis of the addressed properties. In Table 1 the main structures of amine derivatives present in hair detergents are reported.

Table 1.
Fatty amines derivatives, their function in hair detergents and examples.
Interestingly, fatty acid amides, produced from fatty acids and long-chain amines, create the pearlescent effect in shampoos [57].
4. Fatty Amines in Cosmetics
The skin, the body’s largest organ and a component of the integumentary system comprises three primary layers: the epidermis, dermis, and hypodermis. Its critical functions include physical, chemical, and biological protection, as well as thermo- and hydro-regulation. The skin also serves as a significant pathway for drug delivery, allowing topically applied substances to permeate the stratum corneum (SC) and reach deeper skin tissues or systemic circulation. The skin’s extensive surface area facilitates increased active molecules absorption, enabling effective high-payload transdermal drug delivery. However, the SC acts as a barrier, limiting the passive absorption of certain drugs, particularly those with high molecular weight or poor permeability [58].
Due to their peculiar properties, surfactants are very useful molecules and found many application in the cosmetic market: they are used as cleanser, foaming agent, emulsifier, suspending agents, and formula stabilizer. One of their many uses is in detergents and toiletries where the surfactants are exploited to remove fat and dirt particles. Moreover, when used in toothpastes, surfactants are used to help a fast and complete dissolution of the product in our mouth thanks to their capability to improve water dispersion of fatty molecules in water. Finally, it is possible to formulate the right combination of different surfactants to meet all the required characteristics in just one product. For example, the right combination can guarantee high skin tolerance without waiving the dirt removal properties needed [24].
Fatty amines derived from natural fatty acids, such as those found in coconut oil, soybean oil, and other lipid sources, are commonly used in the cosmetics industry. In fact, in cosmetic formulations, these compounds and their derivatives play pivotal roles due to their multifunctional properties. they may act as surfactant, conditioners, anticaking agents, and emulsifiers. Their amphiphilic nature allows them to interact effectively with both hydrophilic and lipophilic substances, making them valuable in a wide range of personal care products [59,60].
Fatty amines and their derivatives are widely used in cosmetic products due to their surfactant properties, which help in cleansing and conditioning. They are often used in formulations for soaps, shampoos, and conditioners to improve texture and stability [60,61]. Additionally, fatty amines and their derivatives are involved in the formulation of makeup products, where they help in fixing water-soluble coloring materials on the skin, enhancing the durability and appearance of makeup [62].
Among the fatty amines derivatives, the most used in the cosmetic field are fatty amine oxide and fatty amine ammonium salts (Figure 12). Amine oxides are interesting products due to their ability to behave as non-ionic surfactants in neutral or alkaline media and due to their high foaming activity where they are appreciated for their solid form and for their ability to be used in water sensitive products [61,62,63]. Ammonium quaternary salts are also used in cosmetics and are appreciated as surfactants, antimicrobial compounds [64], and conditioners [65]. Finally, another emerging application for fatty amines ammonium salts is the functionalization of clays to produce synergistic effect exploitable in skin and hair care.
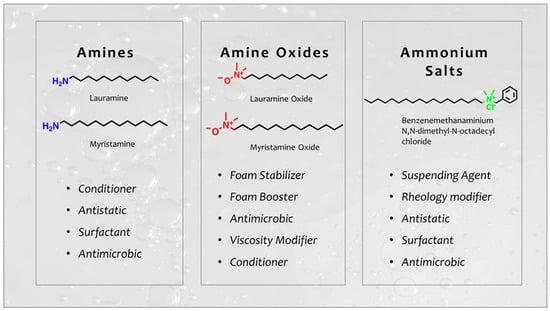
Figure 12.
General structure of amines and ammonium compounds used in cosmetics.
4.1. Functional Roles in Cosmetic Formulations
In cosmetic applications, fatty amines and their derivatives serve several key functions:
Surfactants and Cleansing Agents [66]: They reduce surface tension, aiding in the removal of dirt and oils from skin and hair.
Foam Boosters and Stabilizers [67]: Enhance the formation and stability of foam in products like shampoos and body washes.
Emulsifiers and Emulsion Stabilizers [68,69]: Facilitate the mixing of oil and water phases, ensuring product consistency.
Conditioning Agents [70]: Improve the texture and manageability of hair and skin by providing a soft, smooth feel.
Antistatic Agents [71]: Reduce static electricity, particularly in hair care products, minimizing frizz and flyaway.
Antimicrobial Properties [72,73,74]: Some fatty amine derivatives exhibit antimicrobial activity, which can be beneficial in preserving cosmetic products and ensuring they remain safe for use over time.
Anti-caking Agents [75]: Fatty amines are employed in organo-modified clays for all the function cited before and also in order to keep the powder flowability and softness in time [59].
Finally, fatty amines could help in enhancing the bioavailability of compounds that are not highly hydrosoluble. At the same time, especially in a slightly acidic product, they can act as anti-microbial compounds [76].
4.2. Types of Fatty Amines and Their Uses
4.2.1. Cocamine and Its Derivatives
Cocamine, derived from coconut fatty acids, is commonly used in its oxide form, Cocamine Oxide, in various personal care products. It functions as a mild surfactant, foam booster, and conditioning agent. Its ability to enhance foam and cleansing effectively makes it a staple in shampoos, facial cleansers, and body washes. Additionally, Cocamine Oxide exhibits antistatic properties, contributing to smoother hair texture [77].
4.2.2. Soyamine and Dimethyl Soyamine
Soyamine, obtained from soybean oil, and its derivative, Dimethyl Soyamine, are utilized for their conditioning and antistatic properties. These compounds are particularly effective in hair care formulations, where they help in detangling and reducing static electricity, leading to improved hair manageability [78].
4.2.3. Myristamine and Myristamine Oxide
Myristamine, a fatty amine with a 14-carbon chain, and its oxide form, Myristamine Oxide, are employed in cosmetic products for their surfactant and antimicrobial properties. Myristamine Oxide serves as a foam stabilizer and conditioning agent in shampoos and conditioners. Its antimicrobial activity also contributes to the preservation and hygiene of personal care products [79].
4.2.4. Lauramine and Its Derivatives
Lauramine, derived from lauric acid, and its derivatives, such as Dimethyl Lauramine and Lauramine Oxide, are widely used in cosmetics. Lauramine Oxide functions as an effective foam booster, emulsifier, and viscosity modifier. It is compatible with various surfactants and remains stable across different pH levels, making it suitable for shampoos, facial cleansers, and body washes [80].
4.3. Commercial Applications in Cosmetics
In the commercial landscape, fatty amines and their derivatives are integral to numerous cosmetic products. For instance:
Dimethyl Cocamine is recognized for its antistatic, emulsifying, and conditioning properties, making it valuable in hair care formulations [62].
Lauramine Oxide is utilized for its foam-enhancing and emulsifying capabilities, contributing to the sensory appeal and stability of cleansing products [43].
Myristamine Oxide offers foam stabilization and antimicrobial benefits, enhancing both the performance and shelf-life of personal care items [57].
Stearalkonium hectorite are used as oil phase rheology modifier and emulsifier with the possibility to control the viscosity of the final product and with good suspension capabilities [81].
These applications underscore the versatility and importance of fatty amines in formulating effective and consumer-appealing cosmetic products [52,53,54,55,56,57,58,59,60,61].
5. Fatty Amines and Sustainability
5.1. Alternative Methods of Synthesis
5.1.1. Use of Enzymes
Enzyme catalysis has become a cornerstone of green chemistry, offering significant environmental and economic advantages. Enzymes are derived from renewable sources, are biodegradable, and pose minimal hazard to humans or ecosystems. Importantly, they eliminate reliance on scarce and expensive precious metals, avoiding the need for costly purification of trace metal residues.
These biocatalysts operate effectively under gentle conditions—ambient temperature and pressure in aqueous media—and frequently eliminate the need for protecting-group chemistry or harsh activation steps. Such mild conditions result in more streamlined synthesis with fewer steps, lower waste generation, and reduced energy requirements. Moreover, they can be deployed in conventional batch reactors, removing the necessity for specialized high-pressure or temperature equipment. Enzyme-based reactions can involve either a single catalyst executing a specific transformation or a series of enzymes performing sequential reactions in cascade systems. These multi-step, one-pot processes can be implemented either in living cells or by employing isolated enzymes in vitro, allowing for complex molecular construction with high selectivity [82].
As noted earlier, fatty amines are traditionally produced via the “nitrile route” from fatty acids or by reacting fatty alcohols with ammonia or alkylamines. These processes rely on heterogeneous, often toxic metal catalysts such as nickel, copper, or metal oxides and require harsh reaction conditions including high temperature, pressure, and hydrogen gas. Such methods frequently yield mixtures of primary, secondary, and tertiary amines, complicating downstream processing and necessitating additional safety measures [83].
Citoler et al. [83] developed a one-pot enzymatic cascade that integrates a carboxylic acid reductase (CAR) with a ω-transaminase (ω-TA). This system converts saturated or unsaturated fatty acids (C6–C18) directly into amines, achieving up to 96% conversion. At preparative scale (75 mL, using trilaurin), they isolated laurylamine with a 73% yield after purification.
Similarly, fatty alcohol aminated through combined enzyme cascades—typically pairing alcohol oxidases or dehydrogenases with ω-TAs—generate aldehyde intermediates in situ that are then transaminated. While effective, the process still encounters limitations for longer-chain substrates (C14+), due to low enzyme activity and poor substrate solubility in water. CAR enzymes are especially suited for these cascades. As three-domain proteins driven by ATP and NADPH, CARs derived from Mycobacterium marinum, Segniliparus rugosus, and related strains exhibit broad activity on fatty acids (C6–C18), efficiently generating aldehyde intermediates. Their combination with ω-TAs forms a robust route for fatty amine production.
Successful implementations of similar cascades include systems combining CAR + ω-TA + imine reductase (IRED) for producing piperidines and pyrrolidines with high selectivity, or CAR + ω-TA with adenine recycling and NADPH regeneration for alkaloid biosynthesis [84]. Enzyme-based biocatalysis faces notable limitations—chiefly, enzymes often exhibit insufficient stability under industrial conditions (e.g., elevated temperature, extreme pH, solvents), narrow substrate scopes, low tolerance to product or substrate inhibition, high production costs, low reaction concentrations, and complex scale-up logistics. Yet, the future outlook is promising: advances in protein engineering (including directed evolution, rational design, AI-assisted methods), enzyme immobilization, metagenomic discovery of novel catalysts, and innovative reactor strategies (flow chemistry, microreactors, biphasic systems) are actively addressing these challenges, paving the way for robust, high-yield, cost-effective, and truly sustainable biocatalytic processes.
Enzyme-based biocatalysis presents a sustainable and selective approach to chemical synthesis under mild conditions, but its industrial application is limited by several challenges. These include low enzyme stability under demanding process environments, narrow substrate specificity, reliance on expensive cofactors, and reduced productivity due to low operational concentrations. Scaling up biocatalytic processes is further hindered by complex reactor designs and inefficiencies at large volumes. However, recent advancements are addressing these limitations. Techniques such as protein engineering (e.g., directed evolution, AI-guided design), enzyme immobilization (e.g., CLEAs, nanoparticle supports), integration into continuous-flow systems, and efficient cofactor regeneration are collectively improving enzyme performance and process viability. With these innovations increasingly supported by digital tools and green chemistry principles, enzymatic cascades—such as CAR–ωTA systems—are becoming more feasible for industrial-scale production of bulk chemicals.
5.1.2. Synthesis in Water
Dimethyl alkyl amines are valuable intermediates used in detergents, pharmaceuticals, and as precursors to cationic surfactants. However, their conventional synthesis often relies on activated substrates or involves methylation using hazardous reagents like methyl iodide.
Catalytic alcohol amination offers a highly attractive alternative, as it uses readily available starting materials—aliphatic alcohols and corresponding amines—and generates only water as a byproduct [85]. Catalytic alcohol amination is already used industrially—for example, in the production of methylamines from ammonia and methanol. However, this approach faces limitations when applied to the synthesis of tertiary dimethyl alkyl amines. The commonly used heterogeneous catalyst, while highly active, lacks selectivity and tends to convert dimethylamine (DMA) into a mixture of methyl- and trimethylamines, leading to a complex array of byproducts and downstream processing challenges. The situation is further complicated with fatty alcohols, which typically cannot be processed in the gas phase due to their low volatility, making them unsuitable for use with heterogeneous catalysts.
Unlike heterogeneous systems, homogeneous catalytic amination of alcohols occurs in the liquid phase and presents a promising route for synthesizing dimethyl alkyl amines. This method offers several advantages, including the use of readily available bulk chemicals and robust ruthenium-based catalysts. The reaction proceeds via the “borrowing hydrogen” mechanism (Figure 13), where the alcohol is activated in situ through dehydrogenation. The catalyst plays a dual role—activating the alcohol and hydrogenating the resulting enamine—often requiring high catalyst loadings to ensure complete conversion and minimize reaction time. The concentration of reactive carbonyl intermediates is kept low, reducing side reactions like aldol condensation. The process yields an enamine or imine (depending on the type of amine) and water as the only byproduct, highlighting the sustainability of this reaction pathway.
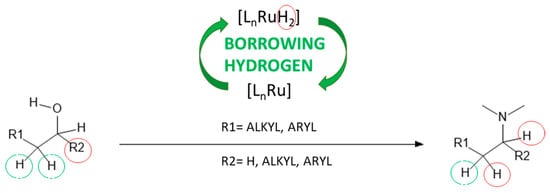
Figure 13.
Mechanism for the amination of alcohols [73].
The amination of long-chain fatty alcohols with dimethylamine in water as the solvent has achieved conversion and selectivity exceeding 99%. This success is attributed to the use of a water-soluble ruthenium-based catalyst and the unique phase-mediating role of dimethylamine. Under these optimized conditions, various fatty alcohols have been efficiently converted into their corresponding tertiary dimethyl alkyl amines. Despite the advantages discussed above, this method faces significant drawbacks, primarily related to catalyst separation and recovery, as well as potential for catalyst deactivation [73].
5.1.3. One-Step Vapor-Phase Thermocatalysis of Triglycerides
In the traditional fatty nitrile process, triglycerides are hydrolyzed, and the resulting fatty acids are catalytically reacted in a liquid-phase reaction with NH3. Shirazi et al. [86], reported a simpler one-step fatty nitrile production method (Figure 14). This method consists of a direct vapor-phase reaction of triglycerides with NH3 catalyzed by heterogeneous solid acid, thus avoiding the hydrolysis step. Catalyst properties were found to influence product yields and the effects of triglyceride/NH3 ratios on product yield were assessed for reactions catalyzed by V2O5.
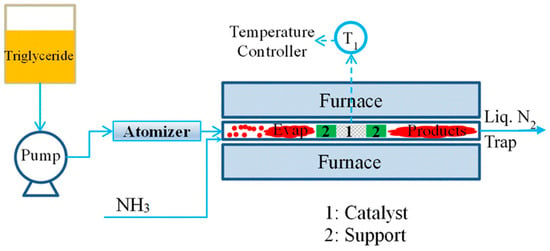
Figure 14.
Schematic diagram of the one-step vapor-phase nitrile reaction system [86].
All experiments were conducted using a continuous vapor-phase reactor system (il-lustrated schematically in Figure 13). Before each run, the reactor was packed with catalyst, placed in a furnace, and purged with pure nitrogen (N2) to eliminate residual moisture and oxygen. When the setpoint temperature was reached, the nitrogen flow was stopped, and NH3 was constantly supplied via a mass flow meter. Micron-sized droplets of coconut oil were then fed into the reactor through an atomizer, maximizing the surface area for efficient heat and mass transfer. The condensed liquid products were separated into an aqueous and an organic phase. Additionally, this process greatly reduces (and even eliminates) the need for complex distillation and purification stages, which are instead necessary in the traditional processes.
Overall, the one-step vapor-phase nitrile process enables the direct conversion of triglycerides into fatty nitriles with yields approaching theoretical limits. It also offers lower energy consumption and the potential for simpler reactor and separation system designs compared to conventional methods. Although one-step vapor-phase thermocatalysis of triglycerides offers benefits such as bypassing the energy-demanding hydrolysis stage, it still presents several drawbacks. These include the necessity for elevated temperatures, which can trigger undesirable reactions like cracking and coke buildup, as well as the risk of catalyst degradation. Moreover, achieving precise control over product selectivity remains difficult, and the method often relies on expensive catalysts that can withstand high thermal stress [83].
5.1.4. Aliphatic Amines from Plastic Waste
Plastic waste can serve as a valuable feedstock for chemical production, and several companies are currently constructing pyrolysis plants with capacities ranging from 5 to 120 kilotons per year. When using polyolefin-rich waste, the resulting pyrolysis oil typically contains a mix of paraffins, aromatics, and olefins (C5 to C25), with olefins and diolefins making up as much as 60 wt%. These long-chain olefins require further processing. Techniques like steam cracking or catalytic conversion can break them down into ethylene, propylene, and aromatics, which can be integrated into existing petrochemical infrastructure. Recent studies have shown that hydroformylation can convert these olefins into aldehydes, which can then be hydrogenated into alcohols—or further transformed into amines via reductive amination.
Aliphatic amines are widely used in solvents, pesticides, feed additives, rubber processing, and water treatment, with solvents and agrochemicals accounting for about 50% of the market. In 2021, their prices exceeded $2000 per metric ton, with diamines costing roughly 1.5 times more than monoamines. Monoamines (e.g., n-butylamine, C4H11N, and C12–C16 primary amines) are primarily produced from natural gas through a series of five steps: steam cracking, oligomerization, distillation, and hydroformylation. In contrast, diamines (e.g., 1,6-hexanediamine, C6H16N2) are typically derived from naphtha via steam cracking, hydrocyanation (which involves hydrogen cyanide, HCN), and hydrogenation.
As an alternative route for producing amines and diamines, Li et al. [87] proposed a tandem process involving hydroformylation followed by reductive amination of olefins found in plastic pyrolysis oil, using ammonia as the nitrogen source. In their study, aldehydes were first generated via hydroformylation of olefins from colored post-consumer recycled high-density polyethylene (PCR-HDPE). These aldehydes were then converted into aliphatic amines using a Ru/C catalyst through reductive amination. Since the hydroformylation step yields only aliphatic aldehydes, the process selectively targets the production of aliphatic amines. The study demonstrated that near-quantitative conversion of aldehydes in hydroformylated pyrolysis oil to amines can be achieved using a 5 wt% Ru/C catalyst at 90 °C and 40 bar H2 in an ammonia–methanol solution. The formation of primary C5–C18 mono- and diamines from PCR-HDPE was confirmed through NMR spectroscopy, gas chromatography, and elemental analysis. Although most metals are removed during pyrolysis, the subsequent hydroformylation and reductive amination steps can still proceed effectively in the presence of trace metal contaminants (<200 ppm). In general, producing aliphatic amines from plastic waste involves complex multi-step processes like pyrolysis and catalytic conversion. While it represents a sustainable approach, it faces key challenges: high energy and operational costs, feedstock contamination, catalyst deactivation, environmental risks, and difficulties in process control due to variable plastic compositions [87].
5.2. A Biocircular Approach
5.2.1. Use of Agro-Food Waste and By-Products as Raw Materials for Synthesizing Fatty Amines
The production of fatty amines from waste biomass is particularly attractive from an industrial perspective, due to the negative value of these starting feedstocks [88] and the high value of the target products [89,90]. However, several bottlenecks must be overcome for the efficient and selective conversion of waste biomass to fatty amines, highlighting its recalcitrance to the one-pot (bio)-chemical conversion strategies [91]. Many biomass sources are available in nature that can be exploited to synthesize nitrogen-containing chemicals, including the fatty amines herein of interest, but very few studies are conducted on the direct and efficient utilization of biomass. Especially when employing complex substrates, the fractionation of useful biomass components remains the preferred strategy, mainly aimed at the isolation of fatty acids as the prevailing components of interest for fatty amine production via chemo-catalytic approach (e.g., via nitrile route). In addition, the protein fraction of the biomass can be fractionated and used as N-donor source for the fatty amine synthesis, via the bio-catalytic approach, thus better developing the bio-circularity concept. These strategies are successful when the chemical composition of the biomass is favorable towards the components of interest. In this context, it becomes of paramount importance to properly consider the chemical composition of the starting feedstock, which should be rich in fatty acids and, even better, in nitrogen-donor species (as proteins/amino acids), especially if the bio-synthetic pathway of fatty amines would be developed. For example, in the field of the agro-food wastes, apple fruits (e.g., from Jonathan and Golden Delicious species) by-products (e.g., as apple pomace and skin), are rich in palmitic, stearic, linoleic and oleic acids as valuable fatty acids, but also amino acids, mainly including leucine, isoleucine, and valine [92]. According to Benítez et al. [93], tomato pomace by-products may represent noteworthy waste biomasses for the production of added-value compounds. In this context, the authors distinguished between fiber (15 wt%), skin (30 wt%) and seed (55 wt%) fractions, developing a fractionation strategy of the biomass components based on alkaline hydrolysis, followed by acidification, thus obtaining a non-hydrolysable solid stream (Rx) and a hydrolyzed liquid one (Hx). The authors claimed that the seed feedstock was the richest in both lipids and proteins (14 and 16 wt%, respectively, evaluated respect to the overall weight of the tomato pomace). Remarkably, Hx pomace from seed and skin differed for the fatty acid composition, the first stream mainly including unsaturated ones (e.g., linoleic acid for ~53%, followed by oleic acid for ~28%), whilst the second one was composed of polyhydroxylated ones (e.g., dihydroxylated C16 acids, that is 10,16-dihydroxypalmitic, for ~43%). Definitely, plant seeds contain the highest quantity of oil and their utilization adds value to these cheap resources. Historically, tallow (with prevailing C16-C18 cut of fatty acids) and coconut oils (with prevailing C8-C14 cut of fatty acids) have been widely used for fatty amine production, according to the nitrile route. Nowadays, other cheap natural oils are used for the same purpose, including those from linseed, canola, palm, soybean, castor (richer in C16-C18 fatty acids) and rapeseed (richer in C20–C22 ones) [94]. From this perspective, the use of soybean oil for fatty amine production, has been specifically preferred, given the corresponding high content of oleic, linoleic and linolenic acids [94]. The high reactivity of this type of substrate to the conversion processes, including the mild bio-catalytic ones, makes this feedstock attractive for the fatty amine production. Remarkably, fish oil is rich in omega-3 fatty acids, specifically eicosapentaenoic and docosahexaenoic acid, at the same time including essential amino acids [95], which are certainly exploitable for fatty amine production. Recently, microalgal oils are attracting growing interest as valuable substitutes for plant oils for oleochemical synthesis, at the same time exploiting their triglycerides, whose chemical profile is often different from other oils (e.g., linseed, soybean, fish), anyway within the cut C14-C18 [96]. Moreover, algal biomass includes nitrogen-based compounds (e.g., proteins, peptides and amino acids), which can be fractionated (generally as hydrolyzed slurries) and further exploited, as in the case of the bio-chemical path for fatty amine production, thus improving the economics, sustainability and circularity of the same process [96]. Lastly, marine bivalve species are well-known animal organisms [94], and the aquaculture industry is strongly promoting their culture in highly controlled aquatic environments. Following the previous criteria, given the high content of fatty acids and proteins, and their high productivity (under highly controlled conditions), it is reasonable that their use for fatty amine production will be evaluated within the near future.
Last but not least mammal and fish animal hides from food industry, slaughterhouses and tanning processes are a valuable source of collagen, the most abundant structural protein of animals [97]. Collagen extraction has been significantly improved within the last years, providing efficient methods (such as chemical hydrolysis, salt solubilization, enzymatic hydrolysis, ultrasound assisted extraction, etc.) [98]. Collagen and its lower molecular weight denaturation product, gelatin, can be exploited, for example, for fatty amine production through the discussed bio-chemical conversion paths, as well as for the synthesis of new bio-based adhesives [99].
5.2.2. Biotechnological Paths for Fatty Amines
As suggested in Section 5.1.1, the enzymatic synthesis of fatty amines has garnered significant attention as a sustainable and efficient alternative to traditional chemical methods. This approach leverages biocatalysts to convert renewable feedstocks into valuable amine compounds under mild conditions. One of the most promising strategies for enzymatic fatty amine synthesis is the use of multi-enzyme cascades. These cascades typically begin with the hydrolysis of triglycerides (from vegetable oils or animal fats) by lipases to generate free fatty acids. These fatty acids are then reduced to their corresponding aldehydes by carboxylic acid reductases (CARs). The final step involves the conversion of these aldehydes to primary amines through the action of transaminases (TAs). This sequential enzymatic approach has been successfully demonstrated with yields of up to 97% in the production of medium- and long-chain fatty amines and was able to isolate laurylamine from trilaurin at a 73% yield in a scaled-up 75 mL reaction [16]. Further expanding on this concept, researchers have explored the use of whole-cell photoenzymatic cascades to synthesize long-chain aliphatic amines and esters from renewable fatty acids. This method utilizes a combination of enzymes, including an alcohol dehydrogenase (ADH), an engineered amine transaminase (Vf-ATA), and a photoactivated decarboxylase (Cv-FAP), to convert fatty acids like ricinoleic acid and oleic acid into amines such as (S,Z)-heptadec-9-en-7-amine and 9-aminoheptadecane. The process has achieved conversions up to 90%, highlighting its effectiveness in producing industrially relevant compounds from renewable resources [100].
Moreover, other enzymatic routes are being explored. ω-Transaminases, for example, can be employed for the amination of ω-functionalized fatty acids, allowing the synthesis of ω-amino fatty acids, which are important intermediates in the production of bioplastics and pharmaceuticals. Imine reductases (IREDs) also represent a valuable class of enzymes capable of performing reductive amination reactions with high stereoselectivity. Moreover, monoamine oxidases (MAOs) have been investigated for their use in deracemization processes, which are critical for the synthesis of enantiomerically pure fatty amines [87].
The advantages of using enzymes for fatty amine synthesis are manifold. Enzymes typically operate under mild temperatures and pressures, which significantly reduces the energy footprint of the process. Their inherent chemo-, regio-, and stereoselectivity leads to fewer by-products and higher product purity, which simplifies downstream processing. Furthermore, because enzymes are biodegradable and reactions often use aqueous media, the overall environmental impact is much lower compared to traditional chemical processes.
Despite these advantages, there are still some challenges to be addressed. Enzyme stability remains a major concern, particularly when reactions are conducted over extended periods or under non-ideal conditions. The scope of substrates that enzymes can effectively transform is also limited, although advances in enzyme engineering and directed evolution are rapidly expanding this range. Additionally, optimizing reaction parameters and enzyme loadings for cost-effective industrial processes is an ongoing area of research. Efficient product recovery and purification from aqueous or complex media can also be technically challenging and may require the development of novel separation technologies.
Looking ahead, the future of enzymatic fatty amine synthesis appears promising. Efforts are currently underway to improve enzyme performance through protein engineering, using rational design and machine learning-guided approaches. These innovations are expected to produce more robust and versatile biocatalysts. At the same time, integrated bioprocessing strategies are being developed to combine enzymatic synthesis with in situ product separation, thereby increasing process efficiency and reducing operational costs.
Within this context, the CBE funded FLEXIZYME project [101], which aims to create modular and flexible enzymatic platforms for the synthesis of fatty acid amines from lipid rich and protein rich by products, aligning the enzymatic synthesis of fatty amines with the principles of circular economy and green chemistry.
In conclusion, enzymatic synthesis of fatty amines represents a compelling alternative to traditional chemical methods, offering significant environmental and technical benefits. While challenges remain in terms of enzyme robustness, scalability, and economic viability, ongoing research and development efforts, including large-scale projects like FLEXIZYME, are paving the way for industrial adoption of these sustainable biocatalytic processes.
5.2.3. Fatty Amines and Cationic Derivatives as Additives in Biobased Formulations
Considering the current tendency to use bio-based materials, use of biobased fatty amines in new formulations of detergents and cosmetics is very promising. The choice of renewable vegetable oils as starting feedstocks is in full agreement with the principles of green chemistry, at the same time avoiding health problems, as instead may occur for tallow oil (e.g., issue of bovine spongiform encephalopathy) [102]. As further proof of these choices, limited supply of tallow is expected, whilst agricultural-based feedstocks are widely available. Use of fatty amines as additives of detergent and cosmetic formulations is already carried out at the industrial level [60]. Anyway, hydrophilic contribution of amino groups is small, if compared with the hydrophobic one of the alkyl chain that prevails at and above pH = 7, and post-modification reactions of fatty amines are required for improving the pristine surfactant properties. For this purpose, cationic surfactants are often synthesized and used in cosmetic and detergent formulations, thus improving the hydrophilic properties of the surfactant, often evaluated in terms of hydrophilic lipophilic balance (HLB) [103], often considering partition coefficient between heptane and water [104]. As already discussed in Section 3, these properties would significantly impact on the micelle packing and interface reactivity, and, consequently, surfactant properties such as solubility, foaming, gelling, dispersion, and emulsification. All these aspects should be properly considered during the development of cosmetic and detergent formulations. Modification of fatty amines to give cationic surfactants may include simple salt formation (e.g., as pseudo cationic amine salts), by their reaction with inorganic (e.g., hydrochloric) or organic (glycolic, lactic, acetic, etc.) acids [105], as reported in Figure 15.
Figure 15.
Synthesis of pseudo-cationic amine salts.
Anyway, this approach is scarcely used in detergent and cosmetic formulations, at least for primary amines, due to the pseudo-cationic properties of these salts only at pH values significantly lower than 7 [106]. As already discussed in Section 3, more profitable choices involve the methylation of primary and secondary fatty amines, according to the Leuckart reaction (e.g., using formic acid as reducing agent) [57] or reductive methylation (in the presence of formaldehyde, hydrogen and Ni-catalyst), as reported in Figure 16 [95]:
Figure 16.
Methylation of fatty amines.
After synthesis of methylated fatty amines, corresponding quaternary ammonium salts can be obtained, which are cationic surfactants of greater interest for the development of cosmetic and detergent formulations, due to the improved hydrophilicity [107]. Alternatively, quaternization can be carried out by reacting fatty amines with alkylating agents, such as methyl chloride, methyl sulfate, or benzyl chloride [108], as reported in Figure 17.
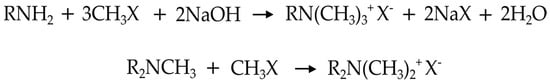
Figure 17.
Synthesis of quaternary ammonium salts.
Reaction of fatty amines with chloroacetic acid results in alkyl betaines (Figure 18). These compounds have important applications in the cosmetics or personal care industries (for instance ingredients in shampoos, conditioners, foaming, and wetting agents), as amphoteric surfactants [109].
Figure 18.
Synthesis of alkyl betaines.
Betaines are particularly interesting surfactants because of their high solubility in water. In fact, in liquid body wash systems, betaines are commonly used as cationic surfactants [110]. Some noteworthy examples are represented by alkylamidopropyldimethyl betaines (APBs) and alkyldimethylbetaines (ABs), that are widely used in cosmetic formulations of cleansers, providing more-stable and richer foam (Figure 19):
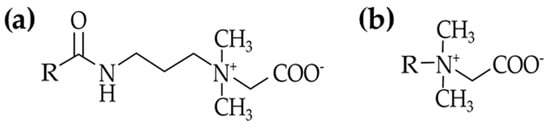
Figure 19.
Examples of structures of (a) alkylamidopropyldimethyl betaine and (b) alkyldimethyl betaine.
In this context, L’Oreal has launched sulfate-free products worldwide, including betaines in body wash and hair shampoo products [100].
Fatty amine alkoxylates and amine oxides represent other classes of fatty amine-derived surfactants, widely employed for improving hydrophilicity of detergent and cosmetic formulations [107]. Regarding fatty amine alkoxylates, these are generally considered as non-ionic surfactants. Ethylene oxide (EO) is the main alkoxylating agent used for this post-modification reaction, although propylene oxide (PO) and mixed alkoxylates have been proposed. Ethoxylation of primary fatty amine backbone leads to the corresponding bis(2-hydroxyethyl) tertiary amine, by this way improving water solubility of the pristine fatty amine [107]. Further addition of EO to the tertiary amine can be pursued, generally in the presence of basic catalysts, such as KOH or NaOH, as reported in Figure 20.
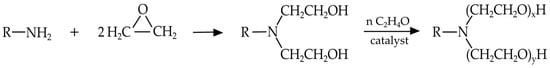
Figure 20.
Synthesis of ethoxylated fatty amines.
Different ethoxylated fatty amines are available for cosmetic and detergent uses, generally up to 50 mol of EO/mol of fatty amine [87]. The use of PO (instead of EO) improves the hydrophobicity of the fatty amine, and its use is required for reducing foaming of the formulation, at the same time obtaining better wetting characteristics and reducing foaming tendency [107].
Regarding the amine oxides, these weak cationic surfactants are manufactured by oxidation of tertiary (dimethyl or ethoxylated) amines with hydrogen peroxide [111], as reported in Figure 21.
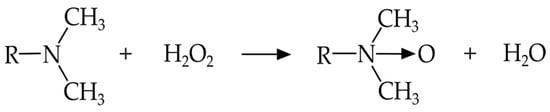
Figure 21.
Synthesis of amine oxides.
Hydrogen peroxide is generally supplied in 35–70% aqueous solution. The reaction is carried out in a water miscible solvent, such as isopropanol, ethanol or propylene glycol [111]. Amine oxides are unstable at high temperature: they degrade via the Cope reaction above 120 °C and by reversion to amine at slightly lower temperatures [111]. Amine oxides can behave as either cationic or non-ionic surfactants, depending on the pH of the environment. In acidic conditions, the amino group becomes protonated, giving the compound cationic properties. In neutral or alkaline solutions, however, amine oxides primarily exhibit non-ionic behavior. Alkyldimethyl amine oxides are commonly used in household cleaners where enhanced detergency is required [110]. When combined with anionic surfactants—especially under alkaline conditions—amine oxides function as effective foam boosters. In shampoos formulated with alkyl sulfates or alkyl ether sulfates (AES), they also act as foam stabilizers, thickeners, and enhance the tactile feel of hair [112]. For example, cocamine oxide is used in small percentages (3–5 wt%, e.g., as secondary or co-surfactants) in shampoo formulations, as foam modifier, enhancing cleansing and viscosity of the product [113]. Its use is mostly in combination with alkyl sulfates, the latter considered as primary surfactants (e.g., 10–15 wt% in the final formulation) [114]. In general, amine oxides are compatible with all other surfactants, and their use is suitable in slightly acidic or neutral formulas [115].
Alkyl polyamines are produced by cyanoethylation (Michael addition) of fatty amines, involving their reaction with acrylonitrile (mononitrile or dinitrile), followed by the hydrogenation to diamines and triamines [108], as reported in Figure 22.
Figure 22.
Synthesis of alkyl polyamines.
The above fatty amine-derived compounds are only some of the most noteworthy examples of surfactants nowadays used in cosmetic and detergent formulations. Remarkably, new classes of amines could be synthesized starting from biomass derivatives. In this context, Xie et al. [116] proposed the synthesis of new amines by catalytic conversion of chitin monomer, e.g., N-acetyl-D-glucosamine (NAG), which could be obtained by hydrolysis of crustacean shells and exoskeleton of insects. After optimization of the deoxygenation (reduction) path of NAG, carried out in the presence of H2 and Ru/C catalyst, the authors claimed maximum yield of about 50% in the new amines (Figure 23).
Figure 23.
New amines obtainable by Ru/C catalyzed deoxygenation of chitin monomer.
Even though this is a preliminary investigation (e.g., a better control of the selectivity parameter would be required), this approach is certainly appealing, developing the valorization of a waste biomass for the production of new classes of value-added amines.
The biorefinery approach is emerging as a sustainable solution for producing additives used in detergents and cosmetics, especially from renewable sources like macroalgae [117]. These compounds include polysaccharides, polyunsaturated fatty acids, antioxidants, and antimicrobial agents, which offer multifunctional benefits such as hydration, anti-aging, and skin protection. While these ingredients offer eco-friendly benefits, their performance may not always meet industrial standards [117]. Combining them with biobased fatty amines—known for their surfactant and antimicrobial properties—can enhance formulation effectiveness and support the transition to greener products. This synergy aligns with circular economy principles and represents a promising direction for future innovation.
A key aspect for the development of new bio-based formulations is the full biodegradability of its components, at the same time guaranteeing the absence of environmental and health issues. Biodegradability of the surfactant is generally evaluated after a 28 day screening test, whereas it should be completely degraded under aerobic conditions [118]. Biodegradation tests and metabolic studies have shown that these cationic surfactants undergo complete degradation [119]. Primary, secondary and tertiary amines are readily degraded, as well as their derivatives. Biodegradation occurs via scission of the alkyl–N bond [108] which can be influenced by the chemical surrounding of the fatty amine (e.g., including both hydrophilic and hydrophobic components). Regarding the hydrophobic component, differences in the chain length distribution do not significantly impact the performance of the surfactants, whilst differences in the degree of unsaturation and isomer distribution affects their oxidative stability, liquidity, and solubility [120]. The oxidative stability is also influenced by the type of unsaturation; for instance it is reported that linolenic acid is about 10 times less oxidatively stable than linoleic acid, which is less stable than oleic acid [109]. The oxidative stability of surfactants can be enhanced by selectively hydrogenating polyunsaturated C18 fatty chains into oleyl derivatives, which maintains fluidity while improving stability [121]. Unsaturation also boosts solubility in hydrocarbons, making oleyl-based compounds a popular choice for surfactant development.
6. Conclusions
This review highlights the multifaceted roles of fatty amines and their derivatives in detergents and cosmetics, emphasizing both their current industrial relevance and their future potential within a sustainability-driven framework. Fatty amines, particularly when protonated or converted into positively charged derivatives, demonstrate strong surfactant activity and antimicrobial properties that are effectively utilized across both sectors.
In the detergent industry, the demand for multifunctional surfactants—especially those with antimicrobial properties—is increasing, driven by the need to clean natural, synthetic, and fossil-derived textiles, as well as hard surfaces and by personal care applications. In cosmetics, fatty amine derivatives are extensively used in hair care formulations to improve shine, manageability, and protection, while also contributing to the stability and texture of creams, lotions, and other liquid products.
From a sustainability standpoint, the synthesis of fatty amines is undergoing a significant transformation. Traditional chemical production methods, which rely on petrochemical feedstocks and toxic catalysts, are energy-intensive and environmentally burdensome. In contrast, greener methodologies such as the valorization of agro-food waste and by-products align with circular economy principles and offer promising alternatives. Notably, enzymatic and biocatalytic processes have emerged as innovative routes for producing fatty amines under milder, safer, and more selective conditions. While challenges related to enzyme stability, scalability, and economic feasibility remain, continued research is steadily advancing these methods toward industrial application.
The integration of biocircular fatty amines into detergent and cosmetic formulations presents promising avenues for product innovation. These compounds can be synergistically combined with bio-based polymers and functional ingredients to develop formulations that are both high-performing and environmentally sustainable. Such innovations offer a viable alternative to conventional products that predominantly rely on fossil-derived ingredients. By aligning efficacy with ecological responsibility, fatty amines emerge as pivotal components in the advancement of next-generation green consumer goods.
In conclusion, the integration of bio-based feedstocks, green chemistry, and biotechnological advancements provides a clear path forward for enhancing the sustainability and functionality of fatty amines. As both consumer demand and regulatory pressures continue to favor sustainable solutions, fatty amines and their derivatives are well-positioned to play a central role in shaping the future of detergents and cosmetics.
Author Contributions
Conceptualization, M.-B.C.; investigation, M.-B.C., D.L. and M.T.; resources, A.S. and M.-B.C.; writing—original draft preparation, M.-B.C., D.L., C.G., M.T. and A.S.; writing—review and editing S.M., P.S., C.E.-C., A.L., R.D. and A.M.R.G.; visualization, C.G. and M.-B.C.; supervision, A.M.R.G.; project administration, A.S. and R.D.; funding acquisition, M.-B.C., A.S. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Circular Bio-based Europe Joint Undertaking and its members, in the framework of project “Construction of a FLEXIble and adaptable enZYMatic biotechnological platform for sustainablE industrial production of bio-based fatty amines from side stream materials” (Flexizyme), Grant agreement ID: 101157528.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This research was funded by the Circular Bio-based Europe Joint Undertaking and its members. In the framework of project “Construction of a FLEXIble and adaptable enZYMatic biotechnological platform for sustainablE industrial production of bio-based fatty amines from side stream materials” (Flexizyme), Grant agreement ID: 101157528. The Chemical Engineering Bachelor student Ginevra Nobili is thanked for contributing to bibliographic research.
Conflicts of Interest
Carolina Galli and Paolo Sonzini the authors are employees of Roelmi HPC srl. Clara Escrivà-Cerdán the author was the employee of Lavantia Nature SL. Sergio Mastroianni the author was the employee of Specialty Operations France (SYENSQO Group). The remain authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Visek, K. Amines, Fatty. In Kirk–Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 2, p. 518. [Google Scholar]
- GlobeNewswire. Surfactants Market Booms, Poised to Hit USD 87.2 Billion by 2033, Asia Pacific to be Dominant Region|Marketresearch.biz. 2024. Available online: https://www.globenewswire.com/news-release/2024/02/21/2832468/0/en/Surfactants-Market-Booms-Poised-to-Hit-USD-87-2-Billion-by-2033-Asia-Pacific-to-be-Dominant-Region-Marketresearch-biz.html (accessed on 13 August 2025).
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability—Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. ACS Sustain. Chem. Eng. 2021, 8, 487–491. [Google Scholar] [CrossRef]
- Foley, P.; Kermanshahi pour, A.; Beach, E.S.; Zimmerman, J.B. Derivation and Synthesis of Renewable Surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Gorouhi, F.; Maibach, H.I. Anti-Aging Topical Peptides and Proteins—Chapter 14. In Cosmeceuticals and Active Cosmetics; Sivamani, R., Jagdeo, J.R., Elsner, P., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2015; p. 36. ISBN 9781482214178. [Google Scholar]
- L’OREAL. Because Our Planet Is Worth It, 2023 Sustainability Report. 2023. Available online: https://www.loreal.com/-/media/project/loreal/brand-sites/corp/master/lcorp/4-brands/consumer-products-division/loreal-paris/prlorealparis-2023.pdf?rev=564eddaeca6247b7b70368785b4c6312&hash=4EC4341DF410442A9FBA9739286A4EE4 (accessed on 18 August 2025).
- Cosmetics Europe. 2025. Available online: https://cosmeticseurope.eu/key-actions/driving-sustainability/ (accessed on 18 August 2025).
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Ball, D.W. The Basics of General, Organic, and Biological Chemistry; LiberTexts: Davis, CA, USA, 2022. [Google Scholar]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Okeke, C.A.V.; Khanna, R.; Ehrlich, A. Quaternary Ammonium Compounds and Contact Dermatitis: A Review and Considerations During the COVID-19 Pandemic. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1721–1728. [Google Scholar] [CrossRef]
- Mekki-Berrada, A.; Bennici, S.; Gillet, J.P.; Couturier, J.L.; Dubois, J.L.; Auroux, A. Ammoniation-Dehydration of Fatty Acids into Nitriles: Heterogeneous or Homogeneous Catalysis? ChemSusChem 2013, 6, 1478–1489. [Google Scholar] [CrossRef]
- Park, J.-H.; Hong, E.; An, S.H.; Lim, D.-H.; Shin, C.-H. Reductive Amination of Ethanol to Ethylamines over Ni/Al2O3 Catalysts. Korean J. Chem. Eng. 2017, 34, 2610–2618. [Google Scholar] [CrossRef]
- Egan, R.R. The Preparation and Properties of Amines and Cationic Surfactants from Fatty Acids. J. Am. Oil Chem. Soc. 1968, 45, 481–486. [Google Scholar] [CrossRef]
- Citoler, J.; Finnigan, W.; Bevinakatti, H.; Turner, N.J. Direct Enzymatic Synthesis of Fatty Amines from Renewable Triglycerides and Oils. ChemBioChem 2022, 23, e202100578. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Kassim, T.A.; Simoneit, B.R.T. Detergents: A Review of the Nature, Chemistry, and Behavior in the Aquatic Environment. Part I. Chemical Composition and Analytical Techniques. Crit. Rev. Environ. Sci. Technol. 1993, 23, 325–376. [Google Scholar] [CrossRef]
- Linke, D. Chapter 34 Detergents: An Overview. In Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 603–617. [Google Scholar]
- Gadberry, J.F. Other Types of surfactants. 6.1 Cationics. In Chemistry and Technology of Surfactants; Farn, R.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 153–165. ISBN 9780470988596. [Google Scholar]
- Stubenrauch, C.; Drenckhan, W. Cleaning Solid Surfaces with Liquid Interfaces and Foams: From Theory to Applications. Curr. Opin. Colloid Interface Sci. 2024, 72, 101818. [Google Scholar] [CrossRef]
- Rushton, H.; Gummer, C.L.; Flasch, H. 2-in-1 Shampoo Technology: State-of-the-Art Shampoo and Conditioner in One. Skin Pharmacol. 1994, 7, 78–83. [Google Scholar] [CrossRef]
- Tomasino, C. 9–Effect of Wet Processing and Chemical Finishing on Fabric Hand. In Effect of Mechanical and Physical Properties on Fabric Hand; Behery, H.M., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2005; pp. 289–341. ISBN 978-1-85573-918-5. [Google Scholar]
- Andres, Y.; Giraud, L.; Gerente, C.; Cloirec, P.L. Antibacterial Effects of Chitosan Powder: Mechanisms of Action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Panariello, L.; Coltelli, M.-B.; Hadrich, A.; Braca, F.; Fiori, S.; Haviv, A.; Miketa, F.; Lazzeri, A.; Staebler, A.; Gigante, V.; et al. Antimicrobial and Gas Barrier Crustaceans and Fungal Chitin-Based Coatings on Biodegradable Bioplastic Films. Polymers 2022, 14, 5211. [Google Scholar] [CrossRef]
- Farn, R.J. Surfactant Manufacturers. In Chemistry and Technology of Surfactants; Farn, R.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 300–309. ISBN 9780470988596. [Google Scholar]
- Ranji, H.; Babajanzadeh, B.; Sherizadeh, S. Detergents and Surfactants: A Brief Review. Open Access J. Sci. 2019, 3, 94–99. [Google Scholar] [CrossRef]
- Salager, J.-L. SURFACTANTS Types and Uses. Master’s Thesis, Universitad de Los Andes, Bogotá, Colombia, 2002. [Google Scholar]
- Hepworth, P. Non-Ionic Surfactants. In Chemistry and Technology of Surfactants; Farn, R.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 133–152. ISBN 9780470988596. [Google Scholar]
- Funes, D.S.H.; Bonilla, K.; Baudelet, M.; Bridge, C. Morphological and Chemical Profiling for Forensic Hair Examination: A Review of Quantitative Methods. Forensic Sci. Int. 2023, 346, 111622. [Google Scholar] [CrossRef]
- Yang, F.-C.; Zhang, Y.; Rheinstädter, M.C. The Structure of People’s Hair. PeerJ 2014, 2, e619. [Google Scholar] [CrossRef] [PubMed]
- Harland, D.P.; Popescu, C.; Richena, M.; Deb-Choudhury, S.; Wichlatz, C.; Lee, E.; Plowman, J.E. The Susceptibility of Disulfide Bonds to Modification in Keratin Fibers Undergoing Tensile Stress. Biophys. J. 2022, 121, 2168–2179. [Google Scholar] [CrossRef]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef]
- Draelos, Z.D. Essentials of Hair Care Often Neglected: Hair Cleansing. Int. J. Trichology 2010, 2, 24–29. [Google Scholar] [CrossRef]
- Cornwell, P.A. A Review of Shampoo Surfactant Technology: Consumer Benefits, Raw Materials and Recent Developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Cebolla-Verdugo, M.; Velasco-Amador, J.P.; Navarro-Triviño, F.J. Contact Dermatitis Due to Hair Care Products: A Comprehensive Review. Cosmetics 2024, 11, 78. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The Biodegradation of Surfactants in the Environment. Biochim. Biophys. Acta-Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Lindberg, J.E.; Gore, R.J.; Sama, S.R.; Galligan, C.J.; Kriebel, D.; Markkanen, P.K.; LeBouf, R.F.; Virji, M.A. Respiratory Quaternary Ammonium and Volatile Organic Compound Exposures Experienced by Home Care Aides during Residential Bathroom Cleaning Using Conventional and Green Products. Ann. Work. Expo. Health 2024, 69, 173–190. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in the Environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Beard, B.C.; Hare, J. Surface Interaction of Quaternary Amines with Hair. J. Surfactants Deterg. 2002, 5, 145–150. [Google Scholar] [CrossRef]
- UL Prospector Staff; ULTRUS. Emerging Trends in Shampoo Formulation: A Comprehensive Guide for Cosmetic Formulators and Raw Materials Suppliers. Available online: https://www.ulprospector.com/knowledge/15610/pcc-emerging-trends-in-shampoo-formulation-a-comprehensive-guide-for-cosmetic-formulators-and-raw-materials-suppliers/ (accessed on 18 August 2025).
- Crutcher, T.; Smith, K.R.; Borland, J.E.; Sauer, J.D.; Perine, J.W. Alkyldimethylamine Oxides as Synergistic Fabric Softeners. J. Am. Oil Chem. Soc. 1992, 69, 682–689. [Google Scholar] [CrossRef]
- Birnie, C.R.; Malamud, D.; Schnaare, R.L. Antimicrobial Evaluation of N-Alkyl Betaines and N-Alkyl-N, N-Dimethylamine Oxides with Variations in Chain Length. Antimicrob. Agents Chemother. 2000, 44, 2514–2517. [Google Scholar] [CrossRef]
- Singh, S.K.; Bajpai, M.; Tyagi, V.K. Amine Oxides: A Review. J. Oleo Sci. 2006, 55, 99–119. [Google Scholar] [CrossRef]
- Govindbhai Mathurdas Patel Application of Surfactants-Fatty Amine Ethoxylates. Available online: https://www.rimpro-india.com/articles1/application-of-surfactants-fatty-amine-ethoxylates.html (accessed on 28 June 2025).
- van Ginkel, C.G.; Stroo, C.A.; Kroon, A.G.M. Biodegradability of Ethoxylated Fatty Amines: Detoxification through a Central Fission of These Surfactants. Sci. Total Environ. 1993, 134, 689–697. [Google Scholar] [CrossRef]
- Naviglio, B.; Calvanese, G.; Tortora, G.; Cipollaro, L. Characterization of Tannery Chemicals: Retanning Agents. 2006. Available online: https://eopcw.com/assets/stores/Leather%20Processing%20II/lecturenote_1533695733retanning%20syntan.pdf (accessed on 18 August 2025).
- Harwood, H.J. Nitrogen-Containing Derivatives of the Fatty Acids. J. Am. Oil Chem. Soc. 1954, 31, 559–564. [Google Scholar] [CrossRef]
- Lim, X. Special to C&EN Questioning Quats’ Safety. C&EN Glob. Enterp. 2020, 98, 28–33. [Google Scholar] [CrossRef]
- Puchta, R. Cationic Surfactants in Laundry Detergents and Laundry Aftertreatment Aids. J. Am. Oil Chem. Soc. 1984, 61, 367–376. [Google Scholar] [CrossRef]
- Ostgard, D.J.; Olindo, R.; Berweiler, M.; Röder, S.; Tacke, T. 128-The Selective Hydrogenatíon of Unstaturated Fatty Nitriles to Unsaturated Primary Amines with Carbon-Templated Activated Nickel Catalysts. In Science and Technology in Catalysis 2006; Eguchi, K., Machida, M., Yamanaka, I., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 172, pp. 537–538. [Google Scholar]
- Pelckmans, M.; Renders, T.; de Vyver, S.; Sels, B.F. Bio-Based Amines through Sustainable Heterogeneous Catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Reck, R.A. Industrial Uses of Palm, Palm Kernel and Coconut Oils: Nitrogen Derivatives. J. Am. Oil Chem. Soc. 1985, 62, 355–365. [Google Scholar] [CrossRef]
- Latypova, T.; Kosovskaya, D.; Lovygin, M.; Evseev, G.; Olkhovskaya, M.; Filatov, V. A Novel Biodegradable Technology for Wool Fabric Restoration and Cotton Color Retention Based on Shikimic Acid and L-Arginine. Textiles 2024, 4, 549–560. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Coiai, S.; Bronco, S.; Passaglia, E. Nanocomposites Based on Phyllosilicates: From Petrochemicals to Renewable Thermoplastic Matrices. In Advanced Nanomaterials; Geckeler, K.E., Nishide, H., Eds.; Wiley: Hoboken, NJ, USA, 2010; Volume 1, pp. 403–458. ISBN 9783527317943. [Google Scholar]
- Scatto, M.; Salmini, E.; Castiello, S.; Coltelli, M.B.; Conzatti, L.; Stagnaro, P.; Andreotti, L.; Bronco, S. Plasticized and Nanofilled Poly(Lactic Acid)-Based Cast Films: Effect of Plasticizer and Organoclay on Processability and Final Properties. J. Appl. Polym. Sci. 2013, 127, 4947–4956. [Google Scholar] [CrossRef]
- Castiello, S.; Coltelli, M.B.; Conzatti, L.; Bronco, S. Comparative Study About Preparation of Poly(Lactide)/Organophilic Montmorillonites Nanocomposites Through Melt Blending or Ring Opening Polymerization Methods. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Maag, H. Fatty Acid Derivatives: Important Surfactants for Household, Cosmetic and Industrial Purposes. J. Am. Oil Chem. Soc. 1984, 61, 259–267. [Google Scholar] [CrossRef]
- Svarc, F.; Hermida, L. Chapter 3—Transdermal and Bioactive Nanocarriers for Skin Care. In Nanocosmetics; Nanda, A., Nanda, S., Nguyen, T.A., Rajendran, S., Slimani, Y., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–58. ISBN 978-0-12-822286-7. [Google Scholar]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Lopez-Galindo, A. Uses of Clay Minerals in Semisolid Health Care and Therapeutic Products. Appl. Clay Sci. 2007, 36, 37–50. [Google Scholar] [CrossRef]
- Kelm, G.R.; Wickett, R.R. Chapter 12—The Role of Fatty Acids in Cosmetic Technology. In Fatty Acids; Ahmad, M.U., Ed.; AOCS Press: Urbana, IL, USA, 2017; pp. 385–404. ISBN 978-0-12-809521-8. [Google Scholar]
- Gervajio, G.C. Fatty Acids and Derivatives from Coconut Oil. In Kirk–Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–38. ISBN 9780471238966. [Google Scholar]
- De Clermonde Gallerenad, H. Functional roles of lipids in make-up products. OCL 2020, 27, 33. Available online: https://www.ocl-journal.org/articles/ocl/pdf/2020/01/ocl200042.pdf (accessed on 20 August 2025). [CrossRef]
- Smith, K.R.; Borland, J.E.; Corona, R.J.; Sauer, J.D. High Active Alkyldimethylamine Oxides. J. Am. Oil Chem. Soc. 1991, 68, 619–622. [Google Scholar] [CrossRef]
- Moore, S.L.; Payne, D.N. Types of Antimicrobial Agents. In Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization; Fraise, A.P., Lambert, P.A., Maillard, J.Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 8–97. ISBN 9780470755884. [Google Scholar]
- Li, S.; Zhang, Z.; Liu, Y.; Wang, J.; Yang, K.; Chen, D.; Liang, Z.; Li, Z. Antibacterial and Biodegradable Keratin-Based Quaternary Ammonium Salt Surfactant Potential as Hair Care Additive. J. Dispers. Sci. Technol. 2023, 44, 2555–2564. [Google Scholar] [CrossRef]
- Corazza, M.; Lauriola, M.M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, Skin Cleansing Protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Luengo, G.S.; Aubrun, O.; Restagno, F. Foams in Cosmetics: New Trends in Detergency, Friction, Coatings. Curr. Opin. Colloid Interface Sci. 2025, 77, 101906. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and Applications of Particle Stabilized Emulsions in Cosmetic Formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Fujii, M.; Inoue, M.; Fukami, T. Novel Amino Acid-Based Surfactant for Silicone Emulsification and Its Application in Hair Care Products: A Promising Alternative to Quaternary Ammonium Cationic Surfactants. Int. J. Cosmet. Sci. 2017, 39, 556–563. [Google Scholar] [CrossRef]
- Mondal, M.G.; Pratap, A.P. Synthesis and Performance Properties of Cationic Fabric Softeners Derived from Free Fatty Acid of Tallow Fat. J. Oleo Sci. 2016, 65, 663–670. [Google Scholar] [CrossRef]
- Baydar, A.; Johnston, R. Novel Quaternary Ammonium Salts Derived from Triglycerides and Their Application in Skin and Hair Products. Int. J. Cosmet. Sci. 1991, 13, 169–190. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial Activity of Saturated Fatty Acids and Fatty Amines against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef]
- Żywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of Bacterial Cellulose with Quaternary Ammonium Compounds Based on Fatty Acids and Amino Acids and the Effect on Antimicrobial Activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef]
- Węgrzyńska, J.; Chlebicki, J.; Maliszewska, I. Preparation, Surface-Active Properties and Antimicrobial Activities of Bis(Ester Quaternary Ammonium) Salts. J. Surfactants Deterg. 2007, 10, 109–116. [Google Scholar] [CrossRef]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Miner. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Boyer, I.; Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; MarksJr, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of PEGs Cocamine and Related Ingredients as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 10S–60S. [Google Scholar] [CrossRef]
- Pellegrene, B.; Hammer, T.J.; Pugh, C.; Soucek, M.D. Maleated Soybean Oil Derivatives as Versatile Reactive Diluents: Synthesis, Characterization, and Evaluation. J. Appl. Polym. Sci. 2022, 139, 51814. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Fernández-Serrano, M.; Fernández-Arteaga, A. Aerobic Biodegradation of Amphoteric Amine-Oxide-Based Surfactants: Effect of Molecular Structure, Initial Surfactant Concentration and PH. Chemosphere 2017, 171, 324–331. [Google Scholar] [CrossRef]
- Jansen, L.M.; den Bakker, P.C.; Venbrux, N.; van Rijbroek, K.W.M.; Klaassen-Heshof, D.J.; Lenferink, W.B.; Lücker, S.; Ranoux, A.; Raaijmakers, H.W.C.; Boltje, T.J. Synthesis and Performance of Bio-Based Amphoteric Surfactants. Chem.-A Eur. J. 2024, 30, e202400986. [Google Scholar] [CrossRef]
- Grigale-Sorocina, Z.; Birks, I. Hectorite and Bentonite Effect on Water-Based Polymer Coating Rheology. C. R. Chim. 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Schmidt-Dannert, C. Multi-Enzymatic Synthesis. Curr. Opin. Chem. Biol. 2010, 14, 174–183. [Google Scholar] [CrossRef]
- Citoler, J.; Derrington, S.R.; Galman, J.L.; Bevinakatti, H.; Turner, N.J. A Biocatalytic Cascade for the Conversion of Fatty Acids to Fatty Amines. Green Chem. 2019, 21, 4932–4935. [Google Scholar] [CrossRef]
- Lee, D.-S.; Song, J.-W.; Voß, M.; Schuiten, E.; Akula, R.K.; Kwon, Y.-U.; Bornscheuer, U.; Park, J.-B. Enzyme Cascade Reactions for the Biosynthesis of Long Chain Aliphatic Amines from Renewable Fatty Acids. Adv. Synth. Catal. 2019, 361, 1359–1367. [Google Scholar] [CrossRef]
- Heider, C.; Menk, M.; Terhorst, M.; Vogt, D.; Seidensticker, T. Synthesis of Tertiary Fatty Amines in Water: The Dual Role of Dimethylamine as Reagent and Phase Mediator in the Homogeneously Catalyzed Fatty Alcohol Amination. ACS Sustain. Chem. Eng. 2023, 11, 11359–11363. [Google Scholar] [CrossRef]
- Shirazi, Y.; Tafazolian, H.; Viamajala, S.; Varanasi, S.; Song, Z.; Heben, M.J. High-Yield Production of Fatty Nitriles by One-Step Vapor-Phase Thermocatalysis of Triglycerides. ACS Omega 2017, 2, 9013–9020. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cuthbertson, A.A.; Alamer, A.A.; Cecon, V.S.; Radhakrishnan, H.; Wu, J.; Curtzwiler, G.W.; Vorst, K.L.; Bai, X.; Landis, C.R.; et al. Aliphatic Amines from Waste Polyolefins by Tandem Pyrolysis, Hydroformylation, and Reductive Amination. Green Chem. 2024, 26, 8718–8727. [Google Scholar] [CrossRef]
- Anal, A.K. Food Processing By-Products and Their Utilization: Introduction. In Food Processing By-Products and Their Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–10. ISBN 9781118432921. [Google Scholar]
- Wang, Y.; Furukawa, S.; Fu, X.; Yan, N. Organonitrogen Chemicals from Oxygen-Containing Feedstock over Heterogeneous Catalysts. ACS Catal. 2020, 10, 311–335. [Google Scholar] [CrossRef]
- Hülsey, M.J.; Yang, H.; Yan, N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 5694–5707. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]
- Benítez, J.J.; Castillo, P.M.; Del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of Tomato Processing By-Products: Fatty Acid Extraction and Production of Bio-Based Materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Sharma, B.K.; Willett, J.L.; Erhan, S.Z.; Cheng, H.N. Soybean Oil as a Renewable Feedstock for Nitrogen-Containing Derivatives. Energy Environ. Sci. 2008, 1, 639–644. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Markham, J.; Templeton, D.W.; Christensen, E.D.; Van Wychen, S.; Vadelius, E.W.; Chen-Glasser, M.; Dong, T.; Davis, R.; Pienkos, P.T. Development of Algae Biorefinery Concepts for Biofuels and Bioproducts; a Perspective on Process-Compatible Products and Their Impact on Cost-Reduction. Energy Environ. Sci. 2017, 10, 1716–1738. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Jafari, H.; Taebnia, N.; Abedi, A.; Amirsadeghi, A.; Niknezhad, S.V.; Alimoradi, H.; Jafarzadeh, S.; Mirzaei, M.; Nie, L.; et al. Protein By-Products: Composition, Extraction, and Biomedical Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9436–9481. [Google Scholar] [CrossRef] [PubMed]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamane, T. Enzymatic Synthesis of Structured Lipids. Adv. Biochem. Eng. Biotechnol. 2004, 90, 151–171. [Google Scholar] [CrossRef]
- FLEXIZYME. Construction of a FLEXIble and Adaptable enZYMatic Biotechnological Platform for SustainablE Industrial Production of Bio-Based Fatty Amines from Side Stream Materials. Available online: https://www.flexizyme.eu/ (accessed on 1 October 2025).
- Müller, H.; Stitz, L.; Riesner, D. Risk Assessment for Fat Derivatives in Case of Contamination with BSE. Eur. J. Lipid Sci. Technol. 2006, 108, 812–826. [Google Scholar] [CrossRef]
- Akzo Nobel Surface Chemistry LLC. HLB and Emulsification. Available online: https://it.scribd.com/doc/71336867/Davies-Chart (accessed on 19 August 2025).
- James, A.D.; Wates, J.M.; Wyn-Jones, E. Determination of the Hydrophilicities of Nitrogen-Based Surfactants by Measurement of Partition Coefficients between Heptane and Water. J. Colloid Interface Sci. 1993, 160, 158–165. [Google Scholar] [CrossRef]
- Behler, A.; Bierman, M.; Hill, K.; Raths, H.C.; Saint Victor, M.E.; Uphues, G. Industrial Surfactant Syntheses—Chapter 1. In Reactions and Synthesis in Surfactant Systems, 1st ed.; Texter, J., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–44. [Google Scholar]
- Gervajio, G.C.; Withana-Gamage, T.S.; Sivakumar, M. Fatty Acids and Derivatives from Coconut Oil. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–45. ISBN 9780471678496. [Google Scholar]
- Tadros, T.F. Surfactants Used in Cosmetic and Personal Care Formulations, Their Properties and Surfactant–Polymer Interaction. In Cosmetic and Personal Care; De Gruyter: Berlin/Heidelberg, Germany; Boston, MA, USA, 2016; pp. 11–50. ISBN 9783110452389. [Google Scholar]
- James, A.D. Cationic Surfactants. In Lipid Technologies and Applications; Padley, F.B., Ed.; Taylor and Fracis: New York, NY, USA, 1997; pp. 232–255. ISBN 9780203748848. [Google Scholar]
- Clendennen, S.K.; Boaz, N.W. Chapter 14—Betaine Amphoteric Surfactants—Synthesis, Properties, and Applications. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Champaign, IL, USA, 2019; pp. 447–469. ISBN 978-0-12-812705-6. [Google Scholar]
- Schick, M.J.; Fowkes, F.M. Foam Stabilizing Additives for Synthetic Detergents. Interaction of Additives and Detergents in Mixed Micelles. J. Phys. Chem. 1957, 61, 1062–1068. [Google Scholar] [CrossRef]
- Franklin, R. Nitrogen Derivatives of Natural Fats and Oils. In Surfactants from Renewable Resources; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 21–43. ISBN 9780470686607. [Google Scholar]
- Tadros, T.F. 10. Formulation of Shampoos. In Cosmetic and Personal Care; De Gruyter: Berlin/Heidelberg, Germany; Boston, MA, USA, 2016; pp. 201–216. ISBN 9783110452389. [Google Scholar]
- Rocafort, C.M. Chapter 21—Functional Materials for Hair. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherland, 2017; pp. 321–335. ISBN 978-0-12-802005-0. [Google Scholar]
- Asadov, Z.H.; Rahimov, R.A.; Mammadova, K.A.; Ahmadova, G.A.; Ahmadbayova, S.F. Synthesis and Colloidal-Chemical Properties of Surfactants Based on Alkyl Amines and Propylene Oxide. Tenside Surfactants Deterg. 2015, 52, 287–293. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S. An Overview of Novel Surfactants for Formulation of Cosmetics with Certain Emphasis on Acidic Active Substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Xie, S.; Jia, C.; Go Ong, S.S.; Wang, Z.; Zhu, M.; Wang, Q.; Yang, Y.; Lin, H. A Shortcut Route to Close Nitrogen Cycle: Bio-Based Amines Production via Selective Deoxygenation of Chitin Monomers over Ru/C in Acidic Solutions. iScience 2020, 23, 101096. [Google Scholar] [CrossRef]
- Coeck, R.; Claes, N.; Cuypers, T.; Bals, S.; De Vos, D.E. The sustainable and catalytic synthesis of N,N-alkylated fatty amines from fatty acids and esters. Green Chem. 2025, 27, 1410–1422. [Google Scholar] [CrossRef]
- Painter, H.A. Biodegradability Testing. In Biodegradability of Surfactants; Karsa, D.R., Porter, M.R., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 65–117. ISBN 978-94-011-1348-9. [Google Scholar]
- van Ginkel, C.G. Biodegradation of Cationic Surfactants: An Environmental Perspective. In Handbook of Detergents: Part B Environmental Impact, Surfactant Science Series; Marcel Dekker, Inc.: New York, NY, USA, 2004; Volume 121, pp. 523–549. [Google Scholar]
- Forno, M.V. Physical Properties of Fats and Fatty Acids. In Baileys Industrial Oil and Fat Products, 4th ed.; Swern, D., Ed.; Wiley: Hoboken, NJ, USA, 1979; Volume 1. [Google Scholar]
- Sonntag, N.O.V. Composition and Characterization of Individual Fats and Oils. In Baileys Industrial Oil and Fat Products; Swern, D., Ed.; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).