Abstract
Nutritional foods are concentrated sources of molecules with a nutritional or physiological effect which contain nutrients. There is a category, “nutricosmetics”, defined as ingestible natural health products that enhance the function and appearance of human skin, nails, and hair. A new variety of Flammulina velutipes (F. velutipes), Oki-Shirayuki 919, was explored to ascertain whether its components have functions of nutricosmetics. We focused on physiological effects for enhancing the human skin condition, such as moisturizing or barrier functions in F. velutipes. A randomized, double-blind, placebo-controlled clinical study was performed between January and March 2022. Among healthy men and women (n = 30) aged 20 to 59 years, the test group (n = 15) took a test product which included F. velutipes dry powder, and the placebo group (n = 15) took a placebo (a similar product in which the F. velutipes dry powder was replaced with plum fruit paste). Since the amount of increase in skin hydration over four weeks in the test group was significantly larger than that in the placebo group, a significant difference between the two groups was observed (p = 0.033). F. velutipe was suggested to have some physiological functions such as improving skin moisture.
1. Introduction
Asian countries have a long tradition of the medicinal use of mushrooms [1]. The ‘Mush-room’ is not a taxonomic category, and has been defined as ‘a macrofungus with a distinctive fruiting body’ [2]. The number of mushroom species on Earth is estimated to be 140,000, suggesting that only 10% are known. Even among the known species, the proportion of well-investigated mushrooms is very low. Mushrooms need antibacterial and antifungal compounds to survive in their natural environment. Therefore, many mushrooms have antimicrobial compounds with strong activities, and they could be of benefit to humans [1]. Mushrooms have great nutritional value since they are quite rich in protein, with an important content of essential amino acids and fiber, and are low in fat, but have a nutritionally significant content of vitamins (B1, B2, B12, C, D, and E) and promote health for the synergistic effects of all the bioactive compounds present [3,4].
- Ingredients of mushrooms and health promotional effects:
Natural cell wall polysaccharides found in yeast and fungi (including mushrooms) possess many health promotional effects on human health, such as anti-tumor, anti-diabetes, anti-infection, lowering blood cholesterol, and immune-modulating properties. In addition, polysaccharides have other skin health and anti-aging effects such as anti-oxidant activity, anti-wrinkle activity, anti-ultraviolet light, wound healing, and moisturizing effect [5]. Glycolipids in which various sugars are bound to ceramides are thought to provide the barrier property of the epidermis [5]. Glycolipids are mainly distributed in animals and fungi, and in small quantities in plants. Although the structure of the lipid part of Glycolipids in fungi and plants is different from that of humans, it has a moisturizing effect on the skin and can be labeled as functional for food [6].
- Mushrooms for ingredients of nutritional food, cosmetics, and nutricosmetics:
Nutritional (food) supplements are concentrated sources of molecules with a nutritional or physiological effect [7]. On the other hand, there is a category, “nutricosmetics”, which is characterized by oral supplementation of nutrients, known as “oral cosmetics”, and defined as ingestible natural health products that enhance the function and appearance of human skin, nails, and hair [8,9]. Mushrooms have been an important part of our diet for years and are now finding uses as ingredients in nutritional food, cosmetics, and nutricosmetics [10]. The classification of nutritional (food) supplements and nutricosmetics depends on each national safety agency, but it is becoming difficult to distinguish between the two [6,8]. Since mushrooms have natural cell wall polysaccharides, glycolipids, antimicrobial compounds, and bioactive compounds, they may be important as medical, nutritional food, cosmetic, and nutricosmetic sources [10,11]. They have been extensively studied and the presence of a wide range of bioactive metabolites in mushrooms, e.g., phenolic compounds, terpenoids, polysaccharides, lectins, steroids, glycoproteins, and several lipid components, has been found [10].
- Effect of mushrooms for skin moisture and barrier:
The stratum corneum (SC) is the outermost layer of the epidermis and plays an important role in maintaining skin moisture and protecting the skin from the external environment. Ceramide and natural moisturizing factor (NMF) are the major SC components that maintain skin moisture [12]. The structure of the SC is generally described by the “brick and mortar” model, in which corneocytes and intercellular lipids represent the bricks and mortar, respectively [13,14]. The intercellular lipids present in the SC play an important role in the regulation of water content. The major skin lipids are ceramides, cholesterol, and free fatty acids (FAs) [15]. The most important lipids that form this permeability barrier are ceramides [16]. Previous studies reported that the oral intake of plant ceramides improved skin moisturizing and the level of transepidermal water loss in healthy human subjects [17,18]. In addition, fungal glucosylceramides were also reported to have reduced skin barrier damage in mice [19]. Glycolipids, a kind of plant ceramide that have a moisturizing effect on the skin, are mainly distributed in animals and fungi. It would therefore be expected that fungi ceramides could be labeled as a functional food.
- Flammulina velutipes
Among the various species of mushrooms, Flammulina velutipes (F. velutipes) is one of the most popular edible fungi [20,21,22,23] and is not only delicious in taste, but also rich in nutrients (carbohydrate, dietary fiber, protein, vitamins, etc.) [24]. One of the main bioactive components in F. velutipes is polysaccharide, and this has functions similar to those of other mushrooms. In recent years, polysaccharides, one of the important components of fungus, have become a research hotspot with excellent prospects for application in food and medicine [24,25]. Many studies have shown that polysaccharides have a variety of bioactivities, such as anti-oxidation, immune regulation, anti-inflammation, liver protection, anti-tumor, anti-hyperlipidemia, memory improvement, decrepitude resistance, and so on [26,27,28,29,30]. F. velutipes has been used as a delicious edible food, as a health-promoting food supplement (nutraceutical), and as a drug in the Asian region [20]. However, as current research on the relationship between the structures and bioactivities of F. velutipe is limited, no general consensus has been established, and clinical studies are insufficient for its use as an authorized drug, a functional food, or as a nutricosmetic worldwide. In recent years, due to advances in cultivation and breeding technology, new varieties have been bred even within F. velutipes, and are expected to have components with further functions beneficial to human health. F. velutipes was reported to have a moisturizing function on the skin in a previous study [31], and to extend these investigations, we conducted a randomized, double-blind, placebo-controlled clinical study focusing on F. velutipes’s effect on skin as a nutricosmetic using a new variety of F. velutipes, namely Oki-Shirayuki 919, and examined its effective functionality in humans.
2. Materials and Methods
2.1. Study Design
This randomized, double-blind, placebo-controlled clinical study was performed between 28 January 2022 and 16 March 2022 in the Laboratory of Systematic Forest and Forest Products, Science Faculty of Agriculture, Kyushu University, Japan. The study included two groups with a 1:1 allocation ratio of the test food or the placebo food. Block randomization of participants was performed to reduce bias.
2.2. Test Food and Intervention
We expected F. velutipes to have components of nutricosmetics, and focused on functions to improve skin moisture or the skin barrier. In a previous study [31], F. velutipes, which is rich in Glucosylceramide (GlcCer), was reported to have a moisturizing function on the skin. In similar previous studies that verified the effect of GlcCer to improve skin quality, 1.2–1.8 mg of glucosylceramide was consumed [17,18]. It was estimated from a previous report that in order to ingest 1.6 mg of GlcCer, 3 g of F. velutipes dry powder was required [31].
F. velutipes dry powder has a unique flavor, and the test food was required to be a food that could be consumed for a long period of time, and to be of a taste and flavor not different from those of the placebo. We blended F. velutipes dry powder into a laver paste which was boiled in soy sauce, a food familiar to Japanese people, and prepared a test food for the intervention. F. velutipes dry powder was obtained from agricultural producers’ cooperative corporation Hakutakekan (Fukuoka, Japan). The laver pastes, which were boiled in soy sauce and plum nuts (150 g), included 15 g of F. velutipes dry powder. A placebo (150 g) was also made with a similar seaweed, replacing the F. velutipes powder with plum fruit paste. The test product also contained plum fruit paste, and it was confirmed that there was no discernible difference in taste or aroma between the two foods, and there was minimal difference between their nutritional values (Table 1). Participants were instructed to consume the 150 g sample over 5 days, and participants in the study group consumed 3 g of F. velutipes by eating 30 g of laver paste per day. The sample consumption design was estimated from a previous report [31], where taking 3 g of F. velutipes dry powder per day enabled the consumption of 1.6 mg of GlcCer per day which was necessary to improve skin quality. The safety of F. velutipes (Oki-shirayuki 919) has been guaranteed via safety of raw materials and cultivating methods, and sales performance over four years with no adverse events or health hazards. Two staff members (neither were the investigator) confirmed that both meals (the test and the placebo) were indistinguishable in appearance. After the preparation of samples, they were kept safely in the laboratory where temperature and humidity were controlled until the study started.

Table 1.
Comparison of the analysis values of nutrient composition of sample food per day.
2.3. Clinical Study and Ethics
The study was performed in accordance with the guidelines of the Declaration of Helsinki, approved by the ethics committee of Kindai University Faculty of Humanity-Oriented Science and Engineering (approval no. 2021002) and was registered in the UMIN-CTR with ID: 000046734.
2.4. Participants and Setting
We referred to a previous investigation that had detected a significant difference in skin moisturization after the oral intake of GlcCer supplement [32,33]. The sample size was calculated with effect size (d) of 1.76, significance level (α) of 0.05, and statistical power (1–β) of 0.95, leading to a required total of 16 subjects in two groups (8 subjects in each group). In addition, the number of participants was set at 30 (15 subjects in each group) to allow for dropouts and noncompliance with the protocol during the study period. Healthy men and women volunteers aged 20–59 years (n = 30) were invited via the network of the laboratory and were checked for eligibility with inclusion and exclusion criteria (Table 2) by a staff member (not the investigator). The volunteers signed an informed consent form stating the purpose, method, compensation, confidentiality, and right of withdrawal from the study. In collaboration with two clinics, we could consult with medical doctors in the case of an adverse event.

Table 2.
Inclusion and exclusion criteria.
2.5. Randomization and Blinding
The randomization was centralized and performed based on a computer-generated list of random numbers by a staff member independent of the investigators. Allocation was performed using the randomization list, and was adjusted by factors including sex, age, and base stratum corneum hydration at base line. Subjects were equally, but randomly, assigned to either the active group or the placebo group (n = 15 per group). An allocation table with the coded test foods was provided to the person in charge of shipping, who sent the test foods to each subject according to the table. The sponsors, principal investigator, entire contract research organization staff, medical doctor, institutional review board members, and others who were related to this study were not aware of the group assignments. The allocation table was locked until the key opening day.
2.6. Study Schedule
All participants took 3 g (dried weight) of F. velutipes by eating 30 g of laver paste per day. To minimize any confounds in the study, all participants were required to refrain from the intake of any similar dietary supplements, quasi drugs, or medicines. They were also prohibited from using any skincare treatments, such as face masks or packs and massages, or from changing their daily skincare cosmetics from the start to finish of the study. Each participant visited the research laboratory twice for assessment: prior to the intake of study formulation at baseline (0 W) and after 4 weeks (4 W) of study formulation intake, for efficacy measurements. The participants were requested to apply daily skin care products in the mornings of the visit days and remove the products at each visit. Measurements were taken on the left cheek (an inner position 5 cm from the lower end of the right earlobe). The skin region of interest was cleansed using a cleansing sheet (Bifesta Cleansing Sheet, Mandom Corporation, Osaka, Japan), wiped with cotton containing a cleansing liquid (Bifesta Face-wash, Mandom Corporation), rinsed with warm water, wiped, and dried for 20 min under stable temperature (20 ± 2 °C) and humidity conditions (50 ± 10%).
2.7. Measurement of Skin Areas and Self-Assessment Scales
Measurement of hydration (arbitrary unit: a.u.) and transepidermal water loss (TEWL) (g/h/m2) were taken using a Corneometer® CM 825 and TEWAMETER® TM 300, respectively (both instruments from Courage and Khazaka, Cologne, Germany) [34]. Each measurement took 1–3 min to complete, and a series of five values or an average value in the measurement time were obtained. Of the five obtained values, the three middle values were utilized to calculate the median value. Intervention effects were clinically evaluated by recording the subjective assessment of the general skin condition, moisture, brightening, skin elasticity, number of wrinkles, and even skin complexion. The self-evaluation of the skin condition was carried out using the visual analog scale (VAS). Three independent researchers compared clinical changes before the series of intervention procedures and after 4 weeks of application. The VAS ranged from 0% to 100%. The lines were vertical. The labels “does not mind at all” and “having greatest worries” were printed at the two end points of the line, respectively. Additionally, mushrooms have many bioactive properties with high contents of carbohydrate, proteins, and fibers, which are considered important nutrients for human health and benefits including improved laxation. Since the consumption of fiber may benefit the gut microbiota, which may also be expected to improve physical and psychological health [35], we employed the Bristol Stool Form Scale [36] and Profile of Mood States 2nd Edition (POMS 2) [37] to infer whether other evaluations would be associated with changes in skin condition or not. The Bristol Stool Form Scale (BSFS), a frequently used measure, categorizes stools into one of seven stool types ranging from type 1 (hard lumps) to type 7 (watery diarrhea). The POMS 2, which includes six negative and one positive mood subscales (anger–hostility, confusion–bewilderment, depression–dejection (DD), fatigue–inertia, vigor–activity, friendliness, and total mood disturbance (TMD) (overall negative mood state)) was used.
2.8. Outcome
The schedules of enrolment, intervention, and assessment are presented in Table 3. The primary outcome was a response to the oral intake of the food containing F. velutipes rich in glucosylceramide with respect to skin hydration and TEWL based on the skin measurement device. The secondary outcomes were self-evaluation of skin condition using VAS, Bristol Stool Form Scale, and POMS 2.

Table 3.
The schedules of enrolment, intervention, and assessment.
2.9. Statistical Analysis
SPSS (version 25.0, Chicago, IL, USA) was used to analyze the data. To compare the quantitative demographic variables of age and weight between the two groups, an independent samples t-test and a Mann–Whitney U test were used. Changes in variables at the end of the study compared to those at the beginning of the study were measured using the Wilcoxon signed rank test. To compare changes in parameters between the two groups, the Mann–Whitney U test was used. Statistical significance was considered at p < 0.05. In light of the assumed limitations for p values, the effect size r was also presented in the result tables as being representative of the effect size. In this study, multiple adjustments were necessary because plural primary outcomes were set. We adopted a closed testing procedure to avoid multiplexity, the analysis was determined in advance to be performed in the order of (1) hydration at the cheek, (2) TEWL at the cheek, (3) skin condition VAS, (4) Bristol Stool Form Scale, and (5) POMS 2, and when the result showed no significant difference in the change in value between the groups, the analysis would end. In addition, since only one male participant was included in each group, we only analyzed female subjects excluding men, adopting the same closed testing procedure.
3. Results
3.1. Participant Flow and Characteristics
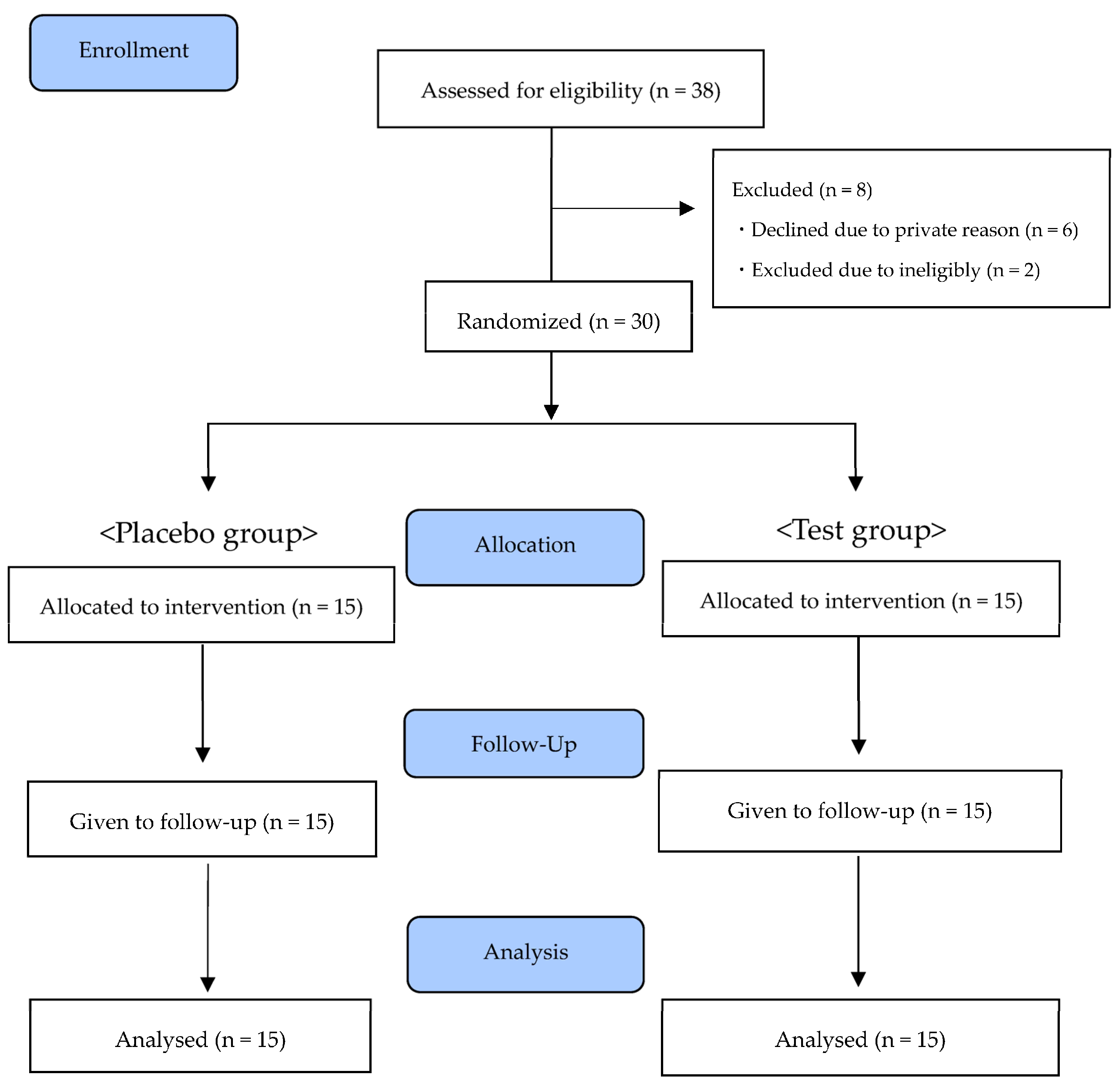
Of the 38 participants recruited, 30 participants who met the eligibility criteria and gave their agreement were enrolled in this study. Thirty participants were allocated to the test group or the placebo group. From January 2022 to March 2022, 30 healthy adults completed the study (Figure 1). The background characteristics for each group are shown in Table 4. There were no significant differences between the two groups in terms of sex, age, body mass index, or other evaluation values at baseline.
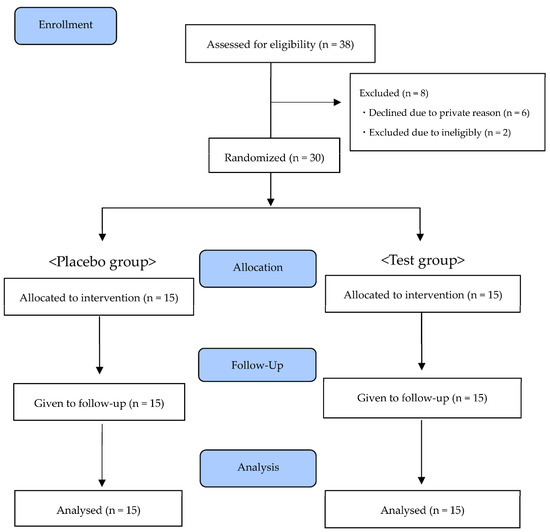
Figure 1.
CONSORT flow diagram.

Table 4.
Background characteristics.
3.2. Effects of F. velutipes on Skin Hydration and TEWL in Total 30 Participants
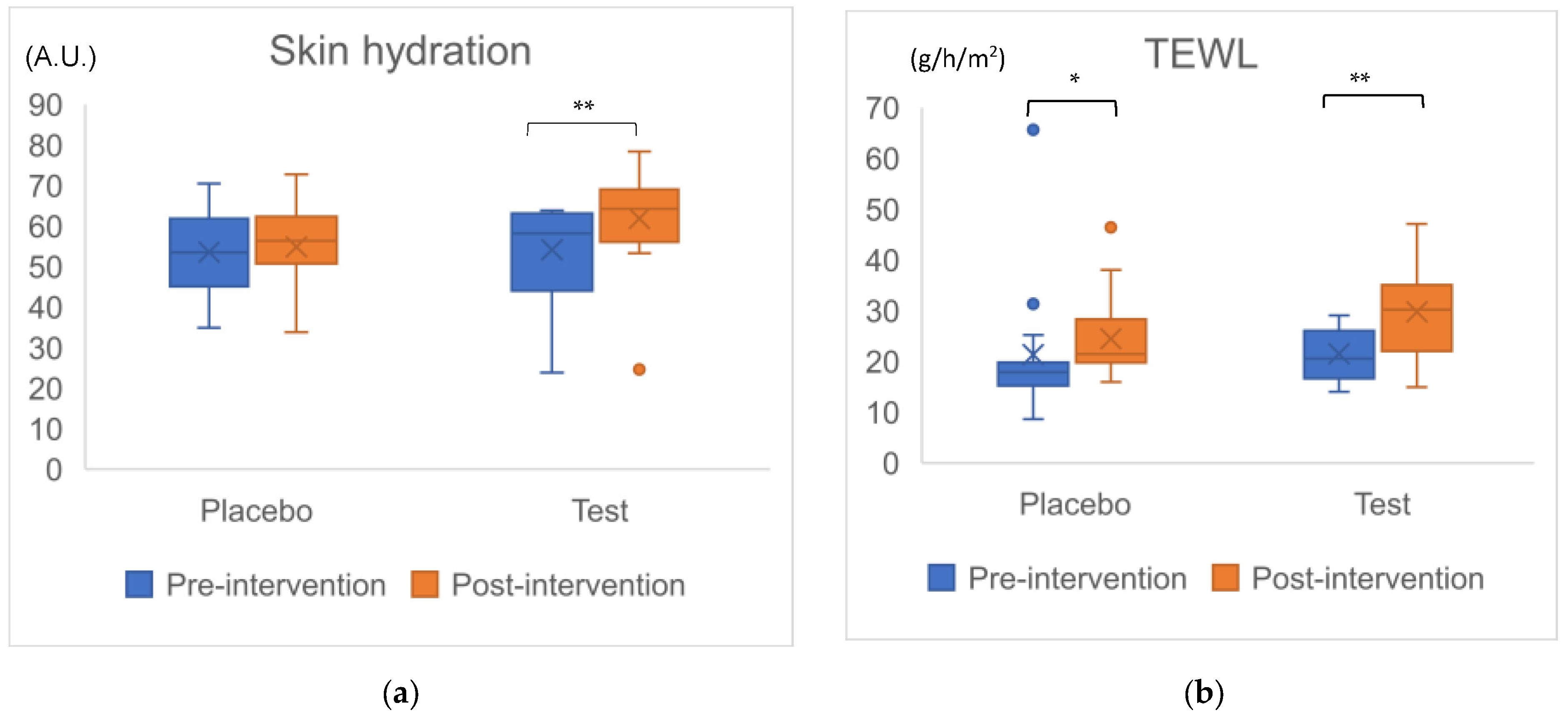
We evaluated hydration and TEWL in the left cheek skin section. First, in the changes within each group during the 4 weeks, although there was no difference in hydration in the placebo group (p = 0.650), there was a significant increase in the test group (p = 0.001) (Figure 2a). Since the amount of increase over 4 weeks in the test group was significantly larger than that in the placebo group, a significant difference between the two groups was observed (p = 0.033, r = 0.39). Second, significant differences in TEWL were observed between each group after 4 weeks: in the placebo group, p = 0.020; in the test group, p = 0.003 (Figure 2b). No significant difference in changes in TEWL over 4 weeks between the two groups was observed (p = 0.161, r = 0.26) (Table 5). Since we adopted the closed testing procedure to avoid multiplexity, when the result of TEWL showed no significant difference in variables between the groups, the analysis ended.
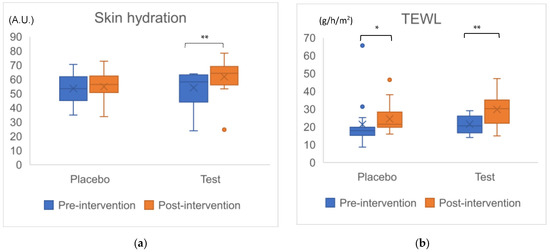
Figure 2.
Change in cheek skin parameters in total 30 participants. Figure (a): Changes in Skin hydration, (b): Changes in TEWL (transepidermal water loss), a.u.: arbitrary unit. Dots beyond the bounds of the whiskers denote outliers. Cross marks and horizontal lines indicate means and medians for each group. Asterisks indicate significant difference in intragroup after the intervention. *: p < 0.05, **: p < 0.01.

Table 5.
Comparisons of items between the placebo group and the test group in total 30 participants.
3.3. Effects of F. velutipes on Skin Hydration and TEWL in Total 28 Female Participants
We evaluated hydration and TEWL in the left cheek skin section in a total of 28 female participants. First, in the changes within each group during the 4 weeks, although there was no difference in hydration in the placebo group (p = 0.975), there was a significant increase in the test group (p = 0.002). Since the amount of increase over 4 weeks in the test group was significantly larger than that in the placebo group, a significant difference between the two groups was observed (p = 0.007, r = 0.50). Second, significant differences in TEWL were observed in each group after 4 weeks: in the placebo group, p = 0.0300; in the test group, p = 0.005. No significant difference in changes in TEWL over 4 weeks between the two groups was observed (p = 0.104, r = 0.31) (Table 6). The result of TEWL showed no significant difference in variables between the groups, and the analysis ended according to the closed testing procedure to avoid multiplexity.

Table 6.
Comparisons of items between the placebo group and the test group in total 28 female participants.
4. Discussion
In the present study, the effects of intake of F. velutipes Oki-Shirayuki 919, which contained more glucosylceramide than existing varieties, on healthy adults’ skin hydration and TEWL, the self-evaluation of skin condition, stool form, and profile of mood states were evaluated. The intake of F. velutipes Oki-Shirayuki 919 over 4 weeks increased skin hydration in the test group. GlcCer, a representative sphingolipid in the cell membranes of plants and fungi, has been reported to have certain benefits, such as the prevention of intestinal impairment and improved skin moisturizing, when consumed [20]. The effects of plant- or fungus-originating sphingolipid bases on colon cancer were reported to have been to the same degree as mammalian compounds via in vitro investigation [38].
Regarding the effect of GlcCer on improving skin function, the mechanism was investigated using special atopic dermatitis-like and damaged skin murine models, and the recovery of the skin barrier function and the change in spingolipid metabolism in the epidermis were reported [39]. The results showed that dietary GlcCer and a spingolipid accelerated the recovery and significantly upregulated the expression of ceramide synthases in the epidermis of the atopic dermatitis-like skin model. In the case of cultured cells, the expression of ceramide synthases in normal human foreskin keratinocytes was significantly upregulated by treatment with spingoid bases. These reports provide the mechanisms of the skin-barrier-improving effect, and findings confirm that the effects of dietary sphingolipids might be due to the activation of ceramide synthesis in the skin, rather than the direct reutilization of dietary sphingolipids. Similarly, another previous study reported that dietary plant and yeast glucosylceramides had been shown to suppress atopic dermatitis-like symptoms in model mice via the regulation of helper T-cell Th1/Th2 balance [17]. To summarize, GlcCer and a spingolipid were suggested to have indirect functions such as the activation of ceramide synthesis in the skin or regulation of helper T-cell Th1/Th2 balance. One potential confound to this explanation is Ergothioneine which is present in F. velutipes. Ergothioneine is a thiol/thione molecule synthesized only by some fungi and bacteria and has powerful antioxidant and cytoprotective properties. Previous reports indicate that F. velutipes contains 34.64 mg of Ergothioneine per kg dry weight [40]. In this study, we did not analyze for ergothioneine in Oki-shirayuki 919. There is a possibility that Ergothioneine in F. velutipes provided a different beneficial mechanism from GlycCer for the skin.
At the same time, a warning about the flammutoxins in F. velutipes should be mentioned. Lin et al. reported that flammutoxin caused lysis of mammalian erythrocytes, swelling of Ehrlich ascites tumor cells, and electrocardiographical changes in parenterally administrated animals [41]. Tung et al. reported that heating extract at 60 °C for 5 min could effectively exclude flammutoxin [42]. Since F. velutipes is usually consumed after heating, the toxicity of flammutoxin is not well known. However, we should acknowledge again these important warnings of the possible side effects of flammutoxin for nutraceutical and nutricosmetic consumption.
In the present study, skin hydration in the test group significantly increased, but TEWL did not improve over 4 weeks, and several reasons could be suggested for this result. The first reason is that since the intervention and observation period was 4 weeks, increased moisture content of the stratum corneum was observed, while the subsequent recovery of the skin barrier function could not be observed due to it requiring more time. Secondly, since the trial was conducted in late winter and early spring, it was strongly affected by the environment due to seasonal fluctuations, which may have delayed the recovery of the barrier function. Regarding the seasonal variations in skin temperature on human skin, it was reported that skin temperature showed seasonal fluctuation, rising in summer and falling in winter, following the fluctuation of outdoor temperature when outdoor temperature was higher than 15 °C [43], and significant correlations were observed between skin temperature and TEWL [44]. The mean outdoor temperature during the five days of intervention significantly changed pre-intervention: 6.7 ± 1.1 °C (mean ± SD), and post-intervention: 16.3 ± 1.7 °C [45]. Lastly, participants in this study were all healthy adults without atopic dermatitis or skin damage, and although they may have experienced dryness of their skin in winter, there were no major skin problems. Although the hydration of the skin improved due to the intake of F. velutipes Oki-Shirayuki 919 which contained more glucosylceramide, it was difficult to increase the barrier function more as shown in the models of animals and cells. However, even in the skin of healthy adults, the skin hydration and subjective evaluation of individuals were significantly improved via the intake of F. velutipes Oki-Shirayuki 919. In the case of temporary instability of the barrier function, it can be greatly expected that the barrier function can be restored via indirect function such as the activation of ceramide synthesis in the skin or regulation of helper T-cell Th1/Th2 balance after a certain period of time.
We expected to improve physical and psychological health via the intake of F. velutipes Oki-Shirayuki 919, but no obvious effect was shown. In a previous study, the in vivo effects of dietary complex sphingolipids were investigated using model mice for colon cancer. The results suggest that dietary complex sphingolipids play a beneficial role in the prevention of inflammatory bowel disease and the effects were specific to cancer cells [46]. Since the results in the present study might also have relied on the characteristics of healthy participants, the effect of the intake of F. velutipes for clinical dermatological-level patients was unknown.
To the best of our knowledge, this is the first study to clinically evaluate the effect of oral intake of F. velutipes Oki-Shirayuki 919, which contained more glucosylceramide than existing varieties, on healthy adults’ skin condition and associated symptoms. This study provides some new clinical evidence along with indicating some limitations and future challenges. First, we intended to evaluate the effect of the gut microbiota from fiber in F. velutipes to improve physical and psychological health, but we could not analyze them in the closed testing procedure to avoid multiplexity. A clinical trial to investigate F. velutipes for improving physical or psychological health as a primary outcome would be required. Second, we evaluated skin hydration and TEWL of people who orally consumed the product, and only skin hydration showed a significant difference compared to the placebo. The sample number was low and the intake period was short. The result of skin hydration in only female participants was even more significant, but there was only one male participant in each group, and so other trials with more male participants would be needed for detailed male results. Third, further clinical trials to be able to check the change in efficacy according to the increase in the concentration of the active ingredient, or to confirm the effect with scaled-up sample sizes and intervention periods, are needed. Finally, we designed this study with reference to a previous report that showed a moisturizing function on the skin [32]. We could not provide the analysis data for glucosylceramide in F. velutipes in the present report. The mechanism of action of the results of this clinical trial was based on the presumption of glucosylceramide from F. velutipes as the main component involved. Further experiments, the component analysis of F. velutipes, or the bioassay experiment and the clinical trial of changes in ceramides in the skin are needed to confirm the results of the present study.
5. Conclusions
Since the amount of increase in skin hydration over four weeks in the test group was significantly larger than that in the placebo group, a significant difference between the two groups was observed. In addition, the result of skin hydration in only female participants was even more significant. GlcCer in F. velutipe was suggested to have an indirect function such as the activation of ceramide synthesis in the skin.
Author Contributions
Data curation, M.N.; formal analysis, M.N.; investigation, M.N., A.I. and S.I.; methodology, M.N. and K.S.; project administration, S.M. and K.S.; resources, S.I., S.M. and K.S.; validation, M.N.; visualization, M.N.; writing—original draft, M.N.; writing—review and editing, M.N. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fukuoka Bioindustry R&D Initiatives 2021–2022.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of Kindai University Faculty of Humani-ty-Oriented Science and Engineering (approval no. 2021002).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We would like to thank Koichiro Onuki of the Faculty of Humanity-Oriented Science and Engineering, Kindai University, and students in the laboratory for their cooperation in carrying out this research.
Conflicts of Interest
The authors declare no conflict of interest. The paper was prepared appropriately and objectively with the understanding of all authors.
References
- Lindequist, U.; Niedermeyer, T.H.J.; Jü, W.-D. The Pharmacological Potential of Mushrooms. eCAM 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Miles, P. Mushroom Biology—A New Discipline. Mycologist 1992, 6, 64–65. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Coderch, L.; Lopez, O.; de la Maza, A.; Parra, J.L. Ceramides and Skin Function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, D.R.; Esko, D.J.; Freeze, H.H.; Stanley, P.; Bertozzi, R.C.; Hart, W.G.; Marilynn, E. (Eds.) Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Piccardi, N.; Manissier, P. Nutrition and Nutritional Supplementation. Derm. -Endocrinol. 2009, 1, 271–274. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and Polyphenols in Nutricosmetics, Nutraceuticals, and Cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A Brief Overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms Extracts and Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics-A Review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin Health Promotion Effects of Natural Beta-Glucan Derived from Cereals and Microorganisms: A Review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, S.H.; Joo, K.M.; Lim, S.H.; Shin, J.H.; Roh, J.; Kim, E.; Park, C.W.; Kim, W. Oral Intake of Enzymatically Decomposed AP Collagen Peptides Improves Skin Moisture and Ceramide and Natural Moisturizing Factor Contents in the Stratum Corneum. Nutrients 2021, 13, 4372. [Google Scholar] [CrossRef]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Investig. Dermatol. 1983, 80, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Norlén, L.; Norlén, L. Stratum Corneum Keratin Structure, Function and Formation-a Comprehensive Review. Int. J. Cosmet. Sci. 2006, 28, 397–425. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Dubbelaar, F.E.R.; Gooris, G.S.; Weerheim, A.M.; Ponec, M. The Role of Ceramide Composition in the Lipid Organisation of the Skin Barrier. Biochim. Biophys. Acta Biomembr. 1999, 1419, 127–136. [Google Scholar] [CrossRef]
- Holleran, W.M.; Takagi, Y.; Uchida, Y. Epidermal Sphingolipids: Metabolism, Function, and Roles in Skin Disorders. FEBS Lett. 2006, 580, 5456–5466. [Google Scholar] [CrossRef]
- Asai, S.; Miyachi, H. Evaluation of Skin-Moisturizing Effects of Oral or Percutaneous Use of Plant Ceramides. Rinsho Byori. 2007, 55, 209–215. [Google Scholar]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral Intake of Glucosylceramide Improves Relatively Higher Level of Transepidermal Water Loss in Mice and Healthy Human Subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef]
- Tsuji, K.; Mitsutake, S.; Ishikawa, J.; Takagi, Y.; Akiyama, M.; Shimizu, H.; Tomiyama, T.; Igarashi, Y. Dietary Glucosylceramide Improves Skin Barrier Function in Hairless Mice. J. Dermatol. Sci. 2006, 44, 101–107. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Advances in the Extraction, Purification, Structural-Property Relationships and Bioactive Molecular Mechanism of Flammulina Velutipes Polysaccharides: A Review. Int. J. Biol. Macromol. 2021, 167, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, Characterization and Anti-Proliferation Activity of Polysaccharides from Flammulina Velutipes. Carbohydr. Polym. 2012, 88, 474–480. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Xiao, N.N.; Deng, Y.L.; He, P.F.; Sun, P.L. Purification and Structural Investigation of a Water-Soluble Polysaccharide from Flammulina Velutipes. Carbohydr. Polym. 2012, 87, 2279–2283. [Google Scholar] [CrossRef]
- Leung, M.Y.K.; Fung, K.P.; Choy, Y.M. The Isolation and Characterization of an Immunomodulatory and Anti-Tumor Polysaccharide Preparation from Flammulina Velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, X.; Xu, X.; Wei, W.; Yu, M.; Jiang, N.; Ye, L.; Yang, Z.; Fei, X. Antioxidant and Immunomodulatory Activities of a Polysaccharide from Flammulina Velutipes. J. Tradit. Chin. Med. 2014, 34, 733–740. [Google Scholar] [CrossRef]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of Edible Mushroom as a Potent Therapeutics for the Diabetes and Obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yao, W.; Yang, X.; Xie, C.; Liu, D.; Zhang, J.; Gao, X. Purification, Characterization and Biological Activity on Hepatocytes of a Polysaccharide from Flammulina Velutipes Mycelium. Carbohydr. Polym. 2007, 70, 291–297. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, J.S.; Feng, T.; Deng, J.; Lin, C.C.; Fan, H.; Yu, W.J.; Bao, H.Y.; Jia, W. Structural Elucidation of a Polysaccharide from Flammulina Velutipes and Its Immunomodulation Activities on Mouse B Lymphocytes. Sci. Rep. 2018, 8, 3120. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Zhang, Y.; Zhang, Z. Bioactivity of the Crude Polysaccharides from Fermented Soybean Curd Residue by Flammulina Velutipes. Carbohydr. Polym. 2012, 89, 1268–1276. [Google Scholar] [CrossRef]
- Wu, D.M.; Qiang, D.W.; Liu, Y.; Cen, Y. Anti-Inflammatory Effect of the Polysaccharides of Golden Needle Mushroom in Burned Rats. Int. J. Biol. Macromol. 2010, 46, 100–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of Extraction Methods on the Antioxidant Activities of Polysaccharides Obtained from Flammulina Velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef]
- Fan, J.; Kamal El-Deen, A.; Ashour, A.; Hassan, A.R.; Amen, Y.; Takemoto, N.; Nagata, M.; Matsumoto, M.; Wang, D.; Ishikawa, S.; et al. The Expansion of the Promising Value of Flammulina Velutipes and the Prospect of Its Sustainable Development. Available online: https://q-aos.kyushu-u.ac.jp/asiaweek2021poster/wp-content/uploads/2021/10/fan.pdf (accessed on 1 March 2023).
- Masatomo, N.; Akinobu, M.; Keiichi, M. Effects of Supplement Containing Glucosylceramide Extracted from Pineapple with Hyaluronic Acid on Skin Conditions in Healthy Japanese with Dry Skin and Itchy Sensation. Med. Consult. New Remedies 2016, 53, 83–90. [Google Scholar]
- Fukunaga, S.; Wada, S.; Sato, T.; Hamaguchi, M.; Aoi, W.; Higashi, A. Effect of Torula Yeast (Candida Utilis)-Derived Glucosylceramide on Skin Dryness and Other Skin Conditions in Winter. J. Nutr. Sci. Vitaminol. 2018, 64, 265–270. [Google Scholar] [CrossRef]
- Instruments from Courage and Khazaka, Cologne, Germany. Available online: https://www.courage-khazaka.de/en/ (accessed on 1 December 2022).
- Hess, J.; Wang, Q.; Gould, T.; Slavin, J. Impact of Agaricus Bisporus Mushroom Consumption on Gut Health Markers in Healthy Adults. Nutrients 2018, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Riegler, G.E.I. Bristol Scale Stool Form. A Still Valid Help in Medical Practice and Clinical Research. Tech. Coloproctol. 2014, 5, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Watanabe, K. Japanese Version POMS-2 Manual: A Profile of Mood States, 2nd ed.; Kanekosyobou Press: Tokyo, Japan, 2015. [Google Scholar]
- Satoshi, H.; Aya, S.; Yuko, H.; Tsuyoshi, M.; Koji, Y.; Amane, K. Dietary Rice Bran Extract Lmproves TEWL of Whole Body. Jpn. Pharmacol. Ther. 2013, 41, 1051–1059. [Google Scholar]
- Duan, J.; Sugawara, T.; Hirose, M.; Aida, K.; Sakai, S.; Fujii, A.; Hirata, T. Dietary Sphingolipids Improve Skin Barrier Functions via the Upregulation of Ceramide Synthases in the Epidermis. Exp. Dermatol. 2012, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A Diet-Derived Antioxidant with Therapeutic Potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Wu, H.L.; Shi, G.Y. Toxicity of the Cardiotoxic Protein, Flammutoxin, Isolated from the Edible Mushroom Flammunlina Velutipes. Toxicon 1975, 13, 323–332. [Google Scholar] [CrossRef]
- Tung, C.H.; Lin, C.C.; Wang, H.J.; Chen, S.F.; Sheu, F.; Lu, T.J. Application of Thermal Stability Difference to Remove Flammutoxin in Fungal Immunomodulatory Protein, FIP-Fve, Extract from Flammulina Velutipes. J. Food Drug Anal. 2018, 26, 1005–1014. [Google Scholar] [CrossRef]
- Keiko, O.; Yasuo, N. Annual Fluctuations of Skin Temperature and Body Temperature Based on Experiments for the Same Thermal Environment. Jpn. J. Biometeor. 1997, 34, 89–95. [Google Scholar]
- Takashi, A.; Junko, M.; Noriko, K.; Seiichi, A. The Seasonal Variations of Skin Temperature, Skin PH, Evaporative Water Loss (TEWL) and Skin Surface Lipid Values on Human Skin. Chem. Pharm. Bull. 1980, 28, 387–392. [Google Scholar]
- Japan Weather Association. Past Weather. Available online: https://tenki.jp/docs/sitemap/ (accessed on 22 August 2022).
- Aida, K.; Kinoshita, M.; Sugawara, T.; Ono, J.; Miyazawa, T.; Ohnishi, M. Apoptosis Inducement by Plant and Fungus Sphingoid Bases in Human Colon Cancer Cells. J. Oleo Sci. 2004, 53, 503–510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).