Abstract
The genus Spondias has two species of native trees from Brazil that deserve to be highlighted: Spondias tuberosa (“umbu”) and Spondias mombin (“cajá”). Their fruit contain bioactive compounds which have been associated with several biological activities. However, they remain little exploited in the development of food and pharmaceutical products. In this perspective, the present review summarizes the literature data about the physicochemical and nutritional characteristics, bioactive compounds, potential health benefits, and industrial applications of these fruit, including their pulp, seed, and peel. The current scenery mapping for scientific articles was performed in the Scopus and Web of Science databases. The study also considered patent applications collected in the Derwent database. Results showed an increase in scientific publications in recent years for both species. Many applications are related to food technology; nevertheless, due to the composition of their non-edible fractions, they have the potential for use in biorefinery, being their use an opportunity for bioeconomy. Thus, this review provides a comprehensive overview of these Brazilian native fruit to offer a theoretical foundation and valuable data for future investigations and exploitation.
1. Introduction
Brazil is renowned for its rich biodiversity and holds the status of a megadiverse country [1]. Owing to its expansive territory and diverse climate conditions, Brazil occupies a prominent position on the global stage as the third-largest fruit producer. The country boasts the world’s most extensive array of flora species, encompassing native fruit that not only offer nutritional value by providing energy and micronutrients but also contain bioactive compounds beneficial to health [2]. According to the FAO, the global fruit production reached 887 million tons in 2020 [3]. Notably, orange and bananas emerge as significant Brazilian commodities [4]. Alongside these, other species like cashew (Anacardium occidentale), pineapple (Ananas comosus), and passion fruit (Passiflora spp.), commercially exploited on a large scale, play a crucial role in the agroindustry. However, some fruit species remain confined to specific geographical areas [5]. Among these fruit trees, the genus Spondias stands out, with species belonging to the Anacardiaceae family. These native trees yield fruit distinguished by exceptional sensory characteristics, chemical composition, and nutritional aspects [6].
Spondias tuberosa is a native tree endemic from Brazil, popularly known as “umbuzeiro” besides other popular names [7]. This species is mainly distributed in areas with a semi-arid climate, and their fruit, known as “umbu”, present a green–yellow color and bittersweet flavor. Due to its bittersweet flavor, the pulp is used in the preparation of many products, especially those with added sugar such as nectars. However, for this purpose, the fruit are depulped, generating a by-product mainly composed of peels and seeds [8,9].
According to the Brazilian Institute of Geography and Statistics [10], it is estimated that 12,771 tons of umbu were produced in 2021, and the States of Bahia and Minas Gerais were the largest producers with 43.9% and 39.7%, respectively. This production was the highest recorded since 2009, with an increase of 34.9% on the previous year.
Spondias mombin is another native fruit tree from Brazil [7], which is distributed in tropical areas of Americas, between Southern Mexico and Brazil, including some Caribbean islands [11]. In Brazil, its fruit is called “cajá”, “taperebá”, “cajá-mirim”, or “cajazinho”, among other local names [7]. The S. mombin fruit show a yellow–orange color [12], and they have a fibrous and smooth texture and sour–sweet and refreshing tastes [13]. They can be consumed in natura or used to produce frozen pulp, juices, ice cream, and liqueurs, among others [14].
Official data on cajá production are unavailable, but it is estimated that between 15,000 and 20,000 tons of this fruit are produced annually. The cultivation is predominantly confined to the northeastern and northern regions of Brazil [15].
Studies report that S. tuberosa and S. mombin, including pulp and by-products (peel and seed), are sources of carbohydrates, protein, dietary fibers, essential minerals, and bioactive compounds such as phenolic compounds and carotenoids, among others [9,16,17,18,19,20,21]. Moreover, several studies have documented health benefits associated with the fruit and their components. Antioxidant and antibacterial actions, acetylcholinesterase inhibition, and cancer chemopreventive activity have already been reported [20,21,22,23,24].
Review articles related to S. tuberosa and S. mombin focusing mainly on general aspects of the trees, fruit characteristics, and on pharmacological aspects have been published. However, they do not bring together, in the same work, information about S. tuberosa and S. mombin, unlike this review, which brings together information about academic research and patents relating to the two fruits. Also, a bibliometric analysis of articles published in the last 20 years is provided herein; these characteristics differ this review from others that are already published [25,26,27,28,29,30,31,32,33].
Considering the published data, this paper aimed to map academic production regarding topics related to S. tuberosa and S. mombin using bibliometric analysis and a literature review, including general information about the physicochemical and nutritional characteristics, bioactive compounds, biological properties, and an evaluation of the intellectual property about the fruit and their parts (pulp, seed, and peel) by patent analyses.
Furthermore, the trends and gaps for future research related to the fruit from these two species of Spondias, so relevant in the Brazilian scenario, are also highlighted in this present review, including potential applications of the waste generated from their fruit processing.
2. Scientific and Technological Monitoring
A scientific paper selection was conducted using the keywords “Spondias tuberosa” and “Spondias mombin”. The search encompassed the Scopus and Web of Science databases, covering the period from 2003 to 2022. Results were filtered based on title, abstract, or keywords. Additionally, the study considered patent applications retrieved from the Derwent database using the keywords “Spondias tuberosa” or “umbu” and “Spondias mombin” or “cajá,” with results filtered by title or abstract. Data processing was carried out using Microsoft Office Excel (version 365), and visual representations of relationship networks were generated using the VOSviewer software (version 1.6.18).
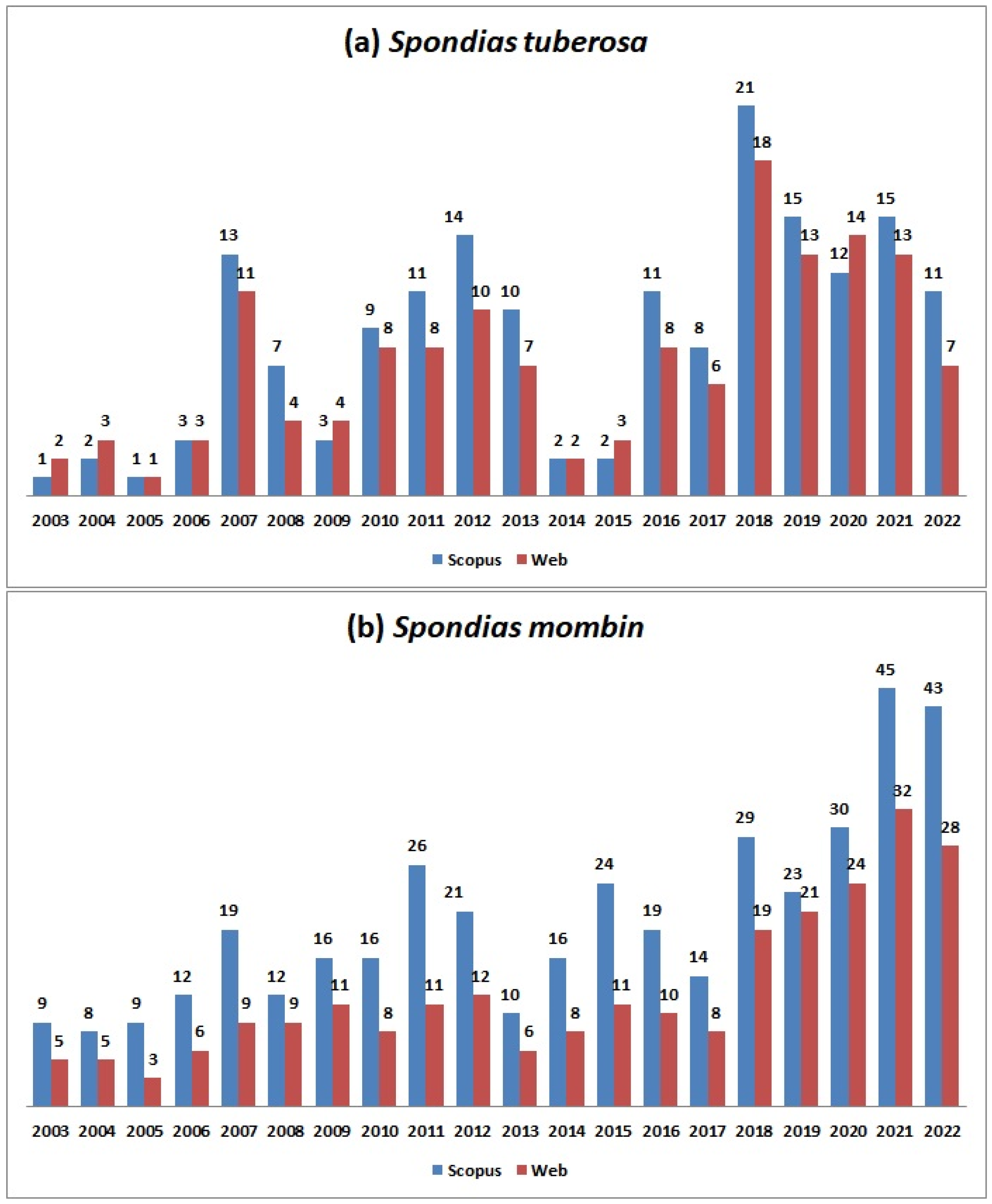
Figure 1a shows the historical series of scientific publications about S. tuberosa from 2003 to 2022. The literature search returned 171 documents in the Scopus database, and 145 in the Web of Science. An increase in publications can be observed between 2016 and 2022, which corresponds to 54% of the total, and in 2018, the highest number of publications was seen. Brazil had the largest share of publications with 98.5% of participation, followed by Germany (3.8%) and Italy (1.7%).
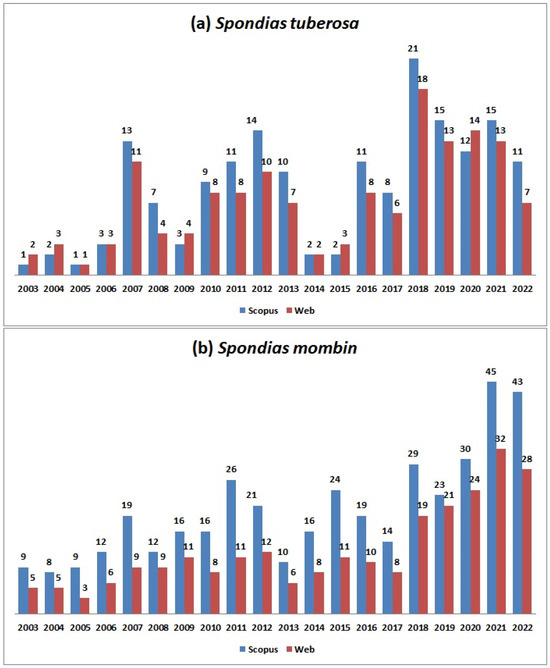
Figure 1.
Historical series of articles on Spondias tuberosa (a) and Spondias mombin (b) between 2003 and 2022. Source: Adapted from Scopus and Web of Science, 2023.
The evolution of scientific research on S. mombin is depicted in Figure 1b. The search retrieved 401 documents in Scopus and 247 in the Web of Science. A noticeable increase in publications over the last five years is evident, reaching its peak in 2021. In terms of publication distribution, Brazil leads with 45.0%, followed by Nigeria (23.0%), Mexico (7.8%), the USA (6.3%), South Africa (3.9%), France (3.2%), India (2.7%), and other countries contributing 8.0%. It is noteworthy that there is a substantial contribution from various regions to the scientific literature on S. mombin, likely due to the fruit being found in different countries.
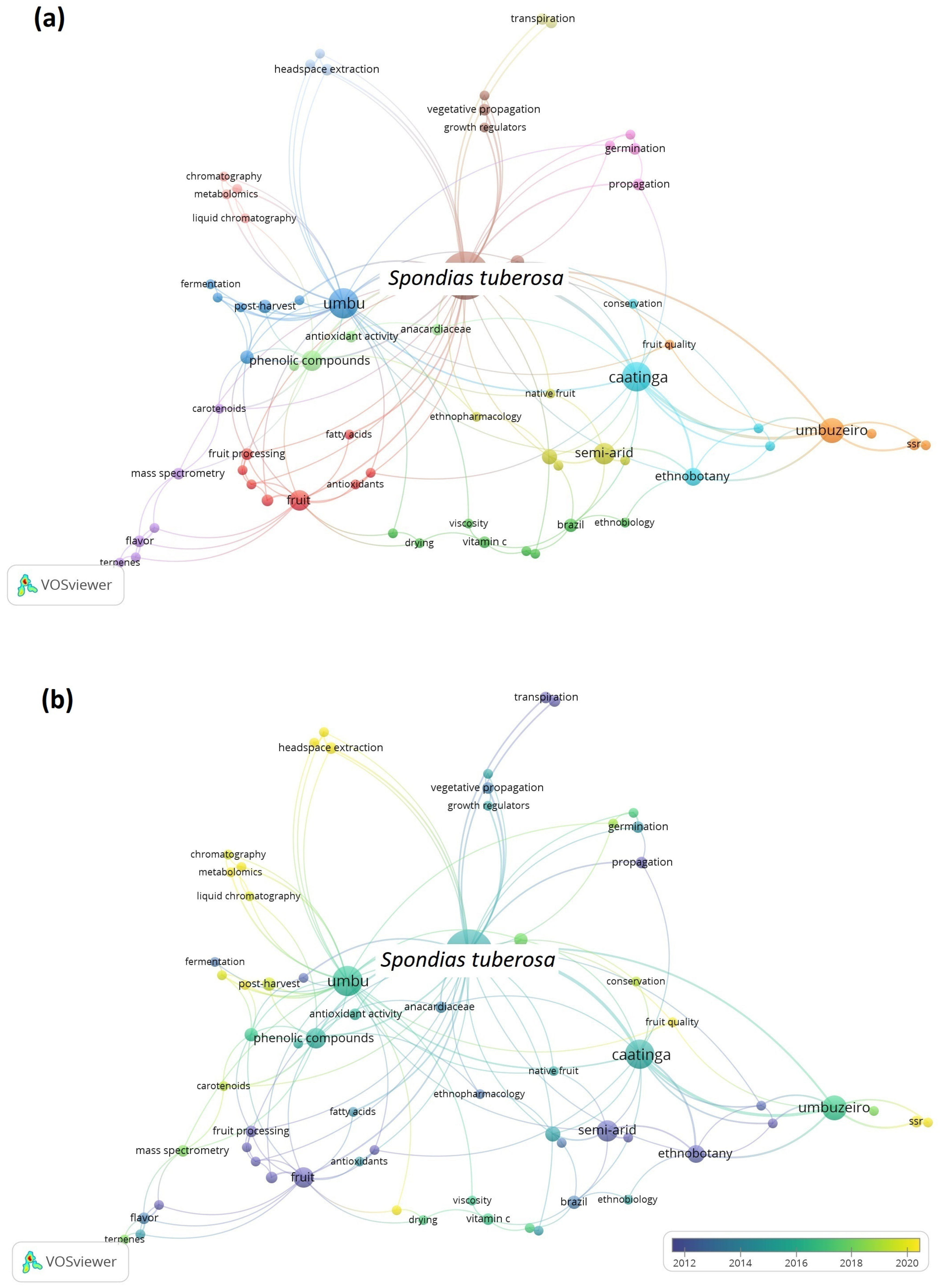
Since the Scopus database contained the most articles, the data retrieved were utilized to generate maps using the VOSviewer software. The co-occurrence maps of author keywords for S. tuberosa and S. mombin are presented in Figure 2 and Figure 3, respectively. The size of the circle corresponds to the frequency of keyword occurrences, while the color signifies the cluster of keywords that are more interconnected.
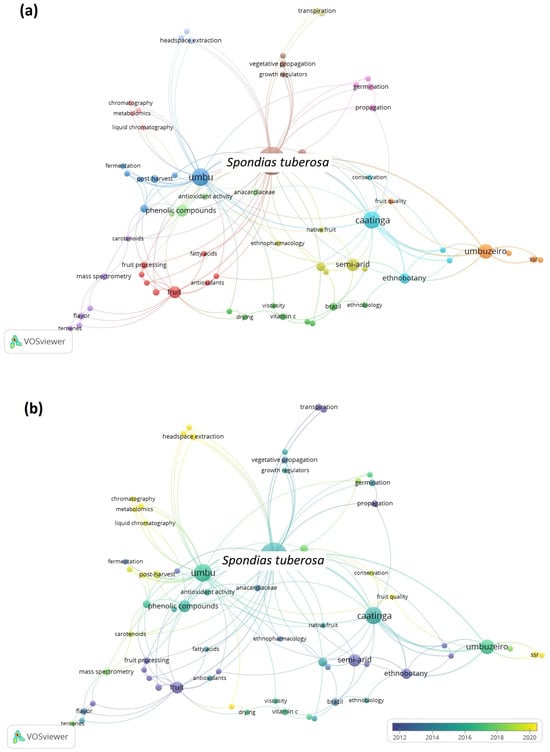
Figure 2.
Maps of co-occurrence of keywords from publications about Spondias tuberosa in the last 20 years.
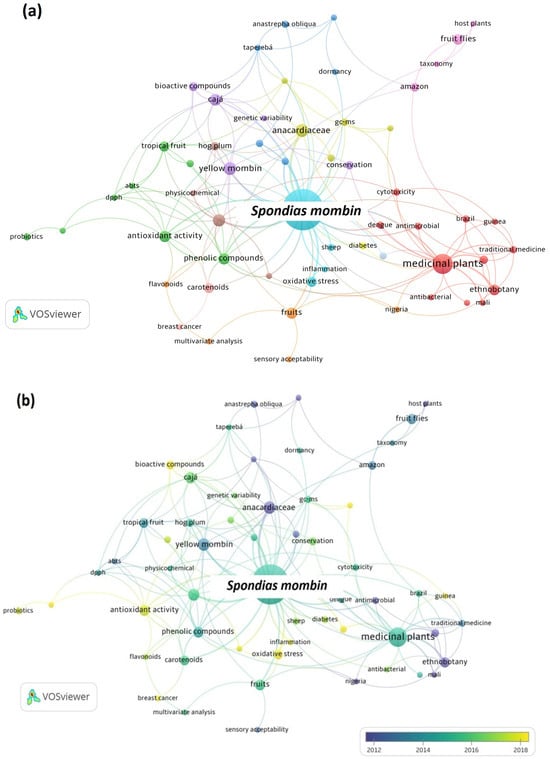
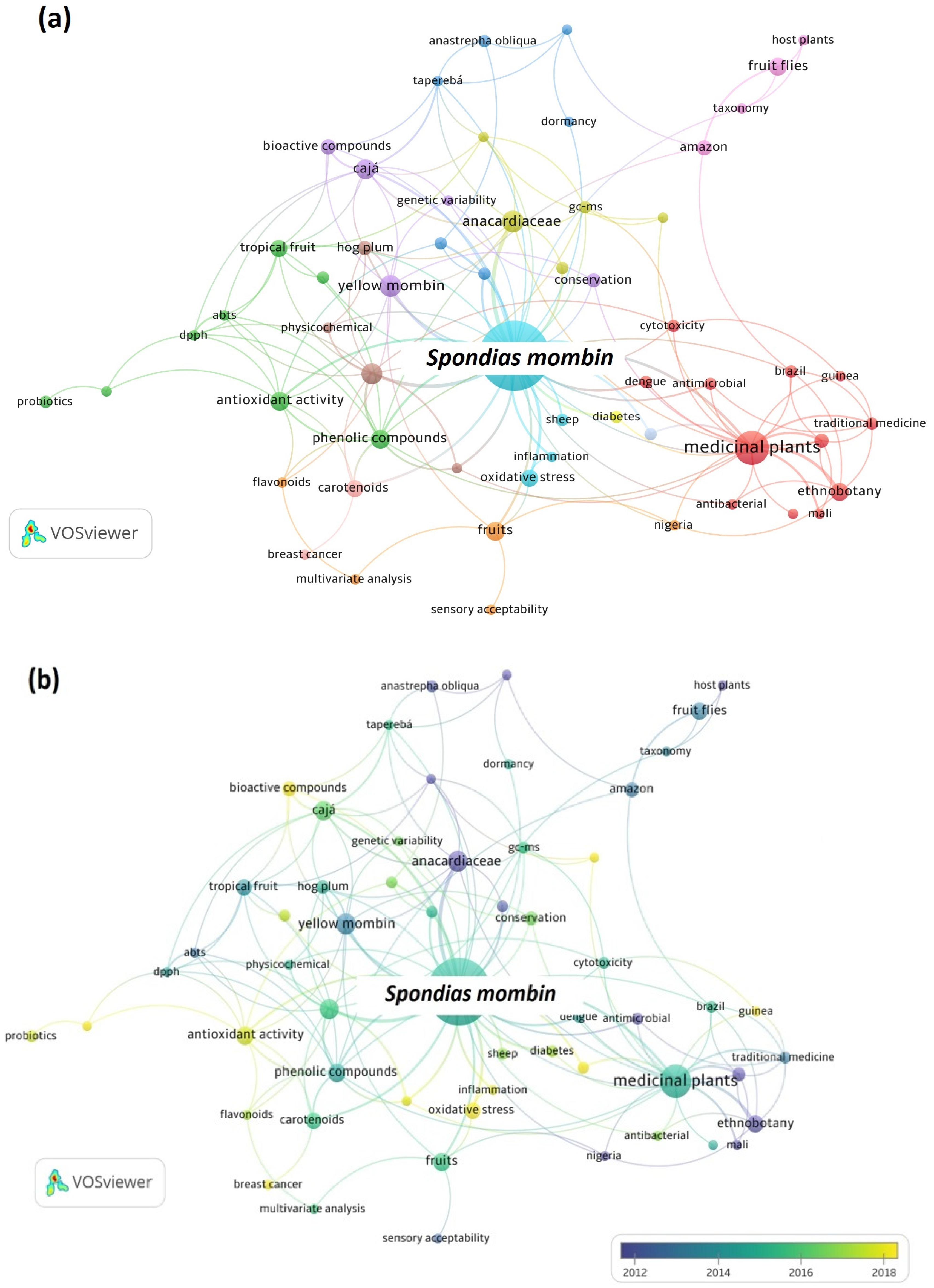
Figure 3.
Maps of co-occurrence of keywords from publications about Spondias mombin in the last 20 years.
As observed in Figure 2a, thirteen clusters of terms were generated, showing the range of studies performed over the years. Among them, the red and the light green clusters highlight studies concerning the chemical composition, phenolic compounds, antioxidant properties, fruit processing, and sensory analysis of the fruits. The lilac cluster comprises mostly publications that explore volatile compounds and flavor. The blue one is related to the postharvest, fermentation, quality, and shelf life. The turquoise cluster is related to studies of conservation and ethnobotany, and the orange cluster is related to plant breeding and fruit quality.
Figure 2b shows the chronological view of publications. The older studies are represented in purple color and recently published ones are given in yellow color. It can be observed that some keywords trending between 2018 and 2022 are aromatic fruit, headspace extraction, metabolomics, terpenes, carotenoids, chromatography, and mass spectrometry, which are in line with the evolution of high-resolution equipment used in the bioprospecting of plant matrices. With the advent of more sensitive and high-resolution analytical techniques, it is becoming increasingly possible to identify new active principles and more reliably relate activity with composition.
In Figure 3a, eleven clusters can be observed in the map of keywords for S. mombin. The largest cluster is the red one and addresses publications involving medicinal plants and biochemical effects. The green cluster is the second largest and addresses publications that are related to antioxidant properties and functional foods, corroborating the trend observed for S. tuberosa. As shown in Figure 3b, the most-studied topics between 2018 and 2022 are bioactive compounds, antioxidant activity, probiotics, and oxidative stress. It is in line with the more sustainable use of vegetable raw materials and the production of healthier foods.
Regarding the sources of scientific publications, the search returned 104 documents for S. tuberosa, and the top 10 journals with the largest number of scientific documents related to the topics selected were Food Chemistry (four articles and 929 citations), Journal of Ethnopharmacology (four articles and 449 citations), Food Research International (five articles and 317 citations), Environmental and Experimental Botany (one article and 120 citations), Brazilian Journal of Plant Physiology (two articles and 76 citations), Economic Botany (one article and 68 citations), Pharmaceutical Biology (two articles and 58 citations), Revista Brasileira de Fruticultura (eleven articles and 56 citations), Journal of Venomous animals and Toxins including Tropical Diseases (one article and 56 citations), and Journal of Arid Environments (one article and 54 citations). For S. mombin, 247 sources were found and the top 10 were the Journal of Ethnopharmacology (nineteen articles and 1282 citations), Food Chemistry (four articles and 1090 citations), Food Research International (nine articles and 722 citations), Revista Brasileira de Fruticultura (eleven articles and 143 citations), Journal of Herbal Medicine (eight articles and 138 citations), Revista Brasileira de Farmacognosia (three articles and 109 citations), Pharmaceutical Biology (five articles and 106 citations), Arabian Journal of Chemistry (one article and 105 citations), Journal of Food Engineering (one article and 104 citations), and Proceedings of the National Academy of Sciences of the United States of America (one article and 102 citations).
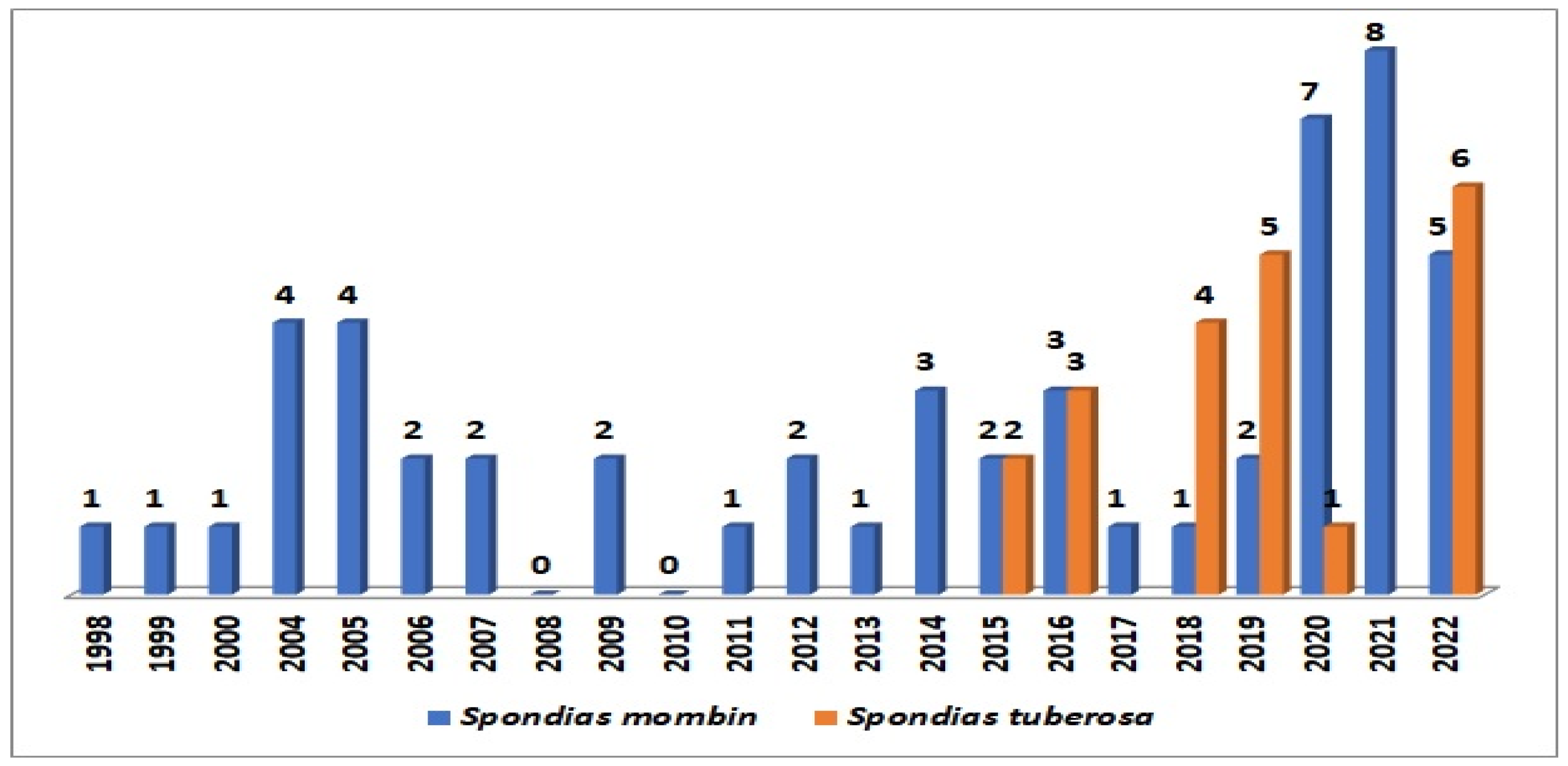
Figure 4 illustrates the historical series of deposited patents in the Derwent database. Notably, 21 patent applications were identified for S. tuberosa, with the initial patents dating back to 2015. The year 2022 stands out with the highest number of granted patents, totaling six. Universities and research institutes from Brazil were accountable for approximately 86% of these patents. A closer examination reveals that the patents are primarily distributed across fields of knowledge such as food science and technology (19), polymer science (9), biotechnology applied microbiology (8), and pharmacology/pharmacy (4).
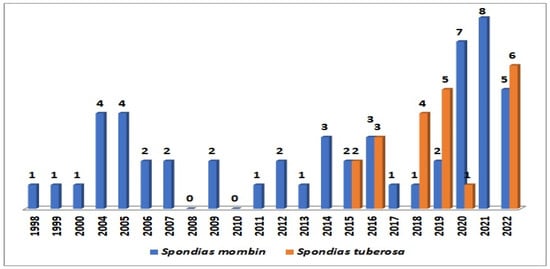
Figure 4.
Historical series of patents for Spondias tuberosa and for Spondias mombin. Source: Adapted from Derwent, 2023.
Regarding S. mombin, the study showed a total of 53 patents between 2002 and 2022. From 2019, there was a substantial increase in publication numbers followed by 2020 and 2021. The areas of knowledge are mainly distributed among biotechnology applied microbiology (33), pharmacology/pharmacy (32), food science and technology (21), and polymer science (17). Some applications are focused on the preparation of functional food and beverages, extraction and isolating active substances such as flavonoids and other phenolic compounds, as well as pharmaceutical and cosmetic formulations.
3. Nutritional and Mineral Composition
Table 1 displays the values of the nutritional composition of S. tuberosa and S. mombin fruit, including pulp, seed, and peel, investigated by different researchers.
According to Galvão et al. [34], ripe S. tuberosa pulp represents about 57.88 ± 0.09% of the fruit mass, while peel and seed represent 27.20 ± 0.07% and 15.99 + 0.11%, respectively. The average weight of the ripe fruit was 18.7 g. Consequently, after depulping, 34% of waste is produced, underscoring the significance of assessing the composition of these traditionally discarded fractions. This evaluation aims to provide a more valuable purpose than the standard disposal method, often utilized for processing waste fruit into animal feed [35].
Sugars and organic acids play an important role in the sensorial acceptance and the soluble solid content of fruit juices [36]. Ribeiro et al. [9] reported that S. tuberosa fruit is rich in carbohydrates, which occurs mainly in the pulp (71.53 g/100 g dry weight-dw) and peel (40.42 g/100 g dw) fractions. The authors stated that the sugar in the pulp is the reason for the sweet taste of the fruit, which makes it an attractive fraction to produce new products. Moreover, this component contributes to the energy value of the fruit. Concerning the organic acids, Omena et al. [37] identified citric acid in the umbu peel (3.78 ± 0.07 μg/g), umbu seeds (3.11 ± 0.06 μg/g), and umbu pulp (2.12 ± 0.04 μg/g), and the quinic acid in the umbu pulp (41.88 ± 0.84 μg/g).
Regarding total dietary fiber, Ribeiro et al. [9] found that S. tuberosa seeds and peels had higher fiber content (65.00 and 49.34 g/100 g dw). Dietary fibers can offer crucial positive effects and health benefits, including reducing the risk of obesity, intestinal diseases, diabetes, and cancer [38]. It is recommended that for a 2000 cal diet, the daily dietary fiber intake should be 28 g [39].
In a study by Cangussu et al. [40] values of insoluble and soluble fibers found for mature peel flour were 41.26 ± 2.29% and 13.78 ± 0.51%, and for the mature pulp flour, 10.19 ± 1.18% and 4.58 ± 0.79%, respectively. According to the authors, the polysaccharide constituents of the pulp and peel were tentatively characterized as arabinoxylans, arabinogalactans, rhamnoarabinogalactans, xyloglucans, and pectin of the rhamnogalacturonan type [40].
Lipid content is the highest in umbu seeds (Table 1). Borges et al. [41] reported a content of 58%. The fatty acid profile is composed of linoleic acid, oleic acid, palmitic acid, stearic acid, and arachidic acid. Costa et al. [42] showed that S. tuberosa seed contains saturated fatty acids (myristic, palmitic, stearic, n-nonadecilic, arachidic, behenic, and n-heneicosoic) and unsaturated acids (oleic majority, 43%) and linoleic (18%), which is in accordance with the data from Borges et al. [41]. Also, sitosterol and stigmasterol were reported by Santos et al. [43], which are important phytosterols found in edible oils.

Table 1.
Proximate and mineral composition of Spondias tuberosa and Spondias mombin fractions determined by different authors.
Table 1.
Proximate and mineral composition of Spondias tuberosa and Spondias mombin fractions determined by different authors.
| S. tuberosa | |||||||
|---|---|---|---|---|---|---|---|
| Analysis | Pulp 1 Paula et al. [44] | Pulp 2,4 Ribeiro et al. [9] | Pulp Silva et al. [19] | Seed 3 Borges et al. [41] | Seed 4 Ribeiro et al. [9] | Peel 4 Ribeiro et al. [9] | Peel Santiago et al. [45] |
| Energy (kcal/100 g) | - | 256.34–307.21 | - | - | 148.03 | 161.49 | - |
| Ash (g/100 g) | 0.41 ± 0.01 | 2.21 ± 0.32–4.17 ± 0.08 | 0.41 ± 0.01 | 4.00 ± 0.31–4.60 ± 0.31 | 2.74 ± 0.07 | 3.38 ± 0.05 | 3.69 ± 0.07 |
| Carbohydrate (g/100 g) | - | 50.17 ± 0.19–71.53 ± 0.65 | - | 7.60 ± 3.11–11.50 ± 3.11 | 14.31 ± 0.08 | 40.42 ± 0.05 | - |
| Fiber (g/100 g) | - | 12.35–35.32 | - | - | 65.00 | 49.34 | - |
| Lipid (g/100 g) | 0.39 ± 0.01 | 4.12 ± 0.61–6.00 ± 1.21 | 0.26 ± 0.01 | 55.00 ± 2.33–58.00 ± 2.33 | 8.92 ± 0.70 | 0.78 ± 0.05 | - |
| Protein (g/100 g) | 0.44 ± 0.01 | 6.18 ± 0.00–7.30 ± 0.01 | 0.50 ± 0.02 | 24.20 ± 0.22–25.10 ± 0.22 | 9.01 ± 0.08 | 6.08 ± 0.05 | 1.66 ± 0.20 |
| Moisture (g/100 g) | 89.48 ± 0.06 | - | 88.05 ± 0.76 | 5.10 ± 0.25–5.60 ± 0.25 | - | - | 10.25 ± 0.46 |
| Total solids (g/100 g) | - | 9.23 ± 0.04–11.09 ± 0.01 | - | - | 96.36 ± 0.01 | 90.33 ± 0.01 | - |
| Potassium (mg/100 g) | - | 1240 ± 12–2164 ± 16 | - | 684.01 ± 16.0–699.14 ± 15.0 | 755 ± 2 | 1491 | - |
| Iron (mg/100 g) | - | 2 ± 0–4 ± 0 | - | 07.50 ± 0.5–10.10 ± 1.0 | 74 ± 4 | 1 ± 0 | - |
| Zinc (mg/100 g) | - | 0.7 ± 0.0–1 ± 0 | 2 ± 0 | 0.7 ± 0.0 | - | ||
| Phosphorous (mg/100 g) | - | 114 ± 3–150 ± 1 | - | 772.38 ± 43.0–825.03 ± 50.0 | 287 ± 20 | 154 ± 1 | - |
| Calcium (mg/100 g) | - | 64 ± 0–171 ± 3 | - | 114.48 ± 5.0–191.02 ± 3.0 | 348 ± 1 | 195 ± 3 | - |
| Magnesium (mg/100 g) | - | 57 ± 1–87 ± 2 | 462.56 ± 10.0–477.59 ± 15.0 | 135 ± 10 | 88 ± 0 | - | |
| Sodium (mg/100 g) | - | 6 ± 0–26 ± 1 | - | 0.14 ± 0.2–00.16 ± 0.02 | 6 ± 0 | 12 ± 0 | - |
| Manganese (mg/100 g) | - | - | - | 1.85 ± 0.1–2.38 ± 0.3 | - | - | - |
| Copper (mg/100 g) | - | - | - | 2.33 ± 0.3–2.62 ± 0.2 | - | - | - |
| Aluminum (mg/100 g) | - | - | - | 0.46 ± 0.2–0.55 ± 0.1 | - | - | - |
| Barium (mg/100 g) | - | - | - | - | - | - | - |
| S. mombin | |||||||
| Analysis | Pulp Adepoju et al. [46] | Pulp 5 Mattietto et al. [47] | Pulp Tiburski et al. [18] | Pulp Nascimento et al. [48] | Pulp Silva et al. [19] | Seed Esua et al. [49] | Peel Pinheiro et al. [50] |
| Energy (kcal/100 g) | - | - | 65.42 | 59.99 ± 1.29 | - | - | 384.09–416.62 |
| Ash (g/100 g) | 1.0 ± 0.02 | 0.58 ± 0.02 | 0.76 ± 0.01 | 0.17 ± 0.05 | 0.59 ± 0.02 | 8.09 ± 0.15 | 5.61 ± 0.04–5.91 ± 0.12 |
| Carbohydrate (g/100 g) | 7.9 ± 0.05 | - | 13.90 ± 0.04 | 14.07 ± 0.34 | - | 40.56 ± 0.27 | 59.19–66.45 |
| Fiber (g/100 g) | 4.2 ± 0.04 | 1.18 ± 0.10 | 1.87 | - | - | 31.86 ± 0.08 | - |
| Lipid (g/100 g) | 2.0 ± 0.05 | 0.26 ± 0.09 | 0.62 ± 0.05 | 0.03 ± 0.02 | 0.41 ± 0.02 | 3.28 ± 0.03 | 7.76 ± 4.46–14.69 ± 6.19 |
| Protein (g/100 g) | 2.6 ± 0.04 | 0.82 ± 0.01 | 1.06 ± 0.04 | 0.86 ± 0.20 | 0.84 ± 0.01 | 7.73 ± 0.12 | 11.90–12.70 |
| Moisture (g/100 g) | 82.3 ± 3.57 | 89.42 ± 0.18 | 83.66 ± 0.04 | 84.87 ± 0.35 | 86.84 ± 0.11 | 8.48 ± 0.03 | 6.30 ± 0.29–8.87 ± 0.13 |
| Total solids (g/100 g) | - | - | - | - | - | - | - |
| Potassium (mg/100 g) | 270.0 ± 14.14 | - | 288.276 ± 23.895 | - | - | - | - |
| Iron (mg/100 g) | 3.2 ± 0.14 | - | 0.327 ± 0.001 | 1.22 ± 0.44 | - | 83.908 ± 0.159 | 15.26 ± 1.09–19.12 ± 9.17 |
| Zinc (mg/100 g) | 0.2 ± 0.01 | - | - | 0.06 ± 0.06 | - | 1.527 ± 0.002 | 11.56 ± 0.14–12.93 ± 0.85 |
| Phosphorous (mg/100 g) | 37.1 ± 0.21 | - | 32.849 ± 2.401 | - | - | - | |
| Calcium (mg/100 g) | 31.8 ± 0.42 | - | 11.038 ± 0.767 | 23.66 ± 3.12 | - | 131.77 ± 2.13 | |
| Magnesium (mg/100 g) | 465.0 ± 21.21 | - | 15.095 ± 0.863 | 45.50 ± 2.12 | - | 49.471 ± 0.051 | |
| Sodium (mg/100 g) | 400.0 ± 12.43 | - | 5.551 ± 2.352 | 4.16 ± 0.68 | - | - | |
| Manganese (mg/100 g) | 0.2 ± 0.01 | - | 0.025 ± 0.001 | 0.42 ± 0.01 | - | 1.793 ± 0.010 | 17.64 ± 0.27–22.57 ± 1.33 |
| Copper (mg/100 g) | 1.0 ± 0.14 | - | 0.118 ± 0.037 | 0.24 ± 0.01 | - | 0.768 ± 0.002 | 19.11 ± 1.80–29.65 ± 0.63 |
| Aluminum (mg/100 g) | - | - | 0.394 ± 0.086 | - | - | - | |
| Barium (mg/100 g) | - | - | 0.069 ± 0.006 | - | - | - | |
1 Values referring to pasteurized commercial pulp. 2 Values referring to commercial pulp and fresh pulp. 3 Values referring to two cultivars in two stages of maturity. 4 Results expressed on a dry weight basis (dw). 5 Results expressed on a wet weight basis. The other authors do not report the basis on which the results were expressed. Moreover, Galvão et al. [34] showed that the ripe umbu pulp oil contained 63.85% of the saturated fatty acids. The composition comprises hexadecanoic acid (30.43 ± 1.24%), 9-octadecenoic acid (27.49 ± 0.21%), n-pentadecanoic acid (13.23 ± 0.12%), octadecanoic acid (13.01 ± 1.23%), and 9,12-octadecadienoic acid (4.82 ± 0.09%), among others.
According to Table 1, minerals are found in the S. tuberosa pulp, including Fe, Zn, Ca, Mg, Na, and Mn, besides K (1240 ± 12 mg/100 g) and P (150 ± 1 mg/100 g), which are the most abundant minerals. Ribeiro et al. [9] also identified in the S. tuberosa seed and peel the same minerals found in the pulp. The seed presented the highest content of Mg, Ca, Zn, and P (135, 348, 2, and 287 mg/100 g dw, respectively) when compared to the other parts of the fruit. Thus, the intake of 100 g of S. tuberosa seed flour can supply 52, 35, 34, and 41% of the recommended daily intake of Mg, Ca, Zn, and P, respectively, as reported by Ribeiro et al. [9].
Menezes et al. [51] determined the influence of the maturation stage (unripe to ripe—three stages) on the physicochemical quality of the umbu fruit. The reported pH values were between 2.79 ± 0.03 and 2.88 ± 0.05, titratable acidity (% citric acid) between 2.05 ± 0.13 and 2.25 ± 0.13, and soluble solids between 10.25 ± 0.28 and 11.00 ± 0.71 °Brix. According to the authors, the quality of S. tuberosa fruit was not influenced by the different stages of fruit maturation. In a study carried out by Ribeiro et al. [52] using a frozen commercial pulp, the reported pH values were 2.49 ± 0.01, soluble solids 5.0 ± 0.1 °Brix, and titratable acidity 1.70 ± 0.00 g/100 g. The high difference on soluble solid content may indicate the addition of water during the fruit pulp production, for example.
Also, it is important to note that the differences found in the characterization of plant matrices can be explained by different agricultural practices, as well as seasonal variations [53]. However, umbu and its fractions can be considered good sources of nutrients as carbohydrates, proteins, lipids, fibers, and minerals.
Considering S. mombin, it is mainly composed of pulp, which corresponds to 56.7–73.22% of the fruit. The peel ranged from 8.4 to 18.7% and 15.7 to 31.0%, respectively, and the fruit weight ranged from 9.25 to 40.0 g as reported by Sacramento et al. [12].
The S. mombin pulp shows an acidic pH range from 2.49 ± 0.1 to 2.83 ± 0.01, a titratable acidity in % citric acid range between 1.21 ± 0.01 and 1.86 ± 0.01, and soluble solids ranging between 9.80 ± 0.10 and 15.17 ± 0.14 °Brix [13,18,47,48,54].
These results align with the limits set by the Brazilian legislation [55]. These physicochemical characteristics support the utilization of the pulp as an ingredient in the preparation of foods like mixed juices and nectars, contributing to an enhanced sensory acceptance.
According to Table 1, carbohydrates are the major nutrient present in S. mombin fruit [18,48,49,56]. Concerning the organic acids, Lucena et al. [24] identified acetic acid (3.34 ± 0.21 mg/g dw) and malic acid (6.00 ± 0.12 mg/g dw) in the S. mombin fruit.
Mattietto et al. [47] determined that S. mombin pulp contains insoluble (0.43 ± 0.12%) and soluble (0.75 ± 0.12%) fibers. In a study by Oladunjoye et al. [57], the dietary fiber content was determined for S. mombin bagasse obtained from juice processing residue. The values found were 19.06 ± 0.11% for insoluble fiber and 6.67 ± 0.06% for soluble fiber. The total fiber content (25.73%) was higher than wheat flour (0.88%).
Rezende et al. [58] showed that the seed contains more unsaturated fatty acids (oleic acid and linoleic acid) with 56.08% of the total fatty acid content, and the main triglyceride present was identified as glycerol 1,3-dioleoyl-2-linoleoyl. In the study performed by Abiodun et al. [59], the methanol extract of S. mombin seed presented dodecanoic acid (22.48%), tetradecanoic acid (17.95%), n-hexadecanoic acid (15.35%), capsaicin (12.11%), and dihydrocapsaicin (5.23%), besides other compounds in lower quantities.
Based on the evaluated literature records, S. mombin peels can also be utilized for industrial application. Recently, Pinheiro et al. [50] reported the presence of lipids, proteins, carbohydrates, and minerals (Fe, Zn, Mn, and Cu) in flour produced from the fruit peel by conventional drying and lyophilization.
S. mombin pulp (Table 1) also exhibits high contents of K and P, although in smaller quantities compared to the S. tuberosa fruit. Additionally, Ba and Al were detected in S. mombin pulp among the minerals. Nevertheless, Tiburski et al. [18] suggest that the amount of Ba in the pulp can be considered negligible. The presence of Al in the pulp is a significant indicator of acidic soils with low fertility characteristics where this fruit usually naturally grows (semi-arid).
4. Bioactive Compounds
Bioactive compounds isolated from edible portions of fruit, or its by-products mainly include tannins (gallotannins and condensed tannins), flavonoids (flavanols, catechins), phenolic acids, alkaloids, vitamins, carotenoids, and volatiles compounds [35]. These substances can present a specific metabolic or physiological activity beneficial to health, such as antioxidant, hypocholesterolemic, anti-inflammatory, and anti-angiogenic [60,61]. The antioxidants can neutralize the excess of free radicals present in biological cells, inhibiting the harmful effects on living organisms [61].
Carotenoids such as α-carotene, β-carotene, and β-cryptoxanthin are precursors of vitamin A, and the dietary deficiency in this nutrient is a global health problem responsible for growth retardation in children and an increased susceptibility to infection, blindness, and death [62]. Moreover, they are natural pigments, health-promoting compounds, and lately, they are also attracting interest in the context of nutricosmetics, as they have been shown to provide cosmetic benefits when ingested in appropriate amounts [63].
As described in Table 2, different researchers present various data of S. tuberosa and S. mombin fruits’ bioactive compounds such as polyphenols, tannins, carotenoids, and vitamins. These different classes of substances increase their applications to areas other than the food industry.

Table 2.
Bioactive compounds in Spondias tuberosa and Spondias mombin (pulp, peel, and seed).
4.1. Spondias tuberosa
According to Table 2, the pulp exhibited a total phenolic content (TPC) ranging from 0.158 to 40.4 mg GAE/g, peels ranged between 12.2 and 52.5 mg GAE/g, and seeds ranged from 2.5 to 202 mg GAE/g. The antioxidant capacity of extracts displayed variability in the results, which can be attributed to the chemical composition of the samples and specific details of the evaluation protocol, such as the standard used to express the results, and the nature of the sample, the type of solvent, among other factors. In a comparative study by Ribeiro et al. [9], the S. tuberosa peel demonstrated the highest antioxidant capacity (142.78 µmol TE/g), followed by commercial pulp (105.24 µmol TE/g), seed (84.30 µmol TE/g), and fresh pulp (25.24 µmol TE/g). The same authors identified two flavonoids (rutin and quercetin) in both samples.
Cangussu et al. [40] identified the presence of p-coumaric acid, ellagic acid, quercetin, procyanidin B2, and protocatechuic acid in the peel and pulp of S. tuberosa flour. Furthermore, the authors reported the presence of trigonelline, an alkaloid found in the plant which presents bioactive activities, including dental cavity prevention, anti-carcinogenicity, and anti-diabetic effect. Pulp samples presented higher contents of these compounds (6.14 ± 0.05 and 2.85 ± 1.00 mg/100 g for mature and semi-mature, respectively) than peel samples (3.26 ± 0.18 and 1.75 ± 0.21 mg/100 g for mature and semi-mature, respectively). In the same study, the authors identified the presence of gallotannins and ellagitannins as the main tannins present in this plant species. Ribeiro et al. [23] reported fifteen compounds in the optimized extract of S. tuberosa peel, which mainly comprised phenolic acids and flavonoids.
According to Table 2, studies indicate that pulp and peel from S. tuberosa contain lutein, zeaxanthin, zeinoxanthin, β-cryptoxanthin, α-carotene, β-carotene, 13-cis-β-carotene, and 9-cis-β-carotene. Among the identified carotenoids, β-carotene was the most abundant in all the samples studied [9,40].
Other compounds have been identified in S. tuberosa fruit. Meinhart et al. [68] identified the presence of chlorogenic acid isomers such as 4-caffeoylquinic acid (1.81 ± 0.02 mg/kg) and 5-caffeoylquinic (1.21 ± 0.25 mg/kg).
In a previous study by Galvão et al. [69], 246 volatile compounds were detected in the ripe fruit pulp, and 80 of them were positively identified. Among them, the following are highlighted: 4-methyl-3-penten-2-one; 1-penten-3-one; 2-nonanol, 2,2-dimethyl-4-octenal; 1- nonanol; 2-pentanol; 2-octanol; 3-methylethyl-2-butanoate; and butyl benzoate.
Ferreira et al. [70] evaluated the volatile organic compounds from fruit and identified ethyl butanoate, α-pinene, myrcene, ethyl caproate, limonene, ocimene cis- and trans-isomers, linalool, nonanal, and p-menth-1-en-4-ol. The major chemical classes identified were terpenes (about 7–72%) and esters (about 14–72%).
Regarding the vitamins present in the S. tuberosa pulp, a previous study by Gouvea et al. [71] reported vitamin C values of 10.06 ± 0.39 mg/100 g. Assis et al. [72] determined riboflavin B2 (0.099–0.455 mg/100 g), nicotinamide B3 (0.344–0.620 mg/100 g), pantothenic acid B5 (1.317–1.615 mg/100 g), biotin B7 (0.018–0.330 mg/100 g), vitamin C (3.81–32.88 mg/100 g), and carotenoid provitamin A (2.1–3.3 μg equivalent of retinol activity (RAE)/100 g). Cangussu et al. [40] reported that mature S. tuberosa pulp and peel flour contain α-tocopherol (1.79 ± 0.09 µg/g for pulp, and 6.86 ± 0.44 µg/g for peel). In addition, the peel also stood out due to its vitamin C content (79 mg/100 g). Based on these results, it is emphasized that the consumption of 100 g of S. tuberosa pulp provides 8.5% to 73% of the recommended daily intake of vitamin C for adults, according to the WHO data [73].
4.2. Spondias mombin
The total phenolic content (TPC) reported for pulp ranged from 0.270 to 13.4 mg GAE/g [13,18,20,64]. The values reported for peel and seed were 5.57 mg GAE/g and 239.5 mg GAE/g, respectively [59,67], showing that seed is the richest fraction in TPC.
Soares et al. [20] reported six phenolic compounds in S. mombin pulp (coumaric acid-O-hexoside II, vanillic acid-O-hexoside I, quercetin-7-O-glucoside I, ellagic acid I, quercetin-7-O-glucoside II, quercetin II). In the study by Brito et al. [67], several phenolic compounds were quantified in S. mombin peel, such as flavonols, phenylpropanoids, benzoic acid derivatives, coumarins, stilbenes, dihydrochalcones, flavones, and flavanones. Among the flavonols, quercetin was the most abundant compound, followed by myricetin and kaempferol-3-glucoside.
The optimized extract from S. mombin by-products (seed, residual pulp, and peel) was obtained by Santana Neto et al. [74] with the following conditions: 70 °C; 35 min; ethanol 55%. The extract showed TPC of 1666.18 ± 127.56 mg GAE 100/g dw, and DPPH• scavenging activity IC50 at 38.03 ± 0.49 µg/mL. The main components of the extract were characterized as 2,5-dihydroxybenzoic acid, salicylic acid, 4-hydroxybenzoic acid, ellagic acids, caffeic acid, rutin, myricetin, catechin, and hesperetin.
Another class of bioactive compounds reported in the literature for S. mombin is the carotenoids. The study performed by Costa et al. [66] shows that the main carotenoids in the frozen pulp of the fruit were (all-E)-β-cryptoxanthin (6.5 µg/g fresh weight (fw)) and (all-E)-zeinoxanthin (3.5 µg/g fw); esters of lutein, β-cryptoxanthin, and zeinoxanthin.
S. mombin pulp is also a source of vitamins. Assis et al. [72] reported nicotinamine B3 (0.156–0.441 mg/100 g), pyridoxine B6 (0.002–0.039 mg/100 g), pantothenic acid B5 (0.035–0.136 mg/100 g), vitamin C (6.44–14.20 mg/100 g), and carotenoid provitamin A (7.8–12.7 μg equivalent of retinol activity (RAE)/100 g). In the study of Aniceto et al. [13], the vitamin C content found was 25.93 ± 1.65 mg/100 g. Costa et al. [66] reported a provitamin A value of 67.3 ± 3.7 mg RAE/100 g.
Some important volatile compounds were found in S. mombin. Neiens et al. [11] suggested that the sweet and fruity aroma of S. mombin pulp is mainly related to ethyl butanoate, 3-methylbutyl acetate, ethyl 3-methylbutanoate, ethyl hexanoate, methyl 3-hydroxybutanoate, and ethyl 2-methylbutanoate, in combination with the sweet caramel-like smell of 4-hydroxy-2,5-dimethylfuran-3(2H)-one. The compounds α-pinene, myrcene, and (Z)-β-ocimene are responsible for the turpentine-like aroma note.
The study by Ampadu et al. [75] showed that the classes of common volatile compounds of S. mombin were esters (49.05%), acids (14.74%), terpenes (7.93%), phenols (2.85%), alcohols (1.45%), ketones (0.345%), and alkanes and alkenes (9.59%).
In view of the rich chemical composition of S. tuberosa and S. mombin, these fruit present potential as raw materials for different applications such as food, cosmetics, nutraceuticals, and pharmaceuticals.
5. Biological Properties
5.1. Spondias tuberosa
Regarding S. tuberosa fractions, Omena et al. [37] demonstrated that ethanol extracts from S. tuberosa seeds also present anti-acetylcholinesterase activity. However, pulp and peel extracts were inactive, and the extracts with concentrations of 100 ppm did not display cytotoxicity in assays using sheep corneal epithelial cells.
In studies by Zeraik et al. [22], the dichloromethane extract of S. tuberosa pulp showed cancer-chemopreventive activity, with a quinone reductase induction in Hepa1c1c7 cells. The induction ratio was 2.8 at 20 µg/mL. Moreover, the viability of these cells was 78.6%, exhibiting very little cytotoxicity in the concentration studied. The authors also reported that methanolic extract (40 µg/mL) inhibited 61% acetylcholinesterase (AChE) activity, in addition to high antioxidant activity in DPPH• (89%), ABTS•+ (97%), and ORAC (64%) assays. Isolated compounds such as gallic acid and 4-methoxyl-5-hydroxymethyl-3-O-β-D-glucopyranoside benzoic acid presented high antioxidant activity and AChE inhibition, with IC50 values less than 13.0 µmol/L.
S. tuberosa pulp has also shown antimicrobial potential. According to Alcântara et al. [76], methanolic extract from pulp showed a minimal inhibitory concentration (MIC) of 500 µg/mL, and no cytotoxicity was observed against immortalized fibroblast-like BGM cells.
According to Ribeiro et al. [23], the extract of S. tuberosa fruit peel inhibited the growth of Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis) and Gram-negative bacteria (Escherichia coli, Acinetobacter baumannii, Psedomonas aeruginosa, Klebsiella pneumonia), with more action on Gram-positive bacteria (MIC values ranging from 0.03 to 0.06 mg GAE/mL) compared to Gram-negative (MIC = 0.12 mg GAE/mL). Furthermore, the optimized extract inhibited α-amylase activity (IC50 value of 0.076 mg GAE/mL), indicating that the extract is a promising candidate for use in diabetes treatments.
5.2. Spondias mombin
The in vivo gastroprotective and ulcer-healing properties of S. mombin juice were assessed by Brito et al. [77]. The authors investigated its effects in indomethacin (100% juice) and ethanol (25%, 50%, and 100% juice) models of acute gastric ulcers in rats. The results indicated a 42.42%, 45.09%, and 98.21% inhibition of ethanol-induced gastric lesions, respectively, and a 58.96% reduction in indomethacin-induced lesions using a solution (100%), compared to the control group. Furthermore, S. mombin juice reduced gastric content and total acidity by 57.35% and 71.97%, respectively, while also effectively speeding up re-epithelialization.
Soares et al. [20] reported that ethanol extract from S. mombin pulp showed in vivo anti-inflammatory activity by inhibiting neutrophil migration. This property can be attributed to the presence of phenolic compounds such as quercetin, epicatechin, and anthocyanins.
According to Pereira et al. [21], S. mombin supplementation attenuated the remodeling process after myocardial infarction in studies with male Wistar rats through reduction in fibrosis, hypertrophy, and an improvement of oxidative stress and the efficiency of energy metabolism, while decreasing lipid hydroperoxide in the myocardium.
The hydrodistilled essential oil of S. mombin peel showed antimicrobial activities against the food-borne pathogenic bacteria (Salmonella spp., Staphylococcus aureus, and Escherichia coli) and fungi (A. niger, and P. oxalicum). According to Plablon et al. [78], the antimicrobial activity of the extract can be related to the presence of monoterpenes, mainly terpene hydrocarbons.
Lucena et al. [24] evaluate the effects of supplementing S. mombin (pulp and peel) on the oxidative, somatic, and lipid parameters of rats fed a high-fat diet. A portion of 400 mg/kg of body weight was supplemented via gavage for 14 days. In summary, the treatment improved glucose tolerance and lipid peroxidation, increased lipid elimination, and protected the liver from oxidative damage caused by the high-fat diet.
6. Technological Applications
Given their nutritional composition and bioactive compounds, the pulp of S. tuberosa and S. mombin fruit are excellent choices as raw materials for producing a variety of food products. Furthermore, with the ongoing scientific monitoring, it is also possible to identify industrial applications for other fractions of the fruit, which are typically discarded after depulping. These applications encompass cosmetic, pharmaceutical, and nutraceutical uses.
6.1. Spondias tuberosa
S. tuberosa pulp can be consumed fresh or processed into juices and ready-to-drink beverages [79,80,81], fermented dairy beverages supplemented with iron [82], fruit juice powder by spray drying [83], jam [84], diet cereal bars [85], and ice cream [86].
According to Ribeiro et al. [52], the pasteurized umbu juice at 86 °C for 25 s remained microbiologically stable during storage, showing that it is possible to preserve it for 90 days without the use of preservatives when submitted to pasteurization and kept under refrigeration. Also, 88.4% of retention of total phenolic compounds, 89.1% of total carotenoids and 83.7% of antioxidant capacity were observed after 90 days of cold storage.
In a study by Ribeiro et al. [80], the clarified umbu juice obtained by microfiltration process in ceramic membranes at 35 °C was stored in incubator at 6 °C for 90 days. The storage of juice in glass bottles at 6 °C allowed for a good preservation of the product’s characteristics, which remained proper for consumption for 90 days. The juice presented antioxidant capacity, probably related to its vitamin C and phenolic compound contents. Also, the microfiltration was able to retain microorganisms.
In the study by Gouvea et al. [71], the enzymatic hydrolysis of the S. tuberosa pulp using the pectinolytic enzyme (Rapidase TF®-100 ppm) at 35 °C by 40 min allowed for an effective viscosity reduction in the S. tuberosa pulp, maintaining the vitamin C content. The authors conclude that this result is an alternative for S. tuberosa processing, and a way to add value to fruit and contribute to the economic development.
Xavier et al. [87] elaborated flour from residues generated by S. tuberosa fruit processing (peels and seeds). The residues were dried (60 ± 2 °C) until reaching constant weight, ground in a knife mill, sieved (20 mesh), and stored at 25 °C. The flour presented high amounts of dietary fiber (insoluble fiber—56.67%), proteins (5.60%), lipids (7.53%), minerals (Ca, P, Mg, Fe, Zn, and Cu), ascorbic acid (44.78 mg/100 g), carotenoids (463.73 µg/100 g), and flavonoids (37.85 mg QE/100 g), as well as antioxidant potential and an absence of potentially toxic substances, causing it to be an option to be exploited in meat, bakery, and dairy products.
The study performed by Santiago et al. [45] showed that dehydrated peels from S. tuberosa can be used as a substrate for the synthesis of exo-polygalacturonase enzyme by solid-state fermentation using Aspergillus niger CCT 0916. The recovery was conducted by a two-phase aqueous system (PEG8000 and potassium phosphate salts) with a yield of 97.14%. This enzyme is used in the industrial process of the clarification of wines and fruit juices.
Among the patents, one worth highlighting pertains to the extraction and isolation of active substances from S. tuberosa pulp for use in functional foods or cosmetics. These active substances have demonstrated antioxidant and acetylcholinesterase inhibitory activities [88] as well as chemopreventive activity [89].
A patent related to obtaining edible flour made with peels and seeds from S. tuberosa fruit was filed by Silva et al. [90]. In addition, Silva et al. [91] published a patent on the production of a type of loaf bread with the addition of flour from the peel and seed of S. tuberosa fruit.
The patent by Trindade et al. [92] refers to the production of a food supplement made with 50% umbu powder and 50% passion fruit powder (Passiflora cincinatta Mast.), without additional mixtures of other substances or ingredients.
In the patent filed by Almeida et al. [93], S. tuberosa pulp and goat milk are used to obtain a powder for food purposes. The product is developed through the lyophilization process in three concentrations of milk and pulp: (i) 40% (umbu) + 60% (milk); (ii): 50% (umbu) + 50% (milk); (iii): 60% (umbu) + 40% (milk).
6.2. Spondias mombin
In the food area, some popular products obtained from S. mombin pulp include beverages [13], jam [94], pulp powder by spray drying [95], sweets [14], and ice cream [96].
Because of the presence of bioactive compounds, Santana Neto et al. [97] developed chicken patties with the addition of bagasse (peel, seed, and residual pulp) extracted from S. mombin. The dried residue was homogenized with 55% ethanol for 5 min and incubated in a water bath (35 min; 70 °C). Then, the mixture was centrifuged (20 min; 8960× g; 10 °C), filtered, and evaporated in a rotary evaporator (180 bar at 45 °C). Two formulations of chicken patties were developed, without and with the antioxidant extract, which were stored under refrigeration at 4 ± 1 °C for 15 days. According to the authors, natural extracts can be used as a potential antioxidant for ready-to-eat chicken patties, inhibiting protein and lipids from oxidative damage during refrigerated storage.
Sousa et al. [98] showed that 1% S. mombin bagasse extract alone or combined with commercial cryoprotectants (sucrose and sorbitol) had a positive effect on the inhibition of lipid and protein oxidation of surumi, corroborating with Santana Neto et al.’s [97] data. The addition of the extract was effective in maintaining the whiteness of the surimi and its gel throughout storage. At the end of 12 days of cold storage at 4 °C, the surimi gel prepared with the addition of 1% extract presented high whiteness compared to the control samples.
Chitosan microparticles containing S. mombin peel extract obtained by spray drying were developed by Brito et al. [67]. The results showed that this approach was able to increase the stability of the extract after 60 days of storage (temperatures of 4 °C, ~25 °C, and ~40 °C), when compared to crude extract. They reported 60% and 35% of retention of phenolic compound content for microencapsulated extract and lyophilized extract in the heat stress temperature, respectively.
Regarding the production of microbial enzymes, Ferraz et al. [99] performed an enzymatic saccharification of food waste using crude enzymatic cellulolytic extract produced by P. roqueforti cultivated in S. mombin residue. Moreover, da Rocha et al. [100] investigated the production of pectinolytic enzymes using S. mombin pulp residue as a substrate and Aspergillus niger IOC 4003. In the study by Marques et al. [101], S. mombin seeds have also been reported as a potential substrate to produce crude extracts containing cellulolytic enzymes.
Pectin extracted from S. mombin residue was used as a film-forming matrix to produce edible coatings using the casting process. Compared with commercial pectin, the film based on natural pectin showed a degree of esterification of 46%, which was higher than commercial (34%), displaying its capacity of forming gels. The film presented a good tensile strength, stretching, and an antimicrobial effect against Gram-negative bacteria [102].
To solve the problem of water pollution, S. mombin seed was used as a raw material in the production of adsorbent for the removal of CrVI ions [103]. The removal efficiency achieved 98% with a concentration of 10 mg·L−1, an adsorbent mass of 0.45 g, pH 2.0, and time of 120 min. According to the authors, this material may be a viable alternative for the treatment of industrial effluents that contain trace elements.
It is interesting to highlight a recent invention related to the production of an antioxidant extract from S. mombin waste (peel, seed, and residual pulp) as a way to add value to the fruit processing industry. According to the inventors, this extract is to be used in food products intended for human and animal consumption [104]. Afterwards, the same inventors published a patent related to the use of natural antioxidants from S. mombin waste in the preparation of hamburgers [105].
A patent disclosed by Souza et al. [106] used the subcritical water extraction process to obtain bioactive compounds from S. mombin fruit to use in the preparation of cosmetic composition for skin treatment.
Silva et al. [107] filed a patent for an invention in which the endocarp of the S. mombin fruit is used in the removal process of contaminants, via adsorption, such as heavy metal, oils, and greases of water or aqueous effluents. Cornelio et al. [108] disclosed an invention involving the use of S. mombin pulp in cosmetic formulations for human skin treatment.
The invention by Pinedo et al. [109] refers to the elaboration of fermented drink based on cajá juice, containing Lactobacillus casei, with application in the food area. Rodrigues et al. [110] published an invention about the process of obtaining Spondias pulp powder from the bacteria Bifidobacterium animalis ssp. lactis by spray drying under different conditions of drying, and use of maltodextrin, and inulin as encapsulant agent.
In summary, most of the applications for S. tuberosa fruit are mainly related to food technology; however, due to the characteristics of the fruit and their fractions discussed here, it is not limited to this area. The traditionally non-edible fractions have potential for biorefinery, with an opportunity for their use in bioeconomy.
7. Conclusions and Future Perspectives
This study is based on the monitoring of the production of academic publications, and patents regarding topics related to S. mombin and S. tuberosa, including data on physicochemical and nutritional characteristics, as well as the potential applications of their fruit (pulp, peel, and seed).
It can be observed that there was an increase in the publication of articles in recent years for both Spondias, and Brazil was accountable for approximately 86% of the deposited patents related to S. tuberosa. An analysis of scientific articles and patents revealed that the fruit contains various compounds such as phenolics, carotenoids, terpenes, vitamins, fatty acids, and polysaccharides, among others. In general, S. tuberosa pulp extracts exhibited activities such as antioxidant, anti-acetylcholinesterase, chemopreventive, and antimicrobial potential. Studies involving extracts of S. tuberosa fruit peel reported antibacterial properties and an inhibitory activity of α-amylase. On the other hand, works on S. mombin fruit extracts primarily demonstrated activities such as anti-inflammatory, gastroprotective, and antimicrobial effects. Therefore, it can be concluded that fruit from S. tuberosa and S. mombin can serve as valuable raw materials for producing new industrial products with innovation and sustainability, including functional foods, cosmetics, and pharmaceuticals, by utilizing the entire fruit.
The present review allowed us to identify many gaps in the scientific literature that addresses the different species of Spondias. It is possible to highlight the lack of studies on the chemical characterization of fractions, using metabolomics approaches, a field that is still very little explored. Another research opportunity in this field is the evaluation of the bioaccessibility of bioactive compounds from Spondias, since data on this topic are scarce. Little has been found about the content of proteins and amino acids, opening space for yet another field of research. The assessment of the chemical, physical, sensorial, and microbiological stability of products is also neglected in most works, which may reduce the possibility of their application in the future.
Consequently, we anticipate that further research will focus on valorizing these native Brazilian fruit. This effort aims to meet the needs of consumers seeking healthier and more sustainable alternatives, while also contributing to reducing the environmental impact caused by the improper disposal of agro-industrial residues from S. tuberosa and S. mombin.
Author Contributions
Conceptualization, L.d.O.R., V.M.d.M. and E.P.J.; methodology, C.N.K., S.P.F. and V.M.d.M.; software, J.S.d.F.; formal analysis, J.S.d.F. and A.d.A.N.; investigation, J.S.d.F. and A.d.A.N.; data curation, C.N.K. and D.d.L.M.; writing—original draft preparation, J.S.d.F., A.d.A.N. and S.P.F.; writing—review and editing L.d.O.R., E.P.J. and D.d.L.M.; supervision, L.d.O.R. and E.P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the support from the National Institute of Technology (INT), Embrapa Food Technology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro Botanical Garden Research Institute, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Programa de Capacitação Intitucional (PCI).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Braga, F.C. Brazilian Traditional Medicine: Historical Basis, Features and Potentialities for Pharmaceutical Development. J. Tradit. Chin. Med. Sci. 2021, 8, S44–S50. [Google Scholar]
- Coradin, L.; Camillo, J. Introdução. In Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas para o Futuro: Região Nordeste; Coradin, L., Camilo, J., Paren, F.G.C., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2018; pp. 19–29. [Google Scholar]
- FAO–Food and Agriculture Organization. Agricultural Production Statistics 2000–2020. Available online: https://www.fao.org/3/cb9180en/cb9180en.pdf (accessed on 18 November 2022).
- FAOSTAT. Food and Agriculture Organization, FAO. Available online: https://www.fao.org/faostat/en/#data (accessed on 18 March 2023).
- da Silva Junior, J.F.; Souza, F.V.D.; Pádua, J.G. A Arca de Noé Das Frutas Nativas Brasileiras; Embrapa: Brasília, Brazil, 2021; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/227633/1/A-arca-de-Noe-das-frutas-nativas-brasileiras-versao-10.pdf (accessed on 12 December 2022).
- Zortéa, K.É.M.; Rossi, A.A.B.; Tiago, A.V.; Cardoso, E.D.S.; Pinto, J.M.A.; Hoogerheide, E.S.S. Spondias mombin (Anarcadiaceae): Molecular Characterization and Conservation. Rev. Biol. Trop. 2021, 69, 1023–1036. [Google Scholar] [CrossRef]
- REFLORA-Plantas Do Brasil: Resgate Histórico e Herbário Virtual Para o Conhecimento e Conservação Da Flora Brasileira. Available online: https://floradobrasil.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/ConsultaPublicaUC.do (accessed on 18 March 2023).
- Oliveira, V.R.; Drumond, M.A.; dos Santos, C.A.F.; de Nascimento, C.E.S. Spondias tuberosa Umbu. In Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas para o Futuro: Região Nordeste; Coradin, L., Camillo, J., Pareyn, F.G.C., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2018; pp. 304–315. [Google Scholar]
- de Ribeiro, L.O.; de Viana, E.S.; de Godoy, R.L.O.; de Freitas, S.C.; Freitas, S.P.; da Matta, V.M. Nutrients and Bioactive Compounds of Pulp, Peel and Seed from Umbu Fruit. Cienc. Rural. 2019, 49, e20180806. [Google Scholar] [CrossRef]
- IBGE-Instituto Brasileiro de Geografia e Estatística. Available online: https://cidades.ibge.gov.br/brasil/pesquisa/16/0 (accessed on 9 December 2023).
- Neiens, S.D.; Geißlitz, S.M.; Steinhaus, M. Aroma-Active Compounds in Spondias mombin L. Fruit Pulp. Eur. Food Res. Technol. 2017, 243, 1073–1081. [Google Scholar] [CrossRef]
- Sacramento, C.K.; de Souza, F.X. Cajá. In Fruticultura Tropical Espécies Regionais e Exóticas; dos Santos-Serejo, J.A., Dantas, J.L.L., Sampaio, C.V., da Coelho, Y.S., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; pp. 85–105. [Google Scholar]
- Aniceto, A.; Montenegro, J.; da Cadena, R.S.; Teodoro, A.J. Physicochemical Characterization, Antioxidant Capacity, and Sensory Properties of Murici (Byrsonima crassifolia (L.) Kunth) and Taperebá (Spondias mombin L.) Beverages. Molecules 2021, 26, 332. [Google Scholar] [CrossRef] [PubMed]
- Jesus, I.G.; Souza, A.C.; Ferreira, I.M.; do Santos, L.V.N.; Silva, A.M.O.; Carvalho, M.G. Caracterização e Aceitação Sensorial de Doce Em Pasta Com Biomassa de Banana e Polpa de Cajá. Segur. Aliment. Nutr. 2019, 26, e019006. [Google Scholar] [CrossRef]
- de Carvalho, J.E.U.; Nascimento, W.M.O. do Cajá. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/215686/1/Editorial-2020.pdf (accessed on 9 March 2023).
- do Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Gregoris, E.; Pereira Lima, G.P.; Fabris, S.; Bertelle, M.; Sicari, M.; Stevanato, R. Antioxidant Properties of Brazilian Tropical Fruits by Correlation between Different Assays. Biomed. Res. Int. 2013, 2013, 132759. [Google Scholar] [CrossRef] [PubMed]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional Properties of Yellow Mombin (Spondias mombin L.) Pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- da Silva, P.B.; Almeida, F.D.; Gomes, J.P.; Silva, S.D.; Barroso, A.J.; Moraes, J.D.; Silva, L.M.; Matos, J.D.; da Silva, L.P.; de Melo, B.A.; et al. Production and Characterization of Lyophilized Powder of Yellow Mombin (Spondias mombin L.) and Umbu (Spondias tuberosa). Aust. J. Crop Sci. 2021, 15, 669–675. [Google Scholar] [CrossRef]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive Characterization of Bioactive Phenols from New Brazilian Superfruits by LC-ESI-QTOF-MS, and Their ROS and RNS Scavenging Effects and Anti-Inflammatory Activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef]
- Pereira, B.L.B.; Rodrigue, A.; de Arruda, F.C.O.; Bachiega, T.F.; Lourenço, M.A.M.; Correa, C.R.; Azevedo, P.S.; Polegato, B.F.; Okoshi, K.; Fernandes, A.A.H.; et al. Spondias mombin L. Attenuates Ventricular Remodelling after Myocardial Infarction Associated with Oxidative Stress and Inflammatory Modulation. J. Cell Mol. Med. 2020, 24, 7862–7872. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Queiroz, E.F.; Marcourt, L.; Ciclet, O.; Castro-Gamboa, I.; Silva, D.H.S.; Cuendet, M.; da Silva Bolzani, V.; Wolfender, J.-L. Antioxidants, Quinone Reductase Inducers and Acetylcholinesterase Inhibitors from Spondias tuberosa Fruits. J. Funct. Foods 2016, 21, 396–405. [Google Scholar] [CrossRef]
- de Ribeiro, L.O.; de Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; dos Santos, A.L.S.; de Moreira, D.L.; do Amaral, B.S.; Jung, E.P.; Kunigami, C.N. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules 2022, 27, 410. [Google Scholar] [CrossRef] [PubMed]
- Lucena, T.L.C.; Batista, K.S.; Pinheiro, R.O.; Cavalcante, H.C.; de Gomes, J.A.S.; da Silva, L.A.; Lins, P.P.; Ferreira, F.S.; Lima, R.F.; dos Lima, M.S.; et al. Nutritional Characterization, Antioxidant, and Lipid-Lowering Effects of Yellow mombin (Spondias mombin) Supplemented to Rats Fed a High-Fat Diet. Foods 2022, 11, 3064. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; de Almeida-Cortez, J.S.; Germer, J.; Sauerborn, J. Umbuzeiro (Spondias tuberosa): A systematic review. Rev. Bras. Ciênc. Ambient. 2015, 36, 179–197. [Google Scholar] [CrossRef]
- Mertens, J.; Germer, J.; Siqueira, J.A.; Sauerborn, J. Spondias tuberosa Arruda (Anacardiaceae), a threatened tree of the Brazilian Caatinga? Braz. J. Biol. 2017, 77, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.N.; de Carvalho Costa, É.K.; Donato, S.L.; Narain, N. Perfil fitoquímico e propriedade antioxidante de diferentes genótipos de frutos do umbuzeiro (Spondias tuberosa Arruda Câmara): Uma revisão. Res. Soc. Dev. 2021, 10, e58101623116. [Google Scholar] [CrossRef]
- Bery, C.C.; Araújo, K.B.; Oliveira, Í.M.; Bery, C.D.; Barretto, L.C.; Freitas, A.G.; Freitas, L.D.; Da Silva, G.F. Technologic Prospection of Bioactive Compounds in Umbu Pulp (Spondias Tuberosa Arruda). Rev. Geintec 2021, 11, 5725–5734. [Google Scholar] [CrossRef]
- Aniceto, A.; Porte, A.; Montenegro, J.; Cadena, R.S.; Teodoro, A.J. A review of the fruit nutritional and biological activities of three Amazonian species: Bacuri (Platonia insignis), murici (Byrsonima spp.), and taperebá (Spondias mombin). Fruits 2017, 72, 317–326. [Google Scholar] [CrossRef]
- Maria, A.C.; Simões, T.R.; Ramos, A.D.; Almeida, M.M.; Silva, M.A.; Cruz, J.D.; Ferreira, J.L.; Silva, J.R.; Amaral, A.C. Spondias mombin L.: An updated monograph. Pharmacogn. Rev. 2022, 16, 45–61. [Google Scholar] [CrossRef]
- Almeida, C.O.; Martinez, R.M.; Figueiredo, M.S.; Teodoro, A.J. Botanical, nutritional, phytochemical characteristics, and potential health benefits of murici (Byrsonima crassifolia) and taperebá (Spondias mombin): Insights from animal and cell culture models. Nutr. Rev. 2023, 82, 407–424. [Google Scholar] [CrossRef]
- Ogunro, O.B.; Oyeyinka, B.O.; Gyebi, G.A.; Batiha, G.E.S. Nutritional benefits, ethnomedicinal uses, phytochemistry, pharmacological properties and toxicity of Spondias mombin Linn: A comprehensive review. J. Pharm. Pharmacol. 2023, 75, 162–226. [Google Scholar] [CrossRef] [PubMed]
- Moke, E.G.; Edje, E.K.; Daubry, T.M.; Nwogueze, B.C.; Ataikiru, O.M.; Umukoro, E.K.; Omorodion, I.L.; Chidebe, E.O.; Demaki, W.E.; Aluya, S.O.; et al. Phytopharmacological Activities of Spondias mombin Linn: A Review. Trop. J. Phytochem. Pharm. Sci. 2024, 3, 117–123. [Google Scholar]
- Galvão, M.S.; Narain, N.; Carnelossi, M.A.G. Post-Harvest Quality Evaluation of Physico-Chemical and Chemical Characteristics in Umbu Fruit at Different Storage Conditions de La Calidad Postcosecha de Las Características Físico-Químicas y Químicas En El Fruto de Umbu a Diferentes Condiciones de Almacenmiento. CYTA J. Food 2010, 8, 103–108. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of Sugar and Organic Acid of Fruit Juices: A Comparative Study and Implication for Authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Omena, C.M.B.; Valentim, I.B.; da Guedes, G.S.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.H.F.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S.; et al. Antioxidant, Anti-Acetylcholinesterase and Cytotoxic Activities of Ethanol Extracts of Peel, Pulp and Seeds of Exotic Brazilian Fruits: Antioxidant, Anti-Acetylcholinesterase and Cytotoxic Activities in Fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of Dietary Fiber on Human Health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- USDA. U.S. Department of Agriculture. Available online: https://www.usda.gov/ (accessed on 13 March 2023).
- Cangussu, L.B.; Fronza, P.; Franca, A.S.; Oliveira, L.S. Chemical Characterization and Bioaccessibility Assessment of Bioactive Compounds from Umbu (Spondias tuberosa A.) Fruit Peel and Pulp Flours. Foods 2021, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.V.; Maia, M.C.A.; de Gomes, R.C.M.; Cavalcanti, N.B. Chemical Composition of Umbu (Spondias tuberosa Arr. Cam) Seeds. Quim. Nova 2007, 30, 49–52. [Google Scholar] [CrossRef]
- Costa, T.A.C.; Campos, V.P.; Menezes, J.S.; Oliva, S.T.; West, C.B. Phytochemical Profile of Seed Extracts of Plants Typical of the Brazilian Semiarid and Their Potential Application in Brackish Water Desalination. J. Braz. Chem. Soc. 2016, 27, 1694–1703. [Google Scholar] [CrossRef]
- Santos, P.A.; de Rezende, L.C.; de Oliveira, J.C.S.; David, J.M.; David, J.P. Chemical Study, Antioxidant and Cytotoxic Activities of Oil Seeds of Spondias tuberosa (Anacardiaceae). Int. J. Fruit Sci. 2019, 19, 246–257. [Google Scholar] [CrossRef]
- de Paula, B.; Carvalho Filho, C.D.; da Matta, V.M.; da Menezes, J.S.; da Lima, P.C.; Pinto, C.O.; Conceição, L.E.M.G. Produção e Caracterização Físico-Química de Fermentado de Umbu. Cienc. Rural 2012, 42, 1688–1693. [Google Scholar] [CrossRef][Green Version]
- Santiago, Â.M.; de Sousa Conrado Oliveira, L.; de Oliveira, P.L.; Almeida, R.L.J.; Santos, N.C.; Galdino, P.O. Production and Recovery of Exo-Polygalacturonase from Umbu (Spondias tuberosa) Residue. Waste Biomass Valorization 2022, 13, 1101–1115. [Google Scholar] [CrossRef]
- Adepoju, O.T. Proximate Composition and Micronutrient Potentials of Three Locally Available Wild Fruits in Nigeria. Afr. J. Agric. Res. 2009, 4, 887–892. [Google Scholar]
- Mattietto, R.D.; Lopes, A.S.; de Menezes, H.C. Caracterização Física e Físico-Química Dos Frutos Da Cajazeira (Spondias mombin L.) e de Suas Polpas Obtidas Por Dois Tipos de Extrator. Braz. J. Food Technol 2010, 13, 156–164. [Google Scholar] [CrossRef]
- Nascimento, A.L.A.A.; Brandi, I.V.; Durães, C.A.F.; de Lima, J.P.; Soares, S.B.; de Carvalho Mesquita, B.M.A. Chemical Characterization and Antioxidant Potential of Native Fruits of the Cerrado of Northern Minas Gerais. Braz. J. Food Technol. 2020, 23, e2019296. [Google Scholar] [CrossRef]
- Esua, O.J.; Makinde, O.O.; Arueya, G.L.; Chin, N.L. Antioxidant Potential, Phytochemical and Nutrient Compositions of Nigerian Hog Plum (Spondias mombin) Seed Kernel as a New Food Source. Int. Food Res. J. 2016, 23, S179–S185. [Google Scholar]
- Pinheiro, G.K.I.; Cabral de Oliveira, D.E.; Resende, O.; Ferreira Junior, W.N.; Cabral, J.C.O.; Quequeto, W.D. Nutritional Properties of Yellow Mombin (’Spondias mombin’ L.) Epicarp Flours by Conventional Drying and Lyophilization. Aust. J. Crop Sci. 2022, 16, 73–78. [Google Scholar] [CrossRef]
- Menezes, P.H.S.; de Souza, A.A.; da Silva, E.S.; de Medeiros, R.D.; Barbosa, N.C.; Garcia Soria, D. Influence of the Maturation Stage on the Physical-Chemical Quality of Fruits of Umbu (Spondias tuberosa). Sci. Agropecu. 2017, 8, 73–78. [Google Scholar] [CrossRef]
- de Ribeiro, L.O.; Pontes, S.M.; de Ribeiro, A.P.O.; Pacheco, S.; Freitas, S.P.; da Matta, V.M. Avaliação Do Armazenamento a Frio Sobre Os Compostos Bioativos e as Características Físico-Químicas e Microbiológicas Do Suco de Umbu Pasteurizado. Braz. J. Food Technol. 2017, 20, e2015095. [Google Scholar] [CrossRef]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2021, 37, 619–655. [Google Scholar] [CrossRef]
- Gadelha, A.J.F.; da Rocha, C.O.; Vieira, F.F.; do Ribeiro, G.N. Avaliação de Parâmetros de Qualidade Físico-Químicos de Polpas Congeladas de Abacaxi, Acerola, Cajá e Caju. Rev. Caatinga 2009, 22, 115–118. [Google Scholar]
- BRASIL. Secretaria de Defesa Agropecuária. Instrução Normativa No 37, de 01 de Outubro de 2018. Estabelece Os Parâmetros Analíticos de Suco e de Polpa de Frutas e a Listagem Das Frutas e Demais Quesitos Complementares Aos Padrões de Identidade e Qualidade Já Fixados. Available online: https://www.legisweb.com.br/legislacao/?id=368178 (accessed on 20 February 2023).
- Pinheiro, G.K.I.; de Oliveira, D.E.C.; Resende, O.; Junior, W.N.F.; Cabral, J.C.O.; Quequeto, W.D.; Silva, L.C.D.M.; Souza, D.G. Physical, Physicochemical and Functional Technological Properties of Flours Produced from Yellow mombin (Spondias mombin L.) Epicarp. Aust. J. Crop Sci. 2022, 16, 177–183. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Eziama, S.C.; Aderibigbe, O.R. Proximate Composition, Physical, Sensory and Microbial Properties of Wheat-Hog Plum Bagasse Composite Cookies. LWT 2021, 141, 111038. [Google Scholar] [CrossRef]
- Rezende, L.; Santos, P.; Riatto, V.; David, J.; David, J. New Alkyl Phenols and Fatty Acid Profile from Oils of Pulped Spondias mombin L. Seed Wastes. Quím. Nova 2018, 41, 540–543. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Nnoruka, M.E.; Tijani, R.O. Phytochemical Constituents, Antioxidant Activity, and Toxicity Assessment of the Seed of Spondias mombin L. (Anacardiaceae). Turk. J. Pharm. Sci. 2020, 17, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Matheus, J.R.V.; de Andrade, C.J.; Miyahira, R.F.; Fai, A.E.C. Persimmon (Diospyros kaki L.): Chemical Properties, Bioactive Compounds and Potential Use in the Development of New Products—A Review. Food Rev. Int. 2022, 38, 384–401. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of Food Processing on Antioxidants, Their Bioavailability and Potential Relevance to Human Health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Ávila, S.; Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C.W.I. The Association between Chromaticity, Phenolics, Carotenoids, and In Vitro Antioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. J. Food Sci. 2014, 79, C510–C516. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.L.; Mazzutti, S.; de Souza, J.A.L.; Ferreira, S.R.S.; Soares, L.A.L.; Stragevitch, L.; Danielski, L. Extraction of Umbu (Spondias tuberosa) Seed Oil Using CO2, Ultrasound and Conventional Methods: Evaluations of Composition Profiles and Antioxidant Activities. J. Supercrit. Fluids 2019, 145, 10–18. [Google Scholar] [CrossRef]
- Costa, G.A.; Mercadante, A.Z. In Vitro Bioaccessibility of Free and Esterified Carotenoids in Cajá Frozen Pulp-Based Beverages. J. Food Compos. Anal. 2018, 68, 53–59. [Google Scholar] [CrossRef]
- de Brito, G.O.; Reis, B.C.; Ferreira, E.A.; Vilela Junqueira, N.T.; Sá-Barreto, L.C.L.; Mattivi, F.; Vrhovsek, U.; Gris, E.F. Phenolic Compound Profile by UPLC-MS/MS and Encapsulation with Chitosan of Spondias mombin L. Fruit Peel Extract from Cerrado Hotspot—Brazil. Molecules 2022, 27, 2382. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Filho, M.J.; da Silva, L.C.; da Constant, L.S.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and Caffeic Acids in 64 Fruits Consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef]
- Galvão, M.S.; Narain, N.; do Socorro Porto dos Santos, M.; Nunes, M.L. Volatile Compounds and Descriptive Odor Attributes in Umbu (Spondias tuberosa) Fruits during Maturation. Food Res. Int. 2011, 44, 1919–1926. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Fidêncio, P.H.; Castricini, A.; Andrade, R.Q.; Silvério, F.O. Comparison of Spondias tuberosa Arruda Accessions by Fruit Volatile Compounds Using Multivariate Analysis. Food Sci. Technol. 2022, 42, e108721. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Ribeiro, L.O.; Souza, É.F.; Penha, E.M.; Matta, V.M.; Freitas, S.P. Effect of Enzymatic Treatment on the Rheological Behavior and Vitamin C Content of Spondias tuberosa (Umbu) Pulp. J. Food Sci. Technol. 2017, 54, 2176–2180. [Google Scholar] [CrossRef]
- Assis, R.C.; de Lima Gomes Soares, R.; Siqueira, A.C.P.; de Rosso, V.V.; de Sousa, P.H.M.; Mendes, A.E.P.; de Alencar Costa, E.; de Góes Carneiro, A.P.; Maia, C.S.C. Determination of Water-Soluble Vitamins and Carotenoids in Brazilian Tropical Fruits by High Performance Liquid Chromatography. Heliyon 2020, 6, e05307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition. Available online: https://www.who.int/publications/i/item/9241546123 (accessed on 22 July 2023).
- Santana Neto, D.C.; da Ferreira, V.C.S.; da Araújo, Í.B.S.; de Meireles, B.R.L.A.; de Cordeiro, Â.M.T.M.; da Silva, F.A.P. Solid–Liquid Extraction of Bioactive Compounds from Spondias mombin L. by-Products: Optimization and Identification of Phenolic Profile. Braz. J. Chem. Eng. 2022, 39, 511–525. [Google Scholar] [CrossRef]
- Ampadu, G.A.A.; Mensah, J.O.; Darko, G.; Borquaye, L.S. Essential Oils from the Fruits and Leaves of Spondias Mombin Linn.: Chemical Composition, Biological Activity, and Molecular Docking Study. Evid. Based Complement. Altern. Med. 2022, 2022, 7211015. [Google Scholar] [CrossRef]
- Alcântara, L.K.S.; Machado, L.F.C.; Ceravolo, I.P.; dos Santos, R.M.; Souza, M.V.D. Phytochemical Aspects, Cytotoxicity and Antimicrobial Activity of t. he Methanolic Extract of Tropical Fruit Pulps on Clinical Isolates of Escherichia Coli. Biointerface Res. Appl. Chem. 2021, 11, 8210–8217. [Google Scholar] [CrossRef]
- Brito, S.A.; Barbosa, I.S.; de Almeida, C.L.; de Medeiros, J.W.; Silva Neto, J.C.; Rolim, L.A.; da Silva, T.G.; Ximenes, R.M.; Menezes, I.R.; Caldas, G.F.; et al. Evaluation of Gastroprotective and Ulcer Healing Activities of Yellow Mombin Juice from Spondias mombin L. PLoS ONE 2018, 13, e0201561. [Google Scholar] [CrossRef] [PubMed]
- Plabon, M.E.; Mondal, S.C.; Or Rashid, M.M.; Chowdhury, M.K.; Saeid, A.; Althobaiti, F.; Dessok, E.S.; Rehmani, M.I.; Mustafa, S.K.; Islam, M.S. Chemical Composition and Anti-Microbial Activity of Hog Plum (Spondias mombin L.) Peel Oil Extracted from Different Regions of Tropical Climates. Horticulturae 2021, 7, 428. [Google Scholar] [CrossRef]
- Moura Neto, L.G.; de Sousa Lira, J.; Torres, M.; Barbosa, I.C.; do Amarante Melo, G.F.; Soares, D.J. Development of a Mixed Drink Made from Hydrosoluble Soybean Extract, Coconut Water and Umbu Pulp (Spondias tuberosa). Acta Sci. Technol. 2016, 38, 371–376. [Google Scholar] [CrossRef]
- Ribeiro, L.O.; Freitas, S.P.; Costa, S.D.O. Bioactive Compounds and Shelf Life of Clarified Umbu Juice. Int. Food Res. J. 2018, 25, 769–775. [Google Scholar]
- de Lima, L.L.A.; Oliveira e Silva, A.M.; Ferreira, I.M.; Nunes, T.P.; de Carvalho, M.G. Néctar Misto de Umbu (Spondias tuberosa Arr. câmera) e Mangaba (Hancornia Speciosa Gomes): Elaboração e Avaliação Da Qualidade. Braz. J. Food Technol. 2018, 21, e2017034. [Google Scholar] [CrossRef]
- Figueiredo, J.S.B.; Santos, G.L.M.; Lopes, J.P.A.; Fernandes, L.B.; Silva, F.N.; Faria, R.B.; Rocha, A.C.S.; Farias, P.K.S.; Lima, W.J.N.; Durães, C.A.F.; et al. Sensory Evaluation of Fermented Dairy Beverages Supplemented with Iron and Added by Cerrado Fruit Pulps. Food Sci. Technol. 2019, 39, 410–414. [Google Scholar] [CrossRef]
- Souza, M.M.B.; Santos, A.M.P.; Converti, A.; Maciel, M.I.S. Optimisation of Umbu Juice Spray Drying, and Physicochemical, Microbiological and Sensory Evaluation of Atomised Powder. J. Microencapsul. 2020, 37, 230–241. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.R.S.; Santos, A.M.; Ferreira, I.M.; e Silva, A.M.O.; Nunes, T.P.; Carvalho, M.G. Elaboração e Avaliação Da Qualidade de Geleia de Umbu (Spondias tuberosa arr. c.) e Mangaba (Hancornia Speciosa g.) Com Alegação Funcional. Segur. Aliment. Nutr. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- da Silva, A.S.; de Santana, L.R.R.; Bispo, E.D.S.; Lopes, M.V. Use of Umbu (Spondias uberosa arr. camara) Pulp for Preparation of Diet Cereal Bar. Rev. Bras. Frutic. 2018, 40, e-540. [Google Scholar] [CrossRef]
- dos Santos Melo, C.; Ferreira, I.M.; Oliveira, A.M.; de Carvalho, M.G. Sorvete de Umbu e Mangaba Com Propriedade Funcional: Processamento e Caracterização. Segur. Aliment. Nutr. 2021, 28, e021028. [Google Scholar] [CrossRef]
- Xavier, V.L.; Feitoza, G.S.; Barbosa, J.; Maria, L.; Araújo, K.S.; Silva, M.V.; Correia, M.T.; Souza, M.P.; Carneiro-da-Cunha, M.D. Nutritional and Technological Potential of Umbu (Spondias tuberosa Arr. Cam.) Processing by-Product Flour. An. Acad. Bras. Cienc. 2022, 94, e20200940. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Queiroz, E.F.; Castro-Gamboa, I.; Siqueira Silva, D.H.; Cuendet, M.; da Silva Bolzani, V.; Wolfender, J.L. Method for Extracting and Isolating Active Substances from Umbu Pulp, Nutraceutical and/or Functional Foods and Cosmetics Comprising Said Active Substances and Uses Thereof. WO 2016/058071A1, 21 April 2016. [Google Scholar]
- Zeraik, M.L.; Queiroz, E.F.; Castro-Gamboa, I.; Siqueira Silva, D.H.; Cuendet, M.; Marcourt, L.; Ciclet, O.; da Silva Bolzani, V.; Wolfender, J.L. Method for Extracting and Isolating Active Substances from Umbu Pulp, Nutraceutical and/or Functional Foods and Cosmetics Comprising Said Active Substances and Uses Thereof. WO 2016/058070A1, 21 April 2016. [Google Scholar]
- Silva, E.C.A.; Conceição, M.M.; Soares, J.K.B.; Silva, M.C.C.; Martins, A.C.S.; Viera, V.B.; Queiroga, R.C.R.E. Elaboração e Processamento de Farinha Da Casca e Do Caroço de Umbu (Spondias tuberosa Arruda Câmara). BR102017026708-3A2, 25 June 2019. [Google Scholar]
- Silva, E.C.A.; Conceição, M.M.; Soares, J.K.B.; Silva, M.C.C.; Queiroga, R.C.R.E.; Santos, A.C. Elaboração e Processamento de Pão de Forma Adicionado Da Farinha Da Casca e Do Caroço Do Umbu (Spondias tuberosa Arruda Câmara). BR 102017026726-1A2, 25 June 2019. [Google Scholar]
- Trindade, A.M.G.; Nunes, K.M.; Ferreira, M.C.A.; Amorim, B.S. Suplemento Alimentar a Base de Frutos da Caatinga. BR 102017010267-0A2, 4 December 2018. [Google Scholar]
- Almeida, M.D.A.; Mata, M.E.R.M.C.M.; Duarte, M.E.M.; Andre, A.M.M.C.N.; Leite Filho, M.T.; Melo, M.O.P.; Sousa, F.M. Umbuzada Em Pó Elaborada Com Leite Caprino. BR 102020019857-2A2, 12 April 2022. [Google Scholar]
- Akinlolu-Ojo, T.; Nwanna, E.E.; Badejo, A.A. Physicochemical Constituents and Anti-Oxidative Properties of Ripening Hog Plum (Spondias mombin) Fruits and the Quality Attributes of Jam Produced from the Fruits. Meas. Food 2022, 7, 100037. [Google Scholar] [CrossRef]
- Moura, L.G.; Rocha, É.M.; Afonso, M.R.; Rodrigues, S.; Costa, J.M. Physicochemical and Sensory Evaluation of Yellow Mombin (Spondias mombin L.) Atomized Powder. Rev. Caatinga 2015, 28, 244–252. [Google Scholar] [CrossRef]
- de Paula, C.M.; dos Santos, K.M.O.; de Oliveira, L.S.; da Silva Oliveira, J.; Buriti, F.C.A.; Saad, S.M.I. Fat Substitution by Inulin in Goat Milk Ice Cream Produced with Cajá (Spondias mombin) Pulp and Probiotic Cultures: Influence on Composition, Texture, and Acceptability among Consumers of Two Brazilian Regions. Emir. J. Food Agric. 2020, 32, 140–149. [Google Scholar] [CrossRef]
- de Santana Neto, D.C.; Cordeiro, Â.M.T.M.; Meireles, B.R.L.A.; Araújo, Í.B.S.; Estévez, M.; Ferreira, V.C.S.; Silva, F.A.P. Inhibition of Protein and Lipid Oxidation in Ready-to-Eat Chicken Patties by a Spondias mombin L. Bagasse Phenolic-Rich Extract. Foods 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.C.A.; Silva, E.L.L.; da Ferreira, V.C.S.; Madruga, M.S.; da Silva, F.A.P. Oxidative Stability of Green Weakfish (Cynoscion virescens) by-Product Surimi and Surimi Gel Enhanced with a Spondias mombin L. Waste Phenolic-Rich Extract during Cold Storage. Food Biosci. 2022, 50, 102021. [Google Scholar] [CrossRef]
- Ferraz, J.L.A.A.; Souza, L.O.; Soares, G.A.; Coutinho, J.P.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Enzymatic Saccharification of Lignocellulosic Residues Using Cellulolytic Enzyme Extract Produced by Penicillium Roqueforti ATCC 10110 Cultivated on Residue of Yellow Mombin Fruit. Bioresour. Technol. 2018, 248, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.C.; da Silva Araújo, J.; de Paiva, W.K.V.; Ribeiro, E.S.S.; de Araújo Padilha, C.E.; de Assis, C.F.; dos Santos, E.S.; de Macêdo, G.R.; de Sousa Junior, F.C. Yellow Mombin Pulp Residue Valorization for Pectinases Production by Aspergillus Niger IOC 4003 and Its Application in Juice Clarification. Biocatal. Agric. Biotechnol. 2020, 30, 101876. [Google Scholar] [CrossRef]
- Marques, G.L.; Aguiar-Oliveira, E. Yellow Mombin and Jackfruit Seeds Residues Applied in the Production of Reducing Sugars by a Crude Multi-Enzymatic Extract Produced by Penicillium Roqueforti ATCC 101110. J. Sci. Food Agric. 2020, 100, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Júnior, S.D.; de Araújo, J.S.; de Asevedo, E.A.; de Medeiros, F.G.M.; dos Santos, V.S.; de Sousa Júnior, F.C.; de Araújo, N.K.; dos Santos, E.S. Exploiting Films Based on Pectin Extracted from Yellow Mombin (Spondias Mombin L.) Peel for Active Food Packaging. Biomass Convers. Biorefin. 2021, 13, 1565–1579. [Google Scholar] [CrossRef]
- Santos, A.; Santos, T.; Lemos, V.; de Souza, A. Yellow Mombin (Spondias mombin L.) Seeds from Agro-Industrial Waste as a Novel Adsorbent for Removal of Hexavalent Chromium from Aqueous Solutions. J. Braz. Chem. Soc. 2021, 32, 437–446. [Google Scholar] [CrossRef]
- Santana Neto, D.C.; da Silva, F.A.P.; da Silva Ferreira, V.C.; da Silva Araújo, I.B.; Fragoso, S.P. Processo de Obtenção de Extrato Antioxidante Derivado de Resíduos Para Aplicação Em Produtos Alimentícios Destinados Ao Consumo Humano e Animal. BR102018067711-0A2, 17 March 2020. [Google Scholar]
- Santana Neto, D.C.; da Silva, F.A.P.; da Silva Ferreira, V.C.; da Silva Araújo, Í.B.; Fragoso, S.P. Hambúrguer Pronto Para Consumo Elaborado Com Antioxidante Natural de Resíduos. BR 102018072527-0A2, 26 May 2020. [Google Scholar]
- Souza, A.K.S.; Costa, C.B.; Stuker, C.Z.; Ferrari, C.R.; de Oliveira, D.H.; Zimbardi, D.; Shigeru Takano, F.; Reigada, J.B.; Arcuri, H.A.; Reigada, J.; et al. Method for Obtaining Bioactive Ingredients, Use of Subcritical-Water Extraction Process, Bioactive Ingredient, Use of Bioactive Ingredients and Cosmetic Composition. WO 2021/000037A1, 7 January 2021. [Google Scholar]
- Silva, M.E.P.; Gadelha, A.J.F.; Silva, J.M.A.; Rocha, C.O.; Batista, F.F.; Silva, H.A. Biossorvente Obtido a Partir Do Endocarpo Do Cajá (Spondias mombin L.) e Processo de Tratamento de Água e Efluentes Utilizando o Mesmo Biossorvente. BR 1020210192739A, 11 April 2023. [Google Scholar]
- Cornélio, M.L.; Guedes, L.M. Processo e Composição Cosmética e Dermatológica Para Hidratação e Elasticidade Da Pele Humana. BR102019017094-8A2, 2 March 2021. [Google Scholar]
- Pinedo, A.A.; Pereira, C.F.; Arévalo, Z.D.S. Elaboração de Bebida Fermentada à Base de Suco de Cajá (Spondias mombin L.). BR 102020002833-2A2, 24 August 2021. [Google Scholar]
- Rodrigues, T.J.A.; ALbuquerque, A.P.; Pasquali, M.A.B.; Rocha, A.T.; Araujo, G.T. Processo de Obtenção de Polpas Probióticas, Em Pó, de Frutas Spondias Por Secagem Em Leito de Jorro. BR 102021006902-3A2, 11 October 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).