Artificial Intelligence in Cosmetic Formulation: Predictive Modeling for Safety, Tolerability, and Regulatory Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. AI in Cosmetic Formulation
3.1.1. Surfactants
3.1.2. Polymers
3.1.3. Fragrances
3.1.4. Preservatives
3.1.5. Antioxidants
3.1.6. Prebiotics
3.1.7. Texture and Sensory Perception
3.1.8. Stability and Microstructure
3.2. Predicting Efficacy, Toxicity, and Skin Tolerability Using In Silico Models
3.2.1. AI for Predicting Clinical Outcomes in Dermatology
3.2.2. AI for Predicting Cosmetic Satisfaction and Consumer Behavior
3.2.3. In Silico Approaches for Acute Dermal Toxicity
3.2.4. Skin Sensitization Prediction and Industry Applications
3.3. Model Performance Summary
3.4. Ethical and Legislative Challenges
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haykal, D.; Garibyan, L.; Flament, F.; Cartier, H. Hybrid cosmetic dermatology: AI generated horizon. Skin Res. Technol. 2024, 30, e13721. [Google Scholar] [CrossRef] [PubMed]
- Young, A.T.; Xiong, M.; Pfau, J.; Keiser, M.J.; Wei, M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020, 140, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Grech, V.S.; Kefala, V.; Rallis, E. Cosmetology in the Era of Artificial Intelligence. Cosmetics 2024, 11, 135. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the standards of proof (of safety) for FDA regulated consumer products and how the generally recognized as safe criteria could be applied to cosmetics. Regul. Toxicol. Pharmacol. 2024, 149, 105603. [Google Scholar] [CrossRef]
- Mays, D.; Friedman, A.; Kennedy, J.; Yiannias, J.; Morgan, J. Non-adherence to Labeling Standards Can Misrepresent Safety of Ingredients in Cosmetic Cleansers. J. Drugs Dermatol. 2023, 22, 98–100. [Google Scholar]
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef]
- Reeder, M.J.; Warshaw, E.; Aravamuthan, S.; Belsito, D.V.; Geier, J.; Wilkinson, M.; Atwater, A.R.; White, I.R.; Silverberg, J.I.; Taylor, J.S.; et al. Trends in the Prevalence of Methylchloroisothiazolinone/Methylisothiazolinone Contact Allergy in North America and Europe. JAMA Dermatol. 2023, 159, 267–274. [Google Scholar] [CrossRef]
- Xin, H.; Virk, A.S.; Virk, S.S.; Akin-Ige, F.; Amin, S. Applications of artificial intelligence and machine learning on critical materials used in cosmetics and personal care formulation design. Curr. Opin. Colloid Interface Sci. 2024, 73, 101847. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Kim, S.; Lee, C.; Lee, J.; Yoo, S. Deep learning-based skin care product recommendation: A focus on cosmetic ingredient analysis and facial skin conditions. J. Cosmet. Dermatol. 2024, 23, 2066–2077. [Google Scholar] [CrossRef]
- Kalicińska, J.; Wiśniowska, B.; Polak, S.; Spiewak, R. Artificial Intelligence That Predicts Sensitizing Potential of Cosmetic Ingredients with Accuracy Comparable to Animal and In Vitro Tests—How Does the Infotechnomics Compare to Other “Omics” in the Cosmetics Safety Assessment? Int. J. Mol. Sci. 2023, 24, 6801. [Google Scholar] [CrossRef]
- Eppler, A.R.; Ma, H. Artificial Intelligence Beauty Revolution—State of the Art and New Trends from the SCC78 Annual Meeting. Cosmetics 2025, 12, 73. [Google Scholar] [CrossRef]
- Thacker, J.C.R.; Bray, D.J.; Warren, P.B.; Anderson, R.L. Can Machine Learning Predict the Phase Behavior of Surfactants? J. Phys. Chem. B 2023, 127, 3711–3727. [Google Scholar] [CrossRef]
- Boukelkal, N.; Rahal, S.; Rebhi, R.; Hamadache, M. QSPR for the prediction of critical micelle concentration of different classes of surfactants using machine learning algorithms. J. Mol. Graph. Model. 2024, 129, 108757. [Google Scholar] [CrossRef]
- Ham, S.; Wang, X.; Zhang, H.; Lattimer, B.; Qiao, R. A GNN-Based QSPR Model for Surfactant Properties. Colloids Interfaces 2024, 8, 63. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Miwake, H.; Nakatake, R.; Arai, N. Predicting the Performance of Functional Materials Composed of Polymeric Multicomponent Systems Using Artificial Intelligence—Formulations of Cleansing Foams as an Example. Polymers 2023, 15, 4216. [Google Scholar] [CrossRef]
- Yan, C.; Li, G. The Rise of Machine Learning in Polymer Discovery. Adv. Intell. Syst. 2023, 5, 2200243. [Google Scholar] [CrossRef]
- Martí, D.; Pétuya, R.; Bosoni, E.; Dublanchet, A.-C.; Mohr, S.; Léonforte, F. Predicting the Glass Transition Temperature of Biopolymers via High-Throughput Molecular Dynamics Simulations and Machine Learning. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Wang, S.; Sadowski, G.; Ji, Y. Strategy of Coupling Artificial Intelligence with Thermodynamic Mechanism for Predicting Complex Polymer Viscosities. ACS Sustain. Chem. Eng. 2024, 12, 4631–4643. [Google Scholar] [CrossRef]
- Tran, H.; Gurnani, R.; Kim, C.; Pilania, G.; Kwon, H.-K.; Lively, R.; Ramprasad, R. Design of functional and sustainable polymers assisted by artificial intelligence. Nat. Rev. Mater. 2024, 9, 866–886. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, H.; Zhuang, Y.; Wang, L.; Liu, L.; Dong, Y.; Du, J.; Xie, W.; Yuan, Z. Odor prediction and aroma mixture design using machine learning model and molecular surface charge density profiles. Chem. Eng. Sci. 2021, 245, 116947. [Google Scholar] [CrossRef]
- Rodrigues, B.C.; Santana, V.V.; Queiroz, L.P.; Rebello, C.M. Harnessing graph neural networks to craft fragrances based on consumer feedback. Comput. Chem. Eng. 2024, 185, 108674. [Google Scholar] [CrossRef]
- Santana, V.V.; Martins, M.A.; Loureiro, J.M.; Ribeiro, A.M.; Rodrigues, A.E.; Nogueira, I.B. Optimal fragrances formulation using a deep learning neural network architecture: A novel systematic approach. Comput. Chem. Eng. 2021, 150, 107344. [Google Scholar] [CrossRef]
- Heng, Y.P.; Lee, H.Y.; Chong, J.W.; Tan, R.R.; Aviso, K.B.; Chemmangattuvalappil, N.G. Incorporating Machine Learning in Computer-Aided Molecular Design for Fragrance Molecules. Processes 2022, 10, 1767. [Google Scholar] [CrossRef]
- Mahmoud, S.; Irwin, B.; Chekmarev, D.; Vyas, S.; Kattas, J.; Whitehead, T.; Mansley, T.; Bikker, J.; Conduit, G.; Segall, M. Imputation of sensory properties using deep learning. J. Comput. Aided Mol. Des. 2021, 35, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.L.; Wang, C.C.; Lin, Y.C.; Tung, C.W. Computational identification of preservatives with potential neuronal cytotoxicity. Regul. Toxicol. Pharmacol. 2021, 119, 104815. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Fan, W.; Chen, X.; Wang, H.; Qin, C.; Jiang, X. Component spectra extraction and quantitative analysis for preservative mixtures by combining terahertz spectroscopy and machine learning. Spectrochim Acta A Mol. Biomol. Spectrosc. 2022, 271, 120908. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Song, L.; Zhu, S.; Fu, X.; Li, X.; He, C.; Li, J. Machine learning assisted rational design of antimicrobial peptides based on human endogenous proteins and their applications for cosmetic preservative system optimization. Sci. Rep. 2024, 14, 947. [Google Scholar] [CrossRef]
- Jung, J.; Moon, J.O.; Ahn, S.I.; Lee, H. Predicting antioxidant activity of compounds based on chemical structure using machine learning methods. Korean J. Physiol. Pharmacol. 2024, 28, 527–537. [Google Scholar] [CrossRef]
- Ho Thanh Lam, L.; Le, N.H.; Van Tuan, L.; Tran Ban, H.; Nguyen Khanh Hung, T.; Nguyen, N.T.K.; Huu Dang, L.; Le, N.Q.K. Machine Learning Model for Identifying Antioxidant Proteins Using Features Calculated from Primary Sequences. Biology 2020, 9, 325. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial Dysbiosis in the Skin Microbiome and Its Psychological Consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Khalid Anwer, M.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Arcaea, L.L.C. Precision Prebiotics—Skin Microbiome Modulation for Cosmetic Benefits. In Proceedings of the 78th Annual SCC Scientific Meeting & Showcase (SCC78), Los Angeles, CA, USA, 11–13 December 2024. [Google Scholar]
- Carrieri, A.P.; Haiminen, N.; Maudsley-Barton, S.; Gardiner, L.J.; Murphy, B.; Mayes, A.E.; Paterson, S.; Grimshaw, S.; Winn, M.; Shand, C.; et al. Explainable AI reveals changes in skin microbiome composition linked to phenotypic differences. Sci. Rep. 2021, 11, 4565. [Google Scholar] [CrossRef] [PubMed]
- Roso, A.; Aubert, A.; Cambos, S.; Vial, F.; Schäfer, J.; Belin, M.; Gabriel, D.; Bize, C. Contribution of cosmetic ingredients and skin care textures to emotions. Int. J. Cosmet. Sci. 2024, 46, 262–283. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Maia Campos, P.M.B.G. Physical-Mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef]
- Franzol, A.; Banin, T.M.; Brazil, T.R.; Rezende, M.C. Rheological Analyses and Artificial Neural Network as Optimization Tools to Predict the Sensory Perception of Cosmetic Emulsions. Mater. Res. 2021, 24, e20210252. [Google Scholar] [CrossRef]
- Phuaksaman, C.; Wangkananon, W. Optimization of Material Selection for Whitening Cream: Artificial Neural Networks and Genetic Algorithm Approach. Key Eng. Mater. 2017, 728, 416–421. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Gasperlin, M.; Podlogar, F.; Sibanc, R. Evolutionary artificial neural networks as tools for predicting the internal structure of microemulsions. J. Pharm. Pharm. Sci. 2008, 11, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Yoo, J.; Lee, M.L.; Han, S.H.; Han, S.K.; Lee, J.Y.; Yi, S.W.; Nam, J.; Kim, D.S.; Yang, Y.S. Deep Learning Model for Cosmetic Gel Classification Based on a Short-Time Fourier Transform and Spectrogram. ACS Appl. Mater. Interfaces 2024, 16, 25825–25835. [Google Scholar] [CrossRef]
- Du, A.X.; Emam, S.; Gniadecki, R. Review of Machine Learning in Predicting Dermatological Outcomes. Front. Med. 2020, 7, 266. [Google Scholar] [CrossRef]
- Emam, S.; Du, A.X.; Surmanowicz, P.; Thomsen, S.F.; Greiner, R.; Gniadecki, R. Predicting the long-term outcomes of biologics in patients with psoriasis using machine learning. Br. J. Dermatol. 2020, 182, 1305–1307. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, Y.H.; Liang, C.W.; Li, Y.C. Assessment of Deep Learning Using Nonimaging Information and Sequential Medical Records to Develop a Prediction Model for Nonmelanoma Skin Cancer. JAMA Dermatol. 2019, 155, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Roffman, D.; Hart, G.; Girardi, M.; Ko, C.J.; Deng, J. Predicting non-melanoma skin cancer via a multi-parameterized artificial neural network. Sci. Rep. 2018, 8, 1701. [Google Scholar] [CrossRef] [PubMed]
- Khozeimeh, F.; Alizadehsani, R.; Roshanzamir, M.; Khosravi, A.; Layegh, P.; Nahavandi, S. An expert system for selecting wart treatment method. Comput. Biol. Med. 2017, 81, 167–175. [Google Scholar] [CrossRef]
- Tan, E.; Lin, F.; Sheck, L.; Salmon, P.; Ng, S. A practical decision-tree model to predict complexity of reconstructive surgery after periocular basal cell carcinoma excision. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 717–723. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, D.U.; Son, E.J.; Oh, S.H.; Kim, W.; Kim, Y.; Kwon, G. Emotion recognition while applying cosmetic cream using deep learning from EEG data; cross-subject analysis. PLoS ONE 2022, 17, e0274203. [Google Scholar] [CrossRef]
- Tong, W.-S.; Tang, C.-K.; Brown, M.S.; Xu, Y.-Q. Example-Based Cosmetic Transfer. In Proceedings of the 15th Pacific Conference on Computer Graphics and Applications (PG’07), Maui, HI, USA, 29 October–2 November 2007; pp. 211–218. [Google Scholar]
- Flament, F.; Zhang, Y.; Yu, Z.; Jiang, R.; Houghton, J.; Sarda Duthil, L.; Arcin, V.; Daniel, R.; Perrier, J.C.; Niviere, J.; et al. Developing an Artificial Intelligence (A.I)-based descriptor of facial appearance that fits with the assessments of makeup experts. Skin Res. Technol. 2021, 27, 1081–1091. [Google Scholar] [CrossRef]
- Shi, C.; Jiang, Z.; Ma, X.; Luo, Q. A Personalized Visual Aid for Selections of Appearance Building Products with Long-term Effects. In Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems, New Orleans, LA, USA, 29 April–5 May 2022; p. 74. [Google Scholar]
- Vatiwutipong, P.; Vachmanus, S.; Noraset, T.; Tuarob, S. Artificial Intelligence in Cosmetic Dermatology: A Systematic Literature Review. IEEE Access 2023, 11, 71407–71425. [Google Scholar] [CrossRef]
- Caiazzo, G.; Di Caprio, R.; Lembo, S.; Raimondo, A.; Scala, E.; Patruno, C.; Balato, A. IL-26 in allergic contact dermatitis: Resource in a state of readiness. Exp. Dermatol. 2018, 27, 681–684. [Google Scholar] [CrossRef]
- Lou, S.; Yu, Z.; Huang, Z.; Wang, H.; Pan, F.; Li, W.; Liu, G.; Tang, Y. In Silico Prediction of Chemical Acute Dermal Toxicity Using Explainable Machine Learning Methods. Chem. Res. Toxicol. 2024, 37, 513–524. [Google Scholar] [CrossRef]
- Selvestrel, G.; Robino, F.; Russo, M.Z. In Silico Models for Skin Sensitization and Irritation. Methods Mol. Biol. 2022, 2425, 291–354. [Google Scholar] [CrossRef]
- Gilmour, N.; Kern, P.S.; Alepee, N.; Boisleve, F.; Bury, D.; Clouet, E.; Hirota, M.; Hoffmann, S.; Kuhnl, J.; Lalko, J.F.; et al. Development of a next generation risk assessment framework for the evaluation of skin sensitisation of cosmetic ingredients. Regul. Toxicol. Pharmacol. 2020, 116, 104721. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.; Zang, Q.; Paris, M.; Lehmann, D.M.; Allen, D.; Choksi, N.; Matheson, J.; Jacobs, A.; Casey, W.; Kleinstreuer, N. Multivariate models for prediction of human skin sensitization hazard. J. Appl. Toxicol. 2017, 37, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Ta, G.H.; Weng, C.F.; Leong, M.K. In silico Prediction of Skin Sensitization: Quo vadis? Front. Pharmacol. 2021, 12, 655771. [Google Scholar] [CrossRef] [PubMed]
- Fortino, V.; Wisgrill, L.; Werner, P.; Suomela, S.; Linder, N.; Jalonen, E.; Suomalainen, A.; Marwah, V.; Kero, M.; Pesonen, M.; et al. Machine-learning-driven biomarker discovery for the discrimination between allergic and irritant contact dermatitis. Proc. Natl. Acad. Sci. USA 2020, 117, 33474–33485. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; El-Dahiyat, F.; Zyoud, S.H.; Jairoun, O.; Al Shayeb, M. Development and Validation of an Instrument to Appraise the Tolerability, Safety of Use, and Pleasantness of a Cosmetic Product. Cosmetics 2023, 10, 15. [Google Scholar] [CrossRef]
- The ICCVAM Skin Sensitization (Murine LLNA) Reference List, National Toxicologic Program. Available online: https://cebs-ext.niehs.nih.gov/datasets/search/llna2013 (accessed on 5 June 2025).
- Ye, L.; Zhang, J.; Chen, T.; Li, D.; Shu, P. Establishing an Evidence-Based System for Cosmetic Safety and Efficacy Evaluation. In Cosmetic Industry—Trends, Products and Quality Control [Working Title]; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Rinwa, P.; Eriksson, M.; Cotgreave, I.; Bäckberg, M. 3R-Refinement principles: Elevating rodent well-being and research quality. Lab. Anim. Res. 2024, 40, 11. [Google Scholar] [CrossRef]
- Georgievskaya, A.; Tlyachev, T.; Danko, D.; Chekanov, K.; Corstjens, H. How artificial intelligence adopts human biases: The case of cosmetic skincare industry. AI Ethics 2023, 5, 105–115. [Google Scholar] [CrossRef]
- Gordon, E.R.; Trager, M.H.; Kontos, D.; Weng, C.; Geskin, L.J.; Dugdale, L.S.; Samie, F.H. Ethical considerations for artificial intelligence in dermatology: A scoping review. Br. J. Dermatol. 2024, 190, 789–797. [Google Scholar] [CrossRef]
- Rokhshad, R.; Keyhan, S.O.; Yousefi, P. Artificial intelligence applications and ethical challenges in oral and maxillo-facial cosmetic surgery: A narrative review. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 14. [Google Scholar] [CrossRef]
- European Commission. Regulation of the European Parliament and of the Council. Laying Down Harmonized Rules on Artificial Intelligence (Artificial Intelligence Act) and Amending Certain Union Legislative Acts. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52021PC0206 (accessed on 5 June 2025).
- Grzybowski, A.; Jin, K.; Wu, H. Challenges of artificial intelligence in medicine and dermatology. Clin. Dermatol. 2024, 42, 210–215. [Google Scholar] [CrossRef]
- McMullen, E.; Grewal, R.; Storm, K.; Maazi, M.; Butt, A.B.; Gupta, R.; Maibach, H. Diagnosing contact dermatitis using machine learning: A review. Contact Dermat. 2024, 91, 186–189. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Yin, X.; Yang, Y.; Ji, B.; Niu, Y.; Ren, L. Epidermal Wearable Biosensors for Monitoring Biomarkers of Chronic Disease in Sweat. Biosensors 2023, 13, 313. [Google Scholar] [CrossRef]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Palaniappan, K.; Lin, E.Y.T.; Vogel, S. Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare 2024, 12, 562. [Google Scholar] [CrossRef]
- Kenig, N.; Monton Echeverria, J.; Rubi, C. Ethics for AI in Plastic Surgery: Guidelines and Review. Aesthetic Plast. Surg. 2024, 48, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ong, K.G.; McGeehan, M.A. Skin Phototype Classification with Machine Learning Based on Broadband Optical Measurements. Sensors 2024, 24, 7397. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Jiang, R.; Houghton, J.; Zhang, Y.; Kroely, C.; Jablonski, N.G.; Jean, A.; Clarke, J.; Steeg, J.; Sehgal, C.; et al. Accuracy and clinical relevance of an automated, algorithm-based analysis of facial signs from selfie images of women in the United States of various ages, ancestries and phototypes: A cross-sectional observational study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fliorent, R.; Fardman, B.; Podwojniak, A.; Javaid, K.; Tan, I.J.; Ghani, H.; Truong, T.M.; Rao, B.; Heath, C. Artificial intelligence in dermatology: Advancements and challenges in skin of color. Int. J. Dermatol. 2024, 63, 455–461. [Google Scholar] [CrossRef]
- Balato, A.; Zink, A.; Babino, G.; Buononato, D.; Kiani, C.; Eyerich, K.; Ziehfreund, S.; Scala, E. The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life 2022, 12, 2026. [Google Scholar] [CrossRef]
- Scala, E.; Kaczmarczyk, R.; Zink, A.; Balato, A.; PSES study group. Sociodemographic, clinical and therapeutic factors as predictors of life quality impairment in psoriasis: A cross-sectional study in Italy. Dermatol. Ther. 2022, 35, e15622. [Google Scholar] [CrossRef]
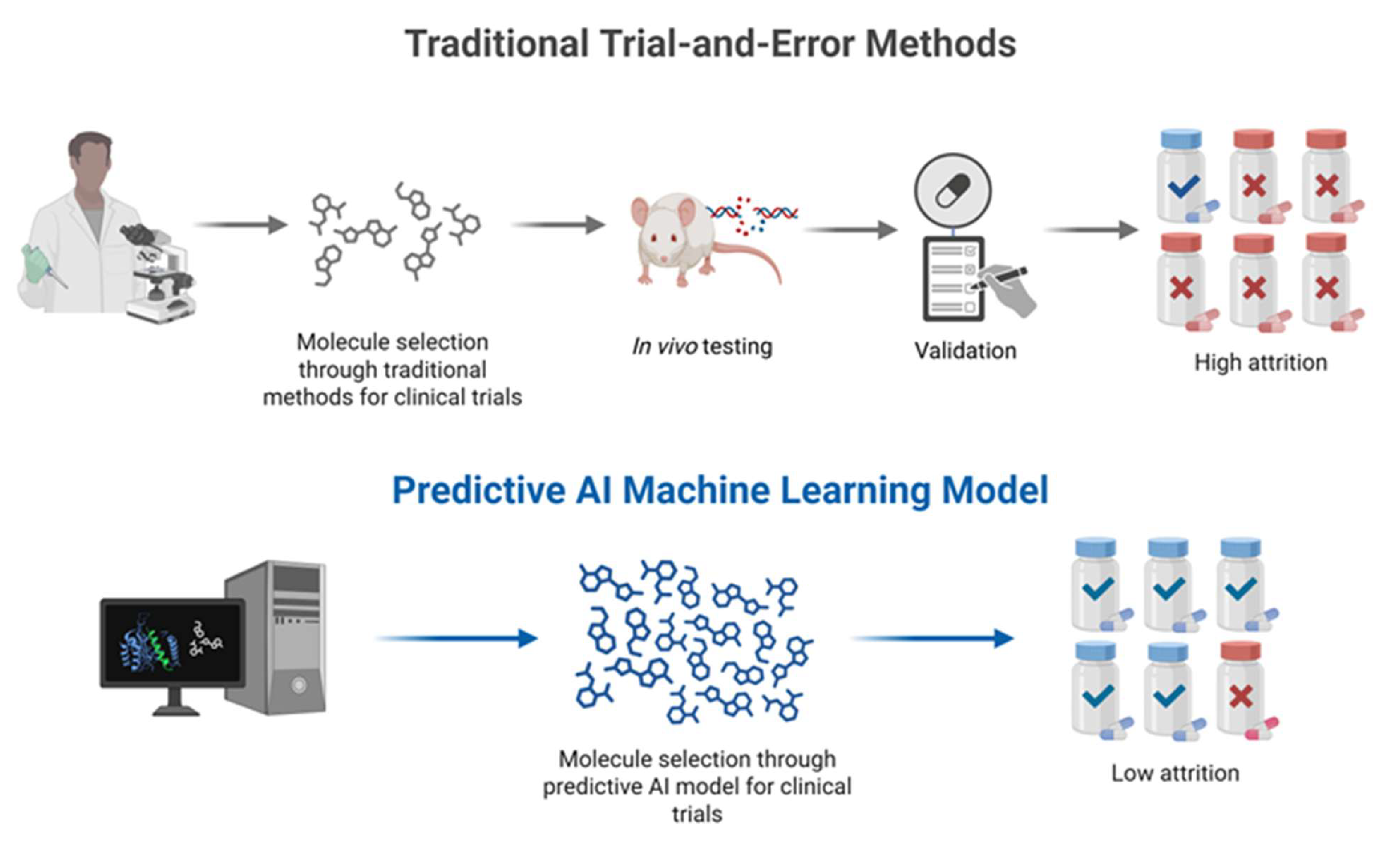
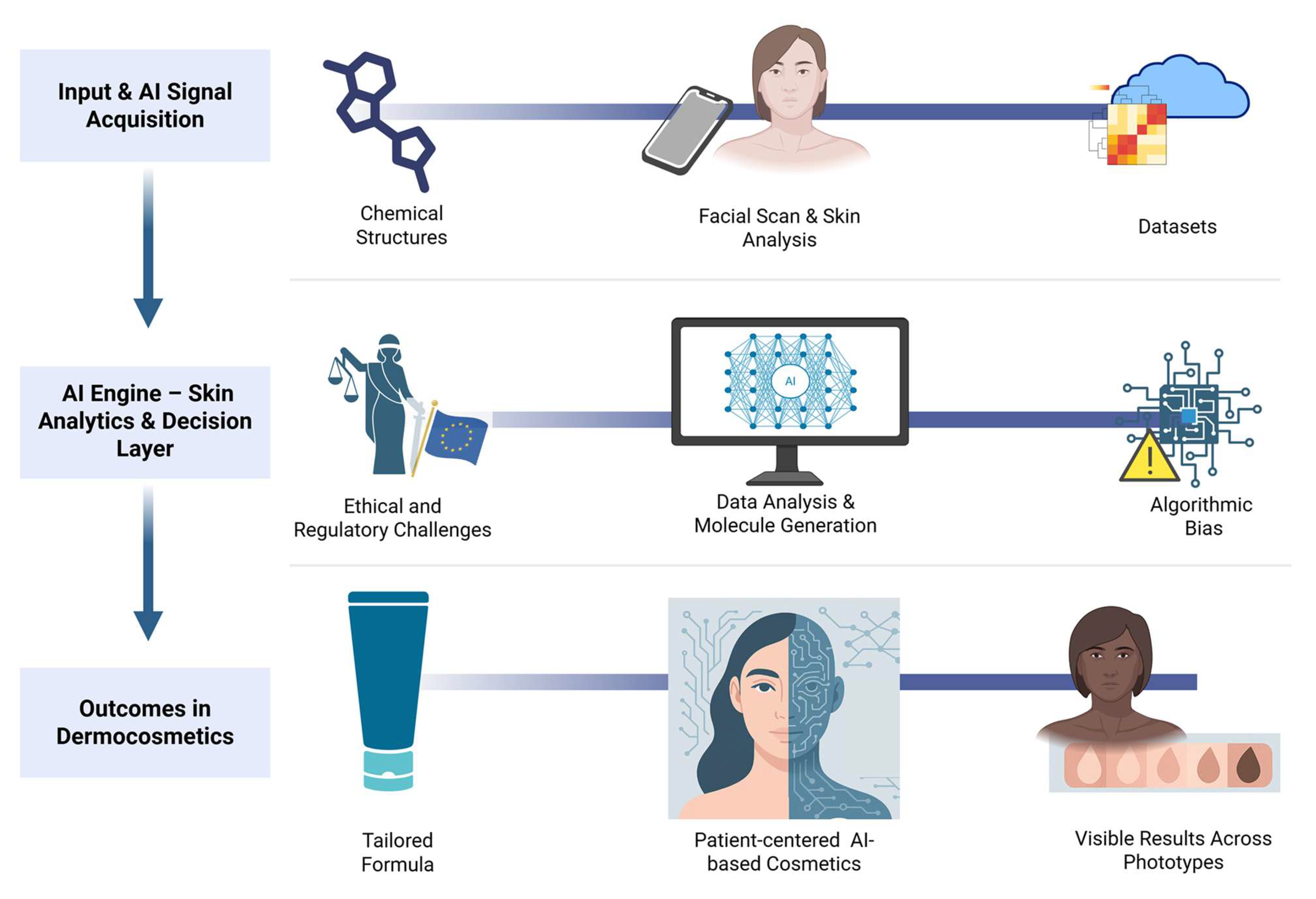
| ML Paradigm | Description | Examples of Techniques | Applications in Cosmetology | Advantages | Limitations |
|---|---|---|---|---|---|
| Supervised Learning | Models are trained on labeled datasets (input + known output) to make predictions. | Support Vector Machines (SVM), Decision Trees, Regression | Prediction of toxicity, solubility, formulation stability, functional efficacy of ingredients | High predictive accuracy, suitable for well-defined endpoints | Requires large, high-quality labeled datasets; manual labeling effort is high |
| Unsupervised Learning | Models analyze unlabeled data to identify hidden patterns or groupings. | K-means Clustering, Hierarchical Clustering, PCA | Consumer segmentation, pattern detection in sensory and formulation parameters | Useful for exploratory analysis; autonomous pattern discovery | No direct output prediction; often less interpretable |
| Semi-supervised Learning | Combines small, labeled datasets with larger unlabeled ones to improve performance. | Self-training, Co-training | Enhancing model performance when labeled data are scarce; label propagation | Reduces labeling effort; improves generalization | Risk of error propagation with incorrect pseudo-labels |
| Reinforcement Learning | An agent learns optimal behavior through interaction with an environment and feedback (rewards/penalties). | Q-learning, Policy Gradient Methods | Adaptive formulation optimization; intelligent recommendation systems | Learns from experience; suitable for dynamic environments | Still mostly experimental in cosmetics; complex to implement in real-world scenarios |
| Aspects | Features |
|---|---|
| Key Functions | Solubilization, foaming, skin feel [12] |
| AI Methods | QSAR, SVR, ANN, GANs [12,13,14] |
| Parameters Modeled | CMC, HLB, toxicity, biodegradability [13,15] |
| Data Sources | SMILES, 3D conformers, molecular descriptors [12,13] |
| Advantages | Virtual screening, de novo design, formulation optimization [12] |
| Limitations | Nonlinear interactions, data quality dependency [15] |
| Aspects | Features |
|---|---|
| Key Functions | Viscosity, film formation, delivery control [16,17] |
| AI Methods | GNNs, polymer-specific fingerprints, active learning [17,18,19] |
| Properties Modeled | Tg, solubility, MW distribution, feel [16,17,18] |
| Key Focus | Bio-based & biodegradable polymers [18,19] |
| Limitations | Sparse polymer-specific databases, insufficient fingerprints [8] |
| Potential | Eco-friendly formulations, sensory optimization [17,18,19] |
| Aspects | Features |
|---|---|
| Key Functions | Sensory appeal, emotional engagement [20] |
| AI Techniques | GNNs, ANN, DNN, RSML-CAMD [20,21,22,23] |
| Targets | Odor prediction, blend optimization, substitution [22,23,24] |
| Unique Aspects | Emotionally tuned blends, activity cliff prediction, odor constraints [22,23,24] |
| Benefits | Faster iteration, customized olfactory profiles [20,23,24] |
| Aspects | Features |
|---|---|
| Function | Antimicrobial protection, shelf-life extension [25] |
| AI Tools | DeepTox, ProTox-II, QSAR (RF+SMOTE), ML + spectroscopy |
| Target Outputs | MICs, cytotoxicity, EDC risk, neurotoxicity [25,26] |
| Innovation | Biomimetic AMPs, synergistic blends [27] |
| Advantages | Safety-focused screening, ethical compliance (no animal testing) [25,26,27] |
| Aspects | Features |
|---|---|
| Function | Reduce oxidative stress, anti-aging [28] |
| AI models | QSAR, Random Forest, SVM, hybrid QM + ML [11,28] |
| Types | Small molecules, antioxidant proteins [11] |
| Application | Natural compound screening, reactivity modeling [11] |
| Benefit | Efficient high-throughput screening [11] |
| Aspects | Features |
|---|---|
| Function | Rebalance microbiota, support skin health [11,30] |
| Outcomes | Odor control, dandruff reduction, hydration prediction [30,31,32] |
| Data Insights | Skin phenotype predictions (age, menopause, smoking) [32] |
| Potential | Personalized skincare, clinical efficacy mapping [32] |
| Aspect | Method/Model | Key Data/Performance | Reference |
|---|---|---|---|
| Texture parameters | Rheological measurements (G′, G″, yield stress, thixotropy) | G′: 10–500 Pa; G″: 5–200 Pa; Yield stress: 1–50 Pa; ANN model predicted sensory pleasantness with 60–84% accuracy | Calixto et al. [35], Franzol et al. [36] |
| Appearance and microstructure | Small-angle X-ray scattering, photon correlation spectroscopy | Transparent systems linked to finely dispersed droplets; milky emulsions linked to larger droplet sizes and higher phase separation risk | Roso et al. [34] |
| Whitening cream optimization | Hybrid ANN–genetic algorithm model | MSE: 6.01 × 10−4; R2: 0.979; Optimal actives: 3.00% Arbutin, 0.658% Aloesin, 0.007% Niacinamide, 0.993% Oxyresveratrol; Melanin content reduced to 0.0824; Sensory panel scores: >80/100 for smoothness and spreadability | Phuaksaman et al. [37] |
| Aspect | Method | Key Data/Performance | Reference |
|---|---|---|---|
| Microstructure detection | Differential Scanning Calorimetry (DSC) | - O/W: freezing peak of supercooled water ~−17 °C-Bicontinuous: bound-water peak ~−50 °C - W/O: lipid-phase solidification ~−8 °C; no water peak | Ravera et al. [38] |
| Prediction of microemulsion type | Artificial Neural Network (ANN) trained on 170 formulations | 90% accuracy; surfactant–cosurfactant ratios of 1:1, 2:1, 1:2; 30–40 wt.% surfactant (Tween 40) and cosurfactant (glyceryl caprylate) | Gasperlin et al. [39] |
| Gel type classification | ResNet-based CNN with STFT spectrograms from rub test data | >90% accuracy; outperformed CWT-based 2D and 1D CNNs; robust across k-fold cross-validation | Sim et al. [40] |
| Application | Study | Sample Size | Algorithms Used | Performance Metric | Notes |
|---|---|---|---|---|---|
| Psoriasis biologic discontinuation | Emam et al. [42] | 681 patients | GLM, RF, ANN | AUC = 0.95 | N/A |
| Non-melanoma skin cancer | Wang et al. [43] | 9494 patients | Semi-supervised CNN | AUC = 0.89 | Sensitivity: 83.1%, Specificity: 82.3% |
| Cryotherapy and immunotherapy response prediction in wart treatment | Khozeimeh et al. [45] | 180 patients | Fuzzy logic | AUC = 0.902 | Accuracy for cryotherapy: 80%, Accuracy for immunotherapy: 98% |
| Surgical complexity following periocular basal cell carcinoma excision | Tan et al. [46] | 156 patients | Naive Bayesian classifier | AUC = 0.854 | Positive predictive value: 38.1%, Negative predictive value: 94.1% |
| Application Area | Sample Size/Data | Algorithm(s) Used | Key Metric(s) | Reference |
|---|---|---|---|---|
| Surfactants property prediction | >500 samples | MLR, RF, ANN, SVR | R2 = 0.77 | Hamaguchi et al. [15] |
| Microemulsion type classification | 170 formulations | ANN | Accuracy: 90% | Gasperlin et al. [39] |
| Gel type classification | N/A | ResNet CNN with STFT | Accuracy: >90%; robust k-fold | Sim et al. [40] |
| Whitening cream optimization | Expert-derived dataset | Hybrid ANN–GA | MSE = 6.01 × 10−4, R2 = 0.979 | Phuaksaman et al. [37] |
| Rheology–sensory mapping | 39 emulsions | ANN | Sensory pleasantness: 60–84% | Calixto et al. [35] |
| Prebiotics microbiome prediction | N/A | Explainable AI | Odor ↓, flakes ↓, redness ↓ | Jensen et al., SCC78 [32] |
| Acute dermal toxicity (in silico) | >3400 data points (animal) | ML + DL + SARpy | AUC: 78% (rabbit), 82% (rat) | Lou et al. [53] |
| Skin sensitization prediction | 157 substances (LLNA data) | Naive Bayes, Random Committee | Accuracy: 86%, Sens: 80%, Spec: 90% | Zhang et al. [20] |
| Clinical outcomes in psoriasis | 681 patients | GLM, RF, ANN | AUC: 0.95 | Emam et al. [42] |
| Non-melanoma skin cancer risk strategy | 9494 patients | Semi-supervised CNN | AUC: 0.89 | Wang et al. [43] |
| Wart treatment response prediction | 180 patients | Fuzzy logic | AUC: 0.902 | Khozeimeh et al. [45] |
| Surgical complexity in BCC excisions | 156 patients | Naive Bayes | AUC: 0.854 | Tan et al. [46] |
| User satisfaction (neurocosmetics) | EEG from cream tests | CNN | Accuracy: 75.4% | Kim et al. [47] |
| Skincare efficacy forecasting | N/A | “SkincareMirror” hybrid model | Higher user confidence scores | Shi et al. [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Guardo, A.; Trovato, F.; Cantisani, C.; Dattola, A.; Nisticò, S.P.; Pellacani, G.; Paganelli, A. Artificial Intelligence in Cosmetic Formulation: Predictive Modeling for Safety, Tolerability, and Regulatory Perspectives. Cosmetics 2025, 12, 157. https://doi.org/10.3390/cosmetics12040157
Di Guardo A, Trovato F, Cantisani C, Dattola A, Nisticò SP, Pellacani G, Paganelli A. Artificial Intelligence in Cosmetic Formulation: Predictive Modeling for Safety, Tolerability, and Regulatory Perspectives. Cosmetics. 2025; 12(4):157. https://doi.org/10.3390/cosmetics12040157
Chicago/Turabian StyleDi Guardo, Antonio, Federica Trovato, Carmen Cantisani, Annunziata Dattola, Steven P. Nisticò, Giovanni Pellacani, and Alessia Paganelli. 2025. "Artificial Intelligence in Cosmetic Formulation: Predictive Modeling for Safety, Tolerability, and Regulatory Perspectives" Cosmetics 12, no. 4: 157. https://doi.org/10.3390/cosmetics12040157
APA StyleDi Guardo, A., Trovato, F., Cantisani, C., Dattola, A., Nisticò, S. P., Pellacani, G., & Paganelli, A. (2025). Artificial Intelligence in Cosmetic Formulation: Predictive Modeling for Safety, Tolerability, and Regulatory Perspectives. Cosmetics, 12(4), 157. https://doi.org/10.3390/cosmetics12040157











