Simple Summary
Pugionium cornutum is a typical xerophyte indigenous to arid and semi-arid regions of northwest China, exhibiting remarkable adaptability to drought and salt stresses. Unlike salt-sensitive plants, maintaining a stable NO3− concentration in the shoots is an important physiological strategy underlying the salt stress adaptation of P. cornutum. This study aimed to investigate the role of PcNPF2.7 from P. cornutum in the NO3− homeostasis and salt tolerance. Our results demonstrated that PcNPF2.7, which was mainly expressed in the stele tissue of the roots of P. cornutum, facilitates root-to-shoot NO3− transport and modulates Na+ accumulation in the shoot, thus contributing to the salt tolerance of the plant. The findings provide a potential candidate gene for improving salt tolerance in crops.
Abstract
Nitrate Excretion Transporter 1 (NAXT1/NPF2.7) is known to regulate NO3− transport in Arabidopsis, a salt-sensitive glycophyte that exhibits a significant reduction in the NO3− content under salt stress. However, its role in the NO3− homeostasis and salt tolerance of xerophytes, which exhibit strong stress tolerance, remains unclear. In the present study, we cloned the NPF2.7 homolog (PcNPF2.7) from the xerophyte Pugionium cornutum, which exhibits stable NO3− content in the shoot under salt stress, and investigated its function in ion homeostasis and salt tolerance. PcNPF2.7 was specifically expressed in the stele tissue of roots and localized to the plasma membrane; its expression level in the roots was significantly induced by NaCl and NaNO3 treatments. PcNPF2.7 overexpression driven by a stelar-specific promoter significantly increased NO3− accumulation and reduced Na+ levels in the shoots of Arabidopsis under 75 mM NaCl or NaNO3 treatments, resulting in an enhanced salt tolerance. Furthermore, PcNPF2.7 overexpression significantly induced AtHKT1;1, which mediates the unloading of Na+ from xylem in the roots. Taken together, our findings showed that PcNPF2.7 facilitates the transport of NO3− from the roots to the shoots and indirectly reduces Na+ accumulation in the shoot, therefore contributing to the salt tolerance in plants.
1. Introduction
Soil salinization is a major abiotic stress that inhibits crop growth and development, posing a serious threat to global food security [1]. Developing stress-tolerant crops through genetic approaches would constitute a sustainable solution for ensuring global food security [2,3]. The exploration of salt-tolerant genetic resources serves as a crucial prerequisite for such breeding practices [3,4]. Given the limited genetic potential of salt tolerance in traditional crops, extremophile plants such as xerophytes that can thrive in harsh environments represent a crucial genetic reservoir that can be utilized to enhance salt tolerance in crops [5,6,7]. Therefore, identifying and utilizing the salt tolerance genes of these plants is critical for enhancing the crop salt tolerance [8,9,10].
The perennial herb Pugionium cornutum L. Gaertn, belonging to the Brassicaceae family, is a typical xerophyte indigenous to the arid and semi-arid regions of northwest China [11,12,13,14,15]. This species exhibits remarkable adaptability to drought and salt stresses [11,12,13,14,15]. The crucial role of P. cornutum in water and soil conservation, as well as its utilization as a vegetable, forage, and traditional Chinese medicinal herb, renders it a crucial ecological barrier and resource plant [16,17]. Previous studies demonstrated that P. cornutum is a typical chloride (Cl−)-tolerant species, as it possesses a strong ability to efficiently absorb and accumulate significant quantities of Cl− in the shoot for osmotic adjustment [12,13,14,18]. The enhanced Cl− uptake and accumulation in most salt-sensitive plants has been shown to be commonly accompanied by a significant reduction in NO3− accumulation under salt stress, which, in turn, inhibits the growth of these plants under saline conditions [19,20]. Unlike most salt-sensitive species, P. cornutum can maintain stable NO3− concentrations in shoots when accumulating large amounts of Cl− for osmotic adjustment under high salt conditions, indicating its strong capacity to maintain shoot NO3− homeostasis in the presence of high Cl− accumulation [12]. However, the molecular mechanisms underlying the maintenance of NO3− homeostasis in P. cornutum under salt stress are poorly understood.
Researchers have identified a series of transporters or channels involved in NO3− transport in plants [21,22,23]. Nitrate Transporter 1/Peptide Transporter Family (NRT1/PTR Family or NPF) has been defined, based on sequence homology and substrate specificity, as NO3− transporters or peptide transporters [24,25]. The model plant Arabidopsis harbors 53 NPF members, including the Nitrate Excretion Transporters (NAXT) subclass members [24,25]. The genes encoding the members of this subclass are tandemly distributed on chromosome 3 and have been proposed to have evolved through gene duplication after speciation events [26]. The NAXT subclass is named after the identification of its first member, NAXT1 (subsequently named NPF2.7), and comprises seven members (named AtNPF2.1~AtNPF2.7) in Arabidopsis. Among them, the functional characteristics of three members, AtNPF2.1, AtNPF2.2, and AtNPF2.6, have not yet been identified. Among the remaining four members, AtNPF2.7 and AtNPF2.3 are reportedly involved in NO3− efflux from the root system and long-distance transport of NO3− from the roots to the shoots, respectively [27,28]. In addition, AtNPF2.5 and AtNPF2.4 are involved in Cl− efflux from the root system and long-distance transport of Cl− from the roots to the shoots, respectively [29,30]. However, current studies on the functions of NAXT subclass members have mainly focused on Arabidopsis, which exhibits a significant reduction in NO3− concentration under salt stress. However, the role of the NAXT members in the NO3− homeostasis and salt tolerance in P. cornutum, which exhibits extremely strong salt tolerance and can maintain stable NO3− levels under salt stress, remains unclear.
In the present study, we isolated the NPF2.7 homolog (PcNPF2.7) from P. cornutum, and analyzed its expression patterns, tissue and subcellular localization, and then investigated its role in the salt tolerance and ion accumulation characteristics after expressing it into Arabidopsis.
2. Materials and Methods
2.1. Isolation of PcNPF2.7 from P. cornutum
After germination at 28 °C for 5 d, uniform P. cornutum seedlings were transferred to plastic pots (6 cm × 6 cm × 5 cm) filled with coarse-grained silica sand, with one seedling per pot. The seedlings were irrigated with 1/2 Hoagland nutrient solution (containing 2 mM KNO3, 0.5 mM KH2PO4, 0.5 mM MgSO4, 0.5 mM Ca(NO3)2, 60 μM Fe-citrate, 50 μM H3BO3, 10 μM MnCl2, 1.6 μM ZnSO4, 0.6 μM CuSO4, and 0.05 μM Na2MoO4, pH = 5.7), and cultivated in a greenhouse as described previously [18]. After 4 weeks, the total RNA was isolated from the root tissues using a RNAprep Pure Plant Kit (TaKaRa, Dalian, China). Then the reverse transcription was performed using a SMART RACE cDNA Kit (Clontech, Mountain View, CA, USA) to create 5′- and 3′-RACE cDNA template. Based on the specific fragment of PcNPF2.7 obtained from the transcriptomic data of P. cornutum [13], the 5′-end cDNA and 3′-end cDNA fragments were cloned with the nested PCR method [18]. The primer pairs for the nested amplification of the 5′-end cDNA were P1/P2 and P3/P4 (Table S1), and those for the nested amplification of 3′-end cDNA were P2/P5 and P4/P6 (Table S1). These three fragments were assembled to obtain the full-length cDNA sequence of PcNPF2.7.
2.2. Bioinformatics Analysis
Multiple alignments were conducted with other NAXT proteins in Brassicaceae plants using the DNAMAN (v6.0) software (Lynnon Biosoft, San Ramon, CA, USA). The phylogenetic tree was generated by the MEGA (v7.0) software (Premier Biosoft International, Palo Alto, CA, USA) using the maximum-likelihood method and 1000 bootstrap replicates. The transmembrane-domain prediction was performed with TMHMM Server v2.0 (http://www.cbs.dtu.dk/services/TMHMM/) (accessed on 10 December 2024).
2.3. Quantitative Real-Time PCR (qRT-PCR) of PcNPF2.7 in Response to NaCl and NaNO3 Treatments
To investigate the response of PcNPF2.7 in P. cornutum to salt treatments, 4-week-old seedlings were exposed to 1/2 Hoagland solution supplemented with either 25 mM or 50 mM NaCl (Cl− stress) and 25 mM or 50 mM NaNO3 (NO3− stress). Roots and shoots were harvested at 0, 3, 6, 12, 24, and 48 h after applying the respective NaCl or NaNO3 treatments. Total RNA in these samples was extracted, and cDNA was synthesized by reverse transcription using PrimeScript™ RT master mix (Perfect Real Time; TaKaRa, Dalian, China). Subsequently, qRT-PCR was performed by a StepOnePlus Real-Time PCR Thermocycler (PRISM 7500, Applied Biosystems, Foster City, CA, USA). PcACTIN2 [15] was used as the reference gene for RNA normalization. TB GreenTM Premix Ex TaqTM mix (Takara, Dalian, China) and the specific primer pairs for PcACTIN2 and PcNPF2.7 (P7/P8 and P9/P10, respectively; Table S1) were used for PCR. Finally, the relative expression level of PcNPF2.7 was calculated using the 2−ΔΔCt method [31]. All reactions were performed with three technical replicates (n = 3), and the experiments were repeated three times.
2.4. In Situ PCR
To investigate the tissue-specific expression of PcNPF2.7 in P. cornutum roots, in situ PcNPF2.7 mRNA levels in the root cross-sections were amplified as described previously [32]. Briefly, roots from 3-week-old seedlings were fixed overnight on ice in a solution containing 63% (v/v) ethanol, 5% (v/v) acetic acid, and 2% (v/v) formaldehyde. The fixed roots were embedded in 5% (w/v) agarose, and 50 µm-thick sections were prepared using a microtome (RM2245, Leica, Wetzlar, Germany). The sections were subjected to genomic DNA digestion using DNase I (TaKaRa, Dalian, China), followed by first-strand cDNA synthesis. Sections not subjected to reverse transcription (NO-RT) served as a negative control. PCR was performed to label the PcNPF2.7 mRNA or 18S RNA (positive control) in the root sections with digoxin. Gene-specific primers for PcNPF2.7 (P11 and P12) and 18S RNA (P13 and P14) are listed in Table S1. Finally, the samples were stained using BM purple AP substrate (Sigma, Darmstadt, Germany) for 30 min, and the signal was observed using a fluorescence microscope (DM6B/DFC7000T, Leica, Wetzlar, Germany).
2.5. Transient Gene Expression in Tobacco
To determine the subcellular localization of PcNPF2.7, its full-length coding sequence was amplified from P. cornutum cDNA via PCR with Primers P15 and P16 (Table S1). Then, the PcNPF2.7 fragment was fused into the plant expression vector p35S-eGFP carrying green fluorescent protein using the In-Fusion HD cloning kit (TaKaRa, Dalian, China) to obtain the recombinant vector p35S-PcNPF2.7-eGFP. Subsequently, the vectors p35S-eGFP and p35S-PcNPF2.7-eGFP were transformed into Agrobacterium tumefaciens (GV3101) via the heat shock method [14], and the bacterial solution was slowly injected into the tobacco (Nicotiana benthamiana) leaves according to the method described previously [33]. The green fluorescent protein (GFP) signal was observed using a confocal laser scanning microscope system (SP8 SR, Leica, Wetzlar, Germany) after infiltration for 3 days.
2.6. Functional Characterization of PcNPF2.7 in Arabidopsis
The effects of PcNPF2.7 on plant growth, ion accumulation, and the expression of key genes involved in NO3− and Na+ transport were investigated by ectopically transforming PcNPF2.7 into Arabidopsis. As PcNPF2.7 is predominantly expressed in the root stele of P. cornutum (as shown in the results), to avoid any potential effects on ion transport caused by non-targeted gene expression in other tissues, a root stelar-specific promoter in Arabidopsis (the promoter of AtNPF2.3, which is only expressed at the root stele and can maintain stable expression levels under NaCl treatment) was used to drive PcNPF2.7 expression [28]. The AtNPF2.3 (pAtNPF2.3) promoter was obtained using the primers P17 and P18 (Table S1). Then, the promoter sequence of pAtNPF2.3, followed by the full-length coding region of PcNPF2.7, was fused into the plant expression vector pBIB-Basta using the In-Fusion HD cloning kit with primer pairs P19 and P20 (Table S1). The recombinant vector was introduced into A. tumefaciens GV3101 and then transformed into wild-type Arabidopsis (Col-0) using the floral-dip method [34]. The relative expression level of PcNPF2.7 in the roots and shoots of 3-week-old T3 homozygous transgenic lines was analyzed by qRT-PCR. Two transgenic lines (labeled as OE1 and OE2) were randomly selected for subsequent experiments.
To analyze the effects of stelar-specific expression of PcNPF2.7 on the salt tolerance of Arabidopsis, the wild-type (WT) and transgenic lines (OE1 and OE2) were grown hydroponically following germination, as previously described [15]. Three-week-old seedlings were then treated with 1/2 Hoagland solution (control) or 1/2 Hoagland solution containing 25 or 75 mM NaCl, or 25 or 75 mM NaNO3. After being treated for 1 week, the dry weight of the shoots and roots were measured. The relative plasma membrane permeability and malondialdehyde (MDA) content in the leaves were measured as described previously [35,36]. Six biological replicates were performed for the abovementioned physiological parameters (n = 6).
The Cl− content in oven-dried tissues was determined using a chloride analyzer (Model 926, Sherwood Scientific Ltd., Cambridge, UK) [30], and the NO3− content was determined by the colorimetric method using a UV spectrophotometer (UV-2102 C, Unico Instrument Co., Ltd., Shanghai, China) as described previously [37]. The K+ and Na+ contents were determined using a flame spectrophotometer (Model 410 Flame; Sherwood Scientific, Ltd., Cambridge, UK), according to the method described previously [15]. Six biological replicates were performed for the abovementioned ion contents (n = 6).
2.7. Analysis of the Abundance of Na+ and NO3− Transport-Related Genes
To analyze the effects of stelar-specific expression of PcNPF2.7 on the expression of key genes involved in Na+ and NO3− transport under salt stress, 3-week-old seedlings of WT, OE1, and OE2 grown in hydroponics were exposed to 1/2 Hoagland solution supplemented with 75 mM NaCl or 75 mM NaNO3 for 24 h. Then, the roots and shoots were immediately harvested, and the relative expression levels of the genes encoding Na+ transporters [AtNHX1 (AT5G27150), AtSOS1 (AT2G01980), and AtHKT1;1 (AT3G62260)] and NO3− transporters [AtNPF2.3 (AT3G45680), AtNRT1.5 (AT1G32450), and AtCLCa (AT5G40890)] were determined by qRT-PCR as described previously in “2.3”. AtACTIN2 (AT3G18780) was used for internal standard, and the specific primers were listed in Table S1. All reactions were performed with three technical replicates (n = 3), and the experiments were repeated three times.
2.8. Data Analysis
All the data are presented as means with standard deviation, and data analysis was performed by analysis of variance using SPSS statistical software (v25.0, SPSS Inc., Chicago, IL, USA). Duncan’s multiple range tests were used to detect differences among means at a significance level of p < 0.05.
3. Results
3.1. Cloning and Characterization of PcNPF2.7
The full-length cDNA of PcNPF2.7 is 1815 bp, comprising a 61-bp 5′ untranslated region, a 1686-bp open reading frame that encodes a protein consisting of 561 amino acid residues, and a 68-bp 3′ untranslated region (Figure S1). Phylogenetic analysis showed that, among the NAXT proteins in Arabidopsis, PcNPF2.7 exhibited a strong evolutionary relationship with AtNPF2.7 (Figure S2). The alignment revealed that PcNPF2.7 shared over 93% sequence identity with NPF2.7 proteins from Brassicaceae species, including A. lyrata subsp., Brassica napus, Eutrema salsugineum, and Capsella rubella, with all proteins containing 12 transmembrane domains (Figure S3).
3.2. The Expression Pattern of PcNPF2.7
Under the control condition (no additional salts), the relative expression level of PcNPF2.7 in P. cornutum roots was significantly higher than in shoots (Figure 1a). Subsequently, the relative PcNPF2.7 levels in the roots of P. cornutum were analyzed after 3, 6, 12, 24, and 48 h of treatment with 25 and 50 mM NaCl. The results showed that the expression abundance of PcNPF2.7 in the roots of P. cornutum first increased, peaking at 24 and 6 h, respectively, and then decreased under 25 and 50 mM NaCl treatment (Figure 1b). Furthermore, we analyzed the changes in the expression level of PcNPF2.7 in the roots of P. cornutum after 25 and 50 mM NaNO3 treatment. The results showed that under 25 mM NaNO3 treatment, the expression abundance of PcNPF2.7 in the roots of P. cornutum first increased, peaking at 6 h, and then decreased to the level before the treatment (Figure 1c). Under 50 mM NaNO3 treatment, the PcNPF2.7 levels in the roots of P. cornutum increased at 3 h, then gradually decreased, and then tended to stabilize with the extension of the treatment time (Figure 1c). These results indicated that NaCl and NaNO3 treatment induced PcNPF2.7 expression in the roots.
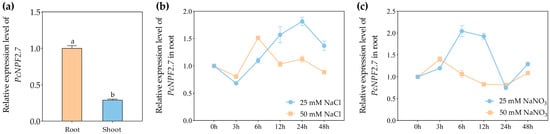
Figure 1.
The expression analysis of PcNPF2.7 in 4-week-old P. cornutum. (a) The expression level of PcNPF2.7 in the root and shoot under control conditions. The expression level in the root was considered “1”. Different letters on the columns indicate significant differences (p < 0.05, n = 3). (b) The expression level of PcNPF2.7 in the root under 25 mM or 50 mM NaCl treatment for 3, 6, 12, 24, and 48 h. (c) The expression level of PcNPF2.7 in the root under 25 mM or 50 mM NaNO3 treatment for 3, 6, 12, 24, and 48 h. The expression level under 25 mM NaCl or NaNO3 at 0 h was considered “1” in (b,c), respectively. All experiments were repeated three times with similar results.
3.3. Tissue and Subcellular Localization of PcNPF2.7
We analyzed the tissue localization of PcNPF2.7 in the roots of P. cornutum using in situ PCR. In the positive (18S) and negative (NO-RT) controls, all and none of the root cells were stained blue, respectively. However, the transcript of PcNPF2.7 was only stained blue in the root stele (Figure 2). This finding indicated that, unlike AtNPF2.7, which is primarily expressed in the cortical cells of the root of Arabidopsis [27], PcNPF2.7 was specifically expressed in the stelar tissue of P. cornutum roots.
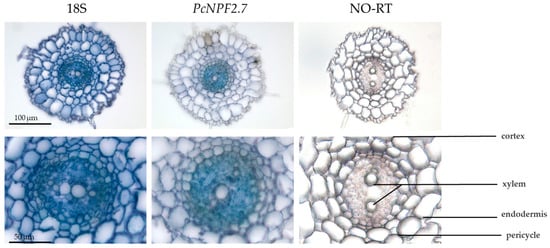
Figure 2.
In situ PCR analysis of PcNPF2.7 in the root of P. cornutum. 18S RNA is the positive control expressed in all cells. NO-RT denotes the negative control without reverse transcription. The blue signal indicates the gene expression location.
In order to determine the subcellular localization of PcNPF2.7, we constructed a GFP-PcNPF2.7 fusion protein and transiently expressed it in tobacco epidermal cells. As shown in Figure 3, the fluorescence of GFP was observed in the cytoplasm, plasma membrane, and nucleus of the leaf epidermal cells of tobacco that were transiently transformed with the empty vector (labeled p35S-eGFP). However, under transient expression of the PcNPF2.7-GFP fusion protein (labeled p35S-PcNPF2.7-eGFP), the green fluorescence signal was localized only on the plasma membrane of tobacco mesophyll cells (Figure 3), indicating that PcNPF2.7 was located on the plasma membrane.
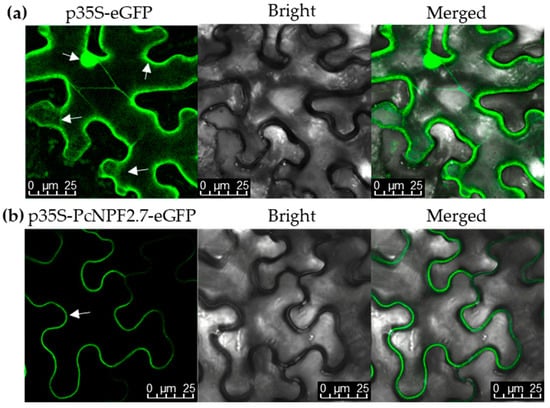
Figure 3.
Subcellular location of PcNPF2.7 in leaf epidermal cells of tobacco. (a) The images of fluorescence signal elicited by the transient expression of empty vector. (b) The images of fluorescence signal elicited by the transient expression of PcNPF2.7, indicating that PcNPF2.7 was localized on the plasma membrane. The p35S-eGFP and p35S-PcNPF2.7-eGFP are the images of fluorescence signal elicited by the transient expression of empty vector and PcNPF2.7-eGFP fusion protein, respectively. Bright field is the image of leaf epidermal cell morphology under bright field condition. Merge is the image after fusing the fluorescence signal with bright field. The arrows show the fluorescence locations.
3.4. Effects of PcNPF2.7 Expression on the Growth of Transgenic Lines of Arabidopsis Under NaCl Treatment
The effect of PcNPF2.7 on salt tolerance and ion accumulation was investigated by overexpressing it in Arabidopsis under the control of the AtNPF2.3 promoter, which is only expressed at the root stele and could maintain stable expression levels under salt stress [37,38]. Two transgenic lines, OE1 and OE2, were randomly selected in the experiments (Figure S4).
Under control conditions, there were no obvious differences in the growth of WT, OE1, and OE2 lines (Figure 4). Under 25 or 75 mM NaCl treatment, the transgenic lines exhibited better growth than WT (Figure 4). Moreover, under the control condition, there was no significant difference in the dry weight of WT, OE1, and OE2 lines (Figure 5a,b). Under 25 mM or 75 mM NaCl treatments, the transgenic lines (OE1 and OE2) exhibited higher shoot dry weight compared to WT (Figure 5a). And the root dry weight of OE1 and OE2 was significantly higher than that of WT under 75 mM NaCl treatment (Figure 5b).

Figure 4.
The growth phenotypes of 3-week-old WT and transgenic lines of Arabidopsis with stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 and 75 mM NaCl treatment for 7 d.
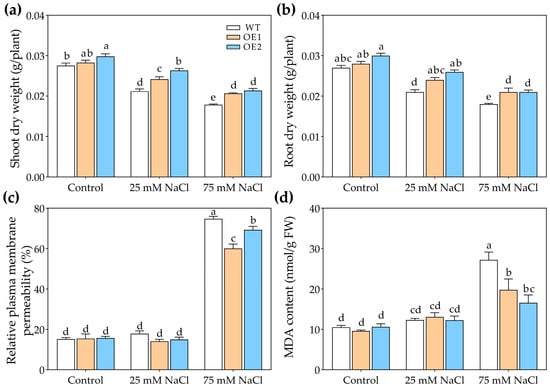
Figure 5.
The growth parameters of 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 mM and 75 mM NaCl for 7 d. (a) Shoot dry weight. (b) Root dry weight. (c) Relative plasma membrane permeability of the leaves. (d) MDA content in the leaves. Six biological replicates were performed for the abovementioned physiological parameters (n = 6). Different letters on the columns indicate significant differences (p < 0.05).
In order to further determine the effects of PcNPF2.7 expression on the salt tolerance of A. thaliana, we measured the relative plasma membrane permeability and malondialdehyde (MDA) content. The results indicated no significant differences in the relative plasma membrane permeability and MDA content of the leaves between the PcNPF2.7 overexpression lines and the WT under control treatment and 25 mM NaCl treatment (Figure 5c,d). While under 75 mM NaCl treatment, the relative plasma membrane permeability and MDA content in the leaves of the PcNPF2.7 overexpression lines were significantly lower than those of the WT (Figure 5c,d), indicating a lower degree of cell membrane damage in the PcNPF2.7 overexpression lines under higher NaCl concentration.
These results indicated that the ectopic expression of PcNPF2.7 enhanced the tolerance of A. thaliana to NaCl stress.
3.5. Effects of PcNPF2.7 Expression on the Cl−, NO3−, Na+, and K+ Ion Accumulation in Transgenic Lines of Arabidopsis Under NaCl Treatment
Regardless of the control or NaCl treatments, no significant difference in the Cl− content of the shoots was observed among OE1, OE2, and WT, whereas the Cl− content in the roots of OE1 and OE2 was lower than that of the WT under NaCl treatments (Figure 6a). Under control conditions, no significant differences were found in the NO3− content in the roots and shoots of OE1, OE2, and WT (Figure 6b). Under 25 or 75 mM NaCl treatments, the NO3− content in the shoots of OE1 and OE2 was higher compared to the WT; although no significant difference was observed in the NO3− content in the roots of WT, OE1, and OE2 (Figure 6b). These results indicated that the ectopic expression of PcNPF2.7 increased NO3− accumulation in the shoot but reduced Cl− accumulation in the root under NaCl stress.
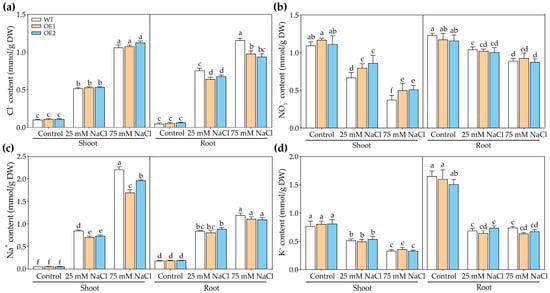
Figure 6.
The ion concentration in 3-week-old WT and transgenic lines of Arabidopsis with stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 and 75 mM NaCl treatment for 7 d. (a) Cl− content. (b) NO3− content. (c) Na+ content. (d) K+ content. Six biological replicates were performed for the abovementioned ion contents (n = 6). Different letters on the columns indicate significant differences (p < 0.05).
Under control conditions, there were no significant differences in the Na+ content in the roots or shoots of OE1, OE2, and WT (Figure 6c). Interestingly, under 25 or 75 mM NaCl treatments, the Na+ content in the shoots of OE1 and OE2 was lower compared to the WT (Figure 6c). Regardless of the control or NaCl treatment, no significant differences were observed in the K+ content in the roots or shoots of OE1, OE2, and WT (Figure 6d). These observations demonstrated that although the stelar-specific overexpression of PcNPF2.7 did not affect K+ accumulation in plants, it reduced Na+ accumulation in the shoots.
3.6. Effects of PcNPF2.7 Expression on the Expression of Genes Related to NO3− and Na+ Transport in Transgenic Lines of Arabidopsis Under NaCl Treatments
Next, we explored the reason underlying the elevation of NO3− content induced by stelar-specific overexpression of PcNPF2.7 in NaCl-treated shoots. We analyzed the expression levels of AtNPF2.3 and AtNRT1.5/AtNPF7.3, which have been shown to mediate xylem loading of NO3− [28,39] in roots of OE1, OE2, and WT under 75 mM NaCl treatment. In addition, we analyzed the expression of AtCLCa, which is involved in vacuolar NO3− compartmentation [40], in the shoots. The results showed that the expression levels of AtNPF2.3 and AtNRT1.5 in the roots and AtCLCa in the shoots of OE1 and OE2 were comparable to those in WT under control conditions or NaCl treatments, (Figure S5). These findings indicated that the increase in NO3− content in the shoots of OE1 and OE2 was directly associated with PcNPF2.7 expression, but it was not associated with the expression of other key genes involved in xylem loading of NO3− and vacuolar NO3− compartmentation.
We also analyzed the reason for the decrease in Na+ content mediated by PcNPF2.7 overexpression in the shoots of NaCl-treated plants. For this purpose, we compared the expression of genes encoding Na+ transporters, including AtNHX1 (involved in vacuolar Na+ compartmentation) [41], AtSOS1 (mediates root Na+ efflux) [42], and AtHKT1.1 (involved in the unloading of Na+ from xylem to parenchyma cells) [43], in OE1, OE2, and WT. The results showed that regardless of control or NaCl treatment, the relative abundances of AtNHX1 in the shoots and AtSOS1 in the roots of OE1 and OE2 were comparable to those observed in the WT (Figure 7a,b). However, under 75 mM NaCl treatment, the AtHKT1;1 level in the roots of OE1 and OE2 were significantly higher than that in WT (Figure 7c), indicating that the stelar-specific PcNPF2.7 overexpression might be induced by the expression of AtHKT1;1 in the roots, leading to more Na+ unloading from xylem to parenchyma cells, thus reducing Na+ transport to the shoots and decreasing Na+ levels in the shoots.
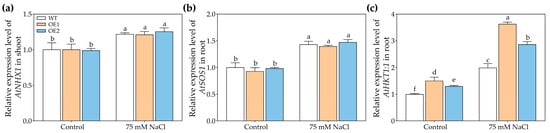
Figure 7.
The expression level of key genes associated with Na+ transport in 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 75 mM NaCl for 24 h. (a) The expression level of AtNHX1 in shoot. (b) The expression level of AtSOS1 in root. (c) The expression level of AtHKT1;1 in root. Different letters on the columns indicate significant differences (p < 0.05, n = 3). All experiments were repeated three times with similar results.
3.7. Effects of PcNPF2.7 Expression on the Growth of Transgenic Lines of Arabidopsis Under NaNO3 Treatments
Since PcNPF2.7 expression in P. cornutum roots was also induced by NaNO3 treatments, we speculated that PcNPF2.7 might play a role in the response of plants to external NO3− changes. Therefore, we compared the differences in the growth and ion accumulation among OE1, OE2, and WT under NaNO3 treatments. The results showed comparable growth of WT, OE1, and OE2 under control conditions (Figure 8). However, the transgenic lines exhibited better growth than WT under NaNO3 treatments (Figure 8). The shoot dry weight and the root dry weight of OE1 and OE2 were higher than those of WT under 25 mM or 75 mM NaNO3 treatments (Figure 9a,b), indicating that PcNPF2.7 overexpression improved the resistance of A. thaliana to high concentrations of NaNO3. Under the control and 25 mM NaNO3 treatment, the relative plasma membrane permeability and MDA content in the leaves were comparable among all three lines (Figure 9c,d). Under 75 mM NaNO3 treatment, the transgenic lines exhibited significantly lower relative plasma membrane permeability and MDA content in the leaves compared to the WT (Figure 9c,d). Therefore, PcNPF2.7 overexpression lines exhibited a lower degree of cell membrane damage under 75 mM NaNO3 treatment.

Figure 8.
The growth phenotypes of 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 mM and 75 mM NaNO3 for 7 d.
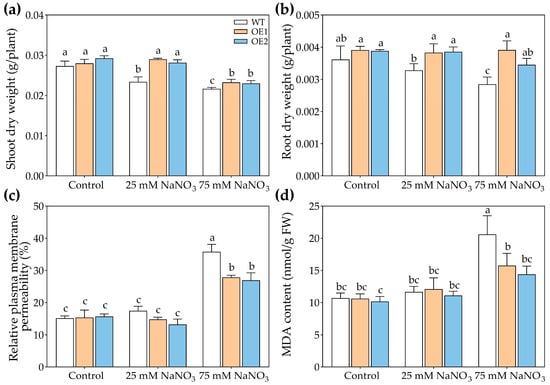
Figure 9.
The growth parameters of 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 mM and 75 mM NaNO3 for 7 d. (a) Shoot dry weight. (b) Root dry weight. (c) Relative plasma membrane permeability of the leaves. (d) MDA content in the leaves. Six biological replicates were performed for the abovementioned physiological parameters (n = 6). Different letters on the columns indicate significant differences (p < 0.05).
3.8. Effects of PcNPF2.7 Expression on the NO3−, Na+, and K+ Ion Accumulation and Salt Tolerance in Transgenic Lines of Arabidopsis Under NaNO3 Treatments
Ion content analysis revealed that under control conditions, the NO3−, Na+, and K+ contents in the roots and shoots of PcNPF2.7 overexpression lines were comparable to those of WT (Figure 10). However, under 25 mM NaNO3 treatment, NO3− contents in the shoots and roots of transgenic lines were significantly higher and lower compared to WT, respectively (Figure 10a). Under 75 mM NaNO3 treatment, the shoots of OE1 and OE2 still exhibited higher NO3− content compared to WT (Figure 10a). Under 25 mM and 75 mM NaNO3 treatment, Na+ content in the shoots of OE1 and OE2 was significantly lower (by 27% and 33%, respectively) compared to WT, with a more prominent decrease under higher NaNO3 concentration (Figure 10b). Taken together, the stelar-specific overexpression of PcNPF2.7 increased NO3− content and decreased Na+ content in the shoots of plants treated with higher NaNO3 concentration. In addition, the K+ contents in the roots and shoots were comparable among WT, OE1, and OE2 under either control or NaNO3 treatments (Figure 10c).
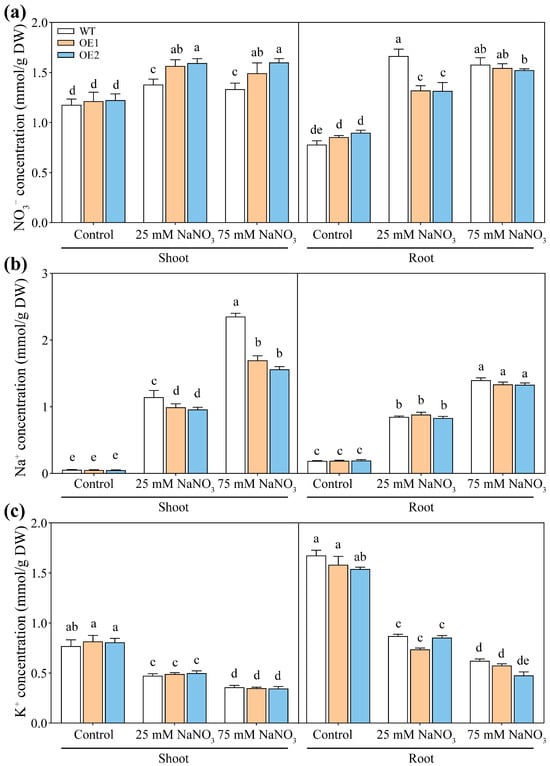
Figure 10.
The ion concentration in 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 25 mM and 75 mM NaNO3 for 7 d. (a) NO3− content. (b) Na+ content. (c) K+ content. Six biological replicates were performed for the abovementioned ion contents (n = 6). Different letters on the columns indicate significant differences (p < 0.05).
3.9. Effects of PcNPF2.7 Expression on the Expression of Genes Related to NO3− and Na+ Transport Under NaNO3 Treatment
In order to investigate the effects of stelar-specific overexpression of PcNPF2.7 on NO3− and Na+ content under NaNO3 treatments, we performed qRT-PCR to analyze the levels of key genes involved in NO3− and Na+ transport in transgenic lines. The results showed that under 75 mM NaNO3 treatment, the expressions of AtNPF2.3 and AtNRT1.5/AtNPF7.3 were comparable in the roots of all three lines (Figure S6a,b); however, the transgenic lines exhibited higher AtCLCa levels in the shoots compared to WT (Figure S6c). Furthermore, under 75 mM NaNO3 treatment, AtNHX1 levels in the shoots and AtSOS1 levels in the roots of OE1 and OE2 were comparable to those of WT (Figure S6d,e), and AtHKT1;1 levels in the roots of OE1 and OE2 were significantly higher than that in WT (Figure S6f).
4. Discussion
The tissue-specific localization of genes encoding ion transport proteins or channels determines their functions in ion transport at the whole-plant level. Even when homologous proteins exhibit identical functions at the cellular level, their roles in ion transport within the plant can vary significantly due to the varied expression patterns of the encoding genes across different plant species [43,44]. For instance, the HKT homologous proteins in some plants mediate Na+ absorption at the cellular level [43]. However, in A. thaliana, the homologous gene AtHKT1;1 is primarily expressed in the parenchyma cells around the xylem of the root, and the encoded protein mainly participates in mediating the unloading of Na+ from the xylem sap to the parenchyma cells [44]. While in rice and wheat, the HKT2;1 homologous genes are predominantly expressed in the epidermal and cortical cells of the root, mediating the absorption of low Na+ concentration by the root from the external medium [44]. The in situ PCR analysis in the present study showed that PcNPF2.7 is primarily expressed in the stelar cells of the root of P. cornutum (Figure 2), different from the tissue localization of AtNPF2.7 in A. thaliana (mainly expressed in the cortical cells of the root) [27]. Thus, the function of PcNPF2.7 in the ion transport in P. cornutum root might vary from that of AtNPF2.7 in A. thaliana. Studies have shown that ion transport proteins or channels located in the xylem tissue of plant roots play a key role in regulating the ion transport and distribution in the roots and shoots of plants [45]. qRT-PCR analysis indicated that PcNPF2.7 expression in the roots of P. cornutum was significantly induced by NaCl and NaNO3 (Figure 1b,c), and thus, we speculated that PcNPF2.7 might participate in the transport and distribution of ions between the root and shoots of P. cornutum under NaCl and NaNO3 treatments.
Studies in A. thaliana have found that among the NAXT family members, AtNPF2.3 can mediate NO3− efflux at the cellular level [28]. Further analysis revealed that AtNPF2.3 is mainly expressed in the stelar cells of the roots, and the encoded protein is located in the plasma membrane [28]. Compared to the WT plants, salt-treated atnpf2.3 mutant lines exhibited significantly decreased NO3− content in the shoots, with significantly lower salt tolerance [28]. This finding indicated that AtNPF2.3 participates in the long-distance transport of NO3− from the roots to the shoots under salt stress, and plays an important role in the salt tolerance of A. thaliana [28]. In the current study, we found that, similar to AtNPF2.3, PcNPF2.7 was located in the plasma membrane (Figure 3) and the coding gene was mainly expressed in the stelar cells of the root (Figure 2). Moreover, overexpressing PcNPF2.7 driven by the root stelar cell-specific promoter in Arabidopsis under NaCl stress led to a significant increase in the NO3− content in the shoots and overall salt tolerance of the plant (Figure 6b). These results indicated that the stelar-specific overexpression of PcNPF2.7 might promote the long-distance transport of NO3− from the roots to the shoots, potentially improving the salt tolerance of the plants. Studies have demonstrated that, in addition to AtNPF2.3, AtNRT1.5/AtNPF7.3, which is localized in the root xylem cells, also mediates the long-distance transport of NO3− from the roots to the shoots of A. thaliana [39]. In the present study, under NaCl stress, the expression levels of AtNPF2.3 and AtNRT1.5 in the roots of PcNPF2.7 overexpression lines were comparable to those in WT plants (Figure S5a,b). Meanwhile, the enhanced vacuolar NO3− compartmentalization in the shoots contributed to NO3− accumulation there [46]. Under NaCl stress, the expression level of AtCLCa, which is responsible for vacuolar NO3− compartmentalization [40], in the shoots of PcNPF2.7 overexpression lines was comparable to the levels in WT (Figure S5c). These results suggested that the increased NO3− content in the shoots of PcNPF2.7 overexpression lines under NaCl stress was directly triggered by the expression of PcNPF2.7, not by AtNPF2.3 and AtNRT1.5 in the roots or AtCLCa in the leaves. Taken together, these findings indicated that PcNPF2.7 is directly involved in the long-distance transport of NO3− from the roots to the shoots under salt stress.
Under higher NaNO3 stress, the NO3− content in the shoots of PcNPF2.7 overexpression lines was significantly higher than that in WT (Figure 10a). Additionally, the levels of AtNPF2.3 and AtNRT1.5 in the roots of PcNPF2.7 overexpression lines were comparable to those in WT (Figure S6a,b), suggesting that PcNPF2.7 also directly mediates the long-distance transport of NO3− from the roots to the shoots under NaNO3 stress. However, excessive cellular accumulation of NO3− can exert toxic effects on cytoplasmic metabolic activities. Interestingly, the AtCLCa levels in the shoots of PcNPF2.7 overexpression lines were significantly higher than those in WT (Figure S6c), potentially facilitating the sequestration of excessive NO3− from the cytoplasm into vacuoles in the shoots, thereby alleviating the toxic effects of excessive NO3− in the leaves of PcNPF2.7 overexpression lines.
In the present study, the stelar-specific overexpression of PcNPF2.7 significantly reduced Na+ accumulation in the shoots of plants under both NaCl and NaNO3 stresses (Figure 6c and Figure 10b). These findings revealed another potential factor contributing to the enhanced salt tolerance observed in PcNPF2.7 overexpression lines compared to WT. There is no evidence to suggest that NPF family proteins are directly involved in Na+ transport at the cellular level in plants. However, previous study found that Arabidopsis AtNRT1.5/AtNPF7.3, which mediates NO3− transport from the roots to the shoots, can affect the Na+ transporter genes AtNHX1 (involved vacuolar in Na+ compartmentation), AtSOS1 (which mainly mediates Na+ efflux in the roots under high salt conditions), and AtHKT1;1 (involved in the unloading of Na+ from xylem to parenchyma cells), thus indirectly affecting the accumulation of Na+ in plants [47]. Furthermore, mutations in the Cl− channel gene AtALMT9 can also affect the expression of AtHKT1;1 and CHX21, potentially affecting Na+ accumulation in the shoots [48]. In the current study, under NaCl or NaNO3 stress, although no significant difference in AtNHX1 levels in the shoots and AtSOS1 levels in the roots was found between the PcNPF2.7 overexpressing lines and WT (Figure 7a,b and Figure S6d,e), the AtHKT1;1 levels in the roots were significantly higher in transgenic lines compared to WT (Figure 7c), which can help to unload Na+ from xylem to parenchyma cells, thereby reducing Na+ transport to the shoots and Na+ accumulation in the shoots [43]. However, the mechanisms underlying the influence of mutations or overexpression of NO3− and other anion transporter genes on the expression of Na+ transporter genes still require further research.
5. Conclusions
This study demonstrated that PcNPF2.7 was primarily expressed in the stele tissue of the roots of the xerophyte P. cornutum, and its expression levels in the roots were significantly induced by salt stress. PcNPF2.7 overexpression in Arabidopsis driven by a stelar-specific promoter revealed that PcNPF2.7 facilitates the transport of NO3− from the roots to the shoots, and modulates Na+ accumulation in the shoots by impacting the expression of AtHKT1;1 in the roots. These results contributed to elucidating the salt tolerance mechanisms in the xerophyte P. cornutum and revealed potential candidate genes for improving salt tolerance in crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14111590/s1, Figure S1: The full-length nucleotide sequence and encoding amino acid sequence of PcNPF2.7 from P. cornutum. Red marks are the start codon (ATG) and stop codon (TGA), respectively; Figure S2: Phylogenetic analysis of PcNPF2.7 with NAXT subclass members in A. thaliana. The identifiers for the AtNPF2.1-2.7 are At3G45720, At3G45690, At3G45680, At3G45700, At3G45710, At3g45660 and At3g45650.; Figure S3: The multiple alignment of amino acid sequence of PcNPF2.7 and NPF2.7 homologs from other plant species in the Brassicaceae. AtNPF2.7 (AT3G45650), AlNPF2.7 (Accession number in GenBank: CAH8267542.1), BnNPF2.7 (Accession number: WZY98940.1), CsNPF2.7 (Accession number: XP_010514891.1) and EsNPF2.7 (Accession number: XP_006419014.1) are the NPF2.7 homologs in A. thaliana, A. lyrata subsp, Brassica napus, Eutrema salsugineum and Camelina sativa, respectively. The red lines indicate the transmembrane domains of PcNPF2.7; Figure S4: RT-PCR analysis of PcNPF2.7 in transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2); Figure S5: The expression level of key genes associated with NO3− transport in 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 75 mM NaCl for 24 h. (a) The expression level of AtNPF2.3 in root. (b) The expression level of AtNRT1.5 in root. (c) The expression level of AtCLCa in shoot. Different letters on the columns indicate significant differences (p < 0.05, n = 3). All experiments were repeated three times with similar results; Figure S6: The expression level of key genes associated with Na+ and NO3− transport in 3-week-old WT and transgenic lines of Arabidopsis with the stelar-specific overexpression of PcNPF2.7 (OE1 and OE2) under 75 mM NaNO3 for 24 h. Different letters on the columns indicate significant differences (p < 0.05, n = 3). All experiments were repeated three times with similar results; Table S1: Primers used in this study.
Author Contributions
Q.M. and X.-X.W. designed experiments; P.-F.R., Z.-M.D. and F.-Z.W. performed experiments; P.-F.R., Z.-M.D., F.-Z.W., H.-R.L. and M.-M.C. analyzed data; Q.M., P.-F.R., Z.-M.D., F.-Z.W. and X.-X.W. wrote the manuscript; H.-R.L., K.-Y.H. and L.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32171677), Gansu Provincial Science and Technology Major Project (23ZDNA009), and the open project of Key Laboratory of Superior Forage Germplasm in the Qinghai-Tibetan Plateau (2025KF001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali, A.; Petrov, V.; Yun, D.J.; Gechev, T. Revisiting plant salt tolerance: Novel components of the SOS pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Sinha, P.; Singh, V.K.; Kumar, A.; Zhang, Q.F.; Bennetzen, J.L. 5Gs for crop genetic improvement. Curr. Opin. Plant Biol. 2020, 56, 190–196. [Google Scholar] [CrossRef]
- Liang, X.Y.; Li, J.F.; Yang, Y.Q.; Jiang, C.F.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Raza, A.; Zaman, Q.U.; Shabala, S.; Tester, M.; Munns, R.; Hu, Z.L.; Varshney, R.K. Genomics-assisted breeding for designing salinity-smart future crops. Plant Biotechnol. J. 2025, 23, 3119–3151. [Google Scholar] [CrossRef]
- Song, J.; Wang, B.S. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Rawat, N.; Wungrampha, S.; Singla-Pareek, S.L.; Yu, M.; Shabala, S.; Pareek, A. Rewilding staple crops for the lost halophytism: Toward sustainability and profitability of agricultural production systems. Mol. Plant 2022, 15, 45–64. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, H.S.; Li, H.J.; Bai, W.P.; Gao, Q.F.; Wu, S.D.; Yin, X.X.; Chen, Q.Q.; Shi, Y.Q.; Gao, T.G.; et al. Genomic analysis reveals phylogeny of Zygophyllales and mechanism for water retention of a succulent xerophyte. Plant Physiol. 2024, 195, 617–639. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, C.C.; Yun, P.; Yu, M.; Zhou, M.X.; Chen, Z.H.; Shabala, S. Climate-resilient crops: Lessons from xerophytes. Plant J. 2024, 117, 1815–1835. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Liu, Q.; Hepworth, S.R.; Li, P.Q.; Huang, J.; Zhang, R.X.; Ma, C.M.; Gao, T.G.; Ma, H.P.; Ke, J.; et al. ZxNHX1 from a xerophyte outperforms AtNHX1 in sequestering Na+ into vacuoles to enhance plant stress resistance and yield. Plant Biotechnol. J. 2025, 23, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.S.; Wang, Q.; Wang, A.L.; Wu, G.L.; Liu, J.Q. Interspecific delimitation and phylogenetic origin of Pugionium (Brassicaceae). J. Syst. Evol. 2010, 48, 195–206. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.T.; Yuan, J.Z.; Wang, F.Z.; Guo, H.; Xia, Z.R.; Wang, S.M.; Ma, Q. Chloride is beneficial for growth of the xerophyte Pugionium cornutum by enhancing osmotic adjustment capacity under salt and drought stresses. J. Exp. Bot. 2020, 71, 4215–4231. [Google Scholar] [CrossRef]
- Cui, Y.N.; Wang, F.Z.; Yang, C.H.; Yuan, J.Z.; Guo, H.; Zhang, J.L.; Wang, S.M.; Ma, Q. Transcriptomic profiling identifies candidate genes involved in the salt tolerance of the xerophyte Pugionium cornutum. Genes 2019, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.N.; Wang, F.Z.; Yuan, J.Z.; Guo, H.; Wang, S.M.; Ma, Q. High concentrations of sodium and chloride ions have opposing effects on the growth of the xerophyte Pugionium cornutum under saline conditions. J. Plant Nutr. Soil Sci. 2021, 184, 88–97. [Google Scholar] [CrossRef]
- Cui, Y.N.; Lin, Z.R.; Cai, M.M.; Liu, R.W.; Wang, S.M.; Ma, Q. PcCLCg is involved in the accumulation of Cl− in shoots for osmotic adjustment and salinity resistance in the Cl−-tolerant xerophyte Pugionium cornutum. Plant Soil 2023, 487, 283–298. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Zhang, C.; Chen, B.; Hui, L.; Shen, Y. Compositional and gastrointestinal prokinetic studies of Pugionium (L.). Food Chem. 2015, 186, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, F.; Yang, J. De novo assembly and analysis of the Pugionium cornutum (L.) Gaertn. transcriptome and identification of genes involved in the drought response. Gene 2017, 626, 290–297. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.Y.; Liu, R.W.; He, Z.H.; Wang, S.M.; Ma, Q. SLAH1 is involved in the long-distance transport of Cl− from roots into shoots in the Cl−-tolerant xerophyte Pugionium cornutum under salt stress. Plant Soil 2022, 479, 631–648. [Google Scholar] [CrossRef]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Gao, N.; Liu, M.; Zhang, C.; Luo, J.; Sun, Y.; Feng, Y. Nitrate transporters and mechanisms of nitrate signal transduction in Arabidopsis and rice. Physiol. Plant. 2024, 176, e14486. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; von Wirén, N. Nitrate signalling: Functions of a nitrate transceptor. Nat. Plants 2015, 1, 15021. [Google Scholar] [CrossRef]
- Claire, C.F.; Benoît, L. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 2017, 68, 3107–3113. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Tsay, Y.F.; Chiu, C.C.; Tsai, C.B.; Ho, C.H.; Hsu, P.K. Nitrate transporters and peptide transporters. FEBS Lett. 2007, 581, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Segonzac, C.; Boyer, J.C.; Ipotesi, E.; Szponarski, W.; Tillard, P.; Touraine, B.; Sommerer, N.; Rossignol, M.; Gibrat, R.M. Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell 2007, 19, 3760–3777. [Google Scholar] [CrossRef] [PubMed]
- Taochy, C.; Gaillard, I.; Ipotesi, E.; Oomen, R.; Leonhardt, N.; Zimmermann, S.; Peltier, J.B.; Szponarski, W.; Simonneau, T.; Sentenac, H.; et al. The Arabidopsis root stele transporter NPF2.3 contributes to nitrate translocation to shoots under salt stress. Plant J. 2015, 83, 466–479. [Google Scholar] [CrossRef]
- Li, B.; Qiu, J.; Jayakannan, M.; Xu, B.; Li, Y.; Mayo, G.M.; Tester, M.; Gilliham, M.; Roy, S.J. AtNPF2.5 Modulates Chloride (Cl−) Efflux from Roots of Arabidopsis thaliana. Front. Plant Sci. 2017, 7, 2013. [Google Scholar] [CrossRef]
- Li, B.; Byrt, C.; Qiu, J.; Baumann, U.; Hrmova, M.; Evrard, A.; Johnson, A.A.T.; Birnbaum, K.D.; Mayo, G.M.; Jha, D.; et al. Identification of a stelar-localized transport protein that facilitates root-to-shoot transfer of chloride in Arabidopsis. Plant Physiol. 2016, 170, 1014–1029. [Google Scholar] [CrossRef]
- Yin, H.J.; Li, M.Z.; Lv, M.H.; Hepworth, S.R.; Li, D.D.; Ma, C.F.; Li, J.; Wang, S.M. SAUR15 promotes lateral and adventitious root development via activating H+-ATPases and auxin biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ogweno, J.O.; Song, X.S.; Hu, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Detached leaves of tomato differ in their photosynthetic physiological response to moderate high and low temperature stress. Sci. Hortic. 2009, 123, 17–22. [Google Scholar] [CrossRef]
- Cao, W.H.; Liu, J.; He, X.J.; Mu, R.L.; Zhou, H.L.; Chen, S.Y.; Zhang, J.S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; Wirén, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar] [CrossRef]
- Zheng, X.; He, K.; Kleist, T.; Chen, F.; Luan, S. Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ. 2015, 38, 474–486. [Google Scholar] [CrossRef]
- Lin, S.H.; Kuo, H.F.; Canivenc, G.; Lin, C.S.; Lepetit, M.; Hsu, P.K.; Tillard, P.; Lin, H.L.; Wang, Y.Y.; Tsai, C.B.; et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Sunarpi; Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori, K.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+ starved roots for growth. EMBO J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Díaz-Rueda, P.; Müller, H.M.; Hürter, A.-L.; Al-Rasheid, K.A.S.; Marten, I.; et al. Silent S-type anion channel subunit SLAH1 gates SLAH3 open for chloride root-to-shoot translocation. Curr. Biol. 2016, 26, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; De Angeli, A. Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).