Abstract
The objective of this work was to characterize a cosmeceutical formulation for the eye area with roasted coffee oil microcapsules (MOF) and evaluate the acceptance and effects of its use by consumers. MOF had 3% microcapsules produced by complex coacervation; a basic formulation (BF) was used for comparison. The addition of microcapsules did not affect the pH (4.52), density (0.99 g mL−1), consistency (0.77 N s), and viscosity index (0.25 N s) of the formulation. However, a reduction in spreadability, firmness, and cohesiveness was observed. The 58 assessors received one kit with the formulations and a notebook with instructions to carry out the tests at home. They were instructed to apply the cream for 28 days and evaluate the attributes of application and treatment effects on 7-point category scales. The effect of oil addition observed in the physical tests was not sensorially perceived for spreadability and tackiness (6.0 and 5.6, respectively), indicating approval and easiness of application. The perception of the benefits (increase in smoothness, hydration, firmness, elasticity, and skin general appearance, and reduction in signs of fatigue and wrinkles/fine lines) was similar comparing MOF and BF. In conclusion, the coffee oil microcapsule is a viable ingredient for dermocosmetics with sensory acceptance.
1. Introduction
The market for cosmeceutical products has recently expanded [1] due to consumer demand for cosmetic products derived from natural sources [2]. The term cosmeceutical, described for the first time in the 1960s and popularized in the late 1970s [3,4], refers to the use of bioactive compounds used in cosmetics to protect the skin and improve its appearance with an anti-aging action [4,5]. Thus, cosmeceutical products must provide therapeutic benefits in addition to the usual actions of a cosmetic [1].
Extracts obtained from coffee (fruit, leaves, and oil) are noted for their biological properties as protectors against UV irradiation and the visible light of the solar spectrum, since they are rich in antioxidant compounds. By preventing aging and reducing the incidence of skin cancer [6,7,8,9], the use of coffee derivatives is gaining special attention for application in cosmetic products.
Roasted coffee is a coproduct of the soluble coffee industry and can be extracted by the mechanical pressing of roasted beans before the extraction of soluble coffee [10]. This oil can be used as a flavoring agent in several products [11] and an alternative to synthetic surfactants [12]. Although green coffee oil is more usually applied in cosmetic products [13,14], roasted coffee oil also has good potential for use since the presence of components such as diterpenes and unsaturated fatty acids [15] may confer antioxidant, nutraceutical, and skin hydration properties.
In the microencapsulation process, particles or droplets are enveloped in a coating or incorporated into a matrix to form small particles called microcapsules [16]. Complex coacervation (CC) stands out among the methods used in the food and cosmetic industries [17] for its reasonable costs and industrial employment feasibility. Microencapsulation may generate innovative and stable products in a competitive sector, such as cosmetics and personal care [18,19], protecting the active ingredient against degradation and controlling its liberation [20]. Considering the oil’s susceptibility to oxygen, microencapsulation can be an alternative to prevent losses by oxidation and hydrolysis, preserving the aroma and the bioactive compounds of interest [21,22,23]. Thus, the oil microencapsulation makes it possible to offer an ingredient in a dry and stable form, facilitating storage and handling during the industrial production process.
Sensory analysis is an essential tool for the cosmetics industry and is applied in the most diverse areas, especially in product development, quality assurance, marketing, and innovation; it is also used to evaluate characteristics, acceptance, and claims evaluations associated with cosmetic products [24]. The use of sensory analysis also allows the evaluation of relationships between the properties of a product measured by physical methods and the perception of these characteristics by the consumers [25]. Therefore, sensory analysis has become an indispensable technique to help the formulator evaluate the quality of new cosmetics during the development stage, avoiding the outlay of launching a product in the market that is not sensorially acceptable, so that rejection could be detected in a preliminary study [26].
In previous studies conducted by our research group, roasted coffee oil showed chemical and physical characteristics of interest for use in cosmeceutical products [15]. In a second study by Böger et al. [27], roasted oil microcapsules were produced by different microencapsulation techniques and characterized; those produced by CC presented high stability (with low aw and moisture, and high glass transition temperature) and good solubility in water, and stand out for the higher content of encapsulated oil. Considering those characteristics, Böger et al. [28] verified in a following study the feasibility of roasted coffee oil as an ingredient in cosmeceutical formulations, comparing the use of oil in the free form and microcapsules produced by CC with a base formulation. The addition of coffee oil in both forms increased antioxidant activity, the sun protection factor and the stability of the product, but use of roasted oil microcapsules allow bioactive compounds to remain protected and available for a slower release in the posterior use of the product in addition to the advantage of using a dry ingredient with easier storage [28]. However, the physical and chemical characteristics and acceptance of the formulations still need to be evaluated. Thus, the objective of this research was to characterize a cosmeceutical formulation for the eye area with microcapsules of roasted coffee oil. Both physical and chemical properties were evaluated as well as a preliminary study of the sensory acceptance and the effects on the skin of the product use described by consumers. A comparison with a basic formulation, without the addition of microcapsules, was carried out.
2. Materials and Methods
2.1. Materials
Microcapsules of roasted coffee oil produced by complex coacervation (CC), prepared as previously described by Böger et al. [27], were applied. Other ingredients of the cosmeceutical formulations are: bidistilled glycerin (Galena, São Paulo, Brazil), cetostearyl alcohol (Infinity Pharma, São Paulo, Brazil), ethoxylated cetostearyl alcohol 20OE (All Chemistry do Brasil, São Paulo, Brazil), glyceryl monostearate (Arte Cheiro, Paraná, Brazil), Butyrospermum parkii (shea) butter (Galena, São Paulo, Brazil), capric/caprylic acid triacylglycerols (Galena, São Paulo, Brazil), Phenogard MP (2-methyl-4-isothiazolin-3-one and 2-phenoxyethanol) (Via Farma, São Paulo, Brazil), Skincolor 2-medium beige pigment (Biotec, São Paulo, Brazil), propylene glycol (Fortquim, Paraná, Brazil) and Double cream essence with floral fragrance (combining white lily, damask rose, Bulgarian rose, and white jasmine) (FAV 105, São Paulo, Brazil).
2.2. Reagents, Standards, and Equipment
The HPLC-grade solvents used were tert-butyl methyl ether (Acrós Organics, Morris Plains, NJ, USA), acetonitrile (Mallinckrodt Baker, Phillipsburg, NJ, USA), methanol (Merck, Darmstadt, Germany), isopropanol (Merck, Darmstadt, Germany), and n-heptane (Sigma-Aldrich, St. Louis, MO, USA). The following analytical grade materials were also used: potassium and sodium hydroxide (Quimex, São Paulo, Brazil), 98% ethanol (J. T. Baker, Phillipsburg, NJ, USA), and hexane (Anidrol, Diadema, Brazil). The water for the preparation of standards and solutions was obtained by the Elga Purelab Option-Q purification and filtration system (Veolia Water Technologies, Saint-Maurice, France). Nylon membranes (0.22 μm) were used for mobile phase filtration (Millipore, Billerica, MA, USA) and samples (Whatman, Maidstone, UK). Standards of fatty acid methyl esters (FAME Mix C4-C24) (Sigma-Aldrich, St. Louis, USA) and kahweol and cafestol (Axxora, San Diego, CA, USA) were used.
The following equipment were used: centrifuge 5804 R (Eppendorf, Hamburg, Germany), digital pH meter FiveEasy F20 (Mettler Toledo, Greifensee, Switzerland), UV–vis spectrophotometer Cary 100 (Agilent Technologies Inc., Santa Clara, CA, USA), vortex shaker MX-S (Phox, Colombo, Brazil), CR-400 colorimeter with D65 illuminant (Konica Minolta Sensing Inc., Osaka, Japan), TA-XT2i texturometer (Stable Micro Systems, Surrey, UK), and rotary evaporator model MA 120 (Marconi, Piracicaba, Brazil).
Fatty acid analysis was performed in a Shimadzu 17A GC equipped with a flame ionization detector (Shimadzu, Kyoto, Japan), and diterpene analysis in a Waters Acquity UPLC with an automatic sample injector, quaternary solvent pumping system, column oven, and diode array detector, controlled by the Empower 3 program (Waters, Milford, MA, USA).
2.3. Characterization of the Fatty Acids Profile and Diterpenes of Microcapsules
To determine fatty acids, oil extraction was first performed according to Böger et al. [29]. Microcapsules (0.5 g) were dissolved in 4 mL of distilled water at 50 °C, vortexed for 10 min, and 15 mL of hexane:isopropanol (3:1, v/v) was added. The dispersion was centrifuged (10 min, 4400× g, 4 °C). After collecting the supernatant, another 10 mL of hexane: isopropanol (3:1, v/v), was added, repeating agitation, centrifugation, and collection of the supernatant. This step was repeated, and the supernatants were evaporated (50 °C, 7 rpm) until constant mass.
The hydrolysis and transesterification of the fatty acids, in genuine triplicate, were performed according to the ISO method 5509 (1978) [30]. N-heptane (1.5 mL) was added to the fat (150 mg) and stirred. 2 M NaOH/Methanol solution (1.5 mL) was added and stirred. After rest, the phase containing n-heptane was separated and stored in an amber vial at −18 °C until analysis.
Fatty acid methyl esters were analyzed in a CP SIL 88 capillary column (100 m × 0.25 mm) (Agilent Technologies Inc., Santa Clara, CA, USA). The column temperature was programmed to: 65 °C for 15 min; 10 °C min−1 to 165 °C and maintained for 2 min; 4 °C min−1 to 185 °C and maintained for 8 min; 4 °C min−1 to 235 °C and held for 5 min. The detector and injector were kept at 260 °C, using a 1/100 split. The gas flow rate was 1.2 mL min−1 for the carrier gas (N2), 30 mL min−1 for the auxiliary gas (H2), 30 mL min−1 and 300 mL min−1 for the flame gases, H2 and synthetic air, respectively. The analysis was performed in duplicate, identification was based on comparison with standards, and results were expressed as relative percentages of fatty acids.
Extraction of diterpenes was performed according to Dias et al. [31] in genuine duplicate. The microcapsules (0.2 g) were saponified with 2.0 mL of 2.5 mol·L−1 potassium hydroxide in ethanol (96% v/v) at 80 °C for 1 h. For the extraction of the unsaponifiable matter, 2.0 mL of distilled water and 2.0 mL of tert-butyl methylether were added. After stirring and centrifugation at room temperature (1562× g/3 min), the organic phase was collected; this step was repeated three times. Distilled water (2 mL) was added for cleaning, and the organic extract was collected and evaporated to dryness in a water bath (70 °C). The extract was resuspended in 4 mL of the mobile phase (45:55, v/v, water: acetonitrile), filtered, and stored in an amber vial at −18 °C until analysis. For analysis, the extract was diluted 1:9 with the mobile phase.
The analysis was performed in duplicate according to Mori et al. [32], using Kinetex C18 column (150 mm × 4.6 mm, 2.6 µm) (Phenomenex, Torrance, CA, USA) at 26 °C and detection at 230 nm (cafestol) and 290 nm (kahweol). Isocratic elution with water:acetonitrile (45:55 v/v) at flow rate of 1.2 mL min−1, and injection volume of 1.4 µL were used. Quantitation was performed by external standardization using 6-point analytical curves in triplicate (r ≥ 0.999, p < 0.001), and the results were expressed as kahweol and cafestol contents (mg 100 g−1).
2.4. Preparation of Cosmeceutical Formulations
The formulations were prepared as a simple emulsion of the O/W type, being a base formulation (BF) without actives (negative control) and a formulation with the addition of roasted coffee oil microcapsules (MOF). As defined in previous research [28], 3% of oil microcapsules were used in the MOF formulation. The microcapsules had an oil content of 42.8%, a light yellow/orange color (L* of 72.5, C* of 23.9, and h* of 79.4), irregular shape with a particle size of 125 µm, apparent density of 0.48 g cm−3, good solubility (58% in water at 95 °C) and wettability (1100 s) [27].
The formulations were prepared according to Böger et al. [28]. Cetostearyl alcohol was used as a thickener, ethoxylated cetostearyl alcohol 20OE and glyceryl stearate as an emulsifier, shea butter and caprylic/capric triacylglycerols as emollients, Phenogard as a preservative, and distilled water as a vehicle. To mask the color and odor of the formulations, avoiding identification in the sensory analysis, the pigment and the essence were added (Table 1).

Table 1.
Ingredients of cosmeceutical formulations (%; w/w).
For MOF preparation, the aqueous and oil phases were heated separately to 75 ± 2 °C. The oil phase (phase 2) was slowly added over the aqueous phase (phase 1) and mixed with a stick. The microcapsules pre-dissolved in distilled water at 50 °C (phase 3) were added and stirred until complete homogenization. Then, the preservative (phase 4), the pigment previously dissolved in propylene glycol (phase 5), and the essence (phase 6) were added and mixed with a stick until reaching a temperature of 40 °C. The same procedure was carried out for BF preparation without adding phase 3.
After preliminary tests, the formulations were evaluated for pre-stability by centrifugation (5 g of sample in graduated tubes and centrifuged at 1317× g/30 min, in triplicate). As the products proved to be stable, they were characterized.
2.5. Characterization of the Formulations
The formulations were characterized by pH, color, density, and spreadability (analysis in triplicate) and texture (analysis in sextuplicate). The results were compared by t test at the 5% significance level using Statistica 7.1 software [33].
The pH was determined using a digital pH meter at room temperature (25 ± 5 °C). For the determinations, 9.0 g of the sample was dissolved in 1.0 mL of distilled water [34].
Color determination was carried out with a colorimeter, and the results were expressed as lightness (L*), chroma (C*) and hue (h*).
For density analysis, the formulation (5.0 g) was diluted in 45.0 mL of distilled water, vortexed until completely dissolved, and kept at 20 °C in water and an ice bath. Density was determined using a volumetric flask (5.0 mL), obtaining the weight with the sample and with water. The absolute density of the diluted formulation and water was estimated, and the result was corrected considering the dilution, according to Equation (1).
The spreadability analysis was performed at room temperature (25 °C) and in triplicate for each sample type and weight. A glass microscope slide was positioned on millimeter paper, its outline was traced, and the center point was marked. The sample (0.50) was placed at the center of the glass slide and covered with another determined ground sheet (23.94 g). After 1 min, the diameter of the circle (mm) formed by the sample spreading was registered. The same procedure was repeated in intervals of 1 m, adding one by one standard weights of mass (2.00, 4.00, and 10.00 g). The results were expressed and graphed as sample spreadability due to the applied mass, according to Equation (2) [35]. The spreadability obtained with a mass of 33.94 g was used to compare the formulations.
where: Ei = sample spreadability for mass i (mm2); d = medium diameter (mm).
Texture analysis was carried out at room temperature by simple compression in a back extrusion test type, according to Calixto; Campos [36] and Tai; Bianchini; Jachowicz [37]. The sample (25 mL) was added to a 50 mL beaker (6 cm height and internal diameter of 4.1 cm). The test was performed using a P25 cylindrical probe, a force of 0.98 N, a penetration distance of 3 mm, and a speed of 2 mm s−1. The firmness (N) was defined as the maximum force of the positive curve; the consistency (N s) was defined as the area of the positive curve; the cohesiveness (N) was defined as the maximum force of the negative curve, and the viscosity index (N s) was defined as the area of the negative curve [36,37].
2.6. Sensory Analysis of the Formulations
Assessors conducted the tests at home over 28 days. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee on Research Involving Human Beings of the Universidade Estadual de Londrina (protocol code 2.587.333 and date of approval 6 October 2017; CAAE: 82054617.9.0000.5231). Before initiating the test, all assessors signed a free and informed consent form agreeing to participate (Figure S1). They received a copy of the form, which contains detailed information on the test (objective, procedures, and duration of the study), and stated that their participation will be voluntary (they may refuse to participate or even withdraw at any time), and their data will be treated with confidentiality (information used only for the purposes of this research and identity preserved). They were informed that they will not need to pay or be remunerated for their participation, but expenses arising from their participation in the research will be reimbursed and in case of any problems, the researchers will undertake to support them.
The freshly prepared formulations were stored in 15 g capacity false-bottomed plastic cream jars with screw caps and stoppers (RN Embalagens, São Paulo, Brazil) with an identification of the eye to which they should be applied. The order of presentation of the samples (BF or MOF) was balanced between assessors. At recruitment, each assessor performed a pre-test; they were instructed to apply the two samples to simulate the test procedure and observe any adverse reactions. After that, they decided whether they wanted to participate in the study.
We initiated the test with 60 assessors; two informed us that they forgot to apply the cream for some days, so their results were not considered. The panel was then composed of 58 women (students, employees, and professors at the university) with a high level of education (10% with elementary or high school education, 12% with incomplete or complete college education, and 78% with graduate degrees). They were all adults with a wide age range—14% under 25, 53% between 25 and 40, 31% between 40 and 60, and 2% over 60. According to self-declaration, most of the assessors were white/pink skinned (74%), followed by dark-skinned (10%), yellow and black (approximately 7% each), and dark brown (2%). Among the assessors, the majority (82%) reported using some skincare product, but with variation in the frequency of use: daily (81%), weekly (17%), or rarely (2.08%). The products used were sunscreen (21%), moisturizer (25%), sunscreen and moisturizer (19%), and anti-wrinkle and/or anti-aging products (35%). Considering the assessors who used anti-wrinkle and/or anti-aging products, more than half (59%) reported also using moisturizer and/or sunscreen.
Evaluation of Application and Allegation Characteristics
Each assessor received a kit containing two cream jars with formulations (one for each eye), a plastic spatula to help during the application and a small spiral notebook with a plastic cover for data registration (Figure S2). The notebook included product use instructions, a calendar for registration and control of the application, a definition of each attribute to be analyzed and the correspondent scale, and answer forms. First, they were instructed to wash and dry their face and hands with a towel. Later, they were instructed to apply the product using the ring finger to the whole area around the eyes, starting from the internal part of the upper eyelids with circular and soft movements until absorption. It was recommended to consistently apply the same amount of cream, approximately one grain of rice; the cream should be taken with the help of a spatula.
The assessors were instructed to apply the creams at night for 28 days. A 7-point category scale (Table 2) was used to evaluate the product application and treatment characteristics [24,38]. Evaluation sheets should be filled on days 7, 14, 21, and 28. Only the final results (28 days) were considered for the data analysis, and the intermediary analyses were used to familiarize the assessors with the attributes and scales and follow the evolution of the product use, considering the long application period. It was suggested that assessors could take pictures of themselves during the test in order to facilitate the comparison of the effect on each eye between the initial condition and after 28 days of use.

Table 2.
Definition of characteristics, evaluation form, and scales.
The spreadability and tackiness attributes associated with the application function were evaluated on a scale ranging from greatly disliked (1) to greatly liked (7). The smoothness, hydration, firmness, elasticity, fatigue signs, and wrinkles/fine lines attributes, corresponding to the treatment function, were evaluated on a scale ranging from greatly decreased (1) to greatly increased (7). The general appearance attribute, also associated with the treatment function, was evaluated on a scale ranging from much worse (1) to much better (7).
Descriptive statistics were used to analyze the results by calculating the mean and the standard deviation and the formulations were compared by the t test at 5% significance [33].
In addition to the acceptance analysis and evaluation of the characteristics, the assessors were asked, if possible, to indicate at the end of the 28 days their preference for one of the formulations. Data were compared by the t test at 5% significance [33]. Some points highlighted as observations by the group were reported as percentages of answers.
3. Results and Discussion
3.1. Characterization of the Fatty Acid and Diterpene Profiles of the Microcapsules
Roasted coffee oil microcapsules presented 2.03 g 100 g−1 total diterpenes, 0.95 g 100 g−1 cafestol and 1.08 g 100 g−1 kahweol (Table 3).

Table 3.
Characterization of diterpenes and fatty acids profile of roasted coffee oil microcapsules.
Kahewol and cafestol are the main coffee diterpenes produced only by plants from the Coffea genus [31]. They have anticarcinogenic, antioxidant, anti-inflammatory and hepatoprotective activity, and skin moisturizing and sun protection effects [39,40], which makes them attractive for application in food and cosmetic products.
The fatty acid profile showed a predominance of unsaturated acids (58.1%), especially linoleic and palmitic acids (Table 3). The comparison with roasted coffee [27] shows that the microencapsulation process did not modify the profile. Among the fatty acids found in the oil, palmitic acid stands out for being applied in skin product formulations such as soap and shaving cream, and linoleic, stearic, and oleic fatty acids are described as excellent cosmetic materials [41].
3.2. Characterization of the Formulations
The addition of microcapsules did not change the pH (p > 0.05); the cosmeceutical formulations showed an average pH of 4.52 (Table 4). This value is close to that described for natural skin pH, which varies from 4.6 to 5.8 [42]. Higher pH values were reported for formulations for the eye area with 0.5 and 1.0% green coffee extract (4.87 to 5.67) [41] and with 10% spent coffee ground oil and green coffee oil (4.89 and 6.63) [43].

Table 4.
Characterization of formulations: base (BF) and with microcapsules of coffee oil (MOF).
The formulations showed no differences regarding chroma (p > 0.05, C* approximately 24), but MOF was slightly clearer (L* of 66) and yellowish (h* of 64) (p ≤ 0.05), even with the pigment addition to mask the presence of the microcapsules (Table 4). Images of the two formulations are shown in Figure 1.
Figure 1.
Images of the cosmeceutical formulations developed: base formulation (BF) and formulation with microcapsules of coffee oil (MOF).
The formulations density was 0.99 g mL−1 (Table 4). Similar values (0.98 to 1.00 g mL−1) were described by Santos et al. [44] for formulations for the eye area with 0.5 and 1.0% green coffee extract and by Figueiredo; Martini; Michelin [45] for phytocosmetics containing green tea extract (0.98 to 1.01 g mL−1).
Spreadability determination is essential for products with topic applications since it relates to applications at the site of action [35] and can affect final acceptance. Spreadability is defined as the necessary force to apply the product on the skin until total absorption (disappearance) of the product, which is directly related to viscosity [46].
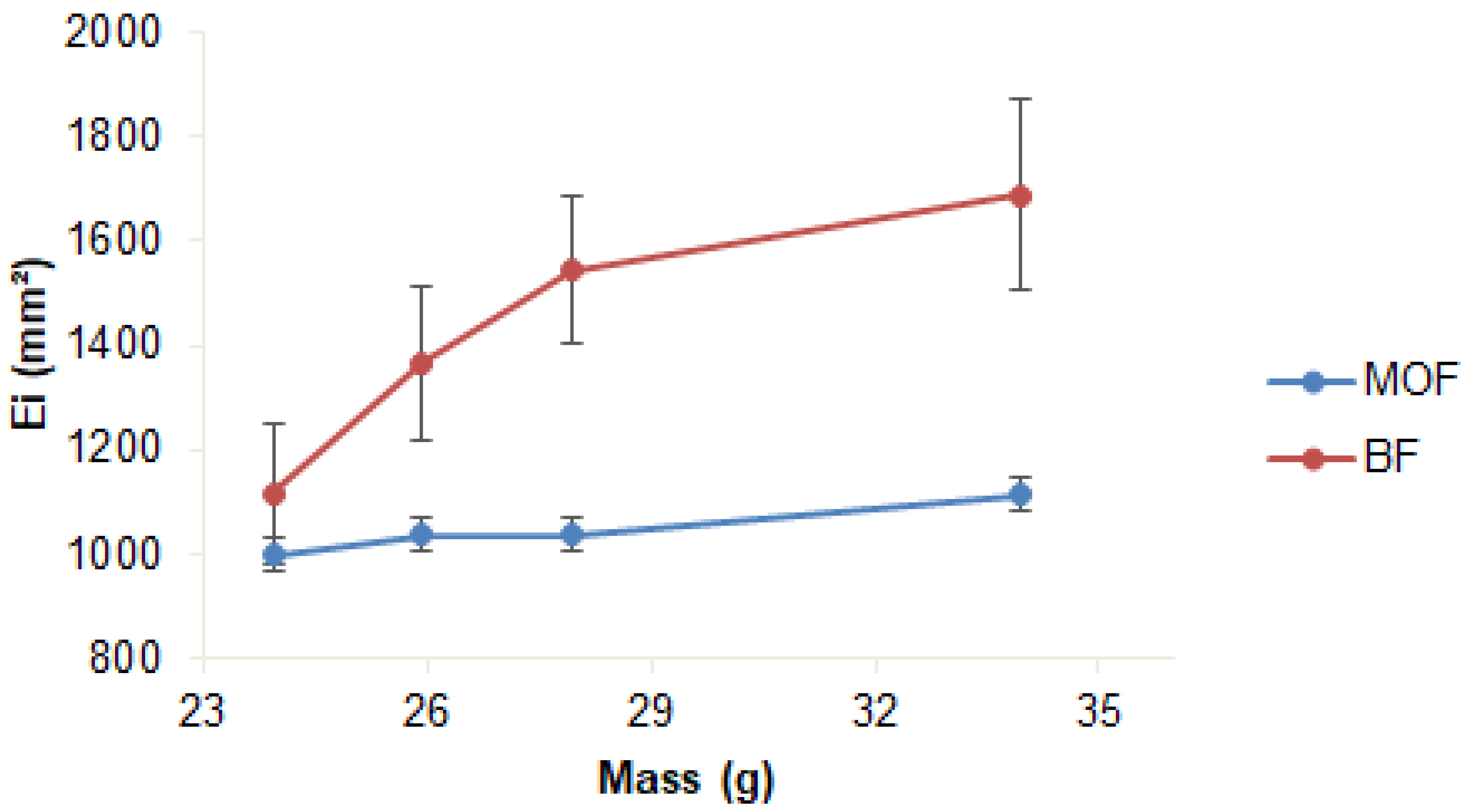
The spreadability of the formulations differed both in final value and in behavior with the application of pressure: BF showed high final spreadability (p > 0.05) but demanded more pressure (33.94 g) than MOF (25.94 g) to reach stability (Table 4 and Figure 2). The lower stability of MOF may be due to the presence of coffee oil microcapsules, which reduced the homogeneity of the mixture, making spreading difficult.
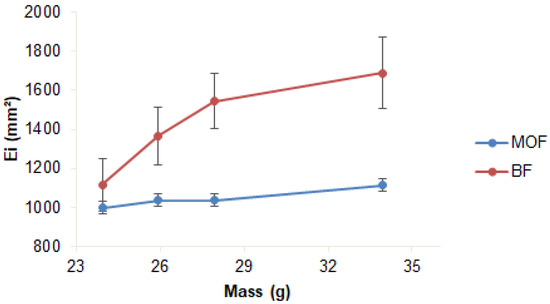
Figure 2.
Spreadability of cosmeceutical formulations: base (BF) and with microcapsules of coffee oil (MOF). Triplicate mean; error bars: standard deviation.
These results agree with those described in the literature. Savian et al. [47] reported a maximum spreadability value of approximately 1600 mm2 for emulsions with green coffee oil evaluated for 90 days of storage at 20 °C. Calixto and Campos [36] reported that adding ingredients (chicory root extract and UV filter) to a gel-cream formulation reduced the spreadability value of the base.
The adequate texture is one of the key properties to improving consumer acceptance of cosmetics [48]. No difference (p > 0.05) regarding consistency and viscosity index (0.77 N s and 0.25 N s, respectively) was observed for the two formulations. However, the MOF presented lower values (p ≤ 0.05) for firmness (0.77 N) and cohesiveness (0.52 N) than BF (Table 4). When the microcapsule was added to the formulation, firmness and cohesiveness decreased, which may account for the lower pressure required for MOF spreading (Figure 2).
Similar behavior was reported by Pinto et al. [48], who observed that an increase in the concentration of Portuguese chestnut extract in gels led to a reduction in firmness (from 0.554 N to 0.288 N).
3.3. Sensory Analysis of the Formulations
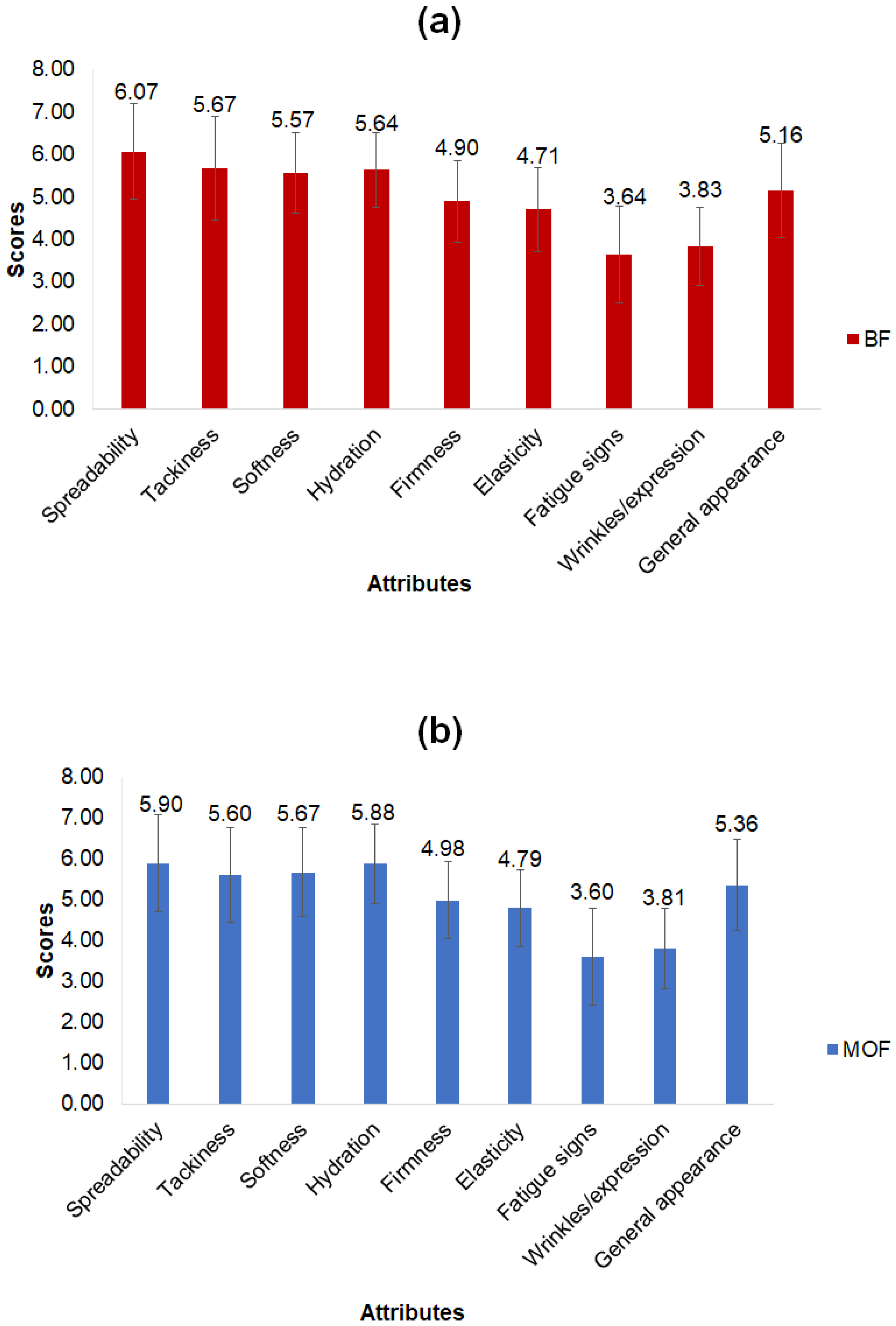
The analysis of the sensory results after 28 days of product application showed no significant difference (p > 0.05) between the formulations for the attributes evaluated (Figure 3). Observations highlighted by the group as important are reported below.
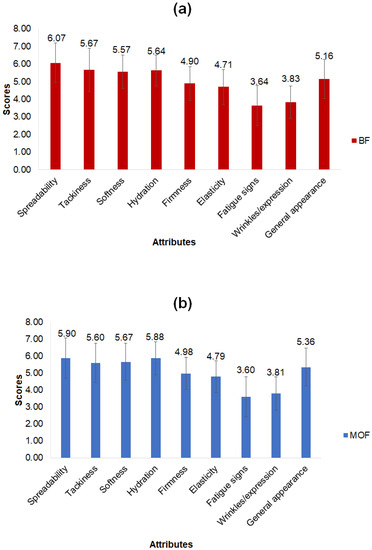
Figure 3.
Results of sensory evaluation of cosmeceutical formulations: base (BF) (a) and with microcapsules of coffee oil (MOF) (b). Mean values of 58 evaluations; error bars: standard deviation; no significant difference (by t test, p > 0.05) between BF and MOF for the attributes evaluated.
The formulations showed average scores of 6.0 and 5.6 (on a scale of 7) for the spreadability and tackiness attributes, respectively, indicating a similar approval (liked moderately) regarding the easiness of application of the formulations (Figure 3). As for product firmness, only two assessors (3% of the panel) reported that the creams seemed firmer as the days went by.
Higher firmness and spreadability were observed for formulation FB considering the physical analysis (Table 4). During the sensory analysis, fourteen assessors (24%) reported better spreadability for BF, stating that it was faster and easier to spread, with faster drying and lower tackiness, while a smaller number (5%) mentioned better spreadability for MOF. It should be considered, however, that probably the movement used to spread the formulation on the skin until absorption is more energetic than that employed during the physical tests, leading to a more effective rupture of the microcapsules, which could justify the lack of significant sensorial difference in the application of the formulations.
Among the attributes associated with treatment function, the perception of an increase (from small to moderate) in skin smoothness and hydration stands out (with average scores of 5.6 and 5.8, respectively), as well as a slight increase in skin firmness and elasticity (average scores of 4.9 and 4.8, respectively) (Figure 3). Fifteen assessors (26%) reported that both creams improved skin smoothness and hydration, with a minor appearance of tired skin, but only 5% noted increased firmness and elasticity (Figure 3).
Some research in the literature evaluated the sensory effect of coffee oil in cosmetic formulations regarding hydration and perception of oiliness immediately after application. Wagemaker et al. [49], in a study with a formulation with 15% green coffee oil, reported that most of the panel (19 assessors) noticed softer and hydrated skin after application but described an oily film on the skin. Ribeiro et al. [43] reported that formulations with 10% spent coffee ground oil and green coffee oil increased skin hydration and oiliness (evaluated by physical measures), and the formulation with coffee ground oil (a roast coffee derivative) scored highest by a group of 10 assessors for texture, oiliness, and skin feel after application. However, it is important to highlight that these products developed for the body used a large amount of oil directly in the emulsion, making their effect noticed immediately after each application.
Average scores for the signs of fatigue and wrinkles/expression attributes (3.6 and 3.8, respectively) indicated no change or very little reduction (Figure 3). Nine assessors (16%) reported a reduction in signs of fatigue and wrinkles/fine lines. Among them, some (9% of the panel) mentioned an improvement in the coloration and depth of the dark circles under the eyes. Interestingly, a small number of assessors (3%) reported a highly expressive reduction in the bags under the eyes with the use of MOF, showing that this could be an evaluation parameter to be considered in future studies.
For general appearance (an average grade of 5.3), the assessors reported a slight and similar improvement using the two formulations (Figure 3). Considering that only half of the panel reported routinely employing moisturizers or anti-aging products, many assessors may have actually seen benefits from daily use, regardless of formulation.
After completing the test, 19 assessors (33%) reported a preference for MOF and 18 (31%) for BF, and 21 assessors did not have or preferred not to state a preference. Among the assessors who preferred BF, the justification for the choice was equally divided between the easier application (better spreadability and less tackiness) (44%) and the perceived skin benefits (reduced signs of fatigue and wrinkles, greater firmness, hydration, and softness) (44%), and 12% did not state a reason. Among the assessors who preferred MOF, the following were mentioned as justification: reduction in signs of fatigue and wrinkles, bags under the eyes, and dark circles (58%); higher absorption, hydration, and softness (21%); greater creaminess of the product and skin brightness (21%).
These preliminary results indicate that the microcapsules can be applied with good sensory acceptance in dermocosmetic products. Thus, considering the size of the panel and the duration of the study, for a more thorough evaluation of the benefits of the presence of roasted coffee oil in the formulation for the eye area, extended studies are suggested.
4. Conclusions
The addition of roasted coffee oil microcapsules allowed the incorporation of bioactive compounds of interest (diterpenes and unsaturated fatty acids) without affecting the characteristics of pH, density, consistency and viscosity index of the cosmeceutical formulation. Physical evaluations showed a reduction in spreadability, firmness and cohesiveness with the addition of the microcapsule, but the effect on spreadability and tackiness was not sensorially perceived.
During the development of the study (28 days), the perception of the formulation benefits with the addition of oil microcapsules (increase in softness, hydration, firmness, elasticity, and general skin appearance and reduction in the fatigue signs and wrinkles/fine lines) was similar to those observed for the basic formulation.
Roasted coffee oil microcapsules proved to be a viable ingredient for dermocosmetic products with good sensory acceptance, so extended sensory studies are suggested to verify the possible benefits of their use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics10010024/s1, Figure S1: Free and informed consent term in the form of an invitation to assessors of cosmeceutical formulations—Acceptance test (English version). Figure S2: Photo of the kit elements delivered to the assessors for sensory analysis of the formulations.
Author Contributions
Conceptualization, B.R.B., A.A.S.G.L. and M.d.T.B.; methodology, B.R.B. and M.d.T.B.; validation, B.R.B.; formal analysis, B.R.B.; investigation, B.R.B.; resources, B.R.B., A.A.S.G.L. and M.d.T.B.; data curation, B.R.B.; writing—original draft preparation, B.R.B.; writing—review and editing, A.A.S.G.L. and M.d.T.B.; visualization, B.R.B.; supervision, A.A.S.G.L. and M.d.T.B.; project administration, M.d.T.B.; funding acquisition, B.R.B., A.A.S.G.L. and M.d.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPq, grant number 408645/2018-0.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee on Research Involving Human Beings of the Universidade Estadual de Londrina (protocol code 2.587.333 and date of approval October 06 2017; CAAE: 82054617.9.0000.5231).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
To Cia. Iguaçu de Café Solúvel (Cornélio Procópio, PR, Brazil) for providing the oil and CNPq and CAPES for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kalouta, K.; Eleni, P.; Boukouvalas, C.; Vassilatou, K.; Krokida, M. Dynamic mechanical analysis of novel cosmeceutical facial creams containing nano-encapsulated natural plant and fruit extracts. J. Cosmet. Dermatol. 2020, 19, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Reed, R. The definition of “cosmeceutical”. J. Soc. Cosmet. Chem. 1962, 13, 103–106. [Google Scholar]
- Newburger, A.E. Cosmeceuticals: Myths and misconceptions. Clin. Dermatol. 2009, 27, 446–452. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Mushroom-based cosmeceutical ingredients: Microencapsulation and in vitro release profile. Ind. Crops Prod. 2018, 124, 44–52. [Google Scholar] [CrossRef]
- Abel, E.L.; Hendrix, S.O.; Mcneeley, S.G.; Johnson, K.C.; Rosenberg, C.A.; Mossavar-Rahmani, Y.; Vitolins, M.; Kruger, M. Daily coffee consumption and prevalence of nonmelanoma skin cancer in Caucasian women. Eur. J. Cancer Prev. 2007, 16, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Lin, T.J.; Chiu, C.Y.; Chang, C.W.; Hsu, K.C.; Fan, P.C.; Wen, K.C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Farris, P. Idebenone, green tea, and Coffeeberry® extract: New and innovative antioxidants. Dermatol. Ther. 2007, 20, 322–329. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Patel, M.; Brody, N.; Jagdeo, J. Caffeine protects human skin fibroblasts from acute reactive oxygen species-induced necrosis. J. Drugs Dermatol. 2012, 11, 1342–1346. [Google Scholar]
- Oliveira, A.L.; Cruz, P.M.; Eberlin, M.N.; Cabral, F.A. Brazilian roasted coffee oil obtained by mechanical expelling: Compositional analysis by GC-MS. Ciênc. Tecnol. Alim. 2005, 25, 677–682. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Coffee oil as a natural surfactant. Food Chem. 2019, 295, 180–188. [Google Scholar] [CrossRef]
- Calligaris, S.; Munari, M.; Arrighetti, G.; Barba, L. Insights into the physicochemical properties of coffee oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1270–1277. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Carvalho, C.R.L.; Maia, N.B.; Baggio, S.R.; Filho, O.G. Sun protection factor, content and composition of lipid fraction of green coffee beans. Ind. Crops Prod. 2011, 33, 469–473. [Google Scholar] [CrossRef]
- Böger, B.R.; Mori, A.L.B.; Viegas, M.C.; Benassi, M.T. Quality attributes of roasted Arabica coffee oil extracted by pressing: Composition, antioxidant activity, sun protection factor and other physical and chemical parameters. Grasas Aceites 2021, 72, e394. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Comp. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Barel, A.; Paye, M.; Maibach, H. Handbook of Cosmetic Science and Technology, 1st ed.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2015, 33, 1–17. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Determination of critical storage conditions of coffee oil microcapsules by coupling water sorption isotherms and glass transition temperature. Int. J. Food Sci. Technol. 2012, 47, 1044–1054. [Google Scholar] [CrossRef]
- Freiberger, E.B.; Kaufmann, K.C.; Bona, E.; Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Gonçalves, O.H. Encapsulation of roasted coffee oil in biocompatible nanoparticles. LWT Food Sci. Technol. 2015, 64, 381–389. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Optimization of coffee oil flavor encapsulation using response surface methodology. LWT Food Sci. Technol. 2016, 70, 126–134. [Google Scholar] [CrossRef]
- Noronha, R.L.F. Análise sensorial de produtos cosméticos. In Cosmetologia e Empreendedorismo: Perspectivas para a Criação de Novos Negócios, 1st ed.; Leonardi, G.R., Spers, V.R.E., Eds.; Pharmabooks: Rio de Janeiro, Brazil, 2015; pp. 289–311. [Google Scholar]
- Chiar, B.; Almeida, M.G.J.; Correa, M.A.; Isaac, V. Cosmetics’ Quality Control. In Latest Research into Quality Control, 1st ed.; Akyar, I., Ed.; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef]
- Del Castillo, A.; Pérez, M.J.; Falqué, E.; Domínguez, H. Stability of Sun Creams Formulated with Thermal Spring Waters from Ourense, Northwest Spain. Cosmetics 2016, 3, 42. [Google Scholar] [CrossRef]
- Böger, B.R.; Acre, L.B.; Viegas, M.C.; Kurozawa, L.E.; Benassi, M.T. Roasted coffee oil microencapsulation by spray drying and complex coacervation techniques: Characteristics of the particles and sensory effect. Innov. Food Sci. Emerg. Technol. 2021, 72, e102739. [Google Scholar] [CrossRef]
- Böger, B.R.; Bigotto, B.G.; Lonni, A.A.S.G.; Benassi, M.T. Eye cosmeceutical formulations with roasted coffee oil in free and microencapsulated forms: Development and preliminary stability study. Eur. J. Lipid Sci. Technol. 2022, 124, e2100168. [Google Scholar] [CrossRef]
- Böger, B.R.; Georgetti, S.R.; Kurozawa, L.E. Microencapsulation of grape seed oil by spray drying. Food Sci. Technol. 2018, 38, 263–270. [Google Scholar] [CrossRef]
- ISO 5509; Animal and Vegetable Fats and Oils: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization (ISO): London, UK, 1978.
- Dias, R.C.E.; Faria-Machado, A.F.; Mercadante, A.Z.; Bragagnolo, N.; Benassi, M.T. Roasting process affects the profile of diterpenes in coffee. Eur. Food Res. Technol. 2014, 239, 961–970. [Google Scholar] [CrossRef]
- Mori, A.L.B.; Kalschne, D.L.; Ferrão, M.A.G.; Fonseca, A.F.A.; Ferrão, R.G.; Benassi, M.T. Diterpenes in Coffea canephora. J. Food Compos. Anal. 2016, 52, 52–57. [Google Scholar] [CrossRef]
- Statsoft. Statistica for Windows: Computer Program Manual, Versão 7.1; Software Inc.: Tulsa, OK, USA, 2006. [Google Scholar]
- Brasil, Ministério da Saúde; Agência Nacional de Vigilância Sanitária (ANVISA). Guia de Controle de Qualidade de Produtos Cosméticos—Uma Abordagem Sobre os Ensaios Físicos e Químicos, 2nd ed.; Agência Nacional de Vigilância Sanitária (ANVISA): Brasília, Brazil, 2007.
- Borghetti, G.S.; Knorst, M.T. Desenvolvimento e avaliação da estabilidade física de loções O/A contendo filtros solares. Rev. Bras. Ciênc. Farm. 2006, 42, 531–537. [Google Scholar] [CrossRef]
- Calixto, L.S.; Campos, P.M.B.G.M. Physical–mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Noronha, R.L.F.; Braghetto, C.P.; Ferreira, L.D.; Passos, J.L. Comprovação de claims relacionados a benefícios sensoriais em produto de maquiagem. Braz. J. Food Technol. 2010, 1, 49–54. [Google Scholar]
- Kim, H.G.; Hwang, Y.P.; Jeong, H.G. Caveol blocks STAT3 phosphorylation and induces apoptosis in human lung adenocarcinoma A549 cells. Toxicol. Lett. 2009, 187, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; Arauz, J. Coffee and liver diseases. Fitoterapia 2010, 81, 297–305. [Google Scholar] [CrossRef]
- Dangarembizi, R.; Chivandi, E.; Dawood, S.; Erlwanger, K.H.; Gundidza, M.; Magwa, M.L.; Muredzi, P.; Samie, A. The fatty acid composition and physicochemical properties of the underutilised Cassia abbreviata seed oil. Pak. J. Pharm. Sci. 2015, 28, 1005–1008. [Google Scholar]
- Leonardi, G.R.; Gaspar, L.R.; Campos, P.M.B.G.M. Estudo da variação do pH da pele humana exposta à formulação cosmética acrescida ou não das vitaminas A, E ou de ceramida, por metodologia não invasiva. An. Bras. Dermatol. 2002, 77, 563–569. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Santos, A.C.F.; Kalschne, D.L.; Viegas, M.C.; Vanini, L.S.; Benassi, M.T.; Lonni, A.A.S.G. Desenvolvimento de uma formulação cosmecêutica para região dos olhos com extrato padronizado de café. Vis. Academ. 2017, 18, 18–33. [Google Scholar] [CrossRef]
- Figueiredo, B.K.; Martini, P.C.; Michelin, D.C. Desenvolvimento e estabilidade preliminar de um fitocosmético contendo extrato de chá verde (Camellia sinensis) (L.) Kuntze (Theaceae). Ver. Bras. Farm. 2014, 95, 770–788. [Google Scholar]
- Czepula, A.I.S. Desenvolvimento de Preparações Semi-Sólidas Contendo Extrato de Sphagneticola trilobata (L.) Pruski (Acmela brasiliensis, Wedelia paludosa) (ASTERACEAE) e Avaliação da Atividade Anti-Inflamatória Tópica in Vivo. Master’s Thesis, Pharmaceutical Sciences-Universidade do Vale do Itajaí, Itajaí, Brazil, 2006. [Google Scholar]
- Savian, A.L.; Varella, F.T.; Athayde, M.L.; Silva, C.D.B. Desenvolvimento e avaliação preliminar da estabilidade de emulsão não-iônica O/A contendo óleo de café verde como potencializador de fator de proteção. Rev. Bras. Farm. 2011, 91, 82–88. [Google Scholar]
- Pinto, D.; Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa Bur: An undervalued by-product but a promising cosmetic ingredient. Cosmetics 2017, 4, 50. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Rosado, C.; Andrade, I.P.; Fernandes, A.S.; Rijo, P.; Campos, P.M.; Rodrigues, L.M. Evaluation of the sensory properties of a cosmetic formulation containing green coffee oil. Biomed. Biopharm. Res. 2013, 10, 101–108. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).